Abstract
Aging is reported to be associated with decline in oral tolerance induction, which is initiated at the intestinal mucosal surface. Herein, we examined the effect of aging in T cells and cytokines at the intestinal mucosa that might be involved in oral tolerance induction. Frequencies of regulatory-type IEL subsets such as TCRγδ+ and TCRαβ+CD8αα+ were lower in aged mice. Mucosal CD4+CD25+Foxp3+ and CD4+LAP+ T cells increased with aging but activated CD44+CD4+ mucosal T cells also augmented. Production of TGF-β and IL-10 in the small intestine of old mice was reduced. Moreover, the ability of mucosal dendritic cells of aged mice to stimulate TGF-β secretion and differentiation of CD4+LAP+ T cells in co-culture studies also declined with aging. Reduction in these regulatory-type cytokines and T cells may help to explain the decline in susceptibility to oral induction during aging. However, not all mucosal regulatory elements were altered by aging and CD4+CD25+Foxp3+ T cells were especially resistant to changes. Persistence of some mechanisms of regulation might play a critical role in maintaining mucosal homeostasis during aging.
Keywords: aging, dendritic cells, intra-epithelial lymphocytes, oral tolerance, TGF-β, IL-10
INTRODUCTION
The gut mucosa is a major route for foreign antigens to contact the immune system. A large and regular amount of dietary antigens reach the gut daily, and a continuous exposure to the autochthonous microbiota provides additional stimulation to the abundant lymphoid tissue located in the intestinal mucosa (Brandtzaeg et al, 1998). This daily antigenic contacts play an important role in the development of the immune system after weaning (Menezes et al, 2003). Antigenic contact initiated by oral route is known to induce oral tolerance, a state of systemic suppression of specific immune responses to subsequent parenteral injections of the same antigen (Vaz et al, 1977; Mowat 2003; Faria and Weiner, 2005). Many subsets of lymphocytes with regulatory phenotype such as CD4+LAP+ and CD4+CD25+Foxp3+ have been described to play a critical role in oral tolerance induction (Faria e Weiner, 2005; Curotto de Lafaille et al, 2008). Intraepithelial cells seem also to be involved in oral tolerance induction due to their ability to produce non-inflammatory and regulatory cytokines such as TGF-β and IL-10 which play an important role in the gut homeostasis (Ke et al, 1997; Saurer et al, 2004).
Oral tolerance is one of many immune functions altered by the aging process. In fact, aging brings about several changes in the immune system. It affects drastically the T cell compartment, cytokine production, antigen-specific antibody responses (Linton and Dorshkind, 2004; Speziali et al, 2009; Santiago et al, 2008) and the composition of lymphoid organs such as gut Peyer’s patches (Kato et al, 2003). Interestingly, development of aging alterations occurs earlier in the mucosal immune system than in the systemic immune compartment (Koga et al, 2000). Our group has previously shown that the two main immunological events initiated at the gut surfaces, IgA production and oral tolerance induction, are differentially affected by the aging process. Susceptibility to oral tolerance decreases with age. Mice that is susceptible to oral tolerance induction by a single feeding of antigen at 8 weeks of age become less susceptible at 24 weeks and totally refractory at 70 weeks of age (Faria et al, 1993). Only a regimen of continuous feeding is able to render 70-week-old mice tolerant to orally administered antigen (Faria et al, 1998; Faria et al, 2003). On the other hand, production of secretory IgA (S-IgA), the most abundant immunoglobulin at mucosal surfaces, is unaltered in old mice (Santiago et al, 2008).
Few accounts are available evaluating immunological parameters such as cell types and cytokine production at the mucosal surfaces during the aging process. Since many regulatory-type elements exist in the gut environment, changes in these cellular and molecular components may be involved in the age-related decline of local immune functions such as oral tolerance induction. Among gut-associated T cells, there seem to be a decrease in CD45+ T cells as well as an increase in intestinal NK1.1+ T cells and double-positive CD4+CD8+ IEL population of aged mice (Ishimoto et al, 2004; Hayashi et al, 2009). Aging-related alterations on dendritic cell (DC) phenotype and function have also been investigated, but the results are still controversial. One study demonstrated that DCs derived from peripheral blood of humans do not show significant changes in phenotype or function with aging (Steger et al, 1996). Another report did not find changes in the percentage on spleen DC expressing CD80 and CD86 in studies conducted using senescence-accelerated prone mice (Hanura et al, 1995). Simioni and co-workers observed an age-related reduction in CD86 expression in spleen DCs, but not in the frequency of cells expressing this molecule (Simioni et al, 2010).
In addition to the paucity of studies that addressed the effects of aging in the mucosal associated lymphoid tissue, the impact of aging in different compartments and regions of the gut-associated lymphoid compartment is not known. Our aim in this study was to perform a systematic evaluation of several regulatory-type cells and cytokines usually present in this tissue at different ages to study the impact of aging in function of mucosal associated lymphoid tissue. Our hypothesis is that putative alterations in immune elements in the gut may help to explain the decreased susceptibility to oral tolerance induction during aging.
MATERIAL AND METHODS
Animals
Female TCR OVA-specific transgenic (DO11.10) and BALB/c mice at ages ranging from 2 to 24 months were obtained from Centro de Bioterismo (CEBIO, Instituto de Ciências Biológicas, UFMG, Belo Horizonte, Brazil) and maintained in our experimental animal facility throughout the experiments. Mice were kept in micro-isolators; autoclaved diet and water were offered ad libidum. All animal procedures were approved by the local ethical committee for animal research (Protocol # 115/2007, CETEA-UFMG, Brazil).
Analysis of Ig isotypes by ELISA
Levels of total immunoglobulins were determined by ELISA. Briefly, 96-well plates (NUNC, Roskilde/Denmark) were coated with 0.1μg goat anti-mouse UNLB antibody, in coating buffer pH 9.8 overnight. Wells were washed and blocked with 200μl of PBS contain 0.25% casein for 1 h at room temperature. Sera were added to the plate and incubated for 1 h at room temperature, plates were washed, then peroxidase- streptavidin goat anti-mouse or rat anti-goat (SOUTHERN BIOTECHNOLOGY, Birmingham, AL/USA) 1:15000 was added, and plates were incubated for 1 h at 37°C. Color reaction was developed at room temperature with 100μl/well of orthophenylenediamine (1mg/ml) (SIGMA), 0.04% H2O2 substrate in sodium citrate buffer. Reaction was interrupted by the addition of 20 μl/well of 2 N H2SO4. Absorbance was measured at 492nm by an ELISA microplate reader (BIO-RAD Model 450, Hercules/CA/USA).
Histomorphometric Analysis
Small intestine was removed and immersed in 10% formaldehyde buffer; tissues were further dehydrated with alcohol-containing solutions using an automatic tissue processor (TITERTEK, Huntsville, AL/USA). Gut tissues were then included in paraffin and 4μm transverses sections obtained by Spencer microtome (SPENCER SCIENTIFIC Co, Derry, NH/USA). Tissues were stained with eosin & hematoxylin and morphologic profile determined using an Olympus microscope. (OLYMPUS, Center Valley, PA/USA). Villous length was measured using millimeter laminule. IEL cells numbers were calculated by manually counting cells in 10 villous and expressed as ratios of IEL/100 epithelial cells as described by Ferguson and Murray (Ferguson and Murray, 1971).
Cell preparation and cytokine assay
Small intestine was separated into duodenum, proximal jejunum, distal jejunum and ileum and placed in buffer solution (1ml/g) containing 10.000UIC/ml of aprotinin. Tissue fragments were homogenized and centrifuged for 15 minutes 600g at 4°c. Supernatants were collected for cytokine assay. Plates were coated with purified monoclonal antibodies reactive with cytokines IL-4, IFN-γ, IL-10 and TGF-β (BD-PHARMINGEN, San Jose, CA/USA) overnight at 4°C. In the following day, wells were washed and supernatants were added and plate was incubated overnight at 4°C. In the third day, biotinylated monoclonal antibodies against cytokines are added and plate was incubated for 1 hour at room temperature. Color reaction was developed at room temperature with 100μl/well of orthophenylenediamine (1mg/ml), 0.04% H2O2 substrate in sodium citrate buffer. Reaction was interrupted by the addition of 20 μl/well of 2 N H2SO4. Absorbance was measured at 492nm by an ELISA reader (BIO-RAD Model 450). Cytokines were also determined in the supernatants of co-cultures of T and dendritic cell (DC)-enriched cells using the same methodology.
T cell and DC separation
Spleen T cell populations were obtained from 8-week-old naive DO11.10 transgenic mice. Spleen and mesenteric lymph nodes DC population were obtained from young (2-month-old) and aged (12-month-old) BALB/c mice. T- and DC-enriched populations were isolated using MACS micro beads (anti-Thy1 and anti-CD11c) and LS MACS Column (MILTENYI BIOTEC, Bergisch Gladbach/Germany) according to the manufacturer instructions. Enriched cell suspensions were monitored by flow cytometry. DC preparations contained approximately 60% CD11c+ cells and T cell contained approximately 95% CD3+ cells. T CD4+ cells from naive DO11.10 mice were 80-90% KJ1.26 positive.
Lymphocyte preparation and flow cytometry analysis
Small intestines and mesenteric lymph nodes were removed from euthanized mice, flushed with cold calcium and magnesium free HBSS. Payer’s patches and fat were then removed from the tissues that were incubated in HEPES-containing HBSS medium (2% HEPES buffer, 1% penicillin/streptomycin and 0,05% gentamicin). Mesenteric lymph nodes were smashed and passed through a 70μm cell strainer (BD FALCON). For intraepithelial lymphocytes (IEL) cells isolation, small intestine was washed, diced and incubated in IEL medium (RPMI containing 2% FBS, 2% HEPES buffer, 1% penicillin/streptomycin and 0.5% gentamicin) for 40min at 37°C in a shaker (150rpm). Cells were filtered through a 70μm cell strainer and supernatant containing IEL fraction kept in ice. For lamina propria (LP) cells isolation, cells were incubated with 100Ug/ml collagenase II for 40min at 37°C in a shaker (150rpm), smashed and passed through a 70μm cell strainer and then resuspended in IEL medium. Both IEL and LP cells were then centrifuged at 300g (5min. at 4°C), resuspended in 4.5ml 44% Percoll. Then, 2.3ml 67% Percoll was laid into the 15ml tube to create a Percoll gradient (BD FALCON). Cells were then centrifuged at 600g for 20min and interface layer was harvested as the lymphocyte population. Cells were stained with FITC, PE or Cy-labeled monoclonal antibodies for CD4, CD8, CD25, CD44, CD62L, LAP, αβ/γδTCR CD11c, F4/80, CD80, CD86 (BD PHARMINGEN) in 1% BSA or with permeabilization buffer (for Foxp3 labeling) and fixed in 4% formaldehyde. Fixed cells were then incubated with PE-labeled anti-Foxp3 monoclonal antibody (BD PHARMINGEN) for 30min. Cells were analyzed with a FACSCan (BECTON & DICKINSON) and data were analyzed by FlowJo (TREESTAR, Ashland, OR/USA).
Statistical analysis
Results expressed as means ± standard deviation. Differences were determined by ANOVA when more than two groups were compared and by Student’s t test when only two groups were compared. A value of p<0.05 was considered to be significant.
RESULTS
Aging affects the frequency of IEL subsets with regulatory phenotypes
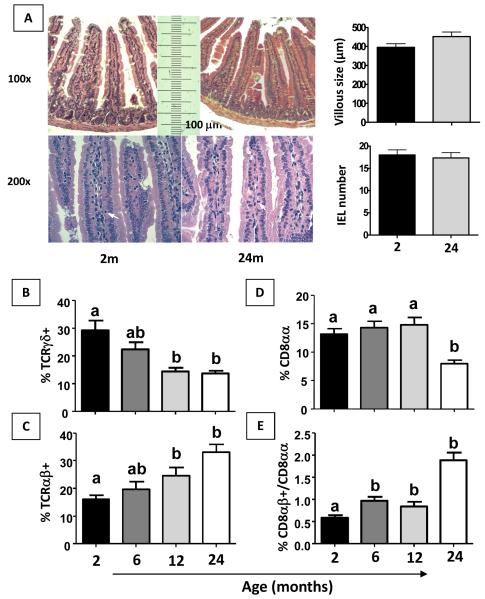
We investigated small intestine morphology in mice of 2 and 24 months of age and found no difference between these groups regarding either villous lenght or numbers of IELs (Figure 1A). We found no significant difference either in the number of cells in other lymphoid organs such as spleen, mesenteric lymph nodes and Peyer’s patches among mice of different age groups (data not shown). IELs are in the first line of mucosal surface intertwined with epithelial cells, and the main subpopulations of IEL, αβ+ and γδ+, are both described as involved in inhibition of cytotoxic T cells (CTL) (Kapp et al, 2004; Lambolez et al, 2007) although only γδ+ IELs are related to oral tolerance induction (Ke et al, 1997) and colitis modulation (Chen et al, 2002). Expression of CD8αα in TCRαβ+ IELs has also been reported as important parameter to define a subpopulation of IEL with modulatory properties (Poussier et al, 2002; Denning et al, 2007). Changes in all these sub-populations were evaluated during aging. Frequencies of TCRαβ, TCRγδ, TCRαβ+CD8αα+ and ratio TCRαβ+CD8αβ/ CD8◻α+ IEL populations were analyzed in non-manipulated 2- to 24-month old mice. There was a decrease in frequencies of αδ+ and an increase in αβ+ T cells in the IEL population in 12- and 24-month-old mice (Figure 1B and C). We also found a reduced percentage of TCRαβ+CD8αα+ IELs in mice at 24 months of age, and the ratio CD8αβ+/CD8αα+ among TCRαβ+ IELs was increased in 6- to 24-month-old mice (Figure 1 D and E).
Figure 1. TCRγδ +, TCRαβ +, CD8αβ+ and ratio CD8αβ/CD8αα IELs are altered in aged mice.
Histology of H&E stained-sections from small intestine of 2 and 24-month-old non-manipulated mice. Villous length and IEL number were evaluated. Original magnification: 100x and 200x (A). Flow cytometry and frequency of IEL isolated from 2- to 24-month old non-manipulated mice stained with fluorescent antibodies to TCRαβ+, TCRγδ+, TCRαβ+CD8αα+ and TCRαβ+CD8αβ+ were gated in total lymphocytes (B-E). Bars represent the mean ± SEM of 5 mice per group. Letters (a, b) represent differences among groups (p< 0.05).
Cytokine secretion at different portions of small intestine changes during aging
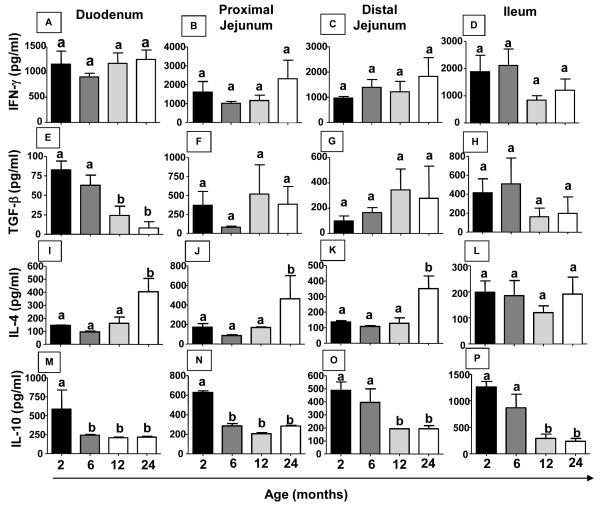
Several studies suggest an important role for cytokines in oral tolerance induction (Faria and Weiner, 2005). Therefore, cytokine secretion at different portions of the small intestine was evaluated during aging. There was no change in IFN-γ production in any region of the small intestine examined throughout life (Figure 2 A-D). However, IL-4 secretion increased in duodenum, proximal and distal jejunum but not in the ileum portion (Figure 2 I-L) in 24-month-old mice. On the other hand, production of regulatory cytokines such as TGF-β and IL-10 decreased in duodenum of aged mice (Figure 2 E and M). IL-10 secretion was also reduced in the proximal and distal jejunum as well as in the ileum portion of the small intestine of 12-24-month-old mice (Figure 2 M-P).
Figure 2. Production of cytokines in the intestinal mucosa changes during aging.
Small intestines from non-manipulated mice from 2 to 24 months of age were removed, separated into duodenum, proximal jejunum, distal jejunum, ileum and homogenized in extract buffer. Extract supernatant was collected for cytokine assay. IFN-γ(A-D), TGF-β(E-H), IL-4(I-L) and IL-10 levels (M-P) were measured by ELISA. Bars represent the mean ± SEM of 5 mice per group. Letters (a, b) represent differences among groups (p< 0.05).
Aging correlates with alterations in regulatory-type, naive and activated T cell populations
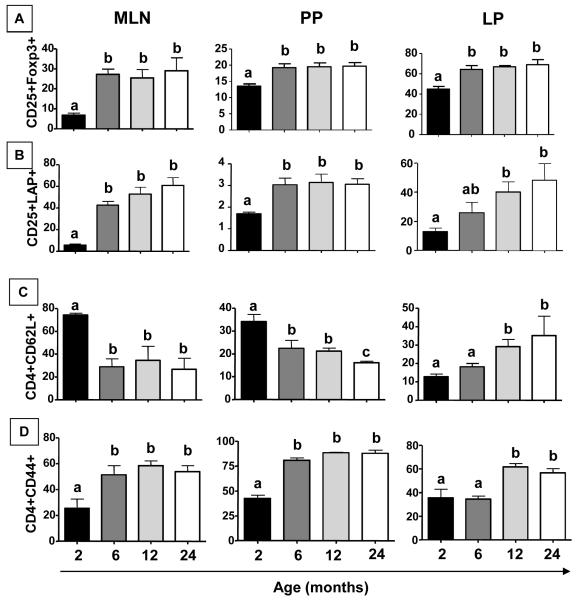
Peripherally induced CD4+CD25+FoxP3+ regulatory T cells have been described as essential for oral tolerance induction (Curotto de Lafaille et al, 2008). CD4+LAP+ T cells are also reported as a distinctive subset of regulatory T cells (Ghandi et al, 2010). Therefore, we investigated frequency of both types of regulatory T cells in non-manipulated mice of 2, 6, 12 and 24 months of age. Our data shows that frequencies of CD4+CD25+Foxp3+ and CD4+LAP+ T cells were increased in mesenteric lymph nodes (MLN), Peyer’s patches (PP) and intestinal lamina propria (LP) of 6-24 month old mice (Figure 3 A-B).
Figure 3. Aging is associated with alterations in regulatory-type, naïve and activated mucosal T cells.
Mesenteric lymph nodes and Peyer’s patches T cells were obtained from non-manipulated mice from 2 to 24 months of age. Frequencies of CD4+CD25+Foxp3+ (A), CD4+LAP+ (B) CD4+CD62L+ (C) and CD4+CD44+ (D) T cells, gated in CD4+ T cells, were assessed by flow cytometry. Bars represent the mean ± SEM of 5 mice per group. Letters (a, b) represent difference between groups (p< 0.05).
The effect of aging in frequencies of naïve and activated T cells in secondary lymphoid organs has been described (Linton and Dorshkind, 2009). Frequencies of CD4+CD44+ and CD4+CD62L+ cells in MLN, PP and LP of non-manipulated mice from 2 to 24 months of age were evaluated. There was an increase in CD4+CD44+ T cells and a concomitant decrease in CD4+CD62L+ T cells in MLN and PP of 6-24-month old mice (Figure 3 C-D). However, an augment in both CD4+CD44+ and CD4+CD62L+ T cells was observed in LP of 12-24-month old mice (Figure 3 C-D).
Aging affects antigen-presenting cells in the gut
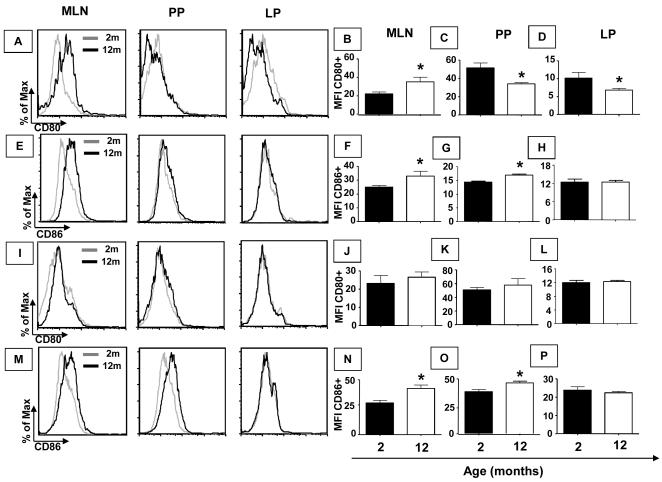
Since frequencies of activated T cells and production of T-cell-related cytokines were altered by aging, our next step was to examine whether compatible changes could be found in antigen presenting cells during aging. Oral tolerance impairment can be observed at 12 months of age (Faria et al, 1998) and most aging-related alterations can be seen before 6 months of age in mice (Ishimoto et al, 2004). Therefore, we used 2 and 12-month-old mice to study antigen presenting cells (APCs). First, expression of co-stimulatory molecules in APCs such as macrophages and dendritic cells from MLN, PP and small intestine LP compartments of non-manipulated 2- and 12-month-old mice was analyzed. A clear augment was detected in the expression of the co-stimulatory molecule CD86 in both macrophages and DCs from MLN and PP of aged mice (Figure 4 E, F, G, M, N and O). No difference was observed in the expression of CD86 in macrophages and DCs from the LP compartment of older mice (Figure 4 E, H, M and P). Expression of CD80 in macrophages from MLN of aged mice was also increased (Figure 4 A and B). In PP and LP of aged mice, expression of CD80 in macrophages was reduced (Figure 4 A, C and D). No aging-related alteration was observed in the expression of CD80 in DCs from MLN, PP and LP of aged mice (Figure 4 I-L).
Figure 4. Expression of co-stimulation molecules CD80/86 in F4/80 and CD11c+ cells is affected by aging.
Mesenteric lymph nodes, Peyer’s patches and small intestine lamina propria cells were obtained from non-manipulated mice from 2 and 12 months of age. Histogram of CD80 (A) and CD86 (E) gated in F4/80+ cells as well as CD80 (I) and CD86 (M) gated in CD11c+ cells. Bars show the median (MFI) of cells stained with fluorescent antibodies anti-CD80 (B, C and D) gated in F4/80+ cells, anti-CD86 (F, G and H) gated in F4/80+ cells, anti-CD80 (J, K and L) and anti-CD86 (N, O and P) gated in CD11c+ cells. Median (MFI) of double positive cells, gated in total granulocytes, was assessed by flow cytometry. Bars represent the mean ± SEM of 5 mice per group (*p< 0.05).
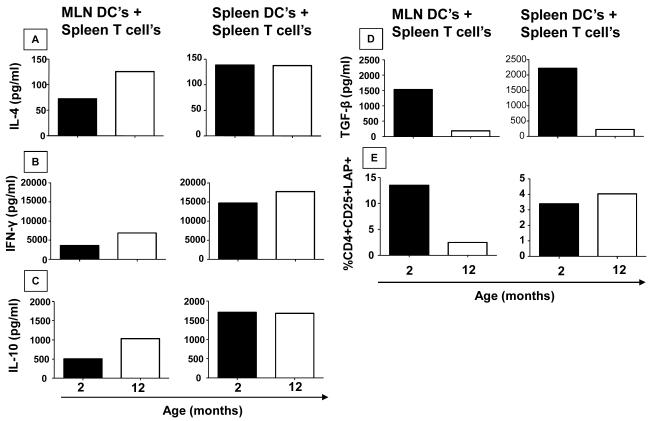
Dendritic cells were reported to be less affected by aging than macrophages (Kovacs et al, 2009). At the same time, they play a key role in oral tolerance induction (Viney et al, 1998; Fleeton et al, 2004). Thus, antigen presenting function of MLN DCs were examined in young (2 months) versus aged (12 months) mice. Spleen and MLN DCs from both spleen and mesenteric lymph nodes of young and old mice were co-cultured with spleen T cells from young DO11.10 mice. Since these T cells are mostly OVA-specific (80-90%), cultures containing OVA and different DCs would provide a method to discriminate their functional abilities as antigen presenting cells (APCs). Production of cytokines and induction of LAP+CD25+CD4+ Treg cells were used as a read out of APC function. Cells were stimulated for 72h at 37°C with OVA (1mg/ml). Co-cultures using DCs isolated from MLN of aged mice, but not from spleen, produce more IL-4, IFN-γ and IL-10 when compared with those isolated from young mice (Figure 5 A, B and C). On other hand, reduced levels of TGF-β seemed to be induced in co-cultures using DCs isolated from both, spleen and MLN, of aged mice (Figure 5 D). Phenotype of co-cultured T cells showed that cultures containing MLN but not spleen-derived DCs from aged mice induced lower frequencies of CD4+CD25+LAP+ T cells (Figure 5 E).
Figure 5. DCs from MLN of aged mice are less efficient to induce TGF-β production and CD4+LAP+ regulatory T cells when co-cultured with OVA-TCR transgenic T cells.
Pooled DCs were obtained from mesenteric lymph nodes and spleen of 5 non-manipulated mice (2 and 12-month-old). Pools of T cells were obtained from spleen of 5 non-manipulated DO11.10 mice (2-month-old). DCs from 2- or 12-month-old mice from MLN or spleen were co-cultured with T cells from 2-month-old DO11.10 mice and stimulated with either medium or OVA (1mg/ml) for 72h at 37°C. Supernatants were collected for cytokine assays. IFN-γ (A), IL-4 (B), IL-10 levels (C) and TGF-β (D) were measured by ELISA, frequency of CD25+LAP+ cells (E), gated in CD4+ T cells, assessed by flow cytometry.
DISCUSSION
The most common outcome for the antigenic contacts occurring at the gut surfaces is not an inflammatory immune response but rather a state of hiporesponsiveness to orally administered antigens named oral tolerance (Vaz et al, 1977; Mowat, 2003; Faria and Weiner, 2005). In addition, mucosal B lymphocytes produce high levels of secretory IgA (S-IgA) that represents another non-Inflammatory immune response typical of mucosal surfaces. Some factors such as aging are known to influence oral tolerance induction but it does not affect S-IgA production (Santiago et al, 2008; Koga et al, 2000; Faria et al, 1993). Usually aging-associated alterations affect the mucosal-associated lymphoid tissues than the systemic immune compartment (Koga et al, 2000). We have shown previously that although antigen-specific antibody response to a soluble antigen declines in 18-month old mice, total levels of serum antibodies as well as frequencies of spleen and bone marrow antibody-producing cells are increased in aged mice. In addition, proliferative response of non-stimulated spleen T cells from aged mice were augmented and insensitive to increasing doses of concanavalin A stimulation as compared to young mice that showed a typical dose-dependent response to mitogen stimulation in vitro (Speziali et al, 2009). These data altogether suggest that the higher activation mode of B and T cells in senescent mice is a result of an increased frequency of cells committed to previous antigenic experiences and with poor ability to respond to novel antigenic challenges. Therefore, it is expected that oral tolerance to OVA would be affected by aging.
To understand the mechanisms involved in the age-associated decline of mucosal immune function, regulatory elements that are reported to participate in oral tolerance were evaluated. Our hypothesis was that some of these elements would be reduced during aging. However, we did not expect to find a general decline in all regulatory functions of the gut mucosa since we have already shown that oral tolerance is still induced in aged mice when a continuous feeding regimen is used (Faria et al, 2003).
Major parameters of gut architecture such as length of the villi or number of intraepithelial lymphocytes (IELs) did not change in mice as old as 24 months when compared with 2-month old mice. No change was observed either in the sizes and cell contents of lymphoid organs such as spleen, mesenteric lymph nodes or Peyer’s patches. However, frequency of TCRγδ+, but not TCRαβ+ IELs was diminished in 12- to 24-month-old mice. Ishimoto and coworkers also found a similar decrease in TCRγδ+ IEL in aged mice (Ishimoto et al, 2004). This subset of IEL was previously described as involved in gut regulatory activities. It has been shown that in vivo blockage (Mengel et al, 1995) or genetic deletion of TCRγδ (Ke et al, 1997) is associated with failure in oral tolerance induction. Moreover, αδ+ IELs are involved in modulation of colitis induced by dextran sodium sulfate (DSS) in mice (Chen et al, 2002) and γδT cell clones from IEL inhibit the development of cytotoxic T cells (Kapp et al, 2004) suggesting that αδ+ IELs have immuno-regulatory properties. In addition, we found a lower frequency of TCRαβ+CD8αα+ IEL in 24-month old mice. This subset of IEL represents thymic-educated T cells that completed its development in the gut (Lambolez et al, 2007). They are described to prevent colitis induced by the transfer of CD4+CD25+CD45high T cells to immune-deficient mice (Poussier et al, 2002). These cells also express constitutive levels of the anti-inflammatory cytokines TGF-β1 and TGF-β3, as well as other modulatory proteins such as LAG-3 and the novel immune coagulant fgl2/fibroleukin both of them found in CD4+CD25+Foxp3+ regulatory T cells (Denning et al, 2007). It is plausible that the reduction of these two regulatory-type IEL subsets in aged mice influence susceptibility to oral tolerance.
By contrast, when regulatory T cells in the gut mucosa were evaluated, an increase in CD4+CD25+Foxp3+ and CD4+CD25+LAP+ cells was found in mesenteric lymph nodes (MLN) and Peyer’s patches (PP) of mice from 6-24 months of age when compared to young control animals (2-month old), suggesting that these T cell subsets augment after sexual maturity but remains stable afterwards. The same result could be seen in lamina propria (LP) but only in mice from 12 to 24 months of age. Frequency of CD4+CD25+Foxp3+ T cells was also augmented in spleen of 24-month old mice (data not shown). Peripherally-induced but not natural CD4+CD25+Foxp3+ T cells have been reported as essential for oral tolerance induction in mice (Mucida et al, 2005; Curotto de Lafaille et al, 2008). We could not distinguish the contribution of peripherally-differentiated versus natural FoxP3+ Tregs for the general increase in the population of FoxP3+ Tregs in the gut mucosa during aging. Candidates of specific markers for thymus-derived Foxp3+ Tregs, such as the transcription factor Helios, have been recently described (Thornton et al, 2010). It would be interesting to perform such a distinction since thymus is one of the most affect lymphoid organ during aging. CD4+LAP+ T cells represent a subset of regulatory T cells expressing TGF-β bound to their membranes in its precursor form (associated with the latent associated peptide, LAP). They participate in the control of intestinal Inflammation in experimental models of colitis (Nakamura et al, 2004; Oida et al, 2003) and they have been recently reported as a distinct subset of T cells with regulatory function (Ghandi et al, 2010). In parallel to the rise in the frequency of these T regulatory subsets, an increase in frequencies of activated CD4+CD44+ cells in MLN, PP and LP, as well as in spleen (data not shown) was detected in aged mice. Our results are in agreement with Han and coworkers who showed increased frequencies of Foxp3+ and CD44+ CD4+ T cells in SJL/J mice. According to this study, Foxp3+ CD4+ T cells are augmented in spleen and MLN but not in thymus suggesting that they are peripherally-induced regulatory T cells (Han et al, 2009). Aging is usually associated with a substantial reduction in the frequency of naive with concomitant augment in memory T cells (Taub and Longo, 2005), a phenomenon that was observed in our study in MLN and PP of 12-month-old mice. It is likely that the increase in frequencies of regulatory adaptive T cells observed in our study is part of the general activated state of lymphocytes in aged mice. Interestingly, it has been shown that the effector T cell pool has the ability to index their expansion to the regulatory T cell pool via IL-2 secretion (Almeida et al, 2006). Thus, it is plausible that the age-related increase in the frequencies of Tregs in our study can be seen as a compensatory mechanism to keep at least some gut homeostasis during a process that affects other regulatory elements in the intestinal mucosa.
Curiously, there was an increased CD4+CD62L+ T cells only in lamina propria of 24-month-old mice. This increase in CD62L+ T cells in LP compartment was not due to a increase in memory/effector T cells as there was no difference in CD4+CD44+CD62L+ cells between aged and young mice (data not shown). One possible explanation is that activated T cells in the lamina propria regain CD62L expression to recirculate back to the gut mucosa.
A major factor influencing oral tolerance induction is regulatory cytokines such as TGF-β and IL-10 (Sonoda et al, 1989; Faria and Weiner, 2006; Rizzo et al, 1999). IL-10 deficient mice develop spontaneous enterocolitis as a sole pathological alteration (Kuhn et al, 1993) and gut inflammation in these mice is associated with breakdown of oral tolerance to intestinal microbiota (Duchmann et al, 1996). TGF-β also has been shown to participate in oral tolerance induction and in modulation of colitis in a series of experimental models (Faria and Weiner, 2006). Both cytokines were decreased in the duodenum of aged mice while IL-4 was augmented and IFN-γ was not altered. Concentration of IL-10 was reduced not only in duodenum but in all portions of small intestine examined. Of note, lymphocytes are not homogeneously distributed in the intestinal mucosa, and duodenum is where most of the diffuse lymphoid tissue is located (Mowat, 2003). In addition to their direct action in IgA class switch and suppression of inflammatory events, IL-10 and TGF-β are linked to the peripheral conversion of regulatory T cell such as CD4+CD25+Foxp3+ T cells (Wan and Flavell, 2006) and CD4+LAP+ T cells (Ghandi et al, 2010). This differentiation role did not seem affected by the aging process since both populations of Tregs were augmented in aged mice. It is possible that reduction in the production of IL-10 and TGF-β in the small intestine in addition to the reduction in the frequencies of TCRγδ+ and TCRαβ+CD8αα+ IELs play a direct rather than an indirect role in the impairment of oral tolerance induction in old mice.
Dendritic cells are the main antigen-presenting cells and they have been associated with deletion or silencing of auto-reactive lymphocytes as well as induction or regulatory T cells leading to peripheral tolerance (Faria et al, 2005; Hawiger et al, 2004). They are also key elements in oral tolerance induction (Viney et al, 1998; Fleeton et al, 2004), and previous studies have shown that mucosal DCs have immune suppressive properties (Iwasaki, 2007; Mucida et al, 2007). Few reports exist describing the aging effects on phenotype and function of DCs and other antigen presenting cells in the gut. We found increased expression of CD86 in DCs as well as macrophages from MLN and PP of aged mice. Simioni and coworkers reported recently that aged is associated with decreased expression of CD86 and impaired ability to stimulate proliferation by spleen DCs (Simioni et al, 2010). These authors did not examine DCs from mucosal sites and mice in their work were fed and immunized whereas mice used in our study were non-manipulated. When tested for their function as antigen presenting cells in co-cultures of T cells and DCs from spleen as well as MLN of young and old mice, DCs from MLN were clearly more affected by aging than those from spleen. This result is in agreement with reports suggesting that the aging impact can be observed earlier in the mucosal immune system than in the systemic immune compartment (Koga et al, 2000). Interesting, concomitant reduction in TGF-β production and in the induction CD4+CD25+LAP+ T cells was observed in co-cultures containing DCs from aged mice.
Both CD25+ and CD25− LAP+ T cells were shown to bear modulatory properties in experimental models of colitis (Nakamura et al, 2004; Oida et al, 2003). Interestingly, murine CD4+ T cells can be induced to express LAP either by forcing Foxp3+ expression or by stimulation with TGF-β in a Foxp3-independent fashion (Oida and Weiner, 2010). Therefore, some of the CD4+LAP+ T cells represent a distinct regulatory T cell subset that does not need Foxp3 expression to exert its modulatory function. In the co-culture studies, we found that aged MLN-derived DCs had a lower ability to induce differentiation of CD25+LAP+ T cells. In spite of that, frequency of CD4+LAP+ T cells was increased in MLN, PP and LP of old mice. This result confirmed our previous finding that global production of immunoglobulins and cytokines is increased in aged mice, but specific recall immune responses are reduced (Speziali et al, 2008). Indeed, CD4+LAP+ T cells measured in lamina propria represent a global in situ analysis of this population whereas its differentiation in cell cultures stimulated with antigen represent a specific recall response. Moreover, augmented production of effector cytokines such as IL-4 and IFN-γ as well as IL-10 seemed to result from co-culture of T cells with DCs from MLN of aged mice. Although T cell compartment is known to be highly affected by aging, our data indicates that antigen-presenting cells, namely DCs, may also play a key role in the decline of regulatory immune function at the gut mucosa.
We cannot rule out the possibility that the methodology used to sort cells for culture studies interfered with our results. We have enriched T cells and DCs by positive selection using magnetic beads coupled to anti-Thy1 and anti-CD11c antibodies. However, others have successfully used positive selection approaches for isolation of cells to study the role of DCs in T cell differentiation in vitro. Recent reports showed that TGF-β and retinoic acid drive the differentiation of naïve CD4+ T cells into CD4+CD25+Foxp3+ T regulatory cells by using anti-CD11c beads to purify DCs from mesenteric lymph nodes and spleens (Mucida et al, 2007; Coombes et al, 2007). The only major difference between these and our co-culture studies was the use of pure naïve CD4+ T cells in the former ones. Xiao and coworkers also used positive selection of CD4+ T cells (isolated by magnetic beads followed by cell sorting) to study the role of retinoic acid in induction of Foxp3 expression and inhibition RORγt (Xiao et al, 2008).
Altogether, our results indicate that several regulatory-type components of the gut mucosa were affected by aging in mice. Namely, frequencies of IEL with regulatory phenotype were lower, production of TGF-β and IL-10 in the small intestine were reduced. In addition, the ability of DCs from MLN of aged mice to stimulate TGF-β-dependent immune responses was also impaired. However, not all regulatory elements were altered by aging and adaptive CD4+CD25+Foxp3+ regulatory T cells at mucosal sites were especially resistant to age-associated changes. It is likely that the alterations of IEL and cytokine production in aged mice correlate to the decline in oral tolerance induction. On the other hand, the persistence of some regulatory mechanisms may explain why oral tolerance can still be fully induced in aged mice by certain optimal regimens such as continuous feeding (Faria et al, 1998; Faria et al, 2003).
ACKNOWLEDGMENTS
We are thankful to Ilda Marçal de Souza for the excellent care of the mice. This work was supported by a grant from Fogarty International Center (FIRCA/NIH TW007636-01). Some of the authors are recipients of scholarships and fellowships from Conselho Nacional para o Desenvolvimento Científico e Tecnológico – CNPq, Brazil (A.F.S., A.C.A., R.M.F., R.P.O., A.M.C.F.).
Abbreviations
- DCs
Dendritic cells
- IEL
Intraephitelial cells
- LP
Lamina propria
- MLN
Mesenteric lymph nodes
- OVA
Ovalbumin
- PP
Peyer’s patches
- S-IgA
Specific secretory IgA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST The authors have no conflicts of interest to be disclosed.
REFERENCES
- Almeida AR, Zaragoza B, Freitas AA. Indexation as a novel mechanism of lymphocyte homeostasis: the number of CD4+CD25+ regulatory T cells is indexed to the number of IL-2-producing cells. J.Immunol. 2006;177:192–200. doi: 10.4049/jimmunol.177.1.192. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Development and basic mechanisms of human gut immunity. Nutr. Rev. 1998;56:S5–18. doi: 10.1111/j.1753-4887.1998.tb01645.x. [DOI] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103◻ DCs induces Foxp3◻ regulatory T cells via a TGF-◻ and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lafaille M.A. Curotto, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille J,J. Adaptive Foxp3+ regulatory T cell- dependent and – independent control of allergic Inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Denning TL, Granger SW, Mucida D, Graddy R, Leclercq G, Zhang W, Honey K, Rasmussen JP, Cheroutre H, Rudensky AY, Kronenberg M. Mouse TCRalphabeta+CD8alphaalpha intraepithelial lymphocytes express genes that down-regulate their antigen reactivity and suppress immune responses. J Immunol. 2007;178(7):4230–9. doi: 10.4049/jimmunol.178.7.4230. [DOI] [PubMed] [Google Scholar]
- Faria AMC, Weiner HL. Oral tolerance and TGF-β- Producing Cells. Inflam Allergy Drug Targets. 2006;5:179–190. doi: 10.2174/187152806778256034. [DOI] [PubMed] [Google Scholar]
- Faria AMC, Ficker SM, Speziali E, Menezes JS, Stransky B, Rodrigues V. Silva, Vaz NM. Aging affects oral tolerance induction but not its maintenance in mice. Mech Ageing Dev. 1998;102:67–80. doi: 10.1016/s0047-6374(98)00024-4. [DOI] [PubMed] [Google Scholar]
- Faria AMC, Garcia G, Rios MJC, Michalaros CL, Vaz NM. Decrease in susceptibility to oral tolerance and occurrence of oral immunization to ovalbumin in 20-38-week old mice. The effect of interval between oral exposures and rate of antigen intake in the oral immunization. Immunol. 1993;78:147–151. [PMC free article] [PubMed] [Google Scholar]
- Faria AMC, Maron R, Ficker SM, Slavin AJ, Spahn T, Weiner HL. Oral tolerance induced by continuous feeding: enhanced up-regulation of transforming growth factor-B/interleukin-10 and suppression of experimental autoimmune encephalomyelitis. J.Autoimmunity. 2003;20:135–145. doi: 10.1016/s0896-8411(02)00112-9. [DOI] [PubMed] [Google Scholar]
- Faria AMC, Weiner HL. Oral tolerance. Immunol. Rev. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A, Murray D. Quantification of intraephitelial lymphocytes in human jejum. Gut. 1971;12:988–994. doi: 10.1136/gut.12.12.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleeton M, Contractor N, Leon F, He J, Wetzel D, Dermody T, Iwasaki A, Kelsall B. Involvement of dendritic cell subsets in the induction of oral tolerance and immunity. Ann.N.Y.Acad.Sci. 2004;1029:60–65. doi: 10.1196/annals.1309.008. [DOI] [PubMed] [Google Scholar]
- Ghandi R, Farez MF, Wang Y, Kozoriz D, Quintana FJ, Weiner HL. Human latency associated peptide+ T cells: a novel regulatory T cell subset. J.Immunol. 2010;184:4620–4624. doi: 10.4049/jimmunol.0903329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han GM, Zhao B, Jeyaseelan S, Feng JM. Age-associated parallel increase of Foxp3(+)CD4(+) regulatory and CD44(+)CD4(+) memory T cells in SJL/J mice. Cell Immunol. 2009;258:188–96. doi: 10.1016/j.cellimm.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruna H, Inaba M, Inaba K, Taketani S, Sugirura K, Fukuba Y, Doi H, Toki J, Tokunaga R, Ikehara S. Abnormalities of B cells and dendritic cells in SAMP1 mice. Eur. J. Immunol. 1995;25:1319–1325. doi: 10.1002/eji.1830250528. [DOI] [PubMed] [Google Scholar]
- Hawiger D, Masilamani RF, Betteli E, Kuchroo VK, Nussenzweig M. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity. 2004;20(6):695–705. doi: 10.1016/j.immuni.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Hayashi O, Katayanagi Y, Ishii K, Kato T. Flow cytometric analysis of age-related changes in intestine intraepithelial lymphocyte subsets and their functional preservation after feeding mice on spirulina. J. Med. Food. 2009;12:982–989. doi: 10.1089/jmf.2008.1260. [DOI] [PubMed] [Google Scholar]
- Ishimoto Y, Tomiyama-Miyaji C, Watanabe H, Yokoyama H, Ebe K, Tsubata S, Aoyagi Y, Abo T. Age-dependent variation in the proportion and number of intestinal lymphocyte subsets, especially natural killer T cells, double-positive CD4+CD8+ cells and B220+ T cells in mice. Immunol. 2004;113:371–377. doi: 10.1111/j.1365-2567.2004.01961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A. Mucosal dendritic cells. Ann.Rev.Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- Kapp JA, Kapp LM, McKenna KC, Lake JP. Gammadelta T-cell clones from intestinal intraepithelial lymphocytes inhibit development of CTL responses ex vivo. Immunology. 2004;111:155–64. doi: 10.1111/j.0019-2805.2003.01793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Fujihashi K, Kato R, Dohi T, Fujihashi K, Hagiwara Y, Kataka K, Kobayashi R, McGhee JR. Lack of oral tolerance in aging is due to sequential loss of Peyer’s patch cell interactions. Int. Immunol. 2003;15:145–158. doi: 10.1093/intimm/dxg011. [DOI] [PubMed] [Google Scholar]
- Ke Y, Pearce K, Lake JP, Ziegler HK, Kapp JA. γδT lymphocytes regulate the induction of oral tolerance. J. Immunol. 1997;158:3610–3618. [PubMed] [Google Scholar]
- Koga T, McGhee J, Kato H, Kato R, Kiyono H, Fujihashi K. Evidence for early aging in the mucosal immune system. J. Immunol. 2000;165:5352–5359. doi: 10.4049/jimmunol.165.9.5352. [DOI] [PubMed] [Google Scholar]
- Kovacs EJ, Palmer JL, Fortin CF, Fülöp T, Jr, Goldstein DR, Linton PJ. Aging and innate immunity in the mouse: impact of intrinsic and extrinsic factors. Trends Immunol. 2009;30(7):319–24. doi: 10.1016/j.it.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75(2):263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Lambolez F, Kronenberg M, Cheroute H. Thymic differentiation of TCR alpha beta(+) CD8 alpha alpha(+) IELs. Immunol. Rev. 2007;215:178–88. doi: 10.1111/j.1600-065X.2006.00488.x. [DOI] [PubMed] [Google Scholar]
- Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nature Immunl. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- Menezes JS, Mucida DS, Cara DC, Alvarez-Leite JI, Russo M, Vaz NM, Faria AMC. Stimulation by food proteins plays a critical role in the maturation of the immune system. Int. Immunol. 2003;15:447–455. doi: 10.1093/intimm/dxg043. [DOI] [PubMed] [Google Scholar]
- Mengel J, Cardillo F, Aroeira LD, Williams O, Russo M, Vaz NM. Anti-γδ T cell antibody blocks the induction and maintenance of oral tolerance to ovalbumin in mice. Immunol.Letters. 1995;48:97–102. doi: 10.1016/0165-2478(95)02451-4. [DOI] [PubMed] [Google Scholar]
- Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, de Lafaille MA Curotto. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005;115(7):1923–33. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- Muller S, Jungo M, Aichele P, Mueller C. CD5± CD8 alpha beta intestinal intraepithelial lymphocytes (IEL) are induced to express CD5 upon antigen-specific activation: CD5- and CD5+CD8 alpha beta IEL do not represent separate T cell lineages. Eur.J. Immunol. 1997;27:1756. doi: 10.1002/eji.1830270724. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kitani A, Fucs I, Pedersen A, Harada N, Nawata H, Strober W. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J.Immunol. 2004;172(2):834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- Oida T, Zhang X, Goto M, Hachimura S, Totsuka M, Kaminogawa S, Weiner HL. CD4+CD25- T cells that express latency-associated peptide on the surface suppress CD4+CD45RBhigh-induced colitis by a TGF-beta-dependent mechanism. J Immunol. 2003;170(5):2516–22. doi: 10.4049/jimmunol.170.5.2516. [DOI] [PubMed] [Google Scholar]
- Oida T, Weiner HL. TGF-β induces surface LAP CD4 T cells independent of Foxp3 induction. PLoS One. 2010;5(11):e15523. doi: 10.1371/journal.pone.0015523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poussier P, Ning T, Banerjee D, Julius M. A unique subset of self-specific intraintestinal T cells maintains gut integrity. J Exp Med. 2002;195(11):1491–7. doi: 10.1084/jem.20011793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo LV, Morawetz RA, Miller-Rivero NE, Choi R, Wiggert B, Chan CC, Morse HC, III., Nussenblatt RB, Caspi RR. IL-4 and IL-10 are both required for the induction of oral tolerance. J. Immunol. 1999;162:2613–2622. [PubMed] [Google Scholar]
- Santiago AF, Fernandes RM, Santos BP, Assis FA, Oliveira RP, Carvalho CR, Faria AMC. Role of mesenteric lymph nodes and aging in secretory IgA production in mice. Cell. Immunol. 2008;253:5–10. doi: 10.1016/j.cellimm.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Saurer L, Mueller C. T cell-mediated immunoregulation in the gastrointestinal tract. Allergy. 2009;64(4):505–519. doi: 10.1111/j.1398-9995.2009.01965.x. [DOI] [PubMed] [Google Scholar]
- Senda S, Cheng E, Kawanishi H. Aging-associated changes in murine intestinal immunoglobulin A and M secretions. Scand J Immunol. 1988;27:157–64. doi: 10.1111/j.1365-3083.1988.tb02334.x. [DOI] [PubMed] [Google Scholar]
- Simioni PU, Fernandes LG, Gabriel DL, Tamashiro WM. Effect of aging and oral tolerance on dendritic cell function. Braz J Med Biol Res. 2010;43:68–76. doi: 10.1590/s0100-879x2009007500024. [DOI] [PubMed] [Google Scholar]
- Speziali E, Santiago AF, Fernandes RM, Vaz NM, Menezes JS, Faria AMC. Specific immune response but not basal functions of B and T cells are impaired in aged mice. Cell. Immunol. 2009;256:1–5. doi: 10.1016/j.cellimm.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Steger MM, Maczek C, Grubeck-Loebenstein B. Morphologically and functionally intact dendritic cells can be derived from the peripheral blood of aged individuals. Clin. Exp. Immunol. 1996;105:544–50. doi: 10.1046/j.1365-2249.1996.d01-790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunol. Reviews. 2005;205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184(7):3433–41. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz NM, Maia LC, Hanson DG, Lynch JM. Inhibiton of homocytotropic antibody responses in adult inbred mice by previous feeding of the specific antigen. Brazilian J. Allergy Clin. Immunol. 1977;60(2):110–115. doi: 10.1016/0091-6749(77)90035-5. [DOI] [PubMed] [Google Scholar]
- Verhagen J, Wraith DC. Comment on “Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells”. J Immunol. 2010;185(12):7129. doi: 10.4049/jimmunol.1090105. [DOI] [PubMed] [Google Scholar]
- Viney J, Mowat AM, O’Malley JM, Williamson E, Fanger NA. Expanding dendritic cells in vivo enhances the induction of oral tolerance. J.Immunol. 1998;160:5815–5825. [PubMed] [Google Scholar]
- Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-β-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J. Immunol. 2008;181:2277–2284. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan YY, Flavell RA. The roles for cytokines in the generation and maintenance of regulatory T cells. Immunol. Rev. 2006;212:114–30. doi: 10.1111/j.0105-2896.2006.00407.x. [DOI] [PubMed] [Google Scholar]