The prenatal critical period for masculinization of the ovine sexually dimorphic nucleus of the hypothalamuspreoptic area occurs later than, and can be separated temporally from, the period for masculinization of the external genitals.
Abstract
Sheep exposed to testosterone during a critical period from gestational day (GD) 30 to GD 90 develop masculine genitals and an enlarged male-typical ovine sexually dimorphic nucleus of the preoptic area (oSDN). The present study tested the hypothesis that separate critical periods exist for masculinization of these two anatomical end points. Pregnant ewes were treated with testosterone propionate (TP) either from GD 30 to GD 60 (early TP) or GD 60 to GD 90 (late TP). Control (C) pregnant ewes were treated with corn oil. Fetuses were delivered at GD 135 and the volume of the oSDN was measured. Early TP females possessed a penis and a scrotum devoid of testes, whereas late TP and C females had normal female genitals. Neither period of TP exposure grossly affected the genitals of male fetuses. Despite masculinized genitals, the mean volume of the oSDN in early TP females (0.32 ± 0.06 mm3) was not different from C females (0.24 ± 0.02 mm3) but was significantly enlarged in late TP females (0.49 ± 0.04 mm3; P < 0.05 vs. C) when the genitals appeared normal. In contrast, the volume of the oSDN in late TP males (0.51 ± 0.02 mm3) was not different from C males (0.51 ± 0.04 mm3) but was significantly smaller in the early TP males (0.35 ± 0.04 mm3; P < 0.05 vs. C). These results demonstrate that the prenatal critical period for androgen-dependent differentiation of the oSDN occurs later than, and can be separated temporally from, the period for development of masculine genitals.
We previously described the presence of a sexually dimorphic nucleus (SDN) in the preoptic area/anterior hypothalamus of the sheep, which was named the ovine SDN (oSDN) (1). The oSDN is a dense group of aromatase-expressing neurons that occupy the central component of the medial preoptic nucleus. The volume of the oSDN is 2- to 3-fold greater in rams that are sexually attracted to ewes (female oriented rams) than in either ewes or rams that are sexually attracted to other rams (male oriented rams) and thus correlates with sexual attraction in rams. Studies in other mammalian species, including humans, have also linked sexual preference to the function of the medial preoptic area/anterior hypothalamus and the size of sexually dimorphic nuclei that exist within this brain area (2–4). However, it remains an open question whether the size of the SDN is the cause or consequence of male- vs. female-typical sexual partner preferences.
One approach we have taken to address this question is to determine whether the oSDN develops early in life and to understand how prenatal exposure to hormones affects its development. We reason that if the oSDN develops before birth and thus well before sexual behaviors are manifested, then it could predispose animals to exhibit either male-typical or female-typical sexual preferences. We recently demonstrated that the oSDN is present in late-gestation fetal lambs when it is 2-fold larger in males than females (5). These results do not prove causality but do strengthen the association between oSDN size and sexual partner preference suggesting that they are either directly related or responding in kind to the same developmental cues.
In sheep the critical period for sexual differentiation has been described as occurring between gestational day (GD) 30–90 of a 147-d pregnancy. During this period of maximum androgen sensitivity, gonadal and systemic concentrations of testosterone (T) are significantly higher in males than females (6, 7). Females exposed to exogenous T during the entire length of the critical period (GD 30–90) are born with masculine external genitals consisting of a functioning pseudopenis and scrotum devoid of gonads (i.e. genital masculinization). In addition, exposure of fetal female lambs to excess androgens results in disordered reproductive physiology (8, 9), which includes disruption of estradiol (E2)-negative and -positive feedback, disrupted progesterone (P)-negative feedback, reduced or absent female-typical receptive behaviors (regarded as defeminization), and enhanced expression of male-typical behaviors (i.e. masculinization). Gonadal steroids, in particular T, organize the development of specific brain areas that control sexually dimorphic reproductive physiological responses and sexual behaviors (10). To address the question of whether gonadal hormones can also be implicated in the causation of same-sex behavior in rams through an organizational effect, we studied the effect of prenatal exposure to T on oSDN volume. We found that after exposure of genetic females to exogenous T from GD 30 to GD 90 the volume of the oSDN was significantly larger than in control females and similar in size to control males (5). Thus, prenatal T exposure masculinizes the oSDN, suggesting it is possible that variations in prenatal T exposure or action can account for the size difference of the oSDN in male-oriented rams compared with female-oriented rams.
Male-oriented rams have normal male genitals yet display a female-typical sexual partner preference. To invoke the hypothesis that same-sex preferences in rams are due to variations in prenatal androgen exposure or action, it must be shown that androgen-dependent differentiation of the brain and the genitals occur at distinct developmental times. The present study tested this possibility by exposing sheep fetuses to T propionate (TP) injected into their mothers and examining the effects on their genitals and oSDN. We predicted that exposure of female fetuses to exogenous T during the first half of the critical period (GD 30–60) would masculinize their genitals without altering the volume of their oSDN, whereas exposure during the second half (GD 60–90) would masculinize the volume of their oSDN without altering their genitals.
Materials and Methods
Experimental animals and treatments
Twenty-one timed pregnant ewes (Ovis aries) of mixed Western breeds were bred at the sheep facility at Oregon State University. The pregnant ewes were treated twice a week with 100 mg TP (Steraloids Inc., Newport, RI) injected im in 2 ml of corn oil either from GD 30–60 (early TP group) or GD 60–90 (late TP group). Control (C) pregnant ewes were treated with oil during the same gestational periods. TP is a long-acting 17-alkylated derivative of testosterone, which is hydrolyzed before acting (11). This androgen dose has been used previously by us and others to masculinize female fetuses and produces concentrations of T in the fetus approximately equal to twice that of control male fetuses (5, 12). All animal procedures were approved by the Oregon State University Institutional Animal Care and Use Committee.
Tissue and blood collection
At GD 135 ± 0.2 (sem), jugular blood samples were taken from the ewes. Anesthesia of the ewes was induced with ketamine and diazepam iv and then maintained with inhaled isoflurane and oxygen. Ewes were subjected to a midventral laparotomy to expose the uterus containing the deeply anesthetized fetus. Blood samples (2–3 ml) were taken from the umbilical artery. Heparin (10,000 U) was given through the umbilical vein, followed by 10 ml of saturated KCL to euthanize the fetus and arrest the heart in diastole. The umbilical cord was severed and the fetus weighed and sexed by visual inspection of the gonads. The crown-rump length (CRL), anogenital distance and anoumbilical distances measured in centimeters were also recorded at this time. The brain was then removed, and a diencephalic block that extended from the anterior margin of the optic chiasm to the mammillary bodies was dissected and immediately immersed in ice-cold 4% buffered paraformaldehyde. After immersion fixation overnight, the brains were cryoprotected in 20% sucrose for 72 h, frozen in isopentane at −55 C, and then stored at −80 C.
In situ hybridization
The fixed brain tissues were sectioned coronally (40 μm thick) into four parallel series that were mounted onto Fisher Superfrost microscope slides (Fisher Scientific, Pittsburgh, PA), desiccated, and stored frozen at −80 C. Expression of cytochrome P450 aromatase mRNA was detected by in situ hybridization using a sheep-specific 33P-labeled riboprobe according to previously published procedures (13). Anatomical landmarks were identified in thionin stained material from an adjacent series of brain sections.
The volume of the oSDN was determined from the thionin-stained brain series. In addition, the volume of the oSDN and anterior periventricular preoptic nucleus (AVPV) were determined from the in situ autoradiograms. All volume measurements were performed with NIH Image J version 1.43 (National Institutes of Health, Bethesda, MD).
Steroid hormone measurements
Serum was harvested from blood clotted overnight at 4 C and centrifuged at 1000 × g for 15 min then stored at −20 C until assayed for steroid concentrations. T, E2, and P were each measured in a single RIA after maternal and fetal sera were extracted with ether and fractionated on LH20 Sephadex (Sigma-Aldrich Corp., St. Louis, MO) columns according to our previously published procedures (14). The mean percentage of recovery, minimum level of sensitivity, and interassay coefficient of variation were as follows: T, 62.3%, 4.5 pg/ml, and 10.5%; E2, 67.4%, 2.4 pg/ml, and 2.8%; P, 71.0%, 2.6 pg/ml, and 4.6%.
Statistical analysis
Comparisons of fetal measurements by sex and treatment group were made by two-way ANOVA after log10 transformation if needed to equalize the variances. Post hoc planned comparisons were by Tukey's test when a significant interaction between major effects was found. Maternal hormone concentrations were analyzed by one-way ANOVA.
Results
External genitals
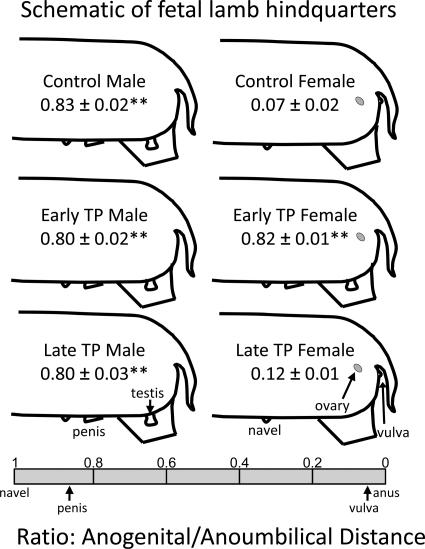
Figure 1 presents schematic views of the external genitals from male (left panel) and female (right panel) GD 135 lamb fetuses. The values represent the average ± sem ratio of anogenital distance to anoumbilical distance. The ratio spans a scale of 0 (position of the anus) to 1 (position of the navel) (see the scale at the bottom of Fig. 1). The ratio in control males is significantly greater than in control females and represents complete masculinization.
Fig. 1.
Schematic representation of fetal lamb hindquarters showing the positions of the gonads and external genitals and the mean anogenital to anoumbilical distance ratio (±sem) for control lambs, lambs treated prenatally with TP from GD 30 to GD 60 (early TP) and lambs treated prenatally with TP from GD 60 to GD 90 (late TP). **, Differences (P < 0.01) compared with control females.
T exposure of female lambs during early gestation alone was sufficient to completely masculinize the external genitals. Early TP exposure of females produced complete masculinization of the external genitals, including development of a penis and empty scrotum. The anogenital to anoumbilical ratio in early TP females was significantly greater than in control females and not different from control males. Early TP exposure did not affect gross morphological development of the genitals in males. In contrast, late TP exposure was without effect on the genitals of GD 135 fetal lambs. Specifically, late TP males and females were indistinguishable from control lambs.
Ovine SDN
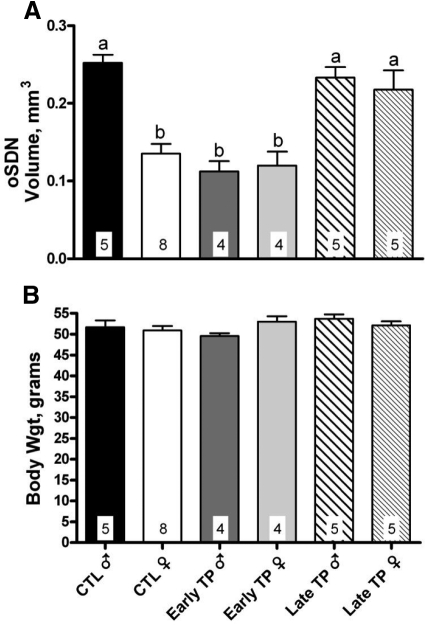
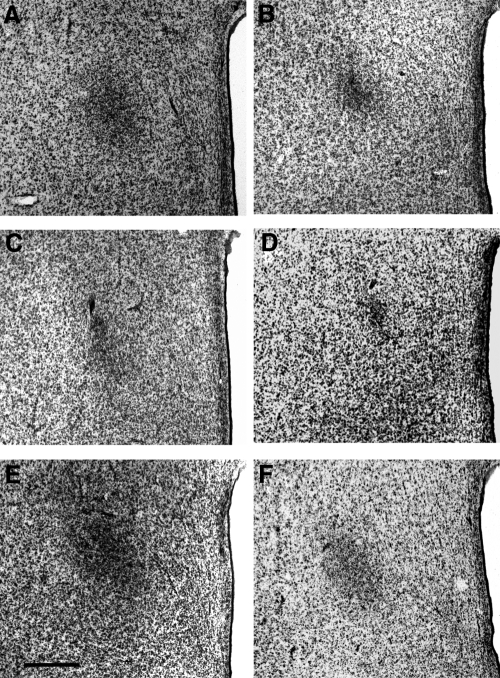
Figure 2A illustrates the effects of sex and T exposure on the volume of the oSDN as defined by Nissl staining. Two-way ANOVA revealed a significant effect of treatment [F (2, 25) = 7.7; P = 0.002], sex [F (1, 25) = 13.2; P = 0.001], and a significant sex × treatment interaction [F (2, 25) = 8.3; P = 0.002]. Representative coronal sections taken through the Nissl-stained oSDN for the control and experimental groups are shown in Fig. 3. The oSDN was significantly larger in GD 135 male lamb fetuses than in female lamb fetuses (Figs. 2 and 3). Planned post hoc comparisons revealed that early TP exposure did not affect the oSDN volume of females but unexpectedly reduced the volume in males. Conversely, late TP exposure masculinized the oSDN volume of females but did not affect the volume of males. There were no significant effects of sex or treatment on brain weights (Fig. 2B) that could have contributed to the morphological features of the oSDN.
Fig. 2.
Effect of early and late TP treatments on oSDN volume (A) and brain weight (B). The oSDN volume was determined from thionin-stained brain sections. Data are presented as means ± sem. Bars with different superscript letters differ significantly (P < 0.05). CTL, Control.
Fig. 3.
Representative coronal sections through the oSDN of GD 135 fetal lambs. A, Control males. B, Control females. C, Early TP males. D, Early TP females. E, Late TP males. F, Late TP females. Magnification, ×50. Bar, 500 μm. See Materials and Methods for description of experimental treatments.
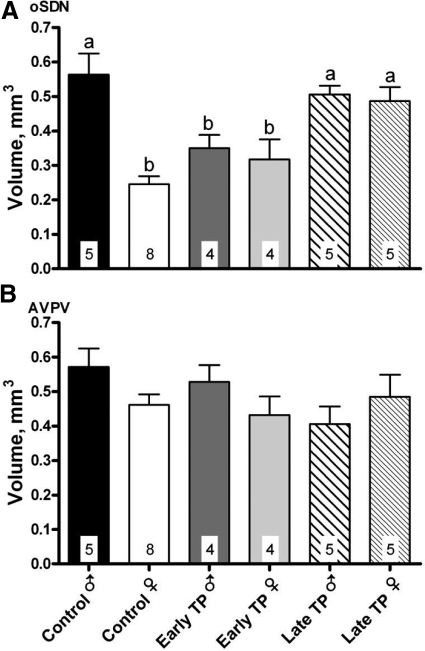
Figure 4A illustrates that the sex difference and treatment effects seen in thionin-stained sections were also apparent when the oSDN volume was measured using the pattern of aromatase mRNA expression, which we reported previously can be used to define the borders of the oSDN (1). Thus, two-way ANOVA revealed significant main effects of sex and treatment and a significant interaction (all P < 0.002), and post hoc comparisons confirmed that late TP, but not early TP, treatment masculinized the oSDN of females.
Fig. 4.
Effect of early and late TP treatments on aromatase mRNA expression volume in the oSDN (A) and AVPV (B). Data are presented as means ± sem. Bars with different superscript letters differ significantly (P < 0.05).
The pattern of aromatase expression was also used to estimate the volume of the AVPV (Fig. 4B). The AVPV was previously shown to be sexually monomorphic in late-gestation lamb fetuses (5) and serves as a tissue control in the current study. As expected, there was not a significant effect of treatment [F (2, 25) = 1.0; P = 0.4], sex [F (1, 25 = 1.1); P = 0.3], or a significant sex × treatment interaction [F (2, 25) = 2.2; P = 0.1] in AVPV. Thus, sex and treatment differences in brain morphology and aromatase expression were specific to the oSDN and not the result of systematic procedural variations.
Physical measures
Table 1 presents the data for body weight and crown-rump length among treatment groups. Two-way ANOVA revealed a significant effect of treatment [F (2, 25 = 5.7); P = 0.009] and a significant interaction [F (2, 25) = 4.6; P = 0.02] between sex and treatment for body weight that can be attributed to a reduced body weight in the early TP females. Similarly, early TP females exhibited a significant treatment effect [F (2, 24) = 10.6, P = 0.0005] due to a shorter CRL.
Table 1.
Sex differences and the effects of early TP and late TP exposure on body weight, CRL, and brain weight of fetal lambs at GD 135
| Measure | Treatment |
|||||
|---|---|---|---|---|---|---|
| Control male | Control female | Early TP male | Early TP female | Late TP male | Late TP female | |
| Body weight (kg) | 3.6 ± 0.4 | 4.0 ± 0.2 | 3.8 ± 0.4 | 2.8 ± 0.1* | 4.1 ± 0.1 | 4.3 ± 0.2 |
| CRL (cm) | 48.6 ± 2.0 | 50.1 ± 1.1 | 46.0 ± 0.9 | 42.6 ± 0.6* | 49.4 ± 0.8 | 49.8 ± 1.7 |
| n | 5 | 8 | 4 | 4 | 5 | 5 |
Data (means ± SEM) were analyzed by two-way ANOVA;
, Differs significantly (P < 0.05) from all other groups.
Hormone levels
There were no significant residual effects of prenatal TP exposure on the concentrations of T, P, and E2 in the serum from umbilical arteries of control, early, and late TP-exposed fetuses (Table 2). There was no significant effect of sex on the concentrations of P or E2 in umbilical arteries. However, there was an overall significant sex difference in testosterone concentrations with males exhibiting higher concentrations than females [F (1, 25) = 5.78; P = 0.02].
Table 2.
Effect of early TP and late TP exposure on steroid concentrations in serum from umbilical artery of fetal lambs at GD 135
| Steroid | Treatment |
|||||
|---|---|---|---|---|---|---|
| Control male | Control female | Early TP male | Early TP female | Late TP male | Late TP female | |
| Testosterone (pg/ml) | 206 ± 31 | 107 ± 12 | 212 ± 41 | 171 ± 45 | 238 ± 124 | 121 ± 30 |
| Progesterone (pg/ml) | 748 ± 213 | 530 ± 108 | 836 ± 56 | 677 ± 175 | 752 ± 118 | 433 ± 146 |
| Estradiol (pg/ml) | 36 ± 5 | 32 ± 9 | 33 ± 8 | 22 ± 3 | 29 ± 3 | 36 ± 7 |
| n | 5 | 8 | 4 | 4 | 5 | 5 |
Data (means ± SEM) were analyzed by two-way ANOVA. Overall sex difference (P < 0.05) observed for T only.
Discussion
The results of the current study indicate that the volume of the oSDN in female lamb fetuses can be enlarged to a size comparable with that of normal males without accompanying genital masculinization, provided T exposure is given from GD 60 to GD 90 (late TP). In contrast, the external genitals are masculinized in females exposed to T from GD 30 to GD 60 (early TP), but the volume of the oSDN in early TP females was not different from C females. These results demonstrate that brain and genital masculinization are independent processes that take place prenatally in sheep.
In sheep, differentiation of the primordial gonad into a testis occurs between GD 25 and GD 35 (15). The fetal testes start to synthesize T around GD 35 (6, 7). Rising blood levels of T program the external genitals to differentiate into the scrotum and penis beginning at GD 40 (16). Throughout midgestation the serum concentrations of T are higher in males than in females and decline after GD 90 (17) but remain higher in males than in females throughout the remainder of gestation and early perinatal life (6) and Roselli, C. E. (unpublished data). Although a detailed study of oSDN ontogeny has not been performed as yet, our preliminary data suggest that this nucleus does not become dimorphic until after GD 85 (18). Thus, in consideration with the current results, it appears that T initiates the program for differentiation of the penis and scrotum earlier in gestation than for masculinization of the oSDN.
Previous studies demonstrated that the discrete critical periods exist in sheep for androgen-regulated sexual differentiation of genital anatomy, behavior, and neuroendocrine function (15, 16, 19). Clarke et al. (16) originally demonstrated that the greatest degree of genital masculinization was achieved in ewes exposed to T from GD 30 to GD 80, whereas the greatest behavioral masculinization and defeminization was seen in ewes exposed to T from either GD 50 to GD 100 or GD 70 to GD 120. Ewes exposed to T from GD 90 to GD 140 exhibited normal estrous cycles and receptivity. These results, together with those of an earlier study by Short (15), demonstrated that the critical period for behavioral masculinization/defeminization occurs later than the sensitive period for genital differentiation. Similar studies in guinea pigs and rhesus macaques also found that genital masculinization occurs earlier in fetal development and largely independent of behavioral masculinization (20, 21). Subsequent studies revealed that copulatory behavior and urinary posture of ewes can be masculinized by exposing female fetuses to T during the latter part of the critical period (16, 22) but defeminization of receptive behavior and the LH surge mechanisms required earlier and longer T exposures (9, 19). Taken together these studies indicate that some aspects of behavioral and neuroendocrine defeminization requires prenatal T exposure that begins earlier and lasts longer than the T exposure required for masculinization.
The function of the oSDN is not yet known, but its volume correlates with sexual partner preferences in sheep. Rams that are sexually attracted to females have on average significantly larger oSDN volumes than rams or ewes that are sexually attracted to rams (1). The size of the oSDN in not affected by serum concentrations of T in adults (23) but is organized prenatally by T (5). The current observation that the oSDN is masculinized later than the genitals makes it possible that a variation in the hormonal environment during midgestation could contribute to same-sex attractions in rams that otherwise have normal male genitals.
Genital masculinization can be separated from brain masculinization in short gestation species such as rodents in which the SDN-preoptic area development is not complete until shortly after birth (24). For instance, neonatally castrated male rats have normal genital development but significantly smaller SDN-preoptic area volumes in comparison with intact controls (25). In contrast, hypothalamic structural dimorphisms in long-gestation mammals such as guinea pigs, ferrets, and monkeys are determined entirely during a prenatal critical period that overlaps with the sensitive period for genital masculinization (26–28). The temporal requirements for differentiation of male-typical SDN and genitals in these species have not been studied in detail and it is not known whether they can be separated. Thus, to our knowledge, the current study is the first to find that masculinization of the sexually dimorphic preoptic structure and genital anatomy has distinct temporal requirements in a long gestation or precocial species.
The oSDN was not affected by late TP exposure in male lamb fetuses. However, we unexpectedly found that early TP exposure reduced oSDN volume. This result suggests that early exposure to exogenous and presumably elevated levels of T and/or its metabolites may have demasculinizing effects in males, possibly by altering the maturation of the hypothalamus or directly affecting testicular steroid synthesis. Interestingly, a previous study by Baum et al. (28) found that fetal exposure to exogenous T tended to reduce the adult preference of male ferrets to approach and mate with female conspecifics, an observation that also points to a demasculinizing effect of exogenous T on the male brain. Alternatively, the effect of early TP may be indirect via metabolic actions on the mother or fetus because we did observe indications of growth suppression in early TP females.
Some perspective on the endogenous and exogenous concentrations of T present during the prenatal treatment can be gained from our previous study that used the same prenatal TP treatment as the present study (5). We found that prenatal treatment produced an average maternal concentration of T (13.5 ng/ml), with much lower concentrations (0.57 ng/ml) present in the fetal serum when measured on GD 85 at 26 h after injection. The elimination half-life of T in pregnant ewes was measured to be 30.8 h. The concentration of T in the serum from untreated male fetuses (0.28 ng/ml) was approximately half that of TP-treated males. Prenatal TP exposure has also been shown to disrupt the serum concentrations of estrogens in sheep fetuses and reduce testis weights (5, 17). It is possible that this disrupted endocrine milieu in the male could have suppressed or altered some component of the hypothalamic-pituitary axis during the developmental window (GD 60–90) when the program for oSDN volume was being established. Previous studies have demonstrated that LH secretion in ovine male fetuses is pulsatile and responsive to gonadal feedback at midgestation (29, 30). Recent evidence also suggests that prenatal T exposure compromises GnRH responsiveness in neonatal male lambs and adversely effects germ cell function in mature testes (31, 32). Further studies will be necessary to determine the reason that early TP exposure in males reduced the volume of the oSDN and whether this produces any permanent behavioral consequences in adults.
In conclusion, our findings support the idea that the critical period for sexual differentiation is not a single entity. In the sheep, genital differentiation by fetal androgens occurs before GD 60 and is succeeded by a later period occurring from GD 60 to GD 90 when the volume of the oSDN is programmed. Thus, these new data help explain how variations in T action during a specific time window of gestation could produce rams that prefer to mate with other rams but still possess normal masculine genitals and other male-typical neuroendocrine and behavioral traits similar to rams that prefer to mate with females.
Acknowledgments
We thank the Oregon State University students who cared for the sheep used in this study. We also wish to acknowledge the expert surgical assistance of Dr. Hernán Montilla and numerous veterinary students who helped with this project.
This work was supported by National Institutes of Health Grant R01 RR014270 (to C.E.R.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AVPV
- Anterior periventricular preoptic nucleus
- CRL
- crown-rump length
- E2
- estradiol
- GD
- gestational day
- oSDN
- ovine SDN
- P
- progesterone
- SDN
- sexually dimorphic nucleus of the preoptic area
- T
- testosterone
- TP
- T propionate.
References
- 1. Roselli CE, Larkin K, Resko JA, Stellflug JN, Stormshak F. 2004. The volume of a sexually dimorphic nucleus in the ovine medial preoptic area/anterior hypothalamus varies with sexual partner preference. Endocrinology 145:478–483 [DOI] [PubMed] [Google Scholar]
- 2. Paredes RG. 2003. Medial preoptic area/anterior hypothalamus and sexual motivation. Scand J Psychol 44:203–212 [DOI] [PubMed] [Google Scholar]
- 3. LeVay S. 1991. A difference in hypothalamic structure between heterosexual and homosexual men. Science 253:1034–1037 [DOI] [PubMed] [Google Scholar]
- 4. Alekseyenko OV, Waters P, Zhou H, Baum MJ. 2007. Bilateral damage to the sexually dimorphic medial preoptic area/anterior hypothalamus of male ferrets causes a female-typical preference for and a hypothalamic Fos response to male body odors. Physiol Behav 2–3:438–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roselli CE, Stadelman H, Reeve R, Bishop CV, Stormshak F. 2007. The ovine sexually dimorphic nucleus of the medial preoptic area is organized prenatally by testosterone. Endocrinology 148:4450–4457 [DOI] [PubMed] [Google Scholar]
- 6. Pomerantz DK, Nalbandov AV. 1975. Androgen levels in the sheep fetus during gestation. Proc Soc Exp Biol Med 149:413–416 [DOI] [PubMed] [Google Scholar]
- 7. Attal JA. 1969. Levels of testosterone, androstenedione, estrone and estradiol-17β in the testis of fetal sheep. Endocrinology 85:280–284 [DOI] [PubMed] [Google Scholar]
- 8. Padmanabhan V, Manikkam M, Recabarren S, Foster D. 2006. Prenatal testosterone excess programs reproductive and metabolic dysfunction in the female. Mol Cell Endocrinol 246:165–174 [DOI] [PubMed] [Google Scholar]
- 9. Roberts EK, Padmanabhan V, Lee TM. 2008. Differential effects of prenatal testosterone timing and duration on phenotypic and behavioral masculinization and defeminization of female sheep. Biol Reprod 79:43–50 [DOI] [PubMed] [Google Scholar]
- 10. Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. 1998. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol 19:323–362 [DOI] [PubMed] [Google Scholar]
- 11. Murad F, Haynes RC. 1985. Androgens. In: Gilman AG, Goodman LS, Rall TW, Murad F. eds. The pharmacological basis of therapeutics. New York: MacMillan Publishing Co.; 1440–1458 [Google Scholar]
- 12. Birch RA, Padmanabhan V, Foster DL, Unsworth WP, Robinson JE. 2003. Prenatal programming of reproductive neuroendocrine function: fetal androgen exposure produces progressive disruption of reproductive cycles in sheep. Endocrinology 144:1426–1434 [DOI] [PubMed] [Google Scholar]
- 13. Roselli CE, Stormshak F, Resko JA. 2000. Distribution of aromatase mRNA in the ram hypothalamus: an in situ hybridization study. J Neuroendocrinol 12:656–664 [DOI] [PubMed] [Google Scholar]
- 14. Resko JA, Ellinwood WE, Pasztor LM, Buhl AE. 1980. Sex steroids in the umbilical circulation of fetal rhesus monkeys from the time of gonadal differentiation. J Clin Endocrinol and Metab 50:900–905 [DOI] [PubMed] [Google Scholar]
- 15. Short RV. Sexual differentiation of the brain of the sheep. In: Forest MG, Bertrand J. eds. Proc International Symposium on Sexual Endocrinology of the Perinatal Period Colloque International Institut National de la Santé et de la Recherche Médicale, Institut National de la Santé et de la Recherche Médicale, Paris, France, 1974, pp 121–142 [Google Scholar]
- 16. Clarke IJ, Scaramuzzi RJ, Short RV. 1976. Effects of testosterone implants in pregnant ewes on their female offspring. J Embryol Exp Morph 36:87–99 [PubMed] [Google Scholar]
- 17. Veiga-Lopez A, Steckler TL, Abbott DH, Welch KB, MohanKumar PS, Phillips DJ, Refsal K, Padmanabhan V. 2011. Developmental programming: impact of excess prenatal testosterone on intra-uterine fetal endocrine milieu and growth in sheep. Biol Reprod 84:87–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reddy RC, Stadelman HL, Roselli CE. A sex specific genetic signature in the developing fetal lamb correlates to the neuroanatomical sexual dimorphism of the preoptic area. Proc Organization for the Study of Sex Differences Program Book 10, Ann Arbor, MI, 2010, p 38 (Abstract A-19) [Google Scholar]
- 19. Wood RI, Mehta V, Herbosa CG, Foster DL. 1995. Prenatal testosterone differentially masculinizes tonic and surge modes of luteinizing hormone secretion in the developing sheep. Neuroendocrinology 62:238–247 [DOI] [PubMed] [Google Scholar]
- 20. Goy RW, Bridson WE, Young WC. 1964. Period of maximal susceptibility of the prenatal female guinea pig to masculinizing actions of testosterone propionate. J Comp Physiol Psychol 57:166–174 [DOI] [PubMed] [Google Scholar]
- 21. Goy RW, Bercovitch FB, McBrair MC. 1988. Behavioral masculinization is independent of genital masculinization in prenatally androgenized female rhesus macaques. Horm Behav 22:552–571 [DOI] [PubMed] [Google Scholar]
- 22. Fabre-Nys C, Venier G. 1991. Sexual differentiation of sexual behaviour and preovulatory LH surge in ewes. Psychoneuroendocrinology 16:383–396 [DOI] [PubMed] [Google Scholar]
- 23. Roselli CE, Estill CT, Stadelman HL, Stormshak F. 2009. The volume of the ovine sexually dimorphic nucleus of the preoptic area is independent of adult testosterone concentrations. Brain Res 1249:113–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Döhler KD, Coquelin A, Davis F, Hines M, Shryne JE, Gorski RA. 1984. Pre- and postnatal influence of testosterone propionate and diethylstilbestrol on differentiation of the sexually dimorphic nucleus of the preoptic area in male and female rats. Brain Res 302:291–295 [DOI] [PubMed] [Google Scholar]
- 25. Gorski RA, Gordon JH, Shryne JE, Southam AM. 1978. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res 148:333–346 [DOI] [PubMed] [Google Scholar]
- 26. Byne W, Bleier R. 1987. Medial preoptic sexual dimorphisms in the guinea pig. I. An investigation of their hormonal dependence. J Neurosci 7:2688–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tobet SA, Zahniser DJ, Baum MJ. 1986. Differentiation in male ferrets of a sexually dimorphic nucleus of the preoptic/anterior hypothalamic area requires prenatal estrogen. Neuroendocrinology 44:299–308 [DOI] [PubMed] [Google Scholar]
- 28. Baum MJ, Erskine MS, Kornberg E, Weaver CE. 1990. Prenatal and neonatal testosterone exposure interact to affect differentiation of sexual behavior and partner preference in female ferrets. Behav Neurosci 104:183–198 [DOI] [PubMed] [Google Scholar]
- 29. Clark SJ, Ellis N, Styne DM, Gluckman PD, Kaplan SL, Grumbach MM. 1984. Hormone ontogeny in the ovine fetus. XVII. Demonstration of pulsatile luteinizing hormone secretion by the fetal pituitary gland. Endocrinology 115:1774–1779 [DOI] [PubMed] [Google Scholar]
- 30. Matwijiw I, Faiman C. 1989. Control of gonadotropin secretion in the ovine fetus. II. A sex difference in pulsatile luteinizing hormone secretion after castration. Endocrinology 124:1352–1358 [DOI] [PubMed] [Google Scholar]
- 31. Rojas-García PP, Recabarren MP, Sarabia L, Schön J, Gabler C, Einspanier R, Maliqueo M, Sir-Petermann T, Rey R, Recabarren SE. 2010. Prenatal testosterone excess alters sertoli and germ cell number and testicular FSH receptor expression in rams. Am J Physiol Endocrinol Metab 299:E998–E1005 [DOI] [PubMed] [Google Scholar]
- 32. Recabarren SE, Lobos A, Figueroa Y, Padmanabhan V, Foster DL, Sir-Petermann T. 2007. Prenatal testosterone treatment alters LH and testosterone responsiveness to GnRH agonist in male sheep. Biol Res 40:329–338 [PubMed] [Google Scholar]