Abstract
Complex dynamic behaviour involves reciprocal influences between emotion and cognition. On the one hand, emotion is a “double-edged sword” that may affect various aspects of our cognition and behaviour, by enhancing or hindering them and exerting both transient and long-term influences. On the other hand, emotion processing is also susceptible to cognitive influences, typically exerted in the form of emotion regulation. Noteworthy, both of these reciprocal influences are subjective to individual differences that may affect the way we perceive, experience, and eventually remember emotional experiences, or respond to emotionally challenging situations. Understanding these relationships is critical, as unbalanced emotion–cognition interactions may lead to devastating effects, such as those observed in mood and anxiety disorders. The present review analyses the reciprocal relationships between emotion and cognition, based on evidence derived from brain imaging investigations focusing on three main topics: (1) the impact of emotion on cognition, (2) the impact of cognition on emotion, and (3) the role of individual differences in emotion–cognition interactions. This evidence will be discussed in the context of identifying aspects that are fundamental to understanding the mechanisms underlying emotion–cognition interactions in healthy functioning, and to understanding changes associated with affective disorders.
Keywords: Affective–cognitive interactions, Amygdala, Event-related potentials, Functional magnetic resonance imaging, Medial-temporal lobe memory system, Neural circuitry, Prefrontal cortex
The ability to process emotional stimuli with increased efficacy depends on neural mechanisms that allow detection, identification, and processing of stimuli and situations that are important for survival (e.g., finding food, avoiding predators) (Anderson & Phelps, 2001; LeDoux, 1996; Ohman, Flykt, & Ludqvist, 2000). These mechanisms have evolved during evolution and are continuously modelled during ontogeny. At a basic level, these mechanisms overlap with those involved in the generalised response to stressful situations, which consist of coordinated neurohormonal changes that are set in motion when a stressor of any kind exceeds specific thresholds (Arnsten, 1998; Selye, 1985). The engagement of the stress system as a result of exposure to uncontrollable stressful situations sets off a chain of events that not only ensure immediate adaptive responses but also trigger mechanisms that will determine our responses in future similar situations.
Emotions may affect various aspects of our cognition and behaviour, by enhancing or hindering them and by exerting both transient and long-term influences. Possibly as a result of their relevance for survival, emotional stimuli tend to capture our attention more easily than none-motional stimuli, and thus may affect different levels of our cognition, from lower level (e.g., perceptual) to higher level (e.g., mnemonic and executive) cognitive processes. The enhanced significance of emotional stimuli can benefit cognitive processes (e.g., better memory for emotional events) (Dolcos, 2010; Dolcos & Denkova, 2008; Dolcos, LaBar, & Cabeza, 2006; McGaugh, 2004; Phelps, 2004), but can also have detrimental effects on behaviour (e.g., increased distractibility to task-irrelevant emotional stimuli) (Dolcos, Diaz-Granados, Wang, & McCarthy, 2008; Dolcos, Kragel, Wang, & McCarthy, 2006; Dolcos & McCarthy, 2006). Also, although some of these effects are transient, influencing online perceptual and executive processes, others produce long-term effects (Dolcos, LaBar, & Cabeza, 2005) that can last for a lifetime (Markowitsch, 2008; Moscovitch, Nadel, Winocur, Gilboa, & Rosenbaum, 2006; Rubin, 2005). Evidence concerning these aspects will be discussed in the first section of this review.
Emotion processing is also susceptible to cognitive influences, as complex behaviour involves reciprocal interactions between affective and cognitive processes. Indeed, the neurobiological theories of cognitive–affective interactions would not be complete without an account of the mechanisms underlying the effect of cognitive processes on emotional processing. Particularly important in this context is the ability to promptly deploy cognitive control of emotion in order to resist momentary emotional distraction and, in a longer run, the ability to cope with emotional responses as a result of recollecting memories of unpleasant events. The engagement of these mechanisms can occur automatically, probably as a result of gradual development during ontogeny, and depend on the attributed personal significance to the potentially emotional situations. For instance, when witnessing a tragic accident some individuals may be more distressed and hence experience and express intense emotions, whereas others are better able to control their emotions (e.g., by reappraising the situation, or by concealing their emotions), and hence overall reduce their emotional experience. Because emotion regulation strategies have a substantial impact on one's emotional experience, leading to different behavioural, cognitive, and neural results (Gross, 2008; Ochsner & Gross, 2005; Richards, 2004), they are an important part of emotion–cognition interactions. Evidence concerning these aspects will be discussed in the second section.
Understanding the mechanisms underlying the reciprocal interactions between emotion and cognition is critical for understanding fundamentals of healthy functioning, as well as changes associated with psychological and emotional disorders. An important aspect in this context concerns the role of individual differences in emotion–cognition interaction, and, related to this, individual variations in the vulnerability to affective disorders, as even within the realm of healthy emotional responses some individuals may tend to be more emotionally responsive and “anxious,” whereas others can control better their emotions. Understanding the role of individual differences can provide insights into the factors that may influence the susceptibility to affective disorders, in which unbalanced interactions between emotion and cognition may lead to devastating effects, such as those observed in depression and anxiety. For example, the tendency to ruminate on negative emotions and memories observed in depressed patients and the intrusive recollection of traumatic memories observed in patients with posttraumatic stress disorder affect tremendously the way these patients think and behave. Therefore, it has become apparent that finding cures for these disorders depends on understanding the brain mechanisms that are responsible for such dramatic changes in the ways emotion interfaces with cognition, as well as on identifying the role of individual differences in mediating these interactions. Evidence concerning these aspects will be discussed in the third section.
To summarise, the present review analyses the reciprocal interactions between emotion and cognition, as derived from brain imaging investigations focusing on the three main topics highlighted earlier: (1) the impact of emotion on cognition, (2) the impact of cognition on emotion, and (3) the role of individual differences in emotion–cognition interactions. First, we will discuss evidence concerning the neural circuitry underlying the enhancing/beneficial and impairing/detrimental impact of emotion on cognitive processes, by considering findings from studies focusing on both lower level perceptual processing and higher level executive functions, and on both transient and longer lasting effects of emotion. Conversely, we will consider the reverse side of these effects, namely the impact of cognition on affective processing, by addressing evidence concerning the neural correlates of emotion regulation and coping with emotional distraction. Finally, we will also discuss the reciprocal interactions between emotion and cognition in the context of highlighting the role of individual differences (i.e., related to personality, sex, and age).
THE IMPACT OF EMOTION ON COGNITION
The impact of emotion on perception and attention
In this section, we will review two main polarised positions concerning emotion processing: one supporting a traditional view according to which emotion processing is automatic and does not depend on the availability of processing resources (Vuilleumier, Armony, Driver, & Dolan, 2001), and the other supporting a competing view, which posits that emotion processing depends on attentional resources (Pessoa, McKenna, Gutierrez, & Ungerleider, 2002). In the following paragraphs, we will discuss the main evidence supporting these two opposing views, along with their reciprocal critiques, as well as emerging evidence supporting the idea that the two views are not mutually exclusive.
The traditional view proposes that, probably because of their relevance for survival, processing of emotional stimuli is generally automatic. An automatic process is fast, unintentional and unconscious, therefore it is not subject to control, and cannot be avoided or interrupted in its course (Schneider & Shiffrin, 1977). Evidence comes from studies showing that emotional stimuli can be detected and processed with increased efficacy, and that this privileged processing depends on the amygdala (AMY), a main brain structure associated with emotion processing. For instance, visual search paradigms reveal more rapid and accurate detection of schematically depicted threatening faces presented among similar distractors, an effect called the “face in the crowd,” “snake in the grass,” or “pop-out” effect (e.g., Ohman, Lundqvist, & Esteves, 2001). Also, evidence from AMY patients shows that intact AMY is needed to observe enhancement of attention by emotional stimuli, which eliminate “attentional blinks” during processing of rapidly succeeding stimuli (Anderson & Phelps, 2001). Further supporting the traditional view and the role of the AMY, brain imaging evidence shows that facial stimuli with emotional expressions (e.g., expressing fear) can be processed even in the absence of awareness (Whalen et al., 1998)
This and other evidence led to the generally accepted notion that processing of emotional information occurs automatically, and does not depend on available attentional resources (LeDoux, 1996; Morris, Ohman, & Dolan, 1999; Ohman, Flykt, & Esteves, 2001; Vuilleumier et al., 2001). Although the exact mechanisms that allow for such automatic processing of emotional information are not fully understood, direct sub-cortical pathways that reach the AMY independently of the typical cortical connections subserving various sensorial modalities are implicated (Vuilleumier, 2005). This is consistent with the evidence that emotional stimuli benefit from enhanced processing due to their ability to “capture attention” and reallocate processing resources (Anderson & Phelps, 2001; LeDoux & Phelps, 2000; Vuilleumier, 2005). As a result of this “parallel processing” that allows for both automatic, nonconscious, preattentional processing and enhanced conscious processing boosted by the engagement of attentional mechanisms, emotional stimuli are processed with increased efficacy (see also Balconi, this issue 2011; Shaw, Lien, Ruthruff, & Allen, this issue 2011; Sutton & Altarriba, this issue 2011).
Strong brain imaging support for the idea that emotion processing is independent of attention comes from an influential fMRI study by Vuilleumier and colleagues (2001). In this study, attention was manipulated by asking subjects to attend either to pairs of houses or faces, which were presented in a four–picture display eccentrically to a fixation point. The pictures in the house-pairs were either identical or different, whereas the faces were either fearful or neutral, and the subjects were asked to attend either to houses or faces and to make same/different judgements. Supporting the view that emotion processing occurs automatically and independently of the attentional focus, fMRI results revealed increased AMY activity to the fearful faces regardless of whether they were attended or not. Also consistent with the traditional view, response times to houses were slower when fearful faces were displayed as distractors.
Although much of the available evidence supports the traditional view concerning the automaticity of emotion processing, there is also evidence supporting an emerging competing view, according to which emotion processing is not automatic, but rather dependent on attentional resources (Pessoa, 2005). Representative evidence for this view comes from work by Pessoa et al. (2002), which challenged Vuilleumier et al.'s (2001) findings and hence triggered an interesting debate concerning the role of attention in early emotion processing (compare Pessoa et al. and Vuilleumier et al.). The main methodological criticism raised by Pessoa et al. was that previous studies failed to reveal evidence for modulation of emotion processing by attention because the tasks used were not demanding enough to reduce the availability of processing resources to be engaged by emotional information—hence, the findings supporting the automaticity of emotion processing.
To address this limitation, Pessoa et al. (2002) devised a more difficult task, in which subjects had to fixate on centrally displayed faces (males or females, with fearful or neutral expressions) and either make a gender judgement or specify if two peripherally displayed bars were oriented in the same direction or not. Thus, similar to Vuilleumier et al.'s (2001) task, the attentional focus was alternating between stimuli with or without emotional content. As expected, in the gender judgement condition (attending faces), fearful faces evoked greater response in a network of regions associated with emotion processing, including the AMY. However, this differential activation was not present when subjects performed the more difficult peripheral (bar-orientation) task, and thus did not attend to faces. Also, there were no differences in response times related to the fearful expression of the face distractors. Based on these findings, Pessoa et al. concluded that emotional stimuli can “capture attention” if there are enough attentional resources “to be captured” and not engaged by other tasks at hand.
Since their initial publication, when the evidence supporting the two views was seen in radical opposition (Pessoa et al., 2002; Vuilleumier et al., 2001), the positions of their proponents have became milder relative to the compatibility of the two views (see Pessoa, 2005; Vuilleumier, 2005). Nevertheless, discussions about the automatic versus attention-dependent nature of emotion processing continue to be a matter of current debate. One possible cause for this continuing debate is the fact that emotional content and task demands were not systematically and impartially manipulated within the same study. Studies providing support for automatic emotion processing could be criticised for not using challenging enough tasks to deplete attentional resources, but those studies supporting the attention–dependent view could be criticised for not using powerful enough emotional stimuli to “capture” attention. The apparent inconsistency between the findings resulted from these investigations could be reconciled by studies concomitantly manipulating both the task demands and the emotional content (Shafer & Dolcos, 2010). Such investigations may not only solve this debate but could also provide evidence that the two views concerning basic emotion processing are not mutually exclusive, and that depending on the circumstances emotional information can be processed automatically and can also benefit from engaging attentional resources, if available (Shafer & Dolcos, 2010).
The impact of emotion on long-term memory
Emotion has not only transient effects on cognitive processing, by influencing initial perceptual processes and attention paid to emotional stimuli or to details surrounding emotional events, but also long-lasting effects, which will eventually lead to better memory for those events. Vivid memories for emotionally charged personal events support this notion, but there is also empirical evidence that emotional events are better remembered than neutral events (e.g., Bradley, Greenwald, Petry, & Lang, 1992; Christianson, 1992). Emotional stimuli (including pictures, words, and faces) are better remembered than neutral stimuli, an effect that tends to be similar for positive and negative stimuli. This finding suggests that the memory-enhancement effect of emotion is driven by emotional arousal (i.e., emotional intensity) rather than by emotional valence (Dolcos, LaBar, & Cabeza, 2006). Also, emotional memories tend to be accompanied by a sense of re–experiencing (Dolcos et al., 2005; Ochsner, 2000), and these recollection benefits are also augmented relative to neutral memories over time (Anderson, Yamaguchi, Grabski, & Lacka, 2006; Ritchey, Dolcos, & Cabeza, 2008; Sharot & Yonelinas, 2008).
Of particular interest are the neural mechanisms believed to make emotional events more memorable, and the extent to which the brain regions involved in initial stages of processing also contribute to the long-term effects of emotion. The modulation hypothesis, inspired from animal research (McGaugh, 2000, 2002, 2004), suggests that emotional events are remembered better than neutral events because the AMY enhances the function of memory-related brain regions.1 Recent studies involving event-related fMRI in humans provide further support for this idea (Dolcos, LaBar, & Cabeza, 2004b; Kensinger & Corkin, 2004; Kilpatrick & Cahill, 2003; Richardson, Strange, & Dolan, 2004; Ritchey et al., 2008; Sergerie, Lepage, & Armony, 2006). These studies are particularly relevant because they involved methods that allowed identification of (1) memory-specific brain activity from activity associated with more general perceptual processing, as a result of using the so-called subsequent memory paradigm (SMP) (Dolcos et al., 2004b; Kensinger & Corkin, 2004; Paller & Wagner, 2002; Ritchey et al., 2008; Sergerie et al., 2006; Shafer, Iordan, Cabeza, & Dolcos, 2011) (also reviewed in Dolcos, 2010; Dolcos & Denkova, 2008; Dolcos, LaBar, & Cabeza, 2006; LaBar & Cabeza, 2006), (2) nearby medial temporal lobe (MTL) subregions involved in the memory-enhancing effect of emotion, as a result of using the so-called anatomical region of interest (ROI) approach (Dolcos et al., 2004b), and (3) support for the idea that the memory-enhancing effect of emotion involves interactions between emotion-based (AMY) and memory-based brain regions (Dolcos et al., 2004b; Kilpatrick & Cahill, 2003; Richardson et al., 2004; Ritchey et al., 2008).
By using the SMP in conjunction with fMRI, we showed that emotion enhances long-term episodic memory (i.e., memory for personal events) by modulating activity in two main memory-related brain regions, i.e., the MTL memory system (Dolcos et al., 2004b) and the prefrontal cortex (PFC) (Dolcos, LaBar, & Cabeza, 2004a). Further, we showed that these effects can occur during one or more memory stages, including the initial encoding of an emotional event (Dolcos & Cabeza, 2002; Dolcos, Graham, LaBar, & Cabeza, 2003; Dolcos et al., 2004a, b) and the retrieval and reexperiencing of emotional memories (Dolcos et al., 2005). For instance, one of our studies focusing on the role of the AMY and MTL memory system (Dolcos et al., 2004b) during encoding yielded two main findings supporting the modulation hypothesis in neurologically intact humans (also reviewed in Dolcos, 2010; Dolcos & Denkova, 2008; Dolcos, LaBar, & Cabeza, 2006). Using anatomically defined ROI to precisely quantify the MTL activity, and the SMP to identify brain activity specifically predicting memory performance, we found enhanced memory for emotional information to be associated with enhanced memory-related activity in (and interactions between) the AMY and the MTL memory regions. Consistent with animal findings, the greatest differences between the emotional and the neutral encoding success activity were found in the basolateral nucleus of the AMY (BLA), the anterior part of the hippocampus (HC) and the entorhinal cortex (EC). Moreover, encoding success activity in AMY and MTL memory regions was more strongly correlated with each other for emotional than neutral stimuli. This finding suggests that these regions interact more intimately during the encoding of emotional stimuli than during the encoding of neutral stimuli, thus providing further support for the modulation hypothesis (McGaugh, 2002).
Evidence from a recent study involving retention interval manipulations also provided insight into the effect of emotion consolidation, by showing that AMY–MTL interactions initiated during encoding contribute to the persistence of emotional memories over time (Ritchey et al., 2008). In this study, participants were scanned while encoding negative and neutral pictures, then postscanning recognition memory was tested after a short delay (20 min after encoding) and a long delay (1 week after encoding). This manipulation revealed that AMY–MTL connectivity during encoding was greater for items retrieved after a longer delay, thus suggesting that AMY–MTL interactions underlie consolidation and persistence of the emotional memories over time (Ritchey et al., 2008).
Studies of memory retrieval also showed that emotional memory is particularly resilient to time, with laboratory enhancements being reported up to 1 year after encoding (Dolcos et al., 2005). Our retrieval study (Dolcos et al., 2005) provided behavioural and functional neuroimaging evidence concerning the effect of emotional arousal on memory retrieval processes after a longer retention interval than typically involved in laboratory-based studies (minutes, days, weeks) (e.g., Cahill, Babinsky, Markowitsch, & McGaugh, 1995; LaBar & Phelps, 1998). In this study, participants encoded high-arousing emotional (pleasant and unpleasant) and low-arousing neutral pictures, and 1 year later memory was tested while undergoing fMRI. Notably, subjects performed a recognition task that distinguishes between recollection–based and familiarity–based responses (Tulving, 1985). One year after initial encoding, emotionally arousing pictures were remembered better and elicited greater recollection than neutral pictures, and fMRI results indicated that emotional content enhances activity in the AMY and MTL memory systems (HC and EC) related to successful retrieval of individual items from long-term storage (hits vs. misses). These findings support the idea that successful retrieval of emotional memories involves MTL mechanisms similar to those identified during successful emotional encoding (Cahill et al., 1996; Dolcos et al., 2004b). Also, in the AMY and HC (but not in the EC), the emotion effect was greater for recollection than for familiarity, which provided initial evidence concerning the neural correlates of the differential effect of emotion on these two types of memory retrieval (Ochsner, 2000).
Although the majority of studies have focused on the interaction between the emotion-based AMY system and memory-related MTL system, the role of the PFC in emotional memory formation has also been investigated. The extant evidence concerning the role of the PFC in emotional memory encoding suggests both arousal-driven effects (e.g., Dolcos et al., 2004a; Sergerie, Lepage, & Armony, 2005) and valence-driven effects (e.g., Kensinger & Corkin, 2004). For instance, we found that emotional arousal enhanced successful encoding activity (Dm effect) in two subregions of the left lateral PFC (i.e., one dorsolateral—Brodman Area [BA] 9/6 and the other ventrolateral—BA 47), in which the Dm effect for highly arousing pictorial items (both pleasant and unpleasant) was greater than for low arousing and neutral items (Dolcos et al., 2004a). Similarly, using a word encoding task with high- and low-arousing negative and neutral stimuli, Kensinger and Corkin (2004) also obtained a regionally specific modulation of PFC activity by valence. These findings are further supported by studies providing evidence for AMY–PFC interactions during emotional memory formation (e.g., Canli, Desmond, Zhao, & Gabrieli, 2002; Kilpatrick & Cahill, 2003). Given the role of left ventrolateral PFC (vlPFC) regions in semantic processing (Poldrack et al., 1999) and the role of the dorsolateral PFC (dlPFC) regions in working memory operations (D'Esposito, Postle, & Rypma, 2000), these findings suggest that arousing events enhance memory in part by promoting semantic (vlPFC) and working memory (dlPFC) processing.
Finally, complementary evidence concerning the mechanisms underlying the formation of emotional memories comes from studies investigating the timing of processing linked to the memory-enhancing effect of emotion (e.g., Dolcos & Cabeza, 2002; Palomba, Angrilli, & Mini, 1997). Using event-related potential (ERP) recordings during encoding, in conjunction with the SMP, we identified that the emotional Dm effect occurs faster than the neutral Dm (Dolcos & Cabeza, 2002): Emotional Dm was greater during an early epoch (400–600 ms poststimulus onset), whereas the Dm effects were similar for emotional and neutral stimuli during a late epoch (600–800 ms). Although given the spatial resolution of ERPs it was difficult to identify the neural generators of this effect, faster emotional Dm suggests that emotional stimuli have privileged access to processing resources leading to subsequent memory, which could be another mechanism conferring a memory advantage to emotional information (Dolcos & Cabeza, 2002).
In sum, the previously mentioned studies provide strong evidence for the notion that emotion enhances long-term episodic memory by modulating activity in two main memory-related brain regions, the MTL memory system and the PFC, which also benefits from faster encoding. The effects of emotion on MTL and PFC regions, however, may be related to different mechanisms. The evidence concerning the functions typically attributed to these brain regions (Moscovitch, 1992; Simons & Spiers, 2003) suggests that in the MTL emotion enhances basic encoding, consolidation, storage, and retrieval of memory representations, whereas in the PFC emotion enhances semantic, strategic, and working memory processes. These led to the proposal that AMY and MTL are part of basic/direct neurohormonal mechanisms underlying the memory-enhancement effect of emotion, whereas PFC has an indirect/mediated involvement in the formation of emotional memories, by enhancing strategic, semantic, and working memory processes (LaBar & Cabeza, 2006) (Figure 1). Finally, evidence also suggests that overall the memory-enhancing effect of emotion is driven by the intensity of emotional events, rather than by their valence, although there is evidence that valence is also a contributing factor (Kensinger & Corkin, 2004; Mickley Steinmetz & Kensinger, 2009; Shaw, Brierley, & David, 2005).
Figure 1.
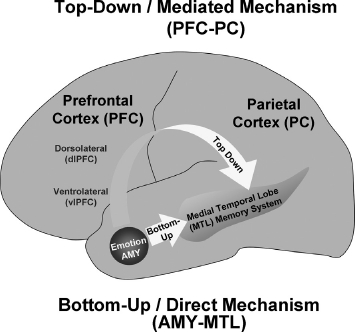
Main mechanisms involved in the memory-enhancing effect of emotion. Emotion enhances long-term episodic memory by modulating activity in two main memory-related brain regions, the medial–temporal lobe (MTL) memory system and the prefrontal cortex (PFC). However, the effects of emotion on MTL and PFC regions may be related to different mechanisms: AMY and MTL are part of basic/direct neurohormonal mechanisms underlying the memory-enhancement effect of emotion (bottom-up mechanism), whereas PFC is part of a mechanism (also including the parietal cortex—PC) that has an indirect/mediated involvement in the formation of emotional memories, by enhancing strategic, semantic, working memory, and attentional processes (top–down mechanism). Adapted from LaBar and Cabeza (2006); courtesy of Dr. Roberto Cabeza. [To view this figure in colour, please visit the online version of this Journal.]
The impact of emotion on working memory and decision making
In addition to influencing initial stages of processing and their long-term consequences (reviewed previously), emotional information also impacts other higher level processes, such as working memory and decision making, which are essential to goal-directed behaviour. Evidence concerning the impact of emotion on these executive processes will be discussed in turn.
Working memory
Working memory (WM) is involved in the active maintenance and manipulation of task-relevant information (Baddeley, 1996). As suggested earlier, maintaining emotional information in WM may be one of the mechanisms contributing to the enhancing effects of emotion on long-term memory (Dolcos et al., 2004a; Kensinger & Corkin, 2004). Although this may imply that emotional stimuli also produce an enhancing effect on WM, the evidence from studies investigating this matter by comparing WM performance for emotional versus neutral stimuli did not yield conclusive findings (Kensinger & Corkin, 2003). Instead, emerging evidence concerning the impact of emotion on WM and the associated neural correlates comes from studies in which emotional stimuli are presented as task-irrelevant distractors (Chuah et al., 2010; Denkova et al., 2010; Dolcos et al., 2008; Dolcos & McCarthy, 2006; Wong et al., in press).
Distraction challenges our ability to maintain focus on goal-relevant information and thus may impair cognitive performance. The existence of neural mechanisms that allow for privileged access of emotional information to processing resources raises the possibility that emotional information may also be a very potent distraction, particularly when task irrelevant. However, up until recently, the neural correlates of the detrimental effects of emotion on cognitive functions had received relatively less attention (Johnson et al., 2005; Most, Chun, Widders, & Zald, 2005). A default assumption, based on the findings concerning the enhancing effect of emotion on memory (where emotion enhanced activity in memory-related brain regions), is that the impairing effect of emotion may be linked to reduced activity in brain regions subserving the functions impaired by emotion. This assumption is supported by evidence from both clinical and non–clinical groups.
Models of affective–cognitive interactions inspired by clinical studies point to dysfunctional interactions between a dorsal executive neural system and a ventral affective system, and propose that impaired executive control and enhanced emotional distractibility observed in depression are linked to hypofunction of the dorsal executive and hyperfunction of the ventral affective systems (Drevets & Raichle, 1998; Mayberg, 1997) (Figure 2). The dorsal system includes brain regions typically associated with “cold” executive (ColdEx) functions, such as the dlPFC and the lateral parietal cortex (LPC), which are critical to active maintenance of goal-relevant information in WM; increased activity in these regions during WM tasks is typically associated with increased performance (Fuster, 1997; Smith & Jonides, 1999). The ventral system includes brain regions involved in “hot” emotion (HotEmo) processing, such as the AMY, the vlPFC, and the medial PFC (Davidson & Irwin, 1999; Davis & Whalen, 2001; Phan, Wager, Taylor, & Liberzon, 2002).
Figure 2.
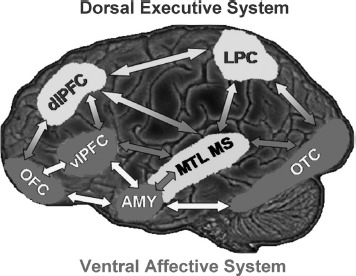
Neural systems involved in cognitive/executive (dorsal) versus emotional (ventral) processing. The dorsal system includes brain regions typically associated with “cold” executive (ColdEx) colour–coded in blue) functions, such as the dorsolateral prefrontal cortex (dlPFC) and the lateral parietal cortex (LPC), which are critical to active maintenance of goal-relevant information in working memory (WM). The ventral system includes brain regions involved in “hot” emotional (HotEmo; colour–coded in red) processing, such as the amygdala (AMY), the ventrolateral PFC (vlPFC), and the medial PFC. Other brain regions that these systems interact with (MTL MS, OTC) are also illustrated. MTL MS = medial temporal lobe memory system, OFC = orbitofrontal cortex, OTC = occipitotemporal cortex. Monochromatic arrows represent connections within the same system, whereas bichromatic arrows represent connections across systems. Although visual cortical areas illustrated here (OTC) are not technically part of the HotEmo system, they are coloured in red because these areas are susceptible to influences from emotion processing regions. This representation does not include all regions that are part of the two systems, as in its present format it does not include medial brain regions. Adapted from figure provided by Dr. Lihong Wang. [To view this figure in colour, please visit the online version of this Journal.]
Findings from recent studies investigating the neural correlates of cognitive interference by emotional distraction in healthy participants provide evidence that interactions between the ColdEx and HotEmo systems are not only reflected in longer lasting altered states, as observed in clinical conditions such as depression, but can also occur transiently, in response to ongoing task irrelevant emotional distractors. A series of studies from our group investigating the neural correlates of the response to emotional distraction presented during the delay between memoranda and probes of a WM task identified dissociable patterns of brain activity in ColdEx versus HotEmo systems, which were specific to transient emotional distractors (Dolcos et al., 2008; Dolcos & McCarthy, 2006; Dolcos, Miller, Jha, & McCarthy, 2007). For instance, Dolcos and McCarthy (2006) provided initial evidence that impaired WM performance in the presence of emotional distraction was linked to increased activity in the HotEmo system and concomitant decreased activity in the ColdEx system.
Follow-up investigations in both healthy (Dolcos et al., 2007, 2008) and clinical (Morey et al., 2009) groups provided further evidence that these patterns of neutral responses are specific to emotional distraction, and that the detrimental effect of emotional distraction reflects bottom-up effects (Chuah et al., 2010; Denkova et al., 2010). In a study comparing the effects of memoranda–confusable and nonemotional distractors with those of emotional distractors (Dolcos et al., 2008), we identified opposing modulation of dlPFC activity linked to the nature of distraction: The memoranda–confusable2 distractors were associated with enhanced dlPFC activity, whereas emotional distractors were associated with decreased dlPFC activity, in conditions where both types of distractors produced similar effects on WM performance. A more recent study (Denkova et al., 2010) investigating the effects of more specific emotional distractors (i.e., anxiety-inducing angry faces) found similar brain imaging effects to those produced by general emotional distractors involved in previous studies (Dolcos et al., 2008; Dolcos & McCarthy, 2006), hence replicating the dissociable patterns of activity in the ColdEx versus HotEmo systems linked to impaired performance in the presence of emotional distraction. This study also provided evidence concerning a dissociation between bottom-up effects observed in face-sensitive brain areas (i.e., fusiform gyrus [FG]) linked to individual variation in trait anxiety and WM performance. Finally, a recent study examining the impact of sleep deprivation on the response to emotional distraction (Chuah et al., 2010) also pointed to bottom-up effects of emotional distraction, by showing that increased distraction by negative emotional pictures following sleep deprivation was associated with increased AMY activation and reduced functional connectivity between this region and cognitive control brain regions.
Collectively, these findings provide strong evidence that the outcome of task-irrelevant emotional distraction depends on dynamic interactions between neural systems that allow the ability to stay focused on task-relevant information and systems involved in the processing of emotional information that may compete with the available processing resources. Possibly as a result of their salience, emotional distractors may produce a bottom-up impact on processing of goal-relevant information by reallocating processing resources (Vuilleumier et al., 2001) and impairing performance. However, as we will see later, the disadvantageous outcomes of this bottom-up impact of emotional distraction can be mitigated by “top-down” interventions from cognitive control regions, which are engaged to regulate emotional responses and cope with emotional distraction (Dolcos, Kragel, et al., 2006; Gray, Braver, & Raichle, 2002; Pessoa, 2008).
Decision making
Recently, the fact that emotion provides essential input for high-level complex processes, like decision making, has become more largely accepted. Initially developed to account for problems of decision making displayed by patients with ventromedial PFC (vmPFC) damage, the influential so-called “somatic marker hypothesis” (SMH) (Damasio, 1994, 1996) argues that decision making is a process shaped by multiple sources of input, not only cognitive, but also somatic-related feedback with emotional/motivational relevance.
Generally speaking, a somatic marker is a type of nonspecific “feeling” that has been linked through learning to predicted outcomes in the future (Damasio, 1994, 1996). Somatic markers can be related to either primary inducers (e.g., seeing a snake or losing a large sum of money) or secondary inducers (e.g., the perspective of losing a large sum of money), which send a biasing signal that crudely affects choice (Damasio, 1995). Using the Iowa Gambling Task (IGT; Bechara, Damasio, Damasio, & Anderson, 1994; Bechara, Tranel, Damasio, & Damasio, 1996), Damasio and colleagues showed that successful performance in the IGT is related to the ability to display anticipatory skin conductance responses (i.e., somatic markers), which are typically present in normal subjects but absent in patients with vmPFC/orbitofrontal cortex (OFC) damage. The implicit ability to use these responses in making advantageous decisions (Bechara, Damasio, Tranel, & Damasio, 1997) suggests that emotion–related signals are important for decision making even when they reside at a nonconscious level. The SMH underlines the contributions of the AMY and vmPFC as components of a neural network with an essential role in judgement and decision making (Damasio, 1994). However, the contributions of the two structures are at different levels—not only do both of them contribute to the integration of exteroceptive and interoceptive somatic/emotional information, but also AMY is critical for processing primary inducers and OFC is critical for processing secondary inducers (Bechara, Damasio, & Damasio, 2003).
Emotional influences on decision–making processes is further supported by fMRI studies using the ultimatum game (e.g., Sanfey, Rilling, Aronson, Nystrom, & Cohen, 2003), whereby two players respond to fair and unfair proposals to split money. Unfair offers elicited activity in brain areas related to both emotion (anterior insula) and cognition (dlPFC). If the decisions are made purely on cognitive grounds, acceptance of even the most unfair offers would lead to gains, whereas in case of rejected offers neither of the players receives anything. Typically, this does not happen, as the tendency for players is to reject unfair offers, despite a detriment to their own gains. Interestingly, activation in the anterior insula (which is commonly associated with processing of negative emotions, particularly disgust), increased for rejected offers, suggesting that in those cases emotion “overruled” cognitive processes, thus highlighting an important role of emotional responses in decision making (see also Nguyen et al., this issue 2011).
THE EFFECT OF COGNITION ON EMOTION PROCESSING
Up to this point, we have reviewed the impact of emotion on cognitive processing. In this section, we focus on the reciprocal influence, namely the effect of cognition on emotion processing. Principally, we review evidence from studies of emotion regulation and investigations of the neural correlates of coping with emotional distraction.
Emotion regulation—background
Understanding the mechanisms by which cognition affects emotion is the focus of a recently emerging field investigating the neural underpinnings of cognitive control of emotion (or emotion regulation), which is an important coping mechanism in the face of emotional stressors (Ochsner & Gross, 2005). Despite recent progress in identifying the neural correlates of emotion regulation, a number of questions remain. Chief among these is the role of different types of emotional control and their associated neural correlates. Most of the previous emotion regulation research has focused on conscious/deliberate forms of regulation, such as instructed reappraisal (Kim & Hamann, 2007; Ochsner, Bunge, Gross, & Gabrieli, 2002), suppression (Jackson et al., 2003), and cognitive distraction (Denkova, Chakrabarty, Dolcos, & Dolcos, in press; Levesque et al., 2003). A large body of evidence shows that the deliberative forms of emotion regulation broadly involve the PFC, especially the ventrolateral aspects of the PFC, as well as the posterior portion of the dorsomedial PFC (dmPFC)/superior frontal gyrus (BA 6/8) (Goldin, McRae, Ramel, & Gross, 2008; Ochsner et al., 2002). In addition, a few studies also found activation in the dlPFC (Goldin et al., 2008; Levesque et al., 2003), and there is inconsistent support for more posterior regions such as the posterior temporal gyri and inferior parietal lobules (Goldin et al., 2008; Ochsner et al., 2004).
Different than deliberate emotion regulation, the nonconscious/automatic forms of emotion regulation and the associated neural correlates have received relatively little empirical attention (cf. Mauss, Cook, & Gross, 2007). Nonconscious emotion regulation is an important part of our behaviour that, similar to conscious forms of emotional control, undergoes ontogenetic transformations. Although emotions are displayed very early in ontogeny, the ability to control them is not fully developed in early childhood and adolescence. As we age, we learn that displaying certain emotions may not be appropriate in specific contexts, and often we find ourselves automatically adjusting, or regulating, our emotional responses to fit the current circumstances. Recent evidence suggests that nonconscious emotion regulation could be as effective as deliberate emotion regulation, while being less costly because of the automatic effortless engagement (Bargh & Williams, 2007). However, because previous research tended to separately investigate the two forms of emotion regulation (Goldin et al., 2008; Mauss et al., 2007) it is not clear whether they are equally effective in controlling the response to emotionally challenging situations, or whether they are differentially effective depending on the intensity of emotional challenge. Moreover, brain imaging studies investigating the neural correlates of nonconscious emotion regulation are generally lacking (but see Dolcos, Sung, Denkova, Dixon, & Dolcos, in press).
Although the brain imaging studies of emotion regulation mentioned above provided evidence of increased activity in cognitive control regions as a result of engaging emotion regulation strategies, these studies could not establish the precise role of these regions in controlling the impact of emotion on ongoing cognitive processes. On the other hand, brain imaging studies in which emotional information was presented as task-irrelevant distraction provided such evidence by identifying neural correlates of coping with distracting emotions. In the next section, we will focus on findings from such studies highlighting the role of lateral and medial PFC regions.
Cognitive control of emotional distraction
Understanding the impact of cognitive control on emotion processing requires identification of the neural mechanisms associated with the ability to cope with distracting emotions. A series of investigations from our group (Chuah et al., 2010; Denkova et al., 2010; Dolcos et al., 2008; Dolcos, Kragel, et al., 2006; Dolcos & McCarthy, 2006) provided evidence that coping with task-irrelevant emotional distraction entails increased activity in and interactions between brain regions involved in basic emotion processing (the AMY) and brain regions associated with cognitive control (particularly lateral and medial PFC). The engagement of the AMY can be seen as having the role of an “emotional detector” that signals the cognitive control regions the presence of potentially distracting, emotional stimuli and thus the need to control their possible detrimental effects on cognitive performance (Dolcos, Kragel, et al., 2006). The anatomical evidence of substantial AMY–vlPFC connections (Amaral, Price, Pitkanen, & Carmichael, 1992) supports this interpretation, and hence it is reasonable to posit that enhanced functional connectivity between the AMY and vlPFC reflects processing that originates in the AMY, which exerts influence upon inferior frontal cortex activity.
These studies provided evidence for enhanced vlPFC–AMY functional coupling during processing of emotional distraction (Figure 3A), evidence concerning the consequence of engaging cognitive control regions in response to task-irrelevant emotional stimuli, as well as identified a hemispheric asymmetry in the vlPFC concerning its involvement in the subjective (right vlPFC) versus objective (left vlPFC) coping with emotional distraction. In our initial study (Dolcos & McCarthy, 2006) we identified a negative covariation between activity in the right vlPFC and subjective indexes of distractibility, suggesting that participants who engaged this region during processing of emotional distractors perceived them as less distracting (Figure 3B). However, activity in this region did not distinguish between correct and incorrect trials, thus suggesting an engagement of this region in coping with the subjective “feeling of being distracted”. On the other hand, further investigation showed that activity in the left vlPFC did in fact distinguish between successful and unsuccessful trials in the presence of emotional distractors, by showing increased activity to correct versus incorrect trials, and hence suggesting that this region is involved in the “actual coping with emotional” distraction by controlling the objective impact of emotional distraction on working memory performance (Dolcos, Kragel, et al., 2006) (Figure 3C). The link between increased activity in the left vlPFC and enhanced WM performance was further confirmed by other studies (Dolcos et al., 2008; Denkova et al., 2010). For instance, in another recent study we showed that increased activity in the left vlPFC was linked to increased WM performance in the presence of emotional, but not neutral, distractors (Denkova et al., 2010).
Figure 3.
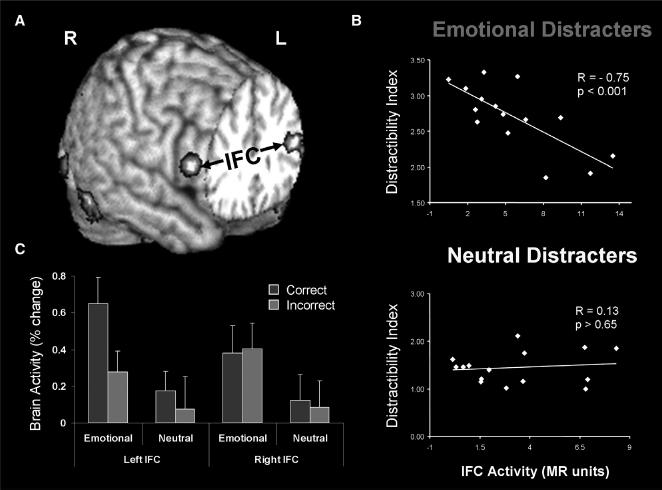
Evidence for the role of lateral PFC in coping with distracting emotions. (A) Brain regions showing enhanced functional coupling with the amygdala during processing of emotional distraction—ventrolateral prefrontal cortex (vlPFC)/inferior frontal cortex (IFC) highlighted. (B) Enhanced correlation between vlPFC activity and subjective emotional distractibility scores. (C) Hemispheric asymmetry in the vlPFC/IFC during successful coping with emotional distraction. Taken together, these findings can also be interpreted as a subjective (right) versus objective (left) dissociation in the role of these regions in coping with the subjective feeling of being distracted (right vlPFC/IFC) versus actually coping with distraction (left vlPFC/IFC). Correct/Incorrect — Remembered/Forgotten items in the working memory task. Adapted from Dolcos, Kragel, et al. (2006) and Dolcos and McCarthy (2006), with permission. [To view this figure in colour, please visit the online version of this Journal.]
Taken together, these investigations provided evidence linking the engagement of activity in these lateral and medial PFC regions not only in controlling the subjective emotional response induced by potentially distracting emotional stimuli (Ochsner & Gross, 2005) but also in diminishing the actual negative impact of distracting emotions on ongoing cognitive processes (Chuah et al., 2010; Denkova et al., 2010; Dolcos et al., 2008; Dolcos, Kragel, et al., 2006). Moreover, the extant evidence also suggests that this engagement involves enhanced functional coupling with activity in the AMY (Chuah et al., 2010; Dolcos, Kragel, et al., 2006).
THE ROLE OF INDIVIDUAL DIFFERENCES IN EMOTION–COGNITION INTERACTIONS
An important aspect in the investigation of emotion–cognition interactions is identification of individual differences that may affect the response to emotional challenge and thus the differential vulnerability to affective disorders. As presented in the previous parts, the interactions between emotion and cognition are apparent at different levels of analysis of human behaviour. The final output of these interactions defines human behaviour as a whole, and is reflected in individual differences, which in the domain of emotion processing seems to be the rule rather than the exception (Eugene et al., 2003). As such, all the effects that have been discussed so far may be influenced by individual differences in the response to emotional and cognitive challenge. A given emotional situation can evoke a wide range of emotional responses across individuals. This suggests that, not only are our responses to emotional contexts influenced by present emotional states, but also our behaviour in the face of emotional challenge is influenced by our personality traits.
In this context, it is important to emphasise that the response to emotional challenge, and thus vulnerability to affective disorders (i.e., depression– and anxiety-related), depend on individual variation in both emotional and cognitive domains, and that the final behavioural outcome depends on the overall “affective–cognitive profile”. It is known, for instance, that the negative affective bias in processing emotional information, the increased susceptibility to emotional distraction, and the affective dysregulation observed in affective disorders are also accompanied by overall impaired cognitive/executive control (e.g., Bishop, 2009; Eysenck, Derakshan, Santos, & Calvo, 2007). Also, these changes could affect not only activity in higher level cognitive control brain regions but also in lower level perceptual processing regions (e.g., Pujol et al., 2009). Investigation of individual differences is also critical for elucidating the neural bases of these phenomena, as it has become apparent that finding cures for these clinical conditions depends not only on integrative understanding of the factors that influence the susceptibility to emotional and cognitive challenge, but also alterations of the brain mechanisms that accompany pathological changes in the emotion–cognition interactions.
In this section, we review behavioural and fMRI findings from studies investigating the role of individual characteristics, such as personality–, sex-, and age-related, in mediating the interactions between emotion and cognition. Investigation of the role of such factors that mediate the response to emotional challenge may prove critical for understanding affective disorders, which are characterised by increased emotional sensitivity and distractibility, have increased incidence in women, and are associated with dysfunctional emotion regulation or emotion dysregulation. Earlier brain imaging investigations have linked individual variation in personality measures reflecting general emotion processing (e.g., extraversion and neuroticism) to brain activity associated with general processing of emotional information (e.g., Canli & Amin, 2002). However, more recent studies (e.g., Denkova et al., 2010) also provided evidence linking individual variation in personality traits reflecting specific emotions (social anxiety) to neural changes in responses to transiently induced specific emotions (e.g., anxiety-inducing distraction). Similarly, studies investigating sex-related differences in emotion processing reported differences in brain activity associated with enhanced emotional reactivity and emotional memory in females compared to males (Cahill, Uncapher, Kilpatrick, Alkire, & Turner, 2004; Canli, Desmond, et al., 2002; Lang, Greenwald, Bradley, & Hamm, 1993). Finally, recent studies investigating changes in emotion processing associated with ageing point to a functional shift consistent with a positivity bias in healthy ageing (Carstensen, Mikels, & Mather, 2006; Mather & Carstensen, 2005), possibly linked to enhanced ability to control emotions (St. Jacques, Dolcos, & Cabeza, 2009; Dolcos, & Cabeza, 2010), as opposed to emotion dysregulation observed in affective disorders. Evidence concerning the role of individual differences in these three main aspects in the context of investigating the neural correlates of emotion processing, in general, and of emotion–cognition interactions, in particular, will now be discussed in turn.
The role of personality–related differences
Recent studies have shown that personality traits, such as extraversion and neuroticism, influence the neural correlates of emotion processing across a range of emotional processes, including experience, perception, attention, and memory (Canli, Sivers, Whitfield, Gotlib, & Gabrieli, 2002; Hamann & Canli, 2004; Touryan et al., 2007). It has also been suggested that individual differences in emotional biases linked to personality might be rooted in an attentional network driven primarily by AMY reactivity during the encoding of emotional stimuli (Haas & Canli, 2008). Extraversion and neuroticism have both been associated with attentional biases (Derryberry & Reed, 1994) but in opposite directions (Rusting, 1999). For example, individuals higher on extraversion exhibit greater attention towards positive- rather than negative–valenced emotional stimuli (Derryberry & Reed, 1994), coupled with longer reaction times when responding to positive relative to neutral words (Haas, Omura, Amin, Constable, & Canli, 2006). Functional MRI studies have demonstrated that regions of the PFC and the AMY exhibit differential responses to emotional stimuli in individuals who describe themselves as highly extraverted versus neurotic (Canli & Amin, 2002; Canli et al., 2001). In those studies, extraversion was positively correlated with the activity of the lateral and medial PFC (including the cingulate gyrus) and the AMY in response to viewing positive (vs. negative) stimuli. Neuroticism, in contrast, was positively correlated with the activity in the PFC when viewing negative (vs. positive) stimuli. Extraversion and neuroticism also affect recollection of personal autobiographical memories. In a recent behavioural study we found that extraversion contributed to remembering more positive personal experiences and to maintaining a positive state, while neuroticism predicted the phenomenological characteristics of negative autobiographical memories (Denkova, Dolcos, & Dolcos, 2011).
Personality-related individual differences were also identified in conditions where emotional information was presented as task-irrelevant distraction. Based on recent findings suggesting individual variation in the response to emotional distraction (Dolcos et al., 2008), in a series of follow-up studies we investigated the factors that may underlie these differences. For instance, an investigation of task performance in conjunction with personality data (Dolcos, 2009) revealed that participants that were more susceptible to emotional distraction in the WM task not only tended to perceive emotional distractors as more emotional (as measured by subjective ratings of the distractors) but also to have higher scores for attentional impulsivity (as measured with the Barratt Impulsiveness Scale [BIS]; Spinella, 2007). Moreover, analyses linking these behavioural differences to brain activity showed that individual variation in the susceptibility to emotional distraction was linked to enhanced activity in brain regions associated with emotional processing (AMY) and to disrupted activity in regions associated with cognitive control (dlPFC) (Dolcos, 2009).
Relationships between brain activity and personality-related differences were identified not only for traits reflecting general aspects of cognitive processing, such as attentional impulsivity discussed earlier, but also for traits reflecting differences in processing and experiencing specific emotions, such as anxiety. Investigations of the role of anxiety-related individual differences show that increased reactivity to potential threat conveyed by socially relevant stimuli (e.g., angry faces) is associated with exacerbated activity in the AMY in both clinical patients with social anxiety disorder, linked to individual variations in the severity of the symptoms (Evans et al., 2008; Goldin et al., 2008; Phan, Fitzgerald, Nathan, & Tancer, 2006), and in nonclinical individuals, linked to variations in the level of anxiety (Bishop, Duncan, & Lawrence, 2004; Ewbank et al., 2009; Stein, Simmons, Feinstein, & Paulus, 2007). These findings suggest that altered functioning of the AMY is not only disorder specific, but can also be observed in individuals prone to develop anxiety disorders (Hariri et al., 2002; Morey et al., 2011). In one of our recent studies (Denkova et al., 2010), we identified brain regions where activity in response to transient anxiety-inducing distraction covaried with individual differences in trait anxiety and cognitive performance. Specifically, activity in the left visual cortex (including the left FG, BA 37) and vmPFC was positively correlated, and activity in the right dlPFC and dmPFC was negatively correlated with anxiety scores. These findings suggest that enhanced trait anxiety is associated with increased sensitivity to transient anxiety-inducing stimulation, which results in enhanced activity in brain regions associated with the perception and experiencing of emotions (FG and vmPFC, respectively), and impaired activity in brain regions associated with the ability to maintain focus on goal-relevant information (dlPFC).
Another important source of interindividual variation in emotion processing is individuals’ tendency to spontaneously engage in emotion regulation, particularly in a context when there is no explicit instruction or awareness of emotion regulation. Spontaneous emotion regulation is typically assessed by evaluating the use of regulation strategies such as reappraisal and suppression, as indexed by the Emotion Regulation Questionnaire (ERQ; Gross & John, 2003). Spontaneous reappraisal tendencies have been associated with lower levels of negative affect, greater interpersonal functioning, and greater psychological and physical well-being (Gross & John, 2003). At the brain level, similar with instructed reappraisal, individual differences in habitual engagement of reappraisal are associated with decreased activation in ventral emotion generative regions, such as AMY, and increased activation in prefrontal control regions, such as dlPFC and dmPFC, in response to negative stimuli (Drabant, McRae, Manuck, Hariri, & Gross, 2009).
The role of sex-related differences
In our society, it is commonly believed that women are more emotional than men. Consistent at least in part with this widely held belief, there is evidence that women are more emotionally reactive (Lang, Greenwald, Bradley, & Hamm, 1993) and expressive (Kring & Gordon, 1998), display more extensive knowledge of emotional experience (Barrett, Lane, Sechrest, & Schwartz, 2000), and recall more emotional autobiographical memories (Seidlitz & Diener, 1998). These sex-related differences observed behaviourally are supported by recent evidence from brain imaging studies pointing to sex-related differences in brain regions that are part of the neural networks involved in emotion processing, such as the AMY and OFC (Goldstein et al., 2001; Gur, Gunning–Dixon, Bilker, & Gur, 2002). These differences have been observed in several domains, including responses to emotionally arousing stimuli, responses to emotional facial expressions, and emotional memory (Cahill & van Stegeren, 2003; Hamann, 2005).
Particular attention have received sex-related differences showing a hemispheric asymmetry in memory-related activity in the AMY (Figure 4), i.e., left-lateralised activity in women and right-lateralised activity in men (Cahill, 2006; Cahill et al., 2004). Further evidence suggests that this lateralisation is confined to the BLA, and is observed only when memory is tested after a delay (e.g., 2 weeks later), but not when tested immediately (Mackiewicz, Sarinopoulos, Cleven, & Nitschke, 2006). Interestingly, these hemispheric differences were not observed for dorsal AMY (Mackiewicz et al., 2006) where activity was associated with anticipatory processes and predicted immediate, but not delayed recognition memory (see also Talmi, Anderson, Riggs, Caplan, & Moscovitch, 2008). These findings suggest that the sex-related lateralisation is specifically related to the BLA and to memory consolidation.
Figure 4.
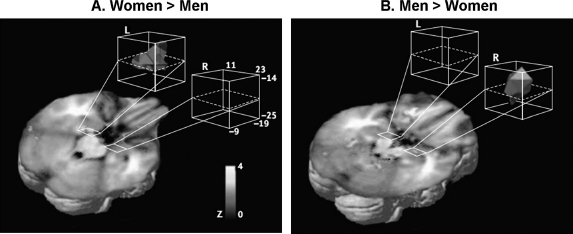
Sex-related differences identified in the amygdala showing a hemispheric asymmetry in memory-related activity. (A) Activity in the left amygdala while viewing emotionally arousing images was more significantly related to subsequent memory for the images in women than in men, and (B) the opposite pattern was observed in the right amygdala, i.e., greater activity in men than in women. Reproduced from Cahill et al. (2004), with permission. [To view this figure in colour, please visit the online version of this Journal.]
Given the function attributed to the left and right hemisphere in verbal/local versus nonverbal/ global processing, it is possible that the pattern revealed by these studies could be due to the use of verbal/local strategies during emotional encoding by women and the use of visual spatial/global strategies by men (Cahill, 2006). However, recent evidence suggests a social account for sex differences in emotional memory. Consistent with this interpretation, recent evidence suggests that the sex-related lateralisation of AMY activity depends on the sex of the faces, in that left AMY is preferentially involved in successful encoding of female faces in women, whereas right AMY is linked to successful encoding of male faces in men (Armony & Sergerie, 2007). This finding highlights the possible social/biological relevance of the relationship between the perceiver's sex and the stimulus properties. Related to this, there is evidence that women seem, indeed, to have a greater preference for socially relevant stimuli (faces and persons vs. scenes; Proverbio, Zani, & Adorni, 2008), and that it is the feminine and masculine roles as established by the society, rather than the biological sex per se, that seem to influence emotional memory (Cahill et al., 2004).
Although investigation of sex-related differences in the cognitive control of emotion have received less attention, the available evidence points to possible differences in both emotion regulation strategies and the associated neural correlates. Indeed, emotional responses and the subsequent memory can be influenced not only by differences in emotional reactivity per se, but also by differences in how emotions are regulated, or some interaction between emotional reactivity and emotion regulation (McRae, Ochsner, Mauss, Gabrieli, & Gross, 2008). Consistent with the idea of possible dissociable engagement of emotion regulation strategies in women and men, in a study by Koch et al. (2007), during a task involving emotion regulation females mainly recruited brain regions associated with emotion processing (i.e., AMY and the OFC), whereas males mainly recruited brain regions typically involved in cognitive processing (i.e., prefrontal and superior parietal regions). Also, a study investigating sex-related differences during cognitive reappraisal found that men showed greater decreases in AMY activity, along with lesser control-related prefrontal activity, whereas women showed greater ventral striatal activity (McRae et al., 2008). These results may suggest that men may be able to use cognitive regulation with less effort than women and that women may use positive affect in the service of down-regulating negative affect to a greater extent than men.
Sex-related differences were also identified in the relationship between the habitual engagement of emotion regulation strategies and the retrieval of emotional autobiographical memories, with reappraisal being related to positive personal memories in men and suppression with negative personal memories in women (Denkova et al., 2011). These findings extend the evidence that men and women differ not only in terms of general emotional processing and memory but also in the engagement of emotion regulation strategies (Matud, 2004; Thayer, Rossy, Ruiz-Padial, & Johnsen, 2003).
In sum, the extant evidence suggests that differential processing of and memory for specific emotional events, along with the associated neural correlates, differ in men and women, and that these differences may be related to variations in the use of encoding and emotion regulation strategies.
The role of age-related differences
Although older age is associated with increasing physical ailments, psychological stress, social losses, and increased dependency, most of the people are in general satisfied in old age and experience relatively high levels of emotional well-being. Supporting this idea, a large body of research indicates that negative affect decreases (Grühn, Jacqui, & Baltes, 2005) and positive affect increases or remains stable throughout the life span (Carstensen, Isaacowitz, & Charles, 1999). This unexpected phenomenon, characterised by improved subjective emotional experience in older age, is called the “paradox” of ageing (Carstensen et al., 1999; Mather et al., 2004) and understanding it in the context of elucidating the role of individual differences in emotion–cognition interactions warrants further investigation (Allen et al., 2005).
Abundant evidence suggests an overall cognitive decline with ageing (Daselaar, Dennis, & Cabeza, 2007; Park et al., 2002; see also Timpe, Rowe, Matsui, Magnotta, & Denburg, this issue 2011), but there is also evidence suggesting the existence of compensatory mechanisms, the engagement of which allow healthy elderly to perform in cognitive tasks as well as their younger counterparts (Cabeza, Dolcos, Graham, & Nyberg, 2002; Dolcos, Rice, & Cabeza, 2002). However, evidence from the affective domain suggests not only overall preservation of ability to process emotional information with ageing (Keightley, Winocur, Burianova, Hongwanishkul, & Grady, 2006; Mather & Knight, 2006), but also an enhanced ability to control emotion (Gross et al., 1997), which is reflected in a positive affective bias, i.e., the tendency to attenuate negative emotions and enhance positive emotions (Mather & Knight, 2006).
Age-related positivity effects have been documented in how people perceive and appraise emotional material, as well as how they remember emotional memories (Mather et al., 2004). Functional neuroimaging evidence suggests that age-related changes in general emotion processing are linked to changes in AMY and frontal cortex activity, and in their interaction. Although some reports suggest age-related decrease in AMY activation (Fischer et al., 2005; Tessitore et al., 2005), others show that diminished AMY activity is limited to negative stimuli, as opposed to positive stimuli (Mather et al., 2004). In a recent investigation of functional connectivity within the emotional network (St. Jacques et al., 2010), we identified evidence for age-related increase in AMY–frontal interactions during processing of negative stimuli, in the context of preserved AMY function (Figure 5A). Specifically, older adults showed enhanced connectivity between activity in the AMY and the anterior cingulate cortex (ACC) while rating the emotional content of negative pictures (Figure 5B). Interestingly, these age-related differences in AMY–ACC interactions were associated with changes in the perceived emotional content of negative pictures, as shown by more “neutral” responses given by the older participants to the negative pictures (St. Jacques et al., 2010). Other studies that explicitly manipulated emotion regulation in older adults also found a coupling between the AMY and both the medial and lateral PFC (Urry et al., 2006; Winecoff, Labar, Madden, Cabeza, & Huettel, 2011)
Figure 5.
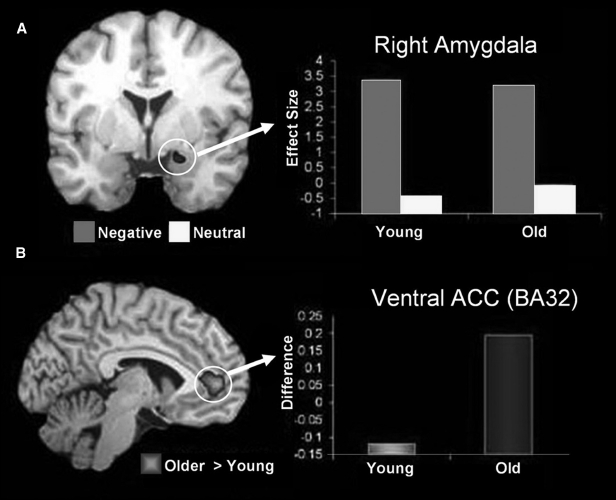
Preserved amygdala function and increased amygdala–ACC connectivity in healthy ageing. (A) Coronal brain view and bar graph showing overlapping activity in the right AMY region during processing of negative pictures (Neg > Neu) in young and older adults. The y–axis represents the difference in activity between negative and neutral conditions and units are in effect size, the difference in the parameter estimates of the activation. (B) Sagittal/lateral brain view and bar graph showing age-related increase in the interaction between AMY and the anterior cingulate cortex (ACC) during processing of negative pictures. The y–axis represents the difference in correlations between negative and neutral conditions. From St. Jacques et al. (2010), with permission. [To view this figure in colour, please visit the online version of this Journal.]
Other reports focusing on age-related changes in emotional memory also provided evidence that preserved emotional enhancement of memory in ageing is associated with relative preservation of AMY structure and function, as well as with changes in the neural circuitry underlying emotion processing in healthy older adults (Kensinger & Schacter, 2008; St. Jacques et al., 2009). In our study investigating age-related differences in emotional memory, for instance (St. Jacques et al., 2009), we identified similar involvement of AMY in emotional memory encoding in both younger and older participants, i.e., greater encoding success activity for emotional than for neutral stimuli. However, the study also identified age-related differences in the formation of emotional memories in both MTL and PFC regions, i.e., a decrease in the MTL contribution (along with other posterior brain regions, including visual cortex) coupled with an increase in the dlPFC involvement. Moreover, these opposing effects of ageing on MTL and dlPFC activity were also observed in the functional connectivity of these brain regions with AMY: decreased for HC, and enhanced for dlPFC during encoding of memories for negative pictures.
These findings suggest an age-related reduction in the contribution of the direct AMY–MTL mechanisms, which is compensated by the enhanced contribution of AMY–PFC mechanisms to the formation of emotional memories. These age-related differences in the emotional network are consistent with a more general pattern of Posterior-Anterior Shift in Ageing (the PASA model), i.e., diminished engagement of the posterior brain regions compensated by enhanced contribution of the PFC regions (Davis, Dennis, Daselaar, Fleck, & Cabeza, 2008). Given that the PASA pattern observed in the cognitive domain is interpreted as reflecting the engagement of compensatory mechanisms (Davis et al., 2008; Grady et al., 1994), the findings in the affective domain mentioned above could also be interpreted as reflecting compensatory engagement of PFC mechanisms as a result of overall diminished contribution of the MTL mechanism. This interpretation is consistent with the behavioural findings that showed overall better memory for emotional than for neutral stimuli in both young and older groups, although the latter group also showed a reduction in the emotional enhancement of memory, possibly as a result of reduced contribution from the direct MTL mechanisms (St. Jacques et al., 2009). However, given the evidence supporting enhanced cognitive control of emotion with ageing (Gross & John, 1997), an alternative account for increased PFC involvement is that it reflects enhanced engagement of emotion regulation strategies in older adults (St. Jacques et al., 2010), which also results in reduced overall enhancing effect of emotion on memory in older adults.
In sum, behavioural and brain imaging evidence supports the idea of age-related differences in emotion–cognition interactions, in the context of relative preservation of the mechanisms responsible for emotional processing. These phenomena are associated with differential involvement of the MTL and PFC mechanisms and relative preservation of anatomical and functional integrity of the AMY. Collectively, these findings suggest a pattern defined by decreased MTL contributions associated with increased PFC involvement and with enhanced AMY–PFC interactions, which could reflect the engagement of compensatory mechanisms and/or enhanced emotion regulation (see also Allen, Kaut, Baena, Lien, & Ruthruff, this issue 2011; Noh & Isaacowitz, this issue 2011).
CONCLUSIONS, OPEN ISSUES, AND FUTURE DIRECTIONS
The present review analysed the neural correlates underlying reciprocal interactions between emotion and cognition, focusing on three main topics: (1) the impact of emotion on cognition, (2) the impact of cognition on emotion, and (3) the role of individual differences in emotion–cognition interactions. As discussed, emotion can produce both enhancing and impairing effects on cognition, can have both immediate and long-term effects that may impact not only lower level (e.g., perceptual) but also higher level (e.g., mnemonic) cognitive processes. An important aspect related to the impact of cognition on emotion processing is the capacity to deploy cognitive control in order to resist momentary emotional distraction and, on a long run, the ability to cope with longer lasting emotions and feelings. The evidence concerning the role of individual differences in these interactions points to factors that may influence susceptibility to mood and anxiety disorders, which are characterised by unbalanced interactions between emotion and cognition that lead to the devastating effects associated with these psychiatric conditions.
Overall, the extant evidence concerning the neural substrate of various aspects of emotion–cognition interactions points to dynamic interplays between separate but interconnected neural systems involved in emotional and cognitive/ executive functions. This evidence also highlights the role of the AMY as central to the impact of emotion on cognition, although its engagement impacts differently activity in brain regions involved in the enhancing (increased) versus impairing (decreased) effects of emotion. Amygdala activity is also the target of top-down control of emotion, exerted by lateral and medial PFC regions. Finally, activity in these neural systems may be linked to individual variation in aspects that potentially influence the sensitivity to emotional information, such as neuroticism, and functional alterations in the way they interface with each other may lead to emotional and cognitive disturbances, such as those observed in affective disorders. Despite a rapidly growing body of literature providing clarification of the neural correlates of emotion–cognition interactions, a number of issues are still unclear. We will now briefly introduce them in relationship with aspects reviewed in this paper.
-
1.
Regarding the impact of emotion on cognition, although the findings reviewed here provide compelling evidence that emotion can produce both enhancing and impairing effects on cognition, the link between these two effects is not clear. For instance, it is unclear to what extent certain brain regions commonly identified in studies investigating the enhancing and impairing effects of emotion on cognitive processing (e.g., AMY) contribute to both effects, or may specifically contribute to one or the other of the effects, depending on the context in which emotional stimuli are presented (i.e., as task relevant or task irrelevant). Simultaneous investigation of the neural correlates of the two opposing effects of emotion on cognition (i.e., the enhancing vs. impairing) would allow identification of the neural mechanisms underlying processing that links the immediate disrupting effects of emotional distraction on short-term/working memory to the enhancing effect of emotion on long-term episodic memory. It is plausible to expect that reallocation of processing resources in the presence of task-irrelevant emotional distraction leads to both impaired WM and enhanced episodic memory for the distractors themselves, but probably through modulation of brain activity in different neural systems. Investigation of these effects within the same subjects is critical, as they tend to cooccur in both normal and clinical conditions.
-
2.
Another open question refers to the role of emotional valence and arousal in both immediate and long-term effects of emotion on cognition. For instance, the majority of studies investigating the impact of task-irrelevant emotional distraction on performance in short-term/working memory tasks (Chuah et al., 2010; Denkova et al., 2010; Dolcos et al., 2008; Dolcos & McCarthy, 2006) used only high-arousing unpleasant stimuli as emotional distractors, and hence it is not known whether the observed effects could be generalised to all emotional stimuli, regardless of valence and level of arousal. Similarly, although most of the evidence from studies of episodic memory suggests that arousal is the main factor influencing the memory-enhancing effect of emotion, the neural correlates underlying the contribution of emotional valence are less specified (but see Mickley Steinmetz & Kensinger, 2009; Talmi, Schimmack, Pater–son, & Moscovitch, 2007). Given that positive and negative emotions have evolved to subserve different functions, it is reasonable to expect that their contribution to the memory-enhancing effect of emotion may be associated with different neural mechanisms, which may work in synchrony with the mechanisms underlying the more general effect of emotional arousal. In this context, it would also be interesting to investigate whether similar mechanisms contribute to the memory-enhancing effect of emotion in the case of specific emotions that may share one or both of the basic emotion properties of arousal and valence (e.g., anger vs. sadness, anger vs. fear).
-
3.
Regarding the neural correlates of emotion control, chief among the open questions is understanding the role of different types of emotional control and their associated neural correlates. Most of the previous emotion regulation research has focused on conscious/deliberate forms of regulation, such as conscious suppression of emotional responses (Jackson et al., 2003), which may require effort in controlling emotional responses, but little is known about nonconscious/automatic forms of emotion regulation and the associated neural correlates (see however Dolcos et al., 2009; Mauss, Evers, Wilhelm, & Gross, 2006). Manipulating the inducement of the goal to control emotional response would be a practical way to directly compare the two forms of emotion regulation. It is reasonable to expect that although automatic regulation might be less effortful and less costly, it might also be less efficient in the face of highly challenging transient emotional situations. It is important to note, however, that typically most of the emotions that we have to deal with in daily life situations are rather “mild,” and thus it is very likely that automatic emotion regulation is more frequently engaged as a regulation strategy in normal circumstances, as opposed to the engagement of deliberate strategies that may become critical in “crisis” circumstances. Therefore, it is important to investigate whether nonconscious and deliberate emotion regulation strategies have similar immediate versus long-term effects on behaviour, whether they share the same neural correlates, and whether they are similarly affected in the case of chronic exposure to emotional challenge, leading to affective dysregulation observed in affective disorders.
-
4.
Concerning the role of individual differences in understanding emotion–cognition interactions and the associated neural correlates, important open questions refer to their link to individual variation in the susceptibility to affective disorders. One way to address this issue is by linking investigations of task performance in conjunction with personality data to investigations of brain activity. Also, in the context of a rapidly accumulating corpus of evidence pointing to sex-related differences in emotion–cognition interactions, an important open question concerns the link between emotional reactivity and distractibility Specifically, it is not known whether the enhanced emotional reactivity observed in female participants also leads to enhanced emotional distractibility, and whether individual variations in emotional reactivity are linked to specific patterns of activity in the neural network involved in the cognitive control of emotion. Thus, it is important to investigate whether sex-related differences in behaviour are reflected in different patterns of brain activity in brain regions associated with emotion processing and cognitive control.
-
5.
Another important area of investigation concerns identification of alterations in the neural correlates of cognition–emotion interaction in clinical conditions, such as major depression and posttraumatic stress disorder (Hayes et al., 2011; Ritchey, Dolcos, Eddington, Strauman, & Cabeza, 2011; Wang, LaBar, et al., 2008), and direct comparisons of findings from populations showing contrasting affective biases. An example of such an “extreme” comparison in the domain of emotion regulation is between healthy ageing, associated with enhanced ability to control emotion, and depression, associated with impaired emotion regulation, or emotion dysregulation. Existing evidence supports the idea of age-related differences in emotion–cognition interactions, in the context of relative preservation of the mechanisms responsible for emotion, and point to AMY–PFC interactions that may reflect the engagement of enhanced emotion regulation (St. Jacques et al., 2009, 2010). However, less is known about the nature of specific automatic engagement of emotion regulation strategies used by the elderly that generates positivity biases in emotion processing and how they may be affected in depression. Recent findings from our studies suggest that similar neural circuitry, which appears to be engaged under conditions of enhanced cognitive control of emotion (in healthy ageing) (St. Jacques et al., 2010), malfunctions in conditions where regulation of emotion is dysfunctional (e.g., in depression) (Wang, Krishnan, et al., 2008; Wang, LaBar, et al., 2008), and that these effects are reversed by treatment (Ritchey et al., 2011). Thus, further investigations and direct comparisons of the neural mechanisms underlying these opposing affective biases could prove fruitful in determining whether the opposing affective biases observed behaviourally are also reflected in the neural responses associated with differences in the ability to control emotions observed in these two groups. These investigations have the potential to also provide an explanation for the evidence suggesting reduced incidence of depression in the elderly (Christensen et al., 1999; Jorm, 2000).
-
6.
Finally, another open issue concerns the investigation of higher level affective phenomena, as well as emotion–cognition interactions in social contexts. Recent methodological refinements have encouraged the empirical approach of emotion–cognition interactions involving superior emotions, such as love (Zeki, 2007), and complex phenomena, such as social interaction (Adolphs, 2009). In both cases, the ability to decode emotional expressions and body language is crucial for the assessment of others’ intentions. Studies investigating the neural correlates of social cognition usually tended to use facial stimuli (e.g., Todorov, 2008). However, there are clear advantages to using also whole-body stimuli, as they can be recognised as reliably as facial expressions, can direct attention to a person's actions, and overall are more ecologically valid (de Gelder, 2009 Sung et al., 2011). Using emotion manipulations with dynamic whole-body stimuli that mimic more closely real-life situations may contribute not only to better understanding of the neural mechanisms of social interactions in healthy behaviour, but also to gaining insight into possible causes of deficits in social behaviour in such clinical conditions as social anxiety and autism (Pelphrey & Morris, 2006).
NOTES
‘Another influential hypothesis concerning the specific role of the AMY is the so-called plasticity hypothesis (Dolcos & Denkova, 2008), which has also been formulated based on animal studies (LeDoux, 2000). This hypothesis proposes that the AMY itself is a site of plasticity underlying learning and memory of fear conditioning. For a comparison of both hypotheses see Dolcos and Denkova (2008).
Memoranda–confusable distraction refers to stimuli that are similar to the memoranda (e.g., novel faces presented as distractors in a WM task for faces), and hence can be potent distractors despite being nonemotional.
REFERENCES
- Adolphs R. The social brain: Neural basis of social knowledge. Annual Review of Psychology. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P. A., Kaut K. P., Baena E., Lien M.-C., Ruthruff E. Individual differences in positive affect moderate age-related declines in episodic long-term memory. Journal of Cognitive Psychology. 2011;23(6):768–779. [Google Scholar]
- Allen P. A., Kaut K. P., Lord R. G., Hall R. J., Grabbe J. W., Bowie T. An emotional mediation theory of differential age effects in episodic and semantic memories. Experimental Aging Research. 2005;31(4):355–391. doi: 10.1080/03610730500206642. [DOI] [PubMed] [Google Scholar]
- Amaral D. G., Price J. L., Pitkanen A., Carmichael S. T. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. New York, NY: Wiley-Liss; 1992. [Google Scholar]
- Anderson A. K., Phelps E. A. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Anderson A. K., Yamaguchi Y., Grabski W., Lacka D. Emotional memories are not all created equal: Evidence for selective memory enhancement. Learning and Memory. 2006;13(6):711–718. doi: 10.1101/lm.388906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armony J. L., Sergerie K. Own-sex effects in emotional memory for faces. Neuroscience Letters. 2007;426(1):1–5. doi: 10.1016/j.neulet.2007.08.032. [DOI] [PubMed] [Google Scholar]
- Arnsten A. F. The biology of being frazzled. Science. 1998;280(5370):1711–1712. doi: 10.1126/science.280.5370.1711. [DOI] [PubMed] [Google Scholar]
- Baddeley A. The fractionation of working-memory. Proceedings of the National Academy of Sciences of the USA. 1996;93(24):13468–13472. doi: 10.1073/pnas.93.24.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balconi M. Frontal brain oscillation modulation in facial emotion comprehension: The role of reward and inhibitory systems in subliminal and supraliminal processing. Journal of Cognitive Psychology. 2011;23(6):723–735. [Google Scholar]
- Bargh J. A., Williams L. E. On the nonconscious regulation of emotion. In: Gross J., editor. Handbook of emotion regulation. Vol. 1. New York, NY: Guilford Press; 2007. pp. 429–445. [Google Scholar]
- Barrett L. F., Lane R., Sechrest L., Schwartz G. Sex differences in emotional awareness. Personality and Social Psychology Bulletin. 2000;26:1027–1035. [Google Scholar]
- Bechara A., Damasio A. R., Damasio H., Anderson S. W. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1–3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio H., Damasio A. R. Role of the amygdala in decision-making. Annals of the New York Academy of Sciences. 2003;985:356–369. doi: 10.1111/j.1749-6632.2003.tb07094.x. [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio H., Tranel D., Damasio A. R. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275(5304):1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bechara A., Tranel D., Damasio H., Damasio A. R. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cerebal Cortex. 1996;6(2):215–225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Bishop S. J. Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience. 2009;12(1):92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Bishop S. J., Duncan J., Lawrence A. D. State anxiety modulation of the amygdala response to unattended threat-related stimuli. Journal of Neuroscience. 2004;24(46):10364–10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M. M., Greenwald M. K., Petry M. C., Lang P. J. Remembering pictures: Pleasure and arousal in memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1992;18(2):379–390. doi: 10.1037//0278-7393.18.2.379. [DOI] [PubMed] [Google Scholar]
- Cabeza R., Dolcos F., Graham F., Nyberg L. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. Neuroimage. 2002;16(2):317–330. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nature Reviews Neuroscience. 2006;7(6):477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Cahill L., Babinsky R., Markowitsch H. J., McGaugh J. L. The amygdala and emotional memory. Nature. 1995;377(6547):295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- Cahill L., Haier R. J., Fallon J., Alkire M. T., Tang C., Keator D., et al. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proceedings of the National Academy of Sciences of the USA. 1996;93(15):8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L., Uncapher M., Kilpatrick L., Alkire M. T., Turner J. Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: An FMRI investigation. Learning and Memory. 2004;11(3):261–266. doi: 10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L., van Stegeren A. Sex-related impairment of memory for emotional events with beta-adrenergic blockade. Neurobiology of Learning and Memory. 2003;79(1):81–88. doi: 10.1016/s1074-7427(02)00019-9. [DOI] [PubMed] [Google Scholar]
- Canli T., Amin Z. Neuroimaging of emotion and personality: Scientific evidence and ethical considerations. Brain and Cognition. 2002;50(3):414–431. doi: 10.1016/s0278-2626(02)00517-1. [DOI] [PubMed] [Google Scholar]
- Canli T., Desmond J. E., Zhao Z., Gabrieli J. D. Sex differences in the neural basis of emotional memories. Proceedings of the National Academy of Sciences of the USA. 2002;99(16):10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T., Sivers H., Whitfield S. L., Gotlib I. H., Gabrieli J. D. Amygdala response to happy faces as a function of extraversion. Science. 2002;296(5516):2191. doi: 10.1126/science.1068749. [DOI] [PubMed] [Google Scholar]
- Canli T., Zhao Z., Desmond J. E., Kang E., Gross J., Gabrieli J. D. An fMRI study of personality influences on brain reactivity to emotional stimuli. Behavioral Neuroscience. 2001;115(1):33–42. doi: 10.1037/0735-7044.115.1.33. [DOI] [PubMed] [Google Scholar]
- Carstensen L. L., Isaacowitz D. M., Charles S. T. Taking time seriously: A theory of socio-emotional selectivity. The American Psychologist. 1999;54(3):165–181. doi: 10.1037//0003-066x.54.3.165. [DOI] [PubMed] [Google Scholar]
- Carstensen L. L., Mikels J. A., Mather M. Aging and the intersection of cognition, motivation and emotion. In: Birren J. E., Schaie K. W., editors. Handbook of the psychology of aging. San Diego, CA: Academic Press; 2006. pp. 343–362. [Google Scholar]
- Christensen H., Jorm A. F., Mackinnon A. J., Korten A. E., Jacomb P. A., Henderson A. S., et al. Age differences in depression and anxiety symptoms: A structural equation modelling analysis of data from a general population sample. Psychological Medicine. 1999;29(2):325–339. doi: 10.1017/s0033291798008150. [DOI] [PubMed] [Google Scholar]
- Christianson S. A. Emotional stress and eyewitness memory: A critical review. Psychological Bulletin. 1992;112(2):284–309. doi: 10.1037/0033-2909.112.2.284. [DOI] [PubMed] [Google Scholar]
- Chuah L. Y. M., Dolcos F., Chen A. K., Zheng H., Parimal S., Chee M. W. L. Sleep deprivation and interference by emotional distracters. SLEEP. 2010;33(10):1305–1313. doi: 10.1093/sleep/33.10.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio A. R. Descartes error: Emotion, reason and the human brain. New York, NY: Avon; 1994. [Google Scholar]
- Damasio A. R. Toward a neurobiology of emotion and feeling: Operational concepts and hypotheses. The Neuroscientist. 1995;1:19–25. [Google Scholar]
- Damasio A. R. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical Transactions of the Royal Society of London: Biological Sciences. 1996;351B(1346):1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Daselaar S. M., Dennis N. A., Cabeza R. Ageing: Age-related changes in episodic and working memory. In: Rombouts S. A. R. B., editor; Barkhof F., Scheltens P., editors. Clinical applications of functional brain MRI. New York, NY: Oxford University Press; 2007. pp. 115–148. [Google Scholar]
- Davidson R. J., Irwin W. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences. 1999;3(1):11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Davis M., Whalen P. J. The amygdala: Vigilance and emotion. Molecular Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Davis S. W., Dennis N. A., Daselaar S. M., Fleck M. S., Cabeza R. Que PASA? The Posterior-anterior shift in aging. Cerebral Cortex. 2008;18(5):1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gelder B. Why bodies? Twelve reasons for including bodily expressions in affective neuroscience. Philosophical Transactions of the Royal Society of London: Biological Sciences. 2009;364B(1535):3475–3484. doi: 10.1098/rstb.2009.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkova E., Chakrabarty T., Dolcos S., Dolcos F. Brain imaging investigation of the neural correlates of emotional autobiographical recollection. Journal of Visualized Experiments (in press). doi: 10.3791/2396. [DOI] [PMC free article] [PubMed]
- Denkova E., Dolcos S., Dolcos F. Reliving personal memories: Affective biases linked to personality and sex-related differences. 2011. Manuscript under review. [DOI] [PubMed]
- Denkova E., Wong G., Dolcos S., Sung K., Wang L., Coupland N., et al. The impact of anxiety-inducing distraction on cognitive performance: A combined brain imaging and personality investigation. PLoS ONE. 2010;5(11):e14150. doi: 10.1371/journal.pone.0014150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derryberry D., Reed M. A. Temperament and attention: Orienting toward and away from positive and negative signals. Journal of Personality and Social Psychology. 1994;66(6):1128–1139. doi: 10.1037//0022-3514.66.6.1128. [DOI] [PubMed] [Google Scholar]
- D'Esposito M., Postle B. R., Rypma B. Prefrontal cortical contributions to working memory: Evidence from event-related fMRI studies. Experimental Brain Research. 2000;133(1):3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- Dolcos F. Neural correlates of opposing modulation of emotion on episodic and working memory processes. 2009. Paper presented at the 2009 annual meeting of the Memory Disorders Research Society.
- Dolcos F. The impact of emotion on memory: Evidence from brain imaging studies. Saarbrücken, Germany: VDM Verlag; 2010. [Google Scholar]
- Dolcos F., Cabeza R. Event-related potentials of emotional memory: Encoding pleasant, unpleasant, and neutral pictures. Cognitive, Affective and Behavioral Neuroscience. 2002;2(3):252–263. doi: 10.3758/cabn.2.3.252. [DOI] [PubMed] [Google Scholar]
- Dolcos F., Denkova E. Neural correlates of encoding emotional memories: A review of functional neuroimaging evidence. Cell Science Reviews. 2008;5(2):78–122. [Google Scholar]
- Dolcos F., Diaz-Granados P., Wang L., McCarthy G. Opposing influences of emotional and non-emotional distracters upon sustained prefrontal cortex activity during a delayed-response working memory task. Neuropsychologia. 2008;46(1):326–335. doi: 10.1016/j.neuropsychologia.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Dolcos F., Graham R., LaBar K., Cabeza R. Coactivation of the amygdala and hippocampus predicts better recall for emotional than for neutral pictures. Brain and Cognition. 2003;51:221–223. [Google Scholar]
- Dolcos F., Kragel P., Wang L., McCarthy G. Role of the inferior frontal cortex in coping with distracting emotions. Neuroreport. 2006;17(15):1591–1594. doi: 10.1097/01.wnr.0000236860.24081.be. [DOI] [PubMed] [Google Scholar]
- Dolcos F., LaBar K. S., Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: An event-related fMRI study. Neuroimage. 2004a;23(1):64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Dolcos F., LaBar K. S., Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004b;42(5):855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Dolcos F., LaBar K. S., Cabeza R. Remembering one year later: Role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proceedings of the National Academy of Sciences of the USA. 2005;102(7):2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F., LaBar K. S., Cabeza R. The memory-enhancing effect of emotion: Functional neuroimaging evidence. In: Uttl B., editor; Ohta N., Siegenthaler A. L., editors. Memory and emotion: Interdisciplinary perspectives. Malden, MA: Blackwell Publishing; 2006. pp. 107–133. [Google Scholar]
- Dolcos F., McCarthy G. Brain systems mediating cognitive interference by emotional distraction. Journal of Neuroscience. 2006;26(7):2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F., Miller B., Jha A., McCarthy G. Regional brain differences in the effect of distraction during a delay interval of a working memory task. Brain Research. 2007;1152:171–181. doi: 10.1016/j.brainres.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F., Rice H. J., Cabeza R. Hemispheric asymmetry and aging: Right hemisphere decline or asymmetry reduction. Cognitive and Biobehavioral Reviews. 2002;26:819–825. doi: 10.1016/s0149-7634(02)00068-4. [DOI] [PubMed] [Google Scholar]
- Dolcos S., Sung K., Denkova E., Dixon R. A., Dolcos F. Brain imaging investigation of the neural correlates of emotion regulation. Journal of Visualized Experiments, doi: 10.3791/2430 (in press) [DOI] [PMC free article] [PubMed]
- Drabant E. M., McRae K., Manuck S. B., Hariri A. R., Gross J. J. Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biological Psychiatry. 2009;65(5):367–373. doi: 10.1016/j.biopsych.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets W. C., Raichle M. E. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. Cognition and Emotion. 1998;12(3):353–385. [Google Scholar]
- Eugene F., Levesque J., Mensour B., Leroux J. M., Beaudoin G., Bourgouin P., et al. The impact of individual differences on the neural circuitry underlying sadness. Neuroimage. 2003;19(2. Pt., 1):354–364. doi: 10.1016/s1053-8119(03)00121-6. [DOI] [PubMed] [Google Scholar]
- Evans K. C., Wright C. I., Wedig M. M., Gold A. L., Pollack M. H., Rauch S. L. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depression and Anxiety. 2008;25(6):496–505. doi: 10.1002/da.20347. [DOI] [PubMed] [Google Scholar]
- Ewbank M. P., Lawrence A. D., Passamonti L., Keane J., Peers P. V., Calder A. J. Anxiety predicts a differential neural response to attended and unattended facial signals of anger and fear. Neuroimage. 2009;44(3):1144–1151. doi: 10.1016/j.neuroimage.2008.09.056. [DOI] [PubMed] [Google Scholar]
- Eysenck M. W., Derakshan N., Santos R., Calvo M. G. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7(2):336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Fischer H., Sandblom J., Gavazzeni J., Fransson P., Wright C. I., Backman L. Age-differential patterns of brain activation during perception of angry faces. Neuroscience Letters. 2005;386(2):99–104. doi: 10.1016/j.neulet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Fuster J. M. Network memory. Trends in Neurosciences. 1997;20(10):451–459. doi: 10.1016/s0166-2236(97)01128-4. [DOI] [PubMed] [Google Scholar]
- Goldin P. R., McRae K., Ramel W., Gross J. J. The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63(6):577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. M., Seidman L. J., Horton N. J., Makris N., Kennedy D. N., Caviness V. S., Jr., et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex. 2001;11(6):490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Grady C. L., Maisog J. M., Horwitz B., Ungerleider L. G., Mentis M. J., Salerno J. A., et al. Age-related changes in cortical blood flow activation during visual processing of faces and location. Journal of Neuroscience. 1994;14(3, Pt. 2):1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. R., Braver T. S., Raichle M. E. Integration of emotion and cognition in the lateral prefrontal cortex. Proceedings of the National Academy of Sciences of the USA. 2002;99(6):4115–4120. doi: 10.1073/pnas.062381899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. J. Emotion regulation. In: Lewis M., editor; Haviland-Jones J. M., Barrett L. F., editors. Handbook of emotions. New York, NY: Guilford Press; 2008. pp. 497–512. [Google Scholar]
- Gross J. J., Carstensen L. L., Pasupathi M., Tsai J., Skorpen C. G., Hsu A. Y. Emotion and aging: Experience, expression, and control. Psychology and Aging. 1997;12(4):590–599. doi: 10.1037//0882-7974.12.4.590. [DOI] [PubMed] [Google Scholar]
- Gross J. J., John O. P. Revealing feelings: Facets of emotional expressivity in self-reports, peer ratings, and behavior. Journal of Personality and Social Psychology. 1997;72(2):435–448. doi: 10.1037//0022-3514.72.2.435. [DOI] [PubMed] [Google Scholar]
- Gross J. J., John O. P. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85(2):348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Grühn D., Jacqui S., Baltes P. B. No aging bias favoring memory for positive material: Evidence from a heterogeneity-homogeneity list paradigm using emotionally toned words. Psychology and Aging. 2005;20(4):579–588. doi: 10.1037/0882-7974.20.4.579. [DOI] [PubMed] [Google Scholar]
- Gur R. C., Gunning-Dixon F., Bilker W. B., Gur R. E. Sex differences in temporo-limbic and frontal brain volumes of healthy adults. Cerebral Cortex. 2002;12(9):998–1003. doi: 10.1093/cercor/12.9.998. [DOI] [PubMed] [Google Scholar]
- Haas B. W., Canli T. Emotional memory function, personality structure and psychopathology: A neural system approach to the identification of vulnerability markers. Brain Research Reviews. 2008;58(1):71–84. doi: 10.1016/j.brainresrev.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas B., Omura K., Amin Z., Constable R. T., Canli T. Functional connectivity with the anterior cingulate is associated with extraversion during the emotional Stroop task. Social Neuroscience. 2006;1(1):16–24. doi: 10.1080/17470910600650753. [DOI] [PubMed] [Google Scholar]
- Hamann S. Sex differences in the responses of the human amygdala. The Neuroscientist. 2005;11(4):288–293. doi: 10.1177/1073858404271981. [DOI] [PubMed] [Google Scholar]
- Hamann S., Canli T. Individual differences in emotion processing. Current Opinion in Neurobiology. 2004;14(2):233–238. doi: 10.1016/j.conb.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Hariri A. R., Mattay V. S., Tessitore A., Kolachana B., Fera F., Goldman D., et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297(5580):400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hayes J. P., LaBar K. S., McCarthy G., Selgrade E., Nasser J., Dolcos F., et al. Reduced hippocampal and amygdala activity predicts memory distortions for trauma reminders in combat-related PTSD. Journal of Psychiatric Research. 2011;45(5):660–669. doi: 10.1016/j.jpsychires.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. C., Mueller C. J., Dolski I., Dalton K. M., Nitschke J. B., Urry H. L., et al. Now you feel it, now you don't: Frontal brain electrical asymmetry and individual differences in emotion regulation. Psychological Science. 2003;14(6):612–617. doi: 10.1046/j.0956-7976.2003.psci_1473.x. [DOI] [PubMed] [Google Scholar]
- Johnson M. K., Raye C. L., Mitchell K. J., Greene E. J., Cunningham W. A., Sanislow C. A. Using fMRI to investigate a component process of reflection: Prefrontal correlates of refreshing a just-activated representation. Cognitive, Affective and Behavioral Neuroscience. 2005;5(3):339–361. doi: 10.3758/cabn.5.3.339. [DOI] [PubMed] [Google Scholar]
- Jorm A. F. Does old age reduce the risk of anxiety and depression? A review of epidemiological studies across the adult life span. Psychological Medicine. 2000;30(1):11–22. doi: 10.1017/s0033291799001452. [DOI] [PubMed] [Google Scholar]
- Keightley M. L., Winocur G., Burianova H., Hongwanishkul D., Grady C. L. Age effects on social cognition: Faces tell a different story. Psychology and Aging. 2006;21(3):558–572. doi: 10.1037/0882-7974.21.3.558. [DOI] [PubMed] [Google Scholar]
- Kensinger E. A., Corkin S. Effect of negative emotional content on working memory and long-term memory. Emotion. 2003;3(4):378–393. doi: 10.1037/1528-3542.3.4.378. [DOI] [PubMed] [Google Scholar]
- Kensinger E. A., Corkin S. Two routes to emotional memory: Distinct neural processes for valence and arousal. Proceedings of the National Academy of Sciences of the USA. 2004;101(9):3310–3315. doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger E. A., Schacter D. L. Neural processes supporting young and older adults’ emotional memories. Journal of Cognitive Neuroscience. 2008;20(7):1161–1173. doi: 10.1162/jocn.2008.20080. [DOI] [PubMed] [Google Scholar]
- Kilpatrick L., Cahill L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. Neuroimage. 2003;20(4):2091–2099. doi: 10.1016/j.neuroimage.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Hamann S. Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience. 2007;19(5):776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Koch K., Pauly K., Kellermann T., Seiferth N. Y., Reske M., Backes V., et al. Gender differences in the cognitive control of emotion: An fMRI study. Neuropsychologia. 2007;45(12):2744–2754. doi: 10.1016/j.neuropsychologia.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Kring A. M., Gordon A. H. Sex differences in emotion: Expression, experience, and physiology. Journal of Personality and Social Psychology. 1998;74(3):686–703. doi: 10.1037//0022-3514.74.3.686. [DOI] [PubMed] [Google Scholar]
- LaBar K. S., Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience. 2006;7(1):54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- LaBar K. S., Phelps E. A. Arousal-mediated memory consolidation: Role of the medial temporal lobe in humans. Psychological Science. 1998;9(6):490–493. [Google Scholar]
- Lang P. J., Greenwald M. K., Bradley M. M., Hamm A. O. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30(3):261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain: The mysterious underpinnings of emotional life. New York, NY: Simon & Schuster; 1996. [Google Scholar]
- LeDoux J. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux J., Phelps E. A. Emotional networks in the brain. New York, NY: Guilford Press; 2000. [Google Scholar]
- Levesque J., Eugene F., Joanette Y., Paquette V., Mensour B., Beaudoin G., et al. Neural circuitry underlying voluntary suppression of sadness. Biological Psychiatry. 2003;53(6):502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Mackiewicz K. L., Sarinopoulos I., Cleven K. L., Nitschke J. B. The effect of anticipation and the specificity of sex differences for amygdala and hippocampus function in emotional memory. Proceedings of the National Academy of Sciences of the USA. 2006;103(38):14200–14205. doi: 10.1073/pnas.0601648103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitsch H. J. Autobiographical memory: A biocultural relais between subject and environment. European Archives of Psychiatry and Clinical Neuroscience. 2008;258(Suppl. 5):98–103. doi: 10.1007/s00406-008-5021-3. [DOI] [PubMed] [Google Scholar]
- Mather M., Canli T., English T., Whitfield S., Wais P., Ochsner K., et al. Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychological Science. 2004;15(4):259–263. doi: 10.1111/j.0956-7976.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- Mather M., Carstensen L. L. Aging and motivated cognition: The positivity effect in attention and memory. Trends in Cognitive Sciences. 2005;9(10):496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mather M., Knight M. R. Angry faces get noticed quickly: Threat detection is not impaired among older adults. Journal of Gerontology: Psychological Sciences and Social Sciences. 2006;61B(1):54–57. doi: 10.1093/geronb/61.1.p54. [DOI] [PubMed] [Google Scholar]
- Matud M. P. Gender differences in stress and coping styles. Personality and Individual Differences. 2004;37:1401–1415. [Google Scholar]
- Mauss I. B., Cook C. L., Gross J. J. Automatic emotion regulation during anger provocation. Journal of Experimental Social Psychology. 2007;43:698–711. [Google Scholar]
- Mauss I. B., Evers C., Wilhelm F. H., Gross J. J. How to bite your tongue without blowing your top: Implicit evaluation of emotion regulation predicts affective responding to anger provocation. Personality and Social Psychology Bulletin. 2006;32(5):589–602. doi: 10.1177/0146167205283841. [DOI] [PubMed] [Google Scholar]
- Mayberg H. S. Limbic-cortical dysregulation: A proposed model of depression. Journal of Neuropsychiatry and Clinical Neurosciences. 1997;9(3):471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- McGaugh J. L. Memory: A century of consolidation. Science. 2000;257(5451):248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh J. L. Memory consolidation and the amygdala: A systems perspective. Trends in Neurosciences. 2002;25(9):456–461. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- McGaugh J. L. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McRae K., Ochsner K. N., Mauss I. B., Gabrieli J. J. D., Gross J. J. Gender differences in emotion regulation: An fMRI study of cognitive reappraisal. Group Processes and Intergroup Relations. 2008;11:143–163. doi: 10.1177/1368430207088035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickley Steinmetz K. R., Kensinger E. A. The effects of valence and arousal on the neural activity leading to subsequent memory. Psychophysiology. 2009;46(6):1190–1199. doi: 10.1111/j.1469-8986.2009.00868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey R. A., Dolcos F., Petty C. M., Cooper D. A., Hayes J. P., LaBar K. S., et al. The role of trauma-related distractors on neural systems for working memory and emotion processing in post-traumatic stress disorder. Journal of Psychiatric Research. 2009;43(8):809–817. doi: 10.1016/j.jpsychires.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey R. A., Hariri A. R., Gold A., Hauser M. A., Munger H.J., Dolcos F., et al. Serotonin transporter gene polymorphisms and brain function during emotional distraction from cognitive processing in posttraumatic stress disorder. BMC Psychiatry. 2011;11(1):76. doi: 10.1186/1471-244X-11-76. http://www.biomedcentral.com/1471-244X/11/76, doi:10.1186/1471-244X-1-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. S., Ohman A., Dolan R. J. A subcortical pathway to the right amygdala mediating “unseen” fear. Proceedings of the National Academy of Sciences of the USA. 1999;96(A):1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working-with-memory: A component process model based on modules and central systems. Journal of Cognitive Neuroscience. 1992;4(3):257–267. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- Moscovitch M., Nadel L., Winocur G., Gilboa A., Rosenbaum R. S. The cognitive neuroscience of remote episodic, semantic and spatial memory. Current Opinion in Neurobiology. 2006;16(2):179–190. doi: 10.1016/j.conb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Most S. B., Chun M. M., Widders D. M., Zald D. H. Attentional rubbernecking: Cognitive control and personality in emotion-induced blindness. Psychonomic Bulletin and Review. 2005;12(4):654–661. doi: 10.3758/bf03196754. [DOI] [PubMed] [Google Scholar]
- Noh S. R., Isaacowitz D. M. Age differences in the emotional modulation of attention: Effects of emotional cues on attentional engagement and disengagement. Journal of Cognitive Psychology. 2011;23(6):709–722. [Google Scholar]
- Nguyen C., Koeniqs M., Yamada T., Teo S.-H., Cavanaugh J., Tranel D., et al. Affective factors predict economic decision-making. Journal of Cognitive Psychology. 2011;23(6):748–759. doi: 10.1080/20445911.2011.575773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K. N. Are affective events richly recollected or simply familiar? The experience and process of recognizing feelings past. Journal of Experimental Psychology: General. 2000;129(2):242–261. doi: 10.1037//0096-3445.129.2.242. [DOI] [PubMed] [Google Scholar]
- Ochsner K. N., Bunge S. A., Gross J. J., Gabrieli J. D. Rethinking feelings: An FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner K. N., Gross J. J. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner K. N., Ray R. D., Cooper J. C., Robertson E. R., Chopra S., Gabrieli J. D., et al. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ohman A., Flykt A., Esteves F. Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology: General. 2001;130(3):466–478. doi: 10.1037/0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Ohman A., Flykt A., Ludqvist D. Unconscious emotion: Evolutionary perspectives, psychophysiological data and neuropsychological mechanisms. In: Lane R. D., Nadel L., editors. Cognitive neuroscience of emotion. New York, NY: Oxford University Press; 2000. pp. 296–327. [Google Scholar]
- Ohman A., Lundqvist D., Esteves F. The face in the crowd revisited: A threat advantage with schematic stimuli. Journal of Personality and Social Psychology. 2001;80(3):381–396. doi: 10.1037/0022-3514.80.3.381. [DOI] [PubMed] [Google Scholar]
- Paller K. A., Wagner A. D. Observing the transformation of experience into memory. Trends in Cognitive Sciences. 2002;6(2):93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Palomba D., Angrilli A., Mini A. Visual evoked potentials, heart rate responses and memory to emotional pictorial stimuli. International Journal of Psychophysiology. 1997;27(1):55–67. doi: 10.1016/s0167-8760(97)00751-4. [DOI] [PubMed] [Google Scholar]
- Park D. C., Lautenschlager G., Trey H., Davidson N. S., Smith A. D., Smith P. K. Models of visuospatial and verbal memory across the adult life span. Psychology and Aging. 2002;17(2):299–320. [PubMed] [Google Scholar]
- Pelphrey K. A., Morris J. P. Brain mechanisms for interpreting the actions of others from biological-motion cues. Current Directions in Psychological Science. 2006;15(3):136–140. doi: 10.1111/j.0963-7214.2006.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. To what extent are emotional visual stimuli processed without attention and awareness? Current Opinion in Neurobiology. 2005;15(2):188–196. doi: 10.1016/j.conb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews Neuroscience. 2008;9(2):148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Pessoa L., McKenna M., Gutierrez E., Ungerleider L. G. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences of the USA. 2002;99(17):11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K. L., Fitzgerald D. A., Nathan P. J., Tancer M. E. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biological Psychiatry. 2006;59(5):424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Phan K. L., Wager T., Taylor S. F., Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phelps E. A. Human emotion and memory: Interactions of the amygdala and hippocampal complex. Current Opinion in Neurobiology. 2004;14(2):198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Poldrack R. A., Wagner A. D., Prull M. W., Desmond J. E., Glover G. H., Gabrieli J. D. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10(1):15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Proverbio A. M., Zani A., Adorni R. Neural markers of a greater female responsiveness to social stimuli. BMC Neuroscience. 2008;9(1):56. doi: 10.1186/1471-2202-9-56. doi:10.1186/1471-2202-1189-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J., Harrison B. J., Ortiz H., Deus J., Soriano-Mas C., Lopez-Sola M., et al. Influence of the fusiform gyrus on amygdala response to emotional faces in the non-clinical range of social anxiety. Psychological Medicine. 2009;39(7):1177–1187. doi: 10.1017/S003329170800500X. [DOI] [PubMed] [Google Scholar]
- Richards J. M. The cognitive consequences of concealing feelings. Current Directions in Psychological Science. 2004;13(4):131–134. [Google Scholar]
- Richardson M. P., Strange B. A., Dolan R. J. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nature Neuroscience. 2004;7(3):278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- Ritchey M., Dolcos F., Cabeza R. Role of amygdala connectivity in the persistence of emotional memories over time: An event-related fMRI investigation. Cerebral Cortex. 2008;18(11):2494–2504. doi: 10.1093/cercor/bhm262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M., Dolcos F., Eddington K. M., Strauman T. J., Cabeza R. Neural correlates of emotional processing in depression: Changes with cognitive behavioral therapy and predictors of treatment response. Journal of Psychiatric Research. 5(5):577–587. doi: 10.1016/j.jpsychires.2010.09.007. doi:10.1016/j.jpsychires.2010.09.007 (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D. C. A basic-systems approach to autobiographical memory. Current Directions in Psychological Science. 2005;14:79–83. [Google Scholar]
- Rusting C. L. Interactive effects of personality and mood on emotion-congruent memory and judgment. Journal of Personality and Social Psychology. 1999;77(5):1073–1086. doi: 10.1037//0022-3514.77.5.1073. [DOI] [PubMed] [Google Scholar]
- Sanfey A. G., Rilling J. K., Aronson J. A., Nystrom L. E., Cohen J. D. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300(5626):1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Schneider W., Shiffrin R. M. Controlled and automatic human information processing: I. Detection, search, and attention. Psychological Review. 1977;84(1):1–66. [Google Scholar]
- Seidlitz L., Diener E. Sex differences in the recall of affective experiences. Journal of Personality and Social Psychology. 1998;74(1):262–271. doi: 10.1037//0022-3514.74.1.262. [DOI] [PubMed] [Google Scholar]
- Selye H. The nature of stress. Basal Facts. 1985;7(1):3–11. [PubMed] [Google Scholar]
- Sergerie K., Lepage M., Armony J. L. A face to remember: Emotional expression modulates prefrontal activity during memory formation. Neuroimage. 2005;24(2):580–585. doi: 10.1016/j.neuroimage.2004.08.051. [DOI] [PubMed] [Google Scholar]
- Sergerie K., Lepage M., Armony J. L. A process-specific functional dissociation of the amygdala in emotional memory. Journal of Cognitive Neuroscience. 2006;18(8):1359–1367. doi: 10.1162/jocn.2006.18.8.1359. [DOI] [PubMed] [Google Scholar]
- Shafer A., Dolcos F. Functional MRI investigation of the role of processing load, emotional content, distraction time, and individual differences in the impact of distraction on a visual perceptual task. 2010 October. Paper presented at the fourth annual meeting of the Social and Affective Neuroscience Society, Chicago, IL.
- Shafer A., Iordan A., Cabeza R., Dolcos F. Brain imaging investigation of the memory-enhancing effect of emotion. Journal of Visualized Experiments. 2011;51 doi: 10.3791/2433. doi: 10.3791/2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharot T., Yonelinas A. P. Differential time-dependent effects of emotion on recollective experience and memory for contextual information. Cognition. 2008;106(1):538–547. doi: 10.1016/j.cognition.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Shaw K., Lien M.-C., Ruthruff E., Allen P. A. Electrophysiological evidence of emotion perception without central attention. Journal of Cognitive Psychology. 2011;23(6):695–708. [Google Scholar]
- Shaw P., Brierley B., David A. S. A critical period for the impact of amygdala damage on the emotional enhancement of memory? Neurology. 2005;65(2):326–328. doi: 10.1212/01.wnl.0000168867.40688.9b. [DOI] [PubMed] [Google Scholar]
- Simons J. S., Spiers H. J. Prefrontal and medial temporal lobe interactions in long-term memory. Nature Reviews Neuroscience. 2003;4(8):637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Smith E. E., Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283(5408):1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Spinella M. Normative data and a short form of the Barratt Impulsiveness Scale. International Journal of Neuroscience. 2007;117(3):359–368. doi: 10.1080/00207450600588881. [DOI] [PubMed] [Google Scholar]
- St. Jacques P. L., Dolcos F., Cabeza R. Effects of aging on functional connectivity of the amygdala for subsequent memory of negative pictures: A network analysis of fMRI data. Psychological Science. 2009;20(1):74–84. doi: 10.1111/j.1467-9280.2008.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Jacques P. L., Dolcos F., Cabeza R. Effects of aging on functional connectivity of the amygdala during negative evaluation: A network analysis of fMRI data. Neurobiology of Aging. 2010;31:315–327. doi: 10.1016/j.neurobiolaging.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M. B., Simmons A. N., Feinstein J. S., Paulus M. P. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. American Journal of Psychiatry. 2007;164(2):318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Sung K., Dolcos S., Flor-Henry S., Zhou C., Gasior C., Argo J., et al. Brain imaging investigation of the neural correlates of observing virtual social interactions. 2011. Journal of Visualized Experiments 53 doi: 10.3791/2379. [DOI] [PMC free article] [PubMed]
- Sutton T. M., Altarriba J. The automatic activation and perception of emotion in word processing: Evidence from a modified dot probe paradigm. Journal of Cognitive Psychology. 2011;23(6):736–747. [Google Scholar]
- Talmi D., Anderson A. K., Riggs L., Caplan J. B., Moscovitch M. Immediate memory consequences of the effect of emotion on attention to pictures. Learning and Memory. 2008;15(3):172–182. doi: 10.1101/lm.722908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmi D., Schimmack U., Paterson T., Moscovitch M. The role of attention and relatedness in emotionally enhanced memory. Emotion. 2007;7(1):89–102. doi: 10.1037/1528-3542.7.1.89. [DOI] [PubMed] [Google Scholar]
- Tessitore A., Hariri A. R., Fera F., Smith W. G., Das S., Weinberger D. R., et al. Functional changes in the activity of brain regions underlying emotion processing in the elderly. Psychiatry Research. 2005;139(1):9–18. doi: 10.1016/j.pscychresns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Thayer J. F., Rossy L. A., Ruiz-Padial E., Johnsen B. H. Gender differences in the relationship between emotional regulation and depressive symptoms. Cognitive Therapy and Research. 2003;27:349–364. [Google Scholar]
- Timpe J. C., Rowe K. C., Matsui J., Magnotta V. A., Denburg N. L. White matter intensity, as measured by diffusion tensor imaging, distinguishes between impaired and unimpaired older adult decision makers. Journal of Cognitive Psychology. 2011;23(6):760–767. doi: 10.1080/20445911.2011.578065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov A. Evaluating faces on trustworthiness: An extension of systems for recognition of emotions signaling approach/avoidance behaviors. Annals of the New York Academy of Sciences. 2008;1124:208–224. doi: 10.1196/annals.1440.012. [DOI] [PubMed] [Google Scholar]
- Touryan S. R., Johnson M. K., Mitchell K. J., Farb N., Cunningham W. A., Raye C. L. The influence of self-regulatory focus on encoding of, and memory for, emotional words. Social Neuroscience. 2007;2(1):14–27. doi: 10.1080/17470910601046829. [DOI] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychology. 1985;26:1–12. [Google Scholar]
- Urry H. L., van Reekum C. M., Johnstone T., Kalin N. H., Thurow M. E., Schaefer H. S., et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26(16):4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: Neural mechanisms of emotional attention. Trends in Cognitive Sciences. 2005;9(12):585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Armony J. L., Driver J., Dolan R. J. Effects of attention and emotion on face processing in the human brain: An event-related fMRI study. Neuron. 2001;30(3):829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Wang L., Krishnan K. R., Steffens D. C., Potter G. G., Dolcos F., McCarthy G. Depressive state-and disease-related alterations in neural responses to affective and executive challenges in geriatric depression. American Journal of Psychiatry. 2008;165(7):863–871. doi: 10.1176/appi.ajp.2008.07101590. [DOI] [PubMed] [Google Scholar]
- Wang L., LaBar K. S., Smoski M., Rosenthal M. Z., Dolcos F., Lynch T. R., et al. Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Research. 2008;163(2):143–155. doi: 10.1016/j.pscychresns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen P. J., Rauch S. L., Etcoff N. L., McInerney S. C., Lee M. B., Jenike M. A. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18(1):411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winecoff A., Labar K. S., Madden D. J., Cabeza R., Huettel S. A. Cognitive and neural contributors to emotion regulation in aging. Social Cognitive and Affective Neuroscience. 2011;6(2):165–176. doi: 10.1093/scan/nsq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G., Dolcos S., Denkova E., Morey R. A., Wang L., McCarthy G., Dolcos F. Brain imaging investigation of the impairing effect of emotion on cognition. Journal of Visualized Experiments, doi: 10.3791/2434 (in press) [DOI] [PMC free article] [PubMed]
- Zeki S. The neurobiology of love. FEBS [Federation of European Biochemical Societies] Letters. 2007;581(14):2575–2579. doi: 10.1016/j.febslet.2007.03.094. [DOI] [PubMed] [Google Scholar]


