SUMMARY
Activation of group I metabotropic glutamate receptors leads to long-term depression (mGluR-LTD). Alterations in this form of plasticity have been linked to drug addiction and cognitive disorders. A key characteristic of mGluR-LTD is its dependence on rapid protein synthesis; however, the identities of the proteins mediating LTD remain elusive. Here, we identify the X-linked mental retardation protein OPHN1 as a molecule essential for mGluR-LTD in the hippocampus. mGluR-LTD induction elicits rapid dendritic OPHN1 synthesis, which is dependent on mGluR1 activation and independent of fragile X mental retardation protein (FMRP). This response is essential for mGluR-LTD, as acute blockade of OPHN1 synthesis impedes LTD. mGluR-induced OPHN1 mediates LTD and associated persistent decreases in surface AMPARs via interactions with Endophilin-A2/3. Importantly, this role of OPHN1 is separable from its effects on basal synaptic strength, which require OPHN1’s Rho-GAP activity and interaction with Homer1b/c. Thus, our data establish a novel role for rapid OPHN1 synthesis in mGluR-LTD.
INTRODUCTION
Activity-dependent changes in the strength of excitatory synapses are thought to be key cellular mechanisms that contribute to the plasticity of neuronal networks underlying learning and memory. Two well-defined cellular models in mammals that measure changes in synaptic strength are long-term potentiation (LTP) and long-term depression (LTD) (Citri and Malenka, 2008; Collingridge et al., 2010; Shepherd and Huganir, 2007). Like memories, they typically occur in two distinct phases: an early phase which usually depends on modification of preexisting proteins, and a late phase which is more persistent and dependent on the synthesis of new proteins (Citri and Malenka, 2008; Costa-Mattioli et al., 2009; Richter and Klann, 2009; Sutton and Schuman, 2006). While the importance of de novo protein synthesis in the long-term nature of both memory and its underlying forms of synaptic plasticity has been known for a while, a major difficulty has been the identification of the locally translated proteins directly linked to changes in synaptic strength.
At hippocampal CA1 synapses, several forms of plasticity that are dependent on protein synthesis have been described, including late-phase NMDA receptor (NMDAR)-dependent LTP and LTD (Citri and Malenka, 2008; Collingridge et al., 2010; Klann and Dever, 2004), and a form of LTD (mGluR-LTD) that relies on the activation of group I metabotropic glutamate receptors, which consist of mGluR1 and mGluR5 (Huber et al., 2000; Oliet et al., 1997). Activation of either mGluR1 or mGluR5 can induce LTD in the hippocampal CA1 area (Hou and Klann, 2004; Volk et al., 2006). Whereas both mGluR-LTD and NMDAR-LTD are mediated by endocytosis and decreased surface expression of postsynaptic AMPARs, the two forms of LTD rely on distinct signaling pathways and do not occlude each other (Carroll et al., 1999; Moult et al., 2006; Oliet et al., 1997; Snyder et al., 2001; Waung et al., 2008). Significantly, in contrast to NMDAR-LTD, where the requirement for protein synthesis is delayed, mGluR-LTD and the associated decreases in surface AMPARs require rapid (within 5–10 min) dendritic protein synthesis (Huber et al., 2000; Snyder et al., 2001). The prevailing model is that group I mGluRs trigger rapid synthesis of new proteins in dendrites (referred to as ‘LTD proteins’) that function to cause LTD by increasing the rate of AMPAR endocytosis at locally active synapses (Luscher and Huber, 2010; Waung and Huber, 2009). A largely remaining challenge, however, is to determine the identity of the ‘LTD proteins’. Recent studies have unveiled a few candidate proteins, which in the hippocampus include tyrosine phosphatase STEP (Zhang et al., 2008), microtubule-associated protein MAP1B (Davidkova and Carroll, 2007), and as the leading candidate, activity-regulated cytoskeleton-associated protein Arc/Arg3.1 (Park et al., 2008; Waung et al., 2008). All three proteins are rapidly synthesized in response to mGluR activation and have been linked to AMPAR endocytosis, which in the case of Arc involves interactions with Endophilin A2/3 and Dynamin (Chowdhury et al., 2006). So far, however, it has only been shown for Arc that acute blockade of its de novo synthesis impedes mGluR-LTD and the associated long-term decreases in surface AMPARs (Waung et al., 2008).
The mechanisms by which mGluRs regulate rapid protein synthesis appear to be multifaceted, involving the regulation of general translation initiation factors (Costa-Mattioli et al., 2009; Richter and Klann, 2009; Waung and Huber, 2009), the elongation factor EF2 (Davidkova and Carroll, 2007; Park et al., 2008), as well as RNA binding proteins, such as the fragile X mental retardation protein (FMRP), the gene product of FMR1 (Bassell and Warren, 2008; Waung and Huber, 2009). FMRP is thought to function as a repressor of mRNA translation that binds to and regulates the translational efficiency of specific dendritic mRNAs, which include, for instance, Map1b and Arc mRNAs, in response to mGluR activation, and especially mGluR5 (Bassell and Warren, 2008; Costa-Mattioli et al., 2009; Darnell et al., 2011; Dolen et al., 2007; Napoli et al., 2008). In the absence of FMRP, this control is lost, leading to excessive and dysregulated translation of FMRP target mRNAs and enhanced mGluR-LTD that is protein synthesis independent (Bassell and Warren, 2008; Dolen et al., 2007; Hou et al., 2006; Huber et al., 2002; Nosyreva and Huber, 2006). Physical interactions between mGluR5 and molecules signaling to the translation machinery have been described, with the Homer scaffolding proteins forming important links to multiple translation control pathways, including initiation and elongation (Giuffrida et al., 2005; Park et al., 2008; Ronesi and Huber, 2008). mGluR5 has also been linked to the regulation of FMRP through direct binding to and rapid activation (within 1 min) of phosphatase PP2A, which causes dephosphorylation of FMRP and rapid translational upregulation of FMRP target mRNAs (Narayanan et al., 2007). With regards to mGluR1-mediated signaling at the CA1 synapse, less is known. The mGluR1α isoform, which contains the Homer binding motif, is reportedly absent in hippocampal pyramidal neurons (Ferraguti and Shigemoto, 2006). Also, the identity of the proteins specifically synthesized upon mGluR1 activation remains elusive.
Here, we examined the requirement of the X-linked mental retardation protein oligophrenin-1 (OPHN1) (Billuart et al., 1998) for mGluR-LTD. OPHN1 is a Rho GTPase-activating protein (Rho-GAP), a negative regulator of Rho GTPases, which, interestingly, besides RhoA, also interacts with Homer 1b/c (Govek et al., 2004) and Endophilin A2/3 family members (see Figure 3), proteins implicated in mGluR-LTD (Chowdhury et al., 2006; Park et al., 2008; Ronesi and Huber, 2008; Waung and Huber, 2009). The OPHN1 protein is highly expressed in the brain throughout development, where it is found in neurons of all major regions, including hippocampus and cortex, and is present in axons, dendrites and spines (Govek et al., 2004). Significantly, loss of OPHN1 function has been causally linked to a syndromic form of mental retardation (MR). Several studies reported the presence of OPHN1 loss-of-function mutations in families with MR associated with cerebellar hypoplasia and lateral ventricle enlargement (Bergmann et al., 2003; des Portes et al., 2004; Philip et al., 2003; Zanni et al., 2005). Moreover, inactivation of ophn1 in mice recapitulates some of the human phenotypes, such as behavioral and cognitive impairments (Khelfaoui et al., 2007). At the hippocampal CA3-CA1 synapse, during early development, postsynaptic OPHN1, through its Rho-GAP activity, plays a key role in activity-dependent maturation and plasticity of excitatory synapses (Nadif Kasri et al., 2009), suggesting the involvement of OPHN1 in normal activity-driven glutamatergic synapse development. Findings presented here demonstrate that OPHN1 also plays a critical role in mediating mGluR-LTD in CA1 hippocampal neurons. We find that OPHN1 expression is translationally induced in dendrites of CA1 neurons within 10 min of mGluR activation, and that this response is essential for mGluR-dependent LTD. Acute blockade of new OPHN1 synthesis impedes mGluR-LTD and the associated long-term decreases in surface AMPARs. Interestingly, the rapid induction of OPHN1 expression is primarily dependent on mGluR1 activation, and is independent of FMRP. Importantly, OPHN1’s role in mediating mGluR-LTD can be dissociated from its role in basal synaptic transmission (Nadif Kasri et al., 2009). Regulation of basal synaptic strength requires OPHN1’s Rho-GAP activity and association with Homer 1b/c proteins, whereas mGluR-LTD and the associated long-term decreases in surface AMPARs are dependent on OPHN1’s interaction with Endophilin A2/3. Thus, our data unveil a critical role for rapid OPHN1 synthesis in mGluR-LTD, providing not only novel insight into the mechanism and function of mGluR-LTD, but also into the cellular basis by which mutations in OPHN1 could contribute to the behavioral and cognitive deficits in OPHN1 patients.
Figure 3. Disruption of OPHN1 Interactions with Homer 1b/c and Endo2/3.
(A) Domain structure of OPHN1. The Homer 1b/c and Endo2/3 binding sites and the R409Q amino acid substitution in GAP domain that abolishes OPHN1’s Rho-GAP activity are indicated.
(B) Homer 1b-GST fusion protein, or GST alone, immobilized on beads was incubated with extracts from HEK293T cells expressing OPHN1WT-EGFP or OPHN1Hom-EGFP. Bound OPHN1 was detected by immunoblotting with anti-GFP antibody. GST-fusion proteins used are indicated by Coomassie Blue (CBB) staining. TL, total lysate.
(C) Homer 1b-GST fusion protein (lower panel, CBB staining) immobilized on beads was first incubated with synaptosomal extracts followed by pep-OPHN1Hom or pep-contHom at indicated concentrations. Bound OPHN1 was detected by immunoblotting with anti-OPHN1 antibody.
(D) Endo2-GST fusion protein, or GST alone (lower panel, CBB staining), immobilized on beads was incubated with extracts from HEK293T cells expressing OPHN1WT, OPHN1-PRD1*, -PRD2*, or -PRD3*(= OPHN1Endo)-EGFP fusion proteins. Bound OPHN1 was detected by immunoblotting with anti-GFP antibody.
(E) Extracts prepared from acute hippocampal brain slices (CA1 regions), pretreated with cycloheximide (CHX, 50 µM), or control vehicle, for 5 min and then treated with DHPG (100 µM), or ACSF, for 10 min were incubated with anti-Endo2 antibody, or control IgG. The immunoprecipitates (IP) and total lysates (TL) were analyzed by immunoblotting with indicated antibodies. Relative amount of co-immunoprecipitated OPHN1 with Endo2 (compared to control vehicle) is shown in right panel. n = 3, *p < 0.01 (unpaired t-test), as compared with control vehicle-treated slices. Error bars represent SEM.
(F) Endo2-GST fusion protein (lower panel, CBB staining) immobilized on beads was first incubated with synaptosomal extracts followed by pep-OPHN1Endo or pep-contEndo at indicated concentrations. Bound OPHN1 was detected by immunoblotting with anti-OPHN1 antibody.
RESULTS
Group I mGluR Activation Induces Rapid Dendritic Synthesis of OPHN1
Our findings that OPHN1 interacts with Homer 1b/c and Endophilin A2/3 (see below), proteins with reported roles in mGluR-dependent LTD, prompted us to explore the involvement of OPHN1 in this form of plasticity. We reasoned that if OPHN1 plays a direct role in mGluR-LTD, its protein levels should be rapidly regulated in response to mGluR activation. Therefore, OPHN1 protein expression was examined by immunocytochemistry in CA1 neurons of acute hippocampal slices treated with DHPG, a selective mGluR1/5 agonist, or control vehicle. We observed that DHPG treatment of acute slices leads to a rapid increase in OPHN1 protein levels (within 10 min) in both the soma and dendrites of CA1 neurons (Figure 1A). Importantly, this increase was blocked by the protein synthesis inhibitors anisomycin and cycloheximide (Figure 1A, and data not shown), but not the DNA transcription inhibitor actinomycin D (Figure S1A available online), implying that mGluRs trigger new synthesis of OPHN1 protein from pre-existing mRNA. Similar results were obtained by Western blot analysis; namely, DHPG treatment of acute hippocampal slices (for 10 min) caused a significant increase in OPHN1 protein levels, and this increase was blocked by anisomycin, but not actinomycin D (Figures 1B and S1B). Neither of the two inhibitors affected basal levels of OPHN1 (Figures 1B and S1B). In contrast to DHPG, treatment of slices with a chemical induction paradigm for NMDAR-LTD did not trigger an increase in OPHN1 protein levels (Figure 1C).
Figure 1. OPHN1 is Rapidly Synthesized in CA1 Dendrites in Response to Group I mGluR Stimulation.
(A1) DHPG treatment (100 µM) of acute hippocampal slices increases OPHN1 immunofluorescence (red) in dendrites of CA1 neurons 10 min after DHPG onset. Pretreatment with anisomycin (20 µM, 30 min) blocks DHPG-induced increases of OPHN1. βIII-tubulin immunoreactivity (green) indicates the presence of dendrites. Scale bar, 50 µm. s.p. stratum pyramidale; s.r. stratum radiatum. (A2) Quantification of OPHN1 immunofluorescence in dendrites. Mean OPHN1 fluorescence intensity was expressed as percentage of control. n = 27–36 slices (40 µm) from 4–5 animals per condition, *p < 0.01 (unpaired t-test), as compared with control. Error bars represent SEM in all panels.
(B1) Western blot of OPHN1 in acute hippocampal slices (CA1 regions) from rats pretreated with anisomycin (20 µM) or control vehicle for 30 min prior to DHPG (100 µM, 10 min) or ACSF treatment. γ-tubulin was used as loading control. (B2) Mean OPHN1 levels in drug treated slices expressed as percentage of control treated slices. OPHN1 levels were normalized to γ-tubulin levels in the same sample. n = 3–6 slices (400 µm) from 3–6 animals per condition. *p < 0.01 (unpaired t-test), as compared with control.
(C) Western blots of OPHN1 in acute hippocampal slices (CA1 regions) from rats treated with DHPG (100 µM, 10 min), NMDA (20 µM, 3 min), or ACSF. ERK2 was used as loading control. NMDA treatment does not increase OPHN1 levels; n = 3 slices (400 µm) from 3 animals; p > 0.05.
(D1) Representative differential interference contrast (DIC) (left) and OPHN1 immunofluorescence (middle) images from CA1 regions where a cut severed the dendrites from the pyramidal cell layer. βIII-tubulin immunoreactivity (right) indicates the presence of dendrites. Top: vehicle treated; bottom: DHPG treated (100 µM, 10 min). Scale bar, 20 µm. (D2) Quantification of OPHN1 immunofluorescence in dendrites that were severed (cut) from the soma and in neighboring uncut dendrites, akin as in A2. n = 5 slices (40 µm) from 5 animals per condition. *p < 0.01 (unpaired t-test), as compared with control. Quantification of βIII-tubulin immunofluorescence reveals no difference in βIII-tubulin levels in cut and uncut dendrites treated with DHPG or control vehicle; p > 0.05.
(E1) Western blots of synaptoneurosome preparation (SN) reveals enrichment of PSD-95 and Synaptophysin (Syn) and a reduction in βIII-tubulin and Histone H3 (H3) in comparison to whole homogenate (Input) or supernatant (Sup). (E2) Western blot of synaptoneurosome preparation pretreated with anisomycin (20 µM) or control vehicle 30 min prior to DHPG treatment (100 µM, 15 min). (E3) Mean OPHN1 levels in drug treated synaptoneurosomes expressed as percentage of control vehicle treated synaptoneurosomes. OPHN1 levels were normalized to ERK2 levels in the same sample. n = 3 independent preparations from 3 animals. *p < 0.01 (unpaired t-test), as compared with control.
(F1) Western blot of OPHN1 in acute hippocampal slices (CA1 regions) from rats pretreated with LY367385 (100 µM), MPEP (10 µM), or control vehicle 30 min before DHPG treatment (100 µM, 10 min). ERK2 was used as loading control. (F2) Mean OPHN1 levels are presented as in E1 above. n = 3–6 slices (400 µm) from 3–6 animals per condition. *p < 0.01 (unpaired t-test), as compared with control.
(G1) Western blot of OPHN1 in acute hippocampal slices (CA1 regions) from 4 to 6 weeks old Fmr1 KO mice and corresponding WT mice treated with DHPG (100 µM, 10 min) or ACSF. βIII-tubulin was used as loading control. (G2) Mean OPHN1 levels are presented as above. n = 4 slices (400 µm) from 4 animals per condition. *p < 0.01 (unpaired t-test), as compared with control treated WT slices.
The observed increase in dendritic OPHN1 levels within 10 min of DHPG application could be the result of new OPHN1 synthesis from preexisting mRNA residing in the dendrites. We note that OPHN1 mRNA is present in dendrites of unstimulated hippocampal neurons (Figure S2). Alternatively, this could be due to rapid transport of OPHN1 from the cell body. To distinguish between these two possibilities, we determined whether DHPG increases OPHN1 protein levels in isolated dendrites. To this end, slices in which the CA1 pyramidal neuron soma had been mechanically severed from the dendrites were treated with DHPG, or control vehicle, for 10 min. DHPG effectively increased OPHN1 protein levels in the isolated dendrites (Figure 1D), implying that OPHN1 is locally synthesized in dendrites. Finally, to determine whether mGluR activation elicits synaptic synthesis of OPHN1, we prepared hippocampal synaptoneurosomes (Figure 1E), and incubated them for 15 min with DHPG or control vehicle. Western blot analysis revealed an increase in OPHN1 protein levels in DHPG-treated synaptoneurosomes, which was blocked by preincubation of the synaptoneurosomes with anisomycin (Figure 1E). Together, these data provide evidence for mGluR-induced rapid dendritic synthesis of OPHN1 protein in CA1 hippocampal neurons.
DHPG-Induced Rapid Increase in OPHN1 Expression Depends on mGluR1 Activation and Occurs in the Absence of FMRP
Group I mGluRs consist of two subtypes, mGluR1 and mGluR5, and both of these receptors contribute to the induction of mGluR-LTD in the CA1 hippocampal area (Hou and Klann, 2004; Volk et al., 2006). To determine which of the group I mGluR subtype(s) is responsible for the rapid DHPG-induced increase in OPHN1, we applied specific mGluR1 or mGluR5 antagonists (LY367385 and MPEP, respectively) to acute hippocampal slices, 30 min before the addition of DHPG. As expected, OPHN1 levels were elevated within 10 min upon application of DHPG alone. This elevation, however, was blocked when LY367385 was present (Figures 1F and S1C). In contrast, MPEP did not appreciably affect the DHPG-induced increase in OPHN1 levels (Figures 1F and S1C). Treatment of slices with either LY367385 or MPEP alone did not alter basal levels of OPHN1 (data not shown). These data indicate that the rapid increase of OPHN1 largely depends on activation of mGluR1, rather than mGluR5.
A key player in the regulation of mGluR-stimulated protein translation is the FMRP protein. In the absence of FMRP, excess basal translation and loss of mGluR-induced translation of selected mRNAs, including those encoding MAP1B and Arc, have been reported (reviewed in Bassell and Warren, 2008). Although loss of FMRP has generally been linked to excessive mGluR5 signaling (Bassell and Warren, 2008; Dolen et al., 2007; Osterweil et al., 2010), at this point, however, a role for FMRP in the regulation of OPHN1 synthesis could not be excluded. To assess this, we prepared acute hippocampal slices from Fmr1 knockout (KO) mice and corresponding wild type mice, and stimulated them with DHPG or control vehicle. OPHN1 expression in control vehicle-treated slices was not considerably different between wild type and Fmr1 KO conditions (Figure 1G). Moreover, DHPG treatment of Fmr1 KO derived slices resulted in a rapid increase in OPHN1 protein levels to an extent similar as seen in wild type DHPG-treated slices (Figure 1G). Thus, loss of FMRP does neither affect basal OPHN1 levels nor the mGluR-induced upregulation of OPHN1, implying that the synthesis of OPHN1 in hippocampal neurons is not subject to FMRP regulation.
OPHN1 Knockdown Impairs mGluR-LTD
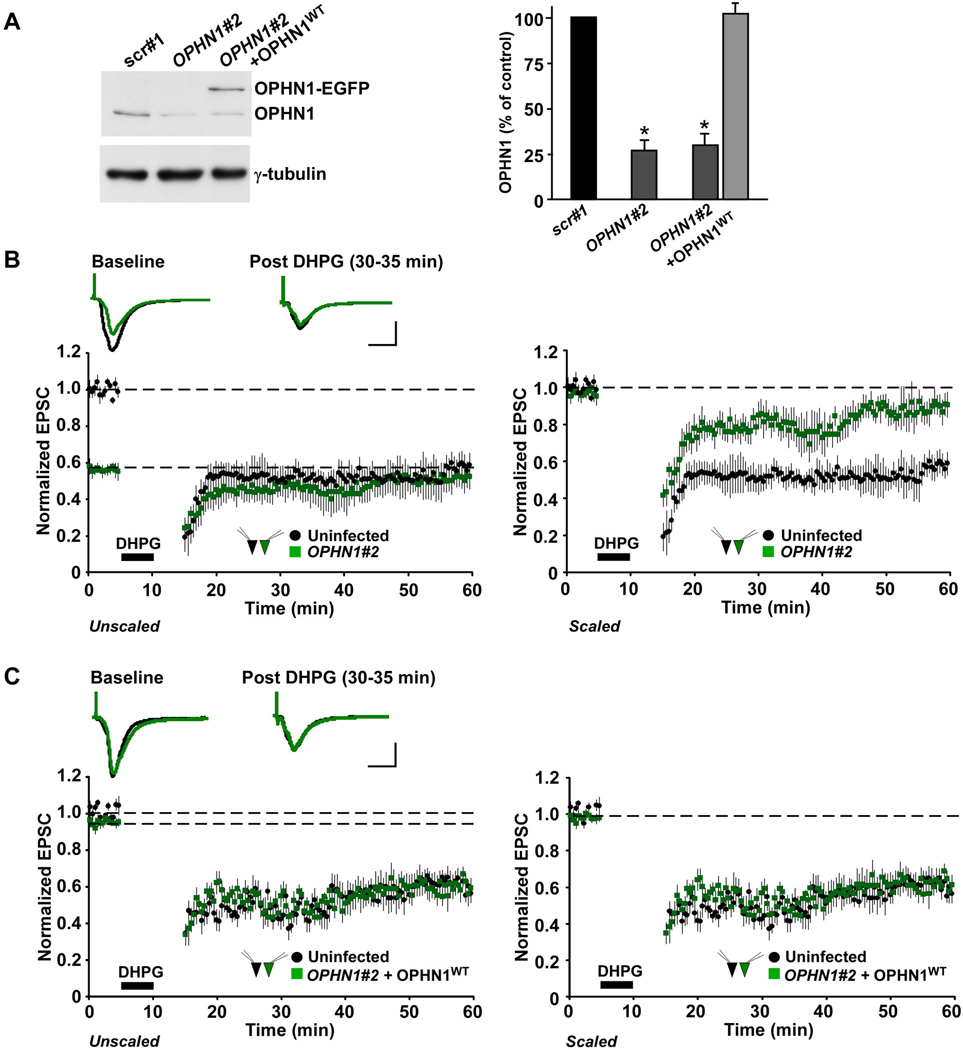
Based on our findings that OPHN1 becomes rapidly upregulated in dendrites of CA1 neurons in response to mGluR activation, we next investigated whether OPHN1 is required for mGluR-mediated LTD at CA1 synapses. To this end, we utilized a lentivirus that coexpresses EGFP and a short-hairpin (sh) RNA (OPHN1#2) to knockdown OPHN1 mRNA and protein (Nadif Kasri et al., 2009). The OPHN1#2 shRNA significantly reduced endogenous OPHN1 protein levels in hippocampal neurons, whereas a control scrambled shRNA (scr#1) was ineffective (Figure 2A, (Nadif Kasri et al., 2009). We opted for this approach because it allows for spatio-temporal regulation of endogenous OPHN1 expression. RNAi-mediated temporal knockdown of OPHN1 selectively in CA1 neurons has no detectable effect on presynaptic function and it minimizes the possibility of developmental compensations (Nadif Kasri et al., 2009); both of these events could affect the induction and expression of mGluR-LTD (Khelfaoui et al., 2007).
Figure 2. Knockdown of OPHN1 Impairs DHPG-Induced LTD.
(A) Left panel: Dissociated hippocampal cultures were infected with lentiviral vectors expressing scr#1 shRNA and EGFP, OPHN1#2 shRNA and EGFP, or OPHN1#2 shRNA and OPHN1WT-EGFP. 8 d post-infection, cell extracts were analyzed by Western blotting with anti-OPHN1 1296 and anti-γ-tubulin antibody as loading control. Right panel: Mean OPHN1 levels in infected neurons presented as a percentage of scr#1 shRNA expressing cells. OPHN1 levels were normalized to γ-tubulin levels in the same sample. OPHN1#2 shRNAs significantly decreased OPHN1 levels. n = 3 in all cases, *p < 0.001, t-test. Error bars represent SEM.
(B–C) DHPG-induced LTD in hippocampal brain slice cultures. Normalized EPSC amplitudes plotted against time from pairs of uninfected and OPHN1#2 shRNA (B) or OPHN1#2 shRNA + OPHN1WT (C) infected CA1 pyramidal neurons during baseline and after LTD induction (100 µM DHPG, 5 min). Top panels: Representative traces of AMPAR-EPSCs before and after DHPG induction for control (black) and infected (green) neurons. Scale bars, 30 ms and 20 pA. Bottom left panels: EPSC amplitudes normalized to the baseline of uninfected neurons (Unscaled). Bottom right panels: EPSC amplitudes normalized to baseline responses of each cell (Scaled). (B) n = 10 pairs; (C) n = 7 pairs. AMPAR EPSCs measured between 35 and 40 min after DHPG treatment, p < 0.01, paired t-test.
CA1 neurons in cultured hippocampal slices were infected with the OPHN1#2 shRNA containing lentivirus, and 8 to 10 days post-infection the magnitude of mGluR-dependent LTD induced in control uninfected and OPHN1#2 shRNA infected cells with bath application of DHPG (100 µM, 5 min) was examined. Consistent with previous studies (Huber et al., 2000; Huber et al., 2001; Volk et al., 2006), DHPG caused a depression of AMPAR-mediated synaptic transmission in control cells, which is protein translation dependent, and, notably, is attenuated by blockade of mGluR1 with LY367385 throughout the experiment (Figures 2B, S3A and S3B). When compared to mGluR-LTD induced in simultaneously recorded control cells, knockdown of OPHN1 greatly reduced the magnitude of mGluR-LTD. A depression in AMPAR-mediated synaptic transmission of approximately 40% was observed in control cells versus 10% in OPHN1#2 shRNA expressing cells, 30 to 35 min after DHPG application (Figure 2B). To ensure that this effect was specifically caused by impaired OPHN1 expression, we performed rescue experiments by using OPHN1 cDNA that is resistant to OPHN1#2 shRNA-mediated RNAi (Nadif Kasri et al., 2009). The levels of OPHN1 expression in hippocampal neurons coexpressing RNAi- resistant OPHN1WT and OPHN1#2 shRNA were restored to normal levels (Figure 2A), and, most importantly, the magnitude of mGluR-LTD was comparable to that of control neurons (Figure 2C). Thus, knockdown of OPHN1 impairs mGluR-LTD.
One possible explanation for the impaired mGluR-LTD is that it is due to reduction in basal synaptic strength, as OPHN1 RNAi depresses glutamatergic synaptic transmission (Figure 2B, left panel before DHPG application, and see Figure 4A), thereby occluding LTD. Alternatively, however, activity-dependent OPHN1 induction could play a critical role in mediating mGluR-LTD independent of its effects on basal synaptic strength. Distinguishing between these two possibilities requires a dissociation of OPHN1’s role in regulating basal synaptic transmission and mGluR-LTD. To determine whether such dissociation can be achieved, we resorted to OPHN1 mutants and synthetic blocking peptides that selectively disrupt the interaction between OPHN1 and OPHN1-binding partners present in dendritic spines; the synaptic effects of these mutants and peptides were subsequently tested.
Figure 4. Replacement of Endogenous OPHN1 with OPHN1Endo, But Not OPHN1GAP or OPHN1Hom, Rescues Basal AMPAR and NMDAR EPSCs.
(A–E) Amplitudes of AMPAR (left panel) and NMDAR (right panel) EPSCs of uninfected neurons are plotted against simultaneously recorded neighboring neurons expressing OPHN1#2 shRNA (A), OPHN1#2 shRNA+OPHN1WT (B), OPHN1#2 shRNA+OPHN1GAP (C), OPHN1#2 shRNA+OPHN1Hom (D) or OPHN1#2 shRNA+OPHN1Endo (E). Gray symbols represent single pairs of recordings; black symbols show mean ± SEM. Inserts in each panel show sample averaged traces; green traces, infected neurons; black traces, uninfected neighboring neurons. Scale bars, 20 ms and 20 pA. See Supplemental Data for quantifications.
(F) Summary (mean ± SEM) of effects of expressing OPHN1#2 shRNA alone or together with OPHN1WT or one of the indicated OPHN1 mutants on AMPAR (left) and NMDAR (right) EPSCs calculated as the averaged ratios obtained from pairs of infected and uninfected neighboring neurons. Data are shown as mean ± SEM. *p < 0.05, paired t-test.
Selective Disruption of OPHN1 Interactions with Its Postsynaptic Binding Partners Homer 1b/c, RhoA, and Endophilin A2/3
We previously described an interaction between OPHN1 and the small GTPase RhoA, as well as Homer 1b/c, at the post-synaptic site of hippocampal neurons (Govek et al., 2004). We showed that OPHN1 through its Rho-GAP domain represses the RhoA/Rho-kinase pathway in spines of CA1 neurons (Govek et al., 2004), and generated an OPHN1 mutant, OPHN1GAP (R409Q) that abolishes its Rho-GAP activity (Nadif Kasri et al., 2009, see Figure 3A). This mutant failed to rescue the OPHN1 RNAi-evoked defects in structural and functional maturation of glutamatergic synapses (Nadif Kasri et al., 2009). With regards to OPHN1 and Homer 1b/c, we demonstrated that these proteins physically interact and co-localize in dendritic spines (Govek et al., 2004, Figure S4A); the importance of this association remained however unknown. Introduction of mutations in the consensus Homer binding motif located in the N-terminus of OPHN1 disrupted its interaction with Homer 1b/c (OPHN1Hom, Figures 3A and 3B; Govek et al., 2004). As an additional tool to acutely disrupt this interaction, we designed a peptide consisting of an OPHN1 sequence that contains the Homer ligand domain (pep-OPHN1Hom, Figure 3C). The peptide was made cell permeable by addition of the human immunodeficiency virus-type 1 Tat sequence. We found that this peptide disrupts the OPHN1-Homer 1b/c interaction (Figure 3C), whereas a control peptide containing three amino acid substitutions in the binding motif did not (pep-contHom, Figure 3C). Notably, pep-OPHN1Hom. did not disrupt the association between Homer 1b/c and Dynamin-3, nor between Homer 1b/c and mGluR5 (Figures S4B and S4C).
A third class of proteins we found to associate with OPHN1 are members of the Endophilin A family, which include Endophilin A1, A2 and A3 (Kjaerulff et al., 2010). In previous studies, we and others demonstrated a direct interaction between OPHN1 and Endophilin A1 (Endo1) (Khelfaoui et al., 2009; Nakano-Kobayashi et al., 2009), which is predominantly expressed in presynaptic nerve terminals, and showed that this interaction is critical for OPHN1’s presynaptic function in synaptic vesicle retrieval (Nakano-Kobayashi et al., 2009). The Endophilin A2 (Endo2) and Endophilin A3 (Endo3) proteins, on the other hand, are enriched in postsynaptic compartments and have been implicated in the regulation of AMPAR endocytosis in hippocampal neurons (Chowdhury et al., 2006). Given that all three family members are highly conserved, containing an N-terminal N-BAR (Bin/Amphiphysin/Rvs) domain and a C-terminal SH3 domain (Kjaerulff et al., 2010), we tested whether OPHN1 also interacts with Endo2 and 3. We found that this is indeed the case (Figures 3D and 3E; S5A and S5B), and that the interaction is mediated via binding of the third proline rich domain (PRD3) of OPHN1 to the SH3 domain of Endo2/3 (Figures 3D and S5B, data not shown). Moreover, coimmunoprecipitation experiments revealed that treatment of hippocampal slices with DHPG leads to increased binding of OPHN1 to Endo2/3, which, notably, is protein synthesis dependent (Figure 3E). Mutations in the PRD3 of OPHN1 (PRD3*) disrupt its association with Endo2/3 (OPHN1Endo, Figures 3D and S5B). Specific disruption of the OPHN1-Endo2/3 interaction was also achieved by employing a peptide consisting of an OPHN1 sequence that contains the Endophilin ligand domain (pep-OPHN1Endo), but not a control peptide containing three amino acid substitutions in the binding motif (pep-contEndo) (Figures 3F and S5C–S5E). Importantly, all three OPHN1 mutants, OPHN1GAP, OPHN1Hom, and OPHN1Endo still resided in spines, as revealed by two-photon microscopy of CA1 neurons of hippocampal slices (Figure S5F). Also, treatment of slices with either pep-OPHN1Hom or pep-OPHN1Endo did not affect the localization of OPHN1 in spines (data not shown).
Regulation of Basal Synaptic Transmission by OPHN1 Requires its Rho-GAP Activity and Interaction with Homer 1b/c, but Not Endo2/3
To determine whether disruption of any of the above-described interactions could dissociate OPHN1’s role in regulating basal synaptic transmission and mGluR-LTD, we began by examining the synaptic effects of replacing endogenous OPHN1 with one of the three OPHN1 mutants using a lentivirus-mediated molecular replacement strategy (Nadif Kasri et al., 2009). To this end, lentiviral vectors that coexpress OPHN1#2 shRNA and RNAi-resistant OPHN1GAP, OPHN1Hom or OPHN1Endo fused to EGFP were generated. We first tested whether any of these mutants could rescue the decrease in basal synaptic strength caused by OPHN1 RNAi in CA1 neurons (Figures 4A and 4F). Coexpression of OPHN1WT with OPHN1#2 shRNA restored basal synaptic strength to normal (Figures 4B and 4F). In contrast, coexpression of OPHN1GAP or OPHN1Hom failed to rescue the OPHN1#2 shRNA-evoked defects in AMPAR- and NMDAR-mediated transmission (Figures 4C, 4D, 4F). Interestingly, coexpression of OPHN1Endo rescued the defects in basal synaptic transmission akin to OPHN1WT (Figures 4E and 4F). Notably, all OPHN1 mutants were expressed at similar levels (Figure S6). These results indicate that OPHN1’s Rho-GAP activity and interaction with Homer 1b/c, but not Endo2/3, are important for regulating basal synaptic strength.
OPHN1 Mediates mGluR-Dependent LTD through Interaction with Endo2/3
Next, we examined the abilities of OPHN1GAP, OPHN1Hom, and OPHN1Endo to rescue the deficit in mGluR-LTD caused by OPHN1 knockdown, using the above described replacement strategy. CA1 neurons coexpressing OPHN1#2 shRNA and OPHN1GAP, or OPHN1Hom, displayed impaired mGluR-LTD to an extent similar to that seen in cells expressing OPHN1#2 shRNA alone (Figures 5A, 5B, and 5D). Most interestingly, neurons coexpressing OPHN1#2 shRNA and OPHN1Endo, although having normal basal synaptic transmission, showed a defect in mGluR-LTD (Figures 5C and 5D). These results indicate that the effects of OPHN1 on basal synaptic transmission and mGluR-LTD are dissociable and involve distinct protein-protein interactions, with the interaction between OPHN1 and Endo2/3 being critical for its role in mGluR-LTD.
Figure 5. Molecular Dissociation of OPHN1’s Role in Regulating Basal Synaptic Strength and mGluR-LTD.
(A–C) Normalized EPSC amplitudes plotted against time from pairs of uninfected and OPHN1#2+OPHN1GAP (A), OPHN1#2+OPHN1Hom (B), or OPHN1#2+OPHN1Endo (C) infected neurons during baseline and after LTD induction (100 µM DHPG, 5 min). Top panels: Representative traces of AMPAR-EPSCs before and after DHPG induction for control and infected neurons. Scale bars, 30 ms and 20 pA. Bottom left panels: EPSC amplitudes normalized to the baseline of uninfected neurons (Unscaled). Bottom right panels: EPSC amplitudes normalized to the baseline responses of each cell (Scaled).
(D) Baseline (DHPG -) and 30–35 min post DHPG (DHPG +) data are summarized. Baseline AMPAR currents and AMPAR currents 30–35 min after DHPG application are normalized to baseline AMPAR currents of adjacent uninfected neurons for each indicated condition. Data are shown as mean ± SEM. *p < 0.005, paired t-test. See Supplemental Data for quantifications.
To corroborate and extent these findings, we next investigated the impact of pep-OPHN1Endo and pep-OPHN1Hom, which disrupt OPHN1-Endo2/3 and OPHN1-Homer interactions, respectively, on mGluR-LTD in acute hippocampal brain slices. Postsynaptic infusion of pep-OPHN1Endo through a whole-cell patch pipette (30 min prior to DHPG application) significantly reduced mGluR-LTD compared to the control peptide (Figure 6A). Infusion of CA1 neurons with pep-OPHN1Endo had no effect on basal synaptic transmission (Figure 6B). These findings indicate that the actions of pep-OPHN1Endo are rapid and corroborate our results obtained with the OPHN1Endo mutant. When pep-OPHN1Hom was included in the pipette, mGluR-LTD and baseline EPSC amplitudes were comparable to those of the control peptide (Figures 6C and 6D). Of note, the lack of an effect on basal synaptic transmission upon short-term disruption of the OPHN1/Homer 1b/c interaction with pep-OPHN1Hom is consistent with previous findings that prolonged, but not short-term, knockdown of OPHN1 reduces basal synaptic transmission (Nadif Kasri et al., 2009). Together, our data indicate that OPHN1 plays a crucial role in mediating mGluR- LTD, and that OPHN1’s interaction with Endo2/3, but not Homer 1b/c proteins, is critical for this event.
Figure 6. OPHN1 Interaction with Endo2/3, But Not Homer 1b/c, Is Required for mGluR-LTD and Associated Decreases in Surface AMPARs.
(A) Using simultaneous dual-patch-clamp recording from two neurons, DHPG-induced mGluR-LTD (100 µM, 5 min) is present in cells infused with control pep-contEndo (10 µM), but is blocked in cells infused with pep-OPHN1Endo (10 µM). Neurons were infused with respective peptides 30 min prior to DHPG application. Scale bars, 30 ms and 20 pA. n = 6 pairs, p < 0.05, paired t-test; see Supplemental data for quantifications.
(B) Average normalized EPSC amplitudes of CA1 neurons from acute hippocampal slices infused with 10 µM pep-contEndo or pep-OPHN1Endo for 60 min (n= 4 pairs). No changes in EPSC amplitudes were observed, paired t-test.
(C) Using simultaneous dual-patch-clamp recording from two neurons, mGluR-LTD (DHPG 100 µM, 5 min) is induced in cells infused with pep-OPHN1Hom (10 µM) and pep-contHom (10 µM). Neurons were infused 30 min prior to DHPG application. Scale bars, 30 ms and 20 pA. n = 5 pairs, p > 0.05, paired t-test; see Supplemental data for quantifications.
(D) Average normalized EPSC amplitudes of CA1 neurons from acute hippocampal slices infused with 10 µM pep-contHom or pep-OPHN1Hom peptide for 60 min (n = 4 pairs). No changes in EPSC amplitudes were observed, paired t-test.
(E) Representative images of surface GluR1 in cultured hippocampal neurons pretreated with no peptide (no pep), pep-contEndo or pep-OPHN1Endo (5 µM) 3 h prior to DHPG or control media (10 min) treatment. 1 h post-treatment, neurons were live-labeled with anti-GluR1 antibody, fixed and processed for surface GluR1 immunofluorescence. Scale bars, 10 µm in top and 2 µm in bottom panels.
(F) Quantification of the number of surface GluR1 puncta presented as percentage of control (i.e. DHPG-unstimulated and no peptide). n = 74–118 neurons from 5 cultures per condition. *p < 0.05 (unpaired t-test), as compared with control. Error bars represent SEM.
Previous studies have shown that activation of group I mGluRs leads to persistent decreases in surface AMPAR expression levels that mediate LTD (Snyder et al., 2001; Waung et al., 2008). Since the OPHN1-Endo2/3 interaction is critical for mGluR-LTD, we directly tested whether it is important for mGluR-induced changes in surface AMPAR expression and endocytosis. To quantify AMPAR surface levels and the degree of AMPAR internalization, we employed a biochemical method to crosslink surface-only AMPAR subunits. Acute slices of hippocampal area CA1 were preincubated with no peptide, pep-contEndo or pep-OPHN1Endo. The CA1 slices were then treated with DHPG or control vehicle (for 10 min), and 50 min later incubated with the membrane-impermeant cross-linking reagent bis (sulfosuccinimidyl) suberate (BS3). Western blotting with an anti-GluR1 antibody revealed a decrease in cell-surface GluR1 expression and an increase in internalized GluR1 levels 1 h after DHPG treatment in the no peptide and control peptide preincubated CA1 slices (Figures S7A and S7B). The DHPG-induced decrease in cell-surface GluR1 expression and increase in internal GluR1 levels were, however, significantly attenuated in CA1 slices that were preincubated with pep-OPHN1Endo (Figures S7A and S7B). Of note, the pep-OPHN1Endo peptide did not affect basal levels of surface GluR1 (Figures S7A and S7B). Similar results were obtained for the GluR2 AMPAR subunit (data not shown). To corroborate these findings, we undertook an immunofluorescence approach to measure AMPAR surface levels. Cultured hippocampal neurons, preincubated with no peptide, pep-contEndo or pep-OPHN1Endo, were treated with DHPG or control vehicle (for 10 min), and 1 h after treatment labeled with an N-terminal directed anti-GluR1 antibody. Consistent with our above biochemical data, pep-OPHN1Endo did not affect basal levels of surface GluR1, but attenuated the decrease of surface GluR1 observed 1 h post-DHPG (Figures 6E and 6F). We conclude that the OPHN1-Endo2/3 interaction plays a key role in mGluR-triggered long-term decreases in surface AMPARs.
Acute Blockade of mGluR-Induced OPHN1 Synthesis Impedes mGluR-LTD
Our data showed that mGluR activation triggers rapid synthesis of OPHN1 and that OPHN1 mediates mGluR-LTD and the associated long-term decreases in surface AMPAR expression through its interaction with Endo2/3. The latter experiments, however, did not address whether new synthesis of OPHN1 in response to mGluR activation is required for these events. To prevent/block mGluR-elicited new synthesis of OPHN1, we employed a previously described siRNA (Ophn1#2 siRNA) (Govek et al., 2004). We reasoned that acute delivery of Ophn1#2 siRNA should only prevent the DHPG-induced rapid increase in OPHN1 expression, without affecting basal levels of OPHN1, given that OPHN1 is a relatively stable protein and there is very little OPHN1 synthesis for a period of up to several hours in the absence of DHPG (Figure S8A, data not shown). To test this, Ophn1#2 siRNA or a non-targeting Ophn1 mismatch siRNA was introduced into cultured hippocampal neurons using lipid mediated transfer. Thirty minutes after siRNA delivery, neurons were treated with DHPG or control vehicle for 10 min, and analyzed by confocal microscopy (Figure 7A). Of note, we know from experiments using fluorescently labeled siRNAs that the siRNAs are effectively taken up by the cells within a 30 min time frame (Figures S8B–8D). DHPG stimulation over a period of 10 min induced a significant increase in dendritic OPHN1 levels in neurons exposed to the mismatch siRNA, and, importantly, this increase was abolished in neurons subjected to the Ophn1#2 siRNA (Figures 7A and 7B, DHPG). Notably, incubation of neurons with Ophn1#2 siRNA for 40 min in the absence of DHPG did not affect the basal levels of OPHN1 (Figures 7A and 7B, control). Thus, these data indicate that acute delivery of Ophn1#2 siRNA can be used to prevent/block new OPHN1 synthesis induced by DHPG.
Figure 7. Acute Blockade of New OPHN1 Synthesis Blocks mGluR-Induced LTD and Long-Term Decreases in Surface AMPARs.
(A) Representative images of OPHN1 immunofluorescence in cultured hippocampal neurons pretreated (30 min in Lipofectamine) with Ophn1#2 siRNA or mismatch siRNA. Neurons were then treated with DHPG (100 µM) or control media, and fixed 10 min after onset of treatment. Scale bars, 10 µm in top and 2 µm in bottom panels.
(B) Quantification of OPHN1 immunofluorescence in dendrites. Mean OPHN1 fluorescence intensity presented as percentage of mismatch siRNA. n = 27–49 cells from 3 cultures per condition, *p < 0.05 (unpaired t-test), as compared with mismatch siRNA. Error bars represent SEM in all panels.
(C) Representative images of surface GluR1 in cultured hippocampal neurons pretreated with Ophn1#2 siRNA or mismatch siRNA 30 min prior to DHPG or control media (10 min) treatment. 1 h post-DHPG or control media, neurons were live-labeled with anti-GluR1 antibody, fixed and processed for surface GluR1 immunofluorescence. Scale bars, 10 µm in top and 2 µM in bottom panels.
(D) Quantification of the number of surface GluR1 puncta presented as percentage of mismatch siRNA. n = 30–35 neurons from 3 cultures per condition, *p < 0.05 (unpaired t-test), as compared with control mismatch siRNA.
(E) Average normalized EPSC amplitudes of CA1 neurons from acute hippocampal slices recorded with pipettes containing 25 nM mismatch siRNA or Ophn1#2 siRNA for 60 min (n= 5 pairs). No changes in EPSC amplitudes were observed, paired t-test.
(F) DHPG applied to the bath resulted in LTD of EPSC amplitudes in neurons filled with mismatch siRNA, but was blocked in neurons filled with Ophn1#2 siRNA. n = 6 pairs.
(G) Representative traces of AMPAR-EPSCs at −60 mV of neurons recorded with pipettes containing 25 nM mismatch siRNA or Ophn1#2 siRNA before and 30–35 min after LTD induction. Scale 20 ms, 20 pA.
(H) Summary graph of AMPAR-EPSCs at −60 mV of neurons recorded with pipettes containing mismatch siRNA or Ophn1#2 siRNA before and 30–35 min after LTD induction. n = 6 pairs for all conditions, **p < 0.01, paired t-test; see Supplemental data for quantifications.
Using the Ophn1#2 and mismatch siRNAs, we then investigated the effects of blocking rapid OPHN1 synthesis on mGluR-induced decreases in surface AMPARs. Thirty minutes after delivery of the siRNAs, neurons were treated with DHPG or control vehicle (for 10 min), and labeled as described above with an N-terminal directed anti-GluR1 antibody 1 h post-treatment. Ophn1#2 siRNA did not affect basal levels of surface GluR1, however, it hampered the decrease of surface GluR1 observed 1 h after DHPG treatment (Figures 7C and 7D). These data indicate that rapid OPHN1 synthesis is important for the mGluR-induced persistent decreases in surface AMPAR expression.
Next, we tested the effect of blocking rapid OPHN1 synthesis on basal synaptic transmission and DHPG-induced mGluR-LTD. We introduced Ophn1#2 siRNA, or mismatch siRNA, into CA1 neurons of acute hippocampal slices via whole-cell recording pipettes, and recorded evoked ESPCs. Similar to the mismatch siRNA, Ophn1#2 siRNA did not affect basal synaptic transmission (Figure 7E). To induce LTD, we subjected the slices to DHPG bath application (100 µM, 5 min) 30 min after breaking into the cells. LTD was observed in cells infused with control mismatch siRNA (Figures 7F–H). In contrast, DHPG failed to induce LTD in neurons infused with Ophn1#2 siRNA. Note, all LTD experiments were performed within the same slice using two simultaneous patch-clamp recordings of neighboring CA1 cells; each pipette was filled with one of the siRNAs. Together, these data indicate that rapid synthesis of OPHN1 is necessary for mGluR-LTD.
Noteworthy, previous studies demonstrated that mGluR-LTD persists in the absence of protein synthesis in Fmr1 KO mice (Hou et al., 2006; Nosyreva and Huber, 2006). Our data indicate that mGluR-induced OPHN1 synthesis is independent of FMRP (Figure 1G), raising the question as to whether mGluR-LTD in Fmr1 KO mice still requires OPHN1 synthesis. To address this, we introduced Ophn1#2 siRNA, or mismatch siRNA, into CA1 neurons of acute hippocampal slices prepared from Fmr1 KO and corresponding wild type mice, and subjected the slices to DHPG bath application 30 min after breaking into the cells. LTD was observed in both wild type and Fmr1 KO cells infused with the control mismatch siRNA (Figures 8A and 8B), which consistent with previous reports was protein synthesis dependent in wild type, but not Fmr1 KO neurons (data not shown). Interestingly, whereas DHPG-induced LTD was inhibited in wild type neurons infused with Ophn1#2 siRNA, LTD was not affected in Fmr1 KO neurons infused with the Ophn1#2 siRNA (Figures 8A and 8B). These data indicate that OPHN1 synthesis is required for mGluR-LTD in wild type, but not Fmr1 KO mice. Likely, the elevated/aberrant protein synthesis caused by loss of FMRP can compensate for the requirement of new synthesis of OPHN1.
Figure 8. OPHN1 Synthesis Is Not Required for mGluR-LTD in Fmr1 KO Mice and Model for Dual Function of OPHN1 in Basal Synaptic Function and mGluR-LTD.
(A–B) DHPG-induced mGluR-LTD was measured in acute hippocampal brain slices of wild type and Fmr1 KO mice in the presence of mismatch siRNA or Ophn1#2 siRNA (25 nM). Top panels: Representative traces of AMPAR-EPSCs at −60 mV of neurons recorded with pipettes containing mismatch siRNA or Ophn1#2 siRNA before (1) and 30–35 min after (2) LTD induction. Scale 40 ms, 20 pA. Bottom panel: AMPAR currents 30–35 min after DHPG application normalized to baseline AMPAR currents before DHPG application. n = 6 pairs for all conditions. (A) p < 0.01, (B) p >0.05, paired t-test; see Supplemental data for quantifications. In all cases neurons were infused with respective siRNA 30 min prior to DHPG application.
(C) Model for effects of OPHN1 on basal synaptic function and mGluR-LTD. Left panel: OPHN1 in basal synaptic function. Synaptic activity through NMDAR activation drives OPHN1 into dendritic spines, where it forms a complex with Homer 1b/c proteins and through its Rho-GAP activity locally suppresses RhoA activity and remodels actin filaments. Via its actions on Homer 1b/c and RhoA, OPHN1 regulates activity-dependent AMPAR synaptic stabilization, as well as maintenance of spine structure, thereby permitting the maturation and strengthening of CA1 excitatory synapses (Nadif Kasri et al., 2009). Right panel: OPHN1 in mGluR-LTD. Activation of mGluR1 triggers a rapid increase in dendritic OPHN1 synthesis. mGluR1-induced OPHN1 then engages in a complex with Endophilin A2/3 and Dynamin to enhance AMPAR internalization, thereby mediating persistent decreases in surface AMPARs and LTD.
DISCUSSION
A common feature for mGluR-LTD in many brain regions is the reliance on rapid and local protein synthesis (Luscher and Huber, 2010; Waung and Huber, 2009). The identities of the newly synthesized proteins that mediate LTD, however, remain largely elusive, with Arc/Arg3.1 being the leading candidate ‘LTD protein’ in the hippocampal CA1 area (Park et al., 2008; Waung and Huber, 2009; Waung et al., 2008). Our study identifies the X-linked mental retardation protein, OPHN1, as a new molecule that is rapidly synthesized upon activity and is required for mGluR-LTD in the hippocampus. Importantly, the role of OPHN1 in mediating mGluR-LTD can be molecularly dissociated from its role in basal AMPAR-mediated synaptic transmission (Nadif Kasri et al., 2009). Whereas the former requires OPHN1’s interaction with Endo2/3, the latter requires OPHN1’s Rho-GAP activity and interaction with the Homer 1b/c proteins (Figure 8C).
Group I mGluR Activation Triggers Rapid and Dendritic OPHN1 Synthesis in an mGluR1 Dependent and FMRP Independent Manner
Our results provide several lines of evidence for rapid dendritic synthesis of OPHN1 in response to group I mGluR stimulation in the hippocampal CA1 area. First, activation of group I mGluRs triggers a fast upregulation (within 10 min) of OPHN1 in hippocampal CA1 neurons, in a process that relies on protein synthesis from preexisting mRNA. Second, the rapid upregulation of OPHN1 not only occurs in dendrites of intact hippocampal CA1 neurons, but also in isolated dendrites that have been severed from their cell bodies, implying that the increased OPHN1 levels in dendrites are not caused by soma-mediated synthesis and transport into the dendrites. Finally, rapid protein synthesis dependent upregulation of OPHN1 is also evident in synaptoneurosomes upon group I mGluR activation. Notably, stimuli that elicit NMDAR-dependent LTD or -LTP, or spontaneous synaptic activity, do not trigger an increase in OPHN1 protein expression (this study and Nadif Kasri et al., 2009), suggesting that OPHN1 induction is rather specific for mGluR-inducing stimuli.
Our results further reveal that the mechanism by which mGluR activity triggers rapid OPHN1 synthesis involves the activation of mGluR1, rather than mGluR5. This is of particular interest, as little is known about how mGluR1 is molecularly linked to the translational machinery, and, most importantly, what its relevant targets are in the hippocampal CA1 area (Waung and Huber, 2009). To our knowledge, OPHN1 is the first protein shown to be rapidly induced by mGluR activity in an mGluR1 dependent manner. In the case of, for instance, STEP, its induction occurs in an mGluR5 dependent manner (Zhang et al., 2008). Intriguingly, our results also indicate that the synthesis of OPHN1 associated with mGluR activation is FMRP independent. In contrast to Arc and MAP1B (Hou et al., 2006; Park et al., 2008), the basal level of OPHN1 is not elevated in the hippocampus of Fmr1 KO mice and it can be increased upon mGluR stimulation. Hence, OPHN1 is not likely a target for FMRP-mediated repression. With regard to this finding, and in light of our finding that OPHN1 synthesis is dependent on mGluR1 activation, it is noteworthy that the function of FMRP in mGluR-stimulated protein synthesis has been linked mainly to mGluR5 (Bassell and Warren, 2008; Dolen et al., 2007; Osterweil et al., 2010). For instance, the excessive protein synthesis observed in Fmr1 KO hippocampus can be corrected by genetic reduction or acute pharmacological inhibition of mGluR5 (Dolen et al., 2007; Osterweil et al., 2010). Together, our data unveil a potential novel FMRP-independent pathway linking mGluR1 to the regulation of OPHN1 synthesis.
mGluR-Induced OPHN1 Mediates Persistent Downregulation of Surface AMPARs and LTD Via Interaction with Endo2/3
To determine whether OPHN1 synthesis is required for mGluR-LTD, we used siRNAs to specifically prevent/block the mGluR-induced rapid increase in OPHN1 levels. Our data show that acute blockade of OPHN1 induction impedes mGluR-LTD, indicating that OPHN1 synthesis is necessary for mGluR-LTD. Consistent with previous reports that mGluR-LTD is mediated by a persistent reduction in surface AMPARs (Moult et al., 2006; Snyder et al., 2001; Waung et al., 2008), we find that acute blockade of OPHN1 synthesis blocks the downregulation of surface AMPARs one hour after mGluR activation. Together, these data imply that mGluR-induced OPHN1 mediates LTD by promoting the internalization of AMPARs.
Further support for these results, and mechanistic insight into how OPHN1 induction could regulate AMPAR endocytosis during mGluR-LTD, were provided by our finding that OPHN1 interacts with N-BAR domain-containing Endo2/3 core components of the postsynaptic clathrin-dependent endocytic machinery (Chowdhury et al., 2006). Interestingly, our data show that mGluR stimulation enhances OPHN1 association with Endo2/3 in a protein synthesis dependent manner. And importantly, disruption of the OPHN1-Endo2/3 interaction impedes both mGluR-elicited persistent decreases in surface AMPARs and LTD. Notably, these effects are not attributable to some general disruption of AMPARs or the machinery that controls their trafficking, because disruption of the OPHN1-Endo2/3 interaction does not affect basal AMPAR levels or basal synaptic function. Thus, these data imply that the downregulation of surface AMPARs during mGluR-LTD requires OPHN1 induction and its ability to bind Endo2/3. Likely, OPHN1 induced upon mGluR activation, via the regulation of Endo2/3’s activities, increases the rate of AMPAR endocytosis.
While our data demonstrate a requirement for OPHN1 synthesis in mGluR-LTD, previous studies have shown that newly synthesized Arc protein is also required for this process (Waung et al., 2008), implying that both mGluR-induced OPHN1 and Arc, and perhaps other proteins, such as MAP1B and STEP (Davidkova and Carroll, 2007; Zhang et al., 2008), are likely to contribute jointly to LTD, and, moreover, that mGluR1/5 must coordinate the various translational control mechanisms involved. Of particular interest is that Arc also interacts with Endo2/3 and this interaction is important for the role of Arc in AMPAR trafficking (Chowdhury et al., 2006). Of note, OPHN1 and Arc interact with different regions of Endo2/3, with OPHN1 binding to the SH3 domain of Endo2/3, and Arc to the C-terminus of the N-BAR domain of Endo2/3 (Chowdhury et al., 2006). Therefore, it is possible that newly synthesized OPHN1 and Arc cooperate at the level of Endo2/3 to promote mGluR-driven AMPAR endocytosis, either by regulating distinct aspects of Endo2/3 function or by promoting/engaging a common mechanism, at least under wild type conditions.
Importantly, a different mode of mGluR-LTD regulation seems to occur upon loss of FMRP. Indeed, previous studies demonstrated that mGluR-LTD in Fmr1 KO mice is distinctly different from that in wild type mice. For instance, whereas mGluR-LTD in wild type mice is protein synthesis dependent, it persists in the absence of protein synthesis in Fmr1 KO mice (Hou et al., 2006; Nosyreva and Huber, 2006). Consistent with this, our data show that acute blockade of OPHN1 synthesis does not affect mGluR-LTD in Fmr1 KO mice, albeit it clearly blocks mGluR-LTD in wild type mice, indicating that OPHN1 synthesis is required for mGluR-LTD under wild type conditions but not upon loss of FMRP. We conjecture that the elevated/aberrant protein synthesis caused by loss of FMRP can compensate for the requirement of new synthesis of OPHN1 and likely other proteins as well.
OPHN1 Serves Multiple Functions at the Hippocampal CA1 Synapse
In a previous study, we demonstrated that postsynaptic OPHN1 controls the maturation and strengthening of CA1 excitatory synapses in response to synaptic activity and NMDAR activation (Nadif Kasri et al., 2009). Combined with our current work, this indicates that OPHN1 carries out multiple functions at the hippocampal CA1 synapse. Our data show that the effects of OPHN1 on mGluR-LTD and basal synaptic strength are dissociable and involve distinct protein-protein interactions. As discussed above, disruption of the OPHN1-Endo2/3 interaction blocks mGluR-induced LTD and the associated long-term decreases in surface AMPARs. Yet, disruption of the OPHN1-Endo2/3 interaction does not interfere with basal synaptic function, or NMDAR-dependent LTP (data not shown), indicating that OPHN1 regulation of mGluR-LTD via its interaction with Endo2/3 is independent of its role in potentiating synaptic strength. We posit that OPHN1, upon induction by mGluR activity, engages in a complex with Endo2/3 to enhance AMPAR internalization, thereby mediating persistent decreases in surface AMPARs and LTD.
On the other hand, we find that OPHN1’s interaction with Homer 1b/c is not required for its role in mGluR-LTD, but that this interaction, as well as the Rho-GAP activity of OPHN1, is important for its role in regulating basal synaptic function. The GAP activity of OPHN1 towards RhoA is also required for its role in controlling structural and functional changes during LTP (Nadif Kasri et al., 2009). As to how OPHN1 could mediate the strengthening of synapses via interactions with Homer 1b/c and RhoA, we previously demonstrated that stabilizing AMPARs at the synapse prevents the defects in synaptic structure and function caused by extended OPHN1 knockdown (Nadif Kasri et al., 2009). Hence, a conceivable scenario is that OPHN1 via its interactions with Homer 1b/c and RhoA regulates the stabilization of AMPARs at the synapse, thereby controlling activity dependent maturation and strengthening of synapses (Figure 8C).
Together, these findings point to a multifunctional role for OPHN1 at CA1 synapses. Independent of its role in activity driven glutamatergic synapse development, regulated OPHN1 synthesis plays a critical role in mGluR-dependent LTD. Thus, it is conceivable that on one hand OPHN1 might play an important role in synapse maturation and circuit wiring during early development, on the other hand the regulated OPHN1 synthesis could operate during adulthood to weaken synapses in response to behaviorally relevant stimuli. In light of the previously reported role for LTD in behavioral flexibility and novelty detection (Kemp and Manahan- Vaughan, 2007; Luscher and Huber, 2010), and the association of OPHN1 loss of function with altered social behavior and novelty-driven hyperactivity (des Portes et al., 2004; Khelfaoui et al., 2007; Zanni et al., 2005), the requirement for OPHN1 in mGluR-LTD could offer an intriguing potential explanation for some of the behavioral deficits exhibited by OPHN1 patients.
EXPERIMENTAL PROCEDURES
DNA Constructs, Virus Production, siRNAs, Peptides, and Fluorescence in Situ Hybridization assay are included in Supplemental Experimental Procedures.
Acute Brain Slices and Hippocampal Slice and Dissociated Cultures
Acute slices were prepared from 21–30-day old rats (Sprague-Dawley), or 3.5–6-week old Fmr1 KO mice (FVB.129P2-Fmr1tm1Cgr/J) and corresponding littermates. Briefly, animals were anesthetized with isoflurane and decapitated; the brains were quickly removed and chilled in ice-cold dissection buffer. Coronal slices (400 µm) were cut in dissection buffer using a VT-1000S vibratome (Leica) and transferred to a storage chamber containing artificial cerebrospinal fluid (ACSF) for 30 min at 32°C. Slices were then incubated at RT for 1 to 3.5 h prior to use in experiments. See Supplemental Experimental Procedures for details on buffers, and for the preparation, infection and transfection of hippocampal slice and dissociated cultures.
Biochemical Analysis, AMPA Receptor Surface Labeling and Immunofluorescence
Western blotting, pull-down assays, coimmunoprecipitations, synaptoneurosome preparation, AMPAR surface labeling, and immunofluorescence were performed largely as described (Govek et al., 2004; Nadif Kasri et al., 2009; Park et al., 2008; Waung et al., 2008). See Supplemental Experimental Procedures for details.
Cut Dendrite Experiments
Dendrites in CA1 region of hippocampal slices were cut at the border of the stratum pyramidale and stratum radiatum with a microdissection knife under a dissection microscope (Huber et al., 2000). Ten minutes after treatment with DHPG or control vehicle (ACSF), slices were fixed, embedded in agarose (3%), resectioned at 40 µm, and processed for immunofluorescence staining with anti-OPHN1 and anti-βIII-tubulin antibodies.
Electrophysiology
Cultured and acute brain slice whole-cell recordings were obtained with Multiclamp 700B amplifiers (Axon Instruments). For the former, cultured slices from P7-P9 rats (Sprague-Dawley) were infected with indicated lentiviruses at DIV1, and 8 to 10 days later, whole-cell recordings were obtained simultaneously from an infected and an adjacent uninfected neuron in the CA1 region under visual guidance using epifluorescence and transmitted light illumination. For the latter, acute hippocampal slices were prepared from 21–30-day old rats or 23–28-day old mice and placed in a recording chamber perfused with artificial cerebrospinal fluid (ACSF) solution. See Supplemental Experimental Procedures for details.
Supplementary Material
ACKOWLEDGMENTS
We thank B. Li, E-E Govek and members of the Van Aelst lab for discussions and critical reading of the manuscript. We are grateful to K. Svoboda and A. Zador for Fmr1 KO mice. This work was supported by NAAR and NIMH grants to LVA. N.N.K was a postdoctoral fellow from FWO Flanders and HFSF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL DATA
The Supplemental Data include Supplemental Experimental Procedures and 8 figures.
REFERENCES
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann C, Zerres K, Senderek J, Rudnik-Schoneborn S, Eggermann T, Hausler M, Mull M, Ramaekers VT. Oligophrenin 1 (OPHN1) gene mutation causes syndromic X-linked mental retardation with epilepsy, rostral ventricular enlargement and cerebellar hypoplasia. Brain. 2003;126:1537–1544. doi: 10.1093/brain/awg173. [DOI] [PubMed] [Google Scholar]
- Billuart P, Bienvenu T, Ronce N, des Portes V, Vinet MC, Zemni R, Roest Crollius H, Carrie A, Fauchereau F, Cherry M, et al. Oligophrenin-1 encodes a rhoGAP protein involved in X-linked mental retardation. Nature. 1998;392:923–926. doi: 10.1038/31940. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Lissin DV, von Zastrow M, Nicoll RA, Malenka RC. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat Neurosci. 1999;2:454–460. doi: 10.1038/8123. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Peineau S, Howland JG, Wang YT. Long-term depression in the CNS. Nat Rev Neurosci. 2010;11:459–473. doi: 10.1038/nrn2867. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, et al. FMRP Stalls Ribosomal Translocation on mRNAs Linked to Synaptic Function and Autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidkova G, Carroll RC. Characterization of the role of microtubule-associated protein 1B in metabotropic glutamate receptor-mediated endocytosis of AMPA receptors in hippocampus. J Neurosci. 2007;27:13273–13278. doi: 10.1523/JNEUROSCI.3334-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- des Portes V, Boddaert N, Sacco S, Briault S, Maincent K, Bahi N, Gomot M, Ronce N, Bursztyn J, Adamsbaum C, et al. Specific clinical and brain MRI features in mentally retarded patients with mutations in the Oligophrenin-1 gene. Am J Med Genet A. 2004;124A:364–371. doi: 10.1002/ajmg.a.20422. [DOI] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326:483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- Giuffrida R, Musumeci S, D’Antoni S, Bonaccorso CM, Giuffrida-Stella AM, Oostra BA, Catania MV. A reduced number of metabotropic glutamate subtype 5 receptors are associated with constitutive homer proteins in a mouse model of fragile X syndrome. J Neurosci. 2005;25:8908–8916. doi: 10.1523/JNEUROSCI.0932-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govek EE, Newey SE, Akerman CJ, Cross JR, Van der Veken L, Van Aelst L. The X-linked mental retardation protein oligophrenin-1 is required for dendritic spine morphogenesis. Nat Neurosci. 2004;7:364–372. doi: 10.1038/nn1210. [DOI] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51:441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Hou L, Klann E. Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2004;24:6352–6361. doi: 10.1523/JNEUROSCI.0995-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Huber KM, Roder JC, Bear MF. Chemical induction of mGluR5-and protein synthesis--dependent long-term depression in hippocampal area CA1. J Neurophysiol. 2001;86:321–325. doi: 10.1152/jn.2001.86.1.321. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci. 2007;30:111–118. doi: 10.1016/j.tins.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Khelfaoui M, Denis C, van Galen E, de Bock F, Schmitt A, Houbron C, Morice E, Giros B, Ramakers G, Fagni L, et al. Loss of X-linked mental retardation gene oligophrenin1 in mice impairs spatial memory and leads to ventricular enlargement and dendritic spine immaturity. J Neurosci. 2007;27:9439–9450. doi: 10.1523/JNEUROSCI.2029-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khelfaoui M, Pavlowsky A, Powell AD, Valnegri P, Cheong KW, Blandin Y, Passafaro M, Jefferys JG, Chelly J, Billuart P. Inhibition of RhoA pathway rescues the endocytosis defects in Oligophrenin1 mouse model of mental retardation. Hum Mol Genet. 2009;18:2575–2583. doi: 10.1093/hmg/ddp189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaerulff O, Brodin L, Jung A. The Structure and Function of Endophilin Proteins. Cell Biochem Biophys. 2010 doi: 10.1007/s12013-010-9137-5. [DOI] [PubMed] [Google Scholar]
- Klann E, Dever TE. Biochemical mechanisms for translational regulation in synaptic plasticity. Nat Rev Neurosci. 2004;5:931–942. doi: 10.1038/nrn1557. [DOI] [PubMed] [Google Scholar]
- Luscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moult PR, Gladding CM, Sanderson TM, Fitzjohn SM, Bashir ZI, Molnar E, Collingridge GL. Tyrosine phosphatases regulate AMPA receptor trafficking during metabotropic glutamate receptor-mediated long-term depression. J Neurosci. 2006;26:2544–2554. doi: 10.1523/JNEUROSCI.4322-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadif Kasri N, Nakano-Kobayashi A, Malinow R, Li B, Van Aelst L. The Rho-linked mental retardation protein oligophrenin-1 controls synapse maturation and plasticity by stabilizing AMPA receptors. Genes Dev. 2009;23:1289–1302. doi: 10.1101/gad.1783809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano-Kobayashi A, Kasri NN, Newey SE, Van Aelst L. The Rho-linked mental retardation protein OPHN1 controls synaptic vesicle endocytosis via endophilin A1. Curr Biol. 2009;19:1133–1139. doi: 10.1016/j.cub.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli I, Mercaldo V, Boyl PP, Eleuteri B, Zalfa F, De Rubeis S, Di Marino D, Mohr E, Massimi M, Falconi M, et al. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E–BP. Cell. 2008;134:1042–1054. doi: 10.1016/j.cell.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Narayanan U, Nalavadi V, Nakamoto M, Pallas DC, Ceman S, Bassell GJ, Warren ST. FMRP phosphorylation reveals an immediate-early signaling pathway triggered by group I mGluR and mediated by PP2A. J Neurosci. 2007;27:14349–14357. doi: 10.1523/JNEUROSCI.2969-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J Neurophysiol. 2006;95:3291–3295. doi: 10.1152/jn.01316.2005. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Malenka RC, Nicoll RA. Two distinct forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neuron. 1997;18:969–982. doi: 10.1016/s0896-6273(00)80336-0. [DOI] [PubMed] [Google Scholar]
- Osterweil EK, Krueger DD, Reinhold K, Bear MF. Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. J Neurosci. 2010;30:15616–15627. doi: 10.1523/JNEUROSCI.3888-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59:70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip N, Chabrol B, Lossi AM, Cardoso C, Guerrini R, Dobyns WB, Raybaud C, Villard L. Mutations in the oligophrenin-1 gene (OPHN1) cause X linked congenital cerebellar hypoplasia. J Med Genet. 2003;40:441–446. doi: 10.1136/jmg.40.6.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter JD, Klann E. Making synaptic plasticity and memory last: mechanisms of translational regulation. Genes Dev. 2009;23:1–11. doi: 10.1101/gad.1735809. [DOI] [PubMed] [Google Scholar]
- Ronesi JA, Huber KM. Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J Neurosci. 2008;28:543–547. doi: 10.1523/JNEUROSCI.5019-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Volk LJ, Daly CA, Huber KM. Differential roles for group 1 mGluR subtypes in induction and expression of chemically induced hippocampal long-term depression. J Neurophysiol. 2006;95:2427–2438. doi: 10.1152/jn.00383.2005. [DOI] [PubMed] [Google Scholar]
- Waung MW, Huber KM. Protein translation in synaptic plasticity:mGluR-LTD, Fragile X. Curr Opin Neurobiol. 2009;19:319–326. doi: 10.1016/j.conb.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59:84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanni G, Saillour Y, Nagara M, Billuart P, Castelnau L, Moraine C, Faivre L, Bertini E, Durr A, Guichet A, et al. Oligophrenin 1 mutations frequently cause X-linked mental retardation with cerebellar hypoplasia. Neurology. 2005;65:1364–1369. doi: 10.1212/01.wnl.0000182813.94713.ee. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Venkitaramani DV, Gladding CM, Kurup P, Molnar E, Collingridge GL, Lombroso PJ. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J Neurosci. 2008;28:10561–10566. doi: 10.1523/JNEUROSCI.2666-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.