Abstract
Tendon tissue engineering with a biomaterial scaffold that mimics the tendon extracellular matrix (ECM) and is biomechanically suitable when combined with readily available autologous cells may provide successful regeneration of defects in tendon. Current repair strategies using suitable autografts and freeze-dried allografts lead to a slow repair process that is sub-optimal and fails to restore function, particularly in difficult clinical situations such as zone II flexor tendon injuries of the hand. We have investigated the effect of GDF-5 on cell proliferation and gene expression by primary rat adipose-derived stromal cells (ADSCs) that were cultured on poly(DL-lactide-co-glycolide) PLAGA fiber scaffold and compared to PLAGA 2D film scaffold. The electrospun scaffold mimics the collagen fiber bundles present in native tendon tissue, and supports the adhesion and proliferation of multipotent ADSCs. Gene expression of scleraxis, the neotendon marker was upregulated 7 – 8 fold at 1 week with GDF-5 treatment when cultured on 3D electrospun scaffold, and was significantly higher at 2 weeks compared to 2D films with or without GDF-5 treatment. Expression of the genes that encode the major tendon ECM protein, collagen type I, was increased by 4 fold starting at 1 week on treatment with 100ng/mL GDF-5, and at all time points the expression was significantly higher compared to 2D films irrespective of GDF-5 treatment. Thus stimulation with GDF-5 can modulate primary ADSCs on PLAGA fiber scaffold to produce a soft, collagenous musculoskeletal tissue that fulfills the need for tendon regeneration.
1. Introduction
Soft tissue injuries involving tendons and ligaments account for 50% of all musculoskeletal injuries reported in the United States each year and are associated with suboptimal healing leading to patient morbidity and loss of function (Calve et al. 2004, Butler et al. 2004a, Butler et al. 2004b). Current treatment for completely lacerated tendon is reattachment of the tendon stumps end-to-end to provide continuity, however the reduction in tendon length restricts the range of limb motion (Maffulli and Ajis 2008). Large tendon gap defects must be reconstructed and augmented with a graft or with prostheses. Currently, tendon transfer surgery uses autografts for chronic ruptures, however acellularized allografts are used for multiple tendon ruptures (Derwin et al. 2006, Chen et al. 2009). In addition to donor scarcity, the use of such grafts has risk factors such as donor-site morbidity, tissue rejection, disease transmission and inadequate repair. The outcomes of current tendon graft procedures are variable and sub-optimal leading to a high risk of failure in tension even with appropriate post-surgical therapy. Strategies to engineer tendon tissue could overcome these shortcomings by regeneration of tissue that is biomechanically, biochemically and histologically similar to the native tendon. Although scaffolds composed of various materials and fabrication techniques have been used to regenerate tendon, there is still the need for an ideal biodegradable scaffold that could mimic the architecture of native tendon extracellular matrix (ECM). The scaffold should have sufficient mechanical properties to provide support, which is critical to the early phase of repair. In addition, biocompatibility of the substrate for cell attachment and proliferation, along with its biological cues for tendon regeneration, are especially important for stem-cell based approaches to tendon regeneration (Sahoo et al. 2007).
Electrospinning has emerged as an efficient technique to fabricate fibers composed of natural and synthetic materials in dimensions that mimic collagen fiber bundles (Calve et al. 2004, Li et al. 2002, Matthews et al. 2002, Park et al. 2007, Zhang et al. 2007). Randomly deposited electrospun nonwoven fiber matrices have been used successfully in wound healing and drug delivery as well as other biomedical applications (Kumbar et al. 2008c). These nano/micro fiber scaffolds combine the advantages of mechanical strength with a large biomimetic surface. The high surface-to-volume ratio and porosity of the scaffold facilitates cell attachment, cell proliferation, and transport of nutrients and wastes through the scaffold (Kumbar et al. 2008a, Kumbar et al. 2008b). A scaffold that provides the requisite mechanical properties could minimize the risk of re-rupture associated with the movement of the tendon gap defect following surgical repair. Limb movement during the early phase of repair helps prevent restrictive adhesions and scar tissue formation which affect range of motion and full recovery of function (Platt 2005).
Various polymers of both synthetic and natural origin have been electrospun successfully into nano/micro nonwoven fibers for a variety of biomedical applications. Polyesters, namely poly(lactide), poly(glycolide) and their copolymers (PLAGA), have been approved by the FDA for clinical use and attracted greater attention as scaffolds for tissue engineering and drug delivery. Recent investigations with PLAGA scaffolds composed of nano- and micro- diameter fibers and seeded with bone marrow stromal cells (BMSCs) for tendon/ligament regeneration shown that the scaffold facilitates cell attachment and proliferation due to the similarity to native tendon ECM (Ouyang et al. 2002, Sahoo et al. 2006). Obtaining tendon fibroblasts requires the collection of healthy donor tissue; moreover, terminally differentiated fibroblasts within this tissue have limited lifespan. By contrast, autologous undifferentiated progenitor cells present an attractive alternative for tissue regeneration strategies because they avoid immune rejection, posses the ability to proliferate in culture and to differentiate into multiple cell types (Kang et al. 2004, Timper et al. 2006, Xu et al. 2008, Liang et al. 2009, Kajiyama et al. 2010). It now is apparent that many adult tissues, including adipose and bone marrow, harbor cells that have can differentiate into multiple cell types when maintained in appropriate culture conditions (BECKER et al. 1963, Friedenstein et al. 1968, Zuk et al. 2001, Zuk et al. 2002). Human adipose tissues are abundant and expendable due to the lack of morbidity, making it relatively easy to isolate adequate numbers of ADSCs under local anesthesia with little associated patient discomfort. The use of ADSCs has been demonstrated in animal models of ischemia revascularization, cardiovascular tissue regeneration, bone/cartilage repair, wound/ulcer healing, and urinary tract reconstruction with a view towards possible human therapies (Mizuno 2009, Hong et al. 2010, Montzka and Heidenreich 2010). It has been hypothesized that the benefits of ADSCs in this instance are due to paracrine effects that stimulate specific cellular events. In addition to recruiting progenitor cells, the ADSCs in turn differentiate to form the building blocks that are needed for tissue regeneration.
Human amniotic fluid stem cells and BMSCs have been reported to differentiate into osteogenic phenotype on nanofiber meshes of 500 – 600 nm diameter range when cultured in an osteogenic media (Kolambkar et al. 2010). Similarly, electrospun PLAGA nanofiber non-woven scaffolds of fiber diameter 760 nm support the differentiation of human BMSCs into osteogenic and chondrogenic phenotypes under appropriate culture conditions (Xin et al. 2007). The undifferentiated human embryonic stem cell (hESC) line SA002, when cultured on non-woven scaffolds of mean fiber diameter of 360 nm, differentiated into neurons when stimulated with neuronal inducing factors (Carlberg et al. 2009). These studies strongly suggest that in addition to a scaffold that mimics the native ECM, adequate biological signals are necessary to encourage the proliferation and direct differentiation of the seeded multi-potent cells in order for the stromal cell strategy for tissue engineering to succeed (Petrigliano et al. 2007, Sahoo et al. 2010). The exact cues necessary for the differentiation of progenitor cells along the tendinogenic pathway into tendon fibroblasts is unknown, however, several cytokines such as transforming growth factor – B, basic fibroblast growth factor, insulin growth factor – I, platelet derived growth factor and Growth/Differentiation Factor −5 have been identified as possible participating factors (James et al. 2008). Among these, Growth/Differentiation factor −5 stimulates cell growth and modulates the repair process in tendons and ligaments (Chhabra et al. 2003, Mikic et al. 2001, Mikic et al. 2009, Mikic et al. 2008). GDF-5 when delivered into ectopic sites in animal models, elicits the formation of neotendinous tissue (Wolfman et al. 1997). Complete tendon lacerations in animals that are repaired using sutures coated with GDF-5 show improved tensile strength and organized ECM compared to repairs done with non-coated sutures (Rickert 2008). Our earlier studies have reported increased proliferation and enhanced ECM gene expression in ADSCs cultured on tissue culture plastic in response to GDF-5 protein treatment (Park et al. 2010). Most growth factors in varying concentrations and combined with other factors, are known to direct differentiation along multiple connective tissue lineages thus necessitating evaluation of tendon differentiation markers during the development of the tendon tissue engineering application.
We hypothesize that electrospun matrices that mimic native ECM will support adhesion and proliferation of ADSCs, and the combinatorial approach using scaffold and GDF-5 protein supplemented culture media will stimulate events leading to increased tendon/neotendon phenotype gene expression and up regulate gene related to tendon ECM components when compared with the appropriate controls. The aim of this study was to characterize the non woven PLAGA scaffold and evaluate the viability, proliferation and gene expression of primary adipose derived stem cells that were maintained on the scaffold in vitro. The effect of GDF-5 on cell proliferation and genetic markers of tendon phenotype and of ECM were determined.
2. Materials and Methods
2.1. PLAGA 65:35 3D electrospun and 2D film fabrication
A 21% (w/v) PLAGA 65:35 (Lakeshore Biomaterials) was prepared in a solvent mixture of THF and DMF in the ratio of 3:1. This solution was electrospun using a syringe pump at a rate of 3.5 mL/h using an 18G blunt needle. Electrospinning was performed using pre-optimized electrospinning parameters of 0.8 kV/cm, ambient temperature and humidity. Fibers were spun on a flat aluminum foil of 13 cm2 to achieve a thickness of ~1 mm and kept desiccated in vacuum at room temperature for a period of 1 week to completely evaporate any residual solvent. 2D films were fabricated by pouring the 21% (w/v) PLAGA solution into a Teflon-coated mold, and placed at −20°C to allow slow solvent evaporation.
2.2. Nanofiber Characterization
2.2.1. Scanning Electron Microscopy
The vacuum stored samples were mounted onto aluminum stubs using carbon tape and sputter coated with gold under vacuum for 120 seconds at a voltage of 25V. The electrospun scaffolds were viewed by a scanning electron microscope (JEOL JSM6400) and imaged at various magnifications using a 15 kV accelerating voltage. The fiber diameters were measured using ImageJ (NIH) software on 3 different samples up to 100 fibers for each sample and the fiber diameter distribution was plotted.
2.2.2. Porosimetry
Median pore diameter and percent porosity, defined as the ratio of scaffold void space to total scaffold volume, was determined using mercury porosimetry (Micromeritics Autopore III porosimeter; Micromeritics, Norcross, GA). For each measurement a sample size of 3 was used.
2.2.3. Mechanical strength
Fiber matrices were cut into dog-bone shape with test-area dimensions of 20.00 × 10.00 mm and thickness measured by digital calipers of ± 0.01mm accuracy. The scaffolds have a thickness of 0.80±0.16 mm and cross-sectional area of 8mm2. The scaffolds were clamped and tested in tension at 0.025 mm/s strain till failure on an Instron 5544 using a 100 N load cell. The stress–strain data was plotted and elastic modulus, ultimate tensile strength, failure load, stiffness, % strain at break were calculated and average values reported. For each measurement a sample size of 8 was used.
2.3. Adipose derived stem cells (ADSCs)
Primary rat adipocytes were isolated from the inguinal fat pad of 6 week old male Fischer 344 rats. The rats were euthanized using CO2 in accordance to an Institutional Animal Care and Use Committee (IACUC) approved protocol. The inguinal fat pads were excised immediately using aseptic techniques, finely minced and washed three times with PBS supplemented with 2% Penicillin/Streptomycin (P/S). The tissue was collected by centrifugation at 500g for 5 min after each wash. Collagenase (0.01%) was added to the minced and washed adipose tissue and incubated under constant agitation at 37°C for 45 min. The aqueous portion (non-adipose) was carefully removed and centrifuged at 500g for 10 min to obtain a cell pellet. The cell pellet was resuspended in basal medium (BM) comprised of Dulbecco’s modified eagle medium (DMEM) containing 10% fetal bovine serum (FBS), 1% Penicillin/Streptomycin (P/S) and plated in 75cm2 cell culture flask maintained at 37°C in a humidified incubator with 5% CO2. Culture media was changed every other day and maintained at subconfluent levels. The adherent cells were passaged using trypsin/EDTA. The primary rat adipocytes (P1 – P2) were seeded onto 24 well plates at a density of 50,000 cells/cm2 and cultured in BM. At 24hrs post-seeding, culture medium was changed either to osteogenic (OM) or adipogenic medium (AM) which has been described in Table 1 and replaced every 3 days (Li et al. 2005). At 14 days post-seeding, the cell layers were rinsed in PBS and fixed in 10% buffered formalin or paraformaldehyde for 30 mins at room temperature (RT). The fixed cells were washed with double distilled water. Calcium deposits were detected by staining with Alizarin Red (STARE100, American MasterTech) and lipid vesicles were detected by staining with Oil Red O (KTORO, American MasterTech).
Table 1.
Composition of basal medium (BM), osteogenic medium (OM) and adipogenic medium (AM).
| Basal Medium (BM) |
Osteogenic Medium (OM) |
Adipogenic Medium (AM) |
|---|---|---|
| DMEM Low glucose 10% FBS 1% P/S |
DMEM Low glucose 10% FBS 1% P/S 0.01 μM Vitamin D3 200 μM Ascorbic Acid 10 mM sodium β-glycerophosphate 10 nM Dexamethasone |
DMEM Low glucose 10% FBS 1% P/S 100 μM Dexamethasone |
2.4. PLAGA Scaffold-Cell In Vitro Culture
Scaffolds were cut into 1 cm squares and sterilized using a combination of 70% ethanol for 5 min and UV light for 1 hr on each side. The sterile scaffolds were soaked in DMEM media under vacuum for 1hr to remove air bubbles. The scaffolds were then incubated overnight at 37°C and 5% CO2. Cells from passage P2 were seeded onto electrospun PLAGA scaffolds at a concentration of 30,000 cells/scaffold and cultured in BM overnight. Culture medium was replaced with BM supplemented with a range of concentrations of GDF-5 protein (CYT-442, ProSpec-Tany TechnoGene Ltd.). GDF-5 protein supplemented BM was replaced every 3 days. Our earlier studies have shown that increasing the concentration of GDF-5 protein above 100 ng/mL had no significant effect on the proliferation of ADSCs, however the expression of genetic markers of tendinogenesis and ECM were inhibited at GDF-5 concentrations higher than 100 ng/mL (Park et al. 2010).
2.5. Characterization of cellular response
2.5.1.Proliferation assay
DNA was measured on cells maintained in culture on PLAGA film and electrospun PLGA scaffolds using the Picogreen DNA Assay (P7589, Molecular Probes) with excitation at 480 nm and emission at 520 nm. ADSCs were treated with 0, 1, 10, and 100ng/mL GDF-5 and proliferation was quantified at 7 and 14 days post-seeding. Briefly, the recovered scaffolds were washed with PBS and DNA was extracted using a freeze-thaw protocol in a 10% solution of sodium dodecyl sulfate (SDS). For each measurement a sample size of 4 was used and experiments were repeated twice.
2.5.2. Confocal and Scanning Electron Microscopy (SEM)
At 2 weeks post-seeding, the cell-scaffold constructs were stained with Live/Dead Assay Kit (L3224, Invitrogen) and viewed under confocal microscopy. Longitudinal sections, 5μm thickness were cut on a cryotome and stained with DAPI (H-1500, Vector Laboratories) for fluorescence imaging. Images of the ADSCs cultured on PLAGA scaffolds in GDF-5 supplemented BM were also obtained by SEM.
2.5.3. Gene expression
ADSCs were cultured on both 3D and 2D PLAGA scaffolds and treated with 0, 10 and 100 ng/mL GDF-5 protein. Scaffolds were retrieved at 3, 7 and 14 days post-seeding. Total RNA was obtained using the RNeasy Mini kit (Qiagen), and cDNA was synthesized from the total RNA using the Reverse Transcription System kit. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR) was then performed with Quantitect SYBR Green PCR master mix (Bio-Rad) using specific primers designed using Primer3 software. Standard curves were generated and quantities of each transcript were normalized to 18S used as the internal control. All data points were normalized to gene expression level on day 3 with the 3D scaffold and 0ng/mL GDF-5 treatment and represented as fold change. Amplification primers used for the real time assay are listed in table 2. For each measurement a sample size of 4 was used and experiments were repeated twice.
Table 2.
Forward and Reverse Primers used for Real Time – PCR assay of ECM and Tendinogenic Genes
| Gene | Primer Sequence |
|---|---|
| Collagen Type I α1 | |
| Gene ID: 29393 | |
| Forward | 5′-AGG GTC ATC GTG GCT TCT C-3′ |
| Reverse | 5′-CTC CAG AGG GGC CTT GTT C-3′ |
| Collagen Type III α1 | |
| Gene ID: 84032 | |
| Forward | 5′-AGG CTT TGA TGG ACG CAA TG-3′ |
| Reverse | 5′-GCG GCT CCA GGA AGA CC-3′ |
| Scleraxis | |
| Gene ID: 680712 | |
| Forward | 5′-CGA AGT TAG AAG GAG GAG GGT C-3′ |
| Reverse | 5′-CGC TCA GAT CAG GTC CAA AG-3′ |
| Tenomodulin | |
| Gene ID: 64104 | |
| Forward | 5′-GGA CTT TGA GCA GGA TGG-3′ |
| Reverse | 5′-CGC TTG CTT GTC TGG TGC-3′ |
| 18S | |
| Gene ID: M11188.1 | |
| Forward | 5′-CGG CGA CGA CCC ATT CGA AC-3′ |
| Reverse | 5′-GAA TCG AAC CCT GAT TCC CCG TC-3′ |
2.6. Statistical analysis
The difference in cell proliferation and relative gene expression were analyzed using two-way ANOVA test. When the analysis of variance indicated significance in the difference among the data, multiple comparisons were performed with Tukey’s post hoc tests. A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Scaffold Properties
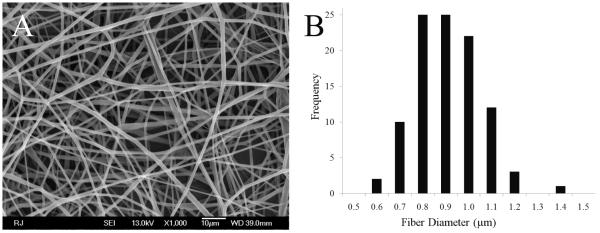
At a pre-optimized concentration of 21% (w/v) in 3:1 THF:DMF and operating parameters of 0.8 kV/cm electric potential, 3.5 mL/hr flow rate and needle size of 18G, a stable Taylor cone formation resulting in the deposition of random bead free fibers with an fiber diameter distribution range of 0.6–1.2μm was achieved (see histogram, Figure1B). The mean fiber diameter size was measured to be 0.8μm. SEM images of the top and bottom surface of the scaffolds showed defect-free non-woven fibers within the measured diameter range. Figure 1A shows PLAGA non-woven scaffold with bead-free fibers in random orientation, and the many interconnected, large size pores. The fabrication of this scaffold with non-woven fiber morphology was reproducible. Scaffolds with beads and other artifacts cannot be reproducibly fabricated and have variable physico-chemical properties. The selected fiber matrices have 50% overall porosity with an average pore diameter of 13.8±3.2μm as determined by mercury intrusion porosimetry. Tensile properties of these fiber matrices were evaluated at a physiologic strain rate of 0.025 mm/second and showed an average modulus of 81±8 MPa, Yield strength of 13.5±1.5 MPa, Ultimate strength of 18±4 MPa, failure load of 20±4.3 N, stiffness and % strain at break to be of 18.71±5.2N/mm and 31.8±6.6 % respectively (n=8).
Figure 1.
(A) SEM micrograph of nonwoven electrospun PLAGA nanofiber scaffold at 1000X. PLAGA 65:35 at a solution concentration of 21% (w/v) in THF:DMF (3:1 ratio) and a potential gradient of 0.8kV/cm resulted in bead free randomly oriented fibers with a diameter ranging between 0.6–1.1μm. (B) Histogram showing the distribution frequency of fiber diameters (n=100). The mean diameter of the electrospun PLAGA fibers is 0.8μm.
3.2. Multi-Potent Adipose Derived Cells
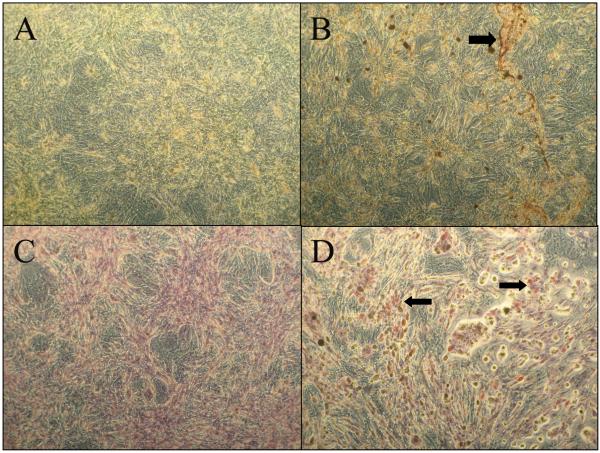
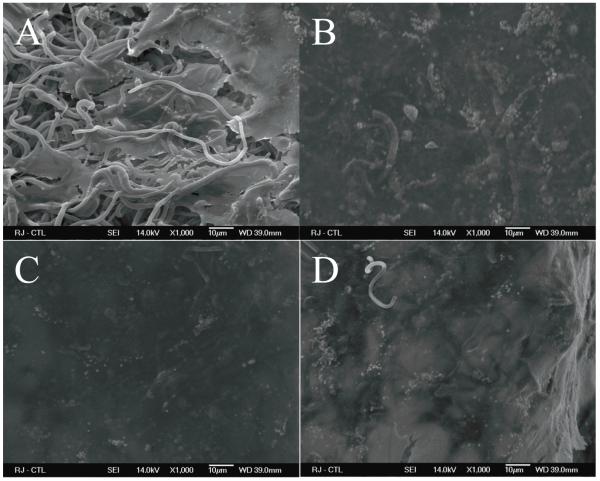
Positive staining with Alizarin Red and Oil Red O suggests differentiation of the primary rat adipose derived cells towards osteogenic and adipogenic phenotypes respectively (Figure 2). Upon stimulation in culture with the appropriate inductive factors for osteogenesis or adipogenesis, the isolated primary adipose derived stromal cells (ADSCs) showed their multipotent characteristic.
Figure 2.
Multipotency of adipose derived stromal cells: Alizarin Red staining of cells treated (A) without and (B) with osteogenic culture media. Positive staining for calcium deposits can be seen in panel B shown by the arrow. Oil Red O staining of cells treated (C) without and (D) with adipogeneic medium shows lipid vesicles depicting adipogenesis seen in D indicated by arrows. All images are at 10X magnification.
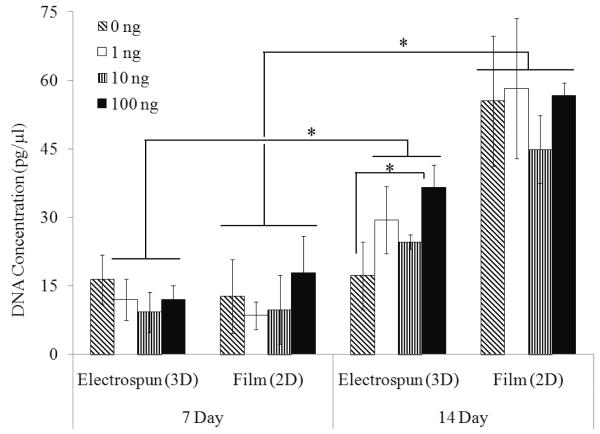
3.3. Increased cell proliferation on the biomimetic electrospun scaffold
ADSCs seeded onto the PLAGA film proliferated significantly with time, and was significantly higher compared to the 3D electrospun scaffold at 14 days post-seeding (Figure 3). At 7 and 14 day time points in culture, there were no differences in cell number on PLAGA film scaffolds with or without GDF-5 treatment. By contrast, cells maintained on PLAGA electrospun scaffolds, showed increased proliferation in a GDF-5 dose and time dependent manner. Cell proliferation was significantly higher with GDF-5 treatment (10ng/mL and 100ng/mL) on 3D scaffolds at 14 days compared to 0ng/mL concentration. Temporal proliferation of ADSCs on 3D scaffolds was significantly higher in the treated groups at 14 days.
Figure 3.
Proliferation of cells maintained on 2D PLAGA film and 3D electrospun scaffold and treated with increasing concentrations of GDF-5: While proliferation on the 2D sheets was significantly higher than on the 3D scaffold by 14 days (p<0.05), the effect of GDF-5 on ADSCs proliferation in vitro is more pronounced with cells cultured on 3D scaffold compared with 2D film.
3.4. Infiltration of cells on the electrospun scaffold
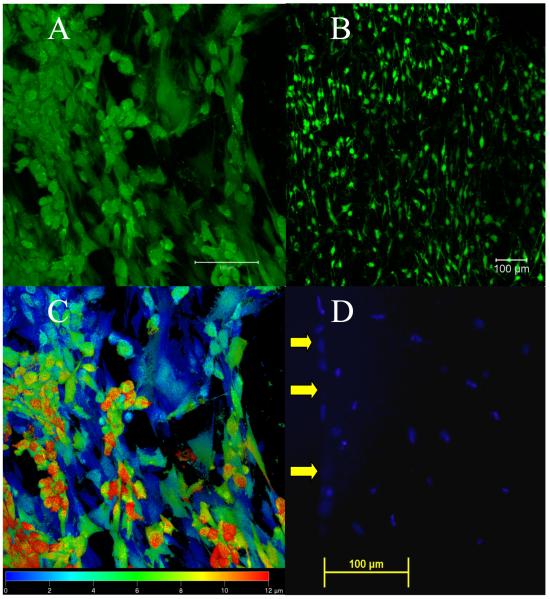
On tissue culture polystyrene (TCPS), the ADSCs were flat with extensive cellular processes, and at higher confluence, cells detached in sheets from the tissue culture surface. Composite confocal microscopy images of cells on the PLAGA electrospun scaffolds at 14 days in culture show viable cells with extending processes (Figure 4A) were adhered and covering the scaffold surface (Figure 4B). Confocal microscopy images of ADSCs on PLAGA electrospun scaffolds showed no differences in cell appearance with or without GDF-5 treatment at the study time points. Furthermore, ADSCs were imaged up to 12μm from the surface of the nanofiber scaffold into the interior as demonstrated by false color imaging (Figure 4C). Depth perception using confocal microscope is limited, and thus DAPI staining of cell nuclei in longitudinal sections of the ADSC-scaffold construct, showed that the cells had migrated even further into the 3D scaffold, and were visible in the interior at least 200 μm from the scaffold surface (Figure 4D).
Figure 4.
Cell Viability and Migration: A, B and C: Confocal images of ADSCs on electrospun PLAGA scaffolds at 14 days post-seeding. (A) Staining with Live/Dead Assay shows cells with a flat morphology and extended processes, (scale bar = 100μm). (B) Staining with Live/Dead Assay shows at low magnification the ADSCs have adhered and proliferated throughout the scaffold surface, (scale bar = 100μm). (C) A false color confocal image indicating the depth of cells from the scaffold surface (20x). Blue is indicative of cells at the surface and red indicates that cells extend to a depth of 12μm. (D) DAPI fluorescence image of cell nuclei showing ADSCs up to 200μm from the scaffold surface. Yellow arrows point to the surface of the scaffold.
SEM image showed that without GDF-5 treatment, the ADSCs were visible between the fibers of 3D scaffolds (Figure 5A). With varying concentrations of GDF-5 (1ng/mL, 10ng/mL and 100ng/mL), the images show extensive cell growth on the 3D scaffold. The ADSCs cover the entire scaffold surface (Figure 5 B, C and D) with few fibers visible in the SEM images.
Figure 5.
SEM images of ADSCs on 3D Scaffold with or without GDF-5. Cells on the 3D scaffold were treated with (A) 0ng/mL, (B) 1ng/mL, (C) 10ng/mL, and (D) 100ng/mL GDF-5. In response to treatment with GDF-5, the cells had formed a dense layer on the 3D scaffold at 14 days with only occasional fibers still partially visible.
3.5. GDF-5 protein increases expression of tendon phenotype and ECM genes
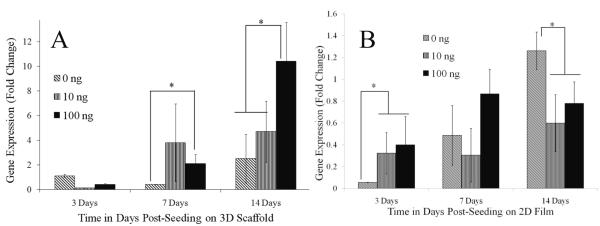
The expression of several ECM and tendinogenesis related genes as measured by RT-PCR showed GDF-5 dose dependence. The results for the different treatment groups at each time point showed that collagen type I gene expression was significantly increased with a 4 fold upregulation starting at day 7 in cells treated with 100 ng/mL GDF-5 (p<0.05, Figure 6A) as compared to treatment without GDF-5. The increase in collagen type I gene expression persisted with at least a 4 fold change at day 14 in the group treated with 100 ng/mL GDF-5 protein. The temporal expression of collagen type I gene increased significantly in the GDF-5 treated 3D scaffold groups compared to the 3 day time point. At the early time point, day 3, collagen I gene expression was higher in ADSCs seeded onto PLAGA film and treated with GDF-5 protein. ADSCs seeded onto PLAGA film showed no difference in collagen gene expression upon treatment with GDF-5 protein at 7 days, and at 14 days gene expression was significantly decreased in the treated groups compared to the untreated groups (Figure 6B). Collagen type I gene expression was significantly reduced in ADSCs seeded onto the 2D PLAGA films as compared to the 3D scaffolds irrespective of GDF-5 treatment. Cells on the 3D scaffold and 2D film treated with GDF-5 showed no statistically significant differences or trends in the expression of collagen type III at the time points studied.
Figure 6.
Expression of collagen type I gene in response to treatment with GDF-5. (A) Cells on 3D scaffold, and (B) on 2D film. Upon treatment with 100 ng/mL GDF-5, collagen type I gene expression in cells on the 3D scaffold increased at 7 and 14 days on treatment with GDF-5, by contrast collagen gene expression by cells on the 2D film had decreased at the end of 2 weeks (p<0.05). Collagen gene expression is lower on the 2D films compared to the 3D electrospun scaffolds. Gene expression data is normalized to the 18s gene and expression of day 3 on 3D scaffold without GDF-5 (0ng/mL).
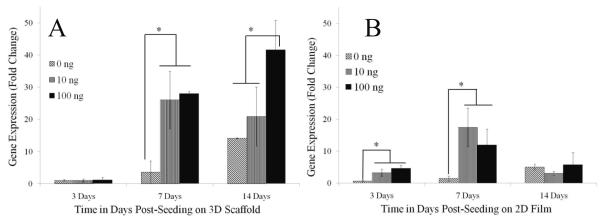
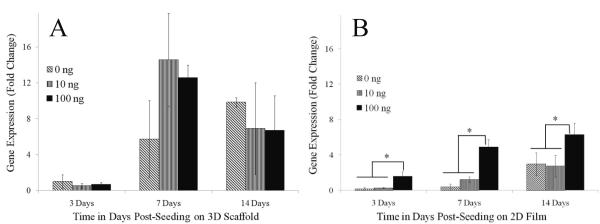
Expression of the genes for the tendinogenic markers scleraxis and tenomodulin showed a dependence on GDF-5 concentration and were increased with the dose of GDF-5 used to treat the cells (Figure 7A). Scleraxis is a neotendon marker that is expressed exclusively at pro-tendon sites in the developing embryo. Scleraxis gene expression increased significantly starting at 7 days of treatment with 10 and 100 ng/mL GDF-5 with an increase of 7 – 8 folds. By day 14, treatment with 100 ng/mL GDF-5 resulted in a 3 fold change compared to the untreated cells. This expression level was significantly higher than in the cells that were treated with 10 ng/mL GDF-5. Temporal scleraxis gene expression was significantly higher starting at 7 days for the GDF-5 treated groups, and at day 14 was significantly higher compared to 3 day in all the groups. ADSCs cultured on 2D films showed an increase in scleraxis gene expression at 3 and 7 day in the GDF-5 supplemented groups, however, scleraxis gene expression was not different at the late time point (Figure 7B). Scleraxis gene expression was significantly reduced at 14 days on the 2D film with GDF-5 supplementation as compared to the 7 day time point. ADSCs on the electrospun scaffold compared to the 2D scaffold showed significantly higher scleraxis gene expression only at the 14 day time point. However, with GDF-5 treatment expression was significantly higher starting at 7 days and the differences increased further at the later time point. Gene expression for tenomodulin, a marker of mature tendon tissue, was higher in all the groups treated with GDF-5 and maintained on the 3D scaffold at 7 days post-seeding; however the difference between the GDF-5 treated groups and without GDF-5 was not significant at the 14 day time point (Figure 8A). By contrast, tenomodulin gene expression of ADSCs cultured on the 2D film was significantly higher only on treatment with 100ng/mL GDF-5 (Figure 8B). Temporal gene expression of tenomodulin was higher at 7 and 14 day time points compared to the day 3 in ADSCs cultured on 3D scaffolds. On 2D films, at 14 days the expression of tenomodulin gene expression was higher compared to 3 days. Tenomodulin expression was higher on the 3D scaffolds compared to the 2D films at 7 and 14 days with or without GDF-5 treatment.
Figure 7.
Scleraxis gene expression by cells treated with GDF-5. (A) Cells on 3D scaffold, and (B) on 2D film. Gene expression for scleraxis, a marker for early tendon phenotype or neotendon, was increased on treatment with 10 and 100 ng/mL GDF-5 starting at 7 days post-seeding on the 3D scaffold (p<0.05). However, in cells on the 2D film, scleraxis gene expression ADSCs increased initially but decreased by 14 days. Gene expression data is normalized to the 18s gene and expression of day 3 on 3D scaffold without GDF-5 (0ng/mL).
Figure 8.
Expression of the tenomodulin gene in response to treatment with GDF-5. (A) Cells on 3D scaffold, and (B) on 2D film. Gene expression for tenomodulin, a marker of the mature tendon phenotype, was not significantly different in cells treated with GDF-5 compared to cells cultured in basal medium without GDF-5 at early and late time points. Tenomodulin gene expression by cells on 3D scaffold increased over time (A), and was higher than expression by cells on 2D film (B) at 7 days. Gene expression data is normalized to the 18s gene and expression of day 3 on 3D scaffold without GDF-5 (0ng/mL).
4. Discussion
Electrospun PLAGA 3D scaffolds supported the adhesion and proliferation of primary ADSCs. Upon treatment with GDF-5, ADSCs cultured on the scaffold showed an increase in cell proliferation with increasing concentrations of GDF-5. Our previous studies showed that GDF-5 at concentration higher than 100ng/mL inhibited expression of tendon and ECM gene markers by ADSCs that are cultured on tissue culture polystyrene (TCPS) (Park et al. 2010). In tendon, the ECM which provides anchorage to and supports tendon cells, sequesters cytokines that regulate the survival, proliferation and differentiation of the cells and maintains an environment within which the cells can perform their function in vivo. Tendon ECM is composed of collagen molecules that are assembled into fibrils (the smallest tendon structural unit) with diameters ranging from 10 to 500nm, depending on species, age, and sample location. Collagen fibrils are further organized into fibers with diameters ranging from 0.5 – 20μm. Tenocytes and tenoblasts reside between the collagen fibers. Adult human tendons are comprised of individual fibrils, ranging in diameter from 60 – 175nm, which form collagen fibers with diameters in the 0.6–1μm range (Dyer and Enna 1976, Wang 2006). By comparison, at 0.8μm, the diameter of the electrospun scaffold falls within the range of diameters of the collagen fiber bundles that occur in tendon. The micro-diameter fibers in the electrospun PLAGA scaffold mimic the structure tendon ECM and provide a large surface area for the attachment of ADSCs.
Collagen fibers in tendon ECM transfer the forces of muscle contraction to bone enabling movement of the extremities. The tensile strength of the scaffold is within the range of forces reported for passive or unresisted motion in flexor tendon of the hand (Mortenson and Urbaniak 1972, Schuind et al. 1992, Kursa et al. 2006). In flexor tendons of the hand, a mean force of 2N has been reported during passive-flexion and 9N during active-flexion against slight resistance (Mortenson and Urbaniak 1972). In active unresisted flexion of the interphalangeal joints of the hand, forces range from 19 – 25 N for the flexor digitorum tendons. The forces exerted during pinching represent strong resistive active movement with a mean force of 83 N and 25 N in the flexor digitorum profundus and flexor digitorum longus respectively (Schuind et al. 1992). The current study shows that the electrospun scaffolds can support tensile force and extension to allow for passive flexion-extension of the flexor tendons. Being mechanically competent, the scaffold would support controlled early or passive motion, in vivo, which is necessary to prevent adhesions and scar tissue formation, thereby promoting faster recovery from injuries in order to restore functionality (Sahoo et al. 2007, Moffat et al. 2009). In addition, early mobilization of the scaffold in vivo would contribute mechanical stimuli to modulate the regenerative process following tendon gap defect repair with a graft (Moreau et al. 2008, Nirmalanandhan et al. 2009). It is expected that as the scaffold degrades and is replaced with the regenerate tissue, tendon strength will increase thereby allowing resistive or active flexor tendon motion. PLAGA scaffolds that are composed of micron diameter fibers have been fabricated by braiding and knitting and shown to have mechanical properties comparable to Achilles tendons and knee ligaments, both of which experience large tensile forces under intensive activity (Cooper et al. 2005, Van Eijk et al. 2004). These braided and knitted scaffolds support cell adhesion and proliferation in vitro. However, the fiber bundles under tensile loading in vivo become tightly packed preventing cellular infiltration (Sahoo et al. 2007, Hutmacher 2001). Recently, the surfaces of these scaffolds were modified by electrospinning biomimetic fibers in order to promote cellular adhesion and proliferation (Sahoo et al. 2007, Sahoo et al. 2006, Ouyang et al. 2003, Ge et al. 2005).
We have reported previously that ADSCs cultured in vitro on TCPS can be modulated to promote cellular events that lead to tendinogenic differentiation (Park et al. 2010). There is an increase in the number of cells on 3D electrospun and 2D film PLAGA that were treated with GDF-5, however, only cells on the biomimetic electrospun scaffold showed an increase in gene expression for the early tendinogenic and ECM. Collagen type I is the predominant component of tendon ECM and its organization is responsible for the tensile strength of tendon (Maffulli and Ajis 2008, James et al. 2008). Collagen type III expression was not different at the GDF-5 concentrations used to treat the cells during the culture periods examined in this study. Relatively low amounts of collagen type III are present in tendon, however, collagen type III increases during the early stages of repair and is located within a disorganized fibrillar matrix that is heavily infiltrated with fibroblastic cells that are responsible for collagen III synthesis (Lin et al. 2004). Our results suggest that the ADSCs on electrospun scaffold supplemented with GDF-5 expressed predominantly collagen type I, and that this increased 5 fold by the end of 2nd week. The absence of a change in collagen type III gene expression suggests the lack of a fibroblastic response. Such a response would otherwise lead to scarring in vivo, and, if not remodeled appropriately will limit functionality. A similar temporal effect of collagen type I expression has been observed in BMSCs on biomimetic scaffolds in response to other cytokines (Nakamura et al. 2003, Jenner et al. 2007). Significant decrease in collagen type I gene expression in ADSCs on the 2D films when treated with GDF-5 could be possibly because of cells having established confluent monolayer and under stimulatory effect of GDF-5 are changing phenotype.
In the developing embryo, the scleraxis gene is identified as the marker for tendon progenitor population within the mesenchyme (Merino et al. 1999, Storm and Kingsley 1999). Mutant mice lacking the scleraxis gene were viable, but showed severe tendon defects, which drastically limited use of all paws and back muscles and a complete inability to move the tail. Tendons from the mutant mice present a reduced and less organized matrix and disorganization at the cellular level (Murchison et al. 2007). Our study demonstrates the potency of GDF-5 treatment on the expression of neotendon phenotype in ADSCs that are cultured on the ECM-like 3D scaffold. Studies using ADSCs cultured on tissue culture plastic have reported between 3 and 6 fold increase in scleraxis gene expression at the early time points and this trend is noted in this study on 2D films (Park et al. 2010). However, on 3D electrospun scaffolds without GDF-5 supplementation, scleraxis gene expression is significantly higher at 14 days compared to 2D PLAGA films, and with GDF-5 supplementation expression is prominently higher at both 7 and 14 days emphasizing the combinatorial effect of the ECM-like 3D scaffold and GDF-5 protein at directing cell differentiation to the tendon phenotype. In the early limb bud the application of Noggin antagonizes endogenous BMP activity and induces ectopic scleraxis expression. However, the abundance of scleraxis positive tendon progenitors did not lead to an increase in tendon number or length, indicating that additional signals are required for the full development of a tendon (Schweitzer et al. 2001, Asou et al. 2002). Tenomodulin is a late marker of tendon formation and expression and is reported to be positively regulated by scleraxis in chick embryo studies (Shukunami et al. 2006). ADSCs did not show maturation into tendon fibroblasts at the time points that were studied. It is important to note that the in vitro study presented here is limited to static cultures, and that stronger effects (expression of tenomodulin) might have been observed in an environment where additional stimulation was present, such as biomechanical stimulation in a bioreactor or additional growth factors that may be present and have their effects in vivo. In vitro studies have shown that optimal differentiation of BMSCs into tendon/ligament phenotype may require simultaneous and sequential administration of biochemical and mechanical cues (Moreau et al. 2005, Moreau et al. 2006). Tendinogenic stimulation with other GDF family proteins have been recently reported with in vitro culture of human BMSCs isolated from the femoral head and virally transected with GDF-6 and GDF-7 cDNA (Haddad-Weber et al. 2010). GDF-5 mutant mice present tendons with collagen fibers of reduced diameter (Chhabra et al. 2003, Mikic et al. 2001, Mikic 2004) The mutant mice also show delayed tendon, however, the tendons heal eventually suggesting a role for other growth factors, in addition to GDF-5, in regenerating tendon defects.
In the literature, most studies report higher cell proliferation on 3D scaffolds as compared to 2D films. However, ADSCs cultured on the 2D films gave significantly higher readings compared to the 3D electrospun scaffolds, and may be due to higher efficiency of cell lysis and DNA extraction from the films as compared to the 3D scaffold (Sahoo et al. 2010). Cord-blood stem cells seeded onto PLAGA electrospun scaffolds show increased expression of osteogenic markers as compared with TCPS culture (Seyedjafari et al. 2010). Additionally, the cord-blood derived stem cells show increased cell proliferation on TCPS compared to electrospun scaffolds. Our studies exhibit a similar trend, where an increased proliferation rate of ADSCs is noted on the 2D films compared to 3D scaffolds. During the same time period, there is increased expression of tendinogenic/neotendon and ECM marker genes by ADSCs on 3D scaffold suggesting lineage differentiation towards the tendon phenotype. In vivo, tendon progenitor cells originating from within the tendon stump and stromal cells migrating to the tendon wound site through the vascular circulation modulate the healing process (Bi et al. 2007, Chamberlain et al. 2007, Salingcarnboriboon et al. 2003). Studies to deliver BMSCs to the injured tendon in order to accelerate repair have yielded variable results (Ouyang et al. 2002, Gulotta et al. 2009, Hankemeier et al. 2009, Pacini et al. 2007, Chong et al. 2007, Hui et al. 2005, Awad et al. 1999). To date, there are no reports of the use of multipotent cells from adipose tissue in combination with growth factors or ECM-like 3D scaffolds for tendon tissue engineering. GDF-5 has a limited effect on the growth and differentiation of BMSC seeded on braided PLAGA constructs (Jenner et al. 2007). There was a small increase in cell proliferation, although GDF-5 have been shown to induce tendon formation ectopically (Wolfman et al. 1997)), and to enhance tendon healing in vivo using gene transfer approaches and sutures coated with GDF-5 (Hankemeier et al. 2009, Lou et al. 2001, Rickert et al. 2001, Rickert et al. 2005, Bolt et al. 2007, Aspenberg 2007, Basile et al. 2008). Lacerated tendon stumps repaired with sutures soaked with GDF-5 protein have shown increased tensile strength and modulus compared to controls that did not receive GDF-5 (Dines et al. 2007). However, histological evaluation revealed isolated sites of cartilage formation associated with regions of suture placement. In this instance, the higher concentration of GDF-5 protein delivered to the suture placement may have contributed to the formation of cartilaginous tissue. These observations are similar to in vitro studies with cells on TCPS with high concentrations of GDF-5 that down-regulate tendinogenic phenotype, and combinatorial effect with other factors promoting differentiation along osteogenic and chondrogenic pathways (Park et al. 2010, Li et al. 2005, Cui et al. 2008).
The limitations of our study may be addressed in future work. ADSCs appear to have more tendinogenic responsiveness to GDF-5 compared to BMSCs and in the absence of other stimulatory factors (Zeng et al. 2006). In addition, autologous ADSCs are more readily available thereby facilitating tendon tissue engineering. So far, no specific cell surface markers that predispose progenitors to a tendon phenotype have been identified in stromal populations to enable pre-selection and cell enrichment. Our studies may be extended to using human adipose-derived stromal cells to evaluate tendinogenic differentiation pathways that are modulated by GDF-5 and by the ECM-like electrospun 3D scaffolds. The tendinogenic effect of ADSCs seeded on electrospun scaffolds was shown with GDF-5 protein supplemented in the culture media. Futures studies could incorporate GDF-5 protein onto the scaffold surface by covalent bonding, include mechanical stimuli and evaluate the composite as a potential graft in vivo for improved regeneration and functional assessment. Our study sets the stage for a better understanding of therapies that would enhance soft musculoskeletal connective tissue regeneration.
Conclusions
Recombinant GDF-5 protein significantly stimulated neotendon phenotype in ADSCs cultured on a 3D PLAGA scaffold that mimics the structure and physical properties of flexor tendon ECM. The 3D ECM-like scaffold supported cell adhesion and proliferation, and, GDF-5 supplementation enhanced collagen type I gene expression. The tensile strength of the scaffold is within the range measured for human flexor digitorum tendons under passive and active unresisted flexion-extension and could provide initial biomechanical stability that is required during the early repair phase.
Acknowledgement
We acknowledge funding from NIH R03 AR052891 (NIAMS) awarded to Abhinav B. Chhabra, M.D. and from NSF-(EFRI-0736002) to Cato T. Laurencin, M.D., Ph.D. Dr. Laurencin was the recipient of a Presidential Faculty Fellow Award from the National Science Foundation.
References
- Asou Y, Nifuji A, Tsuji K, Shinomiya K, Olson EN, Koopman P, Noda M. Coordinated expression of scleraxis and Sox9 genes during embryonic development of tendons and cartilage. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2002;vol. 20(no. 4):827–833. doi: 10.1016/S0736-0266(01)00169-3. [DOI] [PubMed] [Google Scholar]
- Aspenberg P. Stimulation of tendon repair: mechanical loading, GDFs and platelets. A mini-review. International orthopaedics. 2007;vol. 31(no. 6):783–789. doi: 10.1007/s00264-007-0398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad HA, Butler DL, Boivin GP, Smith FN, Malaviya P, Huibregtse B, Caplan AI. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue engineering. 1999;vol. 5(no. 3):267–277. doi: 10.1089/ten.1999.5.267. [DOI] [PubMed] [Google Scholar]
- Basile P, Dadali T, Jacobson J, Hasslund S, Ulrich-Vinther M, Soballe K, Nishio Y, Drissi MH, Langstein HN, Mitten DJ, O’Keefe RJ, Schwarz EM, Awad HA. Freeze-dried tendon allografts as tissue-engineering scaffolds for Gdf5 gene delivery. Molecular therapy : the journal of the American Society of Gene Therapy. 2008;vol. 16(no. 3):466–473. doi: 10.1038/sj.mt.6300395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKER AJ, McCULLOCH EA, TILL JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;vol. 197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nature medicine. 2007;vol. 13(no. 10):1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- Bolt P, Clerk AN, Luu HH, Kang Q, Kummer JL, Deng ZL, Olson K, Primus F, Montag AG, He TC, Haydon RC, Toolan BC. BMP-14 gene therapy increases tendon tensile strength in a rat model of Achilles tendon injury. The Journal of bone and joint surgery.American volume. 2007;vol. 89(no. 6):1315–1320. doi: 10.2106/JBJS.F.00257. [DOI] [PubMed] [Google Scholar]
- Butler DL, Juncosa N, Dressler MR. Functional efficacy of tendon repair processes. Annual Review of Biomedical Engineering. 2004a;vol. 6:303–329. doi: 10.1146/annurev.bioeng.6.040803.140240. [DOI] [PubMed] [Google Scholar]
- Butler DL, Shearn JT, Juncosa N, Dressler MR, Hunter SA. Functional tissue engineering parameters toward designing repair and replacement strategies. Clin.Orthop.Relat.Res. 2004b;vol. (427 Suppl)(no. 427 Suppl):S190–9. doi: 10.1097/01.blo.0000144858.65450.d2. [DOI] [PubMed] [Google Scholar]
- Calve S, Dennis RG, Kosnik PE, Baar K, Grosh K, Arruda E. Engineering of functional tendon. Tissue engineering. 2004;vol. 10(no. 5-6):755–761. doi: 10.1089/1076327041348464. [DOI] [PubMed] [Google Scholar]
- Carlberg B, Axell MZ, Nannmark U, Liu J, Kuhn HG. Electrospun polyurethane scaffolds for proliferation and neuronal differentiation of human embryonic stem cells. Biomedical materials (Bristol, England) 2009;vol. 4(no. 4):045004. doi: 10.1088/1748-6041/4/4/045004. [DOI] [PubMed] [Google Scholar]
- Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem cells (Dayton, Ohio) 2007;vol. 25(no. 11):2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- Chen J, Xu J, Wang A, Zheng M. Scaffolds for tendon and ligament repair: review of the efficacy of commercial products. Expert review of medical devices. 2009;vol. 6(no. 1):61–73. doi: 10.1586/17434440.6.1.61. [DOI] [PubMed] [Google Scholar]
- Chhabra A, Tsou D, Clark RT, Gaschen V, Hunziker EB, Mikic B. GDF-5 deficiency in mice delays Achilles tendon healing. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2003;vol. 21(no. 5):826–835. doi: 10.1016/S0736-0266(03)00049-4. [DOI] [PubMed] [Google Scholar]
- Chong AK, Ang AD, Goh JC, Hui JH, Lim AY, Lee EH, Lim BH. Bone marrow-derived mesenchymal stem cells influence early tendon-healing in a rabbit achilles tendon model. The Journal of bone and joint surgery.American volume. 2007;vol. 89(no. 1):74–81. doi: 10.2106/JBJS.E.01396. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Lu HH, Ko FK, Freeman JW, Laurencin CT. Fiber-based tissue-engineered scaffold for ligament replacement: design considerations and in vitro evaluation. Biomaterials. 2005;vol. 26(no. 13):1523–1532. doi: 10.1016/j.biomaterials.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Cui M, Wan Y, Anderson DG, Shen FH, Leo BM, Laurencin CT, Balian G, Li X. Mouse growth and differentiation factor-5 protein and DNA therapy potentiates intervertebral disc cell aggregation and chondrogenic gene expression. The spine journal : official journal of the North American Spine Society. 2008;vol. 8(no. 2):287–295. doi: 10.1016/j.spinee.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Derwin KA, Baker AR, Spragg RK, Leigh DR, Iannotti JP. Commercial extracellular matrix scaffolds for rotator cuff tendon repair. Biomechanical, biochemical, and cellular properties. The Journal of bone and joint surgery.American volume. 2006;vol. 88(no. 12):2665–2672. doi: 10.2106/JBJS.E.01307. [DOI] [PubMed] [Google Scholar]
- Dines JS, Weber L, Razzano P, Prajapati R, Timmer M, Bowman S, Bonasser L, Dines DM, Grande DP. The effect of growth differentiation factor-5-coated sutures on tendon repair in a rat model. Journal of shoulder and elbow surgery / American Shoulder and Elbow Surgeons …[et al.] 2007;vol. 16(no. 5 Suppl):S215–21. doi: 10.1016/j.jse.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Dyer RF, Enna CD. Ultrastructural features of adult human tendon. Cell and tissue research. 1976;vol. 168(no. 2):247–259. doi: 10.1007/BF00215881. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;vol. 6(no. 2):230–247. [PubMed] [Google Scholar]
- Ge Z, Goh JC, Lee EH. Selection of cell source for ligament tissue engineering. Cell transplantation. 2005;vol. 14(no. 8):573–583. doi: 10.3727/000000005783982819. [DOI] [PubMed] [Google Scholar]
- Gulotta LV, Kovacevic D, Ehteshami JR, Dagher E, Packer JD, Rodeo SA. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. The American Journal of Sports Medicine. 2009;vol. 37(no. 11):2126–2133. doi: 10.1177/0363546509339582. [DOI] [PubMed] [Google Scholar]
- Haddad-Weber M, Prager P, Kunz M, Seefried L, Jakob F, Murray MM, Evans CH, Noth U, Steinert AF. BMP12 and BMP13 gene transfer induce ligamentogenic differentiation in mesenchymal progenitor and anterior cruciate ligament cells. Cytotherapy. 2010;vol. 12(no. 4):505–513. doi: 10.3109/14653241003709652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankemeier S, Hurschler C, Zeichen J, van Griensven M, Miller B, Meller R, Ezechieli M, Krettek C, Jagodzinski M. Bone marrow stromal cells in a liquid fibrin matrix improve the healing process of patellar tendon window defects. Tissue engineering.Part A. 2009;vol. 15(no. 5):1019–1030. doi: 10.1089/ten.tea.2008.0046. [DOI] [PubMed] [Google Scholar]
- Hong SJ, Traktuev DO, March KL. Therapeutic potential of adipose-derived stem cells in vascular growth and tissue repair. Current opinion in organ transplantation. 2010;vol. 15(no. 1):86–91. doi: 10.1097/MOT.0b013e328334f074. [DOI] [PubMed] [Google Scholar]
- Hui JH, Ouyang HW, Hutmacher DW, Goh JC, Lee EH. Mesenchymal stem cells in musculoskeletal tissue engineering: a review of recent advances in National University of Singapore. Annals of the Academy of Medicine, Singapore. 2005;vol. 34(no. 2):206–212. [PubMed] [Google Scholar]
- Hutmacher DW. Scaffold design and fabrication technologies for engineering tissues-- state of the art and future perspectives. Journal of biomaterials science.Polymer edition. 2001;vol. 12(no. 1):107–124. doi: 10.1163/156856201744489. [DOI] [PubMed] [Google Scholar]
- James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. The Journal of hand surgery. 2008;vol. 33(no. 1):102–112. doi: 10.1016/j.jhsa.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Jenner JM, van Eijk F, Saris DB, Willems WJ, Dhert WJ, Creemers LB. Effect of transforming growth factor-beta and growth differentiation factor-5 on proliferation and matrix production by human bone marrow stromal cells cultured on braided poly lactic-co-glycolic acid scaffolds for ligament tissue engineering. Tissue engineering. 2007;vol. 13(no. 7):1573–1582. doi: 10.1089/ten.2006.0208. [DOI] [PubMed] [Google Scholar]
- Kajiyama H, Hamazaki TS, Tokuhara M, Masui S, Okabayashi K, Ohnuma K, Yabe S, Yasuda K, Ishiura S, Okochi H, Asashima M. Pdx1-transfected adipose tissue-derived stem cells differentiate into insulin-producing cells in vivo and reduce hyperglycemia in diabetic mice. The International journal of developmental biology. 2010;vol. 54(no. 4):699–705. doi: 10.1387/ijdb.092953hk. [DOI] [PubMed] [Google Scholar]
- Kang SK, Putnam LA, Ylostalo J, Popescu IR, Dufour J, Belousov A, Bunnell BA. Neurogenesis of Rhesus adipose stromal cells. Journal of cell science. 2004;vol. 117(no. Pt 18):4289–4299. doi: 10.1242/jcs.01264. [DOI] [PubMed] [Google Scholar]
- Kolambkar YM, Peister A, Ekaputra AK, Hutmacher DW, Guldberg RE. Colonization and Osteogenic Differentiation of Different Stem Cell Sources on Electrospun Nanofiber Meshes. Tissue engineering.Part A. 2010 doi: 10.1089/ten.tea.2010.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumbar SG, Nukavarapu SP, James R, Hogan MV, Laurencin CT. Recent Patents on Electrospun Biomedical Nanostructures: An Overview. Recent Patents on Biomedical Engineering. 2008a;vol. 1(no. 1):68–78. [Google Scholar]
- Kumbar SG, James R, Nukavarapu SP, Laurencin CT. Electrospun nanofiber scaffolds: engineering soft tissues. Biomedical materials (Bristol, England) 2008b;vol. 3(no. 3):034002. doi: 10.1088/1748-6041/3/3/034002. [DOI] [PubMed] [Google Scholar]
- Kumbar SG, Nukavarapu SP, James R, Nair LS, Laurencin CT. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials. 2008c;vol. 29(no. 30):4100–4107. doi: 10.1016/j.biomaterials.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kursa K, Lattanza L, Diao E, Rempel D. In vivo flexor tendon forces increase with finger and wrist flexion during active finger flexion and extension. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2006;vol. 24(no. 4):763–769. doi: 10.1002/jor.20110. [DOI] [PubMed] [Google Scholar]
- Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. Journal of Biomedical Materials Research. 2002;vol. 60(no. 4):613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- Li X, Jin L, Cui Q, Wang GJ, Balian G. Steroid effects on osteogenesis through mesenchymal cell gene expression. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2005;vol. 16(no. 1):101–108. doi: 10.1007/s00198-004-1649-7. [DOI] [PubMed] [Google Scholar]
- Liang L, Ma T, Chen W, Hu J, Bai X, Li J, Liang T. Therapeutic potential and related signal pathway of adipose-derived stem cell transplantation for rat liver injury. Hepatology research : the official journal of the Japan Society of Hepatology. 2009;vol. 39(no. 8):822–832. doi: 10.1111/j.1872-034X.2009.00506.x. [DOI] [PubMed] [Google Scholar]
- Lin TW, Cardenas L, Soslowsky LJ. Biomechanics of tendon injury and repair. Journal of Biomechanics. 2004;vol. 37(no. 6):865–877. doi: 10.1016/j.jbiomech.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Lou J, Tu Y, Burns M, Silva MJ, Manske P. BMP-12 gene transfer augmentation of lacerated tendon repair. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2001;vol. 19(no. 6):1199–1202. doi: 10.1016/S0736-0266(01)00042-0. [DOI] [PubMed] [Google Scholar]
- Maffulli N, Ajis A. Management of chronic ruptures of the Achilles tendon. The Journal of bone and joint surgery.American volume. 2008;vol. 90(no. 6):1348–1360. doi: 10.2106/JBJS.G.01241. [DOI] [PubMed] [Google Scholar]
- Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;vol. 3(no. 2):232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- Merino R, Macias D, Ganan Y, Economides AN, Wang X, Wu Q, Stahl N, Sampath KT, Varona P, Hurle JM. Expression and function of Gdf-5 during digit skeletogenesis in the embryonic chick leg bud. Developmental biology. 1999;vol. 206(no. 1):33–45. doi: 10.1006/dbio.1998.9129. [DOI] [PubMed] [Google Scholar]
- Mikic B. Multiple effects of GDF-5 deficiency on skeletal tissues: implications for therapeutic bioengineering. Annals of Biomedical Engineering. 2004;vol. 32(no. 3):466–476. doi: 10.1023/b:abme.0000017549.57126.51. [DOI] [PubMed] [Google Scholar]
- Mikic B, Entwistle R, Rossmeier K, Bierwert L. Effect of GDF-7 deficiency on tail tendon phenotype in mice. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2008;vol. 26(no. 6):834–839. doi: 10.1002/jor.20581. [DOI] [PubMed] [Google Scholar]
- Mikic B, Rossmeier K, Bierwert L. Identification of a tendon phenotype in GDF6 deficient mice. Anatomical record (Hoboken, N.J.: 2007) 2009;vol. 292(no. 3):396–400. doi: 10.1002/ar.20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikic B, Schalet BJ, Clark RT, Gaschen V, Hunziker EB. GDF-5 deficiency in mice alters the ultrastructure, mechanical properties and composition of the Achilles tendon. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2001;vol. 19(no. 3):365–371. doi: 10.1016/S0736-0266(00)90018-4. [DOI] [PubMed] [Google Scholar]
- Mizuno H. Adipose-derived stem cells for tissue repair and regeneration: ten years of research and a literature review. Journal of Nippon Medical School = Nihon Ika Daigaku zasshi. 2009;vol. 76(no. 2):56–66. doi: 10.1272/jnms.76.56. [DOI] [PubMed] [Google Scholar]
- Moffat KL, Kwei AS, Spalazzi JP, Doty SB, Levine WN, Lu HH. Novel nanofiber-based scaffold for rotator cuff repair and augmentation. Tissue engineering.Part A. 2009;vol. 15(no. 1):115–126. doi: 10.1089/ten.tea.2008.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montzka K, Heidenreich A. Application of mesenchymal stromal cells in urological diseases. BJU international. 2010;vol. 105(no. 3):309–312. doi: 10.1111/j.1464-410X.2009.09077.x. [DOI] [PubMed] [Google Scholar]
- Moreau J, Chen J, Kaplan D, Altman G. Sequential growth factor stimulation of bone marrow stromal cells in extended culture. Tissue engineering. 2006;vol. 12(no. 10):2905–2912. doi: 10.1089/ten.2006.12.2905. [DOI] [PubMed] [Google Scholar]
- Moreau JE, Bramono DS, Horan RL, Kaplan DL, Altman GH. Sequential biochemical and mechanical stimulation in the development of tissue-engineered ligaments. Tissue engineering.Part A. 2008;vol. 14(no. 7):1161–1172. doi: 10.1089/ten.tea.2007.0147. [DOI] [PubMed] [Google Scholar]
- Moreau JE, Chen J, Horan RL, Kaplan DL, Altman GH. Sequential growth factor application in bone marrow stromal cell ligament engineering. Tissue engineering. 2005;vol. 11(no. 11-12):1887–1897. doi: 10.1089/ten.2005.11.1887. [DOI] [PubMed] [Google Scholar]
- Mortenson RA, Urbaniak JR. Analysis of tensile strength of tendon anastomosis. Surgical forum. 1972;vol. 23(no. 0):470–471. [PubMed] [Google Scholar]
- Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development (Cambridge, England) 2007;vol. 134(no. 14):2697–2708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Yamamoto M, Tamura M, Izumi Y. Effects of growth/differentiation factor-5 on human periodontal ligament cells. Journal of periodontal research. 2003;vol. 38(no. 6):597–605. doi: 10.1034/j.1600-0765.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- Nirmalanandhan VS, Juncosa-Melvin N, Shearn JT, Boivin GP, Galloway MT, Gooch C, Bradica G, Butler DL. Combined effects of scaffold stiffening and mechanical preconditioning cycles on construct biomechanics, gene expression, and tendon repair biomechanics. Tissue engineering.Part A. 2009;vol. 15(no. 8):2103–2111. doi: 10.1089/ten.tea.2008.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang HW, Goh JC, Mo XM, Teoh SH, Lee EH. The efficacy of bone marrow stromal cell-seeded knitted PLGA fiber scaffold for Achilles tendon repair. Annals of the New York Academy of Sciences. 2002;vol. 961:126–129. doi: 10.1111/j.1749-6632.2002.tb03064.x. [DOI] [PubMed] [Google Scholar]
- Ouyang HW, Goh JC, Thambyah A, Teoh SH, Lee EH. Knitted poly-lactide-co-glycolide scaffold loaded with bone marrow stromal cells in repair and regeneration of rabbit Achilles tendon. Tissue engineering; Tissue engineering. 2003;vol. 9(no. 3):431–439. doi: 10.1089/107632703322066615. [DOI] [PubMed] [Google Scholar]
- Pacini S, Spinabella S, Trombi L, Fazzi R, Galimberti S, Dini F, Carlucci F, Petrini M. Suspension of bone marrow-derived undifferentiated mesenchymal stromal cells for repair of superficial digital flexor tendon in race horses. Tissue engineering. 2007;vol. 13(no. 12):2949–2955. doi: 10.1089/ten.2007.0108. [DOI] [PubMed] [Google Scholar]
- Park A, Hogan MV, Kesturu GS, James R, Balian G, Chhabra AB. Adipose-derived mesenchymal stem cells treated with growth differentiation factor-5 express tendon-specific markers. Tissue engineering.Part A. 2010;vol. 16(no. 9):2941–2951. doi: 10.1089/ten.tea.2009.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Ju YM, Son JS, Ahn KD, Han DK. Surface modification of biodegradable electrospun nanofiber scaffolds and their interaction with fibroblasts. Journal of biomaterials science.Polymer edition. 2007;vol. 18(no. 4):369–382. doi: 10.1163/156856207780424997. [DOI] [PubMed] [Google Scholar]
- Petrigliano FA, English CS, Barba D, Esmende S, Wu BM, McAllister DR. The effects of local bFGF release and uniaxial strain on cellular adaptation and gene expression in a 3D environment: implications for ligament tissue engineering. Tissue engineering. 2007;vol. 13(no. 11):2721–2731. doi: 10.1089/ten.2006.0434. [DOI] [PubMed] [Google Scholar]
- Platt MA. Tendon repair and healing. Clin.Podiatr.Med.Surg.North.Am. 2005;vol. 22(no. 4):553–60. doi: 10.1016/j.cpm.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Rickert M. BMP-14 gene therapy increases tendon tensile strength in a rat model of achilles tendon injury. The Journal of bone and joint surgery.American volume. 2008;vol. 90(no. 2):445. author reply 445-6. [PubMed] [Google Scholar]
- Rickert M, Jung M, Adiyaman M, Richter W, Simank HG. A growth and differentiation factor-5 (GDF-5)-coated suture stimulates tendon healing in an Achilles tendon model in rats. Growth factors (Chur, Switzerland) 2001;vol. 19(no. 2):115–126. doi: 10.3109/08977190109001080. [DOI] [PubMed] [Google Scholar]
- Rickert M, Wang H, Wieloch P, Lorenz H, Steck E, Sabo D, Richter W. Adenovirus-mediated gene transfer of growth and differentiation factor-5 into tenocytes and the healing rat Achilles tendon. Connective tissue research. 2005;vol. 46(no. 4-5):175–183. doi: 10.1080/03008200500237120. [DOI] [PubMed] [Google Scholar]
- Sahoo S, Ang LT, Goh JC, Toh SL. Growth factor delivery through electrospun nanofibers in scaffolds for tissue engineering applications. Journal of biomedical materials research.Part A. 2010;vol. 93(no. 4):1539–1550. doi: 10.1002/jbm.a.32645. [DOI] [PubMed] [Google Scholar]
- Sahoo S, Cho-Hong JG, Siew-Lok T. Development of hybrid polymer scaffolds for potential applications in ligament and tendon tissue engineering. Biomedical materials (Bristol, England) 2007;vol. 2(no. 3):169–173. doi: 10.1088/1748-6041/2/3/001. [DOI] [PubMed] [Google Scholar]
- Sahoo S, Ouyang H, Goh JC, Tay TE, Toh SL. Characterization of a novel polymeric scaffold for potential application in tendon/ligament tissue engineering. Tissue engineering. 2006;vol. 12(no. 1):91–99. doi: 10.1089/ten.2006.12.91. [DOI] [PubMed] [Google Scholar]
- Sahoo S, Toh SL, Goh JC. A bFGF-releasing silk/PLGA-based biohybrid scaffold for ligament/tendon tissue engineering using mesenchymal progenitor cells. Biomaterials. 2010;vol. 31(no. 11):2990–2998. doi: 10.1016/j.biomaterials.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Salingcarnboriboon R, Yoshitake H, Tsuji K, Obinata M, Amagasa T, Nifuji A, Noda M. Establishment of tendon-derived cell lines exhibiting pluripotent mesenchymal stem cell-like property. Experimental cell research. 2003;vol. 287(no. 2):289–300. doi: 10.1016/s0014-4827(03)00107-1. [DOI] [PubMed] [Google Scholar]
- Schuind F, Garcia-Elias M, Cooney WP, 3rd, An KN. Flexor tendon forces: in vivo measurements. The Journal of hand surgery. 1992;vol. 17(no. 2):291–298. doi: 10.1016/0363-5023(92)90408-h. [DOI] [PubMed] [Google Scholar]
- Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development (Cambridge, England) 2001;vol. 128(no. 19):3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- Seyedjafari E, Soleimani M, Ghaemi N, Sarbolouki MN. Enhanced osteogenic differentiation of cord blood-derived unrestricted somatic stem cells on electrospun nanofibers. Journal of materials science.Materials in medicine. 2010 doi: 10.1007/s10856-010-4174-6. [DOI] [PubMed] [Google Scholar]
- Shukunami C, Takimoto A, Oro M, Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Developmental biology. 2006;vol. 298(no. 1):234–247. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Storm EE, Kingsley DM. GDF5 coordinates bone and joint formation during digit development. Developmental biology. 1999;vol. 209(no. 1):11–27. doi: 10.1006/dbio.1999.9241. [DOI] [PubMed] [Google Scholar]
- Timper K, Seboek D, Eberhardt M, Linscheid P, Christ-Crain M, Keller U, Muller B, Zulewski H. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochemical and biophysical research communications. 2006;vol. 341(no. 4):1135–1140. doi: 10.1016/j.bbrc.2006.01.072. [DOI] [PubMed] [Google Scholar]
- Van Eijk F, Saris DB, Riesle J, Willems WJ, Van Blitterswijk CA, Verbout AJ, Dhert WJ. Tissue engineering of ligaments: a comparison of bone marrow stromal cells, anterior cruciate ligament, and skin fibroblasts as cell source. Tissue engineering. 2004;vol. 10(no. 5-6):893–903. doi: 10.1089/1076327041348428. [DOI] [PubMed] [Google Scholar]
- Wang JH. Mechanobiology of tendon. Journal of Biomechanics. 2006;vol. 39(no. 9):1563–1582. doi: 10.1016/j.jbiomech.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Wolfman NM, Hattersley G, Cox K, Celeste AJ, Nelson R, Yamaji N, Dube JL, DiBlasio-Smith E, Nove J, Song JJ, Wozney JM, Rosen V. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. The Journal of clinical investigation. 1997;vol. 100(no. 2):321–330. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin X, Hussain M, Mao JJ. Continuing differentiation of human mesenchymal stem cells and induced chondrogenic and osteogenic lineages in electrospun PLGA nanofiber scaffold. Biomaterials. 2007;vol. 28(no. 2):316–325. doi: 10.1016/j.biomaterials.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Liu L, Li Y, Zhou C, Xiong F, Liu Z, Gu R, Hou X, Zhang C. Myelin-forming ability of Schwann cell-like cells induced from rat adipose-derived stem cells in vitro. Brain research. 2008;vol. 1239:49–55. doi: 10.1016/j.brainres.2008.08.088. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Li X, Choi L, Beck G, Balian G, Shen FH. Recombinant growth/differentiation factor-5 stimulates osteogenic differentiation of fat-derived stromal cells in vitro. Connective tissue research. 2006;vol. 47(no. 5):264–270. doi: 10.1080/03008200600980769. [DOI] [PubMed] [Google Scholar]
- Zhang YZ, Su B, Venugopal J, Ramakrishna S, Lim CT. Biomimetic and bioactive nanofibrous scaffolds from electrospun composite nanofibers. International journal of nanomedicine. 2007;vol. 2(no. 4):623–638. [PMC free article] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Molecular biology of the cell. 2002;vol. 13(no. 12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue engineering. 2001;vol. 7(no. 2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]