The forkhead transcription factor FOXL2 plays key and diverse roles in female sex determination and ovarian development and in the postnatal ovary and follicle maintenance; its mutations can lead to premature ovarian failure and granulosa cell tumor formation.
Abstract
The forkhead transcription factor (FOXL2) is an essential transcription factor in the ovary. It is important in ovarian development and a key factor in female sex determination. In addition, FOXL2 plays a significant role in the postnatal ovary and follicle maintenance. The diverse transcriptional activities of FOXL2 are likely attributable to posttranslational modifications and binding to other key proteins involved in granulosa cell function. Mutations of FOXL2 lead to disorders of ovarian function ranging from premature follicle depletion and ovarian failure to unregulated granulosa cell proliferation leading to tumor formation. Thus, FOXL2 is a key regulator of granulosa cell function and a master transcription factor in these cells.
The forkhead transcription factor (FOXL2) is emerging as a central transcription factor in ovarian development and the growth and maturation of ovarian follicles. FOXL2 is a member of the forkhead/hepatocyte nuclear factor 3 gene family (FKH/HNF3) of transcription factors, the first of which was described in Drosophila (1). Now members have been identified in species ranging from yeast to human (2) and play essential roles in embryogenesis (1, 3), cell differentiation (4–6), and tumorigenesis (7–9). FKH/HNF3 family members are characterized by a conserved winged helix domain that is essential for DNA binding but exhibit divergent transcriptional regulation based on their transactivation or transrepression domains (2, 10, 11).
FOXL2 was first cloned by Crisponi et al. (12) from the Blepharophimosis-Ptosis-Epicanthus Inversus Syndrome (BPES) region on human chromosome 3q23. Heterozygous mutations in FOXL2 lead to a characteristic premature ovarian failure and infertility in females (13). Consistent with this, FOXL2 is expressed in the developing eyelids and ovary in the mouse (12) and is also expressed in adult ovarian follicles (12, 14–16). Unlike the primary ovarian failure from primordial follicle arrest that occurs in mice null for Foxl2 (17, 18), which have complete absence of FOXL2 expression, heterozygous mutations in humans with BPES undergo a complete sequence of follicle development with early depletion of the follicle pool and premature ovarian failure (17, 19). Thus, FOXL2 plays a significant role in early ovarian development and sex determination, as well as a later role in granulosa cell differentiation with subsequent follicle depletion, and mutations of FOXL2 contribute to a variety of conditions and disease states. The functional role of FOXL2 in the ovary will be the focus of this review.
Role of FOXL2 during Early Ovarian Development and Sex Determination
FOXL2 is the earliest known marker of ovarian differentiation in mammals and is expressed in the developing mouse ovary as early as 12.5 d post coitum (12, 17, 20). Several genes have been implicated in early gonadal development and sex determination, including wingless-type MMTV integration site family member 4 (Wnt4) and R-spondin-1 (Rspo1) (21, 22), dosage-sensitive sex reversal, adrenal hypoplasia critical region on chromosome X gene 1 (Dax1) (23), SRY (sex determining region Y)-box 9 (Sox9) (24, 25), and Foxl2. Of these, FOXL2 is restricted to granulosa cells and the early ovarian stroma (17, 20), is excluded from the developing male gonads, and appears to be specifically required for female sex determination (26). Knockout mouse models have shown that ablation of Foxl2 expression blocks ovarian follicle formation and leads to partial ovary-to-testis sex reversal in mice (17, 18), independent of Wnt and Rspo1 (27). Several downstream targets of FOXL2 have been identified through expression profiling (27). These include genes that are down-regulated by FOXL2 and are involved in neuronal or vascular development, such as the transcription factor odd Oz/ten-m homolog 4 (Odz4) (28), plasma transmembrane proteins such as plexin (Plxnc1) (29), the cadherin-domain containing calsyntenin 2 (Clstn2) (30), and the leucine-rich repeat protein (Lrrc4) (31), which may contribute to formation of the ovarian-cortico-medullary axis (28, 32). Genes that are down-regulated by FOXL2 and may contribute to its role in female sex determination were also identified (27), including Grip1, a nuclear repressor required for estrogen receptor (ER)-α activity (33), and the aldo-keto reductase, Akr1c14, which metabolizes the androgen dihydrotestosterone (34). Sox9 and Inhibin B (Inhbb), which are central to testicular development (24, 25, 35, 36), are also down-regulated by FOXL2 (27). Genes that were up-regulated by FOXL2 included P450aromatase (Cyp19) and liver receptor homolog-1 (Lrh-1/Nr5a2) (37), which is related to steroidogenic factor 1 (SF-1), an orphan receptor essential for the formation of female and male gonads (38). Consistent with its role in sexual determination, ablation of Foxl2 in the adult mouse ovary causes immediate induction of the transcription factor SOX9 (39), leading to ovary-to-testis transdifferentiation, with Sertoli cells replacing granulosa cells and seminiferous tubules forming instead of follicles (39).
FOXL2 has been associated with sex reversal in several species, including the frog and several fish species (medaka, Nile tilapia, and Japanese flounder) (40–43), and dysregulated FOXL2 expression has been described in the goat polled intersex syndrome (PIS), in which XX male sex reversal occurs (44); taken together, these demonstrate the highly conserved role of FOXL2 in ovarian development. These effects of FOXL2 may be mediated via control of Cyp19 expression in these species (40–43). For example, during the temperature-sensitive phase of sex differentiation in XX Japanese flounder and Nile tilapia females, high temperatures result in down-regulation of Cyp19 expression, leading to decreased estrogen levels and masculinization. This down-regulation of Cyp19 is mediated by FOXL2 and FSH signaling (43, 45). In the goat, PIS leads to absence of the horns in both sexes in a dominant fashion, and to XX female-to-male sex reversal in the recessive state (44). An 11.7-kb DNA element located upstream of the Foxl2 gene was found to be deleted in PIS, and expression levels of Foxl2 and Cyp19 in the gonads were greatly reduced, resulting in masculinization (46). Thus, FOXL2 plays a highly conserved role in the regulation of early ovarian development and female sex determination across multiple species.
FOXL2 Activity during Folliculogenesis
FOXL2 is expressed in the less differentiated granulosa cells of small and medium follicles (12, 16, 17, 20) and therefore likely also plays a role in granulosa cell differentiation and follicle development and maintenance. The alanine/proline-rich carboxyl (C-) terminus of FOXL2 is characteristic of transcriptional repressors (47) and is found in other forkhead transcription factors that function in repressing cellular differentiation (6, 48–52). FOXL2 was initially found to function as a transcriptional repressor of the steroidogenic acute regulatory (StAR) gene (16), and the entire C terminus of FOXL2 functions as a transrepression domain (16, 53). StAR translocates cholesterol from the outer to the inner membrane of mitochondria, which is the rate-limiting step in steroidogenesis (54–56). StAR is also a marker of granulosa cell differentiation and is expressed in granulosa cells of large preovulatory follicles, but not small and medium immature follicles (57). FOXL2 binds to the human StAR promoter and suppresses its activity (16), suggesting that transcriptional repression by FOXL2 prevents StAR expression in immature follicles. FOXL2 also functions as a transcriptional repressor of Cyp19 (P450aromatase) in rainbow trout gonads (58) and both Cyp19 and Cyp11a (P450scc) in immature mouse ovary follicles (53). P450aromatase converts C-19 androgens to C-18 estrogens, a key product of differentiated granulosa cells, and P450scc cleaves the side chain of cholesterol to produce pregnenolone, the first committed and rate-limiting step in steroid hormone synthesis (56). These results indicate that sex steroids may be important for initiating follicle growth, as previously suggested (59, 60). FOXL2 also represses transcription of cyclin D2, which regulates cyclin-dependent kinases 4 or 6 (Cdk4 or Cdk6) to control G1 phase progression of the cell cycle and is involved in granulosa cell proliferation (61, 62). These results indicate that FOXL2 may function as a suppressor of ovarian follicle progression in small and medium follicles by the prevention of premature differentiation and/or proliferation of granulosa cells, thus preventing the premature depletion of ovarian follicles which is seen when FOXL2 function is altered because of mutations.
In postnatal ovaries of Foxl2+/− heterozygous mice (which have been suggested as a mouse model for the human phenotype of premature ovarian failure) (17, 18), a number of somatic cell genes were found to be repressed to a lesser degree but similar to Foxl2−/− null mice (27), including InhbB, Lrh-1, Cyp11a, and another steroidogenic enzyme and cytochrome P450 family member, 17-hydroxylase (Cyp17a1). This may be the result of Foxl2 dose dependency or, because Foxl2 is expressed in granulosa cells of heterozygotes, these genes may be differentially regulated in heterozygous mice.
The KGN cell line (63) has also been widely used as a model of granulosa cells of the postnatal ovary. These adult granulosa cells are derived from granulosa cell tumors (GCTs) which contain a C402G mutation in FOXL2 (discussed below). Therefore, they may actually reflect changes that occur in the pathological state (see below) rather than normal granulosa cells. However important information has been revealed by studies of this cell line. In these cells, a number of genes have been shown to be regulated by FOXL2 (64). These included immunomodulators such as interferon β 1 (IFNB1) (65), interleukin-12 subunit α (IL12A) (66), and interleukin-29 (IL29) (67); intercellular adhesion molecule 1 (ICAM1) (68); proteins involved in cell proliferation, differentiation, and transformation such as the cellular proto-oncogene c-FOS (69); and antiapoptotic factors such as BCL2(B-cell lymphoma 2)-related protein A1 (BCL2A1) (70) and immediate early response 3 (IER3) (71).
A number of genes that lead to fertility defects also appear to be regulated by FOXL2 in the postnatal ovary. These include prostaglandin-endoperoxide synthase 2/cyclooxygenase-2 (PTGS2/COX-2). PTGS2/COX-2 null mice exhibit ovulation and fertilization defects (72). Although FOXL2 up-regulates PTGS2/COX-2 in the KGN granulosa cell model (64), PTGS2/COX-2 is repressed by FOXL2 through a nonclassical estrogen signaling pathway in vitro (73). Thus, depending on the available binding partners, FOXL2 likely has differential effects on a number of genes imperative for ovarian function and female fertility. FOXL2 up-regulates the SF-1–related gene, Lrh-1, in both the mouse ovary, as noted above (27), and in the KGN cell line (64), and also up-regulates peroxisome proliferator-activated receptor-coactivator 1α (Ppargc1A) in KGN cells (64). Ppargc1A binds to both Lrh-1 and SF-1 and markedly enhances their effects on the transcription of genes involved in progesterone production in granulosa cells (74). However, at the protein level, FOXL2 binds to SF-1 and negatively regulates transcriptional activation of other steroidogenic enzymes in human granulosa cells (75). Thus, FOXL2 plays critical roles in ovarian development, folliculogenesis, and maintenance, and mutations of FOXL2 lead to ovarian failure as well as malignancy, effects which are mediated through the differential regulation of a diverse set of genes in granulosa cells. This differential regulation is likely dependent on the posttranslational modifications and available FOXL2 binding partners that make up the transcriptional complex leading to gene regulation.
Posttranslational Modifications of FOXL2
In keeping with a central role for FOXL2 in the ovary, several posttranslational modifications have shown to be involved in modulating its activity, as summarized below.
Sumoylation
Sumoylation is a key mechanism in transcriptional regulation and acts to influence protein stability and subcellular localization (76, 77). Small ubiquitin-related modifier (SUMO) proteins, structurally related to ubiquitin, covalently bind to substrate proteins at lysine residues, in a three-step conjugation pathway similar to that involved in ubiquitination (78). FOXL2 is sumoylated (79), and we recently showed that it is sumoylated by SUMO1 but not SUMO2/3, and that this sumoylation is mediated by ubiquitin-conjugating enzyme-9 (Ubc9) (80). Further, we found that sumoylation enhances FOXL2's activity as a transcriptional repressor of the StAR promoter (80). Marongiu et al., have recently identified four additional sumoylation sites, all of which may be involved in FOXL2 function (81) because they affect localization, sumoylation, and transcriptional activity. Because sumoylation is a dynamic process, the differences in sumoylation sites identified by the two groups may be attributable to different culture conditions and perhaps other upstream regulators that may influence sumoylation of FOXL2. Nevertheless, the data clearly demonstrate a key role for sumoylation in regulating the functional activity of FOXL2.
Phosphorylation
FOXL2 is also phosphorylated, and this phosphorylation regulates the functional activity of FOXL2 (79, 82). We have shown that FOXL2 is phosphorylated by the serine/threonine kinase large tumor suppressor 1 (Lats1), and that this enhances FOXL2's activity as a repressor (82). Similar to the ovarian failure phenotype in mice null for Foxl2 (17, 18), deletion of Lats1 in mice also result in an ovarian phenotype similar to POF (83). Therefore, FOXL2 and LATS1 likely function through a similar pathway during follicle maintenance, and phosphorylation may be another control mechanism regulating FOXL2 activity in granulosa cell differentiation and hence, follicle maturation.
Acetylation and Cell Stress
Veitia and coworkers (79) demonstrated that cell stress significantly influences the posttranslational modification of FOXL2. They found that oxidative stress and heat shock both increase FOXL2 expression levels in the KGN cell line, and that the FOXL2 protein appears to be hyper-acetylated upon oxidative stress. This resulted in increased recruitment of FOXL2 to target promoters, including several genes involved in stress response or regulation of apoptosis (79). Of these, Manganese Superoxide Dismutase (MnSOD), a key mediator of the oxidative stress response, was found to be specifically and directly up-regulated by FOXL2. The authors also found that both the expression level and transcriptional activity of FOXL2 are down-regulated by the NAD-dependent deacetylase sirtuin 1 (SIRT1), and FOXL2 itself induces SIRT1 transcription, demonstrating the importance of acetylation as a regulator of FOXL2 activity and the existence of a feedback loop controlling FOXL2 activity in these cells (79). This response to oxidative stress in the GCT KGN cell line may actually be a mechanism defining the uncontrolled cell proliferation in the pathological state and warrants further investigation.
Potential FOXL2 Binding Partners
DEAD box-containing protein DP103
The DEAD box-containing protein DP103 is known to bind to SF-1 and more recently identified to bind to FOXL2 (84, 85). Co-expression of DP103 and FOXL2 potentiates cell death. DP103 represses the transcriptional activity of SF-1 (84), a key regulator of steroidogenesis, during fetal ovarian differentiation and reproduction (38, 86). The region of DP103 that interacts with FOXL2 is also necessary for SF-1 binding to DP103 (84), suggesting that DP103, FOXL2, and SF-1 may be components of a complex regulatory mechanism in the ovary.
Steroidogenic factor-1
FOXL2 binds directly to SF-1, and recently the nature of the interaction between FOXL2 and SF-1 has been further elucidated (75). FOXL2 and SF-1 proteins interact in granulosa cells, and FOXL2 negatively regulates the transcriptional activation of CYP17a1 by SF-1, by inhibiting binding of SF-1 to the CYP17 promoter (75). These findings illustrate another potential mechanism by which FOXL2 regulates ovarian steroidogenesis and normal ovarian follicle development.
ERα and SOX9
The transcription factors FOXL2 and SOX9 are required for female and male mammalian gonadal development (see above), and alterations in their expression have been implicated in various disorders of sex development (DSD), discussed below (26). Uhlenhaut et al. (39) demonstrated that FOXL2 represses the testis differentiation program mainly through repression of Sox9-regulatory sequences that are required for its testis-specific expression, and found that FOXL2 and ERα cooperate, through protein–protein interactions, in Sox9 repression in vivo. Conditional loss of FOXL2 in the adult ovary results in ovary-to-testis differentiation (17).
ERα – nonclassical activity
An additional role for FOXL2 in modulating ER activity has also been investigated (73). Although FOXL2 has not been shown to be involved in the classical ERα-mediated transcriptional pathways, in which binding of ligand-activated receptors canonical estrogen response elements (EREs) results in the up- or down-regulation of target genes, a role for FOXL2 in mediating nonclassical tethered transcriptional pathways has been demonstrated (73). Specifically, FOXL2 selectively repressed stimulation of an AP1 reporter through ERα by tamoxifen, likely through FOXL2 binding to ERα (73). PTGS2/COX-2, which is required for ovulation (72) and is induced by the nonclassical ER pathway, is suppressed by FOXL2 (73). These findings illustrate that FOXL2 is likely involved in regulating ovarian function through a number of important binding partners, and the complete transcriptional complex likely alternates depending on the upstream signaling pathways.
SMAD
In addition to its functions in the ovary, FOXL2 localizes to α-glycoprotein subunit- and FSH β-positive cells of the adult mouse pituitary and is present in αT3–1 and LβT2 cells (87), but its role remains largely unknown. Activin is a key regulator of follicle development and initiates granulosa cell proliferation (88). Follistatin is a transcriptional target of activin (89) and also modulates activin action (90). In gonadotropic αT3–1 cells, activin induces follistatin transcription via SMAD3 action at an intronic Smad-binding element (SBE1) (87), and FOXL2 functions as a SMAD3 partner in this SBE1-mediated transcription, binding to a forkhead-binding element (FKHB) downstream of the follistatin gene SBE1 site (87). Activin is also a major physiological regulator of FSH (91), and Corpuz et al. recently demonstrated that FOXL2 is also required for activin induction of both mouse and human FSHβ (92). Interestingly, this induction is SMAD-dependent for the mouse gene, but SMAD-independent for human FSHβ (92). We recently demonstrated expression of all SMADs in human granulosa cells (Kuo et al., submitted), suggesting that FOXL2 may also function as a transcriptional regulator and coordinator of SMAD3 targets in the ovary.
FOXL2 in BPES
BPES is an autosomal dominant disorder and is associated with heterozygous mutations of FOXL2 (12). Patients with BPES type 1 exhibit a characteristic eyelid dysplasia together with premature ovarian failure and infertility in affected females (13). Ovaries from BPES type 1 patients are variable in their histological appearance, ranging from the presence of some primordial follicles with atretic follicles to the complete absence of follicles (19, 93). In contrast, BPES type 2 patients present with the eyelid defects only, and both males and females are fertile (13). In BPES type 1, nonsense mutations in the FOXL2 gene create premature stop codons, which are predicted to result in truncated proteins lacking the C-terminal alanine/proline-rich transrepression domain (12, 16, 94–96), whereas FOXL2 mutations in BPES type 2 are predicted to result in expansion of the polyalanine tract (94). These differences in the FOXL2 mutations associated with BPES type 1 and type 2, and loss of the transrepression domain vs. the polyalanine tract, likely underlie the differences in the resulting ovarian phenotypes.
Potential Mechanisms of FOXL2 Mutants in Premature Ovarian Failure in BPES Type 1
Mice deficient for Foxl2 exhibit follicle arrest between the primordial and primary stages, followed by follicle degeneration (17, 18). They fail to undergo sexual maturation and undergo primary ovarian failure (17, 18). However, this contrasts with the majority of human BPES patients. Unlike the complete loss of Foxl2 alleles in null mice, BPES patients typically have heterozygous FOXL2 mutations and do not exhibit primordial follicle arrest, but rather undergo a complete sequence of follicle development, menstrual cyclicity, and ovulation, followed by premature follicle depletion and subsequent ovarian failure (13, 19, 93). As FOXL2 is expressed in primordial, primary, and larger secondary follicles (12, 14–16), it may play important roles during separate stages of follicle development leading to premature ovarian failure.
Patients with BPES type 1 carry heterozygous mutations of FOXL2 that are predicted to result in truncated proteins lacking the C-terminal transrepression domain. The human Q219 × mutation, common in BPES type 1 (12), produces a truncated FOXL2 protein, FOXL2 (a.a. 1–218), that lacks the entire alanine/proline rich region, however it retains the forkhead binding domain. This mutant fails to repress transcription of the StAR (16), and P450aromatase (53) promoters and functions as a dominant negative for wild-type FOXL2's activity as a transcriptional repressor of these promoters (16, 97), likely attributable to hetero-dimerization of the wild-type and mutant FOXL2 (Kuo et al., in preparation). This effect, leading to loss of transcriptional repression of key genes involved in granulosa cell proliferation, differentiation, and steroidogenesis, may result in accelerated granulosa cell differentiation and premature follicle depletion and thus contribute to the ovarian failure seen in BPES type 1.
In addition to potential heterodimer formation, missense mutations of FOXL2 have also been shown to lead to altered subcellular localization, ranging from a diffuse nuclear distribution to nuclear aggregation with cytoplasmic mislocalization (98). In the KGN cell line, this has been shown to lead to differences in transactivation capacity of these mutants, such as loss-of-function and dominant negative effects (98). Although these results should be viewed with caution, as KGN cells carry a mutation of FOXL2, they nonetheless illustrate that altered subcellular localization of mutant FOXL2 proteins may contribute to the development of BPES phenotypes in some patients.
Role of FOXL2 in GCT
In addition to mutations in FOXL2 leading to granulosa cell and follicle depletion, other mutations of FOXL2 are associated with unregulated granulosa cell proliferation, as in the case of adult onset GCTs. GCTs represent 5% to 10% of all ovarian cancers (99, 100) and are associated with a 20% mortality rate and a 5-year survival rate for advanced stage patients of less than 50% (101). GCTs exhibit some features that are similar to normal granulosa cells, including expression of the FSH receptor (102, 103). They bind FSH (104, 105), synthesize estradiol, inhibin, and anti-müllerian hormone (AMH) (106–110), and exhibit up-regulation of cyclin D2, which is necessary for FSH-stimulated granulosa cell proliferation (103). Shah et al. found that a recurrent heterozygous somatic mutation (C402G) in FOXL2 is present in 97% of adult GCTs (111). The C402G mutation was absent in all other tumors tested, including other ovarian stromal sex cord tumors, epithelial ovarian tumors, and breast cancers (111–114), and is also absent from the COV434 human GCT-derived cell line (115), which may in fact represent a juvenile GCT. This mutation is predicted to cause an amino acid change from cysteine to tryptophan (C134W) in the DNA binding domain of FOXL2 (111) and thus may affect its activity as a transcriptional regulator. However, Benayoun et al. (116) found that the transactivation ability of the mutant protein was similar to that of the wild-type, except for a promoter known to be coregulated by FOXL2 and SMAD3, and they did find that homodimer formation might be affected by the presence of the mutant protein. In juvenile GCTs, loss of protein expression is associated with an aggressive pattern of progression and may be a factor regulating unregulated granulosa cell proliferation.
Role of FOXL2 in DSDs
FOXL2 has been described as a key determinant of sexual differentiation in multiple species, including those that undergo sex differentiation secondary to environmental cues such as temperature (43, 45). In addition, genetic aberrations associated with Foxl2, including mutations/deletions in the mouse (24, 39) and deletions of the upstream region in goat (46), lead to ovary-to-testis transdifferentiation. In humans, SOX9 is expressed in (pre-) Sertoli cells, whereas FOXL2 is expressed in granulosa cells and ovarian stroma. In patients with DSDs and intersex states, when both ovarian and testicular development is present, expression of both FOXL2 and SOX9 can be detected, and if an ovotestis is present (i.e., hermaphrodite), expression of both genes can be detected in the same gonad (26). Interestingly, in one patient with DSD, FOXL2 expression was identified in well-developed seminiferous tubules, but it was never strongly coexpressed with SOX9 in the same cell. In a unique rare juvenile GCT of the testis, expression of FOXL2, along with the extinction of SOX9, was coupled with the transdifferentiation of a testicular cell into a GCT (117). Therefore, although different gonadal cell lineages may exist in these patients, FOXL2 and SOX9 expression is not present in the same cell, likely the result of FOXL2 repression of SOX9 similar to that seen in the mouse model.
Conclusions
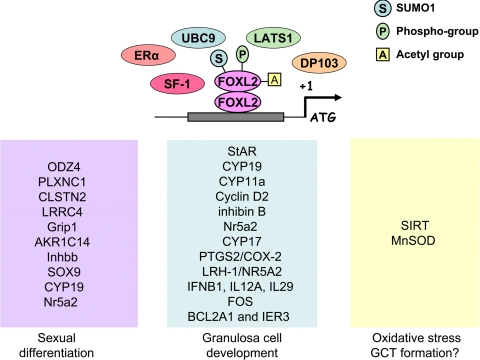
Similar to other forkhead family members, FOXL2 is essential for embryogenesis, cell differentiation, and tumorigenesis; however, unlike other members with individual roles at these stages of regulation, FOXL2 plays an important role in all of these stages (Fig. 1). The highly conserved nature of this gene, and limited expression predominantly in the ovary, suggest that it is a key factor throughout ovarian development, both pre- and postnatally. Because of the vast processes FOXL2 regulates, it is not surprising that its function is modified at the posttranslational level. Further, various binding partners of FOXL2 can make up the transcriptional complex, leading to gene regulation attributable to as yet undefined upstream signaling (Fig. 1). Although we are beginning to develop an understanding of this recently discovered gene, there continues to be a great deal to discover, particularly the upstream cues determining its regulation and the modifications that take place. A better understanding of the functional role of the mutations associated with FOXL2 in pathological states ranging from aberrant sex determination to follicle depletion and cancer may help us develop treatments for these conditions.
Fig. 1.
Activity of FOXL2 as a transcriptional repressor. FOXL2 likely functions as a dimer (118) and requires sumoylation and phosphorylation for its activity. Other factors that may make up the transcriptional complex (Erα, SF-1, and DP103) are indicated, as are the genes known to be regulated by FOXL2. The effects of FOXL2 on gene transcription may differ depending on the stage of ovarian development, including sexual differentiation, granulosa cell development, and pathological states such as GCT formation.
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development and the Office of Research on Women's Health (R01HD047603 to M.P.), and by a grant from the Helping Hands of Los Angeles, Inc. (to M.P.).
Disclosure Summary: M.D.P. is the Medical Editor of the babycenter.com website sponsored by Johnson and Johnson. The other authors have nothing to declare.
Footnotes
- BPES
- Blepharophimosis-Ptosis-Epicanthus Inversus Syndrome
- DSD
- disorders of sex development
- ER
- estrogen receptor
- FOXL2
- forkhead transcription factor L2
- GCT
- granulosa cell tumor
- PIS
- polled intersex syndrome
- SBE
- Smad-binding element
- SF-1
- steroidogenic factor-1
- SUMO
- small ubiquitin-related modifier.
References
- 1. Weigel D, Jurgens G, Kuttner F, Seifert E, Jackle H. 1989. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell 57:645–658 [DOI] [PubMed] [Google Scholar]
- 2. Lehmann OJ, Sowden JC, Carlsson P, Jordan T, Bhattacharya SS. 2003. Fox's in development and disease. Trends Genet 19:339–344 [DOI] [PubMed] [Google Scholar]
- 3. Mahlapuu M, Ormestad M, Enerback S, Carlsson P. 2001. The forkhead transcription factor Foxf1 is required for differentiation of extra-embryonic and lateral plate mesoderm. Development 128:155–166 [DOI] [PubMed] [Google Scholar]
- 4. Nakae J, Kitamura T, Kitamura Y, Biggs WH, 3rd, Arden KC, Accili D. 2003. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell 4:119–129 [DOI] [PubMed] [Google Scholar]
- 5. Brissette JL, Li J, Kamimura J, Lee D, Dotto GP. 1996. The product of the mouse nude locus, Whn, regulates the balance between epithelial cell growth and differentiation. Genes Dev 10:2212–2221 [DOI] [PubMed] [Google Scholar]
- 6. Dottori M, Gross MK, Labosky P, Goulding M. 2001. The winged-helix transcription factor Foxd3 suppresses interneuron differentiation and promotes neural crest cell fate. Development 128:4127–4138 [DOI] [PubMed] [Google Scholar]
- 7. Parry P, Wei Y, Evans G. 1994. Cloning and characterization of the t(X;11) breakpoint from a leukemic cell line identify a new member of the forkhead gene family. Genes Chromosomes Cancer 11:79–84 [DOI] [PubMed] [Google Scholar]
- 8. Teh MT, Wong ST, Neill GW, Ghali LR, Philpott MP, Quinn AG. 2002. FOXM1 is a downstream target of Gli1 in basal cell carcinomas. Cancer Res 62:4773–4780 [PubMed] [Google Scholar]
- 9. Radisavljevic Z. 2003. Nitric oxide suppression triggers apoptosis through the FKHRL1 (FOXO3A)/ROCK kinase pathway in human breast carcinoma cells. Cancer 97:1358–1363 [DOI] [PubMed] [Google Scholar]
- 10. Kaufmann E, Knochel W. 1996. Five years on the wings of fork head. Mech Dev 57:3–20 [DOI] [PubMed] [Google Scholar]
- 11. Carlsson P, Mahlapuu M. 2002. Forkhead transcription factors: key players in development and metabolism. Dev Biol 250:1–23 [DOI] [PubMed] [Google Scholar]
- 12. Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S, Ristaldi MS, Marzella R, Rocchi M, Nicolino M, Lienhardt-Roussie A, Nivelon A, Verloes A, Schlessinger D, Gasparini P, Bonneau D, Cao A, Pilia G. 2001. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet 27:159–166 [DOI] [PubMed] [Google Scholar]
- 13. Zlotogora J, Sagi M, Cohen T. 1983. The blepharophimosis, ptosis, and epicanthus inversus syndrome: delineation of two types. Am J Hum Genet 35:1020–1027 [PMC free article] [PubMed] [Google Scholar]
- 14. Treier M, Gleiberman AS, O'Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG. 1998. Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev 12:1691–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cocquet J, Pailhoux E, Jaubert F, Servel N, Xia X, Pannetier M, De Baere E, Messiaen L, Cotinot C, Fellous M, Veitia RA. 2002. Evolution and expression of FOXL2. J Med Genet 39:916–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pisarska MD, Bae J, Klein C, Hsueh AJ. 2004. Forkhead l2 is expressed in the ovary and represses the promoter activity of the steroidogenic acute regulatory gene. Endocrinology 145:3424–3433 [DOI] [PubMed] [Google Scholar]
- 17. Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, Treier M. 2004. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development 131:933–942 [DOI] [PubMed] [Google Scholar]
- 18. Uda M, Ottolenghi C, Crisponi L, Garcia JE, Deiana M, Kimber W, Forabosco A, Cao A, Schlessinger D, Pilia G. 2004. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet 13:1171–1181 [DOI] [PubMed] [Google Scholar]
- 19. Fraser IS, Shearman RP, Smith A, Russell P. 1988. An association among blepharophimosis, resistant ovary syndrome, and true premature menopause. Fertil Steril 50:747–751 [DOI] [PubMed] [Google Scholar]
- 20. Ottolenghi C, Omari S, Garcia-Ortiz JE, Uda M, Crisponi L, Forabosco A, Pilia G, Schlessinger D. 2005. Foxl2 is required for commitment to ovary differentiation. Hum Mol Genet 14:2053–2062 [DOI] [PubMed] [Google Scholar]
- 21. Maatouk DM, DiNapoli L, Alvers A, Parker KL, Taketo MM, Capel B. 2008. Stabilization of beta-catenin in XY gonads causes male-to-female sex-reversal. Hum Mol Genet 17:2949–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chassot AA, Gregoire EP, Magliano M, Lavery R, Chaboissier MC. 2008. Genetics of ovarian differentiation: Rspo1, a major player. Sex Dev 2:219–227 [DOI] [PubMed] [Google Scholar]
- 23. Sekido R, Lovell-Badge R. 2008. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 453:930–934 [DOI] [PubMed] [Google Scholar]
- 24. Bishop CE, Whitworth DJ, Qin Y, Agoulnik AI, Agoulnik IU, Harrison WR, Behringer RR, Overbeek PA. 2000. A transgenic insertion upstream of sox9 is associated with dominant XX sex reversal in the mouse. Nat Genet 26:490–494 [DOI] [PubMed] [Google Scholar]
- 25. Vidal VP, Chaboissier MC, de Rooij DG, Schedl A. 2001. Sox9 induces testis development in XX transgenic mice. Nat Genet 28:216–217 [DOI] [PubMed] [Google Scholar]
- 26. Hersmus R, Kalfa N, de Leeuw B, Stoop H, Oosterhuis JW, de Krijger R, Wolffenbuttel KP, Drop SL, Veitia RA, Fellous M, Jaubert F, Looijenga LH. 2008. FOXL2 and SOX9 as parameters of female and male gonadal differentiation in patients with various forms of disorders of sex development (DSD). J Pathol 215:31–38 [DOI] [PubMed] [Google Scholar]
- 27. Garcia-Ortiz JE, Pelosi E, Omari S, Nedorezov T, Piao Y, Karmazin J, Uda M, Cao A, Cole SW, Forabosco A, Schlessinger D, Ottolenghi C. 2009. Foxl2 functions in sex determination and histogenesis throughout mouse ovary development. BMC Dev Biol 9:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li H, Bishop KM, O'Leary DD. 2006. Potential target genes of EMX2 include Odz/Ten-M and other gene families with implications for cortical patterning. Mol Cell Neurosci 33:136–149 [DOI] [PubMed] [Google Scholar]
- 29. Worzfeld T, Puschel AW, Offermanns S, Kuner R. 2004. Plexin-B family members demonstrate non-redundant expression patterns in the developing mouse nervous system: an anatomical basis for morphogenetic effects of Sema4D during development. Eur J Neurosci 19:2622–2632 [DOI] [PubMed] [Google Scholar]
- 30. Hintsch G, Zurlinden A, Meskenaite V, Steuble M, Fink-Widmer K, Kinter J, Sonderegger P. 2002. The calsyntenins–a family of postsynaptic membrane proteins with distinct neuronal expression patterns. Mol Cell Neurosci 21:393–409 [DOI] [PubMed] [Google Scholar]
- 31. Zhang Q, Wang J, Fan S, Wang L, Cao L, Tang K, Peng C, Li Z, Li W, Gan K, Liu Z, Li X, Shen S, Li G. 2005. Expression and functional characterization of LRRC4, a novel brain-specific member of the LRR superfamily. FEBS Lett 579:3674–3682 [DOI] [PubMed] [Google Scholar]
- 32. Byskov AG, Guoliang X, Andersen CY. 1997. The cortex-medulla oocyte growth pattern is organized during fetal life: an in-vitro study of the mouse ovary. Mol Hum Reprod 3:795–800 [DOI] [PubMed] [Google Scholar]
- 33. Cvoro A, Tzagarakis-Foster C, Tatomer D, Paruthiyil S, Fox MS, Leitman DC. 2006. Distinct roles of unliganded and liganded estrogen receptors in transcriptional repression. Mol Cell 21:555–564 [DOI] [PubMed] [Google Scholar]
- 34. Hara A, Inoue Y, Nakagawa M, Naganeo F, Sawada H. 1988. Purification and characterization of NADP+-dependent 3 alpha-hydroxysteroid dehydrogenase from mouse liver cytosol. J Biochem 103:1027–1034 [DOI] [PubMed] [Google Scholar]
- 35. Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. 1991. Male development of chromosomally female mice transgenic for Sry. Nature 351:117–121 [DOI] [PubMed] [Google Scholar]
- 36. Brown CW, Houston-Hawkins DE, Woodruff TK, Matzuk MM. 2000. Insertion of Inhbb into the Inhba locus rescues the Inhba-null phenotype and reveals new activin functions. Nat Genet 25:453–457 [DOI] [PubMed] [Google Scholar]
- 37. Sirianni R, Seely JB, Attia G, Stocco DM, Carr BR, Pezzi V, Rainey WE. 2002. Liver receptor homologue-1 is expressed in human steroidogenic tissues and activates transcription of genes encoding steroidogenic enzymes. J Endocrinol 174:R13–R17 [DOI] [PubMed] [Google Scholar]
- 38. Bakke M, Zhao L, Hanley NA, Parker KL. 2001. SF-1: a critical mediator of steroidogenesis. Mol Cell Endocrinol 171:5–7 [DOI] [PubMed] [Google Scholar]
- 39. Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI, Riethmacher D, Schutz G, Cooney AJ, Lovell-Badge R, Treier M. 2009. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 139:1130–1142 [DOI] [PubMed] [Google Scholar]
- 40. Oshima Y, Uno Y, Matsuda Y, Kobayashi T, Nakamura M. 2008. Molecular cloning and gene expression of Foxl2 in the frog Rana rugosa. Gen Comp Endocrinol 159:170–177 [DOI] [PubMed] [Google Scholar]
- 41. Nakamoto M, Wang DS, Suzuki A, Matsuda M, Nagahama Y, Shibata N. 2007. Dax1 suppresses P450arom expression in medaka ovarian follicles. Mol Reprod Dev 74:1239–1246 [DOI] [PubMed] [Google Scholar]
- 42. Wang DS, Kobayashi T, Zhou LY, Paul-Prasanth B, Ijiri S, Sakai F, Okubo K, Morohashi K, Nagahama Y. 2007. Foxl2 up-regulates aromatase gene transcription in a female-specific manner by binding to the promoter as well as interacting with ad4 binding protein/steroidogenic factor 1. Mol Endocrinol 21:712–725 [DOI] [PubMed] [Google Scholar]
- 43. Yamaguchi T, Yamaguchi S, Hirai T, Kitano T. 2007. Follicle-stimulating hormone signaling and Foxl2 are involved in transcriptional regulation of aromatase gene during gonadal sex differentiation in Japanese flounder, Paralichthys olivaceus. Biochem Biophys Res Commun 359:935–940 [DOI] [PubMed] [Google Scholar]
- 44. Pailhoux E, Vigier B, Chaffaux S, Servel N, Taourit S, Furet JP, Fellous M, Grosclaude F, Cribiu EP, Cotinot C, Vaiman D. 2001. A 11.7-kb deletion triggers intersexuality and polledness in goats. Nat Genet 29:453–458 [DOI] [PubMed] [Google Scholar]
- 45. Baroiller JF, D'Cotta H, Bezault E, Wessels S, Hoerstgen-Schwark G. 2009. Tilapia sex determination: where temperature and genetics meet. Comp Biochem Physiol A Mol Integr Physiol 153:30–38 [DOI] [PubMed] [Google Scholar]
- 46. Pannetier M, Servel N, Cocquet J, Besnard N, Cotinot C, Pailhoux E. 2003. Expression studies of the PIS-regulated genes suggest different mechanisms of sex determination within mammals. Cytogenet Genome Res 101:199–205 [DOI] [PubMed] [Google Scholar]
- 47. Hanna-Rose W, Hansen U. 1996. Active repression mechanisms of eukaryotic transcription repressors. Trends Genet 12:229–234 [DOI] [PubMed] [Google Scholar]
- 48. Sullivan SA, Akers L, Moody SA. 2001. foxD5a, a Xenopus winged helix gene, maintains an immature neural ectoderm via transcriptional repression that is dependent on the C-terminal domain. Dev Biol 232:439–457 [DOI] [PubMed] [Google Scholar]
- 49. Sutton J, Costa R, Klug M, Field L, Xu D, Largaespada DA, Fletcher CF, Jenkins NA, Copeland NG, Klemsz M, Hromas R. 1996. Genesis, a winged helix transcriptional repressor with expression restricted to embryonic stem cells. J Biol Chem 271:23126–23133 [DOI] [PubMed] [Google Scholar]
- 50. Bourguignon C, Li J, Papalopulu N. 1998. XBF-1, a winged helix transcription factor with dual activity, has a role in positioning neurogenesis in Xenopus competent ectoderm. Development 125:4889–4900 [DOI] [PubMed] [Google Scholar]
- 51. Xuan S, Baptista CA, Balas G, Tao W, Soares VC, Lai E. 1995. Winged helix transcription factor BF-1 is essential for the development of the cerebral hemispheres. Neuron 14:1141–1152 [DOI] [PubMed] [Google Scholar]
- 52. Hromas R, Ye H, Spinella M, Dmitrovsky E, Xu D, Costa RH. 1999. Genesis, a Winged Helix transcriptional repressor, has embryonic expression limited to the neural crest, and stimulates proliferation in vitro in a neural development model. Cell Tissue Res 297:371–382 [DOI] [PubMed] [Google Scholar]
- 53. Bentsi-Barnes IK, Kuo FT, Barlow GM, Pisarska MD. 2009. Human forkhead L2 represses key genes in granulosa cell differentiation including aromatase, P450scc, and cyclin D2. Fertil Steril 94:353–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Clark BJ, Wells J, King SR, Stocco DM. 1994. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem 269:28314–28322 [PubMed] [Google Scholar]
- 55. Lin D, Sugawara T, Strauss JF, 3rd, Clark BJ, Stocco DM, Saenger P, Rogol A, Miller WL. 1995. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science 267:1828–1831 [DOI] [PubMed] [Google Scholar]
- 56. Stocco DM. 2001. StAR protein and the regulation of steroid hormone biosynthesis. Annu Rev Physiol 63:193–213 [DOI] [PubMed] [Google Scholar]
- 57. Pollack SE, Furth EE, Kallen CB, Arakane F, Kiriakidou M, Kozarsky KF, Strauss JF., 3rd 1997. Localization of the steroidogenic acute regulatory protein in human tissues. J Clin Endocrinol Metabol 82:4243–4251. [DOI] [PubMed] [Google Scholar]
- 58. Baron D, Cocquet J, Xia X, Fellous M, Guiguen Y, Veitia RA. 2004. An evolutionary and functional analysis of FoxL2 in rainbow trout gonad differentiation. J Mol Endocrinol 33:705–715 [DOI] [PubMed] [Google Scholar]
- 59. Vendola K, Zhou J, Wang J, Famuyiwa OA, Bievre M, Bondy CA. 1999. Androgens promote oocyte insulin-like growth factor I expression and initiation of follicle development in the primate ovary. Biol Reprod 61:353–357 [DOI] [PubMed] [Google Scholar]
- 60. Skinner MK. 2005. Regulation of primordial follicle assembly and development. Hum Reprod Update 11:461–471 [DOI] [PubMed] [Google Scholar]
- 61. Sicinski P, Donaher JL, Geng Y, Parker SB, Gardner H, Park MY, Robker RL, Richards JS, McGinnis LK, Biggers JD, Eppig JJ, Bronson RT, Elledge SJ, Weinberg RA. 1996. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature 384:470–474 [DOI] [PubMed] [Google Scholar]
- 62. Moons DS, Jirawatnotai S, Tsutsui T, Franks R, Parlow AF, Hales DB, Gibori G, Fazleabas AT, Kiyokawa H. 2002. Intact follicular maturation and defective luteal function in mice deficient for cyclin- dependent kinase-4. Endocrinology 143:647–654 [DOI] [PubMed] [Google Scholar]
- 63. Nishi Y, Yanase T, Mu Y, Oba K, Ichino I, Saito M, Nomura M, Mukasa C, Okabe T, Goto K, Takayanagi R, Kashimura Y, Haji M, Nawata H. 2001. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology 142:437–445 [DOI] [PubMed] [Google Scholar]
- 64. Batista F, Vaiman D, Dausset J, Fellous M, Veitia RA. 2007. Potential targets of FOXL2, a transcription factor involved in craniofacial and follicular development, identified by transcriptomics. Proc Natl Acad Sci USA 104:3330–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Diaz MO, Ziemin S, Le Beau MM, Pitha P, Smith SD, Chilcote RR, Rowley JD. 1988. Homozygous deletion of the alpha- and beta 1-interferon genes in human leukemia and derived cell lines. Proc Natl Acad Sci USA 85:5259–5263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wolf SF, Sieburth D, Sypek J. 1994. Interleukin 12: a key modulator of immune function. Stem cells (Dayton, Ohio) 12:154–168 [DOI] [PubMed] [Google Scholar]
- 67. Srinivas S, Dai J, Eskdale J, Gallagher GE, Megjugorac NJ, Gallagher G. 2008. Interferon-lambda1 (interleukin-29) preferentially down-regulates interleukin-13 over other T helper type 2 cytokine responses in vitro. Immunology 125:492–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Son EW, Rhee DK, Pyo S. 2006. Gamma-irradiation-induced intercellular adhesion molecule-1 (ICAM-1) expression is associated with catalase: activation of Ap-1 and JNK. J Toxicol Environ Health A 69:2137–2155 [DOI] [PubMed] [Google Scholar]
- 69. Ahrens T, Pertz O, Haussinger D, Fauser C, Schulthess T, Engel J. 2002. Analysis of heterophilic and homophilic interactions of cadherins using the c-Jun/c-Fos dimerization domains. J Biol Chem 277:19455–19460 [DOI] [PubMed] [Google Scholar]
- 70. D'Sa-Eipper C, Chinnadurai G. 1998. Functional dissection of Bfl-1, a Bcl-2 homolog: anti-apoptosis, oncogene-cooperation and cell proliferation activities. Oncogene 16:3105–3114 [DOI] [PubMed] [Google Scholar]
- 71. Wu MX, Ao Z, Prasad KV, Wu R, Schlossman SF. 1998. IEX-1L, an apoptosis inhibitor involved in NF-kappaB-mediated cell survival. Science 281:998–1001 [DOI] [PubMed] [Google Scholar]
- 72. Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. 1997. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 91:197–208 [DOI] [PubMed] [Google Scholar]
- 73. Kim SY, Weiss J, Tong M, Laronda MM, Lee EJ, Jameson JL. 2009. Foxl2, a forkhead transcription factor, modulates nonclassical activity of the estrogen receptor-alpha. Endocrinology 150:5085–5093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yazawa T, Inaoka Y, Okada R, Mizutani T, Yamazaki Y, Usami Y, Kuribayashi M, Orisaka M, Umezawa A, Miyamoto K. 2010. PPAR-gamma coactivator-1alpha regulates progesterone production in ovarian granulosa cells with SF-1 and LRH-1. Mol Endocrinol 24:485–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Park M, Shin E, Won M, Kim JH, Go H, Kim HL, Ko JJ, Lee K, Bae J. 2005. FOXL2 interacts with steroidogenic factor-1 (SF-1) and represses SF-1-induced CYP17 transcription in granulosa cells. Mol Endocrinol 24:1024–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Johnson ES. 2004. Protein modification by SUMO. Annu Rev Biochem 73:355–382 [DOI] [PubMed] [Google Scholar]
- 77. Lin X, Liang M, Liang YY, Brunicardi FC, Feng XH. 2003. SUMO-1/Ubc9 promotes nuclear accumulation and metabolic stability of tumor suppressor Smad4. J Biol Chem 278:31043–31048 [DOI] [PubMed] [Google Scholar]
- 78. Ronen-Fuhrmann T, Timberg R, King SR, Hales KH, Hales DB, Stocco DM, Orly J. 1998. Spatio-temporal expression patterns of steroidogenic acute regulatory protein (StAR) during follicular development in the rat ovary. Endocrinology 139:303–315 [DOI] [PubMed] [Google Scholar]
- 79. Benayoun BA, Batista F, Auer J, Dipietromaria A, L'Hote D, De Baere E, Veitia RA. 2009. Positive and negative feedback regulates the transcription factor FOXL2 in response to cell stress: evidence for a regulatory imbalance induced by disease-causing mutations. Hum Mol Genet 18:632–644 [DOI] [PubMed] [Google Scholar]
- 80. Kuo FT, Bentsi-Barnes IK, Barlow GM, Bae J, Pisarska MD. 2009. Sumoylation of forkhead L2 by Ubc9 is required for its activity as a transcriptional repressor of the Steroidogenic Acute Regulatory gene. Cell Signal 21:1935–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Marongiu M, Deiana M, Meloni A, Marcia L, Puddu A, Cao A, Schlessinger D, Crisponi L. 2010. The forkhead transcription factor Foxl2 is sumoylated in both human and mouse: sumoylation affects its stability, localization, and activity. PloS One 5:e9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pisarska MD, Kuo FT, Bentsi-Barnes IK, Khan S, Barlow GM. 2010. LATS1 phosphorylates forkhead L2 and regulates its transcriptional activity. Am J Physiol Endocrinol Metab 299:E101–E109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. St John MA, Tao W, Fei X, Fukumoto R, Carcangiu ML, Brownstein DG, Parlow AF, McGrath J, Xu T. 1999. Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumours and pituitary dysfunction. Nat Genet 21:182–186 [DOI] [PubMed] [Google Scholar]
- 84. Ou Q, Mouillet JF, Yan X, Dorn C, Crawford PA, Sadovsky Y. 2001. The DEAD box protein DP103 is a regulator of steroidogenic factor-1. Mol Endocrinol 15:69–79 [DOI] [PubMed] [Google Scholar]
- 85. Lee K, Pisarska MD, Ko JJ, Kang Y, Yoon S, Ryou SM, Cha KY, Bae J. 2005. Transcriptional factor FOXL2 interacts with DP103 and induces apoptosis. Biochem Biophys Res Commun 336:876–881 [DOI] [PubMed] [Google Scholar]
- 86. Pelusi C, Ikeda Y, Zubair M, Parker KL. 2008. Impaired follicle development and infertility in female mice lacking steroidogenic factor 1 in ovarian granulosa cells. Biol Reprod 79:1074–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Blount AL, Schmidt K, Justice NJ, Vale WW, Fischer WH, Bilezikjian LM. 2009. FoxL2 and Smad3 coordinately regulate follistatin gene transcription. J Biol Chem 284:7631–7645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bilezikjian LM, Blount AL, Leal AM, Donaldson CJ, Fischer WH, Vale WW. 2004. Autocrine/paracrine regulation of pituitary function by activin, inhibin and follistatin. Mol Cell Endocrinol 225:29–36 [DOI] [PubMed] [Google Scholar]
- 89. DePaolo LV, Mercado M, Guo Y, Ling N. 1993. Increased follistatin (activin-binding protein) gene expression in rat anterior pituitary tissue after ovariectomy may be mediated by pituitary activin. Endocrinology 132:2221–2228 [DOI] [PubMed] [Google Scholar]
- 90. Corrigan AZ, Bilezikjian LM, Carroll RS, Bald LN, Schmelzer CH, Fendly BM, Mason AJ, Chin WW, Schwall RH, Vale W. 1991. Evidence for an autocrine role of activin B within rat anterior pituitary cultures. Endocrinology 128:1682–1684 [DOI] [PubMed] [Google Scholar]
- 91. Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC. 2004. Regulation of gonadotropin subunit gene transcription. J Mol Endocrinol 33:559–584 [DOI] [PubMed] [Google Scholar]
- 92. Corpuz PS, Lindaman LL, Mellon PL, Coss D. 2010. FoxL2 Is required for activin induction of the mouse and human follicle-stimulating hormone beta-subunit genes. Mol Endocrinol 24:1037–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Meduri G, Bachelot A, Duflos C, Bstandig B, Poirot C, Genestie C, Veitia R, De Baere E, Touraine P. 2010. FOXL2 mutations lead to different ovarian phenotypes in BPES patients: Case Report. Hum Reprod 25:235–243 [DOI] [PubMed] [Google Scholar]
- 94. De Baere E, Dixon MJ, Small KW, Jabs EW, Leroy BP, Devriendt K, Gillerot Y, Mortier G, Meire F, Van Maldergem L, Courtens W, Hjalgrim H, Huang S, Liebaers I, Van Regemorter N, Touraine P, Praphanphoj V, Verloes A, Udar N, Yellore V, Chalukya M, Yelchits S, De Paepe A, Kuttenn F, Fellous M, Veitia R, Messiaen L. 2001. Spectrum of FOXL2 gene mutations in blepharophimosis-ptosis-epicanthus inversus (BPES) families demonstrates a genotype–phenotype correlation. Hum Mol Genet 10:1591–1600 [DOI] [PubMed] [Google Scholar]
- 95. Ramirez-Castro JL, Pineda-Trujillo N, Valencia AV, Muneton CM, Botero O, Trujillo O, Vasquez G, Mora BE, Durango N, Bedoya G, Ruiz-Linares A. 2002. Mutations in FOXL2 underlying BPES (types 1 and 2) in Colombian families. Am J Med Genet 113:47–51 [DOI] [PubMed] [Google Scholar]
- 96. De Baere E, Beysen D, Oley C, Lorenz B, Cocquet J, De Sutter P, Devriendt K, Dixon M, Fellous M, Fryns JP, Garza A, Jonsrud C, Koivisto PA, Krause A, Leroy BP, Meire F, Plomp A, Van Maldergem L, De Paepe A, Veitia R, Messiaen L. 2003. FOXL2 and BPES: mutational hotspots, phenotypic variability, and revision of the genotype-phenotype correlation. Am J Hum Genet 72:478–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Pisarska M, Bentsi-Barnes I, Barlow G, Kuo F. 2010. FOXL2 mutations associated with premature ovarian failure (POF) likely dimerize with wild type FOXL2, leading to altered regulation of genes associated with granulosa cell differentiation. The Endocrine Society's 92nd Annual Meeting, San Diego, CA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Beysen D, Moumne L, Veitia R, Peters H, Leroy BP, De Paepe A, De Baere E. 2008. Missense mutations in the forkhead domain of FOXL2 lead to subcellular mislocalization, protein aggregation and impaired transactivation. Hum Mol Genet 17:2030–2038 [DOI] [PubMed] [Google Scholar]
- 99. Schumer ST, Cannistra SA. 2003. Granulosa cell tumor of the ovary. J Clin Oncol 21:1180–1189 [DOI] [PubMed] [Google Scholar]
- 100. Colombo N, Parma G, Zanagnolo V, Insinga A. 2007. Management of ovarian stromal cell tumors. J Clin Oncol 25:2944–2951 [DOI] [PubMed] [Google Scholar]
- 101. Pectasides D, Papaxoinis G, Fountzilas G, Aravantinos G, Pectasides E, Mouratidou D, Economopoulos T, Andreadis C. 2008. Adult granulosa cell tumors of the ovary: a clinicopathological study of 34 patients by the Hellenic Cooperative Oncology Group (HeCOG). Anticancer Res 28:1421–1427 [PubMed] [Google Scholar]
- 102. Fuller PJ, Verity K, Shen Y, Mamers P, Jobling T, Burger HG. 1998. No evidence of a role for mutations or polymorphisms of the follicle-stimulating hormone receptor in ovarian granulosa cell tumors. J Clin Endocrinol Metabol 83:274–279 [DOI] [PubMed] [Google Scholar]
- 103. Chu S, Rushdi S, Zumpe ET, Mamers P, Healy DL, Jobling T, Burger HG, Fuller PJ. 2002. FSH-regulated gene expression profiles in ovarian tumours and normal ovaries. Mol Hum Reprod 8:426–433 [DOI] [PubMed] [Google Scholar]
- 104. Graves PE, Surwit EA, Davis JR, Stouffer RL. 1985. Adenylate cyclase in human ovarian cancers: sensitivity to gonadotropins and nonhormonal activators. Am J Obstet Gynecol 153:877–882 [DOI] [PubMed] [Google Scholar]
- 105. Stouffer RL, Grodin MS, Davis JR, Surwit EA. 1984. Investigation of binding sites for follicle-stimulating hormone and chorionic gonadotropin in human ovarian cancers. J Clin Endocrinol Metabol 59:441–446 [DOI] [PubMed] [Google Scholar]
- 106. Rey RA, Lhomme C, Marcillac I, Lahlou N, Duvillard P, Josso N, Bidart JM. 1996. Antimullerian hormone as a serum marker of granulosa cell tumors of the ovary: comparative study with serum alpha-inhibin and estradiol. Am J Obstet Gynecol 174:958–965 [DOI] [PubMed] [Google Scholar]
- 107. Boggess JF, Soules MR, Goff BA, Greer BE, Cain JM, Tamimi HK. 1997. Serum inhibin and disease status in women with ovarian granulosa cell tumors. Gynecol Oncol 64:64–69 [DOI] [PubMed] [Google Scholar]
- 108. Jobling T, Mamers P, Healy DL, MacLachlan V, Burger HG, Quinn M, Rome R, Day AJ. 1994. A prospective study of inhibin in granulosa cell tumors of the ovary. Gynecol Oncol 55:285–289 [DOI] [PubMed] [Google Scholar]
- 109. Lappohn RE, Burger HG, Bouma J, Bangah M, Krans M, de Bruijn HW. 1989. Inhibin as a marker for granulosa-cell tumors. N Engl J Med 321:790–793 [DOI] [PubMed] [Google Scholar]
- 110. Kaye SB, Davies E. 1986. Cyclophosphamide, adriamycin, and cis-platinum for the treatment of advanced granulosa cell tumor, using serum estradiol as a tumor marker. Gynecol Oncol 24:261–264 [DOI] [PubMed] [Google Scholar]
- 111. Shah SP, Kobel M, Senz J, Morin RD, Clarke BA, Wiegand KC, Leung G, Zayed A, Mehl E, Kalloger SE, Sun M, Giuliany R, Yorida E, Jones S, Varhol R, Swenerton KD, Miller D, Clement PB, Crane C, Madore J, Provencher D, Leung P, DeFazio A, Khattra J, Turashvili G, Zhao Y, Zeng T, Glover JN, Vanderhyden B, Zhao C, Parkinson CA, Jimenez-Linan M, Bowtell DD, Mes-Masson AM, Brenton JD, Aparicio SA, Boyd N, Hirst M, Gilks CB, Marra M, Huntsman DG. 2009. Mutation of FOXL2 in granulosa-cell tumors of the ovary. N Engl J Med 360:2719–2729 [DOI] [PubMed] [Google Scholar]
- 112. Kim MS, Hur SY, Yoo NJ, Lee SH. 2010. Mutational analysis of FOXL2 codon 134 in granulosa cell tumour of ovary and other human cancers. J Pathol 221:147–152 [DOI] [PubMed] [Google Scholar]
- 113. Kim T, Sung CO, Song SY, Bae DS, Choi YL. 2010. FOXL2 mutation in granulosa-cell tumours of the ovary. Histopathology 56:408–410 [DOI] [PubMed] [Google Scholar]
- 114. Schrader KA, Gorbatcheva B, Senz J, Heravi-Moussavi A, Melnyk N, Salamanca C, Maines-Bandiera S, Cooke SL, Leung P, Brenton JD, Gilks CB, Monahan J, Huntsman DG. 2009. The specificity of the FOXL2 c. 402C>G somatic mutation: a survey of solid tumors. PloS One 4:e7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Jamieson S, Butzow R, Andersson N, Alexiadis M, Unkila-Kallio L, Heikinheimo M, Fuller PJ, Anttonen M. 2010. The FOXL2 C134W mutation is characteristic of adult granulosa cell tumors of the ovary. Mod Pathol 23:1477–1485 [DOI] [PubMed] [Google Scholar]
- 116. Benayoun BA, Caburet S, Dipietromaria A, Georges A, D'Haene B, Pandaranayaka PJ, L'Hote D, Todeschini AL, Krishnaswamy S, Fellous M, De Baere E, Veitia RA. 2010. Functional exploration of the adult ovarian granulosa cell tumor-associated somatic FOXL2 mutation p.Cys134Trp (c. 402C>G). PloS One 5:e8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kalfa N, Fellous M, Boizet-Bonhoure B, Patte C, Duvillard P, Pienkowski C, Jaubert F, Ecochard A, Sultan C. 2008. Aberrant expression of ovary determining gene FOXL2 in the testis and juvenile granulosa cell tumor in children. J Urol 180:1810–1813 [DOI] [PubMed] [Google Scholar]
- 118. Lamba P, Fortin J, Tran S, Wang Y, Bernard DJ. 2009. A novel role for the forkhead transcription factor FOXL2 in activin A-regulated follicle-stimulating hormone beta subunit transcription. Mol Endocrinol 23:1001–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]