Abstract
Objective
Outcome and family history data differentiate children with severe mood dysregulation (SMD), a syndrome characterized by chronic irritability, from children with “classic,” episodic bipolar disorder (BD). Nevertheless, the presence of cognitive inflexibility in both SMD and BD highlights the need to delineate neurophysiological similarities and differences between the two patient groups. We used fMRI to examine neural correlates of cognitive flexibility deficits in SMD and BD vs. healthy volunteers (HV).
Method
During fMRI, subjects completed a response reversal task that assesses cognitive flexibility (N=22 SMD, 26 BD, 34 HV). We examined task effects in four regions of interest: caudate, cingulate gyrus, inferior frontal gyrus (IFG), and ventromedial prefrontal cortex.
Results
Diagnosis-by-accuracy interactions emerged in caudate and IFG. In these regions, we calculated the difference in activation between incorrect vs. correct trials. In caudate, this value was smaller in both SMD and BD than in HV. In IFG, however, this value was smaller in SMD than in both BD and HV. Post-hoc analyses indicate that comorbid ADHD in patients may influence the caudate findings. Exploratory whole-brain analysis confirmed the caudate and IFG findings. In addition, other regions differentiating SMD and BD were identified (e.g., superior parietal lobule/precuneus and inferior temporal gyrus).
Conclusions
In response to errors, similar perturbations occur in the caudate for SMD and BD youth relative to HV youth. IFG deficits, in contrast, manifest in SMD, but not BD, youth.
Keywords: functional MRI, response reversal, Bipolar disorder, severe mood dysregulation, pediatric
Introduction
Debate centers on whether severe chronic irritability is a developmental presentation of bipolar disorder (BD). Leibenluft et al. operationalized severe mood dysregulation (SMD) to capture children with chronic, non-episodic irritability1. Outcome and family history data2–4 differentiate youth with SMD from those with ‘classic,’ episodic BD. Nevertheless, the two conditions share some neuropsychological deficits. For example, on behavioral measures of face-emotion labeling and emotion regulation, SMD and BD patients both differ from healthy volunteers (HV). Importantly, however, these shared behavioral deficits reflect disorder-specific neural profiles5,6. The demonstration of disorder-specific neural dysfunction, in the context of similar-appearing behavioral deficits, highlights the importance of utilizing neuroimaging to probe differences between SMD and BD. Examining neural activity in SMD and BD could isolate syndrome-specific dysfunction, ultimately leading to better diagnosis and treatment.
Clinically significant irritability is common in both patient groups, although in SMD it is persistent, whereas in BD it is episodic1. Irritability manifests as a low threshold for anger in response to negative stimuli, and it can be precipitated by frustration7. Frustration occurs when an action fails to elicit an expected outcome8. Individuals with deficits in adapting to changing contingencies are at increased risk for frustration. Such deficits can be identified using cognitive flexibility paradigms, including reversal learning tasks. In such tasks, two stimuli, “A-B”, are presented, and participants learn by trial-and-error that “A,” but not “B,” is rewarded (acquisition phase). Then, without warning, the stimulus/reinforcement relationship reverses, so that participants must learn that now “B,” but not “A,” is rewarded (reversal phase).
Behavioral deficits in reversal learning are prominent in BD youth and may occur in SMD youth9–11. Prior work also finds frontal and parietal hyperactivation in BD vs. HV during reversal learning12. However, since no imaging studies examine reversal learning in SMD, research is needed comparing the neural correlates of reversal learning in SMD and BD.
Imaging studies focused on clearly-defined neuroanatomical circuits often use a region-of-interest (ROI) approach, especially when examining psychopathology13–16. This approach maximizes a study’s ability to identify between-group differences, and it directly extends prior work linking behaviors to specific circuits across a range of species. The anatomical circuit mediating reversal learning has been precisely mapped in humans, non-human primates, and rodents. The circuit encompasses ventromedial prefrontal cortex (vmPFC)8,17–23, cingulate gyrus22,24–27, inferior frontal gyrus (IFG)28–37, and caudate nucleus28,38–40. In this circuit, the vmPFC represents reinforcement information28,41 and encodes the value of actions42. The cingulate gyrus, IFG, and caudate act together to respond to errors28. Specifically, the cingulate gyrus registers the need to resolve response conflict signaled by errors43, whereas IFG facilitates such conflict resolution through motor response selection28, response inhibition44, and attention control45. The caudate nucleus supports motor learning, thus allowing adaptation to errors46. Work implicating these four regions in reversal learning justifies their selection in the current study as a priori ROIs. Nevertheless, for completeness, we also report results from an exploratory whole-brain analysis.
We tested the hypothesis that neural activity during reversal learning differentiates SMD, BD, and HV youths. Given evidence of reversal-learning deficits in SMD and BD9,11, we examined the diagnosis-by-phase-by-accuracy interaction to test for group differences in response to reversal errors. Additionally, we modeled the diagnosis-by-phase interaction to investigate group differences across the reversal phase, including both correct and incorrect trials. Finally, given reports of abnormal error signals47 and contingency-learning deficits in pediatric BD10,48, we examined the diagnosis-by-accuracy interaction to elucidate group differences in response to errors regardless of phase.
Method
Data from 82 subjects were included: 22 SMD, 26 BD, and 34 HV (15 BD and 15 HV published previously12). Subjects provided informed consent/assent. Full recruitment and diagnostic methods are described previously1,5.
Childhood Depression Rating Scale (CDRS)49 and Childhood Global Assessment Scale (CGAS)50 were administered to patients. Young Mania Rating Scale (YMRS)51 was administered to BD, but not SMD as hypo/mania is exclusionary. The Wechsler Abbreviated Scale of Intelligence (WASI)52 and Tanner self-report measure53,54 were administered to all subjects.
In-Scanner Task
This probabilistic response reversal task elicits fronto-striatal activity12,56 using six 6.5-minute, 135-trial runs. Each 2500ms trial (1600ms stimulus presentation, 900ms feedback) presented objects in pairs, and subjects were instructed to choose the “correct” object. Subjects were told that one object would tend to be correct but that the contingencies might reverse, as detailed previously12,56 (and see Supplement 1, available online).
Behavioral Analysis
We used a three (diagnosis: SMD, BD, HV) by two (phase: acquisition, reversal) repeated-measures ANOVA for accuracy. ANOVA also compared groups on number of non-response trials.
MR Imaging
Images were acquired on a 1.5-T GE scanner using gradient echo-planar imaging (TR=2500ms, TE=30ms, 24×24 FOV, flip angle=90°) and 29 4-mm slices, across 147 time-points. A high-resolution scan was acquired (1.5-mm slices, 3-dimensional FSPGR, 20° flip angle, 256×192 matrix, 24cm FOV).
fMRI Analysis
Analysis used Analysis of Functional Neuroimages (AFNI)57. Preprocessing involved (1) despiking, (2) alignment, (3) anatomical coregistration, (4) spatially smoothing data (6mm rms deviation Gaussian blur), (5) masking, and (6) intensity-scaling, producing data in Talairach space. TRs with extreme motion were censored, and motion parameters were included as covariates. TRs n and n-1 were censored if the normed motion vector between time-points was greater than 2mm. Subjects with more than 10% of TRs censored were excluded. SMD had a higher percentage of censored TRs than BD (p=0.005) or HV (p=0.003).
As detailed elsewhere12,56, trials were categorized by phase (acquisition or reversal), accuracy (correct or incorrect, based on subject response), and reinforcement (reward or punishment), yielding nine events: (1) acquisition correct win (“acquisition correct”); (2) acquisition correct lose; (3) acquisition incorrect win; (4) acquisition incorrect lose (“acquisition incorrect”); (5) reversal correct win (“reversal correct”); (6) reversal correct lose; (7) reversal incorrect win; (8) reversal incorrect lose (“reversal incorrect”); (9) no response. For parsimony, correct trials with lose feedback and incorrect trials with win feedback are excluded; therefore, correct-win trials are referred to as “correct,” and incorrect-lose trials as “incorrect.” Individual modeling was performed using a GAM model for each event type regressor plus motion parameters. Beta coefficients and associated t-statistics were calculated for each voxel and each regressor. We were interested in four regressors: acquisition correct, acquisition incorrect, reversal correct, and reversal incorrect.
AFNI’s GroupAna was used to run a 3×2×2 ANOVA: diagnosis (SMD, BD, HV) X phase (acquisition, reversal) X accuracy (correct, incorrect). The following interactions were examined: 1) diagnosis-by-phase-by-accuracy; 2) diagnosis-by-phase; 3) diagnosis-by-accuracy. Given extensive prior literature, group-level analyses adopted two approaches58. First, we examined four ROIs: caudate, cingulate gyrus, IFG, and vmPFC, delimited with bilateral masks created using the Talairach dataset in the AFNI Draw Dataset tool, re-sampled to 3×3×3mm, and thresholded at p<0.005. Monte Carlo simulation determined cluster-extent thresholds at p<0.05 corrected in each ROI: caudate: 7; cingulate: 23; IFG: 23; vmPFC: 20. Because we were interested in event-specific between-group differences, we decomposed the interactions. Mean BOLD signal in each significant cluster was extracted for each subject for all event types of interest. Difference scores for activity to specific events were compared across groups.
As in previous studies, an exploratory whole-brain analysis using a lower statistical threshold than the ROI analyses15 was also conducted. Criteria for statistical significance included individual voxel height intensity of p<0.005 and cluster extent threshold of k>20 voxels59.
Finally, because of a main effect of diagnosis on the number of total correct trials, we re-ran the primary ROI ANOVAs as ANCOVAs, with total correct trials as a covariate. Using Spearman’s correlations, we examined correlations between accuracy and activation.
Post-Hoc Analyses: Comorbidity, Mood, Pubertal Effects, and Age
Given high rates of comorbid Attention Deficit Hyperactivity Disorder (ADHD) and anxiety, and the difference in the rate of Oppositional Defiant Disorder (ODD) between patient groups, we examined possible effects of comorbidity using post-hoc repeated-measures ANOVAs. Again, we decomposed significant interactions by calculating difference scores and comparing them with independent t-tests. We also examined possible effects of puberty and mood on the primary results by conducting Spearman correlations using average Tanner60, mood (CDRS, YMRS) and impairment (CGAS) scales correlated with signal extractions from the significant clusters. As we did not have a priori hypotheses for these analyses, these correlations were Bonferroni-corrected. Because of the subjects’ large age range, we ran Spearman correlations between activation and age across all subjects and in each diagnostic group separately, comparing the latter with Fisher r-to-z transformations.
Results
Participants
Groups did not differ in age, IQ, or gender (Table 1). Groups tended to differ on Tanner scores (ANOVA p=0.096), with lower scores in SMD vs. HV (p=0.062) and BD (p=0.053). SMD had higher rates of ODD (p=0.008) and tended to be more impaired on CGAS (p=0.09) than BD. All SMD and 16 BD were euthymic (YMRS<12, CDRS<40). SMD had lower CDRS scores than BD (p=0.043).
Table 1.
Study Subject Demographics
| Healthy Volunteers (n=34) |
Bipolar Disorder (n=26) |
Severe Mood Dysregulation (n=22) |
|
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Gender | 17 male (50%) | 14 male (53.8%) | 17 male (77.3%) |
| Age | 14.17 ± 2.33 | 14.34 ± 2.59 | 13.25 ± 2.07 |
| Tanner stagea,b | 3.72 ± 1.19 | 3.81 ± 1.47 | 3.00 ± 1.38 |
| IQ | 111 ± 15.10 | 109 ± 16.33 | 109 ± 10.61 |
| Young Mania Rating Scale | --- | 10.12 ± 6.42 | --- |
| Childhood Depression Rating Scalea,c | --- | 28.81 ± 11.18 | 22.05 ± 10.57 |
| Childhood Global Assessment Scalea,d | --- | 53.74 ± 14.01 | 47.73 ± 8.74 |
| No. Comorbid Diagnoses | --- | 1.92 ± 1.55 | 2.36 ± 1.53 |
| N (%) | N (%) | N (%) | |
| Bipolar Disorder-I | --- | 23 (88.5) | --- |
| Bipolar Disorder-II | --- | 3 (11.5) | --- |
| Mood State | |||
| Euthymice | --- | 16 (61.5) | 22 (100) |
| Depressed | --- | 4 (15.4) | 0 (0) |
| Hypomanic/Manic | --- | 8 (30.8) | --- |
| Mixed | --- | 2 (7.7) | --- |
| Medication | |||
| Unmedicated | --- | 6 (23.1) | 8 (36.4) |
| Lithium | --- | 6 (23.1) | 3 (13.6) |
| Antidepressant | --- | 5 (19.2) | 5 (22.7) |
| Stimulant | --- | 9 (34.6) | 9 (40.9) |
| Non-Stim ADHD Med | --- | 2 (7.7) | 3 (13.6) |
| Antipsychotic | --- | 13 (50) | 8 (31.8) |
| Anti-epileptic | --- | 12 (46.2) | 8 (36.4) |
| Anxiolytic/Sedative | --- | 2 (7.7) | 1 (4.5) |
| Other | --- | 5 (19.2) | 6 (27.3) |
| Comorbid Diagnoses | |||
| Any Anxiety Disorder | --- | 12 (46.2) | 10 (45.5) |
| Major Depressive Disorder | --- | --- | 3 (13.6) |
| Obsessive Compulsive Disorder | --- | 2 (7.7) | 2 (9.1) |
| Attention Deficit Hyperactivity Disorder | --- | 14 (53.8) | 17 (77.3) |
| Oppositional Defiant Disorderf | --- | 8 (30.8) | 16 (72.7) |
| Post-Traumatic Stress Disorder | --- | 3 (11.5) | 0 (0) |
| Conduct Disorder | --- | 2 (7.7) | 0 (0) |
Note:
Scores unavailable for Tanner: 5 healthy volunteers (HV), 5 bipolar disorder (BD), 1 severe mood dysregulation (SMD); Childhood Depression Rating Scale (CDRS): 2 SMD; Childhood Global Assessment Scale (CGAS): 3 BD.
Analysis of Variance p=0.096
Between-group p=0.043
Between-group p=0.091
Fisher’s exact test p=0.001
Fisher’s exact test p=0.008
Behavior
Groups did not differ in non-responses. Repeated measures ANOVA revealed no diagnosis-by-phase interaction. There was a main effect of diagnosis (p=0.005); SMD had fewer total correct trials than HV (p=0.001) or BD (p=0.027).
Imaging
ROI
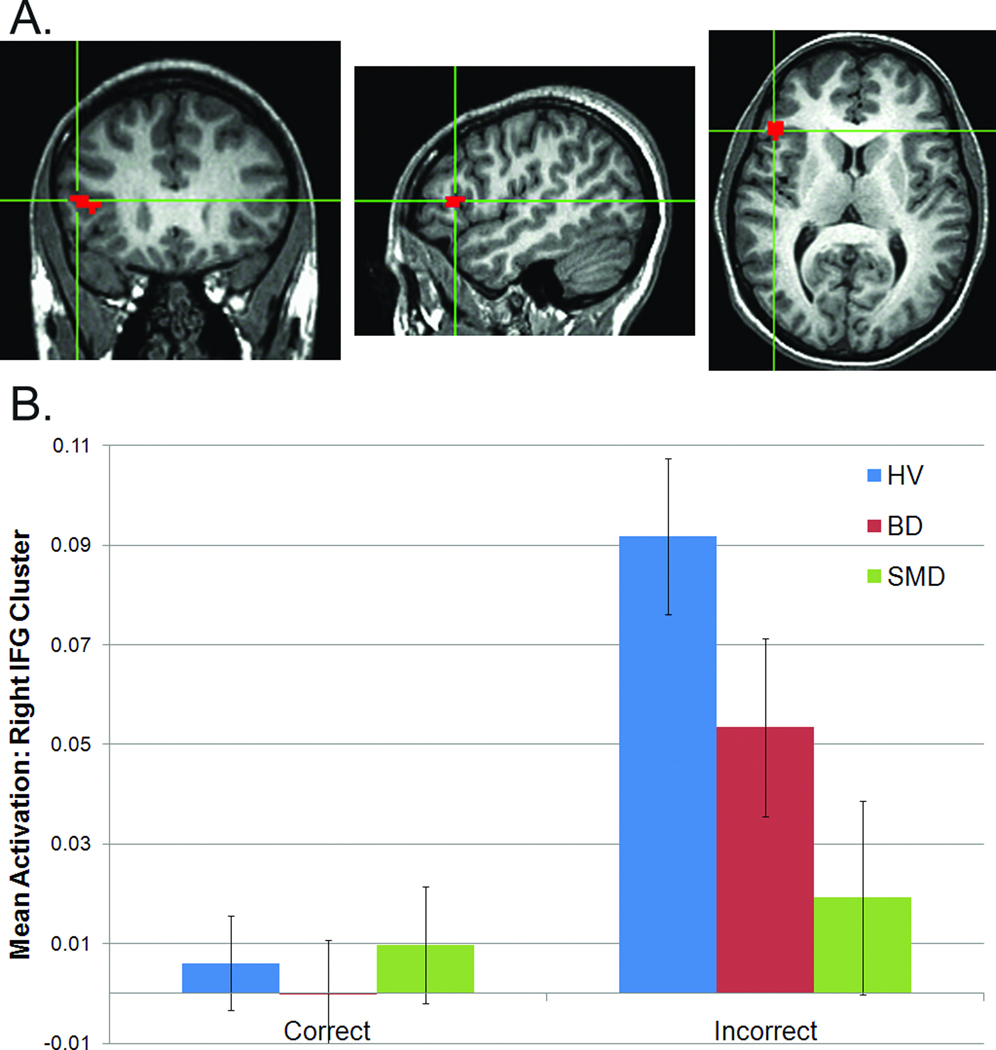
No clusters exhibited diagnosis-by-phase-by-accuracy or diagnosis-by-phase interactions. Two clusters exhibited diagnosis-by-accuracy interactions (Figures 1, 2): right caudate (459mm3, p=0.002) and right IFG (621mm3, p=0.001).
Figure 1.
Diagnosis-by-accuracy interaction in right caudate: (A) location (peak: 11, 11, 2; images in radiological convention, left=right); (B) mean responses to correct and incorrect trials. Note: BD=Bipolar Disorder; HV=Healthy Volunteers; SMD=Severe Mood Dysregulation.
Figure 2.
Diagnosis-by-accuracy interaction in right inferior frontal gyrus (IFG): (A) location (peak: 44, 26, 11; images in radiological convention, left=right); (B) mean responses to correct and incorrect trials. Note: BD=Bipolar Disorder; HV=Healthy Volunteers; SMD=Severe Mood Dysregulation.
Diagnosis-by-accuracy
In caudate, the interaction reflected smaller difference scores (incorrect – correct) in SMD (p<0.001) and BD (p=0.007) relative to HV (Figure 1b); SMD and BD did not differ. In IFG, the interaction reflected an abnormal response only in SMD. Specifically, SMD had smaller difference scores than both BD (p=0.035) and HV (p<0.001; Figure 2b). HV and BD did not differ (p=0.073). In the ANCOVA co-varying for total number of correct trials, both diagnosis-by-accuracy interactions remained significant (caudate: p=0.007, IFG: p=0.006), suggesting that performance differences did not account for the between-group activation differences.
Across all subjects, total-number correct correlated with difference score in both ROIs (IFG: Spearman’s r=0.30, p=0.007; caudate: Spearman’s r=0.35, p=0.001). Thus, engagement of IFG and caudate relates to task performance.
Exploratory Whole-Brain Analysis
Significant clusters were evident for diagnosis-by-phase-by-accuracy, diagnosis-by-phase, and diagnosis-by-accuracy interactions (Table 2 and Table S1, available online).
Table 2.
Exploratory Whole Brain Results
| Cluster Region (Brodmann Area) | No. voxels in cluster (Vol. in mm3) |
Talairach Coords of Cluster Peak |
||
|---|---|---|---|---|
| x | y | z | ||
| Diagnosis-by-phase-by-accuracy: | ||||
| R SPL/Precuneus (7/19) | 56 (1512) | 29 | −73 | 50 |
| L STG (22) and Insula (13) | 30 (810) | −49 | −13 | −7 |
| L SPL/Precuneus (7) | 25 (675) | −25 | −67 | 56 |
| R SPL/Precuneus (7/19) | 21 (675) | 26 | −64 | 59 |
| Diagnosis-by-phase: | ||||
| R STG (38) | 62 (1674) | 32 | 23 | −31 |
| L STG/insula (22/13) | 56 (1512) | −46 | −16 | −13 |
| R ITG/MTG/fusiform (20/21) | 35 (945) | 62 | −19 | −19 |
| L ITG/MTG/fusiform (20/21) | 30 (810) | −49 | −10 | −31 |
| R MFG (6) | 29 (783) | 35 | 2 | 53 |
| R ITG/MTG/fusiform (20/21) | 27 (729) | 47 | −4 | −28 |
| Diagnosis-by-accuracy: | ||||
| R/L superior/medial frontal gyrus (6) | 92 (2484) | 2 | 14 | 65 |
| R IFG/insula (45/13) | 51 (1377) | 35 | 20 | 5 |
| L fusiform/declive (18) | 25 (675) | −19 | −88 | −22 |
| R cerebellum | 23 (621) | 53 | −58 | −28 |
| R caudate | 23 (621) | 8 | 11 | 2 |
Note: Clusters indicated in bold are similar to the two clusters identified from the region of interest (ROI) analysis. ; ITG=Inferior Temporal Gyrus; L=left; MFG=Middle Frontal Gyrus; MTG=Middle Temporal Gyrus; R=right; SPL=Superior Parietal Lobule; STG=Superior Temporal Gyrus.
Diagnosis-by-phase-by-accuracy
Diagnosis-by-phase-by-accuracy interactions were evident in three parietal regions, reflecting aberrant responses in SMD during incorrect reversal trials. In these three regions, SMD had greater activation than HV to incorrect reversal trials; SMD also showed hyperactivation vs. BD in two of these regions. There was also a three-way interaction in superior temporal gyrus (STG)/insula, where SMD and BD showed hyperactivation relative to HV during incorrect acquisition trials.
Diagnosis-by-phase
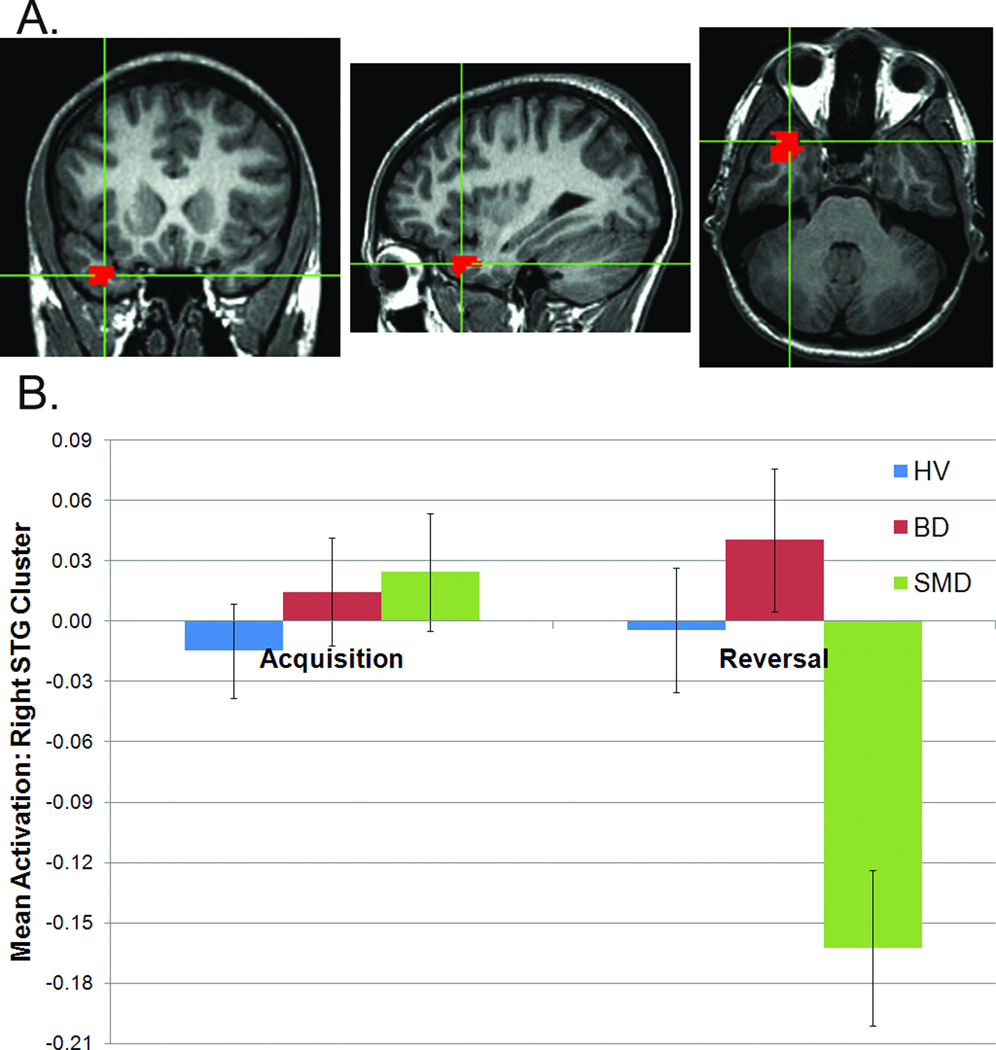
Diagnosis-by-phase interactions were found in five temporal regions and right middle frontal gyrus. Difference scores (acquisition minus reversal) were greater for SMD than HV in every region (e.g., see Figure 3), and in SMD than BD in all regions except left STG/insula.
Figure 3.
Diagnosis-by-phase interaction from whole-brain analysis in right superior temporal gyrus: (A) location (peak: 32, 23, -31; images in radiological convention, left=right); (B) mean responses to acquisition and reversal trials. Note: BD=Bipolar Disorder; HV=Healthy Volunteers; SMD=Severe Mood Dysregulation.
Diagnosis-by-accuracy
Diagnosis-by-accuracy interactions were found in frontal and cerebellar regions and right caudate. Generally, as in the ROI analyses, while HV response differentiated incorrect vs. correct trials, response in SMD did not differentiate incorrect vs. correct trials (in four of five regions), and the pattern in BD was mixed. Notably, the diagnosis-by-accuracy interactions identified in right caudate and right IFG in the ROI analyses were replicated in the whole-brain analysis (as shown in bold in Table 2). Specifically, in caudate, both SMD and BD difference scores (incorrect minus correct) were smaller than those of HV (p<0.001 and p=0.017, respectively). However, in IFG, SMD difference scores were lower than those of both BD and HV (p=0.016 and p<0.001, respectively).
Post-Hoc Analyses
All post-hoc analyses were conducted on the results in caudate and IFG from the primary ROI analysis.
Anxiety
Repeated measures ANOVAs used three groups: HV (n=34), SMD and BD participants with anxiety (+Anx, n=22 [12 BD, 10 SMD]), SMD/BD without anxiety (−Anx, n=26 [14 BD, 12 SMD]). There were group-by-accuracy interactions in both regions (caudate: p=0.003; IFG: p=0.002). In caudate, +Anx and −Anx had smaller difference scores than HV (p=0.008 and p<0.001, respectively); +Anx and −Anx did not differ. In IFG, +Anx and −Anx again had smaller difference scores than HV (p<0.001 and p=0.050, respectively); +Anx and −Anx did not differ. Therefore, group differences in our primary analyses were not driven by comorbid anxiety disorders.
ODD
Repeated measures ANOVAs used three groups: HV (n=34), SMD and BD patients with ODD (+ODD, n=24 [8 BD, 16 SMD]), SMD/BD patients without ODD (−ODD, n=24 [18 BD, 6 SMD]). There were group-by-accuracy interactions in both regions (caudate: p=0.003; IFG: p=0.004). In caudate, both +ODD and −ODD had smaller difference scores than HV (p=0.003 for both comparisons); +ODD and −ODD did not differ. In IFG, +ODD and −ODD had smaller difference scores than HV (p=0.002 and 0.025, respectively); +ODD and −ODD did not differ. These findings suggest that between-group differences in our primary analyses were not driven by comorbid ODD in patients.
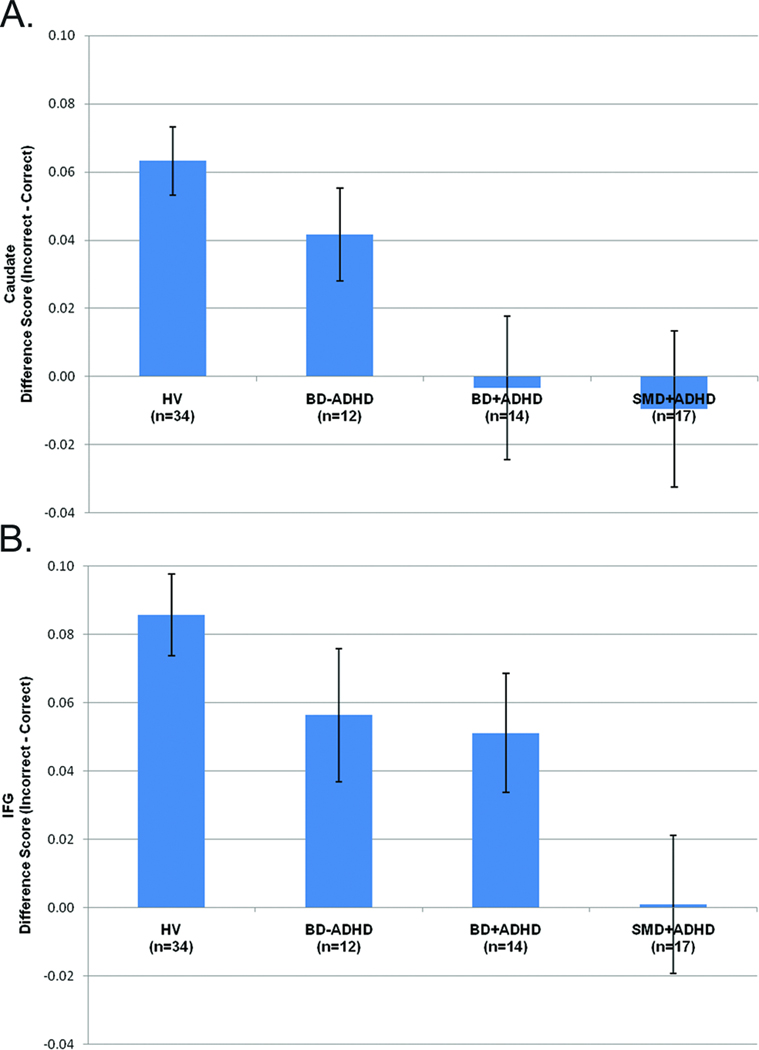
ADHD
Repeated measures ANOVAs compared three groups: HV (n=34); SMD and BD participants with ADHD (+ADHD, n=31 [14 BD, 17 SMD]); SMD/BD participants without ADHD (−ADHD, n=17 [12 BD, 5 SMD]) (Figure 4). There were group-by-accuracy interactions in both regions (p<0.001). In caudate, +ADHD had smaller difference scores than both HV (p<0.001) and −ADHD (p=0.030); −ADHD tended to have smaller difference scores than HV (p=0.084). In IFG, +ADHD had smaller difference scores than HV (p<0.001) but did not differ from −ADHD; −ADHD tended to have a smaller difference scores than HV (p=0.085).
Figure 4.
Difference scores (incorrect minus correct trials) in (A) right caudate and (B) right inferior frontal gyrus (IFG). Note: BD−ADHD=Bipolar Disorder without comorbid Attention Deficit Hyperactivity Disorder; BD+ADHD=Bipolar Disorder with comorbid Attention Deficit Hyperactivity Disorder; HV=healthy volunteers; SMD+ADHD=Severe Mood Dysregulation with Attention Deficit Hyperactivity Disorder.
We also used independent-sample t-tests (without correction for multiple comparison) to compare the difference scores of SMD and BD subgroups based on ADHD comorbidity: SMD with ADHD (SMD+ADHD, n=17), SMD without ADHD (SMD−ADHD; n=5, given small N, this group was excluded from analyses), BD with ADHD (BD+ADHD, n=14), BD without ADHD (BD−ADHD, n=12). SMD+ADHD and BD+ADHD did not differ in caudate, but there was a trend difference in the IFG (p=0.076) (Figure 4). Additionally, SMD+ADHD also tended to have smaller IFG difference scores than BD−ADHD (p=0.068). BD patients with and without ADHD did not differ from each other in either ROI. These results suggest that comorbid ADHD is not likely to be driving the observed differences between SMD and BD in the IFG.
Mood, Impairment, Puberty, and Age
There were no correlations between mean Tanner, CDRS, YMRS, or CGAS scores and activation to correct or incorrect trials in either ROI.
There were no correlations between age and difference scores in caudate, whether this was analyzed across all subjects or separately by group. However, IFG difference scores correlated with age across all subjects (Spearman’s r=0.34; p=0.002), and similar correlations were seen in each group separately (Spearman’s r between 0.29–0.40, p’s between 0.044–0.096). Fisher r-to-z transformation showed that the group correlation coefficients did not differ (all p’s>0.65).
Discussion
This is the first study comparing brain function during reversal learning in SMD, BD, and HV. We examined diagnosis-by-phase-by-accuracy, diagnosis-by-phase, and diagnosis-by-accuracy interactions. In the ROI analyses, only the diagnosis-by-accuracy interaction resulted in significant clusters; subsequent analyses demonstrated that the most salient neural differences between SMD, BD, and HV were in response to errors, regardless of phase. To elucidate these findings, the difference in activation during incorrect vs. correct trials was calculated. In caudate, this value was smaller in SMD and BD than in HV. In IFG, however, this value was smaller in SMD than in both BD and HV. Post-hoc analyses indicated that comorbid ADHD might influence these findings, particularly in caudate. Whole-brain analysis confirmed the IFG and caudate findings.
Behaviorally, SMD made more errors across the entire task than did the other two groups; their deficit was not limited to the reversal phase. Some out-of-scanner work in larger samples demonstrates specific reversal-learning deficits in both SMD and BD9–11; thus, our study resembles others that, across a variety of paradigms, find intact in-scanner performance despite in-clinic deficits56,61. Taken together, current and prior data suggest that behavioral deficits observed in the scanner in SMD and in out-of-scanner testing in SMD and BD9,11 reflect deficient engagement of IFG in SMD and of caudate in both SMD and BD following errors. Neither SMD nor BD exhibited the normative increase in caudate activity to incorrect trials, suggesting both have difficulty learning from errors, an important caudate function46 that could reflect dopamine dysfunction. Dopamine signaling modulates reward-based38 and feedback-dependent62 learning; such learning deficits can result in perseveration63.
Unlike caudate dysfunction, which manifested in both groups, SMD, but not BD, failed to show the expected, normal increase in IFG activity during incorrect trials. Thus, while caudate dysfunction characterized both SMD and BD, frontal dysfunction was unique to SMD. Frontal projections modulate striatal activity, so IFG and caudate are part of a circuit that adjusts behavior following an error28,64. IFG also mediates functions necessary for response selection, including attention maintenance and representation of contingencies, context, and goals. Right IFG plays an important role in response inhibition44, and a recent study indicates that IFG activity is modulated by the response control demands of a motor task65.
Speculatively, chronic SMD symptomatology may result from persistent fronto-striatal dysfunction, while episodic impairment in BD may reflect intermittent prefrontal dysfunction. SMD exhibited IFG and caudate dysfunction, as well as fewer correct trials than the other groups. In BD, on the other hand, IFG response and task performance are more similar to HV; perhaps intact IFG function enables BD subjects to compensate for basal ganglia dysfunction. Most patients were euthymic when scanned; while IFG dysfunction is amongst the most consistently reported finding in BD, a recent metaanalysis suggested such IFG dysfunction may be present during mania but not euthymia or depression66. Future longitudinal studies of BD patients in different mood states are required to test this possibility.
Our results are consistent with prior work. Using the stop signal task in a partially overlapping sample, we found that, relative to controls, BD had hypoactivation in IFG and striatum during unsuccessful attempts to inhibit motor responses47 (i.e., in response to error). Thus, using first a motor inhibition task in BD47 and then a response reversal task in BD and SMD, we identified neural dysfunction that may compromise the ability of both groups to adapt their behavior in response to changing contingencies, causing an increased propensity to experience frustration and irritability67.
Post-hoc analyses suggest that comorbid ADHD may contribute to our caudate findings: patients with ADHD differed from those without ADHD in response to incorrect vs. correct trials, and patients without ADHD more closely resembled controls. In IFG, patients with ADHD did not differ from those without ADHD in response to incorrect vs. correct trials, rendering it less likely that the between-group differences we observed were secondary to ADHD. Our caudate results here are consistent with our previous stop signal findings, where we could not rule out the role of comorbid ADHD in the aberrant neural responses of BD patients during unsuccessful inhibition47. A study using the same response reversal paradigm as here and a different analytic strategy found no neural deficits in ADHD patients56, but other data suggest abnormal activation in ADHD during related cognitive tasks (e.g., behavioral deficits and aberrant IFG and caudate activity in ADHD during response inhibition68–70).
The role of ADHD in our findings is difficult to ascertain because ADHD is present in 77% of the SMD sample; SMD inclusion criteria require three “hyperarousal” symptoms common to ADHD and mania. However, it is unclear whether the pathophysiology of ADHD in the context of SMD or BD is the same as that of ADHD without irritability. Studies report different neural activity5 and neurological symptoms71 in SMD or BD (including those with comorbid ADHD) vs. ADHD alone, again suggesting the importance of using behavioral and neuroimaging data, in addition to symptoms, to differentiate syndromes. Importantly, the current study was not designed to ascertain explicitly the impact of ADHD on the pathophysiology of SMD or BD, since to do so one would need to compare patients with “pure” ADHD without comorbid irritability to patients with SMD, BD, and HV5.
A major strength of this study is the comparison of two clinical populations to each other and a healthy group. Most psychiatric imaging studies compare one clinical population to a healthy group, and thus cannot differentiate disease-unique and disease-common abnormalities in diagnoses with overlapping symptoms. The extent to which SMD and BD children differ from controls but resemble each other indicates overlapping disease substrates. Here, both disorders exhibit striatal dysfunction. On the other hand, differences between clinical groups may indicate disorder-specific abnormalities with important diagnostic and treatment implications; here, we found differences between patient groups in prefrontal cortex function. However, there may be a dimensional component to IFG dysfunction in this task: like SMD, BD may not increase IFG response to incorrect trials as much as HV; the comparison between BD and HV is at a trend level and therefore equivocal. Nonetheless, direct comparison between SMD and BD indicates a categorical between-group difference in IFG activity.
Exploratory whole-brain analyses confirmed the IFG and caudate ROI findings and identified dysfunction in other regions. Like the ROI results, whole-brain results suggest dysfunction in SMD in regions that mediate detecting and learning from errors and executing an alternative response (e.g. superior/medial frontal gyrus72,73, right IFG, caudate). Also similar to the ROI analyses, the whole-brain analysis found that dysfunction was more consistent and pervasive in SMD than BD. Comparing BD and HV, our results are similar to Dickstein et al., who used a partially overlapping sample12. Specifically, in BD vs. HV, both studies found parietal hyperactivation during incorrect reversal trials and hyperactivation to all incorrect trials in superior frontal gyrus.
The study has limitations. First, though larger than those in most pediatric fMRI studies, the sample sizes used here are small. Second, we did not obtain frustration ratings in the scanner, and therefore cannot correlate activation and affective response. Adding such measures would have limited comparability with other studies and altered the psychological processes engaged, perhaps activating top-down regulatory regions. Studies suggest that caudate and IFG mediate switching responses after negative feedback28,64, but we cannot rule out the possibility that the between-group differences we observed are associated with psychological processes not measured by the task, such as increased frustration in response to negative feedback or decreased motivation in patients vs. controls. Also, because we lack dimensional symptom measures for comorbid disorders such as ADHD and ODD, we cannot correlate such symptoms with activation. Finally, many patients were medicated; ethical concerns preclude withdrawing ill children from medication for research. However, data suggest that medication may increase noise, rather than bias towards false positive errors74. While not all subjects were euthymic, most were, and post-hoc analyses suggest that mood state does not account for our findings (see supplement 1, avaliable online). Although post-hoc analyses suggest that the higher ADHD comorbidity, higher error rates, and slightly younger age of the SMD group are not driving between-group differences, these three factors may have interacted to influence the results.
This study is the first to examine the neural underpinnings of response reversal in children with SMD and BD compared to controls, finding deficits in both groups in response to errors. Hypoactivation during incorrect responses occurs in caudate in both SMD and BD and in IFG in SMD. Such hypoactivation to errors may reflect deficits in response inhibition signaling or new response selection, which may result in increased frustration and irritability. Future work should elucidate the role of comorbid ADHD, specifically in the caudate, in error-processing deficits in SMD and BD.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Mental Health (NIMH), National Institutes of Health (NIH).
The authors wish to acknowledge the work of the Section on Bipolar Spectrum Disorders and the NIH functional Magnetic Resonance Imaging (fMRI) facility. Most importantly, we thank the children and families who participated in this research and made it possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental material cited in this article is available online.
Disclosure: Drs. Adleman, Dickstein, Blair, Pine, and Leibenluft, and Mr. Kayser report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Nancy E. Adleman, National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services.
Reilly Kayser, National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services.
Daniel Dickstein, Warren Alpert Medical School of Brown University.
R. James R. Blair, National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services.
Daniel Pine, National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services.
Ellen Leibenluft, National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services.
References
- 1.Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS. Defining clinical phenotypes of juvenile mania. Am J Psychiatry. 2003;160(3):430–437. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- 2.Brotman MA, Kassem L, Reising MM, et al. Parental diagnoses in youth with narrow phenotype bipolar disorder or severe mood dysregulation. Am J Psychiatry. 2007;164(8):1238–1241. doi: 10.1176/appi.ajp.2007.06101619. [DOI] [PubMed] [Google Scholar]
- 3.Brotman MA, Schmajuk M, Rich BA, et al. Prevalence, clinical correlates, and longitudinal course of severe mood dysregulation in children. Biol Psychiatry. 2006;60(9):991–997. doi: 10.1016/j.biopsych.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 4.Stringaris A, Baroni A, Haimm C, et al. Pediatric bipolar disorder versus severe mood dysregulation: risk for manic episodes on follow-up. J Am Acad Child Adolesc Psychiatry. 2010;49(4):397–405. [PMC free article] [PubMed] [Google Scholar]
- 5.Brotman MA, Rich BA, Guyer AE, et al. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am J Psychiatry. 2010;167(1):61–69. doi: 10.1176/appi.ajp.2009.09010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rich BA, Schmajuk M, Perez-Edgar KE, Fox NA, Pine DS, Leibenluft E. Different psychophysiological and behavioral responses elicited by frustration in pediatric bipolar disorder and severe mood dysregulation. Am J Psychiatry. 2007;164(2):309–317. doi: 10.1176/ajp.2007.164.2.309. [DOI] [PubMed] [Google Scholar]
- 7.Leibenluft E, Blair RJ, Charney DS, Pine DS. Irritability in pediatric mania and other childhood psychopathology. Ann NY Acad Sci. 2003;1008:201–218. doi: 10.1196/annals.1301.022. [DOI] [PubMed] [Google Scholar]
- 8.Blair RJ. Psychopathy, frustration, and reactive aggression: the role of ventromedial prefrontal cortex. Br J Psychol. 2010 Aug;101(Pt 3):383–399. doi: 10.1348/000712609X418480. [DOI] [PubMed] [Google Scholar]
- 9.Dickstein DP, Finger EC, Brotman MA, et al. Impaired probabilistic reversal learning in youths with mood and anxiety disorders. Psychol Med. 2010 Jul;40(7):1089–1100. doi: 10.1017/S0033291709991462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickstein DP, Nelson EE, McClure EB, et al. Cognitive flexibility in phenotypes of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(3):341–355. doi: 10.1097/chi.0b013e31802d0b3d. [DOI] [PubMed] [Google Scholar]
- 11.Gorrindo T, Blair RJ, Budhani S, Dickstein DP, Pine DS, Leibenluft E. Deficits on a probabilistic response-reversal task in patients with pediatric bipolar disorder. Am J Psychiatry. 2005;162(10):1975–1977. doi: 10.1176/appi.ajp.162.10.1975. [DOI] [PubMed] [Google Scholar]
- 12.Dickstein DP, Finger EC, Skup M, Pine DS, Blair JR, Leibenluft E. Altered neural function in pediatric bipolar disorder during reversal learning. Bipolar Disord. 2010 Nov;12(7):707–719. doi: 10.1111/j.1399-5618.2010.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrion VG, Haas BW, Garrett A, Song S, Reiss AL. Reduced hippocampal activity in youth with posttraumatic stress symptoms: an FMRI study. J Pediatr Psychol. 2010 Jun;35(5):559–569. doi: 10.1093/jpepsy/jsp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes JP, Labar KS, McCarthy G, et al. Reduced hippocampal and amygdala activity predicts memory distortions for trauma reminders in combat-related PTSD. J Psychiatr Res. 2011 May;45(5):660–669. doi: 10.1016/j.jpsychires.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoning S, Zwitserlood P, Engelien A, et al. Working-memory fMRI reveals cingulate hyperactivation in euthymic major depression. Hum Brain Mapp. 2009 Sep;30(9):2746–2756. doi: 10.1002/hbm.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang TT, Simmons AN, Matthews SC, et al. Adolescents with major depression demonstrate increased amygdala activation. J Am Acad Child Adolesc Psychiatry. 2010 Jan;49(1):42–51. doi: 10.1097/00004583-201001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004 Nov;28(7):771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380(6569):69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 19.Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003 Aug;126(Pt 8):1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- 20.Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cereb Cortex. 2005;15(1):58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- 21.Hornak J, O'Doherty J, Bramham J, et al. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. J Cogn Neurosci. 2004 Apr;16(3):463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- 22.Kringelbach ML, Rolls ET. Neural correlates of rapid reversal learning in a simple model of human social interaction. NeuroImage. 2003 Oct;20(2):1371–1383. doi: 10.1016/S1053-8119(03)00393-8. [DOI] [PubMed] [Google Scholar]
- 23.Roberts AC, Robbins TW, Everitt BJ, Muir JL. A specific form of cognitive rigidity following excitotoxic lesions of the basal forebrain in marmosets. Neuroscience. 1992;47(2):251–264. doi: 10.1016/0306-4522(92)90241-s. [DOI] [PubMed] [Google Scholar]
- 24.D'Cruz AM, Ragozzino ME, Mosconi MW, Pavuluri MN, Sweeney JA. Human reversal learning under conditions of certain versus uncertain outcomes. NeuroImage. 2011 May 1;56(1):315–322. doi: 10.1016/j.neuroimage.2011.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meunier M, Jaffard R, Destrade C. Differential involvement of anterior and posterior cingulate cortices in spatial discriminative learning in a T-maze in mice. Behav Brain Res. 1991 Aug 29;44(2):133–143. doi: 10.1016/s0166-4328(05)80018-x. [DOI] [PubMed] [Google Scholar]
- 26.Nishijo H, Yamamoto Y, Ono T, Uwano T, Yamashita J, Yamashima T. Single neuron responses in the monkey anterior cingulate cortex during visual discrimination. Neurosci Lett. 1997 May 16;227(2):79–82. doi: 10.1016/s0304-3940(97)00310-8. [DOI] [PubMed] [Google Scholar]
- 27.Xue G, Ghahremani DG, Poldrack RA. Neural substrates for reversing stimulus-outcome and stimulus-response associations. J Neurosci. 2008;28(44):11196–11204. doi: 10.1523/JNEUROSCI.4001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Budhani S, Marsh AA, Pine DS, Blair RJ. Neural correlates of response reversal: considering acquisition. NeuroImage. 2007 Feb 15;34(4):1754–1765. doi: 10.1016/j.neuroimage.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 29.Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci. 2002;22(11):4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghahremani DG, Monterosso J, Jentsch JD, Bilder RM, Poldrack RA. Neural components underlying behavioral flexibility in human reversal learning. Cereb Cortex. 2010 Aug;20(8):1843–1852. doi: 10.1093/cercor/bhp247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res. 1970;11(4):376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- 32.Mishkin M, Vest B, Waxler M, Rosvold HE. A re-examination of the effects of frontal lesions on object alternation. Neuropsychologia. 1969;7:357–363. [Google Scholar]
- 33.Mitchell DG, Luo Q, Avny SB, et al. Adapting to dynamic stimulus-response values: differential contributions of inferior frontal, dorsomedial, and dorsolateral regions of prefrontal cortex to decision making. J Neurosci. 2009 Sep 2;29(35):10827–10834. doi: 10.1523/JNEUROSCI.0963-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagahama Y, Okada T, Katsumi Y, et al. Dissociable mechanisms of attentional control within the human prefrontal cortex. Cereb Cortex. 2001 Jan;11(1):85–92. doi: 10.1093/cercor/11.1.85. [DOI] [PubMed] [Google Scholar]
- 35.Remijnse PL, Nielen MM, Uylings HB, Veltman DJ. Neural correlates of a reversal learning task with an affectively neutral baseline: an event-related fMRI study. NeuroImage. 2005;26(2):609–618. doi: 10.1016/j.neuroimage.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. Journal of neurology, neurosurgery, and psychiatry. 1994;57(12):1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rygula R, Walker SC, Clarke HF, Robbins TW, Roberts AC. Differential contributions of the primate ventrolateral prefrontal and orbitofrontal cortex to serial reversal learning. J Neurosci. 2010 Oct 27;30(43):14552–14559. doi: 10.1523/JNEUROSCI.2631-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bellebaum C, Koch B, Schwarz M, Daum I. Focal basal ganglia lesions are associated with impairments in reward-based reversal learning. Brain. 2008 Mar;131(Pt 3):829–841. doi: 10.1093/brain/awn011. [DOI] [PubMed] [Google Scholar]
- 39.Divac I, Rosvold HE, Szwarcbart MK. Behavioral effects of selective ablation of the caudate nucleus. J Comp Physiol Psychol. 1967;63(2):184–190. doi: 10.1037/h0024348. [DOI] [PubMed] [Google Scholar]
- 40.Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. J Cogn Neurosci. 2000 Jan;12(1):142–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- 41.Murray EA, O'Doherty JP, Schoenbaum G. What we know and do not know about the functions of the orbitofrontal cortex after 20 years of cross-species studies. J Neurosci. 2007 Aug 1;27(31):8166–8169. doi: 10.1523/JNEUROSCI.1556-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glascher J, Hampton AN, O'Doherty JP. Determining a role for ventromedial prefrontal cortex in encoding action-based value signals during reward-related decision making. Cereb Cortex. 2009 Feb;19(2):483–495. doi: 10.1093/cercor/bhn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav. 2002 Dec;77(4–5):477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- 44.Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp. 2005 May;25(1):35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002 Mar;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 46.Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- 47.Leibenluft E, Rich BA, Vinton DT, et al. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. Am J Psychiatry. 2007;164(1):52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- 48.Mueller SC, Ng P, Temple V, et al. Perturbed reward processing in pediatric bipolar disorder: an antisaccade study. J Psychopharmacol. 2010 Dec;24(12):1779–1784. doi: 10.1177/0269881109353462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poznanski EO, Cook SC, Carroll BJ. A depression rating scale for children. Pediatrics. 1979;64(4):442–450. [PubMed] [Google Scholar]
- 50.Shaffer D, Gould MS, Brasic J, et al. A children's global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40(11):1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 51.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 52.Wechsler Abbreviated Scale of Intelligence. San Antonio, Texas: The Psychological Corporation; 1999. Wechsler. [Google Scholar]
- 53.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970 Feb;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969 Jun;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dickstein DP, Finger EC, Skup M, Pine DS, Blair RJR, Leibenluft E. Altered neural function in pediatric bipolar disorder during reversal learning. Bipolar Disord. 2010;12(7):707–719. doi: 10.1111/j.1399-5618.2010.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Finger EC, Marsh AA, Mitchell DG, et al. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Arch Gen Psychiatry. 2008;65(5):586–594. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 58.Bar-Haim Y, Fox NA, Benson B, et al. Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychol Sci. 2009 Aug;20(8):1009–1018. doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: rebalancing the scale. Soc Cogn Affect Neurosci. 2009 Dec;4(4):423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoekstra RA, Bartels M, Boomsma DI. Heritability of testosterone levels in 12-year-old twins and its relation to pubertal development. Twin Res Hum Genet. 2006;9(4):558–565. doi: 10.1375/183242706778025071. [DOI] [PubMed] [Google Scholar]
- 61.Nelson EE, Vinton DT, Berghorst L, et al. Brain systems underlying response flexibility in healthy and bipolar adolescents: an event-related fMRI study. Bipolar Disord. 2007 Dec;9(8):810–819. doi: 10.1111/j.1399-5618.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- 62.Cools R, Altamirano L, D'Esposito M. Reversal learning in Parkinson's disease depends on medication status and outcome valence. Neuropsychologia. 2006;44(10):1663–1673. doi: 10.1016/j.neuropsychologia.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 63.Ridley RM. The psychology of perserverative and stereotyped behaviour. Prog Neurobiol. 1994 Oct;44(2):221–231. doi: 10.1016/0301-0082(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 64.Hikosaka O, Isoda M. Switching from automatic to controlled behavior: cortico-basal ganglia mechanisms. Trends Cogn Sci. 2010;14(4):154–161. doi: 10.1016/j.tics.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dodds CM, Morein-Zamir S, Robbins TW. Dissociating inhibition, attention, and response control in the frontoparietal network using functional magnetic resonance imaging. Cereb Cortex. 2011 May;21(5):1155–1165. doi: 10.1093/cercor/bhq187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen CH, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord. 2011 Feb;13(1):1–15. doi: 10.1111/j.1399-5618.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- 67.Ochsner KN. The social-emotional processing stream: five core constructs and their translational potential for schizophrenia and beyond. Biol Psychiatry. 2008 Jul 1;64(1):48–61. doi: 10.1016/j.biopsych.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Durston S, Tottenham NT, Thomas KM, et al. Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry. 2003;53(10):871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- 69.Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am J Psychiatry. 2005;162(6):1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- 70.Vaidya CJ, Bunge SA, Dudukovic NM, Zalecki CA, Elliott GR, Gabrieli JD. Altered neural substrates of cognitive control in childhood ADHD: evidence from functional magnetic resonance imaging. Am J Psychiatry. 2005;162(9):1605–1613. doi: 10.1176/appi.ajp.162.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dickstein DP, Garvey M, Pradella AG, et al. Neurologic examination abnormalities in children with bipolar disorder or attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005 Oct 1;58(7):517–524. doi: 10.1016/j.biopsych.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 72.Rushworth MF, Hadland KA, Paus T, Sipila PK. Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol. 2002 May;87(5):2577–2592. doi: 10.1152/jn.2002.87.5.2577. [DOI] [PubMed] [Google Scholar]
- 73.Zanolie K, Teng S, Donohue SE, et al. Switching between colors and shapes on the basis of positive and negative feedback: an fMRI and EEG study on feedback-based learning. Cortex. 2008 May;44(5):537–547. doi: 10.1016/j.cortex.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 74.Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165(3):313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.