Abstract
Context:
Nodular goiter is common worldwide, but there is still debate over the medical treatment.
Objective:
The objective of the study was the measurement of the effect of a treatment with (nonsuppressive) T4, iodine, or a combination of both compared with placebo on volume of thyroid nodules and thyroid.
Design:
This was a multicenter, randomized, double-blind trial in patients with nodular goiter in Germany [LISA (Levothyroxin und Iodid in der Strumatherapie Als Mono-oder Kombinationstherapie) trial].
Setting:
The study was conducted in outpatient clinics in university hospitals and regional hospitals and private practices.
Participants:
One thousand twenty-four consecutively screened and centrally randomized euthyroid patients aged 18–65 yr with one or more thyroid nodules (minimal diameter 10 mm) participated in the study.
Intervention:
Intervention included placebo, iodine (I), T4, or T4+I for 1 yr. T4 doses were adapted for a TSH target range of 0.2–0.8 mU/liter.
Outcome Measures:
The primary end point was percent volume reduction of all nodules measured by ultrasound, and the main secondary end point was a change in goiter volume.
Results:
Nodule volume reductions were −17.3% [95% confidence interval (CI) −24.8/−9.0%, P < 0.001] in the T4+I group, −7.3% (95% CI −15.0/+1.2%, P = 0.201) in the T4 group, and −4.0% (95% CI −11.4/+4.2%, P = 0.328) in the I group as compared with placebo. In direct comparison, the T4+I therapy was significantly superior to T4 (P = 0.018) or I (P = 0.003). Thyroid volume reductions were −7.9% (95% CI −11.8/−3.9%, P < 0.001), −5.2% (95% CI −8.7/−1.6%, P = 0.024) and −2.5% (95% CI −6.2/+1.4%, P = 0.207), respectively. The T4+I therapy was significantly superior to I (P = 0.034) but not to T4 (P = 0.190).
Conclusion:
In a region with a sufficient iodine supply, a 1-yr therapy with a combination of I and T4 with incomplete suppression of thyrotropin reduced thyroid nodule volume further than either component alone or placebo.
Treatment of uni- or multinodular goiter and/or thyroid nodules remains a significant challenge worldwide. A representative study in Germany determined the prevalence of thyroid nodules greater than 1 cm and goiters as 20 and 36%, respectively, for an age range 20–79 yr (corresponding approximately to 17 million inhabitants with thyroid nodules) (1). Ultrasound screening studies have revealed prevalences of goiter in Denmark of 23% (2) and 30% (3), whereas figures reported for the prevalence of thyroid nodules evaluated by ultrasound across Europe are 31% in Finland (4), 34.7% in France (5), and 33.1% in Italy (6). Corresponding data for North America are scarce; one publication (7) reported thyroid nodules in 67 of 100 normal subjects.
There are insufficient data on medical treatment of nodular goiter because the number of patients in the reported trials (8–18) has been consistently too low to demonstrate even moderate or clinically relevant effects on thyroid nodule volume [maximum number of patients: 123 (18)]. In addition, half of the previous studies on the efficacy of T4 treatment have used a fully TSH-suppressive regimen (TSH < 0.1) with high T4 doses (8–11, 13, 14), which is no longer acceptable because of frequent side effects. Other studies (12, 15–18) aimed to achieve a partially suppressed TSH value (upper limit 0.4). For these treatments, three metaanalyses (19–21) have shown a slight benefit with T4 therapy (vs. placebo).
Koc et al. (22) compared the effect of full TSH suppression (<0.1) with partial TSH suppression (0.4–0.6) and placebo, and they found a similar reduction of nodule volume in both treatment arms. Furthermore, there has been only one placebo-controlled trial (number of patients: 70) evaluating the therapy of nodular goiter with iodine (I) (12).
We therefore performed a national multicenter, prospective, double-blind, randomized, placebo-controlled assessment of the efficacy of T4, I, and T4+I for the therapy of nodular goiters, with a placebo (P) as control, for a period of 12 months [LISA (Levothyroxin und Iodid in der Strumatherapie Als Mono-oder Kombinationstherapie) trial] (23). In this study T4 doses were adapted to a TSH target range of 0.2–0.8 mU/liter under T4 and T4+I therapy.
Materials and Methods
Study objectives
The objective of this study was to evaluate whether the effect of a T4+I combination is superior to either of the monotherapies (T4, I, or P) in a 12-month treatment of nodular goiter.
Primary objective
The primary objective was to compare the change in total volume of all nodules after 12 months of T4+I treatment with the change after 12 month of each of the reference treatments (one of the two active controls or placebo).
Secondary objectives
The secondary objective was to compare the change in goiter volume, number, and echogenicity of nodules after T4+I treatment with that after each reference treatment after 12 months therapy.
Sample size
The number of patients to be included in the study was determined based on previous figures collected from routine data of one of the centers. A change of 10% in total nodule volume corresponding to 0.06 in log total nodule volume was judged to be potentially relevant. Based on practice data, a sd of 0.2 log units was assumed as a rather conservative estimate of the variability of before and after differences in log thyroid volumes determined by the same physician with the same equipment. It was assumed that log total nodule volumes would reveal a similar measurement error. Taking into consideration a dropout rate of 15%, a number of 250 patients per group was fixed to be randomized to receive 212 evaluable patients per group.
Study centers and recruitment of patients:
Sixty German study centers participated (Supplemental Appendix I, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org), with an even geographical distribution across the country. Most of the physicians were members of the German Endocrine Society, and all experienced thyroidologists. The research protocol was approved by the ethic committees of the medical chambers of the concerned federal states; all participants gave written informed consent.
All (consecutively investigated) patients with nodular goiter, who were seen by the participating physicians and were expected to meet the inclusion and exclusion criteria after their previous medical history were screened.
Inclusion and exclusion criteria
Inclusion criteria included the following: Caucasian race; aged 18–65 yr; normal TSH value (range between 0.6 and 3.0 mU/liter); thyroid nodules in a normal-sized or enlarged thyroid; at least one nodule (≤20% of volume with cystic change) with 1.0 cm diameter or greater; for nodules greater than 1.0 cm, the diagnosis must be performed according to the guidelines for diagnostic standards of thyroid disorders to exclude malignancy (24).
Exclusion criteria included the following: thyroid therapy within the last 3 yr; known focal or diffuse autonomous thyroid structure; presence of thyroid cysts; contraindication to iodine; concomitant treatment with iodine-containing medication (i.e. amiodarone) or use of iodine-containing contrast medium within the last 6 wk; presence of thyroid peroxidase antibodies (maximum twice the normal value) or known autoimmune thyreopathy; symptomatic coronary heart disease; endocrine orbitopathy; former radioiodine therapy or surgery; any acute illness or allergy; known pregnancy at time of screening; dermatitis herpetiformis; or pathological laboratory results.
Important changes to inclusion criteria after trial commencements
Age limits changed from 18–55 to 18–65 yr; initial thyroid volume changed from enlarged to normal or enlarged [Amendment 2 (04-09-29)].
Randomization
Centerwise randomization sequences with variable block lengths were generated by the study statistician and sent to the pharmacy that produced unlabeled coded medication packages for the total follow-up period with sufficient medication for the titration process to guarantee concealment and blindness. However, because only licensed drugs were used, the patient but not the physician could have found out what group she/he is in by visiting a pharmacy and comparing his pills with the available drugs.
Visits
Five visits were performed: screening (V1), randomization (V2), and three follow-up visits under therapy after 3 (V3), 6 (V4), and 12 months (V5). Study procedures are listed in Supplemental Table 1.
At each visit sonography was performed with a 7.5- or 10-MHz transducer. Goiter and nodule volumes were calculated by the published method of Brunn et al. (25). All ultrasound images with measurements were photographed and archived as source data.
TSH was measured at V2, V3, V4, and V5 in a central laboratory (luminescence assay, Immulite 2000; Siemens, Erlangen, Germany) under regular external quality control.
Thyroid peroxidase antibodies and clinical laboratory values (at V1 and V5) were measured in the laboratories of the study centers.
Iodine concentration of spot urine was measured centrally at V2 and V5 using a sensitive and specific HPLC method (26).
Medication and adaption of T4 dose
The patients were randomized centrally and assigned at V2 to one of the following four parallel groups: P, placebo; I, 150 μg potassium iodide per day; T4, 75 μg levothyroxine per day; T4+I, combination of 75 μg levothyroxine and 150 μg potassium iodide per day.
At V2 through V5, blood for measurement of TSH was sent to the central laboratory. At V3 and V4, physicians and patients were advised to continue the current medication. After determination of TSH at V3, the central laboratory communicated the results to the Clinical Research Organization and proposed a dose adaptation in the T4 and T4+I arms (if TSH was out of aimed range) or the continuation of the dose (if TSH was in range) by identification of the next-numbered drug package to be used. In the T4 and T4+I arms, if TSH was less than 0.2 mU/liter, the T4 dose was reduced to 50 μg/d, whereas if TSH was greater than 0.8 mU/liter, the T4 dose was increased to 100 μg/d. In the I and P arm, the medication was not changed, but an adaptation was simulated to keep investigators and patients blind. At each follow-up visit, unused medication was collected and counted to estimate patient compliance.
In 38 patients, who stopped medication because of serious adverse events (e.g. hospitalization because of accidents, gynecological operations, infections, etc.), no causal relationship to the medication was assumed by the investigators.
Possible drug-related serious adverse events
Two patients developed atrial fibrillations: both belonged to the P group.
Quality controls
Monitoring
In accordance with International Conference on Harmonization Good Clinical Practice guidelines, the study was monitored by the CRO in regular intervals; the monitors had direct access to the investigator's source documentation to verify consistency and accuracy of the data recorded in the case record forms. The written ultrasound report of the thyroidologist was taken as source documentation.
Sonography
Volumetry by ultrasound is the primary objective of the trial; therefore, two thyroid phantoms with two lobes and six nodules of unknown volume (27) were shipped to the study centers, which were asked to measure the volumes in a blind manner. Of these measurements, total nodular volume per test lobe and lobe volume were calculated using the same formula as they were used for the primary and secondary outcome of the trial. The resulting estimates of the coefficient of variation were 10.9% [95% confidence interval (CI) 7.1–17.0%] or 11.1% (95% CI 6.9–18.2%) for differences of repeated measurements of total nodular volume or thyroid volume, respectively, by the same physician with the same equipment in the same thyroid phantom.
The TSH luminescence assay was regularly controlled by external quality controls.
Statistics
Baseline determinations were compared between randomization groups by one-way ANOVA or χ2 tests. Total nodule volumes were log transformed. The primary outcome was calculated as the difference between the last observation within the 12-month follow-up period and the baseline observation of the same patient. This approach was chosen to preserve the intention-to-treat approach. It is equivalent to change from baseline to 12-month determination in which missing values were conservatively imputed by the last observation carried forward as it was defined in the study protocol. The primary analysis then consisted of the calculation of six pairwise t tests for direct comparison of treatment group. P values were adjusted for multiplicity of comparisons by applying the closure test procedure (28) to keep an experiment-wise type I error rate of 5%. A justification of this approach is given in the Supplemental Appendix II. In an additional post hoc analysis, a two-way ANOVA with the factors iodine and T4 was performed in the total analysis population to study the interaction of the two components for a better understanding of the effect of the combined therapy. We present the results by reporting adjusted P values along with raw (unadjusted) P values and estimates of percent change of total nodule volumes with unadjusted 95% CI for the illustration of effect sizes. Furthermore, sensitivity analyses were performed to study whether methodological decisions were crucial for the result of the trial (reported in Supplemental Appendix II and III).
The secondary analysis on thyroid volume or number of nodules was performed with the same test strategy as the primary analysis. Thyroid volumes were log transformed. Echogenity distributions were compared by χ2 tests.
The association of total nodule volume changes, thyroid volume changes, and treatment effects with variables suggested to be influential on these outcomes was studied with multilevel models. We explored the involvement of the following variable: age, gender, family history, body mass index, baseline TSH greater than 1.0 mU/liter, baseline urine I-excretion I less than 100 μg/liter, baseline thyroid volume greater than 25 ml for men and greater than 18 ml for women, baseline nodular volume greater than 2 ml, the presence of multiple nodules at baseline, and less than 20% cystic largest nodules. Details of the statistical methodology are reported in Supplemental Appendix II.
Results
Analysis populations
One thousand two hundred forty-five consecutive patients with nodular goiter were screened. A total of 1020 patients in 60 centers were randomized (patient flow: Supplemental Fig. 1). Of the randomized patients, seven patients did not receive any dose of the study medication. In another 47 patients, the quantification of nodule sizes at screening or at baseline was insufficient or failed to demonstrate nodules of the required minimum diameter. These patients could not be used for analysis because no reliable reference value was available to determine the changes. Before breaking the blind, a data quality review showed a clustering of out-of-range values of the primary end point in two centers. Because an additional monitoring visit revealed an only fragmentary photographic documentation of sonography results and did not dispel doubts on the validity of measurements, the data of all 172 patients, recruited at these centers were not used for the analysis, after a blind decision of the steering committee. Thus, the primary analysis was performed in the remaining 794 patients. However, a sensitivity analysis in all 1013 patients who received at least one dose of study medication was performed to study whether the inclusion of these patients would have changed the results. For exploration of potential predictors of outcome or effect modification, a complete case analysis population of 600 patients was used.
Patients
Patient characteristics are listed in Table 1: No significant differences were found with respect to demography, thyroid or other diseases, or initial iodine excretion. However, there was a nonsignificant trend toward higher initial iodine excretion in the iodine group and to smaller nodule volumes in the T4 group.
Table 1.
Patient characteristics of the LISA analysis population according to randomization groups, presented as absolute frequencies and percentages or arithmetic means with symmetric 95% CI
| Placebo (n = 199) | Iodine (n = 198) | T4 (n = 206) | T4+Iodine (n = 191) | P value | |
|---|---|---|---|---|---|
| Male gender | 52 (26.1%) | 56 (28.3%) | 59 (28.6%) | 61 (31.9%) | 0.651 |
| Age | 46.1 [44.6–47.5] | 47.0 [45.6–48.4] | 47.1 [45.7–48.5] | 47.4 [46.1–48.8] | 0.570 |
| Family history | 87 (43.7%) | 74 (37.4%) | 84 (40.8%) | 68 (35.6%) | 0.360 |
| Concomitant disease | 120 (60.3%) | 139 (70.2%) | 128 (62.1%) | 120 (62.8%) | 0.180 |
| BMI | 27.0 [26.3–27.8] | 27.3 [26.6–28.1] | 27.3 [26.6–27.9] | 27.0 [26.2–27.7] | 0.871 |
| Thyroid volume (ml) | 18.6 [17.2–20.2] | 18.2 [16.7–19.8] | 18.9 [17.4–20.5] | 18.8 [17.2–20.5] | 0.932 |
| Volume of nodules (ml) | 1.67 [1.43–1.94] | 1.72 [1.49–1.98] | 1.47 [1.26–1.72] | 1.96 [1.67–2.30] | 0.075 |
| Number of nodules | 1.54 [1.42–1.67] | 1.62 [1.51–1.74] | 1.67 [1.55–1.80] | 1.64 [1.50–1.78] | 0.512 |
| Largest nodule <20% cystic | 14.1% | 17.7% | 20.9% | 22.5% | 0.145 |
| TSH (mU/liter) | 1.11 [1.03–1.19] | 1.09 [1.02–1.16] | 1.13 [1.05–1.22] | 1.13 [1.06–1.21] | 0.810 |
| Iodine excretion (μg/liter) | 49.7 [43.0–57.6] | 59.5 [51.1–69.4] | 52.2 [46.4–58.8] | 53.7 [47.5–60.8] | 0.062 |
P values relate to one-way ANOVA (F tests) or χ2 tests. BMI, Body mass index.
T4 dose adaptation and change in TSH levels
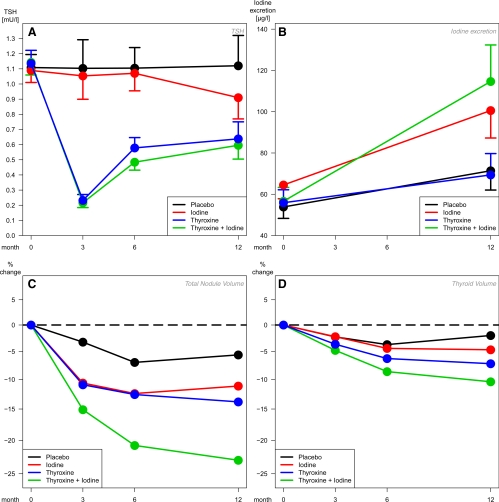
On average, the target ranges of T4 and T4+I treatment as defined in the study protocol were met after dose adaptation (Fig. 1A). Supplemental Table 2 shows the percentages of patients below, within, or above the TSH target range (0.2–0.8 mU/liter) according to visits and treatment.
Fig. 1.
Changes of TSH (A) and iodine (B) levels and corresponding percent changes from baseline of nodule (C) and thyroid (D) volumes in different treatment groups. Marginal means from longitudinal mixed models, with 95% CI (A and B) or presented as percent change from group-specific baseline mean (C and D) Intention-to-treat population, missing value imputation by direct maximum likelihood.
Change in iodine excretion
Iodine excretion increased significantly over the complete study period in all four treatment groups (Fig. 1B and Table 2). The increase was largest in the two groups with iodine medication, and the differences of I to T4 and T4+I to P and T4 were significant.
Table 2.
Change of iodine excretion, nodule and thyroid volumes, and number of nodules from baseline to end of follow-up within 1 yr by randomization group
| Change from baseline (%) | As compared with |
||||||
|---|---|---|---|---|---|---|---|
| Placebo (basis), % | PrawPadjusted | I (basis), % | PrawPadjusted | T4 (basis), % | PrawPadjusted | ||
| Iodine excretion | |||||||
| P | 34.53 | ||||||
| [14.14; 54.92] | |||||||
| I | 63.84 | 29.32 | 0.069 | ||||
| [39.49; 88.20] | [−2.3; 60.93] | 0.069 | |||||
| T4 | 27.16 | −7.37 | 0.594 | −36.68 | 0.017 | ||
| [9.03; 45.28] | [−34.50; 19.77] | 0.594 | [−66.79; −6.58] | 0.038 | |||
| I+T4 | 88.06 | 53.54 | <0.001 | 24.22 | 0.143 | 60.90 | <0.001 |
| [66.55; 109.57] | [24.04; 83.03] | 0.004 | [−8.27; 56.71] | 0.143 | [33.04; 88.76] | <0.001 | |
| Nodule volume | |||||||
| P | −5.2 | ||||||
| [−11.0; 0.9] | |||||||
| I | −9.0 | −3.96 | 0.328 | ||||
| [−13.6; −4.1] | [−11.4; 4.16] | 0.328 | |||||
| T4 | −12.1 | −7.29 | 0.090 | −3.46 | 0.392 | ||
| [−17.4; −6.6] | [−15.04; 1.18] | 0.201 | [−10.97; 4.67] | 0.392 | |||
| I+T4 | −21.6 | −17.26 | <0.001 | −13.85 | 0.001 | −10.76 | 0.018 |
| [−27.1; −15.7] | [−24.75; −9.03] | <0.001 | [−21.14; −5.88] | 0.003 | [−18.79; −1.94] | 0.018 | |
| Thyroid volume | |||||||
| P | −1.9 | ||||||
| [−4.4; 0.6] | |||||||
| I | −4.4 | −2.47 | 0.207 | ||||
| [−7.2; −1.5] | [−6.19; 1.39] | 0.207 | |||||
| T4 | −7.1 | −5.22 | 0.005 | −2.82 | 0.162 | ||
| [−9.5; −4.5] | [−8.67; −1.63] | 0.024 | [−6.63; 1.15] | 0.162 | |||
| I+T4 | −9.7 | −7.94 | <0.001 | −5.61 | 0.013 | −2.87 | 0.190 |
| [−12.8; −6.5] | [−11.78; −3.93] | <0.001 | [−9.80; −1.22] | 0.034 | [−7.02; 1.46] | 0.190 | |
| Number of nodules | |||||||
| P | 0.070 | ||||||
| [−0.006; 0.147] | |||||||
| I | 0.035 | 0.035 | 0.488 | ||||
| [−0.028; 0.099] | [−0.134; 0.064] | 0.488 | |||||
| T4 | −0.005 | −0.075 | 0.200 | −0.040 | 0.463 | ||
| [−0.091; 0.081] | [−0.190; 0.040] | 0.386 | [−0.148; 0.067] | 0.560 | |||
| I+T4 | −0.026 | −0.097 | 0.103 | −0.062 | 0.264 | −0.021 | 0.734 |
| [−0.115; 0.062] | [−0.213; 0.020] | 0.338 | [−0.170; 0.047] | 0.560 | [−0.145; 0.102] | 0.734 | |
Analysis is intention to treat; missing values are imputed by last observation carried forward. Pairwise t tests with nominal (raw) P values (upper entry) followed by P values resulting from a closure test that corrects for the multiplicity of comparisons (lower entry). Multiplicity corrected significant results are in bold. Comparisonwise 95% confidence intervals are in brackets.
Primary objective: change in total nodular volume
Figure 2 (black lines) and Table 2 show the average reductions of the total nodular volume. Significant reductions were observed in the three active treatment groups. However, a nonsignificant trend toward lower values was even seen in P patients. Compared with the initial volume at V2 up to V5, mean volumes were reduced to −5,2% under P, −9.0% under I, −12.1% under T4, and −21.6% under T4+I. Compared with P, nodular volumes reductions were −17.3% (95% CI −24.8%/−9.0%, multiplicity adjusted P < 0.001) in the T4+I group, −7.3% (95% CI −15.0%/+1.2%, P = 0.201) in theT4 group, −4.0% (95% CI −11.4%/+4.2%, P = 0.328) in the I group. In direct comparison, the T4+I therapy was significantly superior to T4 (P = 0.018) or I (P = 0.003). Figure 1C shows the development of the average decrease from visit to visit. The nodular volume reduction in the T4+I group developed gradually and did not come to an end during the observation period. Individual changes were remarkably heterogenous (Fig. 3A). As a result of this high variability, between 42.2% (P) and 26.7% (T4+I) of patients revealed increases in total nodular volumes, although the average volumes decreased (Supplemental Table 3).
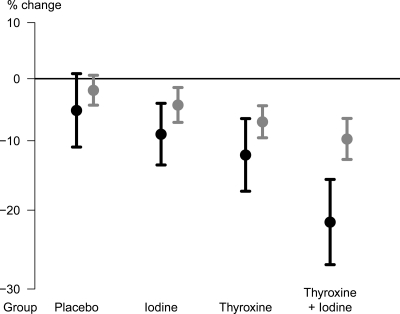
Fig. 2.
Percent change in nodule volume (black lines) and thyroid volume (gray lines) from baseline to end of follow-up within 1 yr by randomization group, with 95% CI.
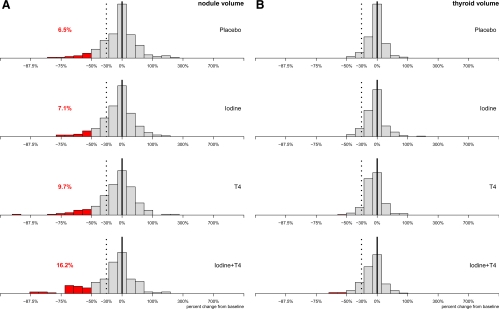
Fig. 3.
Frequency histograms of individual percent changes from baseline to end of follow-up of total nodule volumes (A) and thyroid volumes (B) by randomization group. The scale is logarithmic for optical symmetry of increases and decreases of comparable size. Reductions of more than 50% in nodular volume are marked red and given as percentages. In both panels, reductions of 30% are marked by a broken line.
Additionally the courses of all (1433) individual nodules were evaluated. Decreases of more than 50% were seen in 8.5/9.5/7.6/11.3% of the nodules evaluated in the P/I/T4/T4+I group, respectively.
Secondary objective: change in thyroid volume and number or echogenicity of nodules
Figure 2 (gray lines) and Table 2 show the average reductions of the thyroid volume. As with total nodule volume, significant reductions compared with V2 were observed in the three active treatment groups but to a lesser extent. Reductions were significantly more pronounced with T4+I than with P or I and with T4 than with P. The thyroid volume reduction with T4+I and T4 developed gradually in a similar manner to the total nodule volume reduction but was less pronounced (Fig. 1D). Individual changes in thyroid volumes were heterogenous but less variable than the changes in total nodular volumes (Fig. 3B). Nevertheless, in some patients thyroid volumes increased over the follow-up period.
Table 2 shows the change in number of nodules. The number increased with P or I and decreased with T4 and T4+I. However, changes from baseline or differences between groups were not significant. No group differences were found with respect to echogenicity.
Association of baseline determinations with outcome or treatment effects
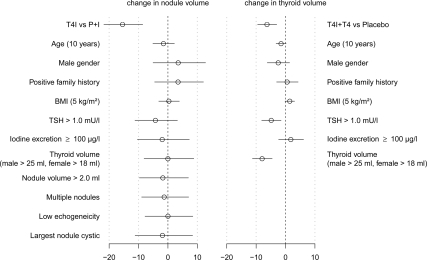
None of the covariates studied was predictive of total nodular volume reduction (Fig. 4, left panel) or modified the effect of T4+I therapy compared with placebo or iodine. Thyroid volume reduction (Fig. 4, right panel) was more pronounced in patients with large thyroids, i.e. baseline thyroid volume greater than 25 ml for men and greater than 18 ml for women (change to baseline of large thyroid compared with normal: −8.4%, 95% CI −11.4/−5.4%, P < 0.001), with TSH greater than 1.0 (change to baseline TSH > 1.0 vs. TSH ≤ 1.0: −5.3%, 95% CI −8.1/−2.5%, P < 0.001) and in older patients (change to baseline −1.8% per 10 yr of age, 95% CI −3.2 /−0.3%, P = 0.016). In patients with a larger body mass index, the reductions were smaller (change to baseline +1.6% per 5 kg/m2, 95% CI +0.2/+3.0%, P = 0.023). These effects were independent of treatment. No covariate that modified the effect of T4 or T4+I therapy compared with placebo was found.
Fig. 4.
Forest plot of the results of two multilevel regression models with identical covariates but different outcomes. BMI, Body mass index.
Discussion
This is the first sufficiently powered trial on the medical treatment of nodular goiter with nonsuppressive levothyroxine and/or iodine compared with placebo. It demonstrates that in formerly iodine-deficient Germany, the volume of benign nodules greater than 1 cm in diameter is significantly more reduced by only partly TSH-suppressive T4 doses in combination with iodine than by T4 alone, iodine alone or placebo (Table 2 and Fig. 2). The average decrease of volumes is smooth and continues to the end of the observation period (Fig. 1C). This could be a result of the low proliferation rate of thyroid epithelial cells or the slow growth of thyroid nodules (29). No significant advantage over placebo could be established for any of the other treatments.
Individual changes of nodular volume were remarkably heterogenous (Fig. 3A). Although the coefficient of variation of thyroid volume changes was about the same as that anticipated on the basis of previous data, the coefficient of variation of nodular volume changes was more than double. Even with T4+I, nodular volume increased in 25% of patients, whereas 16% showed volume decreases greater than 50%, also suggesting heterogeneity of responses and/or pathophysiology (Supplemental Table 3). The intraobserver variability of thyroid nodule volume measurements observed in parallel phantom measurements was high (11%), as previously demonstrated by Brauer et al. (30), thus providing good reason to investigate much higher numbers of patients per group than those enrolled in any previous (lesser powered) trials.
Of note for the design of future studies, several assumptions of our sample size calculation that were based on our experience with total thyroid volumes did not apply to nodular volumes. Due to the unexpectedly wide heterogeneity in nodule volume changes and the reduction of sample size through the exclusion of two centers, the trial was in fact underpowered to detect the assumed 10% change. However, due to the larger-than-expected mean reductions in the nodule volume (exceeding the lowering of thyroid volume reduction by more than twice), the study was sufficiently powered for both end points.
No simultaneous analysis of thyroid nodule and thyroid volume changes in response to treatment has been published to date. It is remarkable that the treatment effects for thyroid nodules, which are most likely caused by unknown somatic mutations inducing increased proliferation (31), are more pronounced than the treatment effects on thyroid volume (Fig. 2).
It is worth noting that several other factors discussed in the literature (such as family history, body mass index, initial thyroid or nodule volume, and multiplicity of nodules) were found not to be associated with the extent of nodule volume reduction (Fig. 4). Furthermore, no subgroup could be identified with larger or smaller treatment effects on nodular volume. In a post hoc analysis, we further studied whether changes in TSH levels and/or iodine excretion were mediators that could explain the heterogeneity in nodular volume changes or the differential treatment effect of T4+I. The analysis was inconclusive (data not shown). Thus, the mechanism underlying our findings could not be explained on statistical grounds.
One possible explanation is offered by the finding that the patients with thyroid nodules included in our trial were clearly iodine deficient (mean urinary iodine excretion 49.7–59.5 μg/liter, Table 1), whereas the recently surveyed German population met the World Health Organization criteria for sufficient iodine supply overall with a mean urinary iodine excretion of 132 μg/liter (32). This is an important argument for the iodine component of the T4+I treatment. Moreover, similar differences in iodine excretion may also be relevant to the etiology of thyroid nodules in other iodine-sufficient countries (33). Given the mean urinary iodine concentration of 164 μg/d determined from the National Health and Nutrition Examination Survey database (34) and assuming similar differences in urinary iodine concentration between the general population and patients with thyroid nodules in North America, patients with thyroid nodules in North America could also be largely iodine deficient [8.8 ± 0.4% of the National Health and Nutrition Examination Survey population were found to have an iodine excretion lower than 50 μg/d (and 28.2 ± 1.2% lower than 100 μg/d) in 2007–2008 (34)]. It would then be important to know whether in areas with better overall iodine supply than Germany, patients with thyroid nodules also constitute a subgroup with lower urinary iodine excretion and probably less efficient iodine conservation mechanisms, arising from some genetic predisposition.
Iodine medication normalized the iodine excretion in the two iodine-taking groups. Iodine excretion also increased in the T4 and placebo groups, probably due to a greater awareness among the patients of the issue of nutritional iodine intake.
TSH adaptation worked in the two T4 groups as intended (Fig. 1A), suggesting the feasibility of subtle TSH-decreasing treatment with low T4 doses (Supplemental Table 2). Together with the similar findings of Koc et al. (22), this is important because it suggests that the complete suppression of TSH by high thyroid hormone doses, which owing to their various side effects are no longer acceptable, is not necessary.
Taken together, the results of this large trial on the medical treatment of nodular goiter will provide the clinician with additional valuable arguments for or against a medical therapy of the individual patient with a nodular goiter. Although limited by its 1 yr duration and lack of data regarding changes of the patient's nodule-related symptoms, our study together with previous data (12) suggests to treat patients with thyroid nodules with T4+I to limit nodule size and to prevent further thyroid nodules. Whether the finding that only the (nonsuppressive) T4+I combination resulted in a significant thyroid nodule volume decrease can be explained by the additional iodine intake alone or an unknown genuine T4-I interaction will need further research.
Supplementary Material
Acknowledgments
We thank Drs. AbdelQader, Adler, Bell, Binder, Bogner, Böhm, Bolsun, Derwahl, Diederich, Dietz, Dohmen, Dröge, Eberhardt, Eilles, Farahati, Feldkamp, Fiesselmann, Finke, Fondis, Fuchs-Hammoser, Glatzel, Graf, Grau, Grosskopf, Grünwald, Monika Grussendorf, Hennig, Hollatz, Ivansevic, Jacopian, Kanitz, Klein, Klemenz, Körber, Laue-Savic, Mann, Michael, Mönig, Nawroth, Palitzsch, Palmai, Pietrek, Reschke, Rövenich, Ruf, Schäfer, Schirrmeister, Schumacher, Schumm-Dräger, Stamm, Ulmer, Vormann, Walter, Wandel, Weber, and Weller for their help in the recruitment and follow-up of the patients. We are also grateful to Dr. R. Vaupel, Berlin, for organizational assistance and to K. Balzer, G. Schön, and Dr. A. Treszl for biometrical support.
This work was supported by Sanofi-Aventis, Berlin, Germany.
Disclosure Summary: M.G. has received fees as principal investigator and for lectures from Sanofi-Aventis. C.R. has nothing to disclose. R.P. has received lecture fees from Sanofi-Aventis and Merck (Germany). K.W. has received fees from Sanofi-Aventis for doing the statistics.
Footnotes
- CI
- Confidence interval
- I
- iodine
- P
- placebo.
References
- 1. Völzke H, Lüdemann J, Robinson DM, Spieker KW, Schwahn C, Kramer A, John U, Meng W. 2003. The prevalence of undiagnosed thyroid disorders in a previously iodine-deficient area. Thyroid 13:803–810 [DOI] [PubMed] [Google Scholar]
- 2. Knudsen N, Bülow I, Jorgensen T, Laurberg P, Ovesen L, Perrild H. 2000. Goitre prevalence and thyroid abnormalities at ultrasonography: a comparative epidemiological study in two regions with slightly different iodine status. Clin Endocrinol (Oxf) 53:479–485 [DOI] [PubMed] [Google Scholar]
- 3. Laurberg P, Jørgensen T, Perrild H, Ovesen L, Knudsen N, Pedersen IB, Rasmussen LB, Carlé A, Vejbjerg P. 2006. The Danish investigation on iodine intake and thyroid disease, DanThyr: status and perspectives. Eur J Endocrinol 155:219–228 [DOI] [PubMed] [Google Scholar]
- 4. Brander A, Viikinkoski P, Nickels J, Kivisaari L. 1989. Thyroid gland: US screening in middle-aged women with no previous thyroid disease. Radiology 173:507–510 [DOI] [PubMed] [Google Scholar]
- 5. Bruneton JN, Balu-Maestro C, Marcy PY, Melia P, Mourou MY. 1994. Very high frequency (13 MHz) ultrasonographic examination of the normal neck: detection of normal lymph nodes and thyroid nodules. J Ultrasound Med 13:87–90 [DOI] [PubMed] [Google Scholar]
- 6. Bartolotta TV, Midiri M, Runza G, Galia M, Taibbi A, Damiani L, Palermo Patera G, Lagalla R. 2006. Incidentally discovered thyroid nodules: incidence, and greyscale and colour Doppler pattern in an adult population screened by real-time compound spatial sonography. Radiol Med 111:989–998 [DOI] [PubMed] [Google Scholar]
- 7. Ezzat S, Sarti DA, Cain DR, Braunstein GD. 1994. Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med 154:1838–1840 [DOI] [PubMed] [Google Scholar]
- 8. Gharib H, James EM, Charboneau JW, Naessens JM, Offord KP, Gorman CA. 1987. Suppressive therapy with levothyroxine for solitary thyroid nodules. A double-blind controlled clinical study. N Engl J Med 317:70–75 [DOI] [PubMed] [Google Scholar]
- 9. Cheung PS, Lee JM, Boey JH. 1989. Thyroxine suppressive therapy of benign solitary thyroid nodules: a prospective randomized study. World J Surg 13:818–821; discussion 822 [DOI] [PubMed] [Google Scholar]
- 10. Reverter JL, Lucas A, Salinas I, Audi L, Foz M, Sanmarti A. 1992. Suppressive therapy with levothyroxine for solitary thyroid nodules. Clin Endocrinol (Oxf) 36:25–28 [DOI] [PubMed] [Google Scholar]
- 11. Papini E, Bacci V, Panunzi C, Pacella CM, Fabbrini R, Bizzarri G, Petrucci L, Giammarco V, La Medica P, Masala M. 1993. A prospective randomized trial of levothyroxine suppressive therapy for solitary thyroid nodules. Clin Endocrinol (Oxf) 38:507–513 [DOI] [PubMed] [Google Scholar]
- 12. La Rosa GL, Lupo L, Giuffrida D, Gullo D, Vigneri R, Belfiore A. 1995. Levothyroxine and potassium iodide are both effective in treating benign solitary solid cold nodules of the thyroid. Ann Intern Med 122:1–8 [DOI] [PubMed] [Google Scholar]
- 13. Mainini E, Martinelli I, Morandi G, Villa S, Stefani I, Mazzi C. 1995. Levothyroxine suppressive therapy for solitary thyroid nodule. J Endocrinol Invest 18:796–799 [DOI] [PubMed] [Google Scholar]
- 14. Lima N, Knobel M, Cavaliere H, Sztejnsznajd C, Tomimori E, Medeiros-Neto G. 1997. Levothyroxine suppressive therapy is partially effective in treating patients with benign, solid thyroid nodules and multinodular goiters. Thyroid 7:691–697 [DOI] [PubMed] [Google Scholar]
- 15. Papini E, Petrucci L, Guglielmi R, Panunzi C, Rinaldi R, Bacci V, Crescenzi A, Nardi F, Fabbrini R, Pacella CM. 1998. Long-term changes in nodular goiter: a 5-year prospective randomized trial of levothyroxine suppressive therapy for benign cold thyroid nodules. J Clin Endocrinol Metab 83:780–783 [DOI] [PubMed] [Google Scholar]
- 16. Zelmanovitz F, Genro S, Gross JL. 1998. Suppressive therapy with levothyroxine for solitary thyroid nodules: a double-blind controlled clinical study and cumulative meta-analyses. J Clin Endocrinol Metab 83:3881–3885 [DOI] [PubMed] [Google Scholar]
- 17. Larijani B, Pajouhi M, Bastanhagh MH, Sadjadi A, Sedighi N, Eshraghian MR. 1999. Evaluation of suppressive therapy for cold thyroid nodules with levothyroxine: double-blind placebo-controlled clinical trial. Endocr Pract 5:251–256 [DOI] [PubMed] [Google Scholar]
- 18. Wémeau JL, Caron P, Schvartz C, Schlienger JL, Orgiazzi J, Cousty C, Vlaeminck-Guillem V. 2002. Effects of thyroid-stimulating hormone suppression with levothyroxine in reducing the volume of solitary thyroid nodules and improving extranodular nonpalpable changes: a randomized, double-blind, placebo-controlled trial by the French Thyroid Research Group. J Clin Endocrinol Metab 87:4928–4934 [DOI] [PubMed] [Google Scholar]
- 19. Castro MR, Caraballo PJ, Morris JC. 2002. Effectiveness of thyroid hormone suppressive therapy in benign solitary thyroid nodules: a meta-analysis. J Clin Endocrinol Metab 87:4154–4159 [DOI] [PubMed] [Google Scholar]
- 20. Richter B, Neises G, Clar C. 2002. Pharmacotherapy for thyroid nodules. A systematic review and meta-analysis. Endocrinol Metab Clin North Am 31:699–722 [DOI] [PubMed] [Google Scholar]
- 21. Sdano MT, Falciglia M, Welge JA, Steward DL. 2005. Efficacy of thyroid hormone suppression for benign thyroid nodules: meta-analysis of randomized trials. Otolaryngol Head Neck Surg 133:391–396 [DOI] [PubMed] [Google Scholar]
- 22. Koc M, Ersoz HO, Akpinar I, Gogas-Yavuz D, Deyneli O, Akalin S. 2002. Effect of low- and high-dose levothyroxine on thyroid nodule volume: a crossover placebo-controlled trial. Clin Endocrinol (Oxf) 57:621–628 [DOI] [PubMed] [Google Scholar]
- 23. Grussendorf M, Vaupel R, Reiners C, Wegscheider K. 2005. LISA-Studiengruppe The LISA trial: a randomized, double-blind, placebo-controlled four-arm study of 1,000 patients with nodular goiter in Germany. Study design and first results of feasibility. Med Klin (Munich) 100:542–546 [DOI] [PubMed] [Google Scholar]
- 24. Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedüs L, Vitti P. 2010. AACE/AME/ETA Task Force on Thyroid Nodules: American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association Medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: executive summary of recommendations. Endocr Pract 16:468–475 [DOI] [PubMed] [Google Scholar]
- 25. Brunn J, Block U, Ruf G, Bos I, Kunze WP, Scriba PC. 1981. Volumetric analysis of thyroid lobes by real-time ultrasound. Dtsch Med Wochenschr 106:1338–1340 [DOI] [PubMed] [Google Scholar]
- 26. Rendl J, Seybold S, Börner W. 1994. Urinary iodide determined by paired-ion reversed-phase HPLC with electrochemical detection. Clin Chem 40:908–913 [PubMed] [Google Scholar]
- 27. Schlögl S, Andermann P, Luster M, Reiners C, Lassmann M. 2006. A novel thyroid phantom for ultrasound volumetry: determination of intraobserver and interobserver variability. Thyroid 16:41–46 [DOI] [PubMed] [Google Scholar]
- 28. Marcus R, Peritz E, Gabriel KR. 1976. On closure testing procedures with special reference to ordered analysis of variance. Biometrika 63:655–666 [Google Scholar]
- 29. Kuma K, Matsuzuka F, Yokozawa T, Miyauchi A, Sugawara M. 1994. Fate of untreated benign thyroid nodules: results of long-term follow-up. World J Surg 18:495–498; discussion 499 [DOI] [PubMed] [Google Scholar]
- 30. Brauer VF, Eder P, Miehle K, Wiesner TD, Hasenclever H, Paschke R. 2005. Interobserver variation for ultrasound determination of thyroid nodule volumes. Thyroid 15:1169–1175 [DOI] [PubMed] [Google Scholar]
- 31. Krohn K, Führer D, Bayer Y, Eszlinger M, Brauer V, Neumann S, Paschke R. 2005. Molecular pathogenesis of euthyroid and toxic multinodular goiter. Endocr Rev 26:504–524 [DOI] [PubMed] [Google Scholar]
- 32. Hampel R, Bennöhr G, Gordalla A, Below H. 2010. Urinary iodide excretion in adults in Germany 2005 meets WHO target. Exp Clin Endocrinol Diabetes 118:254–257 [DOI] [PubMed] [Google Scholar]
- 33. Hegedüs L, Paschke R, Krohn K, Bonnema SJ. 2010. Multinodular goiter. In: Endocrinology. 6th ed Jameson JL, DeGroot L. eds. Philadelphia: Saunders Elsevier; 1636–1649 [Google Scholar]
- 34. Caldwell KL, Makhmudov A, Ely E, Jones RL, Wang RY. 2011. Iodine status of the U.S. population, National Health and Nutrition Examination Survey, 2005–2006 and 2007–2008. Thyroid 21:419–427 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.