Abstract
Primary graft dysfunction (PGD) after lung transplantation may result from ischemia-reperfusion injury (IRI). The innate immune response to IRI may be mediated by Toll-like receptor and IL-1-induced long pentraxin-3 (PTX3) release. We hypothesized that elevated PTX3 levels were associated with PGD. We performed a nested case control study of lung transplant recipients with Idiopathic Pulmonary Fibrosis (IPF) or Chronic Obstructive Pulmonary Disease (COPD) from the Lung Transplant Outcomes Group cohort. PTX3 levels were measured pre-transplant, and 6 and 24 hours post reperfusion. Cases were subjects with grade 3 PGD within 72 of transplantation and controls were those without grade 3 PGD. Generalized estimating equations and multivariable logistic regression was used for analysis. We selected 40 PGD cases and 79 non-PGD controls. Plasma PTX3 level was associated with PGD in IPF but not COPD recipients (p for interaction<0.03). Among patients with IPF, PTX3 levels at 6 and 24 hours were associated with PGD (OR=1.6, p=0.02 at 6hrs; OR=1.4, p=0.008 at 24hrs). Elevated PTX3 levels were associated with the development of PGD after lung transplantation in IPF patients. Future studies evaluating the role of innate immune activation in IPF and PGD are warranted.
Keywords: Primary Graft Dysfunction, Lung transplantation, Long Pentraxin-3, Idiopathic Pulmonary Fibrosis
INTRODUCTION
Ischemia-reperfusion injury (IRI) plays a role in initiating primary graft dysfunction (PGD), activating and altering cellular pathways involved in immune activation and leading to an acute respiratory distress-like (ARDS) clinical phenomenon (1-3). The role of innate immune activation after IRI in lung transplant recipients is poorly understood.
Innate immune activation plays a role in the propagation of IRI in transplanted solid organs (4-7). IRI involves toll-like receptor (TLR) signaling pathways, complement activation and natural killer cell migration in cardiac transplantation models and may lead to decreased allograft tolerance and the later development of accelerated cardiac allograft vasculopathy (4, 5). Innate immune activation as a result of IRI has also been identified to contribute to renal and hepatic allograft dysfunction (6, 7).
Long pentraxin-3 (PTX3) is a member of a phylogenetically conserved group of acute phase reactants that are involved in inflammation and innate immunity. A previous study of plasma cytokines and PGD demonstrated that plasma levels of other acute phase reactants, including tumor necrosis factor alpha and interferon gamma, decreased after transplant and were not associated with risk of PGD (8). PTX3 is produced at the site of inflammation by cells implicated in the pathogenesis of PGD and has been shown to be elevated in other pathologic conditions related to both ischemia and inflammation, notably acute myocardial infarction and acute lung injury (ALI) (9-11).
Abnormal activation of innate immunity in response to IRI may be mediated by Toll-like receptors and IL-1 induced PTX3 release. Consequently, higher PTX3 levels may play a role in PGD pathogenesis or be a marker of innate immune activation. We therefore hypothesized that elevated PTX3 levels would be associated with PGD. Additionally, we sought to identify differences in the association of PGD with PTX3 levels across the two most common indications for lung transplantation, idiopathic pulmonary fibrosis (IPF) and chronic obstructive pulmonary disease (COPD).
MATERIALS AND METHODS
Subject Selection
All study subjects were randomly selected from within the ongoing Lung Transplant Outcomes Group (LTOG) cohort, a thirteen center, prospective study that has been described previously (12, 13). A multicenter, nested case control study design was selected over a cohort study because of the significant expense and sample volume required for evaluating PTX3 plasma concentrations. Cases and controls were randomly selected from the greater than 1100 patients enrolled in the LTOG cohort. Blood samples were collected from all cohort participants prior to transplant and at 6 and 24 hours after transplantation. Patient-level clinical data were collected prospectively and plasma samples were centrifuged within 30 minutes and then stored at -80°C for biomarker measurement. The institutional review boards at each site approved this study. Informed consent was obtained from each subject at the time of enrollment in the cohort.
Subjects with grade 3 primary graft dysfunction that developed within 72 hours of allograft reperfusion were considered as cases. Subjects with grade 0-2 PGD but without grade 3 PGD at any time after lung transplantation were considered as controls. This case definition of PGD has been previously validated and used extensively in the literature (14, 15). Sensitivity analysis was performed using highest PGD grade within the first 72 hours after allograft reperfusion as an ordinal variable.
Patients with a reported predisposing diagnosis of IPF or COPD were eligible for inclusion. In order to eliminate the possibility of small subgroups, ensure the groups were comparable, and to allow for within diagnosis analyses, the cases and controls were frequency matched for these predisposing diagnoses. We chose the two most common indications for transplantation in order to minimize combinations of diagnoses and aid in matching.
PGD Grade Determination
PGD grades were assigned based on International Society for Heart and Lung Transplant (ISHLT) guidelines as previously described (13). Chest x-rays from immediately after transplant and from 24, 48 and 72 hours after transplant were examined independently by two trained physicians with grades assigned for each radiograph (classification kappa=0.95). As defined by the ISHLT guidelines, grade 3 PGD is the presence of diffuse alveolar infiltrates with a PaO2/FiO2 ratio < 200 and the exclusion of secondary causes (14, 16).
Measurement of PTX3 Concentration
Plasma PTX3 concentrations were determined in duplicate using a sandwich enzyme-linked immunosorbent assay (Alexis Biochemicals, Switzerland). The intra-assay coefficient of variation for this assay was 5.7%. All laboratory personnel were unaware of the PGD status of study subjects.
Statistical Analysis
Study subject characteristics were compared using two sample t-tests or Wilcoxon rank sum tests as appropriate. Proportions were compared using two-group proportion tests while characteristics with greater than two variables were assessed with Kruskal-Wallis rank tests as described previously (15). Primary analysis was performed using generalized estimating equations (GEE) to identify differences in PTX3 levels over time and across study subjects. Secondary analyses included using Wilcoxon rank sum tests across groups and multivariable logistic regression modeling. Based on previous studies identifying differences in biomarker levels across predisposing diagnoses, we a priori defined diagnosis leading to transplantation as a possible effect modifier and performed diagnosis specific analyses (15). Recipient and donor age, sex, and race/ethnicity, cardiopulmonary bypass use, transplant surgical type, ischemic time, intra-operative pulmonary artery systolic pressure (PASP), and packed red blood cell (PRBC), platelet, and plasma transfusion volumes were included as possible confounders in multivariable logistic models. A change in odds ratio (OR), after inclusion of a covariate, of greater than 20% was used to identify confounding. Sensitivity analysis evaluating the association of PTX3 concentration with the highest PGD grade on any day was preformed using ordinal logistic regression. A p<0.05 was pre-defined for statistical significance for all tests. We defined the presence of effect modification a priori by a p<0.1. All statistical analyses were performed using Stata 11.1 software (STATA Corp., College Station, TX).
RESULTS
We included 40 PGD cases and 79 non-PGD controls (Table 1). One control sample was excluded from analysis due to spurious results on the standard curve. A higher percentage of PGD cases required cardiopulmonary bypass than controls (54% vs. 30%, p=0.01) and PGD cases received a larger volume of intraoperative PRBCs than controls (1063 ml vs. 696 ml, p=0.04), but cases and controls were otherwise similar. There were 16 PGD cases and 32 non-PGD controls with COPD, and 24 PGD cases and 47 non-PGD controls with IPF. While pre-transplant PTX3 levels were low compared to post-transplant levels in all patients, pre-transplant levels were significantly higher in subjects with IPF (2.1 ng/ml, IQR 1.3, 7.0) compared to subjects with COPD (0.8 ng/ml, IQR 0.5, 2.0, p<0.001).
Table 1.
Subject characteristics stratified for PGD cases and non-PGD controls
| Characteristics | PGD (n=40) | Non-PGD (n=79) | p |
|---|---|---|---|
| Recipient | |||
| Age, yr | 55 (52, 58) | 56 (53, 58) | 0.7 |
| Female Gender | 30% | 46% | 0.1 |
| Race | 0.1 | ||
| Caucasian | 80% | 90% | |
| African American | 13% | 5% | |
| Hispanic | 0% | 4% | |
| Asian | 5% | 1% | |
| Other | 3% | 0% | |
| Donor | |||
| Age, yr | 34 (29, 38) | 33 (30, 36) | 0.7 |
| Female Gender | 48% | 43% | 0.6 |
| Race | 0.2 | ||
| Caucasian | 73% | 62% | |
| African American | 15% | 22% | |
| Hispanic | 13% | 9% | |
| Asian | 0% | 6% | |
| Other | 0% | 1% | |
| Cause of death | 0.9 | ||
| Blunt Trauma | 3% | 4% | |
| Head Trauma | 30% | 35% | |
| Suicide | 3% | 0% | |
| Stroke | 48% | 34% | |
| Anoxia | 0% | 11% | |
| Other | 18% | 15% | |
| Recipient Diagnosis | 0.9 | ||
| COPD | 40% | 41% | |
| IPF | 60% | 59% | |
| Transplant Type, single | 38% | 38% | 0.9 |
| Use of Cardiopulmonary Bypass | 54% | 30% | 0.01 |
| Time on Bypass, min | 235 (201, 270) | 210 (184, 236) | 0.2 |
| Ischemic Time, min | 308 (281, 334) | 282 (262, 302) | 0.1 |
| Pulmonary Artery Systolic Pressure, mmHg | 47 (40, 54) | 41 (36, 46) | 0.2 |
| Packed Red Blood Cells, ml | 1063 (675, 1450) | 696 (538, 854) | 0.04 |
| Fresh Frozen Plasma, ml | 893 (702, 1084) | 1062 (769, 1354) | 0.3 |
| Platelets, ml | 421 (99, 743) | 228 (46, 411) | 0.3 |
PGD is defined as any grade 3 PGD during first 72 hours.
Continuous variables are expressed as means with 95% confidence intervals, while dichotomous and categorical variables are expressed as percentages, which may not exactly total 100% because of rounding.
Among all enrolled subjects, GEE modeling identified a non-significant difference in the trend of PTX3 levels across all the three time points between cases and controls (β=0.09, 95%CI -0.005, 0.2, p=0.06) (Figure 1a). The association between median PTX3 concentration and PGD differed according to predisposing diagnosis (p for interaction<0.03). There was no significant difference in PTX3 concentration between COPD cases and controls at any time point (all p>0.08). GEE longitudinal modeling identified a significant difference in the trend of PTX3 levels across all the three time points between IPF cases and controls (β=0.3, 95%CI 0.1, 0.5, p=0.001) but not COPD cases and controls (β=-0.2, 95%CI -0.5, 0.1, p=0.2) (Figures 1b and 1c). PTX3 levels were significantly higher in IPF patients with PGD compared to non-PGD IPF controls at 6 hours (45.7 ng/ml vs. 18.0 ng/ml, p=0.02) and 24 hours (88.9 ng/ml vs. 22.7 ng/ml, p=0.007) after reperfusion.
Figure 1.
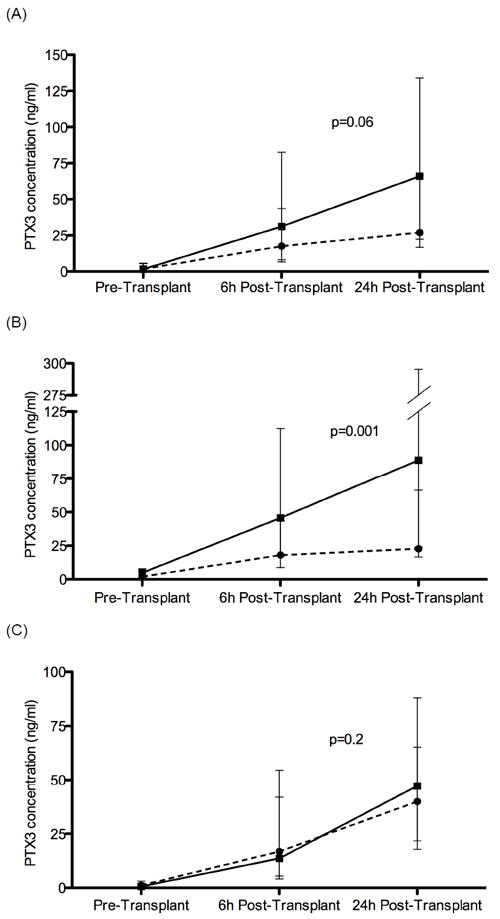
Longitudinal median PTX3 level across the pre-transplant, 6-h post-transplant, and 24-h post-transplant time points.
(a) all study subjects, (b) subjects with IPF, (c) subjects with COPD Solid line represents PGD-cases and dashed line represents PGD-free controls. Error bars represent the 95% CI. P-value reported is from GEE modeling.
When subjects were analyzed according to diagnosis, there was a significant association between PTX3 level and the odds of PGD at 6 hours (OR for each 50 ng/ml higher level =1.6, 95%CI 1.1, 2.5, p=0.02) and 24 hours (OR for each 50 ng/ml higher level =1.3, 95%CI 1.1, 1.7, p=0.008) for IPF patients but no association for subjects with COPD at any time point (p>0.30). Evaluation for possible confounding was performed on the IPF subgroup at 6 and 24 hours post-reperfusion (Table 2). At 6 hours, use of cardiopulmonary bypass attenuated the association of PTX3 level with PGD. There was no attenuation of this relationship by any covariates for PTX3 levels measured at 24 hours after reperfusion. Surgical transplant type was similarly distributed among both COPD and IPF recipients in this study and transplant type was not found to be a confounder of the relationship between PTX3 and PGD.
Table 2.
Odds ratios for the development of Primary Graft Dysfunction in logistic regression models
| Model | Odds Ratio per 50 ng/ml increase in [PTX3], 6 hours (95% CI) | p | Odds Ratio per 50 ng/ml increase in [PTX3], 24 hours (95% CI) | p |
|---|---|---|---|---|
| Unadjusted Base Model (n=119) | 1.1 (0.9, 1.3) | 0.5 | 1.1 (1.0, 1.3) | 0.07 |
| COPD only (n=48) | 0.8 (0.5, 1.3) | 0.3 | 0.9 (0.7, 1.2) | 0.5 |
| IPF only (n=71) | 1.6 (1.1, 2.5) | 0.02 | 1.4 (1.1, 1.7) | 0.008 |
| IPF Adjusted for: | ||||
| Cardiopulmonary Bypass (n=71) | 1.5 (1.0, 2.2) | 0.09 | 1.3 (1.0, 1.6) | 0.02 |
| Transplant Type (n=71) | 1.7 (1.1, 2.6) | 0.02 | 1.4 (1.1, 1.8) | 0.008 |
| Recipient Age (n=71) | 1.7 (1.1, 2.6) | 0.02 | 1.4 (1.1, 1.7) | 0.008 |
| Recipient Sex (n=71) | 1.6 (1.1, 2.5) | 0.03 | 1.4 (1.1, 1.7) | 0.008 |
| Recipient Race/Ethnicity (n=71) | 1.6 (1.0, 2.4) | 0.05 | 1.4 (1.1, 1.9) | 0.01 |
| Donor Age (n=70) | 1.6 (1.0, 2.4) | 0.03 | 1.4 (1.7, 1.7) | 0.009 |
| Donor Mode of Death (n=66) | 1.6 (1.0, 2.4) | 0.04 | 1.3 (1.1, 1.7) | 0.01 |
| Donor Sex (n=71) | 1.6 (1.1, 2.5) | 0.03 | 1.4 (1.1, 1.8) | 0.007 |
| Donor Race/Ethnicity (n=71) | 1.7 (1.0, 2.7) | 0.03 | 1.6 (1.1, 2.2) | 0.01 |
| Total ischemic time (n=67) | 1.9 (1.1, 3.5) | 0.03 | 1.4 (1.1, 1.7) | 0.02 |
| PASP (n=62) | 1.5 (1.0, 2.3) | 0.05 | 1.7 (1.1, 2.5) | 0.01 |
| Packed Red Blood Cells (n=66) | 1.7 (1.1, 2.7) | 0.03 | 1.4 (1.1, 1.7) | 0.01 |
| Platelets (n=55) | 1.9 (1.1, 3.5) | 0.03 | 1.5 (1.1, 2.1) | 0.009 |
Odds ratios for the development of Primary Graft Dysfunction in logistic regression models per 50 ng/ml increase in [PTX3].
COPD = Chronic Obstructive Pulmonary Disease
IPF = Idiopathic Pulmonary Fibrosis
PASP = Pulmonary Arterial Systolic Pressure
A sensitivity analysis using highest PGD grade on any day as an ordinal outcome was performed. There was a significant increase in PTX3 level at 24 hours with increasing severity of PGD (p=0.048) (Figure 2). This relationship was significant in the IPF subgroup (p=0.006) but not the COPD subgroup (p=0.8).
Figure 2.
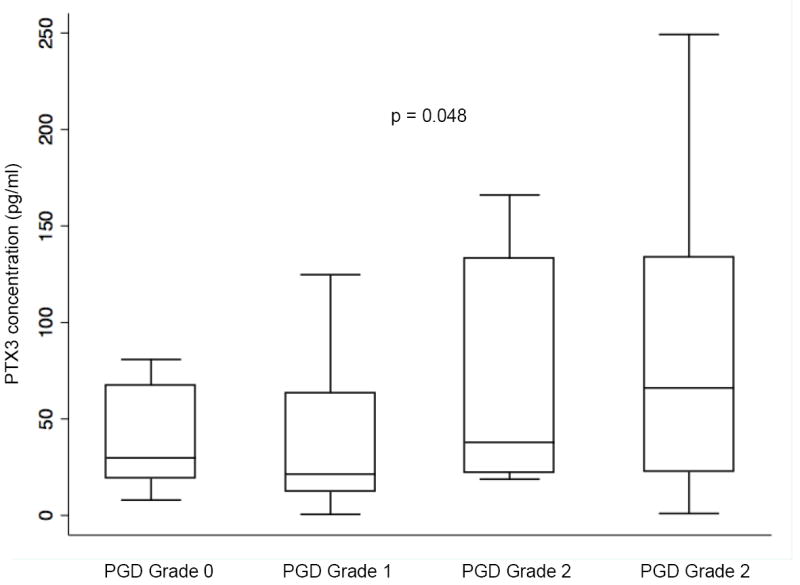
Median plasma PTX3 concentration 24 hours after transplant in all patients stratified by the highest PGD grade developing in the first 72 hours. Horizontal line indicates median concentration. The upper and lower limits of the box indicate the interquartile range. P-value reported is determined from ordinal logistic regression modeling.
Pulmonary hypertension and use of cardiopulmonary bypass have been previously identified as risk factors for PGD (17). While idiopathic pulmonary arterial hypertension patients were not included in this study, PASP among study subjects ranged from 13-111 mmHg. Spearman rank correlation revealed PASP was not significantly associated with PTX3 level prior to transplant (rho=0.15, p=0.1), or at 6 hours (rho=0.14, p=0.2) or 24 hours (rho=0.19, p=0.06) after reperfusion.
DISCUSSION
We found that elevated PTX3 levels were associated with an increased risk of PGD in lung transplant recipients with IPF but not COPD. Longitudinal analysis in the IPF subgroup identified a significant difference in trend over time of PTX3 levels in patients with PGD compared to non-PGD controls. Furthermore, the relationships between PGD and PTX3 levels in the IPF group were independent of most donor, recipient, and surgical characteristics, including PASP, ischemic time, and surgical transplant type, in multivariable modeling. This study provides clinical evidence of a role for activation of innate immune pathways in the pathogenesis of PGD after lung transplantation.
We identified a significant association between PTX3 level and PGD in the IPF subgroup, with pre-transplant PTX3 levels being significantly higher in patients with IPF compared to those with COPD. A registry study demonstrated recipient pre-transplant diagnosis of IPF to be independently associated with increased risk for PGD when compared to subjects with COPD (17). To our knowledge, there are no previous studies investigating the role of PTX3 in IPF patients. We have previously shown that pre-transplant CC16 levels were higher in IPF recipients than in COPD recipients and that post-transplant trends in CC16 levels differed in patients with IPF compared with other indications for transplant (15). The IPF-specific association of elevated PTX3 level and PGD may provide further evidence that post-transplant biomarker profiles and important cellular pathways for the development of PGD are likely dependent on pre-transplant diagnoses.
In the IPF subgroup we identified a significant difference between the longitudinal trend in PTX3 levels between cases and controls, with significant univariate differences at 6 hours and 24 hours after transplant. This time course fits with a previous study demonstrating PTX3 levels peaking at 7.5 hours after CCU admission for myocardial infarction (9). Additionally, continued inflammatory insults can result in prolongation of elevated PTX3 levels as seen in a cohort study of critically ill MICU patients (18). In our study, while the most significant increase in PTX3 occurred in patients with PGD, PTX3 levels increased in all study subjects, regardless of outcome, implying an association with IRI, even at lower levels of severity, in patients without PGD. We do not have measurements of PTX3 levels at later time points to further assess trends over time.
There are several limitations to our study. First, there is the potential for limited generalizability. While our findings were most significant in the subpopulation of subjects with IPF compared to those with COPD, other diagnoses were not included in the study. Given the documented production and release of PTX3 from vascular endothelial cells and the significant association of recipient pulmonary hypertension with PGD, elevated PTX3 plasma levels may be important in PGD pathogenesis in other predisposing diagnoses. Additionally, while the association of PTX3 level and PGD was not significant in the COPD subgroup, the small sample size in the COPD-only group may have led to a false negative result. In this study, we were unable to identify a correlation between PASP and PTX3 level at any time point. There is also the potential for PGD misclassification. While the case control format limits the ability to perform more extensive sensitivity analyses, an analysis with an outcome of highest PGD grade in the 72 hours after reperfusion demonstrated a significant association between worsening grade from 0 to 3 and increased PTX3 plasma level. As with previous biomarker studies, given similar pre-operative PTX3 levels between cases and controls, it is not possible to define a causal relationship of PTX3 production and release leading to PGD. However, the concordance of PTX3 level with PGD supports a mechanistic role for innate immune activation in the development of PGD. While we controlled for identified confounders, we were unable to control for airway infection or pre- and post-transplant bacterial colonization. Given the integral role for PTX3 in innate immune responses and response to infection, it is possible that PTX3 is a marker for infection and that bacterial infection is the link with severe PGD. PTX3 production by antigen presenting cells is inducible by Pseudomonas aeruginosa and Aspergillus fumigatus and PTX3 has been shown to directly bind CMV (19). However, the three day post-operative time frame for developing PGD makes clinically significant airway infection an unlikely explanation for our findings, unless subclinical infection pre-existed in the donor. A previous study demonstrated that positive donor gram stain is not associated with the development of early post-operative pneumonia or worsening oxygenation after transplant (20). Furthermore infection with Pseudomonas, Aspergillus, and CMV occur later in the post-transplant period and are associated with increased rates of BOS, not PGD.
In summary, we identified elevated PTX3 plasma concentrations to be strongly associated with post-lung transplant PGD in patients with IPF. This provides support for a mechanistic role for innate immune activation in the development of severe PGD. Elucidating differences in innate immune activation in IPF compared to COPD in lung transplant recipients is an area of future study.
Acknowledgments
This study was funded by NIH grants HL 087115, HL086919, HL096845, HL081332 and HL088263.
ABBREVIATIONS
- PGD
Primary graft dysfunction
- PTX3
Long Pentraxin-3
- IRI
Ischemia-Reperfusion Injury
- ALI
Acute lung injury
- CRP
C-reactive protein
- SAP
Serum Amyloid P Component
- LTOG
Lung Transplant Outcomes Group
- ISHLT
International Society for Heart and Lung Transplantation
- IPF
Idiopathic pulmonary fibrosis
- COPD
Chronic obstructive pulmonary disease
- BOS
Bronchiolitis obliterans syndrome
- PRBC
Packed Red Blood Cell
- OR
Odds Ratio
- CMV
Cytomegalovirus
- TNF-α
Tissue necrosis factor-alpha
- LPS
Lipopolysaccharide
- TF
Tissue Factor
- PASP
Pulmonary Arterial Systolic Pressure
- GEE
Generalized Estimating Equations
- CC16
Clara Cell Secretory Protein
Footnotes
DISCLOSURE: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Iwata T, Philipovskiy A, Fisher AJ, Presson RG, Jr, Chiyo M, Lee J, et al. Anti-type V collagen humoral immunity in lung transplant primary graft dysfunction. J Immunol. 2008;181(8):5738–5747. doi: 10.4049/jimmunol.181.8.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreno I, Vicente R, Ramos F, Vicente JL, Barbera M. Determination of interleukin-6 in lung transplantation: association with primary graft dysfunction. Transplant Proc. 2007;39(7):2425–2426. doi: 10.1016/j.transproceed.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 3.Diamond JM, Christie JD. The contribution of airway and lung tissue ischemia to primary graft dysfunction. Curr Opin Organ Transplant. 2010;15(5):552–557. doi: 10.1097/MOT.0b013e32833e1415. [DOI] [PubMed] [Google Scholar]
- 4.Millington TM, Madsen JC. Innate immunity and cardiac allograft rejection. Kidney Int. 2010;78(Suppl 119):S18–21. doi: 10.1038/ki.2010.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millington TM, Madsen JC. Innate immunity in heart transplantation. Curr Opin Organ Transplant. 2009;14(5):571–576. doi: 10.1097/MOT.0b013e32832e7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kosieradzki M, Rowinski W. Ischemia/reperfusion injury in kidney transplantation: mechanisms and prevention. Transplant Proc. 2008;40(10):3279–3288. doi: 10.1016/j.transproceed.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Uchida Y, Ke B, Freitas MC, Yagita H, Akiba H, Busuttil RW, et al. T-cell immunoglobulin mucin-3 determines severity of liver ischemia/reperfusion injury in mice in a TLR4-dependent manner. Gastroenterology. 139(6):2195–2206. doi: 10.1053/j.gastro.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffman SA, Wang L, Shah CV, Ahya VN, Pochettino A, Olthoff K, et al. Plasma cytokines and chemokines in primary graft dysfunction post-lung transplantation. Am J Transplant. 2009;9(2):389–396. doi: 10.1111/j.1600-6143.2008.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peri G, Introna M, Corradi D, Iacuitti G, Signorini S, Avanzini F, et al. PTX3, A prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. 2000;102(6):636–641. doi: 10.1161/01.cir.102.6.636. [DOI] [PubMed] [Google Scholar]
- 10.Han B, Haitsma JJ, Zhang Y, Bai X, Rubacha M, Keshavjee S, et al. Long pentraxin PTX3 deficiency worsens LPS-induced acute lung injury. Intensive Care Med. 2010 doi: 10.1007/s00134-010-2067-2. [DOI] [PubMed] [Google Scholar]
- 11.Mauri T, Coppadoro A, Bellani G, Bombino M, Patroniti N, Peri G, et al. Pentraxin 3 in acute respiratory distress syndrome: an early marker of severity. Crit Care Med. 2008;36(8):2302–2308. doi: 10.1097/CCM.0b013e3181809aaf. [DOI] [PubMed] [Google Scholar]
- 12.Covarrubias M, Ware LB, Kawut SM, De Andrade J, Milstone A, Weinacker A, et al. Plasma intercellular adhesion molecule-1 and von Willebrand factor in primary graft dysfunction after lung transplantation. Am J Transplant. 2007;7(11):2573–2578. doi: 10.1111/j.1600-6143.2007.01981.x. [DOI] [PubMed] [Google Scholar]
- 13.Christie JD, Bellamy S, Ware LB, Lederer D, Hadjiliadis D, Lee J, et al. Construct validity of the definition of primary graft dysfunction after lung transplantation. J Heart Lung Transplant. 2010;29(11):1231–1239. doi: 10.1016/j.healun.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christie JD, Shah CV, Kawut SM, Mangalmurti N, Lederer DJ, Sonett JR, et al. Plasma levels of receptor for advanced glycation end products, blood transfusion, and risk of primary graft dysfunction. Am J Respir Crit Care Med. 2009;180(10):1010–1015. doi: 10.1164/rccm.200901-0118OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diamond JM, Kawut SM, Lederer DJ, Ahya VN, Kohl B, Sonett J, et al. Elevated plasma clara cell secretory protein concentration is associated with high-grade primary graft dysfunction. Am J Transplant. 2011;11(3):561–567. doi: 10.1111/j.1600-6143.2010.03431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christie JD, Kotloff RM, Pochettino A, Arcasoy SM, Rosengard BR, Landis JR, et al. Clinical risk factors for primary graft failure following lung transplantation. Chest. 2003;124(4):1232–1241. doi: 10.1378/chest.124.4.1232. [DOI] [PubMed] [Google Scholar]
- 17.Kuntz CL, Hadjiliadis D, Ahya VN, Kotloff RM, Pochettino A, Lewis J, et al. Risk factors for early primary graft dysfunction after lung transplantation: a registry study. Clin Transplant. 2009 doi: 10.1111/j.1399-0012.2008.00951.x. [DOI] [PubMed] [Google Scholar]
- 18.Muller B, Peri G, Doni A, Torri V, Landmann R, Bottazzi B, et al. Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit Care Med. 2001;29(7):1404–1407. doi: 10.1097/00003246-200107000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Ortega-Hernandez OD, Bassi N, Shoenfeld Y, Anaya JM. The long pentraxin 3 and its role in autoimmunity. Semin Arthritis Rheum. 2009;39(1):38–54. doi: 10.1016/j.semarthrit.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Weill D, Dey GC, Hicks RA, Young KR, Jr, Zorn GL, Jr, Kirklin JK, et al. A positive donor gram stain does not predict outcome following lung transplantation. J Heart Lung Transplant. 2002;21(5):555–558. doi: 10.1016/s1053-2498(01)00415-6. [DOI] [PubMed] [Google Scholar]


