Abstract
For many years treatment for advanced or metastatic non-small cell lung cancer (NSCLC) has employed chemotherapy regimens for patient care, with limited effect. Five-year survival rates for these patients are not encouraging. However, for a subgroup of these patients, there have been radical changes over recent years. Our understanding of the basic pathology behind NSCLC at the molecular level has offered up a host of new molecularly targeted therapies, which are revolutionizing this area of cancer care. Results from recent clinical trials provide hope for NSCLC patients harboring oncogenic translocations involving the anaplastic lymphoma kinase (ALK) receptor tyrosine kinase. Just as inhibition of the breakpoint cluster region–ABL complex has changed the face of chronic myeloid leukemia diagnosis, oncogenic ALK fusions offer a step forward in the diagnosis and treatment of ALK-positive NSCLC. This article discusses the current knowledge and potential implications concerning ALK inhibitors and NSCLC.
Lung cancer and a new era of treatment
Figures released by the American Cancer Society for 2008 reported 1.6 million new lung cancer cases worldwide. Indeed, lung cancer is the leading cause of cancer death in men and the second leading cause of cancer death in women, with estimated deaths approaching 1.4 million worldwide in 2008 [1]. Clinically, primary lung cancer is divided into small-cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), and patients receive differential therapy based on these criteria. NSCLC is an umbrella term for a number of tumor types that together account for approximately 80% of lung cancers. These include the three main subtypes of squamous-cell lung carcinoma, large-cell lung carcinoma, and adenocarcinoma [2]. Adenocarcinoma accounts for approximately 40% of all NSCLC and is more prevalent among people who have never smoked [3]. For many years, treatment for advanced or metastatic NSCLC has employed chemotherapy regimens for patient care with limited effect. Five-year survival rates for these patients are not encouraging. However, for a subgroup of these patients, there have been radical changes over recent years. Our understanding of the basic pathology behind NSCLC at the molecular level has offered up a host of new molecularly targeted therapies, which are revolutionizing this area of cancer care. Activating EGFR (epidermal growth factor receptor) mutations in NSCLC provided the first opportunity to generate molecularly defined treatments such as the inhibitors gefitinib and erlotinib [4-7]. Results from recent clinical trials provide hope for NSCLC patients harboring oncogenic translocations involving the anaplastic lymphoma kinase (ALK) receptor tyrosine kinase. Just as inhibition of the BCR–ABL (breakpoint cluster region–c-abl oncogene 1, non-receptor tyrosine kinase) complex has changed the face of chronic myeloid leukemia diagnosis, oncogenic ALK fusions offer a step forward in the diagnosis and treatment of ALK-positive NSCLC. Recent advances in drug development, particularly those targeting ALK, which will be discussed here, have led to significant changes in the way we view this patient population and their future therapeutic prospects.
ALK was first described as an oncogene in human cancer in the 1990s, with the description of the nucleophosmin–ALK (NPM-ALK) fusion gene in anaplastic large-cell lymphoma (ALCL), resulting in the acronym ALK [8,9]. Since then, a large number of ALK translocations in a growing variety of tumor types have been described, in which the uniting theme is the dimerization and inappropriate ligand-independent activation of ALK tyrosine kinase activity by the fusion partner in question [10-13]. As well as a role in hematological malignancies, ALK translocations are also found in a number of solid tumor types, including NSCLC, squamous cell carcinoma, and more recently thyroid cancer [14-18]. While initially considered to be rather unusual, the identification of fusions such as TMPRSS2–ERG (transmembrane protease, serine 2–ETS-related gene) in prostate cancer [19] suggest that we may have underestimated their occurrence in solid tumors and may find more of these translocations in coming years with the application of the latest sequencing technologies.
ALK and NSCLC
The appearance of ALK fusion oncoproteins in NSCLC was first described in 2007 in two independent studies with quite different approaches [15,16]. While Soda et al. [15] used classical tumor DNA library transformation assays to identify echinoderm microtubule-associated protein-like 4 (EML4)–ALK, Rikova et al. [16] carried out one of the initial global phosphotyrosine proteomic analyses of NSCLC cell lines, identifying a number of oncogenic lesions including EML4–ALK and TRK-fused gene–ALK (TFG-ALK).
Prior to the identification of ALK fusion proteins in NSCLC, the patient population presenting with ALK fusions, such as NPM–ALK in ALCL, was limited. This number changed significantly with the consideration of an estimated 3–13% of NSCLC patients [15,16,20-23]. Calculated at a rate of 5% of ALK translocations and based on 2008 American Cancer Society figures [1], NSCLC cases amenable to ALK-directed therapies would be predicted to reach in the order of 80,000 new lung cancer patients per year worldwide.
The NSCLC patient group presenting with ALK translocations is somewhat different from the more commonly appreciated smoking-related lung cancer population. It is now recognized that there is an increasing population of ‘non-smoking-associated lung cancer’ NSCLC patients in which aberrations such as EML4–ALK and activating EGFR mutations are enriched. This population is generally predominantly female and tumors are often adenocarcinomas [21,24,25].
In an attempt to better appreciate the frequency of various defined mutations in NSCLC of the adenocarcinoma type, the National Cancer Institute’s Lung Cancer Mutation Consortium is examining 1,000 tumors for a number of driver mutations, including ALK translocations. Their most recent results, based on 830 patients, suggest that 60% of tumors exhibit driver mutations including 25% KRAS (v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog), 23% EGFR, and 6% ALK rearrangements [23]. This also means that, in 40–50% of NSCLC, there are as yet unknown drivers, perhaps as a result of loss of tumor suppressor genes and epigenetic misregulation, serving as a stern reminder that there are still many questions to be answered.
ALK translocations, fusion proteins, and diagnostics in NSCLC
As mentioned above, many molecularly different ALK translocations have been described in a number of tumor types. While the complete picture is far from clear, the data thus far indicate that different tumor types have their own particular patterns of ALK fusion partners. This is certainly true for ALK fusions in NSCLC, where by far the most common fusion partnership is EML4–ALK [15,16], with others such as TFG [9] and kinesin family member 5B (KIF5B) [26,27] being less frequently observed. The EML–ALK translocation fusions are particularly complex with a number of different break points [28]. While one might envision that other ALK translocation partners may be identified in future studies, a comprehensive study argues against involvement of the common partners such as NPM in NSCLC [29]. To date, a number of studies suggest that together these ALK translocations account for 3–13% of NSCLC [15,16,20-23,29-34].
One critical area of activity is the development of robust and accurate diagnostics for the routine identification of ALK translocations in lung adenocarcinoma. Currently, fluorescence in situ hybridization, immunohistochemistry, and reverse transcriptase-PCR-based strategies are employed; however, the diagnosis of oncogenic ALK fusions is challenging due to the large number of different EML4–ALK variants [28] and the possibility of alternative partners, such as TFG and KIF5B [9,27]. The presence of EML4–ALK is generally considered to be mutually exclusive to EGFR or KRAS mutations (although one exception has been reported of a NSCLC patient with both EML-ALK and EGFR [22]). Given this, one can envision that future clinical investigation of NSCLC may include a standard panel of diagnostic tests aimed at identifying patient populations with driver mutations such as KRAS, EGFR and ALK translocations. While treatment options for patients with KRAS mutations are limited, those falling into EGFR mutant or ALK translocation categories can be offered tailored molecular therapeutic intervention.
ALK inhibitors
There are now a considerable number of interesting ALK inhibitors (see Figure 1 and Table 1). Two of these—NVP-TAE684 [35] and crizotinib (PF-2341066) [36]—are familiar names in the ALK field and have already been employed in a substantial number of scientific studies.
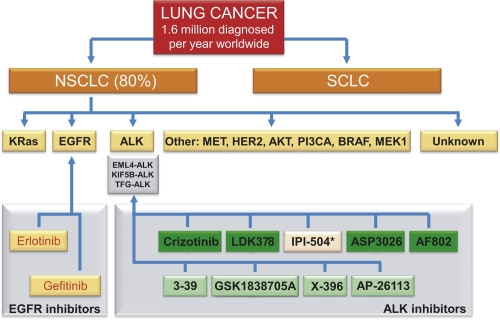
Figure 1. Schematic overview of potential tyrosine kinase inhibitor in non-small cell lung cancer (NSCLC).
Lung cancer is divided into two clinically important groups: NSCLC, which accounts for approximately 80% of lung cancer; and small-cell lung cancer (SCLC). Within NSCLC, a number of ALK kinase inhibitors are shown in green, with the exception of IPI-504 (marked with an asterisk), which is an Hsp90 inhibitor. AKT; v-akt murine thymoma viral oncogene homolog 1; ALK, anaplastic lymphoma kinase; BRAF, v-raf murine sarcoma viral oncogene homolog B1; EGFR, epidermal growth factor receptor; EML, echinoderm microtubule-associated protein-like; HER2, human epidermal growth factor receptor 2; KIF5B, kinesin family member 5B; KRas; v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog; MEC1, mitosis entry checkpoint 1; MET, met proto-oncogene (hepatocyte growth factor receptor); PI3CA, phosphatidylinositol-3 kinase catalytic subunit alpha; TFG, TRK-fused gene.
Table 1. Inhibitors for anaplastic lymphoma kinase (ALK) in or expected to go to clinical trial, 2011.
| Company | Inhibitor | Clinical trial; phase | G/W | Aims of investigation |
|---|---|---|---|---|
| Pfizer | Crizotinib | NCT00932893; III | No | Crizotinib versus standard of care in patients with advanced NSCLC |
| (Xalkori) | NCT01154140; III | No | Randomized, open-label study of the efficacy and safety of crizotinib versus pemetrexed/cisplatin or pemetrexed/carboplatin in previously untreated patients | |
| NCT00939770; I/II | No | Young patients with relapsed or refractory solid tumors, ALCL, CNS, or NBs. | ||
| NCT01121588; I/II | No | Safety and efficacy in patients with tumors except NSCLC that are positive for ALK | ||
| Novartis | NVP-TAE684 | N/A | Not developed | |
| LDK378 | NCT01283516; I | Yes | Safety in ALK-positive/genetically abnormal tumors; no available data. | |
| 3-39 | preclinical | Yes | ||
| Chugai | AF802 (CH5424802) | JapicCTI-101264; I/II | Yes | I. Safety, tolerability, and pharmacokinetic in NSCLC patients with ALK-fusion gene. II. Efficacy and safety of AF802. |
| Infinity | IPI-504* | NCT01228435; II | N/A | Inhibitor of Hsp90, which protects other proteins from being destroyed, possibly also EML4–ALK fusion proteins in NSCLC patients. |
| Astrella | ASP3026 | NCT01284192; I | ND | Safety and tolerability of ASP3026. No preclinical data available but aim for advanced malignancies, B-cell lymphoma, solid tumors, and ALK. |
| Ariad | AP-26113 | preclinical | Yes | AP-26113 abrogates crizotinib-resistant mutations in EML4–ALK. Clinical development 2011 likely. |
| Xcovery | X-396 | preclinical | Yes | X-396 inhibits two ALK point mutations, C1156Y and L1196M, and works in synergy with rapamycin. May initiate clinical trials by the end of 2011. |
| GlaxoSmithKline | GSK-1838705A | preclinical | Yes | Abrogates ALK, and growth of ALCL, some NBs, and a subset of NSCLC. |
*IPI-504 is not an ALK inhibitor, but an Hsp90 inhibitor. ALCL, anaplastic large-cell lymphoma; CNS, central nervous system; EML4, echinoderm microtubule-associated protein-like 4; G/W, ability to inhibit gateway mutation; NB, neuroblastoma, N/A, not applicable; ND, not determined; NSCLC, non-small cell lung cancer.
NVP-TAE684 was presented in 2007 as highly potent and selective ALK ATP-competitive inhibitor, and was shown to block growth in cell lines and in a mouse model of ALCL [35]. Cells expressing oncogenic variants of ALK or EML4–ALK fusion proteins show reduced growth when treated with NVP-TAE684 [37,38]. Also, the ALK inhibitor NVP-TAE684 successfully inhibited tumors in a mouse model of EML4–ALK lung cancer [37], with mice overexpressing EML4–ALK developing tumors with malignant characteristics. This result confirms both the potent oncogenic activity of the fusion kinase and the therapeutic potential of targeted inhibitors [37]. While scientific reports in both cell lines and mouse models have shown NVP-TAE684 to be effective against ALK fusion oncogenes, it is not currently in any clinical trial. Whether this is due to pharmacologic issues with NVP-TAE684 that prevented further clinical development by Novartis, or for other reasons, is not clear.
Like NVP-TAE684, crizotinib (now FDA-approved as Xalkori) is an ATP-competitive small molecule ALK inhibitor, which also displays activity against the c-Met receptor tyrosine kinase [36]. The rapid clinical development of crizotinib is in part a reflection of lessons learnt in preceding years of tyrosine kinase inhibitor development. Crizotinib (initially known as PF-2341066) was first described in 2007, and by 2010 the first clinical trial results had reported promising initial results in NSCLC patients carrying ALK translocations [39]. At the American Society of Clinical Oncology (ASCO) meeting 2011 in Chicago, a follow-up study from this Phase I study of crizotinib was presented, showing progression-free survival in patients with ELM4–ALK-positive NSCLC [40]. This trial has been carried out in 119 enrolled patients with advanced NSCLC, 44% of whom have received more than three treatments before receiving oral crizotinib. Two patients displayed a complete response (disappearance of target lesions), 69 patients had a partial response, and 31 patients were considered to have stable disease, implying that crizotinib treatment has very real patient benefit [39,40].
Currently, Phase III trials with crizotinib are ongoing. Importantly, in response to ethical concerns, these Phase III trials will allow crossover from the chemotherapy control arm to crizotinib on failure to respond, allowing these patients to benefit from ALK-inhibitor therapy. While the crossover aspect of this trial will make it difficult to assess the true impact on overall survival in response to crizotinib, it will allow for patients from the chemotherapy control arm to receive ALK-inhibitor therapy upon failure to respond to chemotherapy. Follow up of the 82 ALK-positive patients reported by Kwak et al. [39], suggest a significant increase in overall survival in response to crizotinib (64% at 2 years) [41].
The results thus far suggest that while we are not yet at the stage of ‘curing’ ALK-positive NSCLC, we may be approaching the scenario of chronic disease management. This brings an additional set of challenges, not least drug toxicity. Results from ALK knockout mice, which are viable, suggest that loss of ALK activity is not life threatening [42]. Oral crizotinib at a therapeutic dose of 250 mg twice a day appears to be relatively well tolerated with most complaints being Grade 1 nausea and diarrhea. Interestingly, a significant proportion of these patients report mild visual disturbances while taking crizotinib [39,40]. While no function in visual development has been described in the mouse, alterations in behavior indicate a role for this receptor in the adult brain [42]. A potential role for ALK in the human visual system is supported by its involvement in the maturation of the optic lobe in the Drosophila brain [43] and the robust expression of ALK within the lens and the neural and pigment layer of the mouse retina [44]. The speed of clinical application of crizotinib in NSCLC since its initial description in 2007 is impressive, and it is now being investigated for ALK inhibition in neuroblastoma and ALCL (Table 1). In neuroblastoma, the ALK mutations are activating kinase domain point mutations in the context of the full length receptor, rather than oncogenic fusions as in NSCLC, and they are also sensitive to ALK inhibitors [45-49]. Furthermore, knowledge gained from the crizotinib experience will hopefully pave the way for the next wave of ALK inhibitors.
Resistance and next generation ALK inhibitors
The development of therapeutic tools for use in ALK-driven cancers has benefited from the experience gained from kinase inhibitors already in clinical use, such as BCL–ABL and EGFR inhibitors. However, the prolonged survival seen with these drugs necessitates long-term treatment, which presents a new set of problems. One such challenge with kinase inhibitors is the development of drug resistance, and particularly appearance of “gatekeeper” mutations that block crizotinib binding. Acquired inhibitor resistance is a serious complication in cancer treatment, where the objective is a chronic maintenance of tumor control rather than a “quick fix”. Indeed, this has already been documented for a patient with NSCLC who relapsed after the appearance of C1156Y and L1196M mutations in EML4–ALK [50]. L1196M represents a mutation of the “gatekeeper” residue, similar to the T790M gefitinib-resistance mutations observed in EGFR, and T315I mutations in ABL. Mutations in the gatekeeper site are thought to increase the affinity for ATP significantly, outcompeting the effects of ATP competitive inhibitors [51]. The effect of the C1156Y mutation is unclear, although it may have an indirect effect on crizotinib binding, and further studies will be required to establish its mechanism.
A number of ALK inhibitors that are able to inhibit ALK variants with “gatekeeper” mutations at L1196M have been developed. One of these is AP26113 from Ariad, which inhibits the growth of crizotinib-resistant H3122 cell lines and xenograft mouse models that carry the L1196M EML4–ALK mutation [52] (Table 1). In a recent publication, high-throughput screening and scaffold modification resulted in CH5424802 (AF802), which inhibits ALK activity in vitro and in mouse xenograft models [53]. This inhibitor proved effective against both C1156Y- and L1196M-resistant EML4–ALK mutants [50]. The structure of the ALK kinase domain in various forms, including several ALK-inhibitor complexes, has recently been reported [54,55] and comparison of the unliganded ALK catalytic domain structure with the structure of the ALK–CH5424802 complex shows that the inhibitor binds in the ATP pocket in DFG-in mode, with some notable differences in comparison with bound crizotinib [53] providing rationalization of the ability of CH5424802 to inhibit forms of EML–ALK that are less sensitive to crizotinib.
Two additional ALK-specific small molecule tyrosine kinase inhibitors, X-376 and X-396, have been identified and biologically characterized [56]. X-396 is also able to inhibit ELM4–ALK (L1196M) and ELM4–ALK (C1156Y), and is active in animal models of NSCLC and neuroblastoma. These data, in conjunction with preliminary toxicology and pharmacokinetic data, suggest that X-396 should be an effective, well-tolerated oral treatment for ALK-positive NSCLC, lymphoma, and neuroblastoma.
Other preclinical ALK inhibitors
A number of other promising ALK inhibitors exist. GSK1838705A has been shown to inhibit ALK (as well as insulin-like growth factor receptor and insulin receptor), inhibiting the proliferation of cancer cell lines and growth of tumor xenografts in nude mice [57] (Table 1). A crystal structure of the ALK kinase domain in a complex with PHA-E429 has been described [55], and F91873 and F91874 were identified as multikinase inhibitors with activity against ALK in a biochemical screen in ALCL cell lines and xenograft models [58]. Cephalon have developed CEP-28122, for which little information currently exists, and ASP3026 is an inhibitor made by Astrella Pharma Inc. that is in Phase I clinical trials for ALK-related malignancies (Table 1). Likewise, few details exist concerning LDK378, an ALK inhibitor developed by Novartis, such as its relationship to the Novartis preclinical compound 3-39 [37,59,60]. LDK378 is currently in Phase I trials for patients with tumors characterized by genetic abnormalities in ALK (Table 1).
In addition to the ALK inhibitors discussed above, new molecules continue to be described, such as NMS-E628 [61], SJ-08-0025 [62], tetrahydropyridopyrazines [63], and compounds from structural-based virtual screening approaches [64]. Other compounds, such as the Hsp90-inhibitor geldenamycin derivatives IPI-504 and 17-AAG, appear to have effects in NSCLC patients with ALK translocations, and this effect appears to extend to ELM4–ALK (L1196M) suggesting they may be useful in overcoming crizotinib-resistant tumors [52,65,66]. A number of clinical trials are in progress (Table 1) and the results of these eagerly awaited.
ALK inhibitors and NSCLC: future and reflection
A great deal of progress has been made since the early days of ALK inhibitors [67], and a substantial number of patent applications for ALK inhibitors have been filed [68], some of which have now been translated into realistic options for clinical use. The rapid pace of ALK drug development is being accompanied by similar progress in robust diagnostics and coordinated approaches to NSCLC treatments. Many questions and challenges remain for the future, especially in terms of use of ALK inhibitors in combination with other signaling inhibitors and the rational design of trials to test these. In spite of the increasing body of impressive data and elegant studies published, we should remember that the response of patients to ALK inhibitors will probably throw up a multitude of unexpected questions and challenges. The human body and the complex interplay with the evolving and adapting tumors never cease to confound scientists and clinicians alike and the unpredictable can be expected. Finally, it is important to bear in mind that if ALK inhibitors work in patients, we should heartily thank all those who have tirelessly worked over the years to bring them to therapeutic realization. Such efforts allow us to look forward to a more optimistic era of treatment for NSCLC patients based on molecular treatments tailored to their tumor type.
Acknowledgments
The authors would like to thank Tony Hunter for critical reading and valuable comments. This work has been supported by grants from the Swedish Cancer Society, the Children’s Cancer Foundation, the Swedish Research Council, Lions Cancer Society, Umeå, and the Association for International Cancer Research. RHP is a Swedish Cancer Foundation Research Fellow.
Abbreviations
- ABL
c-abl oncogene 1, non-receptor tyrosine kinase
- ALCL
anaplastic large cell lymphoma
- ALK
anaplastic lymphoma kinase
- BCR
breakpoint cluster region
- EGFR
epidermal growth factor receptor
- EML4
echinoderm microtubule-associated protein-like 4
- KIF5B
kinesin family member 5B
- KRAS
v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog
- NSCLC
non-small cell lung cancer
- NPM
nucleophosmin
- SCLC
small-cell lung cancer
- TFG
TRK-fused gene
Competing interests
The authors declare that they have no competing interests.
The electronic version of this article is the complete one and can be found at: http://f1000.com/reports/m/3/21
References
- 1.American Cancer Society Global Cancer Facts & Figures, 2nd Edition. Atlanta: American Cancer Society; 2011. [Google Scholar]
- 2.Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC. World Health Organisation Classification of Tumors: Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon, France: International Agency for Research on Cancer (IARC) Press; 2004. [Google Scholar]
- 3.Wakelee HA, Chang ET, Gomez SL, Keegan TH, Feskanich D, Clarke CA, Holmberg L, Yong LC, Kolonel LN, Gould MK, West DW. Lung cancer incidence in never smokers. J Clin Oncol. 2007;25:472–8. doi: 10.1200/JCO.2006.07.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 4.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]; F1000 Factor 25Evaluated by Ruth Palmer 14 Oct 2011, Charles Brenner 02 Dec 2004, Alfred Wittinghofer 15 Sep 2004, Charles Streuli 01 Jul 2004, Joachim Herz 27 May 2004, Patricia C Weber 14 May 2004, William Kaelin 10 May 2004.
- 5.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]; F1000 Factor 19Evaluated by Ruth Palmer 14 Oct 2011, Charles Brenner 02 Dec 2004, Michael B Yaffe 03 Jun 2004, Joachim Herz 27 May 2004, William Kaelin 10 May 2004.
- 6.Cataldo VD, Gibbons DL, Pérez-Soler R, Quintás-Cardama A. Treatment of non-small-cell lung cancer with erlotinib or gefitinib. N Engl J Med. 2011;364:947–55. doi: 10.1056/NEJMct0807960. [DOI] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 7.Engelman JA, Zejnullahu K, Gale CM, Lifshits E, Gonzales AJ, Shimamura T, Zhao F, Vincent PW, Naumov GN, Bradner JE, Althaus IW, Gandhi L, Shapiro GI, Nelson JM, Heymach JV, Meyerson M, Wong KK, Jänne PA. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 2007;67:11924–32. doi: 10.1158/0008-5472.CAN-07-1885. [DOI] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 8.Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, Look AT. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1994;263:1281–4. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 9.Shiota M, Fujimoto J, Semba T, Satoh H, Yamamoto T, Mori S. Hyperphosphorylation of a novel 80 kDa protein-tyrosine kinase similar to Ltk in a human Ki-1 lymphoma cell line, AMS3. Oncogene. 1994;9:1567–74. [PubMed] [Google Scholar]
- 10.Hallberg B, Palmer RH. Crizotinib--latest champion in the cancer wars? N Engl J Med. 2010;363:1760–2. doi: 10.1056/NEJMe1010404. [DOI] [PubMed] [Google Scholar]
- 11.Palmer RH, Vernersson E, Grabbe C, Hallberg B. Anaplastic lymphoma kinase: signalling in development and disease. Biochem J. 2009;420:345–61. doi: 10.1042/BJ20090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 2008;8:11–23. doi: 10.1038/nrc2291. [DOI] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 13.Barreca A, Lasorsa E, Riera L, Machiorlatti R, Piva R, Ponzoni M, Kwee I, Bertoni F, Piccaluga PP, Pileri SA, Inghirami G, European T-Cell Lymphoma Study Group Anaplastic lymphoma kinase (ALK) in human cancer. J Mol Endocrinol. 2011;47:R11–23. doi: 10.1530/JME-11-0004. [DOI] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 14.Murugan AK, Xing M. Anaplastic Thyroid Cancers Harbor Novel Oncogenic Mutations of the ALK Gene. Cancer Res. 2011;71:4403–11. doi: 10.1158/0008-5472.CAN-10-4041. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 factor 7Evaluated by Ruth Palmer 14 Oct 2011, Giancarlo Vecchio 01 Jun 2011, Barry Nelkin 25 May 2011
- 15.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H, Bando M, Ohno S, Ishikawa Y, Aburatani H, Niki T, Sohara Y, Sugiyama Y, Mano H. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 16.Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, Hu Y, Tan Z, Stokes M, Sullivan L, Mitchell J, Wetzel R, Macneill J, Ren JM, Yuan J, Bakalarski CE, Villen J, Kornhauser JM, Smith B, Li D, Zhou X, Gygi SP, Gu TL, Polakiewicz RD, Rush J, Comb MJ. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 17.Du XL, Hu H, Lin DC, Xia SH, Shen XM, Zhang Y, Luo ML, Feng YB, Cai Y, Xu X, Han YL, Zhan QM, Wang MR. Proteomic profiling of proteins dysregulted in Chinese esophageal squamous cell carcinoma. J Mol Med. 2007;85:863–75. doi: 10.1007/s00109-007-0159-4. [DOI] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 18.Jazii FR, Najafi Z, Malekzadeh R, Conrads TP, Ziaee AA, Abnet C, Yazdznbod M, Karkhane AA, Salekdeh GH. Identification of squamous cell carcinoma associated proteins by proteomics and loss of beta tropomyosin expression in esophageal cancer. World J Gastroenterol. 2006;12:7104–12. doi: 10.3748/wjg.v12.i44.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 19.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–8. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]; F1000 Factor 9Evaluated by Ruth Palmer 14 Oct 2011, Jenny Ting 04 Nov 2004.
- 20.Wong DW, Leung EL, So KK, Tam IY, Sihoe AD, Cheng LC, Ho KK, Au JS, Chung LP, Pik Wong M, University of Hong Kong Lung Cancer Study Group The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009;115:1723–33. doi: 10.1002/cncr.24181. [DOI] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 21.Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, Solomon B, Stubbs H, Admane S, McDermott U, Settleman J, Kobayashi S, Mark EJ, Rodig SJ, Chirieac LR, Kwak EL, Lynch TJ, Iafrate AJ. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247–53. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011, Antonella De Luca and Nicola Normanno 02 Nov 2009
- 22.Koivunen JP, Mermel C, Zejnullahu K, Murphy C, Lifshits E, Holmes AJ, Choi HG, Kim J, Chiang D, Thomas R, Lee J, Richards WG, Sugarbaker DJ, Ducko C, Lindeman N, Marcoux JP, Engelman JA, Gray NS, Lee C, Meyerson M, Jänne PA. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14:4275–83. doi: 10.1158/1078-0432.CCR-08-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 23.Kris MG, Johnson BE, Kwiatkowski DJ, Iafrate AJ, Wistuba II, Aronson SL, Engelman JA, Shyr Y, Khuri FR, Rudin CM, Garon EB, Pao W, Schiller JH, Haura EB, Shirai K, Giaccone G, Berry LD, Kugler K, Minna JD, Bunn PA. Identification of driver mutations in tumor specimens from 1,000 patients with lung adenocarcinoma: The NCI’s Lung Cancer Mutation Consortium (LCMC) J Clin Oncol. 2011;29(Suppl):CRA7506. [Google Scholar]
- 24.Yano T, Haro A, Shikada Y, Maruyama R, Maehara Y. Non-small cell lung cancer in never smokers as a representative 'non-smoking-associated lung cancer': epidemiology and clinical features. Int J Clin Oncol. 2011;16:287–93. doi: 10.1007/s10147-010-0160-8. [DOI] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 25.Suda K, Tomizawa K, Yatabe Y, Mitsudomi T. Lung cancers unrelated to smoking: characterized by single oncogene addiction? Int J Clin Oncol. 2011;16:294–305. doi: 10.1007/s10147-011-0262-y. [DOI] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 26.Wong DW, Leung EL, Wong SK, Tin VP, Sihoe AD, Cheng LC, Au JS, Chung LP, Wong MP. A novel KIF5B-ALK variant in nonsmall cell lung cancer. Cancer. 2011;117:2709–18. doi: 10.1002/cncr.25843. [DOI] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 27.Takeuchi, K, Choi YL, Togashi Y, Soda M, Hatano S, Inamura K, Takada S, Ueno T, Yamashita Y, Satoh Y, Okumura S, Nakagawa K, Ishikawa Y, Mano H. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res. 2009;15:3143–9. doi: 10.1158/1078-0432.CCR-08-3248. [DOI] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 28.Sasaki T, Rodig SJ, Chirieac LR, Jänne PA. The biology and treatment of EML4-ALK non-small cell lung cancer. Eur J Cancer. 2010;46:1773–80. doi: 10.1016/j.ejca.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 29.Shinmura K, Kageyama S, Tao H, Bunai T, Suzuki M, Kamo T, Takamochi K, Suzuki K, Tanahashi M, Niwa H, Ogawa H, Sugimura H. EML4-ALK fusion transcripts, but no NPM-, TPM3-, CLTC-, ATIC-, or TFG-ALK fusion transcripts, in non-small cell lung carcinomas. Lung Cancer. 2008;61:163–9. doi: 10.1016/j.lungcan.2007.12.013. [DOI] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 30.Martelli MP, Sozzi G, Hernandez L, Pettirossi V, Navarro A, Conte D, Gasparini P, Perrone F, Modena P, Pastorino U, Carbone A, Fabbri A, Sidoni A, Nakamura S, Gambacorta M, Fernández PL, Ramirez J, Chan JK, Grigioni WF, Campo E, Pileri SA, Falini B. EML4-ALK rearrangement in non-small cell lung cancer and non-tumor lung tissues. Am J Pathol. 2009;174:661–70. doi: 10.2353/ajpath.2009.080755. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 31.Fukuyoshi Y, Inoue H, Kita Y, Utsunomiya T, Ishida T, Mori M. EML4-ALK fusion transcript is not found in gastrointestinal and breast cancers. Br J Cancer. 2008;98:1536–9. doi: 10.1038/sj.bjc.6604341. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 32.Inamura K, Takeuchi K, Togashi Y, Nomura K, Ninomiya H, Okui M, Satoh Y, Okumura S, Nakagawa K, Soda M, Choi YL, Niki T, Mano H, Ishikawa Y. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol. 2008;3:13–7. doi: 10.1097/JTO.0b013e31815e8b60. [DOI] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 33.Perner S, Wagner PL, Demichelis F, Mehra R, Lafargue CJ, Moss BJ, Arbogast S, Soltermann A, Weder W, Giordano TJ, Beer DG, Rickman DS, Chinnaiyan AM, Moch H, Rubin MA. EML4-ALK fusion lung cancer: a rare acquired event. Neoplasia. 2008;10:298–302. doi: 10.1593/neo.07878. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 34.Rodig SJ, Mino-Kenudson M, Dacic S, Yeap BY, Shaw A, Barletta JA, Stubbs H, Law K, Lindeman N, Mark E, Janne PA, Lynch T, Johnson BE, Iafrate AJ, Chirieac LR. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res. 2009;15:5216–23. doi: 10.1158/1078-0432.CCR-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 35.Galkin AV, Melnick JS, Kim S, Hood TL, Li N, Li L, Xia G, Steensma R, Chopiuk G, Jiang J, Wan Y, Ding P, Liu Y, Sun F, Schultz PG, Gray NS, Warmuth M. Identification of NVP-TAE684, a potent, selective, and efficacious inhibitor of NPM-ALK. Proc Natl Acad Sci U S A. 2007;104:270–5. doi: 10.1073/pnas.0609412103. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 36.Christensen JG, Zou HY, Arango ME, Li Q, Lee JH, McDonnell SR, Yamazaki S, Alton GR, Mroczkowski B, Los G. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther. 2007;6:3314–22. doi: 10.1158/1535-7163.MCT-07-0365. [DOI] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 37.Soda M, Takada S, Takeuchi K, Choi YL, Enomoto M, Ueno T, Haruta H, Hamada T, Yamashita Y, Ishikawa Y, Sugiyama Y, Mano H. A mouse model for EML4-ALK-positive lung cancer. Proc Natl Acad Sci U S A. 2008;105:19893–7. doi: 10.1073/pnas.0805381105. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 38.McDermott U, Iafrate AJ, Gray NS, Shioda T, Classon M, Maheswaran S, Zhou W, Choi HG, Smith SL, Dowell L, Ulkus LE, Kuhlmann G, Greninger P, Christensen JG, Haber DA, Settleman J. Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res. 2008;68:3389–95. doi: 10.1158/0008-5472.CAN-07-6186. [DOI] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 39.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, Ou SH, Dezube BJ, Jänne PA, Costa DB, Varella-Garcia M, Kim WH, Lynch TJ, Fidias P, Stubbs H, Engelman JA, Sequist LV, Tan W, Gandhi L, Mino-Kenudson M, Wei GC, Shreeve SM, Ratain MJ, Settleman J, Christensen JG, Haber DA, Wilner K, Salgia R, Shapiro GI, Clark JW, Iafrate AJ. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 40.Camidge DR, Bang Y, Kwak EL, Shaw AT, Iafrate AJ, Maki RG, Solomon BJ, Ou SI, Salgia R, Wilner KD, Costa DB, Shapiro G, LoRusso P, Stephenson P, Tang Y, Ruffner K, Clark JW. Progression-free survival (PFS) from a phase I study of crizotinib (PF-02341066) in patients with ALK-positive non-small cell lung cancer (NSCLC) J Clin Oncol. 2011;29(Suppl):2501. [Google Scholar]
- 41.Shaw AT, Yeap BY, Solomon BJ, Riely GJ, Iafrate AJ, Shapiro G, Costa DB, Butaney M, Ou SI, Maki RG, Bang Y, Varella-Garcia M, Salgia R, Wilner KD, Kulig K, Selaru P, Tang Y, Kwak EL, Clark JW, Camidge DR. Impact of crizotinib on survival in patients with advanced, ALK-positive NSCLC compared with historical controls. J Clin Oncol. 2011;29(Suppl):7507. [Google Scholar]
- 42.Bilsland JG, Wheeldon A, Mead A, Znamenskiy P, Almond S, Waters KA, Thakur M, Beaumont V, Bonnert TP, Heavens R, Whiting P, McAllister G, Munoz-Sanjuan I. Behavioral and neurochemical alterations in mice deficient in anaplastic lymphoma kinase suggest therapeutic potential for psychiatric indications. Neuropsychopharmacology. 2008;33:685–700. doi: 10.1038/sj.npp.1301446. [DOI] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 43.Bazigou E, Apitz H, Johansson J, Lorén CE, Hirst EM, Chen PL, Palmer RH, Salecker I. Anterograde Jelly belly and Alk receptor tyrosine kinase signaling mediates retinal axon targeting in Drosophila. Cell. 2007;128:961–75. doi: 10.1016/j.cell.2007.02.024. [DOI] [PubMed] [Google Scholar]; F1000 Factor 8Evaluated by Talila Volk 20 Mar 2007
- 44.Vernersson E, Khoo NK, Henriksson ML, Roos G, Palmer RH, Hallberg B. Characterization of the expression of the ALK receptor tyrosine kinase in mice. Gene Expr Patterns. 2006;6:448–61. doi: 10.1016/j.modgep.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Mosse YP, Laudenslager M, Longo L, Cole KA, Wood A, Attiyeh EF, Laquaglia MJ, Sennett R, Lynch JE, Perri P, Laureys G, Speleman F, Kim C, Hou C, Hakonarson H, Torkamani A, Schork NJ, Brodeur GM, Tonini GP, Rappaport E, Devoto M, Maris JM. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008;455:930–5. doi: 10.1038/nature07261. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 factor 7Evaluated by Ruth Palmer 14 Oct 2011, Frans Van Roy 24 Sep 2008.
- 46.Janoueix-Lerosey I, Lequin D, Brugières L, Ribeiro A, de Pontual L, Combaret V, Raynal V, Puisieux A, Schleiermacher G, Pierron G, Valteau-Couanet D, Frebourg T, Michon J, Lyonnet S, Amiel J, Delattre O. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature. 2008;455:967–70. doi: 10.1038/nature07398. [DOI] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 47.George RE, Sanda T, Hanna M, Fröhling S, Luther W, 2nd, Zhang J, Ahn Y, Zhou W, London WB, McGrady P, Xue L, Zozulya S, Gregor VE, Webb TR, Gray NS, Gilliland DG, Diller L, Greulich H, Morris SW, Meyerson M, Look AT. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature. 2008;455:975–8. doi: 10.1038/nature07397. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 48.Chen Y, Takita J, Choi YL, Kato M, Ohira M, Sanada M, Wang L, Soda M, Kikuchi A, Igarashi T, Nakagawara A, Hayashi Y, Mano H, Ogawa S. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008;455:971–4. doi: 10.1038/nature07399. [DOI] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 49.Caren H, Abel F, Kogner P, Martinsson T. High incidence of DNA mutations and gene amplifications of the ALK gene in advanced sporadic neuroblastoma tumours. Biochem J. 2008;416:153–9. doi: 10.1042/BJ20081834. [DOI] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 50.Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, Yatabe Y, Takeuchi K, Hamada T, Haruta H, Ishikawa Y, Kimura H, Mitsudomi T, Tanio Y, Mano H, ALK Lung Cancer Study Group EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 2010;363:1734–9. doi: 10.1056/NEJMoa1007478. [DOI] [PubMed] [Google Scholar]
- 51.Yun C. H., Mengwasser K. E., Toms A. V., Woo M. S., Greulich H., Wong K. K., Meyerson M., Eck M. J. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A. 2008;105:2070–5. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katayama R, Khan TM, Benes C, Lifshits E, Ebi H, Rivera VM, Shakespeare WC, Iafrate AJ, Engelman JA, Shaw AT. Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4-ALK. Proc Natl Acad Sci U S A. 2011;108:7535–40. doi: 10.1073/pnas.1019559108. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 53.Sakamoto H, Tsukaguchi T, Hiroshima S, Kodama T, Kobayashi T, Fukami TA, Oikawa N, Tsukuda T, Ishii N, Aoki Y. CH5424802, a Selective ALK Inhibitor Capable of Blocking the Resistant Gatekeeper Mutant. Cancer Cell. 2011;19:679–90. doi: 10.1016/j.ccr.2011.04.004. [DOI] [PubMed] [Google Scholar]; F1000 factor 6Evaluated by Ruth Palmer 14 Oct 2011
- 54.Lee CC, Jia Y, Li N, Sun X, Ng K, Ambing E, Gao MY, Hua S, Chen C, Kim S, Michellys PY, Lesley SA, Harris JL, Spraggon G. Crystal structure of the ALK (anaplastic lymphoma kinase) catalytic domain. Biochem J. 2010;430:425–37. doi: 10.1042/BJ20100609. [DOI] [PubMed] [Google Scholar]
- 55.Bossi RT, Saccardo MB, Ardini E, Menichincheri M, Rusconi L, Magnaghi P, Orsini P, Avanzi N, Borgia AL, Nesi M, Bandiera T, Fogliatto G, Bertrand JA. Crystal structures of anaplastic lymphoma kinase in complex with ATP competitive inhibitors. Biochemistry. 2010;49:6813–25. doi: 10.1021/bi1005514. [DOI] [PubMed] [Google Scholar]
- 56.Lovly CM, Heuckmann JM, de Stanchina E, Chen H, Thomas RK, Liang C, Pao W. Insights into ALK-driven cancers revealed through development of novel ALK tyrosine kinase inhibitors. Cancer Res. 2011;71:4920–31. doi: 10.1158/0008-5472.CAN-10-3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sabbatini P, Korenchuk S, Rowand JL, Groy A, Liu Q, Leperi D, Atkins C, Dumble M, Yang J, Anderson K, Kruger RG, Gontarek RR, Maksimchuk KR, Suravajjala S, Lapierre RR, Shotwell JB, Wilson JW, Chamberlain SD, Rabindran SK, Kumar R. GSK1838705A inhibits the insulin-like growth factor-1 receptor and anaplastic lymphoma kinase and shows antitumor activity in experimental models of human cancers. Mol Cancer Ther. 2009;8:2811–20. doi: 10.1158/1535-7163.MCT-09-0423. [DOI] [PubMed] [Google Scholar]
- 58.Kruczynski A, Mayer P, Marchand A, Vispé S, Fournier E, Annereau JP, Brel V, Barret JM, Delsol G, Imbert T, Fahy J, Bailly C. Antitumor activity of pyridoisoquinoline derivatives F91873 and F91874, novel multikinase inhibitors with activity against the anaplastic lymphoma kinase. Anticancer Drugs. 2009;20:364–72. doi: 10.1097/CAD.0b013e32832a2ed9. [DOI] [PubMed] [Google Scholar]
- 59.Choi YL, Takeuchi K, Soda M, Inamura K, Togashi Y, Hatano S, Enomoto M, Hamada T, Haruta H, Watanabe H, Kurashina K, Hatanaka H, Ueno T, Takada S, Yamashita Y, Sugiyama Y, Ishikawa Y, Mano H. Identification of novel isoforms of the EML4-ALK transforming gene in non-small cell lung cancer. Cancer Res. 2008;68:4971–6. doi: 10.1158/0008-5472.CAN-07-6158. [DOI] [PubMed] [Google Scholar]
- 60.Garcia-Echeverria C, Kanazawa T, Kawahara E, Masuya K, Matsuura N, Miyake T, Ohmori O, Umemura I, Steensma R, Chopiuk G, Jiang J, Wan Y, Ding Q, Zhang Q, Gray NS, Karanewsky D. 2,4-Pyrimidinediamines useful in the treatment of neoplastic disease, inflammatory and immune system disorders. Novartis AG, Novartis Pharma. 2005 GmbH, Patent number: WO/PCT 2005016894. [Google Scholar]
- 61.Ardini E, Menichincheri M, De Ponti C, Amboldi N, Saccardo MB, Texido G, Russo M, Orsini P, Bandiera T, Lombardi Borgia A, Isacchi A, Pesenti E, Colotta F, Magnaghi P, Galvani A, Nerviano Medical Characterization of NMS-E628, a small molecule inhibitor of anaplastic lymphoma kinase with antitumor efficacy in ALK-dependent lymphoma and non-small cell lung cancer models. Mol Cancer Ther. 2009;8(Suppl 1):A243. [Google Scholar]
- 62.Jake Slavish P, Jiang Q, Cui X, Morris SW, Webb TR. Design and synthesis of a novel tyrosine kinase inhibitor template. Bioorg Med Chem. 2009;17:3308–16. doi: 10.1016/j.bmc.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Milkiewicz KL, Weinberg LR, Albom MS, Angeles TS, Cheng M, Ghose AK, Roemmele RC, Theroff JP, Underiner TL, Zificsak CA, Dorsey BD. Synthesis and structure-activity relationships of 1,2,3,4-tetrahydropyrido[2,3-b]pyrazines as potent and selective inhibitors of the anaplastic lymphoma kinase. Bioorg Med Chem. 2010;18:4351–62. doi: 10.1016/j.bmc.2010.04.087. [DOI] [PubMed] [Google Scholar]
- 64.Okamoto M, Kojima H, Saito N, Okabe T, Masuda Y, Furuya T, Nagano T. Virtual screening and further development of novel ALK inhibitors. Bioorg Med Chem. 2011;19:3086–95. doi: 10.1016/j.bmc.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 65.Sequist LV, Gettinger S, Senzer NN, Martins RG, Jänne PA, Lilenbaum R, Gray JE, Iafrate AJ, Katayama R, Hafeez N, Sweeney J, Walker JR, Fritz C, Ross RW, Grayzel D, Engelman JA, Borger DR, Paez G, Natale R. Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non-small-cell lung cancer. J Clin Oncol. 2010;28:4953–60. doi: 10.1200/JCO.2010.30.8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Z, Sasaki T, Tan X, Carretero J, Shimamura T, Li D, Xu C, Wang Y, Adelmant GO, Capelletti M, Lee HJ, Rodig SJ, Borgman C, Park SI, Kim HR, Padera R, Marto JA, Gray NS, Kung AL, Shapiro GI, Jänne PA, Wong KK. Inhibition of ALK, PI3K/MEK, and HSP90 in murine lung adenocarcinoma induced by EML4-ALK fusion oncogene. Cancer Res. 2010;70:9827–36. doi: 10.1158/0008-5472.CAN-10-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li R, Morris SW. Development of anaplastic lymphoma kinase (ALK) small-molecule inhibitors for cancer therapy. Med Res Rev. 2008;28:372–412. doi: 10.1002/med.20109. [DOI] [PubMed] [Google Scholar]
- 68.Milkiewicz KL, Ott GR. Inhibitors of anaplastic lymphoma kinase: a patent review. Expert Opin Ther Pat. 2010;20:1653–81. doi: 10.1517/13543776.2010.527332. [DOI] [PubMed] [Google Scholar]