Abstract
Background
Chronic intravascular hemolysis leads to nitric oxide (NO) depletion and pulmonary hypertension in sickle cell disease. To test whether this pathophysiology occurs in malaria, we examined 53 children admitted to hospital with severe malaria (excluding cerebral malaria) and 31 age-matched controls in Mali.
Methods
Severity of hemolysis was assessed from plasma free hemoglobin (Hb) and arginase-1 levels. NO metabolism was assessed by whole blood nitrite levels and plasma NO consumption. Effects on the cardiovascular system and endothelial function were assessed by using echocardiography to measure peak tricuspid regurgitant jet velocity (TRV) and from plasma levels of N-terminal prohormone brain natriuretic peptide (NT-proBNP) and soluble vascular cell adhesion molecule-1 (sVCAM-1).
Results
Children with severe malaria had higher plasma Hb and arginase-1 levels, reduced whole blood nitrite levels and increased NO consumption relative to controls. They also had increased pulmonary arterial pressures (p < 0.05) with elevated levels of NT-proBNP and sVCAM-1 (p < 0.001).
Conclusions
Children with severe malaria have increased pulmonary pressures and myocardial wall stress. These complications are consistent with NO depletion from intravascular hemolysis, and indicate that the pathophysiologic cascade from intravascular hemolysis to NO depletion and its cardiopulmonary effects is activated in children with severe malaria.
Keywords: malaria, Plasmodium falciparum, nitric oxide (NO), intravascular hemolysis, whole blood nitrite, NO consumption, tricuspid regurgitation velocity (TRV), NT-proBNP, myocardial wall stress, sVCAM-1, endothelial dysfunction
Introduction
More than a century after Laveran’s discovery of the malaria parasite [1], malaria mortality remains 1–2 million per year [2–4]. Although malarial pathogenesis results primarily from parasite invasion of the erythrocyte with secondary hemolysis [5], disease appears related to cytokines such as TNF [6] and the adhesion of parasitized red cells to endothelial cell receptors in post-capillary venules of the brain, heart, lungs, kidneys and other organs [7]. In addition, because of the microvascular pathology observed in malaria, there has been substantial interest in the potential effects of nitric oxide (NO), as a master regulator of blood flow, thrombosis, adhesion and vascular homeostasis. However, despite the potential importance of NO, its role and the role of its metabolism in malarial pathogenesis remain unclear [8–10].
The central unresolved paradox is the combination of upregulated NO production in severe malaria, with elevated levels of inducible NO synthase (iNOS) and downstream NO metabolites comprised largely of nitrate (NO3−), that are collectively termed NOx [10–15]. Some investigators have interpreted these findings as suggesting that elevated levels of NO and its metabolites drive malarial pathogenesis. However, genetic polymorphisms that increase iNOS activity are associated with protection, and thus suggest that the effects of NO on intracellular signaling may be beneficial [15], although the published data are conflicting. A potential solution to this paradox comes from studies of sickle cell disease and other hemolytic anemias [16–19]. In those conditions, intravascular hemolysis releases hemoglobin (Hb) from red cells into plasma, where it reacts rapidly with NO produced by NOS to form biologically inactive nitrate. This leads to NO depletion with elevated pulmonary pressures, and is accompanied by the elevation of biochemical markers normally suppressed by NO (e.g., soluble vascular cell adhesion molecule-1, sVCAM-1). According to this model [17–18], levels of NO synthase and nitrate reflect the synthesis of NO, but not its bioavailability. Thus, intravascular hemolysis produces a state of NO resistance mediated/driven by NO catabolism, which is linked to pulmonary hypertension in sickle cell disease [20]. Finally, the depletion of NO by free Hb from intravascular hemolysis is exacerbated by the simultaneous release of erythrocyte arginase-1 into plasma, which reduces plasma levels of arginine and thus decreases its availability as a substrate for NO production by NOS [18].
Consistent with that model, the severity of murine malaria is related to free plasma Hb, but not the parasite load [21]. In mice, endothelial NOS (eNOS) deficiency increases the severity of malaria, thus suggesting that NO depletion (not NO production) exacerbates malaria. Consistent with increased arginase-1 from intravascular hemolysis, plasma arginine is low in human malaria, and arginine supplementation partially normalizes peripheral blood flow [22–24]. Likewise, Anstey, Weinberg and their colleagues have found that patients with severe malaria have low levels of exhaled NO and evidence of vascular dysfunction when hemolysis is severe [23]. Although there are major differences between cerebral malaria in humans and mice, the administration of inhaled NO to mice with cerebral malaria produces marked improvement and increases their survival [21].
Based on these observations, we have hypothesized that intravascular hemolysis in severe malaria leads to elevated plasma Hb and arginase levels with scavenging of NO by free plasma Hb, and to increased pulmonary arterial pressures with elevated levels of markers for myocardial wall stress. To test this hypothesis, we performed a case-control study of Malian children with severe malaria to evaluate the relationship of increased pulmonary pressures, myocardial wall stress and endothelial dysfunction to intravascular hemolysis and NO depletion.
Methods
Study Design and Case Definitions
This study compared children with severe malaria [25–26] to children without malaria (age-matched controls). To reduce confounding by individual differences, it also compared cases at admission to cases at the time of discharge. Recruitment of cases from the urban and surrounding peri-urban and rural regions of Bamako was performed at the Hôpital Gabriel Touré - the major pediatric hospital in Mali. This study was conducted from October to December 2007 near the end of the rainy season, which coincides with the peak incidence of severe malaria in Mali. The protocol was approved by Institutional Review Boards for the University of Bamako, the National Heart, Lung, and Blood Institute (NIH) and Tulane University.
Inclusion criteria for cases included: 1] a positive thick smear for Plasmodium falciparum parasites, 2] asexual parasite count ≥ 2,000 per µL of blood, 3] blood Hb < 5 gms per dL, 4] age 1–5 years and 5] informed consent. Children with Hb levels 5.0–6.9 gms per dL were eligible if they had respiratory distress (tachypnea with respirations > 40 per minute, alar flaring or intercostal retractions) [27]. However, children were excluded if they had: 1] cerebral malaria (convulsions witnessed by an investigator or a Blantyre coma score ≤ 2) [28], 2] hypoglycemia (blood glucose < 2.2 mmols per L), 3] malaria parasites other than P. falciparum or 4] medical conditions other than malaria. Antimalarial treatment was provided free of charge to children who were recruited, regardless of their participation in the study.
Controls from Bamako and the surrounding region were frequently patient visitors or relatives. Inclusion criteria included: 1] negative thick smear for P. falciparum parasites, 2] temperature ≤ 37.5° C with no history of fever during the previous 2 weeks, 3] no signs or symptoms of malaria (no headache, muscle aches, malaise), 4] age 1–5 years, 5] no anti-malarial medications in the previous 2 weeks, 6] no known medical conditions and 7] informed consent.
Calculation of Sample Sizes
Sample sizes for this study were based on the numbers of cases and controls necessary to detect 25–30% differences in plasma Hb, arginase, sVCAM-1, NT-proBNP, and 0.5 meters per second in the initial tricuspid regurgitant velocity (TRV) – assuming that the magnitude of these differences between children with severe malaria and controls in Mali would be similar to the differences between adults with sickle cell disease and controls in the U.S. [18].
Clinical and Laboratory Evaluation and Follow-Up
All participants had a directed history and physical examination at the time of admission, plus a fingerstick for a parasite count, Hb and glucose; additional blood specimens were obtained by venipuncture. Echocardiograms were performed by a single investigator (JJJ) using a GE Vivid i ultrasound machine (GE Healthcare – Chicago, IL) with a 7S-RS (3.3 – 8.0 MHz) pediatric cardiac probe [20]. Previous validation studies have shown that pulmonary arterial pressures estimated by echocardiography correlate well with measurements performed during cardiac catheterization (r=0.77, p<0.001) [20]. The echocardiograms were analyzed in Bethesda by investigators who did not know whether individual recordings were from cases or controls.
Follow-up examinations were performed at the time of discharge (3–5 days after admission). Studies obtained at that time included repeat cardiac ultrasound, blood smears, chemistry and hematology panels, venous blood gases, NT-proBNP, sVCAM-1, arginase, plasma Hb, whole blood nitrite and NO consumption.
Treatment
Children with severe malaria received artemether by intramuscular injection: 3.2 mg per kg on day 1 and 1.6 mg per kg on days 2, 3 and 4; followed by 3 days of oral Coartesiane: 4 mg artemether and 24 mg lumefantrine per kg per day on days 5–7 (as two divided doses per day). Blood transfusions were provided for severe malarial anemia (Hb < 5 gms per dL; 20 ml whole blood per kg × 2 at 1–2 day intervals) to produce Hb levels ≥ 6 gms per dL.
Laboratory Assays
Thick smears for malaria parasites were obtained by fingerstick, stained with Giemsa and examined using oil immersion magnification (1000×). Each slide was examined by two microscopists who counted the number of asexual P. falciparum parasites in fields with 300 white blood cells, and multiplied by 25 to estimate the number of parasites per µL [29]. Slides for which there was disagreement on the parasitemia (≥ 10%) or on whether the slide was positive were re-examined by a senior investigator (OAK).
Whole blood Hb levels were measured using a portable spectrophotometer system (HemoCue 2001+, HEMOCUE AB - Angelholm, SWEDEN). Chemistry panels for kidney and liver function were performed with a Piccolo analyzer (Abaxis Medical Diagnostics - Union City, CA) and venous blood gas measurements with an i-STAT handheld analyzer (Abbott Laboratories - East Windsor, NJ). N-terminal prohormone brain natriuretic peptide (NT-proBNP), creatine kinase muscle band (CK-MB) and troponin-T assays were performed with the Elecsys 2010 (Roche Diagnostics – Indianapolis, IN); ELISA assays were performed with commercially available kits for plasma Hb (Bethyl Laboratories - Montgomery, TX), haptoglobin (Alpco Diagnostics - Windham, NH), sVCAM-1 (R & D Systems - Minneapolis, MN) and arginase-1 (Cell Sciences - Canton, MA). P. falciparum parasite antigen was detected using a rapid diagnostic test based on parasite LDH (Standard Diagnostics - Suwon City, SOUTH KOREA). Hb types were identified using 5 µl of whole blood hemolysate for cellulose acetate electrophoresis, followed by staining with Ponceau S and comparison with controls for Hb A, C, F and S (Helena Laboratories - Beaumont, TX).
In the NO consumption assay [16, 30], Fe2+ Hb reacts stoichiometrically with NO at near the diffusion limit to produce nitrate: i.e., one molecule of Fe2+ Hb destroys one molecule of NO. NO consumption was measured with a gas-phase chemiluminescence NO analyzer (Figure 1B) [30] after diluting patient samples 1:10 and 1:50 to prevent complete scavenging of all the NO in the system. Whole blood nitrite measurements [31–32] were performed by mixing blood samples initially (at the time of collection) with a ferricyanide buffer to prevent the conversion of nitrite to nitrate or iron-nitrosylhemoglobin by oxidizing Fe2+ Hb to Fe3+ metHb [31–32]. This buffer also contains EDTA and N-ethylmaleimide to inhibit reactions with trace metals and thiols. Extensive validation studies have shown that this buffer preserves nitrite levels in whole blood and that measurements of whole blood nitrite reflect NO bioavailability [31].
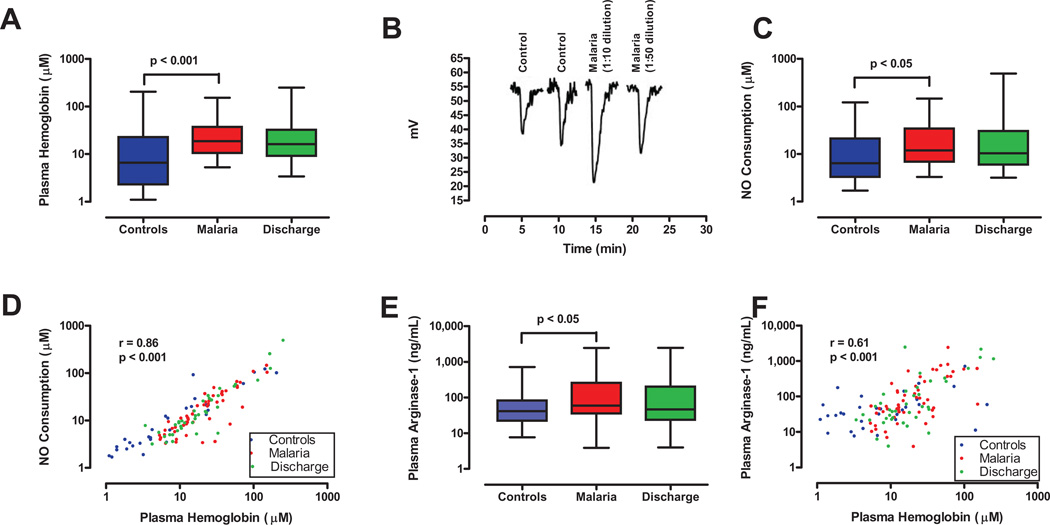
Figure 1. Intravascular hemolysis and nitric oxide resistance in malaria.
A. Comparison of free plasma Hb levels for controls (n=31) at the time of admission, and for cases at the times of admission (n=52) and discharge (day 3–5, n=38). Plasma Hb levels were higher in cases than controls at the time of admission (p<0.001). B. NO consumption by plasma from a control subject and two dilutions of plasma from a patient with severe malaria. The downward displacement of the tracing from the NO analyzer represents the consumption of NO generated from the NO donor agent in the reaction vessel. These four tracings were obtained on the same day and combined in one figure for illustrative purposes. C. Comparison of NO consumption by plasma from controls (n=31) at the time of admission, and cases at the times of admission (n=52) and discharge (n=38). NO consumption was greater for cases than controls at the time of admission (p=0.04). D. Comparison of plasma free Hb and NO consumption results. Plasma Hb results from panel A are plotted on the abscissa with NO consumption results from panel C on the ordinate, demonstrating a positive correlation between the two (p<0.001). E. Comparison of plasma arginase-1 results for controls (n=31) and for cases at the times of admission (n=52) and discharge (n=37). Arginase levels were higher in cases than controls at admission (p<0.05), and showed a non-significant decline at the time of discharge. F. Comparison of plasma free Hb and arginase-1 results. Plasma Hb results from panel A are plotted on the abscissa with arginase-1 results from panel E on the ordinate, and demonstrate a positive correlation (p<0.001).
Statistical Analyses
Data are provided as means and standard deviations, or as medians with interquartile ranges. Between-group differences were examined with the Mann-Whitney or Fisher’s Exact tests. Within-group differences were examined with the Wilcoxon signed rank test and correlations with Spearman’s rank correlation coefficient. Significance levels were set at a 2-sided alpha of 0.05 and analyses were performed using SPSS software (version 15.0 - Chicago, IL).
Results
Baseline Comparison of Cases and Controls
During the eight week study period, 53 children with severe malaria and 31 controls were enrolled who had significant differences in their parasite counts and blood Hb levels (p<0.001 for both, Table 1). Children with severe malaria [25–26] also had higher plasma levels of lactate dehydrogenase (LDH), indirect bilirubin and plasma free Hb (p<0.001 for all, Table 2). Testing for P. falciparum antigen (parasite LDH) was negative in the 31 uninfected controls and positive in 10 samples from cases (p<0.001, Table 1).
Table 1.
BASIC DEMOGRAPHIC DATA FOR CASES AND CONTROLS @
| Severe Malaria | Controls | p-Value | |
|---|---|---|---|
| Number of Subjects | 53 | 31 | NA |
| Age in months | 30.1 ± 16.2 (n=53) |
36.9 ± 16.6 (n=31) |
0.05 |
| Gender (M/F) (percent Male) |
21/32 (40%) |
19/12 (62%) |
0.07 |
| Height in cm | 89.1 ± 13.3 (n=48) |
92.5 ± 14.1 (n=31) |
0.02 |
| Weight in kg | 11.2 ± 2.8 (n=52) |
13.1 ± 3.6 (n=31) |
0.26 |
| Parasite Count (parasites per µl blood) |
35,733 ± 45,365 (n=53) |
000 ± 000 (n=31) |
< 0.001 |
|
P. falciparum Antigen Test (species-specific LDH) |
10 of 10 | 0 of 31 | < 0.001 |
| Hemoglobin (gms per dL) |
4.2 ± 1.3 (n=53) |
8.5 ± 1.1 (n=31) |
< 0.001 |
| Hemoglobin Types (starch gel electrophoresis) |
46 AA, 5 AC, 1 AS (n=52) |
27 AA, 1 AC, 3 AS (n=31) |
Data on age, height, weight, parasite count and blood Hb levels are provided as means with standard deviations.
Table 2.
INTRAVASCULAR HEMOLYSIS, NO CONSUMPTION AND ECHOCARDIOGRAPHY &
| Severe Malaria Cases | Controls | p-Value | |
|---|---|---|---|
| Plasma free Hb by ELISA (µM) |
18.8 (10.8, 37.2) (n=52) |
6.6 (2.3, 22.7) (n=31) |
< 0.001 |
| Plasma Haptoglobin (mg per dL) |
2.1 (1.0, 11.5) (n=10) |
189.8 (97.2, 235.5) (n=31) |
< 0.001 |
| Arginase-1 (ng per mL) |
59 (36, 250) (n=52) |
42 (22, 83) (n=31) |
0.03 |
| Indirect Bilirubin (mg per dL) |
1.10 (0.50. 1.35) (n=40) |
0.4 (0/3, 0.5) (n=27) |
< 0.001 |
| Lactate Dehydrogenase (LDH) (International Units per L) |
645 (504, 839) (n=37) |
338 (273, 579) (n=25) |
< 0.001 |
| NO Consumption by Plasma Hb (µM) |
11.9 (7.0, 34.1) (n=52) |
6.4 (3.3, 21.2) (n=31) |
0.04 |
| Whole Blood Nitrite (µM) |
0.52 (0.39, 0.75) (n=50) |
0.72 (0.51, 0.94) (n=15) |
0.02 |
| Tricuspid regurgitant velocity (TRV) (meters/second) |
2.5 (2.3, 2.8) (n=36) |
2.0 (1.9, 2.4) (n=17) |
< 0.001 |
| Pulmonary arterial systolic pressure (mm Hg) |
31 (26, 36) (n=36) |
21 (19, 27) (n=17) |
< 0.001 |
|
NT-proBNP (ng per mL) |
669 (303, 1689) (n=50) |
100 (56, 150) (n=31) |
< 0.001 |
| sVCAM-1 (ng per mL) |
3444 (2647, 4107) (n=52) |
891 (724, 1504) (n=31) |
< 0.001 |
| Left ventricular ejection fraction (percent) |
64% (60%, 65%) (n=30) |
65% (58%, 65%) (n=12) |
0.54 |
| Troponin T ^ (pg per mL) |
10 (10, 10) (n=50) |
10 (10, 10) (n=31) |
0.29 |
| CK MB (ng per mL) |
4.31 (1.90, 9.34) (n=50) |
3.97 (3.25, 5.12) (n=31) |
0.42 |
Data in Table 2 are provided as medians with 25th and 75th percentiles.
For troponin T, results below the threshold of detection (< 10 pg per mL) were entered as 10 pg per mL.
Plasma Hb and the Consumption of Nitric Oxide (NO)
Consistent with intravascular hemolysis, free plasma Hb was higher in cases than controls (medians of 18.9 vs. 6.6 µM based on heme concentration, p<0.001, Figure 1A) and haptoglobin was lower (medians of 2.16 vs. 189.8 µM, p<0.001, Table 2). To determine whether free plasma Hb scavenged NO, aliquots of plasma from cases were injected into an NO assay system. The results indicate NO consumption was greater with plasma from cases than controls (p<0.05, Figure 1C). Consistent with a stoichiometric reaction in which one molecule of Fe2+ Hb reacts with one of NO, the plasma free Hb by ELISA correlated with the NO concentration scavenged (Figure 1D; r=0.86, p<0.001) [16,19], and was greater for cases than controls (medians of 11.9 vs. 6.4 µM, p=0.04). NO consumption by plasma from cases was lower at discharge (day 3–5) than admission, but not significantly so (p>0.05, Figure 1C).
Plasma Arginase-1 Levels and Intravascular Hemolysis
Arginase-1 is an erythrocyte enzyme that catabolizes arginine to ornithine in the urea cycle. In hemolytic disorders such as sickle cell disease and thalassemia, arginase released into plasma by intravascular hemolysis catabolizes arginine, and thus reduces arginine availability for NO synthesis. In this study, plasma arginase levels were higher in children with severe malaria than controls (p<0.05, Figure 1E). Consistent with simultaneous release of arginase and Hb from red cells during intravascular hemolysis, plasma arginase correlated with plasma free Hb (Figure 1F; r=0.61, p<0.001).
Endothelial Activation and NO Bioavailability
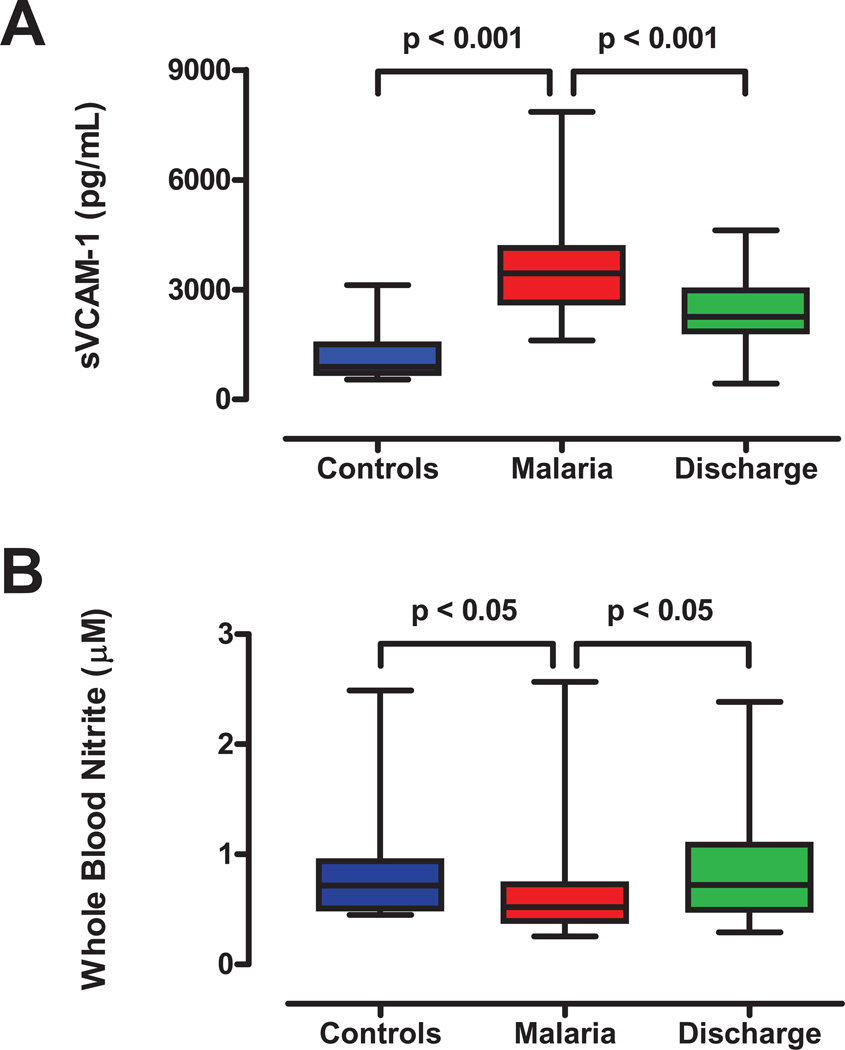
To assess endothelial and vascular function, we measured two biomarkers. The first, sVCAM-1, is associated with NO depletion and endothelial dysfunction, and increases with intravascular hemolysis in sickle cell disease [33]. In this study, sVCAM-1 levels was elevated in cases relative to controls and fell in cases between the times of admission and discharge (Figure 2A; p<0.001 for both comparisons). The second biomarker, whole blood nitrite (Figure 2B), provides a correlate of NO bioavailability in blood [31, 34]. Consistent with reduced NO bioavailability, whole blood nitrite was lower in cases than controls (p<0.05, Figure 2B), correlated negatively with plasma free Hb (r=−0.249, p=0.05), plasma arginase-1 (r=−0.392, p<0.01), indirect bilirubin and LDH (p<0.01, Table 2), and rose with treatment and recovery between times of admission and discharge (p<0.05, Figure 2B).
Figure 2. Markers of circulatory stress during severe malaria.
A. Soluble vascular cell adhesion molecule (sVCAM-1), a marker of endothelial activation and adhesiveness. Plasma levels of sVCAM-1 were higher in cases (n=52) than controls (n=31, p<0.001) at admission, and decreased by the time of discharge (day 3–5, n=38, p<0.001). B. Comparison of whole blood nitrite levels in controls at admission, and in cases at times of admission and discharge. Whole blood nitrite levels, a marker of NO bioavailability, were lower in cases (n=50) than controls at admission (n=15; p<0.05), and rose by the time of discharge (p<0.05, n=26). Box and whisker plots indicate median, range and interquartile boundaries.
Increased Pulmonary Pressures and Myocardial Wall Stress
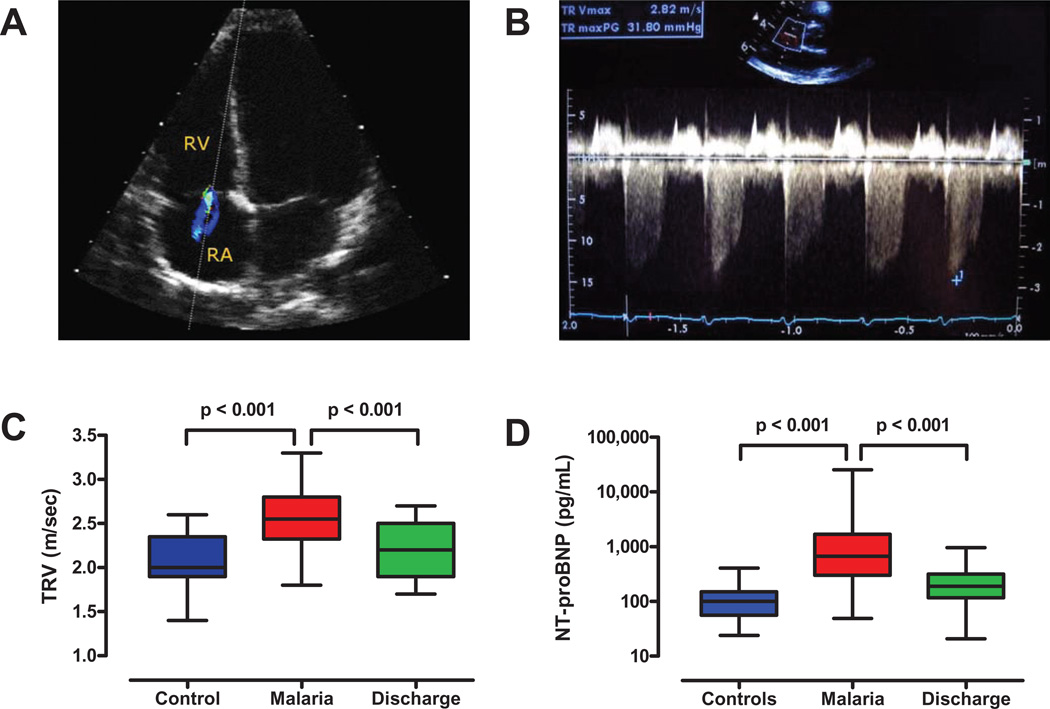
To determine whether acute intravascular hemolysis in severe malaria increased pulmonary arterial pressures or produced myocardial wall stress, we used Doppler echocardiography (Figure 3A) to measure the TRV (Figures 3B–C) [20], and measured plasma levels of NT-proBNP, a biomarker released from cardiac myocytes under the stress of pressure or volume overload (Figure D) [33, 35–37]. Both these measures are elevated during left or right ventricular pressure overload and correlate with pulmonary arterial systolic pressures and cardiovascular risk [35–36]. The TRVs (Figure 3C) and the pulmonary arterial pressures calculated from the TRVs were higher in cases than controls (Table 2, both p<0.001). Although increased pulmonary pressures can be produced secondarily by left ventricular dysfunction, there was no evidence for left ventricular dysfunction in either the cases or controls (based on the normal left ventricular ejection fractions and the normal CK-MB and Troponin T levels, Table 2). Consistent with the increased pulmonary arterial pressures, cases had higher plasma NT-proBNP levels than controls (p<0.001, Figure 3D) [38]. Although increased cardiac output from anemia could increase pulmonary pressures, the only reports we have found which examined this question found no evidence anemia increased pulmonary pressures in sickle cell disease [39] and reported that iron deficiency anemia increased NO production [40].
Figure 3. Echocardiographic evidence of elevated pulmonary arterial pressures in malaria.
A. An ultrasound probe was used to visualize tricuspid regurgitation in Malian children with severe malaria. The dotted line indicates the path of sound waves from the probe across the tricuspid valve, where the regurgitant jet from the right ventricle (RV) to the right atrium (RA) is shown in color. B. Doppler echocardiogram demonstrating tricuspid regurgitation in a child with severe malaria. The lowest point of the downward deflection indicates the initial tricuspid regurgitant jet velocity (TRV) at the peak of right ventricular systole (scale is on the right side of the panel). This echocardiogram was interpreted as having a peak initial TRV of 2.8 meters per second. C. Tricuspid regurgitant jet velocities (TRVs) for controls (n=17) and cases (n=36) at the times of admission and discharge (day 3–5, n=19). TRVs for cases were greater than for controls (p<0.001) and fell between admission and discharge (p<0.001). D. N-terminal prohormone brain natriuretic peptide (NT-proBNP), a marker of cardiac muscle stretch and mechanical stress. Levels of NT-proBNP were higher in cases (n=50) than controls (n=31) at the time of admission (p<0.001), and fell in cases by the time of discharge (n=38, p<0.001).
Effects of Treatment on Pulmonary Pressures, NT-proBNP and sVCAM-1
Coincident with treatment and recovery, children with severe malaria had increases in blood Hb levels with transfusion and decreases in parasite count (with negative blood smears at the time of discharge), TRV, pulmonary arterial pressure, sVCAM-1 and NT-proBNP (p<0.001 for all comparisons), but not the left ventricular ejection fraction (p=0.139) – i.e., there was no evidence of left ventricular dysfunction. In relation to whole blood nitrite, plasma arginase, plasma Hb, blood Hb and the left ventricular ejection fraction, the only significant correlation for TRV or NT-proBNP was a negative correlation between NT-proBNP and the blood Hb (r=−0.46, p<0.001).
Conversely, parameters that did not decrease between admission and discharge included plasma Hb, NO consumption, and plasma arginase (Figures 1A, 1C, 1E). The fact that these three parameters related to intravascular hemolysis did not decrease is consistent with: 1] a normal host response (increased NO synthesis) during treatment and recovery that reduced TRVs, pulmonary pressures, NT-proBNP and sVCAM-1, and increased whole blood nitrite, 2] continuing low-level intravascular hemolysis from transfusion for severe anemia during the first several days of hospitalization, and 3] the time required to replete haptoglobin levels depleted by intravascular hemolysis in severe P. falciparum malaria. Because 80% of cases (42 of 53) were transfused and 6 of the 11 who were not transfused died (n=4) or left the hospital against advice (n=2), there was insufficient statistical power to examine the effects of transfusion on other parameters. Among the controls, there were no significant changes in TRVs, pulmonary arterial pressures, or plasma levels of NT-proBNP or sVCAM-1 between admission and discharge.
Discussion
Pathophysiologic Overview
These results demonstrate that severe malaria in children produces central cardiopulmonary effects (increased pulmonary pressures and myocardial wall stress). Thus, they have the potential to change current thinking about severe malaria (both its pathophysiology and treatment) from a focus on cytoadherence in peripheral blood vessels to its effects on central cardiopulmonary function.
Diagnosis of Malaria
Malaria was diagnosed in the 53 cases by examining thick blood smears. Although the mean blood Hb levels were low in the smear-negative controls (8.5 gms per dL, Table 1), those children had negative tests for parasite antigen. Therefore, the cases and controls examined in this study should provide a valid comparison between children with severe malaria and uninfected controls in Mali.
Intravascular Hemolysis and NO Depletion in Severe Malaria
Six lines of evidence indicate that intravascular hemolysis was greater in cases than controls (total and plasma free Hb, arginase-1, indirect bilirubin, LDH, haptoglobin; Figure 1, Tables 1–2). As a result, both NO consumption by free plasma Hb (Figures 1B–C) and arginase-1 levels were greater in cases than controls (Figure 1E). NO consumption and arginase inhibit vascular NO signaling by: 1] directly consuming NO produced by eNOS and 2] catabolizing arginine, which is normally utilized as a substrate by NOS [16–17]. Evidence for NO depletion in severe malaria included reduced whole blood nitrite and elevated sVCAM-1 levels (Figure 2), a marker of endothelial dysfunction normally repressed by NO [33, 38]. Evidence for cardiopulmonary effects included elevated pulmonary arterial systolic pressures based on TRVs and increased NT-proBNP levels –a biomarker for myocardial wall stress [37] (Figure 3, Table 2). Taken together, these results provide a link between intravascular hemolysis and NO depletion in severe malaria similar to the one in sickle cell disease [16–20, 37]. However, in contrast to the lifelong intravascular hemolysis in sickle cell disease, effects in children with severe malaria were transient. Whole blood nitrite, TRV, NT-proBNP and sVCAM-1 levels improved within 3–5 days as these children were treated and recovered.
Common Mechanisms in Severe Malaria and Sickle Cell Disease
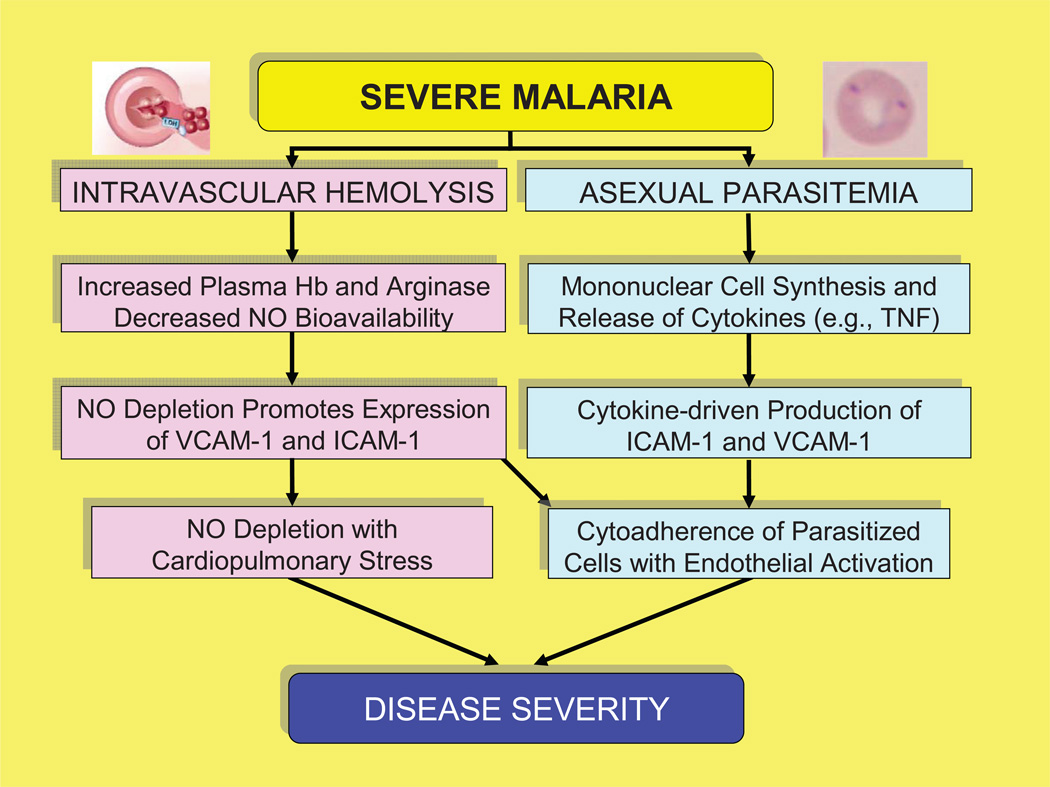
In both malaria and sickle cell disease, elevated levels of proinflammatory cytokines such as TNF lead to increased production of adhesion molecules (VCAM-1, ICAM-1) [6, 41–42]. This effect is amplified by NO depletion and facilitates the cytoadherence of abnormal red cells to endothelial cells [6–7]. In contrast, normal NO levels inhibit the production of adhesion molecules, and reduce the production of proinflammatory cytokines, thus decreasing the cytoadherence of abnormal red cells [38, 43]. This information suggests that two key pathways (cytokine release in response to the parasite [6, 41–42] and NO depletion from intravascular hemolysis [16–20]) converge in severe malaria and sickle cell disease to drive cytoadherence and endothelial activation (Figure 4). In addition, the differences between these diseases may reflect both the rate and duration of hemolysis. For example, massive hemolysis in severe malaria produces an acute potentially life-threatening illness. Conversely, the less intense hemolysis in sickle cell disease produces a chronic illness that becomes life-threatening over decades rather than days [20].
Figure 4. Schematic diagram of the pathophysiology of severe malaria.
Intravascular hemolysis produces increases in VCAM-1 and ICAM-1 from NO depletion that lead to increased pulmonary pressures and myocardial stress. In addition, both cytokine production in response to parasite factors and NO depletion from intravascular hemolysis lead to greater production of ICAM-1 and VCAM-1, which in turn increases the cytoadherence of parasitized red cells and produces endothelial activation.
Pathophysiology of Severe Malaria
Because malaria parasites lyse the red cells they infect, the elevated pulmonary pressures and myocardial wall stress in severe malaria likely result from the high-grade parasitemias associated with P. falciparum infection and the resulting massive intravascular hemolysis. Because NO plays a critical role in down-regulating the expression of adhesion molecules [43] and maintaining blood flow, the catabolism of NO and arginine from intravascular hemolysis in malaria likely promotes inflammatory adhesive events and myocardial wall stress, potentially including circulatory failure. Hemolysis-associated NO catabolism and endothelial dysfunction likely further increase systemic afterload and pulmonary pressures, increasing hemodynamic stress on both the left and right ventricles. In contrast, these effects are likely to be less severe with other human malarias (P. vivax, P. ovale, P. malariae) because they have lower parasitemias [44] and therefore produce less severe intravascular hemolysis.
The microvascular effects of cytoadherence [7] are consistent with the pathology of severe P. falciparum infection, the elevated levels of markers such as angiopoietin-2 [45–46] and vascular endothelial growth factor (VEGF), and the decreases in microvascular responsiveness (vasodilatation) that have been reported in adults with severe malaria [23, 45]. In addition, the results reported here provide: 1] evidence for central cardiopulmonary effects of NO depletion in severe malaria which has not been available previously, 2] stepwise documentation of the pathophysiologic cascade from intravascular hemolysis to NO depletion in severe malaria, and 3] demonstrate that this pathophysiology is present in children in sub-Saharan Africa for whom malaria is a fundamentally different and more severe disease than for adults.
In conclusion, these results bring a new dimension to our understanding of severe malaria. They demonstrate that NO depletion from intravascular hemolysis in severe malaria is associated with increased pulmonary pressures and myocardial wall stress (Figure 4). These mechanisms amplify and complicate the inflammatory pathways defined previously in severe malaria. Taken together, the evidence that NO treatment reduces malaria mortality in mice [21], and the results reported here and by other investigators [23, 45] suggest that it may be possible to reduce the deaths that occur early in the hospital course of children with severe malaria [47] by using treatment strategies that increase NO bioavailability.
Acknowledgments
We thank McWilson Warren for insightful discussions of severe malaria; Belco Maiga, Yacouba Abba Coulibaly, Mamadou Seydou Koné, Aboubacar Sangaré and Adama Koné for providing superb care for these severely ill children; Konia Diarra for assistance with informed consent in Mali; Mary Hall for help with protocol review at NIH; Tracey Bosworth for performing the NT-proBNP assays, Wen Li for determining TRVs and other parameters from the echocardiographic recordings and Thea McAreavey for guidance on the analysis of myocardial function; Ibrah Mahamadou, Fatou Sall, Hervé Dembélé, Aliou Sissako, Ayouba Diarra and Ousmane H. Cissé for reading blood smears, and performing chemistry, hematology and blood gas determinations; Professors Mamadou M. Kéita and Toumani Sidibé and the director of the Hôpital Gabriel Touré, Abdoulayé Néné Coulibaly, for their support; Kadiatou Bamba for administrative support; Frances J. Mather and John Lefante for statistical advice; Hans Ackerman, Daniel G. Bausch, Carlos C. Campbell, Frank B. Cogswell, Elizabeth S. Didier, Seydou O. Doumbia, M. Azam Hadi, Barbara L. Herwaldt, Robert C. Moellering, Jr., David M. Mushatt and Thomas E. Wellems for their reviews of the manuscript; and Standard Diagnostics (Suwon City, SOUTH KOREA) for providing the malaria antigen detection kits.
Funding Statement
These studies were supported by the Critical Care Medicine Division of the Clinical Center and the National Heart, Lung and Blood Institute (NIH), the Faculties of Medicine and Science of the University of Bamako, and by Tulane Research Enhancement grants to FM and DJK. The funders had no role in study design, data collection and analysis, the decision to publish or the preparation of the manuscript.
Abbreviations
- Ang-2
angiopoietin-2
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- LDH
lactate dehydrogenase
- metHb
methemoglobin
- NO
nitric oxide
- NOS
nitric oxide synthase
- NT-proBNP
N-terminal prohormone brain natriuretic peptide
- sVCAM-1
soluble vascular cell adhesion molecule-1
- TNF
tumor necrosis factor
- TRV
tricuspid regurgitant velocity (at onset of right ventricular systole)
- VEGF
vascular endothelial growth factor
Footnotes
Conflict of Interest
The authors declare that they do not have commercial or other associations that might pose a conflict of interest.
Prior Oral Presentations
The results reported here have been presented in part at the 48th annual meeting of the Infectious Diseases Society of America in Washington, DC (Abstracts P-3603 and - 3605, November 2008) and the 57th annual meeting of the American Society of Tropical Medicine and Hygiene in New Orleans (Abstract 1182, December 2008), for which JJJ received the Young Investigator Award.
Author Contributions
The original study design involved JJJ, MTG, OAK, FM and DJK, as did preparation for and presentations to IRBs at NIH (NHLBI; JJJ, MTG, FM, DJK), Tulane University (DJK, FM) and the University of Bamako (OAK, DJK). Doppler echocardiographic recordings were obtained in Mali at the Hôpital Gabriel Touré by JJJ with support from DJK and KS, and were interpreted by VS and Wen Li at the NIH Clinical Center. Direction of the care of these severely ill children at the Hôpital Gabriel Touré was provided by BT, KS and OAK. Clinical coordination of the care and follow-up of these children was provided by JMT who was also responsible for ensuring informed consent prior to enrollment, for maintaining those records, and for the initial antimalarial treatment; she was supervised by OAK and BT. Laboratory studies performed on-site in Mali were directed by LS and supervised by OAK. Laboratory studies performed at NIH included the NO consumption assay (XW) and the arginase-1, haptoglobin, plasma Hb and sVCAM-1 assays (LM); the results of those assays were reviewed and interpreted by GJK and MTG. Those and other laboratory and clinical data were collated and reviewed by FM and DJK. FM created a coded data base, and provided statistical guidance beginning at the time of study design and through preparation of the final manuscript. This manuscript was prepared in draft form by DJK with support from JJJ, MTG, GJK, OAK, FM and HM – who provided invaluable guidance and support throughout the planning, performance and submission of these studies. The manuscript has been provided to all authors for their review, comments and corrections prior to submission for publication.
References
- 1.Laveran CL. Classics in infectious diseases: A newly discovered parasite in the blood of patients suffering from malaria. Parasitic etiology of attacks of malaria: Charles Louis Alphonse Laveran (1845–1922) Rev Infect Dis. 1982;4(4):908–911. doi: 10.1093/4.4.908. [DOI] [PubMed] [Google Scholar]
- 2.Geneva: World Health Organization; 2009. [Accessed 26 February 2010]. Roll Back Malaria: Global Malaria Action Plan: Key Facts, Figures and Strategies. Available at: http://www.rollbackmalaria.org/gmap/. [Google Scholar]
- 3.Hay SI, Guerra CA, Gething PW, et al. A world malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med. 2009;6(3):e1000048. doi: 10.1371/journal.pmed.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korenromp EL, Williams BG, Gouws E, Dye C, Snow RW. Measurement of trends in childhood malaria mortality in Africa: an assessment of progress toward targets based on verbal autopsy. Lancet Infect Dis. 2003;3:349–358. doi: 10.1016/s1473-3099(03)00657-1. [DOI] [PubMed] [Google Scholar]
- 5.Pasvol G. Treatment of complicated and severe malaria. Br Med Bull. 2005;75–76:29–47. doi: 10.1093/bmb/ldh059. [DOI] [PubMed] [Google Scholar]
- 6.Kwiatkowski D, Sambou I, Twumasi P, et al. TNF concentrations in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990;336(8725):1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- 7.Su XZ, Heatwole VM, Wertheimer SP, et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82(1):89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 8.Taylor AM, Day NP, Sinh DX, et al. Reactive nitrogen intermediates and outcome in severe adult malaria. Trans R Soc Trop Med Hyg. 1998;92(2):170–175. doi: 10.1016/s0035-9203(98)90733-7. (1998) [DOI] [PubMed] [Google Scholar]
- 9.Weiss G, Thuma PE, Biemba G, Mabeza G, Werner ER, Gordeuk VR. Cerebrospinal fluid levels of biopterin, nitric oxide metabolites, and immune activation markers and the clinical course of human cerebral malaria. J Infect Dis. 1998;177(4):1064–1068. doi: 10.1086/515229. [DOI] [PubMed] [Google Scholar]
- 10.Anstey NM, Weinberg JB, Hassanali MY, et al. Nitric oxide in children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J Exp Med. 1996;184(2):557–567. doi: 10.1084/jem.184.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kun JF, Mordmuller B, Perkins DJ, et al. Nitric oxide synthase 2 (Lambarene) (G-954C), increased nitric oxide production, and protection against malaria. J Infect Dis. 2001;184(3):330–336. doi: 10.1086/322037. [DOI] [PubMed] [Google Scholar]
- 12.Chiwakata CB, Hemmer CJ, Dietrich M. High levels of inducible nitric oxide synthase mRNA are associated with increased monocyte counts in blood and have a beneficial role in Plasmodium falciparum malaria. Infect Immun. 2000;68(1):394–399. doi: 10.1128/iai.68.1.394-399.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perkins DJ, Kremsner PG, Schmid D, Misukonis MA, Kelly MA, Weinberg JB. Blood mononuclear cell nitric oxide production and plasma cytokine levels in healthy Gabonese children with prior mild or severe malaria. Infect Immun. 1999;67(9):4977–4981. doi: 10.1128/iai.67.9.4977-4981.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anstey NM, Granger DL, Hassanali MY, Mwaikambo ED, Duffy PE, Weinberg JB. Nitric oxide, malaria, and anemia: inverse relationship between nitric oxide production and hemoglobin concentration in asymptomatic, malaria-exposed children. Am J Trop Med Hyg. 1999;61(2):249–252. doi: 10.4269/ajtmh.1999.61.249. [DOI] [PubMed] [Google Scholar]
- 15.Hobbs MR, Udhayakumar V, Levesque MC, et al. A new NOS2 promoter polymorphism associated with increased nitric oxide production and protection from severe malaria in Tanzanian and Kenyan children. Lancet. 2002;360(9344):1468–1475. doi: 10.1016/S0140-6736(02)11474-7. [DOI] [PubMed] [Google Scholar]
- 16.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle cell disease. Nature Med. 2002;12(8):1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 17.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293(13):1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 18.Morris CR, Kato GJ, Poljakovic M, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294(1):81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minneci PC, Deans KJ, Zhi H, et al. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized deoxyhemoglobin. J Clin Invest. 2005;115(12):3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350(9):886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 21.Gramaglia I, Sobolewski P, Meays D, et al. Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nature Med. 2006;12(12):1417–1422. doi: 10.1038/nm1499. [DOI] [PubMed] [Google Scholar]
- 22.Lopansri BK, Anstey NM, Weinberg JB, et al. Low plasma arginine concentrations in children with cerebral malaria and decreased nitric oxide production. Lancet. 2003;361(9358):676–678. doi: 10.1016/S0140-6736(03)12564-0. [DOI] [PubMed] [Google Scholar]
- 23.Yeo TW, Lampah DA, Gitawati R, et al. Impaired nitric oxide bioavailability and L-arginine-reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med. 2007;201(11):2693–2704. doi: 10.1084/jem.20070819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinberg JB, Lopansri BK, Mwaikambo E, Granger DL. Arginine, nitric oxide, carbon monoxide, and endothelial function in severe malaria. Curr Opin Infect Dis. 2008;21(5):468–475. doi: 10.1097/QCO.0b013e32830ef5cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bejon P, Berkley JA, Mwangi T, et al. Defining childhood severe falciparum malaria for intervention studies. PLoS Med. 2007;4(8):e251. doi: 10.1371/journal.pmed.0040251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imbert P, Gérardin P, Rogier C, et al. Severe falciparum malaria in children: a comparative study of 1990 and 2000 WHO criteria for clinical presentation, prognosis and intensive care in Dakar, Senegal. Trans R Soc Trop Med Hyg. 2002;96(3):278–281. doi: 10.1016/s0035-9203(02)90099-4. [DOI] [PubMed] [Google Scholar]
- 27.English M, Waruiru C, Amukoye E, et al. Deep breathing in children with severe malaria: indicator of metabolic acidosis and poor outcome. Am J Trop Med Hyg. 1996;55(5):521–524. doi: 10.4269/ajtmh.1996.55.521. [DOI] [PubMed] [Google Scholar]
- 28.Taylor TE, Molyneux ME, Wirima JJ, Fletcher KA, Morris K. Blood glucose levels in Malawian children before and during the administration of intravenous quinine for severe falciparum malaria. N Engl J Med. 1998;319(16):1040–1047. doi: 10.1056/NEJM198810203191602. [DOI] [PubMed] [Google Scholar]
- 29.Payne D. Use and limitations of light microscopy for diagnosing malaria at the primary health care level. Bull WHO. 1988;66(5):621–626. [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Tanus-Santos JE, Reiter CD, et al. Biologic activity of nitrite in the plasmatic compartment. Proc Natl Acad Sci USA. 2005;101(31):11477–11481. doi: 10.1073/pnas.0402201101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dejam A, Hunter CJ, Pelletier MM, et al. Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood. 2005;106(2):734–749. doi: 10.1182/blood-2005-02-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacArthur PH, Shiva S, Gladwin MT. Measurement of circulating nitrite and S-nitrosothiols by reductive chemiluminescence. J Chromatogr B: Analyt Technol Biomed Life Sci. 2006;851(1–2):93–105. doi: 10.1016/j.jchromb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Kato G, Martyr S, Blackwelder WC, et al. Levels of soluble endothelium-derived adhesion molecules in patients with sickle cell disease are associated with pulmonary hypertension, organ dysfunction and mortality. Br J Haematol. 2005;130(6):943–953. doi: 10.1111/j.1365-2141.2005.05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lauer T, Preik T, Rassaf T, et al. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci USA. 2001;98(22):12814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sachdev V, Machado RF, Shizukuda Y, et al. Diastolic dysfunction is an independent risk factor for death in patients with sickle cell disease. J Am Coll Cardiol. 2007;49(4):472–479. doi: 10.1016/j.jacc.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. N Engl J Med. 2008;359(21):2254–2265. doi: 10.1056/NEJMra0804411. [DOI] [PubMed] [Google Scholar]
- 37.Machado RF, Anthi A, Steinberg MH, et al. N-terminal pro-brain natriuretic peptide levels and risk of death in sickle cell disease. JAMA. 2006;296(3):310–318. doi: 10.1001/jama.296.3.310. [DOI] [PubMed] [Google Scholar]
- 38.DeCaterina R, Libby P, Peng HB, et al. Nitric oxide decreases cytokine-induced endothelial activation: nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96(1):60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anthi A, Machado RF, Jison ML, et al. Hemodynamic and functional assessment of patients with sickle cell disease and pulmonary hypertension. Am J Respir Crit Care Med. 2007;175:1272–1279. doi: 10.1164/rccm.200610-1498OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi JW, Pai SH, Kim SK, Ito M, Park CS, Cha YN. Iron deficiency anemia increases nitric oxide production in healthy adolescents. Ann Hematol. 2002;81(1):1–6. doi: 10.1007/s00277-001-0409-4. [DOI] [PubMed] [Google Scholar]
- 41.Turner GD, Ly VC, Nguyen TH, et al. Systemic endothelial activation occurs in both mild and severe malaria: correlating dermal microvascular endothelial cell phenotype and soluble cell adhesion molecules with disease severity. Am J Pathol. 1998;152(6):1477–1487. [PMC free article] [PubMed] [Google Scholar]
- 42.Min J-K, Kim Y-M, Kim SW, et al. TNF-related activation-induced cytokine enhances leukocyte adhesiveness: induction of ICAM-1 and VCAM-1 via TNF receptor-associated factor and protein kinase C-dependent NF-κB activation in endothelial cells. J Immunol. 2005;175(1):531–540. doi: 10.4049/jimmunol.175.1.531. [DOI] [PubMed] [Google Scholar]
- 43.Serirom S, Raharjo WH, Chotivanich K, Looareesuwan S, Kubes P, Ho M. Anti-adhesive effect of nitric oxide on Plasmodium falciparum cytoadherence under flow. Am J Pathol. 2003;162(5):1651–1660. doi: 10.1016/S0002-9440(10)64299-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaur D, Mayer DC, Miller LH. Parasite ligand-host receptor interactions during invasion of erythrocytes by Plasmodium merozoites. Intl J Parasitol. 2004;34(13–14):1413–1429. doi: 10.1016/j.ijpara.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 45.Yeo TW, Lampah DA, Gitawati R, et al. Angiopoietin-2 is associated with decreased endothelial nitric oxide and poor clinical outcome in severe falciparum malaria. Proc Natl Acad Sci U S A. 2008;105(44):17097–17102. doi: 10.1073/pnas.0805782105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goettsch W, Cryczka C, Korff T, et al. Flow-dependent regulation of angiopoietin-2. J Cell Physiol. 2008;214(2):491–503. doi: 10.1002/jcp.21229. [DOI] [PubMed] [Google Scholar]
- 47.Allen SJ, O’Donnell A, Alexander ND, Clegg JB. Severe malaria in children in Papua New Guinea. Quart J Med. 1996;89(10):779–788. doi: 10.1093/qjmed/89.10.779. [DOI] [PubMed] [Google Scholar]