Abstract
Objective
The associations between prenatal exposure to antidepressants and preterm delivery and fetal growth restriction are controversial and poorly understood. We studied the relation between antidepressant use and these outcomes.
Methods
Analysis included women with nonmalformed infants interviewed in the Slone Epidemiology Center Birth Defects Study between 1998 and 2008. We estimated odds ratios (ORs) and 95% confidence intervals (CIs) for premature and small-for–gestational age (SGA) offsprings, adjusting for sociodemographic, lifestyle, medical, and reproductive factors.
Results
The frequencies of preterm delivery were 7.3% among the 5710 nonusers (reference), 8.9% among the 192 selective serotonin reuptake inhibitor (SSRI) users (OR, 1.1; 95% CI, 0.6–2.0), and 15.3% among the 59 non-SSRI antidepressant users (OR, 2.2; 95% CI, 1.0–4.9); the respective frequencies of delivering an SGA offspring were 7.2%, 10.9% (OR, 1.7; 95% CI, 1.0–2.7), and 13.6% (OR, 2.2; 95% CI, 1.0–4.9). Compared with nonusers, the frequencies of preterm delivery (7.6%) and SGA offspring (5.7%) were not increased among the 106 women who discontinued SSRIs before the end of the first trimester. Among women who continued SSRIs beyond the first trimester, 10.5% delivered a preterm infant (OR, 1.3; 95% CI, 0.6–2.8) and 17.4% had an SGA offspring (OR, 3.0; 95% CI, 1.7–5.5).
Conclusions
Women treated with SSRIs late in pregnancy had a higher frequency of delivering SGA infants, and women receiving non-SSRI antidepressants were more likely to deliver premature and SGA offsprings. The findings suggest an effect of underlying mood disorder or an effect common to both drug classes. In any case, prenatal antidepressant use may help identify women at elevated risks of delivering preterm and SGA infants.
Keywords: antidepressants, preterm delivery, fetal growth restriction, epidemiology
It is estimated that the prevalence of depression during pregnancy is 7% to 13%,1 and 3% to 13% of pregnant women are treated with antidepressants, most commonly selective serotonin reuptake inhibitors (SSRIs).2–4 Antidepressants can effectively control mood and reduce the risks of serious consequences associated with untreated depression for both the mother and her offspring.5 However, use of antidepressants during pregnancy has been associated with adverse pregnancy outcomes. First trimester exposure to certain SSRIs has been associated with some specific birth defects,6–8 whereas use of SSRI and non-SSRI antidepressants, particularly later in pregnancy, has been associated with preterm delivery,9–14 low birth weight,11,12 birth weight small for gestational age (SGA),15,16 gestational hypertension and preeclampsia,17 and various neonatal complications.9–13,18–20 Evidence on the effects of maternal antidepressant use on gestational length and fetal growth, however, is conflicting,21 as some studies did not find such effects.22–27
Although not as dramatic as major birth defects, preterm delivery and fetal growth restriction are leading causes of perinatal mortality and morbidity28,29 and are associated with enormous societal burdens.30 Previous studies often had certain limitations,21 including small sample sizes that limit the power to detect an effect,25–27 inadequate control of potential confounding by lifestyle factors such as cigarette smoking and alcohol intake,10,12,15,26 limited information on exposure timing,10 or potential selection bias with the inclusion of women who actively sought reproductive safety information regarding antidepressant use and whose risks of adverse perinatal outcomes might be different from other antidepressant users.9,22–24,26 This study was undertaken to assess the relation between antidepressant use and both preterm delivery and SGA.
Materials and Methods
Study Population
We used data from the Slone Epidemiology Center Birth Defects Study (BDS), a multi-center case-control surveillance program of birth defects in relation to environmental exposures, particularly medications. Since its inception in 1976, the BDS has interviewed more than 35,000 mothers of babies with and without birth defects from the greater metropolitan areas of Philadelphia, San Diego, and Toronto and selected regions in Iowa, Massachusetts, and New York state. Study subjects are identified through review of admissions and discharges at major birth and pediatric referral hospitals and clinics, logbooks in newborn intensive care units, through weekly telephone contact with collaborators at newborn nurseries in community hospitals, and through collaborations with state birth defects registries. Since 1998, the study has also included a random sample of Massachusetts births. Institutional review board approval is obtained, as appropriate, from each of the participating institutions, and mothers provide informed consent before participation. We restricted our analyses to 6026 women, ascertained at either the hospital-based centers or the Massachusetts birth registry, who gave birth to nonmalformed live-born infants between 1998 and 2008.
Exposure Ascertainment and Definitions
Within 6 months of delivery, trained study nurses unaware of study hypotheses conduct a 45- to 60-minute telephone interview of the study mothers. The interview covers the period 2 months before through the end of pregnancy and includes information on demographic, reproductive, and medical factors; cigarette smoking; alcohol consumption; occupational exposures; and dietary intake. It also uses a series of increasingly detailed questions to collect information on medications (prescription, over the counter, vitamins/minerals, and herbal products) used anytime from 2 months before conception through the pregnancy.31 Standardized questions prompt women recall with a list of indications and specific conditions (eg, depression) and specific drugs (eg, Prozac [fluoxetine; Eli Lilly, Indianapolis, Ind]). When possible, reported medications are verified by asking the subject to read the information from the medication container. Identification of timing of drug exposure is facilitated by use of a 12-month calendar covering periods before and after pregnancy; special dates (eg, last menstrual period and holidays) are marked to help enhance recall. Data are collected on starting and stopping dates, duration, frequency, indication, form, and number of pills per day. The interview also elicits information on last menstrual period, whether based on maternal recall or ultrasound examination, which allows estimation of the approximate conception date and gestational age at birth.
We considered these women to be a retrospective cohort of completed pregnancies. Among them, we identified women who reported using SSRI and non-SSRI antidepressants (including tricyclic antidepressants [TCAs], serotonin norepinephrine reuptake inhibitors [SNRIs], and other antidepressants) at 2 months before pregnancy. We separately categorized users of each group of antidepressants into those who stopped their treatment before the end of the first trimester and those who continued to use their medications after the first trimester. Women who used both SSRIs and non-SSRI antidepressants (n = 18), and those who initiated antidepressive medications during pregnancy (n = 47), were excluded, resulting in 5961 eligible women.
Outcome Ascertainment and Definition
Outcomes of interest were preterm delivery (gestational age at birth <37 completed weeks) and SGA (birth weight <10th percentile for gestational age). Gestational age is estimated from last menstrual period, whereas birthweight is self-reported by the mothers. The sex-specific percentiles used to classify SGA are obtained from a United States reference.32
Statistical Analysis
We compared the risks of preterm delivery and SGA among women with different antidepressant exposure categories using as the reference group women with no antidepressant use from 2 months before pregnancy through delivery. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using logistic regression models. Potential confounders, listed in Table 1, were taken into account, as were region and birth year. We retained in the multivariable models maternal age and race/ethnicity and variables that changed the estimates of anti-depressant use by 10% or more in bivariate models. We also examined the associations according to the sex of the baby, parity (nulliparity and multiparity), maternal prepregnancy body mass index (<25 and ≥25 kg/m2), and cigarette smoking (smoking and no smoking during pregnancy).
Table 1. Maternal Characteristics by Antidepressant Use During Pregnancy (Slone Epidemiology Center BDS, 1998–2008).
| Characteristics | Nonantidepressant Users (n = 5,710)* | SSRI Users (n = 192) | Non-SSRI Antidepressant Users (n = 59)* | |
|---|---|---|---|---|
| Discontinuers (n = 106)* | Continuers (n = 86)* | |||
| Maternal age, yr | ||||
| <25 | 1081 (18.9) | 23 (21.7) | 13 (15.1) | 5 (8.5) |
| 25–29 | 1331 (23.3) | 26 (24.5) | 16 (18.6) | 15 (25.4) |
| 30–34 | 2032 (35.6) | 27 (25.5) | 26 (30.2) | 22 (37.3) |
| ≥35 | 1238 (21.7) | 29 (27.4) | 30 (34.9) | 16 (27.1) |
| Maternal race/ethnicity | ||||
| Non-Hispanic White | 4100 (71.8) | 87 (82.1) | 75 (87.2) | 49 (83.1) |
| Hispanic | 772 (13.5) | 7 (6.6) | 5 (5.8) | 3 (5.1) |
| African American | 410 (7.2) | 8 (7.6) | 4 (4.7) | 3 (5.1) |
| Other | 426 (7.5) | 4 (3.8) | 2 (2.3) | 4 (6.8) |
| Maternal education, yr | ||||
| ≤12 | 1533 (26.9) | 32 (30.2) | 22 (25.6) | 15 (25.4) |
| 13–15 | 1364 (23.9) | 27 (25.5) | 26 (30.2) | 15 (25.4) |
| >15 | 2810 (49.2) | 47 (44.3) | 38 (44.2) | 29 (49.2) |
| Married or living with baby's father | 5125 (89.8) | 90 (84.9) | 78 (90.7) | 50 (84.8) |
| Family income | ||||
| <45,000 US dollars/yr | 1565 (27.4) | 33 (31.3) | 28 (32.6) | 14 (23.7) |
| ≥45,000 US dollars/yr | 3673 (64.3) | 65 (61.3) | 54 (62.8) | 41 (69.5) |
| Diabetes mellitus | 283 (5.0) | 5 (4.7) | 6 (7.0) | 4 (6.8) |
| Prepregnancy hypertension | 137 (2.4) | 2 (1.9) | 2 (2.3) | 4. (6.8) |
| Age at menarche, yr | ||||
| <12 | 1040 (18.2) | 15 (14.2) | 16 (18.6) | 9 (15.3) |
| ≥12 | 4455 (78.0) | 89 (84.0) | 67 (77.9) | 50 (84.8) |
| Fertility treatment | 391 (6.9) | 6 (5.7) | 11 (12.8) | 5 (8.5) |
| No. fetuses | ||||
| Single | 5549 (97.2) | 104 (98.1) | 81 (94.2) | 58 (98.3) |
| 2 or more | 161 (2.8) | 2 (1.9) | 5 (5.8) | 1 (1.7) |
| Male sex of the baby | 2838 (49.7) | 52 (49.1) | 45 (52.3) | 20 (33.9) |
| Gravidity | ||||
| Primigravidae | 1763 (30.9) | 33 (31.1) | 23 (26.7) | 18 (30.5) |
| Multigravidae | ||||
| No history of abortion/miscarriages/stillbirths | 3433 (60.1) | 67 (63.2) | 48 (55.8) | 38 (64.4) |
| History of abortion/miscarriages/stillbirths | 514 (9.0) | 6 (5.7) | 15 (17.4) | 3 (5.1) |
| Illicit dug use | 100 (1.8) | 3 (2.8) | 4 (4.7) | 3 (5.1) |
| Other psychotherapeutic drug use | 28 (0.5) | 6 (5.7) | 4 (4.7) | 9 (15.3) |
| Prepregnancy body mass index, kg/m2 | ||||
| <18.5 | 266 (4.7) | 11 (10.4) | 5 (5.8) | 3 (5.1) |
| 18.5–24.9 | 3574 (62.6) | 59 (55.7) | 48 (55.8) | 23 (39.0) |
| 25.0–29.9 | 1127 (19.7) | 19 (17.9) | 18 (20.9) | 16 (27.1) |
| ≥30 | 644 (11.3) | 16 (15.1) | 15 (17.4) | 14 (23.7) |
| Smoking during pregnancy | ||||
| Never smoked | 3392 (59.4) | 42 (39.6) | 39 (45.4) | 26 (44.1) |
| Past smoker | 1416 (24.8) | 28 (26.4) | 24 (27.9) | 8 (13.6) |
| Smoked <10/d during pregnancy | 447 (7.8) | 18 (17.0) | 9 (10.5) | 9 (15.3) |
| Smoked ≥10/d during pregnancy | 455 (8.0) | 18 (17.0) | 14 (16.3) | 16 (27.1) |
| Alcohol intake during pregnancy | ||||
| Never drank | 2640 (46.2) | 43 (40.6) | 35 (40.7) | 22 (37.3) |
| Past drinker | 2892 (50.7) | 58 (54.7) | 45 (52.3) | 36 (61.0) |
| Drank during pregnancy | 178 (3.1) | 5 (4.7) | 6 (7.0) | 1 (1.7) |
| Coffee drinking during pregnancy | ||||
| Never drank | 2832 (49.6) | 51 (48.1) | 28 (32.6) | 23 (39.0) |
| Past drinker | 2100 (36.8) | 43 (40.6) | 45 (52.3) | 23 (39.0) |
| Drank during pregnancy | 778 (13.6) | 12 (11.3) | 13 (15.1) | 13 (22.0) |
| Weight gain, mean (SD), lb | 33.4 (14.8) | 34.0 (16.5) | 37.0 (17.9) | 28.3 (20.7) |
| Gestational age, mean (SD), wk | 39.3 (1.9) | 39.4 (1.8) | 38.7 (1.7) | 38.9 (2.5) |
| Birth weight, mean (SD), g | 3411 (563) | 3372 (533) | 3246 (622) | 3240 (722) |
Numbers might not add up due to missing data. Numbers in parentheses represent proportions of all women in each category; independent categories were created for women with missing values.
Nonusers were women who were not exposed to any antidepressants from 2 months before pregnancy through delivery; SSRI discontinuers were women who used only SSRIs before pregnancy but stopped taking them after the first trimester; SSRI continuers were women who used only SSRIs before pregnancy and continued to use them after the first trimester; non-SSRI antidepressant users included users of SNRIs, TCAs, or other antidepressants.
Results
Of the 5961 women, 5710 had no exposures to antidepressants from 2 months before conception through delivery. At 2 months before pregnancy, 192 women were using only SSRIs; 106 of them discontinued their medication before the end of the first trimester (SSRI discontinuers), and 86 remained on their treatment beyond the first trimester (SSRI continuers). Most women maintained their exposure status after the first trimester: In later trimesters, 12 SSRI discontinuers (11.3%) restarted their treatment and 7 SSRI continuers (8.1%) stopped taking their medications. Among 59 women with prepregnancy exposure to non-SSRI antidepressants only, 17 used SNRIs. Owing to small numbers, non-SSRI antidepressant users were not analyzed separately by their treatment continuation status.
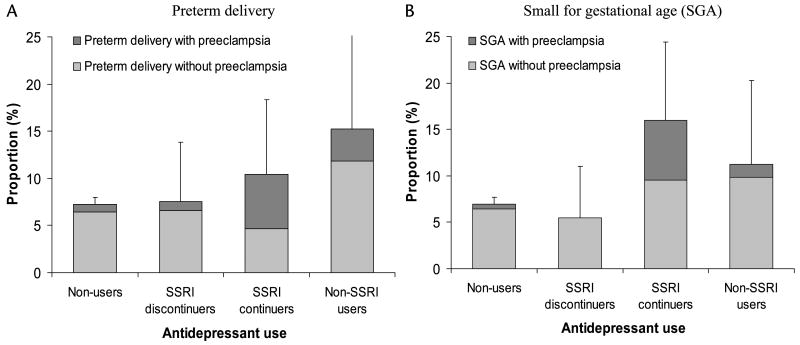
In crude analyses, compared with women who did not use antidepressants, there was no indication that SSRI discontinuers had a greater frequency of delivering a premature or SGA offspring, but SSRI continuers and non-SSRI antidepressant users had a higher frequency of having infants with both outcomes (Fig. 1). A larger proportion of women giving birth to babies with these outcomes also had preeclampsia during pregnancy. When we considered gestational length as a continuous outcome, it was decreased by a mean of 4.5 days (95% CI, 1.6– 7.3) for SSRI continuers compared with nonusers.
Figure 1.
Risks of preterm delivery (A) and SGA (B), with and without preeclampsia, according to antidepressant use during pregnancy (Slone Epidemiology Center BDS, 1998–2008). Nonusers (n = 5710) were women who were not exposed to antidepressants from 2 months before pregnancy through delivery; SSRI discontinuers (n = 106) were women who used only SSRIs before pregnancy but stopped taking them after the first trimester; SSRI continuers (n = 86) were women who used only SSRIs before pregnancy and continued to use them after the first trimester; non-SSRI antidepressant users (n = 59) included users of TCAs, SNRIs, or other antidepressants. The error bars represent the upper limits of the 95% confidence intervals for the risks.
Younger maternal age, nonwhite race, prepregnancy hypertension, smoking at least 10 cigarettes per day during pregnancy, use of other psychotherapeutic medications, and multiple gestations were associated with an increased risk of preterm delivery or SGA and also changed the effect estimates of antidepressants by more than 10% in bivariate models. As shown in Table 2, adjusting for these potential confounders modestly changed the ORs associated with SSRI use. Compared with no use of antidepressants, use of non-SSRI antidepressants was associated with increased risks of both preterm delivery and SGA after adjusting for potential confounders.
Table 2. Risks of Preterm Delivery and SGA According to Antidepressant Use During Pregnancy (Slone Epidemiology Center BDS, 1998–2008).
| No. Cases (%) | Crude OR (95% CI) | Adjusted OR* (95% CI) | |
|---|---|---|---|
| Preterm delivery | |||
| Nonusers | 415 (7.3) | Reference | Reference |
| Users | |||
| SSRIs | 17 (8.9) | 1.24 (0.75–2.06) | 1.12 (0.64–1.95) |
| Discontinuers | 8 (7.5) | 1.04 (0.50–2.16) | 1.01 (0.47–2.19) |
| Continuers | 9 (10.5) | 1.49 (0.74–3.00) | 1.27 (0.59–2.76) |
| Non-SSRI antidepressants | 9 (15.3) | 2.30 (1.12–4.70) | 2.23 (1.02–4.88) |
| SGA | |||
| Nonusers | 408 (7.1) | Reference | Reference |
| Users | |||
| SSRIs | 21 (10.9) | 1.60 (1.00–2.54) | 1.68 (1.03–2.74) |
| Discontinuers | 6 (5.7) | 0.78 (0.34–1.79) | 0.81 (0.35–1.90) |
| Continuers | 15 (17.4) | 2.75 (1.56–4.84) | 3.01 (1.65–5.47) |
| Non-SSRI antidepressants | 8 (13.6) | 2.04 (0.96–4.33) | 2.20 (0.99–4.90) |
Nonusers (n = 5710) were women who were not exposed to antidepressants from 2 months before pregnancy through delivery; SSRI discontinuers (n = 106) were women who used only SSRIs before pregnancy but stopped taking them after the first trimester; SSRI continuers (n = 86) were women who used only SSRIs before pregnancy and continued to use them after the first trimester; non-SSRI antidepressant users (n = 59) included users of TCAs, SNRIs, or other antidepressants.
Adjusted for maternal age, race/ethnicity, prepregnancy hypertension, cigarette smoking, use of other psychotherapeutic drugs, and number of fetuses.
When we further classified non-SSRI antidepressants by their specific drug classes, the crude ORs for preterm delivery were 2.13 (95% CI, 0.89–5.08) for TCAs and 2.32 (95% CI, 0.51–10.50) for SNRIs; the ORs for SGA were 1.37 (95% CI, 0.49–3.85) for TCAs and 5.78 (95% CI, 1.77–18.84) for SNRIs. In general, there was no strong evidence suggesting that the effects of antidepressants on these outcomes varied between male and female infants or by maternal prepregnancy body mass index and smoking status (data not shown), but our statistical power to assess effect modification was low. Results did not change when we restricted the analysis to singleton births.
Discussion
In this study, women who were using SSRIs at the outset of pregnancy but stopped before the end of the first trimester did not have elevated risks of delivering either a premature or SGA offspring; by contrast, women who continued use of SSRIs later in pregnancy had an increased risk of delivering SGA infants, and women who used non-SSRI antidepressants had an increased risk of both preterm delivery and SGA. Our findings were consistent with some,9–12,14–16 but not all,22–27 previous studies. The more recently introduced SNRIs may have similar effects as SSRIs.33 In our crude analysis, the ORs associated with SNRIs appeared to be greater than that of SSRIs and TCAs, but we did not have sufficient numbers of exposed cases to examine the outcomes associated with these medications in further detail.
Comparability of Users and Nonusers of Antidepressants
Compared with previous studies, we were able to more comprehensively consider potentially important confounders related to socioeconomic status and lifestyle behaviors. Furthermore, we extended our analyses to focus on the clinically important question faced by women with depression and their health care providers, that is, stop or continue antidepressant treatment during pregnancy. Our findings suggest increased risks of SGA for SSRI continuers and increased risks of both preterm delivery and SGA for users of non-SSRI antidepressants. Although drug exposure may account for these results, it is also possible that characteristics associated with such exposures might at least partially explain our findings. Antidepressant users differed from nonusers with respect to several known risk factors for the study outcomes (Table 1). Although SSRI discontinuers and continuers did not differ in most baseline variables, these women might still differ in other unmeasured characteristics that might explain our findings.
Confounding by Depression
Confounding by depression deserves special attention because the condition itself may be associated with study outcomes,34–36 possibly through mechanisms that involve hyperactivity of the hypothalamic-pituitary-adrenal axis and increased release of corticotropin-releasing hormone from the placenta.5 Few studies measured depression severity during pregnancy. In a study of 62 women,25 among those with mild/moderate depression, gestational age at birth was similar between babies with and without prenatal fluoxetine exposure; mean birthweight was lower for babies born to treated women than to untreated women but was similar to those born to women without depression. In another study37 that measured monthly depression and stress status of 90 pregnant women, those who took antidepressants for at least 50% of their pregnancy for their major depressive disorder had a higher risk of preterm delivery (14%) compared with similarly depressed women with little or no antidepressant exposure during pregnancy (0%) and with women with no psychiatric history (5.3%); birthweight was similar among these 3 groups. These findings suggest that there may be an effect of antidepressants independent of underlying depression, but the sample size was too small to draw definitive conclusion.
Because we did not identify mood disorders in study participants who did not report drug treatment, we were unable to compare antidepressant use with nonuse among women with depression. However, although it might appear that comparing use and nonuse among women with depression would be ideal to separate the effect of antidepressants from underlying depression, the severity of depression may well differ between users and nonusers of antidepressants, resulting in residual confounding. Similarly, comparisons among women treated with different antidepressive agents or even among continuers and discontinuers of the same drug might be confounded by depression severity. Women in our cohort who continued antidepressant treatment after learning about their pregnancy might have had a more severe depression, but they might also have had better control of their symptoms. Thus, such comparisons might at best reduce but not eliminate confounding by depression severity.
Several studies have found increased risks of preterm delivery or low birthweight among both women receiving SSRIs and those receiving other antidepressants during pregnancy.10,11 These findings, like ours, could be interpreted either as reflecting similar effects of different drug classes or as confounding by underlying depression. Unfortunately, without randomization or information on depression severity, one cannot distinguish between these 2 scenarios.
Information Bias
Studies10,12,13,15 using prescribing or dispensing data might not capture accurate information on actual use or on time of use during pregnancy, leading to potential misclassification of exposure. Our data on antidepressant use were based on drug use reported by the mothers, and timing of use was carefully collected with both interviewers and mothers unaware of the study hypotheses. However, we relied on maternal recall, which might be incomplete. We do not believe incomplete recall was a major concern because antidepressants are used on a regular basis and for nontrivial reasons. Although underreporting of antidepressant use is possible, as women might be reluctant to disclose such information, it is reassuring that the prevalence of SSRI use in our study was consistent with those reported by studies where data were prospectively collected.4,15 Gestational age and birthweight are usually remembered well by mothers during the months after delivery, and because other gestational information was carefully collected, misclassification of outcomes was therefore unlikely.
Conclusions
We found a higher risk for SGA among the offspring of women who continued SSRIs beyond the first trimester and a higher risk of both preterm delivery and SGA for women who used non-SSRI antidepressants in pregnancy. Confounding by underlying depression or severity of depression, however, cannot be ruled out. Many women need antidepressants to control their mood disorders during pregnancy, and optimal antidepressive treatment may help reduce the risks of adverse events associated with untreated depression.38 More research is needed to understand whether the risks found among antidepressant users in the current and previous studies are due to the medications themselves, the underlying conditions, or a combination of both. However, independent of whether antidepressants may play a causal role, exposure to these medications during pregnancy may help identify women at greater risks of giving birth to premature or SGA infants.
Acknowledgments
The authors thank Dawn Jacobs, RN, MPH; Fiona Rice, MPH; Rita Krolak, RN; Kathleen Sheehan, RN; Karen Bennett Mark, RN; Clare Coughlin, RN; Nastia Dynkin; Nancy Rodriguez-Sheridan; and Meghan Malone-Moses, MPH, for their assistance in data collection and computer programming and the staff of the Massachusetts Department of Public Health; the authors also thank all the mothers who participated in the study.
The study was not directly financed by any organization. Drs Mitchell, Louik, Werler, and Hernández-Dìaz were partially supported by grant R01 HD046595 from the National Institute of Child and Human Development (NICHD).
References
- 1.Bennett HA, Einarson A, Taddio A, et al. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol. 2004;103:698–709. doi: 10.1097/01.AOG.0000116689.75396.5f. [DOI] [PubMed] [Google Scholar]
- 2.Reefhuis J, Rasmussen SA, Friedman JM. Selective serotonin-reuptake inhibitors and persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354:2188–2190. 2188–2190. doi: 10.1056/NEJMc060602. author reply. [DOI] [PubMed] [Google Scholar]
- 3.Cooper WO, Willy ME, Pont SJ, et al. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol. 2007;196:544.e1–544.e5. doi: 10.1016/j.ajog.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 4.Andrade SE, Raebel MA, Brown J, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198:194.e1–194.e5. doi: 10.1016/j.ajog.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 5.Bonari L, Pinto N, Ahn E, et al. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry. 2004;49:726–735. doi: 10.1177/070674370404901103. [DOI] [PubMed] [Google Scholar]
- 6.Alwan S, Reefhuis J, Rasmussen SA, et al. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med. 2007;356:2684–2692. doi: 10.1056/NEJMoa066584. [DOI] [PubMed] [Google Scholar]
- 7.Louik C, Lin AE, Werler MM, et al. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med. 2007;356:2675–2683. doi: 10.1056/NEJMoa067407. [DOI] [PubMed] [Google Scholar]
- 8.Wogelius P, Norgaard M, Gislum M, et al. Maternal use of selective serotonin reuptake inhibitors and risk of congenital malformations. Epidemiology. 2006;17:701–704. doi: 10.1097/01.ede.0000239581.76793.ae. [DOI] [PubMed] [Google Scholar]
- 9.Chambers CD, Johnson KA, Dick LM, et al. Birth outcomes in pregnant women taking fluoxetine. N Engl J Med. 1996;335:1010–1015. doi: 10.1056/NEJM199610033351402. [DOI] [PubMed] [Google Scholar]
- 10.Davis RL, Rubanowice D, McPhillips H, et al. Risks of congenital malformations and perinatal events among infants exposed to antidepressant medications during pregnancy. Pharmacoepidemiol Drug Saf. 2007;16:1086–1094. doi: 10.1002/pds.1462. [DOI] [PubMed] [Google Scholar]
- 11.Kallen B. Neonate characteristics after maternal use of antidepressants in late pregnancy. Arch Pediatr Adolesc Med. 2004;158:312–316. doi: 10.1001/archpedi.158.4.312. [DOI] [PubMed] [Google Scholar]
- 12.Wen SW, Yang Q, Garner P, et al. Selective serotonin reuptake inhibitors and adverse pregnancy outcomes. Am J Obstet Gynecol. 2006;194:961–966. doi: 10.1016/j.ajog.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 13.Simon GE, Cunningham ML, Davis RL. Outcomes of prenatal antidepressant exposure. Am J Psychiatry. 2002;159:2055–2061. doi: 10.1176/appi.ajp.159.12.2055. [DOI] [PubMed] [Google Scholar]
- 14.Ericson A, Kallen B, Wiholm B. Delivery outcome after the use of antidepressants in early pregnancy. Eur J Clin Pharmacol. 1999;55:503–508. doi: 10.1007/s002280050664. [DOI] [PubMed] [Google Scholar]
- 15.Oberlander TF, Warburton W, Misri S, et al. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry. 2006;63:898–906. doi: 10.1001/archpsyc.63.8.898. [DOI] [PubMed] [Google Scholar]
- 16.Malm H, Klaukka T, Neuvonen PJ. Risks associated with selective serotonin reuptake inhibitors in pregnancy. Obstet Gynecol. 2005;106:1289–1296. doi: 10.1097/01.AOG.0000187302.61812.53. [DOI] [PubMed] [Google Scholar]
- 17.Toh S, Mitchell AA, Louik C, et al. Selective serotonin reuptake inhibitor use and risk of gestational hypertension. Am J Psychiatry. 2009;166:320–328. doi: 10.1176/appi.ajp.2008.08060817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chambers CD, Hernández-Díaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354:579–587. doi: 10.1056/NEJMoa052744. [DOI] [PubMed] [Google Scholar]
- 19.Kallen B, Olausson PO. Maternal use of selective serotonin re-uptake inhibitors and persistent pulmonary hypertension of the newborn. Pharmacoepidemiol Drug Saf. 2008;17:801–806. doi: 10.1002/pds.1570. [DOI] [PubMed] [Google Scholar]
- 20.Moses-Kolko EL, Bogen D, Perel J, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA. 2005;293:2372–2383. doi: 10.1001/jama.293.19.2372. [DOI] [PubMed] [Google Scholar]
- 21.Kallen B. The safety of antidepressant drugs during pregnancy. Expert Opin Drug Saf. 2007;6:357–370. doi: 10.1517/14740338.6.4.357. [DOI] [PubMed] [Google Scholar]
- 22.Pastuszak A, Schick-Boschetto B, Zuber C, et al. Pregnancy outcome following first-trimester exposure to fluoxetine (Prozac) JAMA. 1993;269:2246–2248. [PubMed] [Google Scholar]
- 23.Nulman I, Rovet J, Stewart DE, et al. Neurodevelopment of children exposed in utero to antidepressant drugs. N Engl J Med. 1997;336:258–262. doi: 10.1056/NEJM199701233360404. [DOI] [PubMed] [Google Scholar]
- 24.Kulin NA, Pastuszak A, Sage SR, et al. Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors: a prospective controlled multicenter study. JAMA. 1998;279:609–610. doi: 10.1001/jama.279.8.609. [DOI] [PubMed] [Google Scholar]
- 25.Suri R, Altshuler L, Hendrick V, et al. The impact of depression and fluoxetine treatment on obstetrical outcome. Arch Womens Ment Health. 2004;7:193–200. doi: 10.1007/s00737-004-0057-5. [DOI] [PubMed] [Google Scholar]
- 26.Cohen LS, Heller VL, Bailey JW, et al. Birth outcomes following prenatal exposure to fluoxetine. Biol Psychiatry. 2000;48:996–1000. doi: 10.1016/s0006-3223(00)00877-5. [DOI] [PubMed] [Google Scholar]
- 27.Pearson KH, Nonacs RM, Viguera AC, et al. Birth outcomes following prenatal exposure to antidepressants. J Clin Psychiatry. 2007;68:1284–1289. doi: 10.4088/jcp.v68n0817. [DOI] [PubMed] [Google Scholar]
- 28.Halbreich U. The association between pregnancy processes, preterm delivery, low birth weight, and postpartum depressions—the need for interdisciplinary integration. Am J Obstet Gynecol. 2005;193:1312–1322. doi: 10.1016/j.ajog.2005.02.103. [DOI] [PubMed] [Google Scholar]
- 29.McIntire DD, Bloom SL, Casey BM, et al. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med. 1999;340:1234–1238. doi: 10.1056/NEJM199904223401603. [DOI] [PubMed] [Google Scholar]
- 30.Petrou S, Sach T, Davidson L. The long-term costs of preterm birth and low birth weight: results of a systematic review. Child Care Health Dev. 2001;27:97–115. doi: 10.1046/j.1365-2214.2001.00203.x. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell AA, Cottler LB, Shapiro S. Effect of questionnaire design on recall of drug exposure in pregnancy. Am J Epidemiol. 1986;123:670–676. doi: 10.1093/oxfordjournals.aje.a114286. [DOI] [PubMed] [Google Scholar]
- 32.Oken E, Kleinman KP, Rich-Edwards J, et al. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lennestal R, Kallen B. Delivery outcome in relation to maternal use of some recently introduced antidepressants. J Clin Psychopharmacol. 2007;27:607–613. doi: 10.1097/jcp.0b013e31815ac4d2. [DOI] [PubMed] [Google Scholar]
- 34.Orr ST, James SA, Blackmore Prince C. Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. Am J Epidemiol. 2002;156:797–802. doi: 10.1093/aje/kwf131. [DOI] [PubMed] [Google Scholar]
- 35.Dayan J, Creveuil C, Marks MN, et al. Prenatal depression, prenatal anxiety, and spontaneous preterm birth: a prospective cohort study among women with early and regular care. Psychosom Med. 2006;68:938–946. doi: 10.1097/01.psy.0000244025.20549.bd. [DOI] [PubMed] [Google Scholar]
- 36.Li D, Liu L, Odouli R. Presence of depressive symptoms during early pregnancy and the risk of preterm delivery: a prospective cohort study. Hum Reprod. 2009;24:146–153. doi: 10.1093/humrep/den342. [DOI] [PubMed] [Google Scholar]
- 37.Suri R, Altshuler L, Hellemann G, et al. Effects of antenatal depression and antidepressant treatment on gestational age at birth and risk of preterm birth. Am J Psychiatry. 2007;164:1206–1213. doi: 10.1176/appi.ajp.2007.06071172. [DOI] [PubMed] [Google Scholar]
- 38.Cohen LS, Rosenbaum JF. Psychotropic drug use during pregnancy: weighing the risks. J Clin Psychiatry. 1998;59(suppl 2):18–28. [PubMed] [Google Scholar]