Kisspeptin gene and protein expression are down-regulated in a state of leptin deficiency and in response to diet-induced obesity.
Abstract
The hormone leptin modulates a diverse range of biological functions, including energy homeostasis and reproduction. Leptin promotes GnRH function via an indirect action on forebrain neurons. We tested whether leptin deficiency or leptin resistance due to a high-fat diet (HFD) can regulate the potent reproductive neuropeptide kisspeptin. In mice with normalized levels of estradiol, leptin deficiency markedly reduced kisspeptin gene expression, particularly in the arcuate nucleus (ARC), and kisspeptin immunoreactive cell numbers in the rostral periventricular region of the third ventricle (RP3V). The HFD model was used to determine the effects of diet-induced obesity and central leptin resistance on kisspeptin cell number and gene expression. DBA/2J mice, which are prone to HFD-induced infertility, showed a marked decrease in kisspeptin expression in both the RP3V and ARC and cell numbers in the RP3V after HFD. This is the first evidence that kisspeptin can be regulated by HFD and/or increased body weight. Next we demonstrated that leptin does not signal (via signal transducer and activator of transcription 3 or 5, or mammalian target of rapamycin) directly on kisspeptin-expressing neurons in the RP3V. Lastly, in leptin receptor-deficient mice, neither GnRH nor kisspeptin neurons were activated during a preovulatory-like GnRH/LH surge induction regime, indicating that leptin's actions on GnRH may be upstream of kisspeptin neurons. These data provide evidence that leptin's effects on reproductive function are regulated by kisspeptin neurons in both the ARC and RP3V, although in the latter site the effects are likely to be indirect.
Fertility and the hypothalamic-pituitary-gonadal axis are closely linked to nutritional status. For women, a lean body type can decrease GnRH production, resulting in cessation of ovulation (1). Obesity can also have detrimental effects on conception and implantation rates (2, 3). Multiple metabolic signals (such as glucose and a raft of metabolic hormones) connect nutritional status and reproductive function (1, 4). Leptin is a metabolic hormone secreted from fat cells. Its primary role is to regulate energy intake and expenditure. However, animals with dysfunctional leptin signaling also have marked suppression of fertility (5, 6). Leptin replacement can overcome fasting-induced suppression of LH pulses in both rodents and primates (7, 8). The primary site of leptin's effects on the reproductive system is central; however, we recently demonstrated that leptin does not directly act on GnRH neurons (9). A number of neuropeptides could feasibly provide an interneuronal link between the leptin receptors that sense an individual's energy balance and the GnRH system. Proopiomelanocortin, neuropeptide Y, and cocaine- and amphetamine-regulated transcript (10–12) have all been proposed as possible intermediaries. More recently, kisspeptin joined this list as a potential intermediary neuropeptide linking metabolic status and reproductive function (13).
Kisspeptins act in the brain to control fertility. The kisspeptin peptides are an expression of the Kiss1 gene. Originally discovered as a 54-amino acid peptide, kisspeptin-54 or metastin was found to inhibit metastatic cancer cells (14). Kisspeptin-54 or shorter products such as kisspeptin-10 have since been found to potently and directly activate GnRH neurons (15). In rodents, kisspeptin-expressing neurons are predominately found in two discrete populations within the hypothalamus: the rostral periventricular region of the third ventricle [RP3V: composed of the anteroventral periventricular nucleus (AVPV) and the preoptic periventricular nucleus] and the arcuate nucleus (ARC) (16).
Although a role for kisspeptin in body weight regulation is unlikely (17), kisspeptin may mediate the effects of leptin on fertility. For example, lactating (a state of negative energy balance) rats have decreased amounts of kisspeptin mRNA and protein in the ARC (18, 19). Smith et al. (20) found approximately 40% of kisspeptin-expressing neurons in the ARC express the gene for leptin receptor. Additionally, male mice lacking functional leptin displayed less Kiss1 mRNA in the ARC as measured by in situ hybridization (20). In rats, mice, and sheep, food restriction has been shown to reduce hypothalamic kisspeptin mRNA levels (13, 21–23). These results provide evidence for a leptin receptor → kisspeptin neuron → GnRH neuron relay. Importantly, females have a much larger RP3V kisspeptin population than males, and these neurons exhibit a pronounced estrogen-induced change in kisspeptin expression (24). The effects of leptin deficiency (as opposed to negative energy balance) on kisspeptin expression have not previously been investigated in females. At the other extreme, it is unknown how the kisspeptin system responds to diet-induced obesity (DIO) and thus high circulating levels of leptin, which ultimately leads to a state of central leptin resistance.
The aim of this paper was to measure the effect of leptin deficiency on kisspeptin mRNA levels and cell numbers expressing kisspeptin protein in the RP3V and ARC of female mice with different levels of circulating estrogens. We also investigated the effect of DIO on kisspeptin cell numbers and mRNA expression using a high-fat diet (HFD) model. Additionally, we attempted to determine whether leptin could regulate kisspeptin neurons directly by assessing leptin-signaling pathways in kisspeptin neurons using dual label immunohistochemistry. Three signaling pathways were measured: the signal transducer and activator of transcription (STAT) 3 and STAT5 pathways, and the mammalian target of rapamycin complex 1 (mTORC1)-dependant pathway. Whereas phosphorylation of STAT3 (pSTAT3) is widely used as a marker of leptin signaling (25, 26), pSTAT5 and mTORC1 have more recently been implicated in hypothalamic leptin signaling (27, 28). Lastly, we investigated the effect of leptin deficiency on kisspeptin neuronal activation during the GnRH/LH surge.
Materials and Methods
Animals
All mice were obtained from the University of Otago animal breeding facility. Mice were group housed (individual housing was used after surgery) and maintained on a 12-h light, 12-h dark cycle (lights on at 0600 h) at a constant temperature (22 ± 1 C). Mice had free access to food and water. The University of Otago Animal Ethics Committee approved all animal protocols.
Experiment 1: effect of leptin deficiency on kisspeptin mRNA levels and cell numbers
This study used adult female leptin-deficient homozygous ob/ob mice (8–10 wk old; body weight ∼40 g), with wild-type littermates (body weight ∼20 g) serving as controls. Three different levels of circulating estradiol were used: ovariectomized (OVX), OVX with low-dose replacement estradiol (OVX+lowE; to mimic estrogen's negative feedback effects), and OVX with low-dose replacement estradiol and then injected with estradiol benzoate (OVX+surgeE; to mimic the estrogenic state leading up to the preovulatory LH surge). Mice in the OVX+lowE and OVX+surgeE groups were treated as described previously (9, 29). Briefly, mice were OVX and had a silicone capsule (internal diameter, 1.0 mm; external diameter, 2.1 mm; Dow Corning, Midland, MI) filled with silicone adhesive (Dow Corning) containing 17-β estradiol (0.1 mg/ml adhesive; Sigma-Aldrich, St Louis, MO) implanted sc. Each mouse was given a 1-cm capsule/20 g body weight. Six days after ovariectomy, OVX+surgeE mice received an sc injection of estradiol benzoate (0.05 mg/kg; Sigma-Aldrich) at 0800 h. At 1800 h the following day (the surge peak time), brains were collected from all three groups for quantitative RT-PCR (qRT-PCR) analysis. Trunk blood was collected from OVX+lowE and OVX+surgeE mice for LH and/or estradiol assay.
Experiment 2: effect of HFD on kisspeptin mRNA levels and cell numbers
Female mice (DBA/2J and C57BL/6J) were obtained at weaning and randomly assigned to receive either a low-fat standard diet (4.8%; Specialty Feeds, Glen Forrest, Australia) or a diet containing 23% fat (SF03-020, Specialty Feeds). In previous work it has been shown that DBA/2J mice are prone to HFD-induced infertility whereas C57BL/6J mice are not (Ref. 30 and our own unpublished observations). All mice remained on their respective diets until 24 wk of age. For mRNA analysis, mice were weighed and OVX 7 d before being killed at the age of 24 wk. Brains were collected for qRT-PCR and trunk blood was collected for leptin assay. A separate group of DBA/2J female mice were raised on HFD or standard chow and then subjected to the OVX+surgeE protocol above. At 19 wk of age brains were collected and prepared for immunohistochemistry.
Experiment 3: measurement of leptin-induced signaling in kisspeptin neurons
Mice (C57BL/6J) were fasted overnight to reduce endogenous circulating leptin concentrations, and 50 μg of bromocriptine mesylate (Tocris Biosciences, Ellisville, MI) were given sc at 0900 h to reduce activation of the STAT5 pathway by prolactin (31). Recombinant mouse leptin (5 mg/kg) (National Hormone and Peptide Program, Torrance, CA) or vehicle (0.2 ml PBS, pH 7.8) was injected ip at 1200 h. Mice were perfused 2 h later via the heart with 20 ml of 4% paraformaldehyde, and brains were collected for immunohistochemistry.
Experiment 4: effect of deficient leptin signaling on kisspeptin neuronal activation during a preovulatory-like LH surge
This experiment used a forebrain neuron-specific leptin receptor knockout mouse that was part of another study (9). Neuron-specific leptin receptor (Lepr) knockout mice were generated by crossing Lepr flox mice with CamKIIα-iCre BAC mice (Leprfl/fl, CamKIIα-Cre) as described elsewhere (9). Littermates homozygous for Lepr flox (Leprfl/fl), but with no CamKIIα-iCre expression, served as controls. Mice were subjected to an OVX+surgeE protocol as described above. A group of control mice (Leprfl/fl) subjected to an OVX+lowE protocol were included to serve as nonsurging controls. Mice were killed and tissue and blood were collected at 1800 h the following day. Brains were processed for immunohistochemical detection of kisspeptin and c-Fos (an early-intermediate gene used as a marker of neuronal activation), and blood sera were processed to assess LH concentration.
Kiss1 mRNA analysis by qRT-PCR
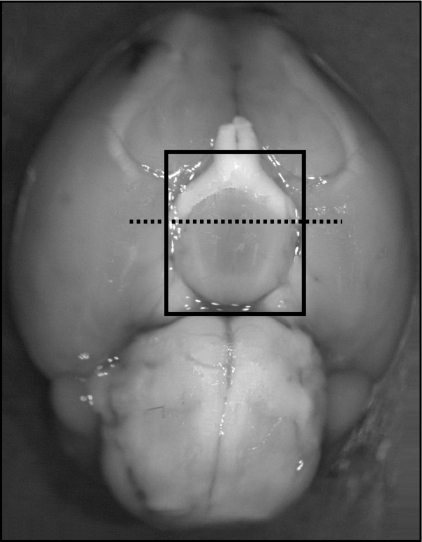
For measurement of Kiss1 mRNA, mice were decapitated, and brains were immediately removed from the skull and frozen at −80 C for later use. Hypothalami were dissected along the following boundaries: laterally 2 mm either side of the third ventricle from the optic chiasm to the posterior border of the mammillary bodies, and the thalamus dorsally. Hypothalami were then bisected in the coronal plane immediately anterior to the pituitary stalk. The anterior dissection contained all of the RP3V, and the posterior dissection contained the entire ARC (Fig. 1). To control for the amount of tissue dissected per mouse, and thus entering the reverse transcription reaction, hypothalamic tissue blocks were weighed. No significant differences in hypothalamic tissue block weight were noted between comparable experimental groups (P > 0.4).
Fig. 1.
Ventral view of mouse brain. The hypothalamus was dissected out along the limits of the black square. Anterior and posterior hypothalamic blocks were separated from each other (dotted line).
These tissues were thoroughly homogenized with sterile plastic pestles and sonicated in TRIzol solution (Invitrogen, Calsbad, CA). Total RNA was separated from excess protein and genomic DNA by chloroform phase separation and then further cleaned using the standard protocol for RNeasy Mini Spin Columns (QIAGEN, Valencia, CA).
RNA quality was assessed by spectrophotometry and agarose gel electrophoresis. The total amount of RNA in each sample was quantified using an ND-1000 spectrophotometer (NanoDrop; Thermo Scientific, Waltham, MA), and 500 ng of total RNA were subjected to deoxyribonuclease I treatment (Ambion, Austin, TX) according to manufacturer's instructions. Total RNA was then reverse transcribed with SuperScript III reverse transcriptase (Invitrogen) using random hexamers as primers.
Triplicate uniplex reactions for measurement of Kiss1 mRNA levels were carried out on cDNA samples using the following primers: forward, 5′-CCTTCCTCCCAGAATGATCTC-3′; and reverse, 5′-GATCCAGGCTTCACTTTTGC-3′ (NCBI reference sequence NM_178260.3). Primers specific for three reference genes were tested over our experimental samples: β-actin, polymerase (RNA) II (DNA directed) polypeptide A (Polr2a), and tata-box binding protein. The reference gene Polr2a was chosen in this experiment due to its minimal variation across experimental groups: forward, 5′-GCACCACGTCCAATGATAT-3′ and reverse, 5′-GTGCTGCTGCTTCCATAA-3′ (NCBI reference sequence NM_009089.2) (32). Reactions (20 μl) were prepared in 96-well optical reaction plates with optical adhesive covers (ABI Prism; Applied Biosystems, Foster City, CA) using SYBR Green PCR Master Mix (Applied Biosystems). Using an ABI PRISM 7300 qPCR thermocycler (Applied Biosystems), samples were heated to 50 C for 2 min, then 95 C for 10 min before 40 cycles of 95 C for 15 sec and 60 C for 1 min as per the manufacturer's instructions. Prior validation experiments were performed to demonstrate that amplification efficiencies of Kiss1 (0.9) were approximately equal to that of Polr2a (1.0).
Quantitative PCR data were analyzed using the comparative Cq method in which Cq is the quantification cycle number at which the fluorescence reading is first recorded above background levels (33). The ranges of Cq values for Polr2a and Kiss1 were similar (Cq = 22–32 and 25–34, respectively). Subtracting the average Cq value for the gene of interest from the average Cq value for the reference gene gives the ΔCq. The comparative Cq method is a relative measure of mRNA expression; thus a fixed arbitrary number can be can be used as a calibrator. Subtracting the calibrator from the ΔCq for all samples gave the relative change (ΔΔCq). Finally the arithmetic formula 2−ΔΔCq was used to achieve relative quantitation (for detailed information see Applied Biosystems' User Bulletin 2).
Immunohistochemistry for kisspeptin- and leptin-signaling molecules
Mice were perfused with 4% paraformaldehyde in 0.1 m phosphate buffer for brain collection and immunohistochemical staining. Coronal (30 μm thick) sections throughout the RP3V and ARC were cut from each brain on a sliding microtome with a freezing stage to provide three sets of consecutive sections (90 μm apart). One set of sections was labeled for kisspeptin by first incubating in polyclonal rabbit antikisspeptin primary antibody (Chemicon AB9754; Millipore, Billerica, MA; 1:20,000 dilution), followed by biotinylated goat antirabbit IgG secondary antibody (Vector Laboratories, Inc., Burlingame, CA; 1:500 dilution). The signal was amplified using avidin-biotin peroxidase (Elite Vectorstain; Vector Laboratories) and then diaminobenzidine solution to visualize the kisspeptin immunoreactivity (brown staining). Kisspeptin neurons in three sections through the RP3V (Figs. 26–32 in Ref. 34). and three sections through the ARC (Figs. 43–52 in Ref. 34) per animal were counted.
For dual-label immunohistochemistry, kisspeptin was first stained as above, and then remaining peroxidase activity was removed by washing with H2O2. Subsequently, sections were incubated in polyclonal rabbit anti-pSTAT3 (Tyr750), anti-pSTAT5 (Tyr694), anti-pS6 (Ser235/236; Cell Signaling Technology, Danvers, MA; 1:2000, 1:1000, and 1:100 dilution, respectively), or anti-c-Fos primary antibodies (AB5; Calbiochem, San Diego, CA; 1:20,000 dilution). Phospho-S6 is the phosphorylated form of the ribosomal protein S6 kinase1, which is a downstream target of mTORC1. Before incubation with the pSTAT5 primary antibody, sections were heated for 5 min at 90 C in 0.01 m Tris (pH 10) to unmask antigens. For anti-pSTAT3, 5 and c-Fos, goat antirabbit horseradish peroxidase (Dako P0449; Glostrup, Denmark; 1:500 dilution) was the secondary antibody used, and immunoreactivity was visualized using nickel-enhanced diaminobenzidine to generate a blue-black nuclear stain. Kisspeptin neurons were counted as pSTAT3-, pSTAT5-, or c-Fos-positive if they had distinct blue-black stained nuclei surrounded by a brown cytoplasmic stain.
Dual-label immunohistochemistry for kisspeptin and mTORC1 differed in that after incubating tissues with both antikisspeptin (sheep antimouse kindly provided by Dr. A. Caraty) and anti-pS6 (rabbit antimouse), tissues were incubated in biotinylated donkey antirabbit (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA; 1:200 dilution) secondary. This was followed by incubation in Texas Red donkey antisheep IgG (Jackson; 1:200 dilution) secondary and conjugated-Fluorescein green strepavidin DCS (Cell sorting grade; Vector Laboratories Inc.; 1:200). Sections mounted with Vectashield Mounting Medium (Vector Laboratories, Inc.) were visualized in the 540–580 and 460–490 excitation wavelength ranges, respectively.
An operator blind to the treatment groups performed all cell counting. Omission of any of the primary anybodies resulted in complete absence of staining.
Hormone assays
Trunk blood was obtained at death and allowed to clot at room temperature for 30 min and then centrifuged at 2500 × g for 15 min at 4 C. Blood sera were then transferred into clean tubes and stored at −20 C until required. An ELISA was used to measure mouse serum leptin (EZML-82K, Millipore) as per manufacturer's instructions. Serum LH concentrations were measured by RIA. Values are expressed in terms of the rat standard National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)-rat LH-RP-3. The tracer, iodinated hormone (NIDDK-rat LH-I-10) was used, and the primary antiserum was NIDDK-rabbit antirat LH-S11 (final dilution 1:500,000). Serum estradiol levels were quantified using an estradiol RIA (DSL-4800, Diagnostic Systems Laboratories, Inc. Webster, TX) as per manufacturer's instructions except an overnight incubation was used after addition of [125I]estradiol. Intraassay coefficient of variation for the LH assays was 16%; those for leptin ELISA and estradiol RIA were less than 5%, and the sensitivities of the assays were 0.2 ng/ml, 0.2 ng/ml, and 0.8 pg/ml, respectively. All samples were analyzed in single assays. LH levels from the mice used in experiment 4 have been reported previously (9).
Statistical analysis
All data were analyzed by Student's t test or one-way ANOVA followed by Bonferroni post hoc analysis where appropriate. The threshold level for statistical significance was set at 5% (P < 0.05).
Results
Experiment 1a: levels of Kiss1 mRNA in the ARC are reduced during leptin deficiency
The replacement estradiol protocol produced circulating estradiol concentrations that were only slightly above assay detection levels in OVX+lowE animals but markedly elevated in OVX+surgeE animals (OVX+lowE: 4.1 ± 0.9 pg/ml; n = 24 compared with OVX+surgeE: 63.7.5 ± 24.4 pg/ml; n = 22; P < 0.05). There were no significant differences in circulating estradiol levels between fat and thin animals treated with the same estradiol regime. Wild-type control animals (n = 15) that underwent OVX+surgeE had LH levels significantly higher than ob/ob mice (n = 13; 4.4 ± 1.0 ng/ml vs. 0.6 ± 0.1 ng/ml, respectively; P < 0.01), indicating that leptin-deficient mice were unable to mount an LH surge even in the presence of high levels of estrogens.
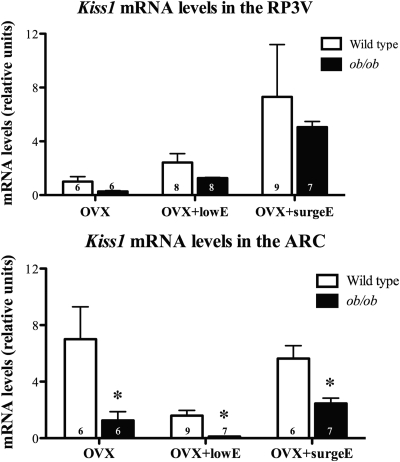
Kiss1 mRNA levels were measured in the RP3V and the ARC under three estradiol conditions in ob/ob mice and wild-type littermates. Kiss1 mRNA increased in the RP3V with increasing levels of estradiol in both wild-type and ob/ob mice under all conditions. There was a nonsignificant decrease in the amount of Kiss1 mRNA found in leptin-deficient ob/ob mice in all groups (Fig. 2, top panel). As expected there was more Kiss1 mRNA in the ARC of OVX animals than in those with low-dose estradiol replacement (Fig. 2, bottom panel). The amount of Kiss1 mRNA under surge conditions was between those found in OVX and OVX+lowE treated animals. The amount of Kiss1 mRNA detected in the ARC of leptin-deficient ob/ob mice was significantly less than wild-type mice in all estrogenic states (P < 0.05; Fig. 2, bottom panel).
Fig. 2.
Effects of leptin deficiency on Kiss1 mRNA in the RP3V (top) and ARC (bottom) in female mice with normalized estrogen levels. The number of animals per group is given at the base of each column. *, P < 0.05.
Experiment 1b: kisspeptin-immunopositive neurons in the RP3V are fewer in leptin-deficient mice
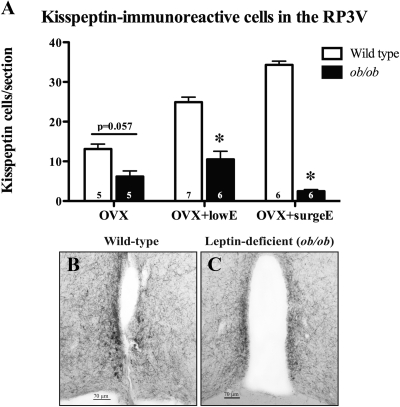
Similarly to Kiss1 mRNA levels in the RP3V, the number of kisspeptin-immunoreactive cell bodies in the RP3V of wild-type mice increased with the amount of estradiol exposure (Fig. 3A). However, ob/ob mice had markedly less kisspeptin-immunoreactive cells under all estrogenic conditions tested (P = 0.057 for OVX and P < 0.05 for other groups; Fig. 3, A–C).
Fig. 3.
Effects of leptin deficiency on numbers of immunoreactive kisspeptin neurons in the RP3V in female mice with normalized estrogen levels (A). Representative examples of immunoreactive kisspeptin neurons in the RP3V (B and C) in wild-type (B) and leptin-deficient (C) mice are shown. Examples shown are from OVX+lowE mice. The number of animals per group is given at the base of each column. *, P < 0.05.
As previously reported by others (35), it was very difficult to visualize kisspeptin-immunoreactive cell bodies in the ARC due to the dense immunopositive fiber network. On average less than one cell was identified per section; thus significant conclusions could not be drawn from these data.
Experiment 2a: hypothalamic Kiss1 mRNA decreases in DBA/2J mice after DIO
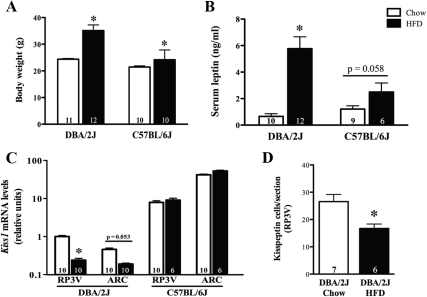
Female DBA/2J mice (and, to a lesser extent, C57BL/6J mice) showed significant weight gain after 21 wk on a HFD (Fig. 4A). This weight gain was proportional to circulating serum leptin levels recorded at death (Fig. 4B).
Fig. 4.
Effects of HFD on body weight (A), serum leptin concentration (B) Kiss1 mRNA levels (C), and RP3V kisspeptin-immunoreactive cell numbers (D) in adult female OVX mice. The number of animals per group is given at the base of each column. *, P < 0.05.
Kiss1 mRNA levels were measured in the RP3V and the ARC in standard chow and HFD animals in both strains. It is of note that DBA/2J mice, which are highly susceptible to obesity-induced infertility (30), had 10-fold less Kiss1 mRNA compared with their C57BL/6J counterparts (Fig. 4C). Kiss1 mRNA was markedly decreased in both the RP3V and ARC of DBA/2J mice fed a HFD (Fig. 4C, left). In contrast, the amount of Kiss1 mRNA did not change in either the RP3V or the ARC of C57BL/6J mice fed a HFD (Fig. 4C, right).
Experiment 2b: kisspeptin-immunopositive neurons in the RP3V decrease in DBA/2J mice after DIO
Female DBA/2J mice subjected to an OVX+surgeE protocol showed a 35% decrease in the number of kisspeptin-expressing cells in the RP3V in response to 16 wk of HFD exposure (Fig. 4D).
Experiment 3: kisspeptin neurons of the RP3V do not colocalize with leptin-induced pSTAT signaling
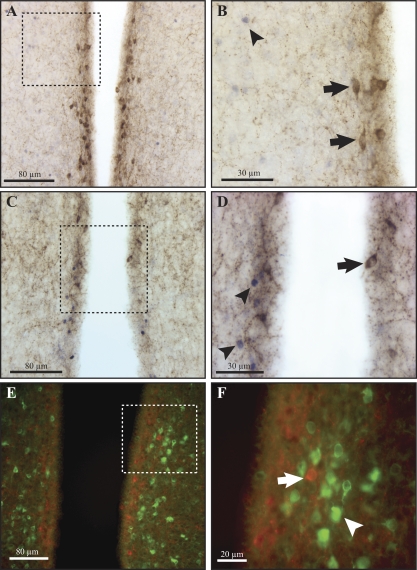
Leptin administered ip induced a marked increase in nuclear pSTAT3 and pSTAT5 immunoreactivity in the RP3V and other hypothalamic regions, particularly the ventromedial hypothalamus and the ARC. There was a striking absence of pSTAT colocalization within kisspeptin neurons in the RP3V (0/385 kisspeptin neurons colocalized with pSTAT3, and 1/280 kisspeptin neurons colocalized with pSTAT5; n = 7; Fig. 5, A–D). Leptin also induced mTORC1 pathway signaling as evidenced by pS6 immunoreactivity with a similar distribution to pSTAT3 and 5. Colocalization of pS6 with kisspeptin-expressing neurons in the RP3V was also completely absent (Fig. 5, E and F).
Fig. 5.
Leptin signaling in kisspeptin-immunopositive neurons in response to an acute injection of leptin (5 mg/kg, ip). Phosphorylated STAT3 (A and B), pSTAT5 (C and D), or pS6 (E and F) were not colocalized with kisspeptin-expressing neurons in the RP3V of adult female mice. The dotted square on the left is magnified on the right. Arrows indicate a lack of pSTAT3/5 or pS6 immunoreactivity in kisspeptin-expressing cells. Arrowheads indicate pSTAT3/5 or pS6 (green) immunoreactivity in unidentified cells.
As noted above, kisspeptin-expressing cell bodies in the ARC were very difficult to distinguish among the dense kisspeptin-positive fiber network. Definition of these cells was even more difficult after leptin treatment and pSTAT immunostaining.
Experiment 4: kisspeptin neurons are not activated by a preovulatory-like LH surge in leptin receptor-deficient mice
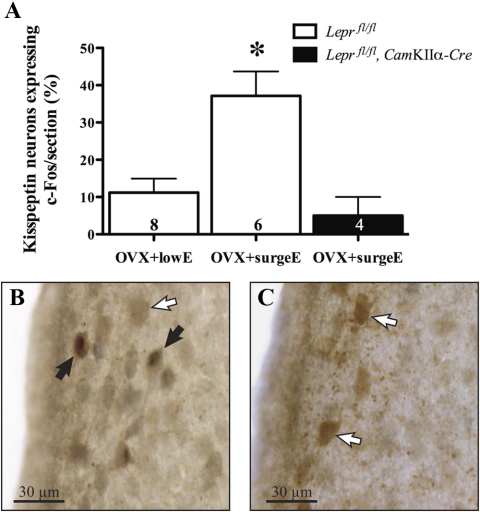
As described elsewhere, neuron-specific Lepr knockout mice were significantly heavier and infertile compared with control mice (9). After wild-type mice were exposed to preovulatory surge-like levels of estradiol LH surge-like levels (OVX+surgeE = 63.8 pg/ml compared with OVX+lowE = 6.8 pg/ml), and GnRH neuronal activation was observed in wild-type but not Lepr knockout mice (9). In wild-type mice, the percentage of RP3V kisspeptin cells expressing c-Fos was significantly increased compared with mice receiving only low-level estradiol treatment (37 ± 6.5% vs. 11 ± 3.8%; P < 0.01). Significantly, this increase was not observed in Lepr knockout mice receiving preovulatory surge-like levels of estradiol (5 ± 6%; Fig. 6).
Fig. 6.
Kisspeptin-immunoreactive neurons within the preoptic periventricular nucleus showing c-Fos signaling in response to a preovulatory-like surge induction protocol compared with control littermates (A). Representative examples show kisspeptin-immunoreactive neurons (brown cytoplasmic staining) and cFos (black nuclear staining) in neuron-specific Lepr knockout (B) and control (C) mice. Solid arrows indicate examples of colocalized neurons; open arrows indicate examples of noncolocalized neurons. The number of animals per group is given at the base of each column. *, P < 0.05 vs. both other groups.
Discussion
The present study aimed to determine whether kisspeptin gene expression is sensitive to changes in the amount of circulating leptin and DIO in female mice. Additionally, leptin signaling was examined in kisspeptin-expressing neurons in the RP3V and ARC. We have shown here that leptin deficiency decreases kisspeptin gene expression predominantly in the ARC, whereas there are fewer kisspeptin-expressing neurons in the RP3V of leptin-deficient mice. Moreover, DBA/2J mice, which are prone to obesity-induced infertility, showed a marked decrease in kisspeptin gene expression in both the RP3V and ARC and a decrease in the number of kisspeptin-immunoreactive neurons in the RP3V after DIO. This is the first evidence that kisspeptin can be regulated by HFD and/or increases in body weight.
Due to leptin's profound effect on the reproductive system, much interest has focused on finding the link between leptin signaling and female fertility. Recently we showed that this link was central, occurring via an indirect neuronal pathway to activate GnRH neurons (9). A number of lines of evidence suggest that kisspeptin could be the intermediary linking leptin signaling to GnRH neurons. It is well known that estrogens regulate kisspeptin gene expression (36); hence, we used three experimental groups with differing circulating levels of estradiol. Kiss1 mRNA and kisspeptin-expressing neurons increased in the RP3V in response to increasing estradiol levels in wild-type mice, as in previous studies (37, 38). Interestingly, ob/ob Kiss1 mRNA levels in the RP3V also increased from a state of low estrogen to surge-like levels of estrogen, indicating that estradiol can promote at least some transcription of Kiss1 even in the absence of leptin.
Previously, female OVX rats with low-dose estrogen replacement fasted for 48 h displayed less Kiss1 mRNA than fed controls in the AVPV (a subregion of the RP3V) but not in the ARC (22). Another report found that prepubertal rats treated with rapamycin (which blocks mTORC1 signaling and suppresses appetite) showed decreased Kiss1 mRNA in both the AVPV and ARC regions of the hypothalamus (28). We report here that Kiss1 mRNA levels decreased in both the RP3V and ARC in response to leptin deficiency, although not significantly in the former region.
Consistent with the literature, Kiss1 mRNA in the ARC was highest under OVX conditions. However, unlike other studies (16, 37), whereas ARC Kiss1 mRNA expression dropped under conditions of low estradiol replacement, it was only slightly reduced under preovulatory-like surge protocol conditions. This discrepancy may be due to different surge protocols; the mice in the study by Smith et al. (16) study and the rats in the study by Adashi et al. (37) had chronically (≥7 d) elevated circulating estradiol concentrations of 200–500 pg/ml, whereas our OVX+surgeE mice had circulating estradiol concentrations of approximately 60 pg/ml for only 33 h.
The present qRT-PCR results suggest leptin deficiency impairs Kiss1 gene expression in the ARC under all estrogenic states. Changes in ARC Kiss1 mRNA expression in response to leptin deficiency agree with previous literature that used fasted prepubertal female rats or male mice (13, 23). The work presented here is consistent with data from male ob/ob mice that also display less Kiss1 mRNA in the ARC, as measured by in situ hybridization (20). Importantly, the work of Smith et al. (20) showed that Lepr and Kiss1 mRNA were coexpressed in the ARC (at least in male mice). The physiological role of the kisspeptin-expressing ARC neurons is still unclear, but they have been proposed to mediate the negative feedback effects of sex steroids on gonadotropin secretion (16, 39), possibly by regulating GnRH pulses at or near GnRH nerve terminals (40). Neuronal tract-tracing studies have shown that unidentified cells in the ARC send projections forward directly to GnRH neurons (29). More recently, it has been shown that colocalized kisspeptin/neurokinin B neurons from the ARC project not only to the GnRH fibers in median eminence but also forward to the periventricular area of the third ventricle (40). However, very few of these rostral projections extend to the preoptic area where the GnRH neurons reside (40). Taken together with the present results, it is likely that leptin acts directly on ARC kisspeptin neurons and thus may contribute positively to GnRH neuronal output at the median eminence, but whether this is essential for fertility remains to be proven.
In studying kisspeptin protein expression, we found that kisspeptin-immunoreactive cell numbers were markedly decreased in leptin-deficient mice in the RP3V under all three estrogenic states tested. This effect was most marked in the OVX+surgeE group of mice. Kiss1 expression in these mice, although tending to be lower in ob/ob animals, was not significantly reduced. This apparently conflicting result with the mRNA data could be due to posttranscriptional deficits in leptin-deficient mice or due to the high degree of variability in the control group (Fig. 2, top panel). These RP3V kisspeptin neurons probably mediate the preovulatory GnRH/LH surge (41), and thus an impairment of this function would be predicted in response to leptin deficiency. Accordingly, we showed that mice with deficient leptin signaling were unable to generate a preovulatory-like GnRH/LH surge (9) or the associated increase in kisspeptin activation in the RP3V (current data) in response to exogenous estrogen.
As has been noted by others (35), accurate quantification of kisspeptin-immunoreactive neurons in the ARC of mice is not possible because positive cells are obscured among a dense kisspeptin-positive fiber network that exists in the ARC. Extending the period of OVX or using colchicine pretreatment (a drug that leads to an accumulation of secretory material within the perikarya of neurosecretory neurons) did not increase definition of kisspeptin-expressing neurons (data not shown). It may be that kisspeptin protein expression in the ARC is very dynamic, with a high turnover and/or rapid transportation out of the cells. Thus in the ARC, mRNA measurements may be a more relevant measure for this neurosecretory product.
Our data do not allow us to distinguish as to whether the reduction in kisspeptin expression is a direct consequence of leptin deficiency, or simply a side effect of failure to initiate puberty in ob/ob mice. To clarify this it would be necessary to replace leptin daily in ob/ob mice until pubertal development is achieved, and then, after allowing leptin levels to subside, repeat the kisspeptin measurements. However, leptin-deficient animals that were stimulated to go through puberty in this way were unable to reproduce after leptin withdrawal, suggesting that adult fertility is indeed reliant on continuous leptin availability (42). Additionally, food restricted postpubertal sheep have reduced kisspeptin gene expression, and leptin infusion can partially reverse this (21), suggesting the effects are not simply a reflection of prepubertal state.
DIO due to HFD exposure for 21 wk was most noticeable in the DBA/2J strain of mice, with an average increase in body weight of about 11 g compared with an approximately 3 g increase for C57BL/6J mice. It has been previously noted that C57BL/6J female mice are resistant to DIO compared with DBA/2J mice (30, 43). The DBA/2J female mouse is sensitive to HFD-induced hypothalamic hypogonadism, showing a decreased GnRH gene expression, a 60% reduction in pregnancy rate, and relatively inactive ovaries (30). We have observed that the diet-induced obese DBA/2J mouse exhibits central resistance to leptin signaling in both the RP3V and ARC and an impaired ability to generate a preovulatory-like GnRH/LH surge (our unpublished data). The data here are the first to show that kisspeptin cell numbers and mRNA expression are decreased by exposure to a HFD in female DBA/2J mice. Luque et al. (23) used male C57BL/6J mice on a HFD for 16 wk; these mice did become approximately 10 g heavier but did not display any changes in hypothalamic Kiss1 gene expression. This was similar to our findings in C57BL/6J HFD-fed female mice (23). Because the most marked effects were seen in female DBA/2J mice, female C57BL/6J mice may not be susceptible to leptin resistance (30) and therefore the associated fertility perturbations. Because our ob/ob mice, which are on a C57BL/6J background strain, had reduced kisspeptin gene expression and were infertile, HFD-induced infertility may occur by different mechanisms than infertility caused by leptin deficiency. Our data reinforce the use of the HFD-fed DBA/2J mouse model to study the neuropeptide changes underpinning DIO-related infertility.
Anatomically, dual label in situ hybridization has been used to show Lepr and Kiss1 colocalization only in the ARC of male mice, and additionally in the preoptic area and ARC of female sheep (20, 21). In our study we observed no evidence for leptin signaling in the RP3V kisspeptin-expressing neurons. The possibility that leptin acts directly on RP3V kisspeptin neurons in rodents to drive the preovulatory GnRH/LH surge has not been investigated until now. Using a functional immunohistochemical approach, leptin-induced pSTAT3, pSTAT5, and pS6 were investigated in kisspeptin-expressing neurons. Virtually no colocalization of any of the these leptin-signaling molecules occurred in kisspeptin-expressing cells within the RP3V, although nearby unidentified cells showed STAT3, STAT5, and S6 activation. Although leptin's actions via other signaling pathways remain possible (44), the STAT3 pathway has generally been thought to be dominant because neuronal STAT3 deletion recapitulates the infertility, obesity, and thermal dysregulation of global leptin and leptin receptor knockout mice (45). However, others have described a leptin receptor knock in transgenic mouse strain that is unable to specifically activate STAT3 signaling; in these mice 43% of the females were fertile (46). One alternative signaling pathway leptin may use to affect fertility is the mTORC1 pathway (27), because the blockade of this pathway with rapamycin inhibits the ability of leptin to stimulate puberty onset (28). Indeed, we observed leptin-induced pS6 (evidence of mTORC1 signaling) in the RP3V with a similar distribution to pSTAT3, but no colocalization with kisspeptin immunoreactivity. Our data therefore strongly indicate that RP3V kisspeptin-expressing cells are not directly activated by leptin. Future studies are required to determine the leptin-responsive inputs to these cells that cause them to become impaired in states of leptin deficiency. In this regard, it has recently been shown that cells from the ventral premammillary nucleus (a region that appears to be critical for leptin's effects on puberty initiation) project to Kiss1 cells in the AVPV (47).
In summary, both leptin deficiency and DIO impair kisspeptin expression. This impairment extends to kisspeptin neuronal activation in the RP3V during a preovulatory-like surge. The work here suggests that whereas the effects of leptin on the ARC kisspeptin neurons may be direct, its effects in the RP3V appear to be indirectly mediated by unknown upstream leptin-sensitive neurons. The identity and location of these leptin-sensitive neurons, which feed into the kisspeptin/GnRH system, are unknown and warrant systematic investigation.
Acknowledgments
We thank Hayden McEwen (University of Otago, Dunedin, New Zealand) for providing HFD animal tissues for immunohistochemistry.
This work was supported by The Royal Society of New Zealand FastStart program (to J.H.Q.), and The Health Research Council of New Zealand (to G.M.A and D.R.G.). J.H.Q. was supported by The Health Sciences Career Development Program Postdoctoral Fellowship from the University of Otago. Grant 1PO1DK26687 from The New York Obesity Research Center (to S.C.) funded the development of the floxed leptin receptor mouse.
Disclosure Statement: The authors have nothing to disclose.
Footnotes
- ARC
- Arcuate nucleus
- AVPV
- anteroventral periventricular nucleus
- DIO
- diet-induced obesity
- HFD
- high-fat diet
- mTORC1
- mammalian target of rapamycin complex 1
- OVX
- ovariectomized
- qRT-PCR
- quantitative RT-PCR
- RP3V
- rostral periventricular region of the third ventricle
- STAT
- signal transducer and activator of transcription.
References
- 1. Schneider JE. 2004. Energy balance and reproduction. Physiol Behav 81:289–317 [DOI] [PubMed] [Google Scholar]
- 2. Brewer CJ, Balen AH. 2010. The adverse effects of obesity on conception and implantation. Reproduction 140:347–364 [DOI] [PubMed] [Google Scholar]
- 3. Marsh EE, Barnes RB. 2008. Obesity and conception. In: Rees M, Karoshi M, Keith L. eds. Obesity and pregnancy. London: The Royal Society of Medicine Press Limited; 78–88 [Google Scholar]
- 4. I'Anson H, Sundling LA, Roland SM, Ritter S. 2003. Immunotoxic destruction of distinct catecholaminergic neuron populations disrupts the reproductive response to glucoprivation in female rats. Endocrinology 144:4325–4331 [DOI] [PubMed] [Google Scholar]
- 5. Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, Clifton DK, Steiner RA. 1996. Leptin is a metabolic signal to the reproductive system. Endocrinology 137:3144–3147 [DOI] [PubMed] [Google Scholar]
- 6. Todd BJ, Ladyman SR, Grattan DR. 2003. Suppression of pulsatile luteinizing hormone secretion but not luteinizing hormone surge in leptin resistant obese Zucker rats. J Neuroendocrinol 15:61–68 [DOI] [PubMed] [Google Scholar]
- 7. Finn PD, Cunningham MJ, Pau KY, Spies HG, Clifton DK, Steiner RA. 1998. The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey. Endocrinology 139:4652–4662 [DOI] [PubMed] [Google Scholar]
- 8. Nagatani S, Guthikonda P, Thompson RC, Tsukamura H, Maeda KI, Foster DL. 1998. Evidence for GnRH regulation by leptin: leptin administration prevents reduced pulsatile LH secretion during fasting. Neuroendocrinology 67:370–376 [DOI] [PubMed] [Google Scholar]
- 9. Quennell JH, Mulligan AC, Tups A, Liu X, Phipps SJ, Kemp CJ, Herbison AE, Grattan DR, Anderson GM. 2009. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology 150:2805–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cone RD. 2005. Anatomy and regulation of the central melanocortin system. Nat Neurosci 8:571–578 [DOI] [PubMed] [Google Scholar]
- 11. Rondini TA, Baddini SP, Sousa LF, Bittencourt JC, Elias CF. 2004. Hypothalamic cocaine- and amphetamine-regulated transcript neurons project to areas expressing gonadotropin releasing hormone immunoreactivity and to the anteroventral periventricular nucleus in male and female rats. Neuroscience 125:735–748 [DOI] [PubMed] [Google Scholar]
- 12. Schioth HB, Kakizaki Y, Kohsaka A, Suda T, Watanobe H. 2001. Agouti-related peptide prevents steroid-induced luteinizing hormone and prolactin surges in female rats. Neuroreport 12:687–690 [DOI] [PubMed] [Google Scholar]
- 13. Castellano JM, Navarro VM, Fernández-Fernández R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. 2005. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology 146:3917–3925 [DOI] [PubMed] [Google Scholar]
- 14. Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. 2001. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 411:613–617 [DOI] [PubMed] [Google Scholar]
- 15. Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. 2005. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A 102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. 2005. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- 17. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 18. Xu J, Kirigiti MA, Grove KL, Smith MS. 2009. Regulation of food intake and gonadotropin-releasing hormone/luteinizing hormone during lactation: role of insulin and leptin. Endocrinology 150:4231–4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yamada S, Uenoyama Y, Kinoshita M, Iwata K, Takase K, Matsui H, Adachi S, Inoue K, Maeda KI, Tsukamura H. 2007. Inhibition of metastin (kisspeptin-54)-GPR54 signaling in the arcuate nucleus-median eminence region during lactation in rats. Endocrinology 148:2226–2232 [DOI] [PubMed] [Google Scholar]
- 20. Smith JT, Acohido BV, Clifton DK, Steiner RA. 2006. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol 18:298–303 [DOI] [PubMed] [Google Scholar]
- 21. Backholer K, Smith JT, Rao A, Pereira A, Iqbal J, Ogawa S, Li Q, Clarke IJ. 2010. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide y and proopiomelanocortin cells. Endocrinology 151:2233–2243 [DOI] [PubMed] [Google Scholar]
- 22. Kalamatianos T, Grimshaw SE, Poorun R, Hahn JD, Coen CW. 2008. Fasting reduces KiSS-1 expression in the anteroventral periventricular nucleus (AVPV): effects of fasting on the expression of KiSS-1 and neuropeptide Y in the AVPV or arcuate nucleus of female rats. J Neuroendocrinol 20:1089–1097 [DOI] [PubMed] [Google Scholar]
- 23. Luque RM, Kineman RD, Tena-Sempere M. 2007. Regulation of hypothalamic expression of KiSS-1 and GPR54 genes by metabolic factors: analyses using mouse models and a cell line. Endocrinology 148:4601–4611 [DOI] [PubMed] [Google Scholar]
- 24. Clarkson J, Herbison AE. 2006. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anderson KD, Lambert PD, Corcoran TL, Murray JD, Thabet KE, Yancopoulos GD, Wiegand SJ. 2003. Activation of the hypothalamic arcuate nucleus predicts the anorectic actions of ciliary neurotrophic factor and leptin in intact and gold thioglucose-lesioned mice. J Neuroendocrinol 15:649–660 [DOI] [PubMed] [Google Scholar]
- 26. Ladyman SR, Grattan DR. 2004. Region-specific reduction in leptin-induced phosphorylation of signal transducer and activator of transcription-3 (STAT3) in the rat hypothalamus is associated with leptin resistance during pregnancy. Endocrinology 145:3704–3711 [DOI] [PubMed] [Google Scholar]
- 27. Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. 2006. Hypothalamic mTOR signaling regulates food intake. Science 312:927–930 [DOI] [PubMed] [Google Scholar]
- 28. Roa J, Garcia-Galiano D, Varela L, Sánchez-Garrido MA, Pineda R, Castellano JM, Ruiz-Pino F, Romero M, Aguilar E, López M, Gaytan F, Diéguez C, Pinilla L, Tena-Sempere M. 2009. The mammalian target of rapamycin as novel central regulator of puberty onset via modulation of hypothalamic Kiss1 system. Endocrinology 150:5016–5026 [DOI] [PubMed] [Google Scholar]
- 29. Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne HJ, Todman MG, Korach KS, Greiner E, Pérez CA, Schütz G, Herbison AE. 2006. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 52:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tortoriello DV, McMinn J, Chua SC. 2004. Dietary-induced obesity and hypothalamic infertility in female DBA/2J mice. Endocrinology 145:1238–1247 [DOI] [PubMed] [Google Scholar]
- 31. Brown RS, Kokay IC, Herbison AE, Grattan DR. 2010. Distribution of prolactin-responsive neurons in the mouse forebrain. J Comp Neurol 518:92–102 [DOI] [PubMed] [Google Scholar]
- 32. Greenwood AD, Horsch M, Stengel A, Vorberg I, Lutzny G, Maas E, Schädler S, Erfle V, Beckers J, Schätzl H, Leib-Mösch C. 2005. Cell line dependent RNA expression profiles of prion-infected mouse neuronal cells. J Mol Biol 349:487–500 [DOI] [PubMed] [Google Scholar]
- 33. Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622 [DOI] [PubMed] [Google Scholar]
- 34. Paxinos G, Franklin KBJ. 2004. The mouse brain in stereotaxic coordinates. San Diego: Elsevier Academic Press [Google Scholar]
- 35. Clarkson J, d'Anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE. 2009. Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol 21:673–682 [DOI] [PubMed] [Google Scholar]
- 36. Navarro VM, Castellano JM, Fernández-Fernández R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. 2004. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology 145:4565–4574 [DOI] [PubMed] [Google Scholar]
- 37. Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. 2007. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 53:367–378 [DOI] [PubMed] [Google Scholar]
- 38. Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. 2005. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 146:4431–4436 [DOI] [PubMed] [Google Scholar]
- 39. Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. 2009. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci 29:3920–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. True C, Kirigiti M, Ciofi P, Grove KL, Smith MS. 2011. Characterisation of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. J Neuroendocrinol 23:52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. 2008. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci 28:8691–8697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chehab FF, Lim ME, Lu R. 1996. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet 12:318–320 [DOI] [PubMed] [Google Scholar]
- 43. Tortoriello DV, McMinn JE, Chua SC. 2007. Increased expression of hypothalamic leptin receptor and adiponectin accompany resistance to dietary-induced obesity and infertility in female C57BL/6J mice. Int J Obes 31:395–402 [DOI] [PubMed] [Google Scholar]
- 44. Morris DL, Rui L. 2009. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab 297:E1247–E1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, Fu XY. 2004. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci U S A 101:4661–4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr 2003. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421:856–859 [DOI] [PubMed] [Google Scholar]
- 47. Donato J, Jr, Cravo RM, Frazao R, Gautron L, Scott MM, Lachey J, Castro IA, Margatho LO, Lee S, Lee C, Richardson JA, Friedman J, Chua S, Jr, Coppari R, Zigman JM, Elmquist JK, Elias CF. 2011. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest 121:355–368 [DOI] [PMC free article] [PubMed] [Google Scholar]