Abstract
Reduction in synaptic transmission and plasticity in mice lacking the hippocampus-enriched AMPAR auxiliary subunit, TARP©-8, could be due to reduction in AMPAR expression or a direct role of ©-8. Here, we generated TARP©-8Δ4 knock-in mice lacking the C-terminal PDZ ligand. We found that synaptic transmission and AMPAR were reduced without changes in extrasynaptic AMPAR expression, but LTP was unaltered. Our findings indicate distinct TARP-dependent mechanisms for synaptic transmission and LTP.
TARPs are auxiliary subunits of AMPARs that modulate expression, channel properties and localization of AMPARs in the brain1. Genetic disruption of TARPs causes reduction of AMPARs2-4, and in the ©-8 knockout (γ-8−/−, also known as CACNG8−/−) mouse, cell-surface AMPAR function and NMDA receptor-mediated LTP are severely impaired3. Interestingly, these phenotypes were also observed in mice lacking the AMPAR subunit, GluA15. Indeed, expression of GluA1 and GluA2/3 in hippocampus of γ-8−/− mice is reduced to 20-30% of wild type levels3,4. Therefore, it remains unclear whether the impaired synaptic transmission and plasticity observed in γ-8−/− mice are caused directly by loss of ©-8 or indirectly by reduction in AMPAR expression. Furthermore, although the TARP/AMPAR complex is proposed to localize at synapses by interacting with PSD-95 through the C-terminal PDZ ligand6,7, overexpression of ©-8 lacking the PDZ ligand (⊗4) increases AMPAR activity in stargazer cerebellar granule cells8. Therefore, it remains unclear whether the PDZ ligand is the only domain responsible for synaptic AMPAR activity.
TARP©-8 and ©-2 were preferentially identified in the Triton X-100 solubilized synaptosomal fraction (extrasynaptic) and the PSD fraction of rodent hippocampus, respectively (Supplementary Fig. 1a)9. AMPAR expression is severely reduced in γ-8−/− mice3,4, and this reduction was more obvious with age (Supplementary Fig. 1b). We found a greater reduction of AMPAR expression in the Triton-solubilized synaptosomal fraction than in the PSD fraction and specific loss of EndoH-resistant AMPARs without a change in the amount of EndoH-sensitive AMPARs (Supplementary Fig. 1c,d). These results indicate that ©-8 is more critical for extrasynaptic AMPA receptors, and that AMPAR reduction in γ-8−/− mice is due to destabilization of mature AMPARs.
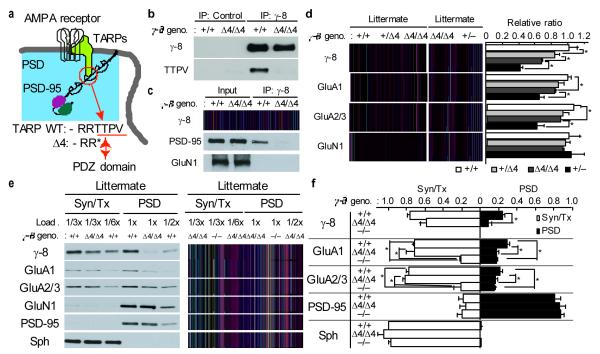
Due to the severe reduction in AMPAR expression in γ-8−/− mice, it remains unclear whether the phenotype is caused by the loss of AMPAR expression or by the loss of ©-8 itself. To circumvent this issue, we generated a knock-in mouse (γ-8Δ4/Δ4) in which the ©-8 PDZ ligand (i.e., 4 amino acids) was deleted (Fig. 1a and Supplementary Fig. 2a–d). Deletion of the last 4 amino acids of ©-8 was confirmed by an antibody (TTPV) (Fig. 1b). Furthermore, PSD-95 was co-immunoprecipitated with ©-8, but not ©-8⊗4 (Fig. 1c). The γ-8Δ4/Δ4 mice are viable and no phenotype was obvious except difficulty in breeding using homozygotes. Compared to stargazer mice (γ-2stg/stg), all double mutant mice (γ-2stg/stg; γ-8Δ4/Δ4) showed severe growth defects and most died by P28 (Supplementary Fig. 3). This result suggests a critical role of the last 4 amino acids of TARP©-8 in survival.
Figure 1.
TARP©-8 PDZ binding is necessary for synaptic localization of AMPARs. (a) diagram of the synaptic AMPAR/TARP/PSD-95 complex. The PDZ ligand (–TTPV) is deleted. (b) The anti-©-8 antibody recognized ©-8 in both γ-8+/+ and γ-8Δ4/Δ4, whereas anti-TTPV antibody recognized ©-8 only in WT. Brain lysates were immunoprecipitated with normal rabbit IgG (control) or anti ©-8 antibody, followed by western blotting. All full and uncropped blots are shown in Supplementary Figure 7. (c) PSD-95 is not associated with ©-8⊗4 in vivo. PSD-95 was co-immunoprecipitated with ©-8 in γ-8+/+, but not in γ-8Δ4/Δ4 mice. (d) Protein levels of ©-8, GluA1, and GluA2/3 were somewhat decreased in hippocampi in a ©-8⊗4 gene dosage-dependent manner (n=4). (e, f) Protein levels of ©-8, GluA1, and GluA2/3 in the PSD fraction from hippocampus were reduced in γ-8Δ4/Δ4 mice, but not in the Triton X-100-solublized synaptosome fraction (Syn/Tx). In contrast, expression of ©-8, GluA1, and GluA2/3 in the Syn/Tx fraction was significantly reduced in γ-8−/− mice, but not in γ-8Δ4/Δ4 mice. (f) Protein levels were normalized to those from γ-8+/+ mice (n=4). Synaptophysin (Sph) was used as a non-PSD protein. All data are given as mean ± s.e.m.; * P < 0.05.
In the γ-8Δ4/Δ4 mice, we observed a slight reduction in AMPAR expression without changes in ©-8 mRNA (Fig. 1d and Supplementary Fig. 2d). Immunohistochemistry showed no obvious difference in ©-8 distribution, but co-localization of AMPARs with PSD-95 was significantly reduced in the γ-8Δ4/Δ4 mice (Supplementary Fig. 4). In the PSD fraction, but not in the Triton X-100 solubilized synaptosome fraction, ©-8⊗4, GluA1, and GluA2/3 were all significantly reduced in γ-8Δ4/Δ4 mice to an extent similar to that observed in γ-8−/− mice, without changes in PSD-95 or GluN1 expression (Fig. 1e,f). Since γ-8−/− and γ-8Δ4/Δ4 mice have a nearly identical PSD phenotype, we conclude that only the last 4 amino acids of TARP are required for synaptic localization of AMPARs. In contrast, our data suggest that the rest of the molecule (©-8⊗4) is sufficient to chaperone or stabilize AMPARs at non-PSD sites, a function that is lost in the complete ©-8 knockout.
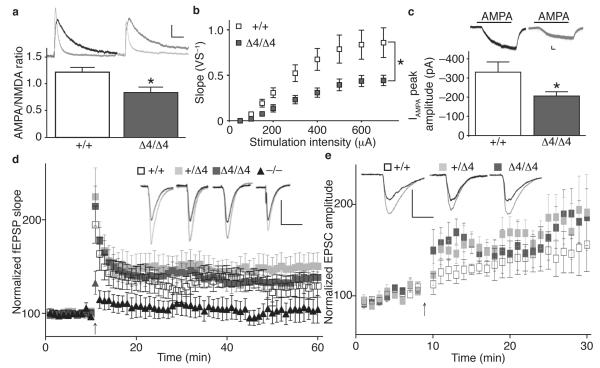
In γ-8−/− mice, both synaptic AMPAR function and LTP are compromised3. We found that the ratio of AMPAR to NMDAR-mediated EPSCs (Fig. 2a), the input-output curve (Fig. 2b), and AMPA-evoked whole cell currents were all significantly reduced in γ-8Δ4/Δ4 mice compared to γ-8+/+ (Fig. 2c). The I-V relationship of synaptic AMPARs and paired-pulse ratio were similar in γ-8Δ4/Δ4 and γ-8+/+ mice (Supplementary Fig. 5). Although other TARPs might contribute to a component of the residual basal transmission observed in γ-8−/− and γ-8Δ4/Δ4 mice, the reduction in basal transmission to similar levels in both mice demonstrates that the ©-8 PDZ ligand is required for basal transmission.
Figure 2.
The ©-8 PDZ ligand modulates AMPAR-mediated basal transmission, but not LTP. (a) Ratio of AMPAR- to NMDAR-EPSCs is reduced by ~30% in CA1 pyramidal cells from γ-8Δ4/Δ4 slices (n=11) compared with those from γ-8+/+ slices (n=5). Representative examples of averaged EPSCs (AMPAR current=light trace; NMDAR current=dark trace). Calibration: 100 ms, 20 pA (+/+) and 16 pA (⊗4/⊗4). (b) Ratio of stimulus intensity (input) to the EPSP slope (output). Input-output was significantly reduced in slices from γ-8Δ4/Δ4 (n=6) compared to those from γ-8+/+ mice (n=10). * P < 0.05 paired t-test. (c) 100 nM AMPA-evoked whole cell currents are reduced by ~38% in γ-8Δ4/Δ4 (n=8) compared to γ-8+/+ mice (n=7). Inset, AMPA-evoked current from representative cells are shown. Calibration: 1 min, 50 pA. (d) Extracellular recordings of field EPSPs before and after tetanic stimulation of Schaffer collaterals (arrow). LTP was elicited in γ-8+/+ (open squares; n=6), γ-8Δ4/Δ4 (gray squares; n=10) and γ-8+/Δ4 slices (light gray squares; n=9) to a similar degree, but attenuated in γ-8−/−slices (black triangles; n=4). Inset, averaged fEPSPs before (dark trace) and during LTP (light trace). Calibration: 10 ms, 0.5 mV (+/+), 0.45 mV (⊗4/⊗4), 0.38 (+/⊗4) and 0.5 mV (−/−). (e) Whole-cell recordings from CA1 pyramidal cells before and after a pairing protocol (arrow). LTP was induced in slices from γ-8+/+ (n=5), γ-8+/Δ4 heterozygote (n=4) and γ-8Δ4/Δ4 homozygote mice (n=6). Inset, representative examples of averaged EPSCs recorded before and during LTP. Calibration: 20 ms, 200 pA (+/+), 160 pA (⊗4/ ⊗4) and 200 pA (+/⊗4). All data are given as mean ± s.e.m.; * P < 0.05.
PDZ binding of TARPs to PSD-95 is proposed to stabilize AMPAR/TARP complexes at synapses during NMDAR-dependent LTP, as LTP was impaired in γ-8−/− mice3, while PSD-95 overexpression occluded LTP10-12. We confirmed a marked reduction in LTP in γ-8−/− mice as reported3, but LTP was normal in the γ-8Δ4/Δ4 mice using field potential recordings (Fig. 2d) and a pairing protocol in individual neurons (Fig. 2e). Robust LTP expression was observed even at 90 min post-induction in both γ-8+/Δ4 (Fig. 2d; 136 ± 21% of control) and γ-8Δ4/Δ4 mice (142 ± 9%). These results indicate that the PDZ ligand of ©-8 is not necessary for LTP, although ©-8 itself plays a critical role in LTP.
In addition to the TARPs, several transmembrane proteins were recently reported as AMPAR-interacting proteins1. Expression of one of such molecules, CNIH2, was reduced in γ-8−/− mice13, suggesting that the AMPAR/©-8 complex interacts with CNIH2 in hippocampus. To test which transmembrane interactors are included in the TARP/AMPAR complex at synapses, we compared protein expression in the PSD fraction. We found a selective reduction in CNIH2, but not SynDIG1 and GluN1 (Supplementary Fig. 6). This suggests that the synaptic AMPAR/©-8 complex contains CNIH2 in hippocampus.
In summary, our results indicate that ©-8 has two distinct roles at synapses. First, without the PDZ ligand of ©-8, synaptic transmission is reduced in a ©-8 gene dosage-dependent manner, indicating that instead of acting as a “dominant negative” mutation (as observed in neurons overexpressing TARPγ-2⊗46,7), the γ-8Δ4/Δ4 mice instead produces a loss-of-function. Second, the PDZ binding sequence of ©-8 is not required for LTP expression. We also find that the synaptic AMPAR/©-8 complex contains CNIH2 in hippocampus. However, in contrast with previous results showing a requirement of the TARP PDZ ligand in synaptic plasticity10,11,14, our data illustrate that hippocampal LTP does not require the PDZ ligand of ©-8. Our findings strongly suggest that distinct mechanisms control the synaptic localization of AMPA receptors during basal transmission and during LTP. Furthermore, our data indicate that the reduction in LTP seen in the ©-8 knockout mouse3 may be due to the reduction in AMPA receptor expression, (as in the GluA1 knockout5) rather than to the loss of ©-8. It is also possible, however, that even at 90 min post-induction, LTP may not require stabilization of AMPARs at synapses. Instead, during the first hour of LTP, perhaps AMPARs insert into synapses, and therefore the total number of AMPARs at a given time, is increased without stabilization through PDZ interaction.
Supplementary Material
Acknowledgement
We thank Dr. Roger Nicoll for providing γ-8−/− mouse. The monoclonal antibody SynDIG1 was developed by and/or obtained from the UC Davis/NIH NeuroMab. This work is supported by NIH/NIMH MH077939 (S.T.) and NIH/NS050570 and NIH/NS065251 (J.A.K.).
Footnotes
These authors contributed equally to this work.
Author Contributions. S.T. and J.A.K. conceived the project and wrote the manuscript. A.S., T.B., M.Y., A.K., D.S.B., and S.T. performed all of the experiments and analyzed results. All authors contributed to the final version of the manuscript.
References
- 1.Jackson AC, Nicoll RA. The Expanding Social Network of Ionotropic Glutamate Receptors: TARPs and Other Transmembrane Auxiliary Subunits. Neuron. 2011;70:178–199. doi: 10.1016/j.neuron.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hashimoto K, et al. Impairment of AMPA receptor function in cerebellar granule cells of ataxic mutant mouse stargazer. J Neurosci. 1999;19:6027–6036. doi: 10.1523/JNEUROSCI.19-14-06027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rouach N, et al. TARP gamma-8 controls hippocampal AMPA receptor number, distribution and synaptic plasticity. Nat Neurosci. 2005;8:1525–1533. doi: 10.1038/nn1551. [DOI] [PubMed] [Google Scholar]
- 4.Fukaya M, et al. Abundant distribution of TARP gamma-8 in synaptic and extrasynaptic surface of hippocampal neurons and its major role in AMPA receptor expression on spines and dendrites. Eur J Neurosci. 2006;24:2177–2190. doi: 10.1111/j.1460-9568.2006.05081.x. [DOI] [PubMed] [Google Scholar]
- 5.Zamanillo D, et al. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, et al. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- 7.Schnell E, et al. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci U S A. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milstein AD, Nicoll RA. TARP modulation of synaptic AMPA receptor trafficking and gating depends on multiple intracellular domains. Proc Natl Acad Sci U S A. 2009;106:11348–11351. doi: 10.1073/pnas.0905570106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inamura M, et al. Differential localization and regulation of stargazin-like protein, gamma-8 and stargazin in the plasma membrane of hippocampal and cortical neurons. Neurosci Res. 2006;55:45–53. doi: 10.1016/j.neures.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Stein V, House DR, Bredt DS, Nicoll RA. Postsynaptic density-95 mimics and occludes hippocampal long-term potentiation and enhances long-term depression. J Neurosci. 2003;23:5503–5506. doi: 10.1523/JNEUROSCI.23-13-05503.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehrlich I, Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J Neurosci. 2004;24:916–927. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu W, et al. Molecular dissociation of the role of PSD-95 in regulating synaptic strength and LTD. Neuron. 2008;57:248–262. doi: 10.1016/j.neuron.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato AS, et al. Hippocampal AMPA receptor gating controlled by both TARP and cornichon proteins. Neuron. 2010;68:1082–1096. doi: 10.1016/j.neuron.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Opazo P, et al. CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron. 2010;67:239–252. doi: 10.1016/j.neuron.2010.06.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.