Abstract
Gout is a chronic arthritic disease associated with high levels of urate in blood. Recent advances in research have permitted the identification of several new and common genetic factors underlying the disease. Among them, a polymorphism in the ABC transporter gene ATP-binding cassette transporter isoform G2 has been highlighted. ATP-binding cassette transporter isoform G2 was found to be involved in renal urate elimination, and the presence of the Q141K polymorphism to induce a 2-fold decrease in urate efflux. The Q141K variant has been shown to have impaired trafficking, leading to its intracellular retention, whereas the wild type protein is expressed on the cell surface. Several agents are being studied for the purpose of improving folding, trafficking and function of various ABC transporters, including ATP-binding cassette transporter isoform G2. If successful, this strategy opens doors to potential new therapies for gout.
Introduction
Gout is a common chronic form of arthritis reported to afflict about 8.3 million people in the United States, 6.4 million in the European Union, and 2.9 million in Japan in 2008 [1,2]. This disease is characterized by high blood urate levels that provoke painful acute inflammatory attacks. In recent years, efforts have been made to determine the genetic causes of gout. Different polymorphisms have been identified as associated with elevated serum urate levels and gout. Among them, a polymorphism in the ATP-binding cassette transporter ABCG2 (ATP-binding cassette transporter isoform G2), initially known because of its involvement in chemotherapy drug efflux, was highlighted. This review aims to discuss the genetic causes of gout in the population, the association of the ABCG2 C421A polymorphism with gout, and the characterization of the ABCG2 variant protein in which a lysine replaces a glutamine. We will address potential new treatment strategies involving ABCG2 variant rescue.
Causes of hyperuricemia and gout
Gout is a progressive disorder caused by impaired urate metabolism, leading to high serum urate levels (hyperuricemia) and the formation of urate crystals that accumulate in joints. Although asymptomatic hyperuricemia is the precursor state to the development of gout, many people with high serum urate never develop gout, the risk varying depending on the degree of hyperuricemia.
Urate is the final metabolite of dietary and endogenous purine metabolism. Food contains little urate, which is mainly produced in the liver and, to a lesser extent, in the small intestine. A third of the urate is excreted via the gastrointestinal tract, and two-thirds via the kidneys, although 90% of urate filtered by the kidneys is reabsorbed [3].
High serum urate levels and gout have been attributed to different causal factors: genetics, nutrition, medication, gender, age, and environment. About 90% of hyperuricemic individuals present with impaired renal urate excretion while around 10% of them overproduce urate [4]. Urate over-production can be due to acquired causes such as eating purine-rich food, being overweight, fructose ingestion, and alcohol intake—the latter two inducing acceleration of ATP degradation to AMP, a precursor of uric acid. Some diseases (i.e. myeloproliferative and lymphoproliferative disorders, psoriasis, and hemolytic anemia) are associated with enhanced turnover of nucleic acid, which can also lead to hyperuricemia. The presence of mutations in enzymes involved in purine metabolism (i.e. hypoxanthine-guanine phosphoribosyltransferase deficiency and PRPP [phosphoribosyl pyrophosphate] synthetase superactivity) have also been identified as rare genetic causes of hyperuricemia and gout [3,4].
Multiple etiologies have also been described for urate excretion failure. Hereditary renal disorders with rare mutant alleles cause clinically distinct forms of familial gout. For example, mutations in the uromodulin and renin genes have been associated with hyperuricemia, gout, and progressive renal failure. A mutation in the aldolase B gene causes the recessive disease hereditary fructose intolerance in patients, most of them also presenting hyperuricemia and gout. Hypertension has also been described as a risk factor for hyperuricemia or gout, partly due to decreased renal urate excretion. Diuretic use, lead exposure, and cyclosporine immunosuppressive therapy also affect urate renal excretion [3,4].
Other factors increase gout incidence. Men have a 2-fold higher risk of gout compared to women, and those over the age of 65 are the most affected population. People who migrate to a westernized country have a higher risk of developing gout than those who remain in their native country, as do people who undergo westernization in their country, due to changes in lifestyle and diet [5,6].
Genetic causes of gout
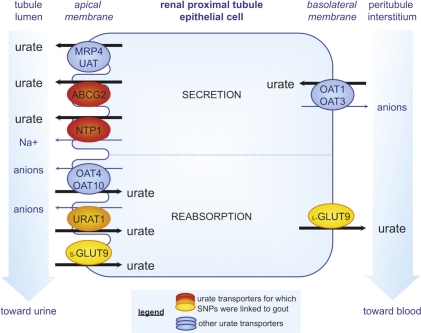
A genome-wide search for genes affecting serum uric acid levels had demonstrated that the heritability of serum urate levels is about 63% [7]. Until recently, our knowledge of the genetics of gout was limited to the rare genetic mutations discussed above. In the last few years, development of new tools to investigate the human genome has permitted important advances in our understanding of disease. Among them, genome-wide association studies (GWAS) are a recently developed research technique to identify DNA polymorphisms distributed across different large populations, and they permit the determination of common genetic factors that influence health and disease. Since 2008, different GWAS identified nine DNA loci associated with serum urate concentration, with four linked to gout in several populations [8-11]. These new data led researchers to determine the function of genes associated with the loci and their relationship with urate levels and gout, along with the relevant single nucleotide polymorphisms (SNPs) for each involved gene. The four loci associated with gout all correspond to urate transporters located in the epithelial cells of renal proximal tubules (Figure 1).
Figure 1. Urate transporters in proximal tubule epithelial cells.
Urate transporters are involved in both urate tubular secretion and postsecretory reabsorption, which determine the net urate excretion. In the urate secretion mechanism, the anion transporters OAT1 and OAT3 (organic anion transporter 1 and 3), localized on the basolateral membrane, have been shown to have the ability to transport urate depending on the gradients for exchanged anions. On the apical membrane, 4 transporters are involved in secretion: UAT (uric acid transporter), NPT1 (sodium phosphate transport protein 1), and the ATP-binding cassette transporters MRP4 (multidrug resistance related protein 4) and ABCG2 (ATP-binding cassette transporter isoform G2). Various ABCG2 and NPT1 variants with impaired function have been linked to increased risk of gout, and were described to decrease urate excretion, explaining the hyperuricemia. In renal reabsorption, the apical urate-anion exchanger URAT1 is considered to be essential in urate homeostasis and has been estimated to be responsible for 50% of urate reabsorption. OAT4 and OAT10 (organic anion transporter 4 and 10) are also apical mediators of this. In addition to these transporters, GLUT9 (glucose transporter 9) may play a dominant role in reabsorption. The short isoform, s-GLUT9, localizes exclusively to the apical membrane while the long isoform, l-GLUT9, is thought to mediate basolateral efflux of urate. GLUT9 and URAT1 single nucleotide polymorphisms (SNPs) have been identified by GWAS to be significantly associated with decreased and increased risk of gout, respectively. While some GLUT9 SNPs would be linked to the decrease in urate reabsorption leading to hypouricemia, the mechanism by which the URAT1 SNPs cause hyperuricemia and gout remains to be elucidated.
The locus most strongly linked to gout corresponds to the glucose transporter 9 (GLUT9), also known as the solute carrier 2A9 (SLC2A9). In contrast to the three other loci that are linked to an increased risk of gout, several variants of GLUT9 are linked to a reduced risk. The transporter is predominantly expressed in liver and kidney but is also found in chondrocytes from human cartilage, where, in gout, urate is deposited. It was initially identified as a glucose/fructose transporter [12], then functional studies demonstrated that SLC2A9 was also able to transport urate in renal reabsorption [13]. SLC2A9 exists as two isoforms that differ by the length of their cytoplasmic domain and by their apical or basolateral localization in renal epithelial cells [14]. Several variants of SLC2A9 are associated with hypouricemia and a lower risk of gout [13,15]. It seems that instead of impairing the protein function, the variants might influence the relative expression level of the two isoforms, leading to reduced reabsorption and increased excretion of uric acid [13,15].
The second locus linked to gout corresponds to the gene encoding the urate transporter 1, URAT1 (also named solute carrier 22A12). The gene was cloned in 2002 and immediately identified as a urate-organic anion exchanger, re-absorption being triggered by high intracellular loads of lactate and several other organic anions [16]. Localized in the apical membrane of kidney cells, URAT1 is one of the essential transporters involved in urate reabsorption. URAT1 SNPs were first linked to hypouricemia [16,17]. Since then, several mutations in the URAT1 gene have been linked to hyperuricemia and gout in different populations [18-20], and a GWAS performed in 2009 linked the locus to an increased risk of gout [9]. The molecular mechanism causing this urate level increase has still not been elucidated.
The third locus linked to gout encodes the renal sodium phosphate transport protein 1 (NPT1, or solute carrier 17A1), which is localized at the apical membrane of renal proximal tubules. It was initially identified in 1993 as a sodium-phosphate cotransporter [21]. Following its linkage to gout by GWAS [9], it was shown to also be a voltage-driven urate transporter involved in urate secretion [22]. The studied protein variant exhibited lower urate transport activity compared with the wild-type protein [22]. Then, several other SNPs in the NPT1 gene were associated with increased risk of gout in humans [23].
The last locus linked to gout corresponds to the ABCG2 gene, a transporter initially known for its involvement in resistance to chemotherapy. We discuss below the unexpected association between ABCG2 SNPs and gout. As a transporter previously associated with multidrug resistance, the identification of a physiological role for ABCG2 has opened up new avenues in which this protein could become an important target for clinical therapy.
ABCG2 variants and their association with gout in various populations
ABCG2 was identified as a new locus related to urate levels and gout from 2008 onwards [8,9]. GWAS associated the missense ABCG2 C421A SNP (Q141K), which replaces a glutamine with a lysine at amino acid 141, with elevated urate levels and gout in both Caucasian-Americans and African-Americans, with a stronger effect in men than women [8]. It was also established that the ABCG2 Q141K variant might be a potential causal candidate for a 70% elevation in gout risk. Six months later, another GWAS conducted from 14 studies in Europe confirmed the Q141K polymorphism as a common variant associated with the disease [9]. The C421A allele frequency is very high in the Japanese and Chinese population (between 27 and 34%), while it is approximately 10% for Caucasians and less than 5% in the African population [24].
Following the linkage by GWAS, a functional assay was performed to evaluate causality between gout and ABCG2 Q141K. The transporter had been previously shown to be expressed in the human kidney proximal tubule apical membrane [25]. In 2009, Woodward et al. [26] demonstrated that ABCG2, already known as a xenobiotic efflux pump, can also transport urate. Moreover, they observed that the presence of the Q141K mutation reduced urate transport by 54% compared with the wild-type form when tested in Xenopus oocytes. They then showed a significant association between gout and the SNP in an American population-based study and determined that among the Caucasian study participants, 10% of gout cases could be attributed to the Q141K mutation.
A study conducted in a Japanese population highlighted a second ABCG2 SNP strongly linked to gout [27]. C376T (Q126X) is a missense mutation encoding a stop codon instead of a glutamine, which prevents the expression of ABCG2. It was observed that, in patients carrying the Q126X variant, the risk of gout was dramatically increased. A functional study also showed that urate transport capacity was nearly eliminated in cells transfected with the Q126X variant compared to those with the wild-type protein, and confirmed that the Q141K polymorphism halved urate efflux. A combination of these two SNPs led to a more than 75% reduction of protein function. Although this combination was found in 10% of gout patients involved in this study, the frequency of the Q126X SNP only reaches 1–2% in the Asian population and is not found in African and Caucasian populations.
In 2010, two other studies were reported relating to the C421A SNP and gout [28,29]. The first one, conducted on a Chinese Han ethnic group, confirmed the association between C421A polymorphism and the disease. The second study was done in New Zealand, where gout rates are high, and revealed an association between C421A SNP and gout in New Zealand Caucasians and Pacific Island populations, but not in the indigenous Maori population, underlining sub-population differences.
ABCG2 C421A characterization
ABCG2 was described for the first time in 1998. It is a member of the ATP-binding cassette (ABC) transporter family. Its structure consists of one intracytoplasmic ATP binding domain, followed by a six transmembrane domain. ABCG2 is an energy-dependent efflux transporter that must dimerize to function, and may exist as a tetramer or higher order oligomer. It is mainly expressed in placenta and hematopoietic stem cells, but is also found in the brain, testis, uterus, digestive system, liver, kidneys, and the mammary gland during lactation. It can transport numerous chemotherapeutics, but also antivirals, antibiotics, carcinogens and toxins out of the cell. Moreover, it has been identified as a transporter of endogenous compounds such as steroids, porphyrins, heme and vitamins in different tissues. Likely physiological roles include control of oral bioavailability, protection in the blood-brain and maternal-fetal barrier, drug elimination, and normal stem cell protection [30].
The C421A SNP leads to an amino-acid change localized in the ATP-binding domain. The ATPase activity of the variant protein is reduced by about 1.3- to 1.8-fold, along with a reduction in its transport efficacy (compared with that of the wild-type protein). Moreover, ABCG2 Q141K protein expression is decreased 2-fold to 4-fold compared with the wild-type, whereas the mRNA level is equal, suggesting that the variant protein is unstable or degraded. A decreased surface expression of the variant has also been observed, coupled with incomplete trafficking to the cell membrane in some studies. In vitro, cells harboring the SNP are less resistant to ABCG2 substrate anticancer drugs than cells bearing the wild-type protein. This correlates with clinical studies showing that patients harboring the Q141K polymorphism display reduced clearance of substrate drugs, including anticancer drugs, antibiotics, or cholesterol-lowering drugs [30,31].
Variant rescue by the pharmacological chaperones
In recent years, it has been shown that the trafficking and function of some ABC transporter variants can be rescued by the use of pharmacological chaperones. These compounds are small molecules that function much like protein chaperones by promoting interactions between protein domains during the folding process. This helps to stabilize the protein by improving its folding, which results in a recovery of the function originally lost due to mutation.
The efficacy of these pharmacological chaperones was first demonstrated with rescue of the Y490 P-glycoprotein mutant, which was retained in the endoplasmic reticulum. The addition of a drug substrate improved the targeting of neosynthetized protein to the cell surface in an active conformation [32].
Since then, this method has been fruitfully tested in vitro with the ABC transporter CFTR (cystic fibrosis transmembrane conductance regulator) and has gone on to yield agents with activity in preclinical and clinical studies in the treatment of cystic fibrosis lung disease. Indeed, cystic fibrosis is caused by various mutations in CFTR, and the use of the corrector VX-809 and the potentiator VX-770 enhance the function of the CFTR variants [33].
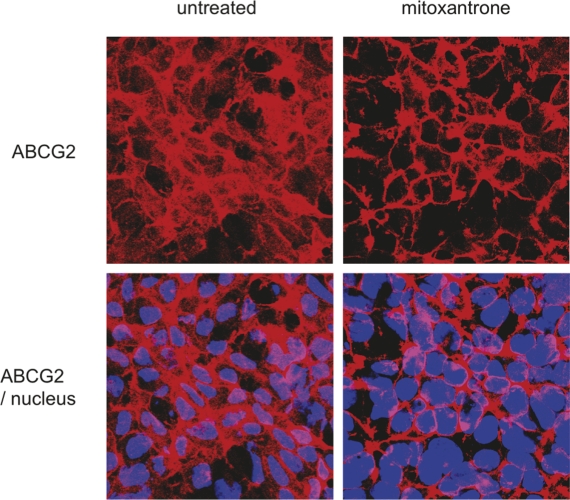
In the same way, in vitro experiments showed that mitoxantrone, an ABCG2 ligand, was able to improve trafficking of an ABCG2 T402L-G406L-G410L mutant that was misfolded and intracellularly retained [34]. As illustrated in Figure 2, the ABCG2 mutant is partly retained in the cytoplasm of the untreated cells while 24-hour treatment with mitoxantrone induces a relocalization of the transporter mainly to the cell surface. While recognizing that in vitro rescue is a simpler objective and a long way from a clinical therapy, these experiments could be extended to the Q141K variant to identify and determine the ability of correctors and potentiators to recover normal trafficking and function.
Figure 2. Impact of mitoxantrone on ABCG2 T402L-G406L-G410L mutant localization.
HEK293 cells transfected with the ABCG2 (ATP-binding cassette transporter isoform G2) T402L-G406L-G410L dimerization mutant were submitted to 24-hour exposure of 5 µM mitoxantrone before indirect immunostaining with the anti-ABCG2 antibody bxp21 according to the manufacturer’s instructions (Kamiya Biomedical, Seattle, WA), and DAPI nuclear stain. An overlay of the two stains is shown by confocal microscopy in the lower panels. Before treatment, the ABCG2 variant is partly retained in the cytoplasm. Mitoxantrone rescued the trafficking defect of the variant, leading to a significant increase in the amount of ABCG2 on the cell surface.
Conclusion: toward a new target for gout therapy?
Gout may not be curable, but it can be treated. Two alternative treatments exist, according to the type of gout. In the case of acute gout, anti-inflammatory drugs (including non-steroidal anti-inflammatory drugs, steroids, and colchicine) are used to reduce the crystal-caused inflammation and ensuing pain. Chronic gout is treated with urate-lowering medication, which aims to prevent gout attacks. In this case, allopurinol (a xanthine-oxidase inhibitor) decreases urate production whereas uricosuric agents, such as probenecid and benzbromarone, are prescribed to increase urate elimination [1].
However, among the 8 million gout-afflicted Americans, conventional treatment is contraindicated or has been ineffective for many patients. For example, it has been recently shown in a clinical study that 35–40% of patients had strong contraindications to multiple gout medications [35]. The increasing understanding of the pathophysiology of hyperuricemia and gout has led to a better comprehension of the failure of some therapeutics for patients with difficult-to-treat gout. For example, the uricosuric agents probenecid and benzbromarone, recently shown to inhibit urate movement transduced by URAT1 and GLUT9, were found to be ineffective in patients bearing a homozygous loss-of-function mutation on URAT1 [17,36]. These newer molecular insights offer the promise of adding to the therapeutic armamentarium. The recent findings on the role of the Q141K polymorphic ABCG2 in gout may open the door to the use of a pharmacological chaperone treatment for ABCG2 as a potential new target for gout therapy.
Acknowledgments
We acknowledge Julian Bahr’s contribution to the drafting of the manuscript and Akina Tamaki for the immunofluorescence data. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research
Abbreviations
- ABCG2
ATP-binding cassette transporter isoform G2
- CFTR
cystic fibrosis transmembrane conductance regulator
- GLUT9/SLC2A9
glucose transporter 9/solute carrier 2A9
- GWAS
genome-wide association studies
- NPT1/SLC17A1
sodium phosphate transport protein 1/solute carrier 17A1
- OAT
organic anion transporter
- SNP
single nucleotide polymorphism
- URAT1/SLC22A12
urate transporter 1/solute carrier 22A12
Competing interests
The authors declare that they have no competing interests.
The electronic version of this article is the complete one and can be found at: http://f1000.com/reports/b/3/23
References
- 1.Brook RA, Forsythe A, Smeeding JE, Lawrence Edwards N. Chronic gout: epidemiology, disease progression, treatment and disease burden. Curr Med Res Opin. 2010;26:2813–21. doi: 10.1185/03007995.2010.533647. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: The National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63:3136–41. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 3.Richette P, Bardin T. Gout. Lancet. 2010;375:318–28. doi: 10.1016/S0140-6736(09)60883-7. [DOI] [PubMed] [Google Scholar]
- 4.Riches PL, Wright AF, Ralston SH. Recent insights into the pathogenesis of hyperuricaemia and gout. Hum Mol Genet. 2009;18:R177–84. doi: 10.1093/hmg/ddp369. [DOI] [PubMed] [Google Scholar]
- 5.Choi HK, Zhu Y, Mount DB. Genetics of gout. Curr Opin Rheumatol. 2010;22:144–51. doi: 10.1097/BOR.0b013e32833645e8. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Susan Bates 14 Oct 2011
- 6.Merriman TR, Dalbeth N. The genetic basis of hyperuricaemia and gout. Joint Bone Spine. 2011;78:35–40. doi: 10.1016/j.jbspin.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 7.Yang Q, Guo CY, Cupples LA, Levy D, Wilson PW, Fox CS. Genome-wide search for genes affecting serum uric acid levels: the Framingham Heart Study. Metabolism. 2005;54:1435–41. doi: 10.1016/j.metabol.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Dehghan A, Kottgen A, Yang Q, Hwang SJ, Kao WL, Rivadeneira F, Boerwinkle E, Levy D, Hofman A, Astor BC, Benjamin EJ, van Duijn CM, Witteman JC, Coresh J, Fox CS. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet. 2008;372:1953–61. doi: 10.1016/S0140-6736(08)61343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Susan Bates 14 Oct 2011
- 9.Kolz M, Johnson T, Sanna S, Teumer A, Vitart V, Perola M, Mangino M, Albrecht E, Wallace C, Farrall M, Johansson A, Nyholt DR, Aulchenko Y, Beckmann JS, Bergmann S, Bochud M, Brown M, Campbell H, EUROSPAN Consortium. Connell J, Dominiczak A, Homuth G, Lamina C, McCarthy MI, ENGAGE Consortium. Meitinger T, Mooser V, Munroe P, Nauck M, Peden J, et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009;5:e1000504. doi: 10.1371/journal.pgen.1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Susan Bates 14 Oct 2011
- 10.Li S, Sanna S, Maschio A, Busonero F, Usala G, Mulas A, Lai S, Dei M, Orrù M, Albai G, Bandinelli S, Schlessinger D, Lakatta E, Scuteri A, Najjar SS, Guralnik J, Naitza S, Crisponi L, Cao A, Abecasis G, Ferrucci L, Uda M, Chen WM, Nagaraja R. The GLUT9 gene is associated with serum uric acid levels in Sardinia and Chianti cohorts. PLoS Genet. 2007;3:e194. doi: 10.1371/journal.pgen.0030194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace C, Newhouse SJ, Braund P, Zhang F, Tobin M, Falchi M, Ahmadi K, Dobson RJ, Marcano AC, Hajat C, Burton P, Deloukas P, Brown M, Connell JM, Dominiczak A, Lathrop GM, Webster J, Farrall M, Spector T, Samani NJ, Caulfield MJ, Munroe PB. Genome-wide association study identifies genes for biomarkers of cardiovascular disease: serum urate and dyslipidemia. Am J Hum Genet. 2008;82:139–49. doi: 10.1016/j.ajhg.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Susan Bates 14 Oct 2011
- 12.Phay JE, Hussain HB, Moley JF. Cloning and expression analysis of a novel member of the facilitative glucose transporter family, SLC2A9 (GLUT9) Genomics. 2000;66:217–20. doi: 10.1006/geno.2000.6195. [DOI] [PubMed] [Google Scholar]
- 13.Vitart V, Rudan I, Hayward C, Gray NK, Floyd J, Palmer CN, Knott SA, Kolcic I, Polasek O, Graessler J, Wilson JF, Marinaki A, Riches PL, Shu X, Janicijevic B, Smolej-Narancic N, Gorgoni B, Morgan J, Campbell S, Biloglav Z, Barac-Lauc L, Pericic M, Klaric IM, Zgaga L, Skaric-Juric T, Wild SH, Richardson WA, Hohenstein P, Kimber CH, Tenesa A, et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet. 2008;40:437–42. doi: 10.1038/ng.106. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Susan Bates 14 Oct 2011
- 14.Augustin R, Carayannopoulos MO, Dowd LO, Phay JE, Moley JF, Moley KH. Identification and characterization of human glucose transporter-like protein-9 (GLUT9): alternative splicing alters trafficking. J Biol Chem. 2004;279:16229–36. doi: 10.1074/jbc.M312226200. [DOI] [PubMed] [Google Scholar]
- 15.Döring A, Gieger C, Mehta D, Gohlke H, Prokisch H, Coassin S, Fischer G, Henke K, Klopp N, Kronenberg F, Paulweber B, Pfeufer A, Rosskopf D, Völzke H, Illig T, Meitinger T, Wichmann HE, Meisinger C. SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat Genet. 2008;40:430–6. doi: 10.1038/ng.107. [DOI] [PubMed] [Google Scholar]
- 16.Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T, Igarashi T, Matsuo H, Kikuchi Y, Oda T, Ichida K, Hosoya T, Shimokata K, Niwa T, Kanai Y, Endou H. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417:447–52. doi: 10.1038/nature742. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Susan Bates 14 Oct 2011
- 17.Ichida K, Hosoyamada M, Hisatome I, Enomoto A, Hikita M, Endou H, Hosoya T. Clinical and molecular analysis of patients with renal hypouricemia in Japan-influence of URAT1 gene on urinary urate excretion. J Am Soc Nephrol. 2004;15:164–73. doi: 10.1097/01.ASN.0000105320.04395.D0. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Susan Bates 14 Oct 2011
- 18.Graessler J, Graessler A, Unger S, Kopprasch S, Tausche AK, Kuhlisch E, Schroeder HE. Association of the human urate transporter 1 with reduced renal uric acid excretion and hyperuricemia in a German Caucasian population. Arthritis Rheum. 2006;54:292–300. doi: 10.1002/art.21499. [DOI] [PubMed] [Google Scholar]
- 19.Shima Y, Teruya K, Ohta H. Association between intronic SNP in urate-anion exchanger gene, SLC22A12, and serum uric acid levels in Japanese. Life Sci. 2006;79:2234–7. doi: 10.1016/j.lfs.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 20.Tu HP, Chen CJ, Lee CH, Tovosia S, Ko AM, Wang SJ, Ou TT, Lin GT, Chiang SL, Chiang HC, Chen PH, Chang SJ, Lai HM, Ko YC. The SLC22A12 gene is associated with gout in Han Chinese and Solomon Islanders. Ann Rheum Dis. 2010;69:1252–4. doi: 10.1136/ard.2009.114504. [DOI] [PubMed] [Google Scholar]
- 21.Chong SS, Kristjansson K, Zoghbi HY, Hughes MR. Molecular cloning of the cDNA encoding a human renal sodium phosphate transport protein and its assignment to chromosome 6p21.3-p23. Genomics. 1993;18:355–9. doi: 10.1006/geno.1993.1476. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Susan Bates 14 Oct 2011
- 22.Iharada M, Miyaji T, Fujimoto T, Hiasa M, Anzai N, Omote H, Moriyama Y. Type 1 sodium-dependent phosphate transporter (SLC17A1 Protein) is a Cl(-)-dependent urate exporter. J Biol Chem. 2010;285:26107–13. doi: 10.1074/jbc.M110.122721. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Susan Bates 14 Oct 2011
- 23.Urano W, Taniguchi A, Anzai N, Inoue E, Kanai Y, Yamanaka M, Endou H, Kamatani N, Yamanaka H. Sodium-dependent phosphate cotransporter type 1 sequence polymorphisms in male patients with gout. Ann Rheum Dis. 2010;69:1232–4. doi: 10.1136/ard.2008.106856. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Susan Bates 14 Oct 2011
- 24.Noguchi K, Katayama K, Mitsuhashi J, Sugimoto Y. Functions of the breast cancer resistance protein (BCRP/ABCG2) in chemotherapy. Adv Drug Deliv Rev. 2009;61:26–33. doi: 10.1016/j.addr.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Huls M, Brown CD, Windass AS, Sayer R, van den Heuvel JJ, Heemskerk S, Russel FG, Masereeuw R. The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int. 2008;73:220–5. doi: 10.1038/sj.ki.5002645. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Susan Bates 14 Oct 2011
- 26.Woodward OM, Kottgen A, Coresh J, Boerwinkle E, Guggino WB, Kottgen M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci U S A. 2009;106:10338–42. doi: 10.1073/pnas.0901249106. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 12Evaluated by Robert Robey and Susan Bates 29 Jun 2009, Fabien Sohet and Dominique Eladari 25 Jun 2009, Richard J Naftalin 25 Jun 2009
- 27.Matsuo H, Takada T, Ichida K, Nakamura T, Nakayama A, Ikebuchi Y, Ito K, Kusanagi Y, Chiba T, Tadokoro S, Takada Y, Oikawa Y, Inoue H, Suzuki K, Okada R, Nishiyama J, Domoto H, Watanabe S, Fujita M, Morimoto Y, Naito M, Nishio K, Hishida A, Wakai K, Asai Y, Niwa K, Kamakura K, Nonoyama S, Sakurai Y, Hosoya T, et al. Common defects of ABCG2, a high-capacity urate exporter, cause gout: a function-based genetic analysis in a Japanese population. Sci Transl Med. 2009;1:5ra11. doi: 10.1126/scitranslmed.3000237. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Susan Bates 14 Oct 2011
- 28.Wang B, Miao Z, Liu S, Wang J, Zhou S, Han L, Meng D, Wang Y, Li C, Ma X. Genetic analysis of ABCG2 gene C421A polymorphism with gout disease in Chinese Han male population. Hum Genet. 2010;127:245–6. doi: 10.1007/s00439-009-0760-4. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Susan Bates 14 Oct 2011
- 29.Phipps-Green AJ, Hollis-Moffatt JE, Dalbeth N, Merriman ME, Topless R, Gow PJ, Harrison AA, Highton J, Jones PB, Stamp LK, Merriman TR. A strong role for the ABCG2 gene in susceptibility to gout in New Zealand Pacific Island and Caucasian, but not Maori, case and control sample sets. Hum Mol Genet. 2010;19:4813–9. doi: 10.1093/hmg/ddq412. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Susan Bates 14 Oct 2011
- 30.Polgar O, Robey R, Bates S. ABCG2: structure, function and role in drug response. Expert Opin Drug Metab Toxicol. 2008;4:1–15. doi: 10.1517/17425255.4.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Sissung TM, Baum CE, Kirkland CT, Gao R, Gardner ER, Figg WD. Pharmacogenetics of membrane transporters: an update on current approaches. Mol Biotechnol. 2010;44:152–67. doi: 10.1007/s12033-009-9220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Susan Bates 14 Oct 2011
- 32.Loo TW, Clarke DM. Correction of defective protein kinesis of human P-glycoprotein mutants by substrates and modulators. J Biol Chem. 1997;272:709–12. doi: 10.1074/jbc.272.2.709. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Susan Bates 14 Oct 2011
- 33.Grasemann H, Ratjen F. Emerging therapies for cystic fibrosis lung disease. Expert Opin Emerg Drugs. 2010;15:653–9. doi: 10.1517/14728214.2010.517746. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Susan Bates 14 Oct 2011
- 34.Polgar O, Ierano C, Tamaki A, Stanley B, Ward Y, Xia D, Tarasova N, Robey R, Bates S. Mutational analysis of threonine 402 adjacent to the GXXXG dimerization motif in transmembrane segment 1 of ABCG2. Biochemistry. 2010;49:2235–45. doi: 10.1021/bi902085q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keenan RT, O’Brien WR, Lee KH, Crittenden DB, Fisher MC, Goldfarb DS, Krasnokutsky S, Oh C, Pillinger MH. Prevalence of contraindications and prescription of pharmacologic therapies for gout. Am J Med. 2011;124:155–63. doi: 10.1016/j.amjmed.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Terkeltaub R. Gout. Novel therapies for treatment of gout and hyperuricemia. Arthritis Res Ther. 2009;11:236. doi: 10.1186/ar2738. [DOI] [PMC free article] [PubMed] [Google Scholar]