Abstract
Hyperandrogenism and hyperinsulinism have both been suggested as etiologic factors behind functional ovarian hyperandrogenism or polycystic ovary syndrome. Females with congenital adrenal hyperplasia provide a clinical model to evaluate the contribution of pre- and post-natal hyperandrogenism on ovarian structure and function.
Study Objective
To evaluate glucose tolerance, and structure and androgen production of the ovaries in young females with classic congenital adrenal hyperplasia.
Design, Setting, Participants
Cross-sectional study, including the enrollment of participants, ages 8 to 20 years, recruited from the pediatric endocrinology clinical program of a tertiary pediatric referral center.
Interventions
Ten participants had oral glucose tolerance testing, adrenal and ovarian androgen measurements, and pelvic ultrasound studies performed.
Main Outcome Measures
Presence of altered response to glucose challenge, ovarian hyperandrogenism, or presence of polycystic ovaries by ultrasound.
Results
Measurements of fasting blood glucose, post-challenge glucose, and insulin resistance were normal in this sample. There was no evidence of ovarian hyperandrogenism after adrenal suppression with dexamethasone. All participants had normal ovarian structure without evidence of polycystic ovaries.
Conclusions
Females with classic congenital adrenal hyperplasia (21-hydroxylase deficiency) and normal glucose tolerance appear to have normal ovarian structure and function during the peripubertal period.
Keywords: CAH, Ovarian structure, Glucose metabolism, PCOS
Introduction
Congenital adrenal hyperplasia (CAH) results in excess androgen production due to inherited alterations in cortisol and aldosterone pathways.1 Newborn screening programs have been developed in many parts of the United States to detect CAH and the children diagnosed through these programs are now entering adolescence. Despite therapy during childhood, full suppression of androgenic precursors is usually not possible without inducing an iatrogenic Cushing’s syndrome, or limiting growth and development.2 Therefore, appropriately diagnosed and treated individuals are exposed to a lifetime of glucocorticoid therapy and a hyperandrogenic state.
Polycystic ovary syndrome (PCOS), defined as clinical and laboratory hyperandrogenism with menstrual dysfunction, is the most common cause of hyperandrogenism in adolescent girls.3,4 There remains controversy as to whether hyperinsulinism or hyperandrogenism is the primary precipitant in the perimenarchal onset of this disorder. Impaired glucose tolerance or diabetes is present in 30–40% of women and adolescents with PCOS.5,6 The insulin resistance (IR) is prevalent in both lean and obese women with PCOS,7 and in prepubertal girls with early adrenarche.8 The prevalence of IR in PCOS and premature adrenarche suggests that hyperinsulinism may be a primary etiologic component. Classic CAH provides a model to evaluate the effects of a hyperandrogenic state on ovarian function and to examine the hypothesis that IR is an essential contributor to the development of PCOS.
Materials and Methods
Subjects
Ten girls and young women ages 8–20 years with CAH diagnosed based on ambiguous genitalia, newborn screen, ACTH stimulation testing, and/or genotyping during the first year of life participated. The subjects were recruited from the Endocrinology Clinical Program of a tertiary pediatric referral center, Children’s Hospital Boston (CHB), between September, 2004, and June, 2005. All participants were healthy and were receiving no medications, other than glucocorticoids, known to affect glucose, insulin, or androgen metabolism. Participants were not excluded for the use of oral contraceptive therapy, but no participants were currently prescribed hormonal medications. The study was approved and all subjects gave written informed consent or assent with parental consent, according to the guidelines of the Committee on Clinical Investigation at CHB.
Study Design
The study was a cross-sectional evaluation of adrenal and ovarian function, and glucose metabolism. All participants had a standard medical history and physical examination completed by a pediatric endocrinologist (AF, CG). Heights were obtained in triplicate on a stadiometer (Perspective Enterprises, Kalamazoo, Mich.). Body mass index (BMI; kg/meters squared) and body surface area (meters squared) were calculated using the measured weight and height. BMI percentile was calculated using age specific tables provided by the Centers for Disease Control (CDC), 2004. Bone age radiographs were performed on all participants under the age of 16 years. The studies were interpreted by a pediatric radiologist and reviewed by a pediatric endocrinologist (AF).
Subjects had an intravenous line placed for phlebotomy and urine was collected in the General Clinical Research Center, CHB. Baseline samples were obtained between 8 and 11 AM after an overnight fast, prior to the morning glucocorticoid dose. Subjects collected a 24-hour urine sample in the week prior to the study visit. An oral glucose tolerance test (OGTT) was performed after a 10-hour fast and 3 days of 300–400 gram carbohydrate intake, as a 1.75 g/kg oral glucose load (75 gram maximum). IR was determined using the homeostasis model assessment (HOMA-IR) method as previously described.9 HOMA-IR has been demonstrated to correlate with euglycemic clamp measures of insulin sensitivity.10
Ultrasound studies of the pelvis were performed using an Acuson Sequoia 512 (Siemens, Malvern, PA) and a variable combination of curved (4C1, 6C2, 8C2) and vector (4V1) array transducers with frequencies ranging from 2.0 to 8.0 MHz. Studies were scheduled and performed without regard to menstrual cycle phase. Transverse and sagittal images of the ovaries were acquired using a transabdominal approach in all patients. Ovarian width measurements were obtained from transverse images and length and thickness measurements were obtained from para-sagittal images. Transverse and sagittal images of the uterus, kidneys and adrenal regions were also routinely obtained. Images were stored electronically using a picture archiving and communication system (PACS; Synapse, Fujifilm Medical Systems USA, Inc., Stamford, Conn).
Two pediatric radiologists (HP, JC) independently reviewed the ultrasound images and arrived at a consensus. Ovarian volume was calculated using the formula for a prolate ellipsoid: length × width × thickness × 0.52.11 The largest measurement obtained for each ovarian dimension was recorded and used for volume calculation. The number and internal architecture of follicles ≥ 1 cm in at least one dimension was noted. Volume measurements were compared to previously published norms stratified by subject age.11,12
A dexamethasone suppression test was performed with 0.5 mg of dexamethasone given orally four times daily. FSH, LH, total and free testosterone were evaluated from a morning sample on day 5.
Assays
Glucose was measured by hexokinase reaction, and hemoglobin A1C by turbidometric analysis in the CHB chemistry laboratory on the Hitachi automated clinical analyzer, Roche, Indianapolis, IN. Insulin, LH, FSH, estradiol, and prolactin were analyzed by electrochemiluminescence immunoassay on the Elecsys analyzer, Roche. DHEA, 17-hydroxyprogesterone, and androstendione were analyzed by HPLC and then by RIA in the CHB laboratory. DHEAS was analyzed by competitive binding chemiluminescence immunoassay on Nichols Advantage analyzer, Nichols, San Clemente, CA. ACTH was measured by chemiluminescent immunoassay at ARUP laboratories, Salt Lake City, Utah. Urinary 17-ketosteroids were assessed in a 24-hour urine sample by spectrophotometry at ARUP laboratories, Salt Lake City, Utah. Urinary pregnanetriol was assessed by colorimetric gas chromatography by Esoterix Inc. laboratory services, Austin, Texas. Total testosterone was determined by HPLC tandem mass spectrometry, free testosterone by equilibrium dialysis, and SHBG by binding capacity performed at Esoterix Inc., Austin Texas.
Data Analysis
Utilizing SPSS statistical software, we tabulated standard parametric and non-parametric descriptive statistics. Data were expressed as mean ± standard deviation. We calculated the percentage of subjects meeting the dichotomous criteria with binomial 95% CI. Pearson or Spearman correlation analyses were carried out as appropriate according to the distributions of the outcome variables.
Results
Clinical characteristics are described in Table 1. Eight participants were diagnosed during the neonatal period due to ambiguous genitalia. One participant had a history of neonatal ambiguous genitalia, but was not immediately diagnosed due to lack of health care services. One participant was diagnosed initially by newborn screening evaluation. One of the participants had confirmatory genetic testing. Eight of 10 (80%) participants required corrective genital surgery. The subjects’ mean height, weight and BMI percentile values were consistent with normal standards for age and gender. The seven participants who were under age 16 years and had bone ages performed had an average bone age of 10.9 years (standard deviation 2.7) with an average chronologic age of 11.0 years (standard deviation 2.9). One participant had a bone age more than one year advanced and one participant had a bone age more than one year delayed. The remaining bone ages were within one standard deviation of the chronologic age of the participant.
Table 1.
Participant Characteristics
| Mean | Standard Deviation | Range | Normative Data | |
|---|---|---|---|---|
| Age and Body Composition | ||||
| Age (years) | 13.7 | 4.5 | 8.7–20.3 | |
| Height (cm) | 151.0 | 16.7 | 128.5–173.4 | |
| Height Percentile | 49.2 | 36.4 | 1.0–95.0 | |
| Weight (kg) | 48.4 | 15.8 | 25.2–64.5 | |
| Weight Percentile | 56.7 | 31.6 | 0.0–99.0 | |
| BMI (kg/m2) | 20.7 | 4.1 | 15.1–27.8 | |
| BMI Percentile | 56.7 | 28.4 | 2.0–98.0 | |
| Glucocorticoid Dose | ||||
| mg/kg/day | 0.45 | 0.18 | 0.24–0.83 | |
| mg/m2 | 15.01 | 6.50 | 7.88–29.36 | |
| Glucose Metabolism | ||||
| Fasting blood glucose (mmol/L) | 4.2 | 0.3 | 3.8–4.5 | <5.5 |
| 2 hour blood glucose (mmol/L) | 4.8 | 1.0 | 3.4–6.1 | <7.8 |
| Fasting Insulin (pmol/L) | 56.4 | 31.2 | 31.3–127.8 | <70 |
| HOMAIR | 1.40 | 0.87 | 0.64–3.26 | <2.0 |
| Hemoglobin A1C (%) | 5.1 | 0.2 | 4.8–5.4 | 4–6% |
| Androgen Status | ||||
| 17 hydroxyprogesterone (pmol/L) | 25883 | 108724 | <60–83025 | * |
| Androstenedione (pmol/L) | 3220 | 2763 | 419–7227 | * |
| Urinary 17-ketosteroids (mmol/day) | 13.4 | 7.9 | 3.8–27.7 | * |
| Free testosterone (pmol/L) | 7.7 | 8.4 | 1.0–24.3 | * |
BMI = body mass index in kilograms per meters squared; mg/kg/day = milligrams per kilogram per day; mg/m2 = milligrams per body surface area; HOMAIR = homeostasis model assessment for insulin resistance
Normative values vary by age and pubertal stage
Glucocorticoid Therapy and Adrenal Metabolism
The subjects’ daily glucocorticoid doses are reported in Table 1. The wide range of daily doses was consistent with an unselected sample. The baseline morning 17-hydroxyprogesterone concentrations showed large variability, with the average indicating lack of adequate adrenal suppression. However, the samples were obtained in the morning after withholding morning glucocorticoid therapy, which may have increased the morning assessment. In addition, the 24-hour urine collections for ketosteroids had only 1 of the seven samples higher than normal for age. The mean value was normal. Therefore, the data reveal variable adrenal suppression. Seven of 10 (70%) participants were receiving fludrocortisone, as well as glucocorticoid therapy. No participants had received prenatal glucocorticoid therapy.
Glucose Tolerance
All participants had normal fasting blood glucose levels and normal 2-hour values post OGTT, without evidence of diabetes or impaired glucose tolerance based on American Diabetes Association reference values.13 The fasting insulin levels were also normal in 9/10 (90%) patients. One of 10 (10%) patients had a HOMA-IR value above 2, the standard adult threshold for insulin resistance.14 This patient had an elevated fasting insulin level. The HOMA-IR and fasting insulin values correlated with the BMI percentiles (Spearman correlations of 0.749, P = 0.02 and 0.731, P = 0.025, respectively). There was no significant correlation between glucocorticoid dose and glucose homeostasis. The glucose values did not show correlations with anthropometric data or androgen levels (Table 1).
Ovarian Androgen Production
In the setting of normal glucose tolerance, the ovarian androgen production was assessed by dexamethasone suppression in 6 participants. The average decrease in free testosterone was 6.6 pmol/L and all patients suppressed free testosterone into the normal range, indicative of limited ovarian androgen production. LH values and LH:FSH ratios were not elevated at baseline or post suppression testing in any subject.
Pelvic Ultrasound Studies
Both ovaries were identified and measured in all 10 subjects (Table 2). In 8 of 10 (80%) patients ovarian volume fell within two standard deviations of the mean11,12 (Figure 1). A unilateral solid adrenal mass measuring 3.0 × 4.0 × 3.0 cm was identified in patient 6 and was thought to represent an adrenal adenoma. No other abnormality was noted. There were no patients with increased follicular structures consistent with polycystic ovaries.
Table 2.
Ovarian Structure
| Subject | Age (yrs) | Right Ovarian Volume (cc)*/Architecture | Left Ovarian Volume (cc)/Architecture | Other |
|---|---|---|---|---|
| 1 | 9 | 0.5/normal | 0.4/normal | - |
| 2 | 8 | 2.1/normal | 1.1/normal | - |
| 3 | 17 | 30.2; hemorrhagic cyst 3.9 cm greatest diameter | 5.3/normal | - |
| 4 | 20 | 4.0/normal | 8.7/normal | - |
| 5 | 15 | 6.7/normal; single follicle | 1.6/normal | - |
| 2.0 cm greatest diameter | ||||
| 6 | 18 | 5.8/normal | 9.0/normal; single follicle 1.6 cm greatest diameter | Solid, echogenic left adrenal mass 3.0 × 4.0 × 3.0 cm |
| 7 | 14 | 2.2/normal | 5.7/normal | - |
| 8 | 8 | 1.5/normal | 2.1/normal | - |
| 9 | 8 | 6.2/normal; single follicle 1.7 cm greatest diameter | 5.8/normal | - |
| 10 | 14 | 2.3/normal | 9.4/normal | - |
Volume = length × width × thickness × 0.52; cc = cubic centimeter; cm = centimeter
Fig. 1.
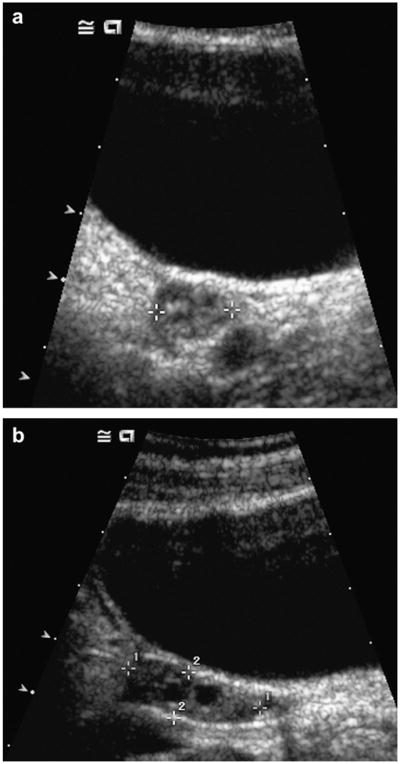
Transverse (a) and longitudinal (b) images of a representative patient demonstrate a normal appearing right ovary. Eight of ten study patients had ovarian volumes within two standard deviations of the mean for age.
Discussion
Young women with 21-hydroxylase deficiency who are exposed to a hyperandrogenic state throughout development may be predisposed to ovarian hyperandrogenism. However, with the advent of newborn screening programs in many areas, the majority of the current adolescent population in the United States will have been treated during the immediate postnatal period, optimizing suppression of adrenal metabolites. These data provide evidence that early treatment of CAH may be protective of both glucose tolerance and ovarian function.
The reported prevalence of abnormal ovarian morphology in females with CAH varies. Some reports note no increase in rates of abnormal ovarian structure over the general population, while other studies find the presence of abnormalities in up to 40% of postpubertal patients and 83% of adult patients.15–17 There is also variability in functional ovarian assessments. In previous studies, women with classic CAH were more likely to exhibit subnormal suppression of free testosterone and elevation of LH after dexamethasone.18 Those women who did not meet these criteria had been treated for CAH since early infancy. The data suggest that women with classic CAH can develop dysregulation of the hypothalamic/pituitary/ ovarian axis, which may be prevented by early and appropriate therapy. In our sample, the dexamethasone suppression test did not reveal abnormal LH secretion or evidence of ovarian hyperandrogenism, suggesting that these are not consistent findings in young women with CAH.
This study revealed normal glucose and insulin values based on OGTT testing in all subjects with the exception of hyperinsulinism in one patient. The participants had variable anthropomorphic data. Previous data on glucose metabolism in adult women with classic CAH have shown reduced insulin sensitivity when compared to both normal women and women with non-classic CAH.19 In addition, studies of children with CAH report higher fasting serum insulin levels and IR, without documenting a correlation with glucocorticoid dosage. The most reliable predictor of IR was BMI.20 Our data from individuals with a wide range of BMI suggest the lack of consistency of IR. Therefore, the questions about whether the reported increase in IR is related directly to the altered body composition in a subset of patients with CAH, and whether this can be alleviated by close monitoring of anthorpomorphic data and/or lifestyle modifications remain unresolved.
The scope of this study was limited by sample size and by its cross-sectional design. In addition, there may have been selection bias introduced due to the voluntary recruitment from a pediatric endocrinology program in a tertiary care center. These factors limit the generalizability of these findings.
In conclusion, the current data suggest that both ovarian structure and function, and glucose tolerance are normal in young women with CAH who have received treatment since infancy. The study provides evidence in support of the hypothesis that IR is a significant contributor to the development of PCOS. In addition, the findings provide optimism regarding the reproductive function and glucose homeostasis of children with CAH treated in the era of early diagnosis and therapy.
Acknowledgments
Funding: This project was supported by an investigator initiated grant from Pfizer, Inc., and the Clinical Investigator Training Program: Harvard-MIT Health Sciences and Technology—Beth Israel Deaconess Medical Center, in collaboration with Pfizer Inc. and Merck & Company, Inc., and Nitt grant M01-RR-2172 from the National Institutes of Health to Children’s Hospital Boston General Clinical Research Center.
References
- 1.Speiser PW, White PC. Congenital adrenal hyperplasia. N Engl J Med. 2003;349:776. doi: 10.1056/NEJMra021561. [DOI] [PubMed] [Google Scholar]
- 2.Migeon CJ, Wisniewski AB. Congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Growth, development, and therapeutic considerations. Endocrinol Metab Clin North Am. 2001;30:193. doi: 10.1016/s0889-8529(08)70026-4. [DOI] [PubMed] [Google Scholar]
- 3.Zawadsku JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. Blackwell Scientific Publications; 1992. [Google Scholar]
- 4.The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 5.Legro RS, Kunselman AR, Dodson WC, et al. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 6.Palmert MR, Gordon CM, Kartashov AI, et al. Screening for abnormal glucose tolerance in adolescents with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:1017. doi: 10.1210/jcem.87.3.8305. [DOI] [PubMed] [Google Scholar]
- 7.Dunaif A, Segal KR, Futterweit W, et al. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- 8.Ibanez L, de Zegher F, Potau N. Premature pubarche, ovarian hyperandrogenism, hyperinsulinism and the polycystic ovary syndrome: from a complex constellation to a simple sequence of prenatal onset. J Endocrinol Invest. 1998;21:558. doi: 10.1007/BF03350781. [DOI] [PubMed] [Google Scholar]
- 9.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 10.Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 11.Orsini LF, Salardi S, Pilu G, et al. Pelvic organs in premenarcheal girls: real-time ultrasonography. Radiology. 1984;153:113. doi: 10.1148/radiology.153.1.6473771. [DOI] [PubMed] [Google Scholar]
- 12.Cohen HL, Tice HM, Mandel FS. Ovarian volumes measured by US: bigger than we think. Radiology. 1990;177:189. doi: 10.1148/radiology.177.1.2204964. [DOI] [PubMed] [Google Scholar]
- 13.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2005;28(Suppl 1):S37. doi: 10.2337/diacare.28.suppl_1.s37. [DOI] [PubMed] [Google Scholar]
- 14.Uwaifo GI, Fallon EM, Chin J, et al. Indices of insulin action, disposal, and secretion derived from fasting samples and clamps in normal glucose-tolerant black and white children. Diabetes Care. 2002;25:2081. doi: 10.2337/diacare.25.11.2081. [DOI] [PubMed] [Google Scholar]
- 15.Hague WM, Adams J, Rodda C, et al. The prevalence of polycystic ovaries in patients with congenital adrenal hyperplasia and their close relatives. Clin Endocrinol (Oxf) 1990;33:501. doi: 10.1111/j.1365-2265.1990.tb03887.x. [DOI] [PubMed] [Google Scholar]
- 16.Salardi S, Orsini LF, Cacciari E, et al. Pelvic ultrasonography in girls with precocious puberty, congenital adrenal hyperplasia, obesity, or hirsutism. J Pediatr. 1988;112:880. doi: 10.1016/s0022-3476(88)80208-7. [DOI] [PubMed] [Google Scholar]
- 17.Stikkelbroeck NM, Hermus AR, Schouten D, et al. Prevalence of ovarian adrenal rest tumours and polycystic ovaries in females with congenital adrenal hyperplasia: results of ultrasonography and MR imaging. Eur Radiol. 2004;14:1802. doi: 10.1007/s00330-004-2329-x. [DOI] [PubMed] [Google Scholar]
- 18.Barnes RB, Rosenfield RL, Ehrmann DA, et al. Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders: evidence for perinatal masculinization of neuroendocrine function in women. J Clin Endocrinol Metab. 1994;79:1328. doi: 10.1210/jcem.79.5.7962325. [DOI] [PubMed] [Google Scholar]
- 19.Paula FJ, Gouveia LM, Paccola GM, et al. Androgen-related effects on peripheral glucose metabolism in women with congenital adrenal hyperplasia. Horm Metab Res. 1994;26:552. doi: 10.1055/s-2007-1001755. [DOI] [PubMed] [Google Scholar]
- 20.Charmandari E, Weise M, Bornstein SR, et al. Children with classic congenital adrenal hyperplasia have elevated serum leptin concentrations and insulin resistance: potential clinical implications. J Clin Endocrinol Metab. 2002;87:2114. doi: 10.1210/jcem.87.5.8456. [DOI] [PubMed] [Google Scholar]


