To understand the amount of salvaged myocardium after reperfusion of acute myocardial infarction (MI), it is essential to know the area of myocardium at risk (AAR) prior to reperfusion in order to assess salvaged myocardium as AAR minus final infarct size. Measuring myocardial salvage offers tremendous potential for development of novel pharmacologic agents that can reduce reperfusion injury and thereby increase myocardial salvage in reperfused MI. In animal studies, dyes such as pthalocyanine blue injected directly into the coronary circulation have been used to assess AAR. In humans, contrast echocardiography has been used in the catheterization lab prior to reperfusion to make this measurement(1). Although an accurate and useful technique, this technology is available in very few laboratories around the world on a moment's notice when a patient presents with an MI. A technique to measure AAR in MI that could be applied after reperfusion would be ideal. T2-weighted (W) imaging by CMR appears to be just such a technique as demonstrated in the studies presented in the iForum piece by Matthias Friedrich MD below. T2-W imaging is sensitive to myocardial edema and it is thought that the area of edema can mark the original AAR. T2-W imaging was first applied in the mid-1990's, but it is only in the last few years that it has begun to hit its clinical stride.
But hold on…. Han Kim MD and Raymond Kim MD point out that T2-W imaging may not be ready for prime time. They present data that raises questions about the presence of edema within the area of salvaged, reversibly injured myocardium. In addition, there are several technical issues with some of the CMR pulse sequences used for this technique and how the data is acquired. There is no consensus yet within the CMR community in regards to how this data is optimally acquired and quantitatively analyzed. A newer quantitative technique called T2-mapping may be one answer in this regard(2). Regardless, the further development and application of T2-W imaging in the measure of myocardial salvage is a fascinating advance in the field of cardiovascular imaging that our readers should watch as the story unfolds in the next few years.
Relevance of the Area at Risk in Patients After Acute Myocardial Infarction
The area at risk represents the myocardial perfusion bed which is directly affected by ischemia due to the acute occlusion of a coronary artery. The tissue is subject to a cascade of reversible and irreversible injury, following a centrifugal expansion in an endo-towards-epicardial “wavefront”. Importantly, the wave of irreversible injury (oncosis, followed by necrosis) is preceded by reversible injury of the entire area at risk(3). Accordingly, if blood flow is restored before, persistent ischemic damage can be prevented. Depending on the total ischemia time of reperfused myocardial infarction, more or less of the area at risk is salvaged. Since the injury in the salvaged region is reversible, there is functional recovery, which is deemed beneficial for the patient with respect to quality of life and longevity. The presence and extent of myocardial salvage therefore indicates the success and relevance of acute revascularization.
The clinical impact of this information depends on the clinical scenario. In symptomatic patients with late presentation after acute MI or with apparently unsuccessful thrombolysis, for example, the absence or presence of salvageable myocardium would be helpful to guide therapy with respect to the indication for a late or “rescue” coronary intervention. Furthermore, the timeliness of revascularization could be retrospectively assessed.
Currently, however, the main value of assessing the area at risk and myocardial salvage, may be its utility as an endpoint in clinical trials. Although acute revascularization is routinely performed, markers indicating its success are scarce and not well defined. Because of a lack of strong surrogate markers, clinical trials need large samples, resources, and time. Given the highly dynamic nature of acute coronary syndrome before, during and after revascularization, it remains challenging to relate clinical and peri-procedural factors to outcome. Especially, there is a need for a better understanding of the impact of late or repeated reperfusion on myocardial salvage. While infarct size certainly is an excellent prognostic marker, clinical studies on the impact of any therapeutic intervention suffer from scatter introduced by the varying size of the perfusion bed. The proportional size of myocardial salvage within the initial myocardium at risk therefore would be a much stronger marker for the efficacy of novel perfusion strategies.
Important Pathophysiological Aspects of the Area at Risk in Acute Myocardial Ischemia
Acute ischemia leads to a multitude of metabolic changes with immediate reversible and subsequent irreversible consequences. Within minutes after its onset, there is a conformational change of intracellular macromolecules, transforming water from its “bound” (gel-like) form into a “free”, fluid state. Furthermore, the breakdown of the ATPase-dependent K+/Na+ exchange leads to inflow of free water into cells, followed by a loss of endothelial membrane integrity with subsequent net water inflow into the area at risk. All these processes add to an increased free water fraction.
Any acute myocardial injury is associated with multiple metabolic and structural changes. Within minutes of no-flow ischemia, the lack of oxygen leads to edema in the affected perfusion bed, before the onset of irreversible injury and significant vascular damage (3). Edema is not only an inherent and essential component of the early pathophysiologic response to no-flow ischemia, but may also itself have significant implications for reperfused myocardium, leading to LV dysfunction and expansion of tissue injury (4).
Of note, edema is more pronounced after reperfusion (5). Furthermore, due to conformational changes, proteins and other macromolecules a release water, accompanied by a change of intracellular water from its gel-like bound to a fluid, free state. Importantly, this is associated with up to 100 times higher T2 relaxation times (6), a contrast-generating effect which can be used by MRI.
Area at Risk Imaging with CMR
The rapid change of T2 relaxation times in the area at risk during acute ischemia was studied in several animal models (7), but only after the introduction of a clinically more robust triple-inversion recovery black blood turbo spin echo sequence in 1996, T2-weighted imaging became useful for clinical research. Subsequent reports showed that T2-weighted CMR differentiates acute from chronic myocardial infarction (8) and is useful in patients with acute myocardial disease of unclear etiology or with suspected acute coronary syndrome (9).
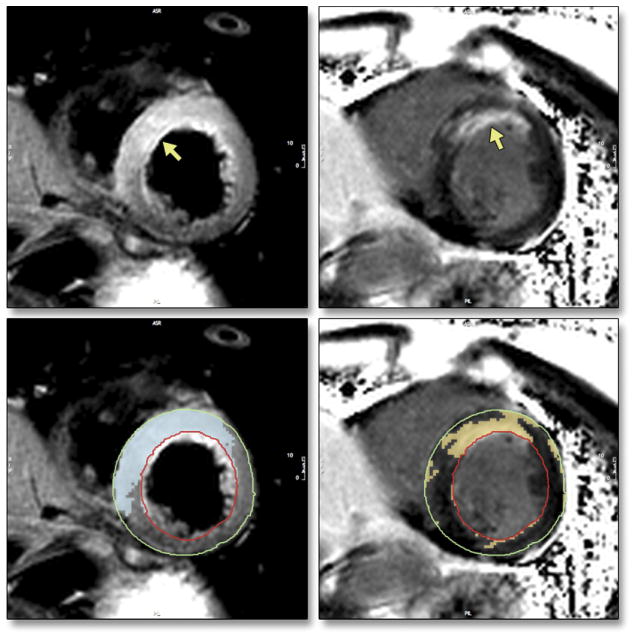
Importantly, high signal intensity in T2-weighted CMR, in the absence of abnormal late Gd enhancement in the same area, reflects reversible ischemic injury (10), making it useful in diseases without overt necrosis. As such a reversibly injured myocardium, the salvaged area at risk after acute revascularization was identified as an important diagnostic target for T2-weighted CMR (11). The accuracy of this approach has been validated in canine (12) and murine (13) models. The extent of the area at risk is more accurately defined by T2-weighted imaging than by using the endocardial extent of bright signal intensity areas in late Gd enhancement images (14). Figure 1 shows a clinical example of myocardial salvage as visualized by CMR.
Figure 1. Myocardial salvage as visualized by CMR using the body coil.
Left upper panel: T2-weighted CMR image showing bright signal intensity in the anterior, anteroseptal and inferoseptal segments, combined with thickened myocardium.
Right upper panel: Late Gd enhancement image of the same slice: Bright signal intensity, mostly endocardial, in the anteroseptal segment.
Lower panels: Quantitative evaluation of signal intensity of both images using the same thresholding algorithm based on automated identification of two signal intensity classes (normal/abnormal), without user interaction.
Myocardial savage as defined by high signal intensity in T2-weighted images with absent late Gd enhancement abnormalities has been used as an endpoint in several clinical trials on revascularization (15) and has shown prognostic value (16).
Other clinical studies included acute myocarditis (17) and hypertrophic cardiomyopathy (18). Recently, a multi-center trial identified myocardial edema to be one of the four essential criteria, in combination with a lack of a significantly brighter signal intensity in late Gd enhancement images (reflecting irreversible injury) (19). Moreover, the assessment of myocardial edema as an in vivo marker for acute myocardial injury also improves risk stratification in patients with acute chest pain and triage them to appropriate treatment (9). These examples underscore the incremental value of T2-weighted CMR in addition to contrast-enhanced CMR.
Specific advantages of T2-weighted CMR include its safety profile (lack of radioactivity or radiation, no contrast agent needed) and high spatial resolution. Most importantly however, the comprehensive assessment of ventricular mass, volumes, function, as well as acute tissue injury, and - combined with contrast-enhanced CMR - infarct size, pericardiac and valvular pathology during one single scan is unparalleled in its diagnostic, workflow- and cost-related efficiency. The accuracy and reproducibility of CMR not only recommend it for clinical research but also for various clinical scenarios and follow-up studies.
The method offers several advantages over single-photon emission computed tomography (20), which is limited by poor spatial resolution and the need for two separate scans, and echocardiography, because functional abnormalities often are not sharply defined and it is difficult to distinguish active motion from tethering.
Current Limitations of T2-weighted CMR
Spin echo sequences used for T2-weighted imaging are often limited by a low signal-to-noise ratio, motion artifacts and incomplete blood suppression. The signal intensity in T2-weighted images is also subject to the coil sensitivity profile of surface coils. Therefore, accurate and diagnostic T2-weighted CMR requires the use of the body coil or a coil sensitivity correction algorithm, as well as careful and consistent selection of sequence parameters. Arrhythmia treatment during the scan may be considered.
Newer sequences such as T2-prepared gradient echo protocols (21), hybrid sequences (22), bright blood approaches (23) or additional adiabatic pre-pulses (24) further improve image quality.
A mere visual assessment of the signal intensity in T2-weighted images is subjective and may give inaccurate results and a quantitative analysis is preferable.
Direct measurement of T2 relaxation times (“T2 mapping”) may be less dependent on confounders affecting signal intensity (2). T1 mapping may also be useful for assessing myocardial edema, yet published data are lacking.
Finally, myocardial edema is not specific for the underlying etiology. While the patient's history and the regional distribution of edema most often are conclusive, the pathology itself may be multifactorial. CMR of edema, its regional distribution and relation with necrosis and dysfunction, however, allow for an integrated analysis of the findings.
Conclusion
T2-weighted imaging is emerging as the diagnostic tool of choice to assess and quantify the myocardial area at risk after acute myocardial infarction. The technique not only allows for a better understanding of post-revascularization pathophysiology, but also for quantifying the success of acute reperfusion in individual clinical settings. Despite existing yet addressable, limitations, the diagnostic information provided by T2-weighted CMR is comprehensive, unique, incremental and relevant.
T2-CMR for delineating myocardium at risk (MAR) is thought to be ready for prime time (25). At some centers, it is being used to inform patient management decisions, and a search of major registries (Clinicaltrials.gov and Current Controlled Trials) shows T2-CMR is being used to provide the primary or secondary endpoint in 15 trials including 3000 patients worldwide (26). However, there are several troubling aspects of the available evidence. Given the serious ramifications we critically review the literature and examine the physiological and technical assumptions underlying this application.
Validation Studies?
Three studies are widely quoted as proof that T2 hyperintense regions delineate the MAR following acute MI. Garcia-Dorado demonstrated in 15 ex-vivo pig hearts that the area of T2 hyperintensity was comparable to the MAR defined by fluorescein staining (27). However, the infarcted region was never delineated, and it is unclear whether there might have been an equally good correlation between T2 and infarct size. Additionally, it is notable that the 3 largest MAR measurements (which primarily drive the correlation) were all from hearts with nonreperfused infarcts, the group in which T2 signal was only minimally elevated in the ischemic zone. Aletras showed in 9 canines with acute reperfused MI that the area of T2 hyperintensity was similar to the MAR measured by fluorescent microspheres, and both were larger than infarct size defined by pathology (28). However, the MAR map had poor spatial resolution because large, transmural tissue blocks were used for microsphere counting. Tilak reported in 14 canines with nonreperfused MI that the area of T2 on post-MI day 2 correlated well with the area of hypoperfusion delineated by first-pass CMR on day 0, and both were larger than infarct size measured by pathology (29). Fluorescent microspheres were administered in 12 animals but not used to measure the MAR. Thus, all 3 studies were small, and the conclusions were based primarily on size comparisons between CMR images or CMR and pathology. None showed any images or data directly comparing the shape and contour of the T2 abnormality with the shape and contour of the MAR as delineated by pathology.
There are numerous other studies that conclude that T2-CMR can identify the MAR, but these do not provide an appropriate pathology reference of the MAR. Rather, they infer T2-CMR depicts the MAR since the T2 hyperintense region is measured to be larger than infarct size as determined by delayed-enhancement-CMR (DE-CMR) or pathology. This type of evidence is indirect (30) and beset with concerns as discussed below.
Usually not discussed are studies that suggest T2-CMR delineates the area of acute infarction rather than the MAR. Johnston studied 19 canines undergoing coronary occlusion with or without reperfusion (31). Despite a clear transmural reduction in blood flow in the ischemic zone as verified by microspheres (i.e. MAR is transmural), T2 measured by spectroscopy was elevated only in tissue from the endocardial half of the ischemic zone and not from the epicardial half. Miller reported on 16 canines with reperfused MI that hyperintense regions on T2-CMR were usually subendocardial and matched regions of infarction delineated by pathology rather than viable ischemic regions defined by microspheres (32). Likewise, T2 as measured by spectroscopy was elevated only in infarcted tissue and not in viable at-risk myocardium. Ryan demonstrated in 16 canines with variable coronary occlusion times that only animals with infarction demonstrated hyperintense regions on T2-CMR, whereas none of the animals without infarction had T2 abnormalities despite regional systolic dysfunction (stunning) at the time of imaging (33). Moreover, T2 hyperintense regions correlated with infarct size but not the MAR as delineated by contrast echocardiography.
Edema in Irreversible Versus Reversible Ischemic Injury
T2 signal is linearly related to myocardial water content (27,38) However, from a mechanistic viewpoint, the assumption that T2-CMR depicts the MAR because of myocardial edema is questionable. The bulk of experimental evidence points to substantial edema occurring in the infarcted region, with minimal edema occurring in the MAR portion with reversible injury. For instance, Whalen observed a 44% increase in water content in myocardium exposed to ischemia sufficient to result in infarction of approximately half the tissue (34). Shorter ischemic periods leading to reversible injury did not lead to changes in water content. From this data one can calculate that a pure sample of irreversibly injured myocardium would have an 88% increase in water content. Jennings reported a 9.6% increase in water content in myocardium reversibly damaged by 15 minutes of ischemia followed by 20 minutes of reperfusion (35). However, up to 40% of the increase in tissue water was secondary to reactive hyperemia, which would be expected to resolve 1-2 days later. Thus, following ischemic injury, the difference in edema between infarcted and salvaged (reversibly injured) myocardium should be ∼9-fold (88%/9.6%=9.2) or higher.
Therefore, if T2-CMR truly tracked edema, signal differences between infarcted and reversibly injured myocardium should be far greater than between reversibly injured and normal myocardium. Assuming to first-order increases in T2 signal are linearly related to increases in edema, (27,38) one might expect for a 50% increase in signal within the infarct zone that there should be a 0%–5% increase in signal for salvaged myocardium within the MAR. Thus, even if salvaged myocardium could be distinguished from normal tissue, T2-CMR images should not depict the MAR as a region of homogeneous hyperintensity.
What about published statements that point to prior physiology studies as evidence that substantial edema occurs in salvaged, reversibly injured myocardium? In general, the studies quoted describe edema in the setting of ischemia but do not distinguish between irreversible and reversible injury (27, 36). Since they involve severe ischemic injury in which substantial infarction is expected or shown, the data are equally consistent with much or all of the edema arising from irreversible injury.
Perhaps due to this conundrum concerning the lack of edema in salvaged myocardium, it has been proposed that changes in fractions of water (protein-bound versus ‘free’) rather than total water may explain the findings (25). Certainly, T2 can change with alterations in protein structure (smaller or larger assemblies) without changes in overall protein mass or water volume (37), however, the proposed mechanism is suspect for several reasons. First, there are no data that show fractions of water are changed in salvaged myocardium. Second, if salvaged myocardium without increased total water could have significantly elevated T2, this is then inconsistent with the totality of data published so far showing a tight, nearly linear relationship between T2 and total water (27,38). Third, if ischemia sufficient to result in reversible injury without edema can greatly elevate T2, why then does ischemia that results in irreversible injury with a large amount of edema not have further increases in T2? It would seem that this would require yet a second unproved mechanism to explain why all the edema in infarcted tissue has no additional effect on T2. Finally, T2 (and T1) relaxation values of biological tissues should reflect the composition and microenvironment of that tissue. Yet, following ischemia and reperfusion, the large differences in edema between myocardium suffering irreversible compared with reversible injury are mirrored by similar large differences in electrolytes, high-energy phosphates, and fine structure by light and electron microscopy (34,35, 39-42). In brief, there are dramatic changes in all these characteristics in irreversibly injured tissue compared with minimal-to-no changes in reversibly injured tissue (Figure 2). Given these findings, it is perplexing that T2-CMR apparently can delineate between normal and reversibly injured tissue, but not between reversibly injured and infarcted tissue.
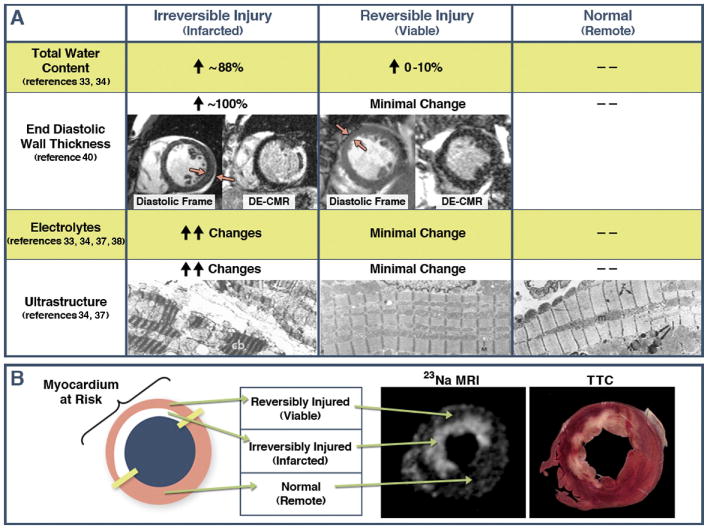
Figure 2.
Panel A summarizes expected changes in several biological characteristics in myocardium following ischemic injury and reperfusion, with comparisons between irreversibly injured, reversibly injured, and normal tissue. Red arrows point to regions that are akinetic on cine-CMR from temporary occlusion to the left circumflex and LAD coronaries, respectively. Note, near doubling of end-diastolic wall thickness in the patient with transmural infarction but no increase in wall thickness in the patient with reversible injury. This is consistent with substantial edema occurring in irreversible but not reversible injury, and consistent with published studies showing acute increases in end-diastolic wall thickness after MI correlates directly with the transmural extent of infarction (reference 42). Electron microscopy images were reproduced from references 35 and 39 with permission. Irreversibly injured tissue shows greatly distorted ultrastructure with formation of vacuoles, large subsarcolemmal blebs, contraction bands (cb) and swollen mitochondria containing dense bodies. In reversibly injured tissue, ultrastructure is virtually indistinguishable from control (normal) tissue. Panel B demonstrates that total myocardial sodium content as reflected by 23Na MRI is substantially elevated in infarcted regions but not in salvaged myocardium at-risk, which is consistent with changes in total water content measured in pathology studies. Reproduced from reference 41 with permission. Given these findings, it is perplexing that T2-CMR apparently can delineate between normal and reversibly injured tissue, but not between reversibly injured and infarcted tissue.
T2-CMR Technical Issues
There are a myriad of reasons why conventional, dark-blood T2-CMR may overestimate infarct size without the need to surmise that T2 hyperintensity delineates the MAR (30). Surface coils can lead to hyperintense regions simply based on proximity to the coil (Figure 3A). Bright signal from static cavity blood may mimic edema, and motion-related signal loss in one region may cause other regions to appear hyperintense despite no actual changes in T2 (Figure 3B-C). The latter two artifacts are particularly pernicious in that these may be associated with physiological changes occurring post-MI rather than occurring randomly. Specifically, injured myocardium is likely hypokinetic, which is often associated with adjacent stagnant cavity blood. Likewise, hypokinetic myocardium, even without edema, may appear hyperintense in comparison with normal regions that have suffered signal loss because of vigorous motion. Newer bright-blood techniques with or without T2 mapping may substantially reduce these artifacts, however, there are no data validating these techniques in comparison with an appropriate pathology reference of the MAR. Moreover, all T2 techniques are limited by the relatively small changes in T2 expected for edematous myocardium. Aletras reported a contrast-to-noise ratio (CNR) between hyperintense and normal regions of only 2.9 for T2-CMR, (28) which is substantially less than the 19 that is typical for DE-CMR (43).
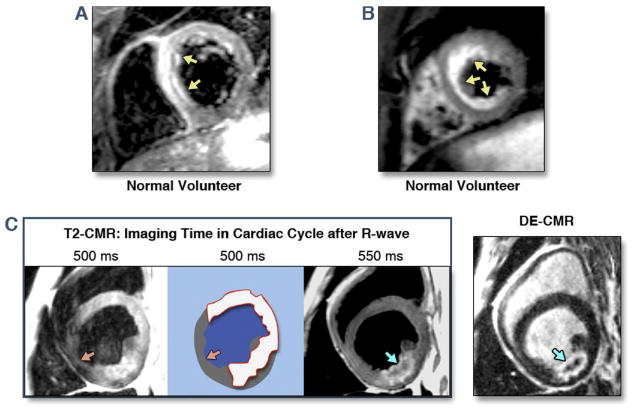
Figure 3.
A and B: On conventional T2-CMR hyperintense regions may be visualized in normal volunteers because of inhomogeneities in myocardial signal and slow cavity blood flow. C: Simply changing the timing of T2 imaging during the cardiac cycle can lead to different regions of hyperintensity. If a region of signal loss (orange arrows) is considered normal myocardium, the extent of hyperintensity may be large (red contour). Also note that the region of apparent T2 hyperintensity extends far beyond the lateral borders of the infarcted region as delineated by DE-CMR. In the T2 image taken 550 ms after the R-wave, the hyperintense region (blue arrow) is not fully transmural and the shape and contour appears to exactly match that of the infarcted region on DE-CMR.
Another technical issue concerns the method of quantifying the size of hyperintense regions. Many studies have arbitrarily defined “bright” myocardium as regions with signal >2 SDs above remote on T2-CMR but >5 SDs above remote on DE-CMR (30). By using a lower threshold for T2-CMR, one increases the likelihood that edema size will often be substantially greater than infarct size simply from partial volume (30). Results using full-width at half maximum (FWHM) methods may be more reproducible, but not necessarily more accurate (44). The addition of one or two very bright pixels within the hyperenhancement zone may substantially reduce measured infarct size by raising the FWHM threshold and rendering a majority of the “grey zone” (admixture of infarcted and viable myocardium) as part of normal tissue.
So how can we know whether the consistent overestimation of infarct size by T2-CMR is artifactual or real? Some clues are found by returning to bedrock physiological principles. Following coronary occlusion, cell death is not simultaneous throughout the MAR but progresses as a “wavefront” from endocardium to epicardium over several hours (45.) Without timely reperfusion, infarction becomes transmural reflecting an absence of salvageable myocardium within the MAR. Thus, if MAR size measured by T2-CMR were larger than infarct size in the setting of transmural infarction, this would be non-physiological. It is puzzling, therefore, that Berry using bright-blood T2 techniques report similar amounts of substantial salvage for both transmural and nontransmural infarction (46). Of the other investigations that report edema size is greater than infarct size, we are unaware of any that describe this data as a function of infarct transmurality.
In their landmark study of the wavefront phenomenon, Reimer and Jennings describe a corollary principle–that is, there is no wavefront circumferentially since there is no perfusion gradient in that direction (45). Reperfusion after only 40 minutes of ischemia resulted in a confluent subendocardial infarct (∼28% transmural) which extended to within 1-2 mm of the lateral edge of the MAR. Although some investigators initially suggested the existence of a wide lateral “border zone” of intermediate-level perfusion, later studies showed this was a partial volume artifact due to the limited resolution of the techniques used, and with progressively higher levels of sampling resolution, investigators have concluded that there is no zone of intermediate perfusion (or injury) at the lateral border (45,47). This is consistent with anatomical studies showing “end-capillary loops” without microvascular connections between adjacent vascular beds in both dogs and man (48,49). The implications of this is that there should be no meaningful salvage at the lateral borders of the infarct, yet the literature is replete with examples of T2 hyperintensity extending laterally, usually in both directions, far beyond the lateral border of the infarct delineated by DE-CMR (25,28). One caveat should be mentioned. In patients with multivessel disease, it is possible that the infarct-related artery prior to occlusion provided substantial collateral flow to a second coronary artery perfusion territory. Thus, during infarction a second vascular bed becomes ischemic. Theoretically, this could result in an MAR that extends laterally beyond the infarct. However, in this situation, (1) the entire circumference of the second vascular bed should be equally ischemic, (2) the level of ischemia needs to be within a narrow range that does not result in subendocardial infarction within the second bed, (3) realistically the MAR should extend laterally only on one side of the infarct, and finally, (4) this is known to be very rare even in the setting of chronic, diffuse multivessel disease (50).
References
- 1.Micari A, Belcik TA, Balcells EA, et al. Improvement in Microvascular Reflow and Reduction of Infarct Size With Adenosine in Patients Undergoing Primary Coronary Stenting. Am J Cardiol. 2005;96:1410–5. doi: 10.1016/j.amjcard.2005.06.090. [DOI] [PubMed] [Google Scholar]
- 2.Verhaert D, Thavendiranathan P, Giri S, et al. Direct T2 Quantification of Myocardial Edema in Acute Ischemic Injury. JACC: Cardiovascular Imaging. 2011;4:269–78. doi: 10.1016/j.jcmg.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kloner RA, Rude RE, Carlson N, Maroko PR, DeBoer LW, Braunwald E. Ultrastructural evidence of microvascular damage and myocardial cell injury after coronary artery occlusion: which comes first? Circulation. 1980;62(5):945–952. doi: 10.1161/01.cir.62.5.945. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Dorado D, Oliveras J. Myocardial oedema: a preventable cause of reperfusion injury? Cardiovascular Research. 1993;27(9):1555–1563. doi: 10.1093/cvr/27.9.1555. [DOI] [PubMed] [Google Scholar]
- 5.Whalen DA, Hamilton DG, Ganote CE, Jennings RB. Effect of a transient period of ischemia on myocardial cells. I. Effects on cell volume regulation. Am J Pathol. 1974;74(3):381–397. [PMC free article] [PubMed] [Google Scholar]
- 6.Foster KR, Resing HA, Garroway AN. Bounds on “bound water”: transverse nuclear magnetic resonance relaxation in barnacle muscle. Science. 1976;194(4262):324–326. doi: 10.1126/science.968484. [DOI] [PubMed] [Google Scholar]
- 7.García-Dorado D, Oliveras J, Gili J, et al. Analysis of myocardial oedema by magnetic resonance imaging early after coronary artery occlusion with or without reperfusion. Cardiovascular Research. 1993;27(8):1462–1469. doi: 10.1093/cvr/27.8.1462. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Aty H, Zagrosek A, Schulz-Menger J, et al. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. 2004;109(20):2411–2416. doi: 10.1161/01.CIR.0000127428.10985.C6. [DOI] [PubMed] [Google Scholar]
- 9.Cury RC, Shash K, Nagurney JT, et al. Cardiac magnetic resonance with T2-weighted imaging improves detection of patients with acute coronary syndrome in the emergency department. Circulation. 2008;118(8):837–844. doi: 10.1161/CIRCULATIONAHA.107.740597. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Aty H, Cocker M, Meek C, Tyberg JV, Friedrich MG. Edema as a very early marker for acute myocardial ischemia: a cardiovascular magnetic resonance study. J Am Coll Cardiol. 2009;53(14):1194–1201. doi: 10.1016/j.jacc.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 11.Friedrich MG, Abdel-Aty H, Taylor A, Schulz-Menger J, Messroghli D, Dietz R. The salvaged area at risk in reperfused acute myocardial infarction as visualized by cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51(16):1581–1587. doi: 10.1016/j.jacc.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Aletras AH, Tilak GS, Natanzon A, et al. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113(15):1865–1870. doi: 10.1161/CIRCULATIONAHA.105.576025. [DOI] [PubMed] [Google Scholar]
- 13.Beyers RJ, Smith RS, Xu Y, et al. T(2) -weighted MRI of post-infarct myocardial edema in mice. Magn Reson Med. 2011 doi: 10.1002/mrm.22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ubachs JFA, Engblom H, Erlinge D, et al. Cardiovascular magnetic resonance of the myocardium at risk in acute reperfused myocardial infarction: comparison of T2-weighted imaging versus the circumferential endocardial extent of late gadolinium enhancement with transmural projection. J Cardiovasc Magn Reson. 2010;12:18. doi: 10.1186/1532-429X-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francone M, Bucciarelli-Ducci C, Carbone I, et al. Impact of Primary Coronary Angioplasty Delay on Myocardial Salvage, Infarct Size, and Microvascular Damage in Patients With ST-Segment Elevation Myocardial Infarction. J Am Coll Cardiol. 2009;54(23):2145–2153. doi: 10.1016/j.jacc.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Eitel I, Desch S, Fuernau G, et al. Prognostic significance and determinants of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. J Am Coll Cardiol. 2010;55(22):2470–2479. doi: 10.1016/j.jacc.2010.01.049. [DOI] [PubMed] [Google Scholar]
- 17.Abdel-Aty H, Boyé P, Zagrosek A, et al. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005;45(11):1815–1822. doi: 10.1016/j.jacc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 18.Abdel-Aty H, Cocker M, Strohm O, Filipchuk N, Friedrich MG. Abnormalities in T2-weighted cardiovascular magnetic resonance images of hypertrophic cardiomyopathy: regional distribution and relation to late gadolinium enhancement and severity of hypertrophy. J Magn Reson Imaging. 2008;28(1):242–245. doi: 10.1002/jmri.21381. [DOI] [PubMed] [Google Scholar]
- 19.Eitel I, Knobelsdorff-Brenkenhoff von F, Bernhardt P, et al. Clinical Characteristics and Cardiovascular Magnetic Resonance Findings in Stress (Takotsubo) Cardiomyopathy: A Multicenter Series in Europe and North America. Journal of the American Medical Association. JAMA. doi: 10.1001/jama.2011.992. in press. [DOI] [PubMed] [Google Scholar]
- 20.Markis JE, Malagold M, Parker JA, et al. Myocardial salvage after intracoronary thrombolysis with streptokinase in acute myocardial infarction. N Engl J Med. 1981;305(14):777–782. doi: 10.1056/NEJM198110013051401. [DOI] [PubMed] [Google Scholar]
- 21.Kellman P, Aletras AH, Mancini C, Mcveigh ER, Arai AE. T2-prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magn Reson Med. 2007;57(5):891–897. doi: 10.1002/mrm.21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aletras AH, Kellman P, Derbyshire JA, Arai AE. ACUT2E TSE-SSFP: a hybrid method for T2-weighted imaging of edema in the heart. Magn Reson Med. 2008;59(2):229–235. doi: 10.1002/mrm.21490. [DOI] [PubMed] [Google Scholar]
- 23.Payne AR, Casey M, McClure J, et al. Bright-blood T2-weighted MRI has higher diagnostic accuracy than dark-blood short tau inversion recovery MRI for detection of acute myocardial infarction and for assessment of the ischemic area at risk and myocardial salvage. Circulation: Cardiovascular Imaging. 2011;4(3):210–219. doi: 10.1161/CIRCIMAGING.110.960450. [DOI] [PubMed] [Google Scholar]
- 24.Cocker MS, Shea SM, Strohm O, Green J, Abdel-Aty H, Friedrich MG. A New Approach Towards Improved Visualization of Myocardial Edema Using T2-Weighted Imaging – A Cardiovascular Magnetic Resonance (CMR) Study. J Magn Reson Imaging. doi: 10.1002/jmri.22622. in press. [DOI] [PubMed] [Google Scholar]
- 25.Friedrich MG. Myocardial edema--a new clinical entity? Nat Rev Cardiol. 2010;7:292–6. doi: 10.1038/nrcardio.2010.28. [DOI] [PubMed] [Google Scholar]
- 26.Accessed 4/18/2011, at http://clinicaltrials.gov/ and http://www.controlled-trials.com/
- 27.Garcia-Dorado D, Oliveras J, Gili J, et al. Analysis of myocardial oedema by magnetic resonance imaging early after coronary artery occlusion with or without reperfusion. Cardiovasc Res. 1993;27:1462–9. doi: 10.1093/cvr/27.8.1462. [DOI] [PubMed] [Google Scholar]
- 28.Aletras AH, Tilak GS, Natanzon A, et al. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113:1865–70. doi: 10.1161/CIRCULATIONAHA.105.576025. [DOI] [PubMed] [Google Scholar]
- 29.Tilak GS, Hsu LY, Hoyt RF, Jr, Arai AE, Aletras AH. In vivo T2-weighted magnetic resonance imaging can accurately determine the ischemic area at risk for 2-day-old nonreperfused myocardial infarction. Invest Radiol. 2008;43:7–15. doi: 10.1097/RLI.0b013e3181558822. [DOI] [PubMed] [Google Scholar]
- 30.Wince WB, Kim RJ. Molecular imaging: T2-weighted CMR of the area at risk--a risky business? Nat Rev Cardiol. 2010;7:547–9. doi: 10.1038/nrcardio.2010.124. [DOI] [PubMed] [Google Scholar]
- 31.Johnston DL, Brady TJ, Ratner AV, et al. Assessment of myocardial ischemia with proton magnetic resonance: effects of a three hour coronary occlusion with and without reperfusion. Circulation. 1985;71:595–601. doi: 10.1161/01.cir.71.3.595. [DOI] [PubMed] [Google Scholar]
- 32.Miller DD, Johnston DL, Dragotakes D, et al. Effect of hyperosmotic mannitol on magnetic resonance relaxation parameters in reperfused canine myocardial infarction. Magn Reson Imaging. 1989;7:79–88. doi: 10.1016/0730-725x(89)90327-5. [DOI] [PubMed] [Google Scholar]
- 33.Ryan T, Tarver RD, Duerk JL, Sawada SG, Hollenkamp NC. Distinguishing viable from infarcted myocardium after experimental ischemia and reperfusion by using nuclear magnetic resonance imaging. Journal of the American College of Cardiology. 1990;15:1355–64. doi: 10.1016/s0735-1097(10)80026-9. [DOI] [PubMed] [Google Scholar]
- 34.Whalen DA, Jr, Hamilton DG, Ganote CE, Jennings RB. Effect of a transient period of ischemia on myocardial cells. I. Effects on cell volume regulation. Am J Pathol. 1974;74:381–97. [PMC free article] [PubMed] [Google Scholar]
- 35.Jennings RB, Schaper J, Hill ML, Steenbergen C, Jr, Reimer KA. Effect of reperfusion late in the phase of reversible ischemic injury. Changes in cell volume, electrolytes, metabolites, and ultrastructure. Circ Res. 1985;56:262–78. doi: 10.1161/01.res.56.2.262. [DOI] [PubMed] [Google Scholar]
- 36.Reimer KA, Jennings RB. The changing anatomic reference base of evolving myocardial infarction. Underestimation of myocardial collateral blood flow and overestimation of experimental anatomic infarct size due to tissue edema, hemorrhage and acute inflammation. Circulation. 1979;60:866–76. doi: 10.1161/01.cir.60.4.866. [DOI] [PubMed] [Google Scholar]
- 37.Chen EL, Kim RJ. Magnetic resonance water proton relaxation in protein solutions and tissue: T(1rho) dispersion characterization. PLoS One. 2010;5:e8565. doi: 10.1371/journal.pone.0008565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins CB, Herfkens R, Lipton MJ, et al. Nuclear magnetic resonance imaging of acute myocardial infarction in dogs: alterations in magnetic relaxation times. Am J Cardiol. 1983;52:184–8. doi: 10.1016/0002-9149(83)90093-0. [DOI] [PubMed] [Google Scholar]
- 39.Kloner RA, Ganote CE, Whalen DA, Jr, Jennings RB. Effect of a transient period of ischemia on myocardial cells. II. Fine structure during the first few minutes of reflow. Am J Pathol. 1974;74:399–422. [PMC free article] [PubMed] [Google Scholar]
- 40.Jennings RB, Sommers HM, Kaltenbach JP, West JJ. Electrolyte Alterations in Acute Myocardial Ischemic Injury. Circ Res. 1964;14:260–9. doi: 10.1161/01.res.14.3.260. [DOI] [PubMed] [Google Scholar]
- 41.Kim RJ, Judd RM, Chen EL, Fieno DS, Parrish TB, Lima JA. Relationship of elevated 23Na magnetic resonance image intensity to infarct size after acute reperfused myocardial infarction. Circulation. 1999;100:185–92. doi: 10.1161/01.cir.100.2.185. [DOI] [PubMed] [Google Scholar]
- 42.Haendchen RV, Corday E, Torres M, Maurer G, Fishbein MC, Meerbaum S. Increased regional end-diastolic wall thickness early after reperfusion: a sign of irreversibly damaged myocardium. Journal of the American College of Cardiology. 1984;3:1444–53. doi: 10.1016/s0735-1097(84)80283-1. [DOI] [PubMed] [Google Scholar]
- 43.Simonetti OP, Kim RJ, Fieno DS, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215–23. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 44.Kim HW, Farzaneh-Far A, Kim RJ. Cardiovascular magnetic resonance in patients with myocardial infarction: current and emerging applications. Journal of the American College of Cardiology. 2009;55:1–16. doi: 10.1016/j.jacc.2009.06.059. [DOI] [PubMed] [Google Scholar]
- 45.Reimer KA, Jennings RB. The “wavefront phenomenon” of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Invest. 1979;40:633–44. [PubMed] [Google Scholar]
- 46.Berry C, Kellman P, Mancini C, et al. Magnetic resonance imaging delineates the ischemic area at risk and myocardial salvage in patients with acute myocardial infarction. Circ Cardiovasc Imaging. 2010;3:527–35. doi: 10.1161/CIRCIMAGING.109.900761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Axford-Gatley RA, Wilson GJ. The “border zone” in myocardial infarction. An ultrastructural study in the dog using an electron-dense blood flow marker. Am J Pathol. 1988;131:452–64. [PMC free article] [PubMed] [Google Scholar]
- 48.Okun EM, Factor SM, Kirk ES. End-capillary loops in the heart: an explanation for discrete myocardial infarctions without border zones. Science. 1979;206:565–7. doi: 10.1126/science.493960. [DOI] [PubMed] [Google Scholar]
- 49.Factor SM, Okun EM, Minase T, Kirk ES. The microcirculation of the human heart: end-capillary loops with discrete perfusion fields. Circulation. 1982;66:1241–8. doi: 10.1161/01.cir.66.6.1241. [DOI] [PubMed] [Google Scholar]
- 50.Lee JT, Ideker RE, Reimer KA. Myocardial infarct size and location in relation to the coronary vascular bed at risk in man. Circulation. 1981;64:526–34. doi: 10.1161/01.cir.64.3.526. [DOI] [PubMed] [Google Scholar]