Abstract
The determinants of HIV-associated cardiovascular disease (CVD) are not well understood. Periodontal disease (PD) has been linked to CVD but this connection has not been examined in HIV infection. We followed a cohort of HIV-infected adults to ascertain whether PD was associated with carotid artery intima media thickness (IMT) and brachial artery flow-mediated dilation (FMD). We performed a longitudinal observational study of HIV-infected adults on HAART for <2 years with no known heart disease. PD was characterized clinically and microbiologically. Cardiovascular disease was assessed by IMT/FMD. Linear mixed models assessed cross-sectional and longitudinal associations between PD and FMD/IMT. Forty three HIV+ adults completed a median of 24 (6–44) months on the study. Defining delta to be the change in a variable between baseline and a follow-up time, longitudinally, on average and after adjusting for change in time, CVD-specific and HIV-specific potential confounding covariates, a 1-log10 increase in delta Porphyromonas gingivalis was associated with a 0.013 mm increase in delta IMT (95% CI: 0.0006–0.0262; p=0.04). After adjusting for the same potential confounding covariates, a 10% increase in delta gingival recession was associated with a 2.3% increase in delta FMD (95% CI: 0.4–4.2; p=0.03). In a cohort of HIV-infected adults, an increase in subgingival Porphyromonas gingivalis, a known periodontal pathogen, was significantly associated with longitudinal increases in IMT, while increased gingival recession, which herein may represent PD resolution, was significantly associated with longitudinal improvement in FMD. In the context of HIV infection, PD may contribute to CVD risk. Intervention studies treating PD may help clarify this association.
Introduction
HIV infection has been linked to adverse cardiovascular outcomes in several large epidemiological studies.1–3 Numerous clinical studies have examined the impact of HIV infection on cardiovascular disease (CVD) risk as assessed by carotid artery intima media thickness (IMT) and/or brachial artery flow-mediated dilation (FMD).4–17 CVD progression is reportedly accelerated in HIV-infected subjects,14 though this finding has not been consistent across studies.15 While highly active antiretroviral therapy (HAART) may itself exacerbate CVD risk,7 the relative contributions of HIV infection18–20 and its resulting long-term immune,6 inflammatory, and coagulation effects21–23 have not been completely elucidated. The underlying mechanisms relating HIV to worsened FMD and IMT are not well understood.12
Periodontal disease (PD) has been linked to CVD by numerous epidemiological and animal studies.24–27 PD-related bacterial genomes have been identified in excised human vascular tissue.28 Recently, prospective studies treating PD in HIV-negative cohorts have reported improvements in FMD and IMT directly related to improved periodontal health.29,30 Our group has previously found high levels of severe PD in our HIV-infected cohort.31 This study examined whether severity of PD in HIV-infected adults was associated with an accelerated progression of FMD and IMT.
Materials and Methods
Subject selection
Adult patients receiving primary HIV care at University Hospitals Case Medical Center (UHCMC), The Cleveland Clinic Foundation (CCF), and MetroHealth Medical Center (Metro) were recruited into this IRB-approved study by physician referral or response to posted advertisements. All subjects provided written informed consent prior to any study procedures. Clinical data were obtained from an electronic database (UH/CMC) or electronic/paper medical records (Metro and CCF). Eligible subjects had HIV infection and were age 18 years or older. To increase cohort homogeneity, we included those who were planning to initiate HAART in the next 2 months or had been treated for less than 2 years. Subjects with clinical evidence of CVD manifested as myocardial infarction, cerebrovascular accident, transient cerebral ischemia, or angina pectoris were excluded as were patients who were pregnant, required antibiotic prophylaxis before dental procedures,32,33 had fewer than 20 teeth, a diagnosis of cancer in the past 5 years, other uncontrolled systemic illnesses, evidence of ongoing medication noncompliance, or diabetes mellitus. Study subjects were seen between May 2005 and March 2009.
Study design
This was a prospective longitudinal study. All subjects were seen at baseline to perform ultrasound and PD measurements and to obtain 12-hour fasting blood work. These measures were repeated during one or more final follow-up study visits at a median of 24 months after enrollment (range: 6–44). Dental care was discussed in detail; information on sources of dental care was provided, but no dental treatment (i.e., periodontal treatment, prophylaxis, restorations, or extractions) was provided by the investigator throughout the study. Dental visits were as per self-report at recall visits and tooth loss was determined using raw data and examination of digital photographs.
Assessment of endothelial function
Endothelium-dependent FMD was obtained on all subjects by one experienced registered vascular technician (RVT), following an established protocol.34 Subjects were seen in the morning after a 12-hour fast and 8 h after their last cigarette. Before testing, subjects rested supine for 20 min. Baseline flow velocity and vessel diameter measurements were obtained on the right brachial artery approximately 4.5 cm proximal to the elbow crease. Optimal longitudinal arterial sections were determined using an Echo-Doppler ultrasound system (Acuson L10, Aspen Advanced, GPS Medical, Indianapolis, IN) with a 10-MHz multifrequency linear array transducer. Reactive hyperemia (RH) was induced above the antecubital fossa using a blood pressure/tourniquet cuff (Model SC5, Hokanson, Bellevue, WA). Absence of arterial flow was verified using color Doppler flow and maintained for 5 min. RH-induced velocity and vessel diameter were measured 10–15 s and 60 s after cuff deflation, respectively. Arterial diameter was determined off-line using edge-detection software (Vascular Research Tools 5, Medical Imaging Applications, Coralville, IA). We obtained 60 image frames over 3 s (approximately 3–5 heart beats) from the cine-loop video clip. Low-quality images (based on edge detection software) were identified on-line and deleted prior to automated analyses. For longitudinal assessments, stored baseline images were visualized on a laptop computer to identify anatomical landmarks and obtain images from the same vessel location. Blood pressure (BP) was measured using an automated monitor (Vital Signs Monitor 300, Welch/Allyn, Skaneateles Falls, NY) applied to the left arm before and after ultrasound testing.
Assessment of arterial structure
B-mode ultrasonographic examination was performed on the right common carotid artery, just proximal to the carotid bulb, on all subjects using the ultrasound system described above. Measurements of the carotid artery IMT were obtained on the far wall by the same designated sonographer certified by a core laboratory for the Study of Fat Redistribution and Metabolic Change in HIV infection (FRAM)35 using an established protocol.36 Carotid artery IMT was determined off-line using the same edge-detection software as above. Both IMT and FMD images were saved on magnetic optical disks (230 MB, Verbatim Optical, Charlotte, NC), and edge-detection software results were saved on disk. The one designated ultrasound technician was trained and certified for FMD and IMT at core training laboratories (Tufts-New England Medical Center, Boston, MA) prior to the study initiation.
Measurement of periodontal disease
Periodontal disease measurements were performed at six sites per tooth on all teeth by one dentist (L.T.V.) as previously described.31 PD was quantified as the percent of teeth (0–100%) with ≥1 of 6 sites meeting or exceeding the following cut-points: ≥5.0 mm for periodontal probing depth (PPD), >0 mm (i.e., loss of gingival tissue relative to the cemento-enamel junction) for gingival recession (REC), and ≥4.0 mm for clinical attachment level (CAL).31 Bleeding on probing (BOP) was defined as the percent of teeth with ≥4 of 6 sites exhibiting BOP. We also collected subgingival dental plaque samples and quantitated DNAs for specific bacteria associated with severe PD,37 i.e., Porphyromonas gingivalis (Pg), Treponema denticola (Td), and Tanneralla forsythia (Tf), by real-time PCR in units of log genome copy number per μg total DNA as previously reported (NCBI accession numbers D64081.1, U29399.1, and AY423857.1, respectively).31,38 We also measured total bacterial DNA levels by quantifying gene copies for the bacterial 23S ribosomal RNA using real-time PCR as we reported previously.31 A pooled plaque sample of eight sites was collected from two predetermined sites in each quadrant of the mouth as previously described.31 PD data, which were collected either on or after the ultrasound examination dates, were obtained more often than ultrasound data. Since PD data were collected within 1 month of ultrasound data on 96% of subjects, and PD data in this observational study were not expected to change significantly within 1 month, the PD data collection date was set to the ultrasound data collection date in the final analyses.
Cardiovascular risk factors
At the baseline visit and subsequent study visits, the number of pack per day years (ppd-yrs) of cigarette smoking, compliance with HAART, and use of antihypertensive and lipid-lowering medication were recorded; family history of CVD was obtained at baseline. Fasting blood work included a lipid panel (i.e., total cholesterol, HDL, LDL, VLDL cholesterol, HDL/total cholesterol ratio, and triglycerides), insulin, glucose, and high sensitivity C-reactive protein (hs-CRP); all values were measured as previously reported.31 Time since date of first known HIV seropositivity, nadir CD4+ T cell count, and months on HAART were abstracted from the medical record. All CD4+ T cell counts and HIV RNA levels were measured within 4 months of the baseline study visit; these prebaseline data were used as a surrogate for baseline values. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as previously reported.39
Statistical analyses
Baseline variables were summarized using standard descriptive statistics. To characterize disease progression during the study and to adjust for variability of time on study, we estimated the average change in study variables from baseline to 24 months using a linear mixed-effects model that included a random intercept and slope to model the subject-specific trajectory over time, assumed an unstructured within-subject correlation structure, and adjusted for the change in time between baseline and follow-up times.
For each measure of PD, a linear mixed-effects repeated-measures model was also used to jointly estimate the association between PD at baseline and FMD or IMT at baseline (termed the cross-sectional effect) as well as the association between delta PD and delta FMD or IMT (termed the longitudinal effect), where delta was defined to be the change in the variable between baseline and the follow-up time. Random effects were also incorporated into each model using a random intercept and slope and unstructured within-patient correlation structure. Because time on study varied, time was treated as a continuous variable and the change in time between baseline and follow-up time was adjusted for in all models. For both FMD and IMT models, baseline covariates in each model included CVD-specific variables: age, male gender, total smoking exposure, body mass index (BMI), systolic BP, and hs-CRP as well as HIV-specific baseline covariates: HAART duration, CD4+ T cell count, HIV RNA, and time since first seropositive. We selected these variables based on prior published studies.4,5,10,13–17,40,41
Results
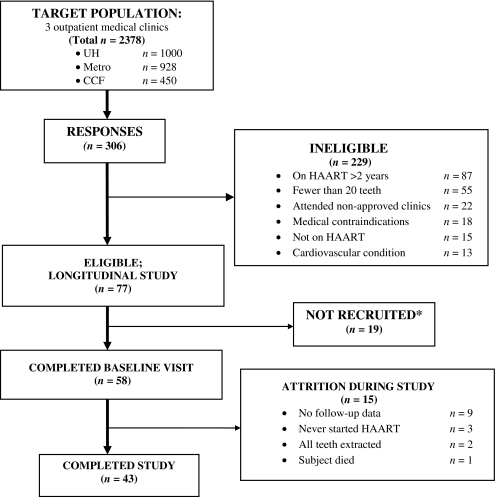
Among the 58 enrolled subjects, 11 did not have follow-up visits, two did not definitively initiate HAART, and two had full-mouth extractions during the study, resulting in an analytic sample of 43 subjects. For these 43 subjects, the median time on study was 24 months (range: 6–44), with 81% completing one ultrasound follow-up visit and 19% completing two or more ultrasound follow-up visits, resulting in a total of 94 visits. During the study, 33 subjects (77%) reported one or more dental visits; however, only seven subjects (16%) reported any scaling and root planning visits. As well, eight subjects (19%) had a total of 18 teeth extracted (excluding seven teeth with loss of clinical crown but retained root); median tooth loss was one tooth (range: one to eight teeth) extracted and was primarily due to dental caries. The subject recruitment flow diagram is depicted in Fig. 1.
FIG. 1.
Subject recruitment flow diagram. *Reasons for non-recruitment: could not be reached by phone, subject lost interest, scheduling conflicts.
Subjects had a mean (±SD) age of 38 (±8) years. Most subjects were black (57%), males (77%) who received federally-funded medical insurance (70%), and had other risk factors for heart disease: a smoking history (72%) and a family history of premature cardiovascular disease (CVD) (21%) (Table 1). The baseline median CD4+ T cell count was 363 cells/μl and the baseline median nadir CD4+ T cell count was 230 cells/μl. The median plasma HIV RNA level for the four subjects who were not on HAART at baseline was 55,552 copies/ml, and 879 copies/ml for the remaining 39 subjects on HAART at baseline. At baseline, 39 (91%) were on HAART and four (9%) were naive to antiretroviral therapy. Of those on HAART at baseline, most (98%) were on a “backbone” nucleoside/nucleotide analogue (NUC), with 54% on a protease inhibitor (PI)-containing regimen and 46% receiving NUCs and a nonnucleotide reverse transcriptase inhibitor (NNRTI). During the entire study, 34 subjects (79%) remained on HAART, one subject (2%) was off HAART for less than 2 weeks, and eight subjects (19%) were off HAART for more than 2 weeks. For those off HAART during the study, the mean (median) time off HAART was 9.2 (10) months.
Table 1.
Patient Characteristics at Baseline (n=43)
| Characteristic | |
|---|---|
| Age, years | 37.5 (30.7, 44.5) |
| Male, n (%) | 33 (77%) |
| Black race, n (%) | 24 (57%) |
| Ever smoked, n (%) | 31 (72%) |
| Smoking amount, pack per day years | 3.9 (0, 9.1) |
| ≤ High school graduate/GED, n (%) | 21 (49%) |
| Medicaid/care or Ryan White insurance, n (%) | 30 (70%) |
| Hypertension medication, n (%) | 6 (14%) |
| Months on HAART | 2.5 (1.1, 7.3) |
| Family history of CVD, n (%) | 9 (21%) |
| Months since first HIV-positive test | 25.0 (11.7, 77.1) |
| CD4+ T cell count (cells/μl) | 363 (207, 453) |
| Plasma HIV RNA level (copies/ml) | 1059 (60, 31017) |
| Nadir CD4+ T cell count (cells/μl) | 230 (135, 305) |
| Months since nadir CD4 | 5.1 (1.8, 9.5) |
Data are shown as median (first and third quartiles), unless otherwise specified.
HAART, highly active antiretroviral therapy; CVD, cardiovascular disease.
Table 2 shows the estimated average change in key variables over 24 months. In terms of PD progression over 24 months, PPD, CAL, and BOP decreased significantly by 11.9±2.2 (p<0.0001), 11.2±2.6 (p≤0.001), and 13.6±3.0 (p≤0.001) percent of teeth exceeding cut points (see Materials and Methods; measurement of PD), respectively; meanwhile, bacterial measures of PD were reduced insignificantly over time. Mean CD4+ T cell count increased significantly (140.3±29.8 cell/μl; p<0.0001) and mean plasma HIV RNA levels decreased significantly by 1.6±0.4 log copies/μl (p < 0.001). HDL cholesterol increased by 5.7±2.5 mg/dl (p=0.03) and the cholesterol/HDL ratio decreased significantly by 0.65±0.22 (p=0.006). Levels of glucose, insulin, and HOMA-IR decreased insignificantly (p>0.32 for all variables) over the estimated 24-month period, while levels of triglycerides and VLDL increased insignificantly (p≥0.91 for both variables; full data not shown).
Table 2.
Mean Characteristics at Baseline and the Estimated Mean Change in Characteristics Between Baseline and 24 Months
| Characteristic | Mean (SD) at baseline (BL) | Estimated mean change (SE) between BL and 24 months | p-value |
|---|---|---|---|
| Ultrasound examinations | |||
| IMT (mm) | 0.6 (0.2) | 0.007 (0.021) | 0.76 |
| FMD (% change) | 12.3 (9.0) | 0.7 (1.9) | 0.72 |
| Immune/inflammatory variables | |||
| CD4+ T cell count (cells/μl) | 357.4 (193.2) | 140.3 (29.8) | <0.0001 |
| HIV RNA (copies/ml) (in log scale) | 7.4 (2.9) | −1.6 (0.4) | <0.001 |
| Hs-CRP (mg/liter) (in log scale) | 0.7 (1.5) | −0.3 (0.2) | 0.11 |
| Periodontal disease measures | |||
| PPD ≥5.0 mm (% teeth) | 31.3 (20.8) | −11.9 (2.2) | <0.0001 |
| REC >0 mm (% teeth) | 47.1 (30.6) | −5.6 (3.1) | 0.08 |
| CAL ≥4 mm (% teeth) | 43.4 (31.8) | −11.2 (2.6) | <0.001 |
| BOP on ≥4 sites/tooth (% teeth) | 39.4 (23.7) | −13.6 (3.0) | <0.001 |
| Porphyromonas gingivalis* | 3.9 (1.9) | −0.15 (0.35) | 0.67 |
| Treponema denticola* | 4.6 (1.7) | −0.21 (0.33) | 0.52 |
| Tanneralla forsythus* | 5.4 (1.3) | −0.067 (0.22) | 0.76 |
| Total 23S rRNA* | 7.6 (0.4) | −0.046 (0.07) | 0.54 |
| Vascular measures | |||
| Systolic BP (mm Hg) | 117.0 (16.8) | 3.3 (2.3) | 0.16 |
| Diastolic BP (mm Hg) | 70.5 (12.4) | 3.1 (1.6) | 0.06 |
| Lipid measures | |||
| Total cholesterol (mg/dl) | 171.1 (35.6) | 4.7 (5.0) | 0.35 |
| Cholesterol/HDL (ratio) | 4.8 (1.8) | −0.65 (0.22) | 0.006 |
| HDL cholesterol (mg/dl) | 39.7 (16.2) | 5.7 (2.5) | 0.03 |
| LDL cholesterol (mg/dl) | 106.0 (28.8) | −1.3 (4.0) | 0.76 |
IMT, carotid artery intima media thickness; FMD, brachial artery flow-mediated dilation; Hs-CRP, high sensitivity C-reactive protein; PPD, periodontal probing depth; REC, gingival recession; CAL, clinical attachment level; BOP, bleeding on probing; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein. *Units are in log genome copy number per μg DNA.
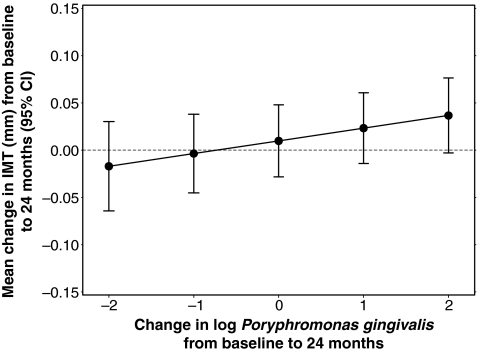
Overall, we found that IMT increased by 0.007±0.021 mm (p=0.76) over 24 months (Table 2). In the longitudinal analysis, after adjustment for change in time and the CVD and HIV-specific potential confounding covariates, we found that a 1-log10 increase in delta Porphyromonas gingivalis was associated with a 0.0134 mm increase in delta IMT (95% CI: 0.0006–0.0262; p=0.04, Fig. 2 and Table 3). In cross-sectional and longitudinal analyses, no other markers of PD were significantly associated with IMT (Table 3).
FIG. 2.
Longitudinal effect of subgingival Porphyromonas gingivalis on intima media thickness (IMT).
Table 3.
Cross-Sectional and Longitudinal Effect of Periodontal Disease Measures on IMT
|
Exposure variable |
Mean estimate (95% CI) |
p-value |
Mean estimate (95% CI) |
p-value |
|---|---|---|---|---|
| Clinical measures of PD | Increase in baseline IMT per 10% increase in baseline PD | Increase in delta IMT per 10% increase in delta PD | ||
| PPD | −0.0010 (−0.0133, 0.0113) | 0.85 | 0.0033 (−0.0128, 0.0195) | 0.64 |
| REC | 0.0008 (−0.0076, 0.0093) | 0.82 | −0.0040 (−0.0176, 0.0097) | 0.50 |
| CAL | 0.0006 (−0.0067, 0.0080) | 0.84 | −0.0100 (−0.0245, 0.0045) | 0.15 |
| BOP | 0.0026 (−0.0124, 0.0175) | 0.70 | −0.0031 (−0.0150, 0.0088) | 0.56 |
| Microbiological measures of PD | Increase in baseline IMT per 1-log10 increase in baseline PD | Increase in delta IMT per 1-log10 increase in delta PD | ||
|---|---|---|---|---|
| P. gingivalis | −0.0082 (−0.0237, 0.0072) | 0.25 | 0.0134 (0.0006, 0.0262) | 0.04 |
| T. denticola | −0.0122 (−0.0266, 0.0023) | 0.09 | 0.0099 (−0.0021, 0.0218) | 0.09 |
| T. forsythia | −0.0126 (−0.0300, 0.0047) | 0.13 | 0.0136 (−0.0054, 0.0326) | 0.13 |
| 23S rRNA | 0.0190 (−0.0593, 0.0974) | 0.58 | 0.0264 (−0.0274, 0.0801) | 0.28 |
PD, periodontal disease; IMT, carotid artery intima media thickness; PPD, periodontal probing depth; REC, gingival recession; CAL, clinical attachment level; BOP, bleeding on probing; P. gingivalis, Porphyromonas gingivalis; T. denticola, Treponema denticola; T. forsythia, Tannerella forsythia; 23S rRNA, 23S ribosomal RNA. Model adjusted for change in time and the CVD and HIV-specific potential confounding covariates.
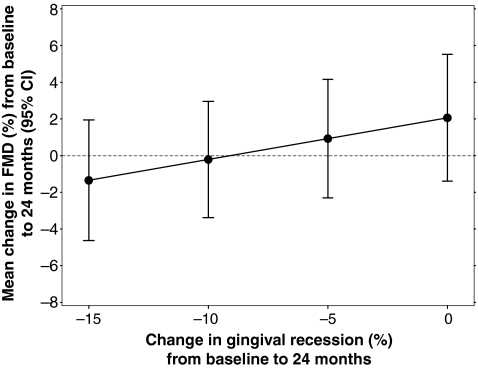
We also found that FMD increased by 0.7±1.9% (p=0.72) over 24 months (Table 2). In the longitudinal analysis, after adjustment for change in time and the CVD and HIV-specific potential confounding covariates, we found that a 10% increase in delta gingival recession was associated with a 2.27% increase in delta FMD (95% CI: 0.37–4.17; p=0.03, Fig. 3 and Table 4). There was no evidence of a cross-sectional or longitudinal association between any other markers of PD and FMD (Table 4).
FIG. 3.
Longitudinal effect of gingival recession (REC) on flow-mediated dilation (FMD).
Table 4.
Cross-Sectional and Longitudinal Effect of Periodontal Disease Measures on FMD
|
Exposure variable |
Mean estimate (95% CI) |
p-value |
Mean estimate (95% CI) |
p-value |
|---|---|---|---|---|
| Clinical measures of PD | Increase in baseline FMD per 10% increase in baseline PD | Increase in delta FMD per 10% increase in delta PD | ||
| PPD | 0.1565 (−0.8082, 1.1212) | 0.71 | −0.0089 (−2.2691, 2.2513) | 0.99 |
| REC | −0.7439 (−1.5748, 0.0869) | 0.07 | 2.2715 (0.3693, 4.1737) | 0.03 |
| CAL | −0.4308 (−1.2453, 0.3836) | 0.24 | 1.0044 (−1.8924, 3.9012) | 0.43 |
| BOP | −0.0760 (−1.1384, 0.9864) | 0.87 | −0.6643 (−2.2971, 0.9685) | 0.36 |
| Microbiological measures of PD | Increase in baseline FMD per 1-log10 increase in baseline PD | Increase in delta FMD per 1-log10 increase in delta PD | ||
|---|---|---|---|---|
| P. gingivalis | −0.2393 (−1.5029, 1.0242) | 0.67 | 0.4232 (−1.1749, 2.0213) | 0.55 |
| T. denticola | −0.4002 (−1.8366, 1.0361) | 0.53 | −0.7024 (−3.2934, 1.8886) | 0.54 |
| T. forsythia | −0.5988 (−2.1249, 0.9272) | 0.37 | −0.9759 (−3.2610, 1.3093) | 0.34 |
| 23S rRNA | −3.5102 (−9.1375, 2.1172) | 0.18 | 1.2753 (−3.0946, 5.6452) | 0.51 |
PD, periodontal disease; IMT, carotid artery intima media thickness; PPD, periodontal probing depth; REC, gingival recession; CAL, clinical attachment level; BOP, bleeding on probing; P. gingivalis, Porphyromonas gingivalis; T. denticola, Treponema denticola; T. forsythia, Tannerella forsythia; 23S rRNA, 23S ribosomal RNA. Model adjusted for change in time and the CVD and HIV-specific potential confounding covariates.
Discussion
In our HIV-infected cohort, we found a statistically significant longitudinal association between increased levels of P. gingivalis and increased (worsened) levels of carotid artery IMT. Furthermore, we found a statistically significant longitudinal association between increased gingival REC and increased (improved) levels of brachial artery FMD. CD4+ T cell count and level of HIV RNA improved significantly during the study, implying successful therapy and immune reconstitution on HAART.
The longitudinal association between levels of P. gingivalis and levels of IMT emerged only after adjusting for preselected CVD-specific and HIV-specific potential confounding covariates (see Supplementary Tables S1A and B; Supplementary Data are available online at www.liebertonline.com/aid). P. gingivalis, an anaerobic gram-negative bacterium, has consistently been associated with severe PD in HIV-uninfected cohorts.37 We previously reported that P. gingivalis was a statistically significant predictor of periodontal disease in HIV+ adults (standardized β=0.290, p<0.001, adjusted R2=0.509).31 In an ApoE-/- mouse model, oral infection with P. gingivalis resulted in accelerated atheroma formation, which could be prevented by immunization to P. gingivalis prior to oral P. gingivalis challenge.42,43 These and other studies suggest that P. gingivalis may be able to induce specific innate immune inflammatory pathways and maintain a state of chronic inflammation at sites distant from oral infection.44 Exploring such mechanisms was beyond the scope of this study; however, murine models suggest that major and minor fimbriae of P. gingivalis can directly or indirectly (through platelets and/or antigen-presenting cells) interact with endothelial cells eliciting inflammatory cascades that promote atherogenesis.44–46 Our data concerning the association between P. gingivalis and increased carotid artery IMT support the growing literature showing a connection between periodontal disease and atherosclerosis in general populations.47
Historically, clinical measures of PD were developed to quantify clinical signs of PD,48 not the mucosal exposure to microbes and their byproducts that could trigger systemic effects. Therefore, it is not surprising that clinical measures of PD in our cohort were unrelated to IMT; these findings are consistent with a study of almost 5000 subjects by Beck et al. in the Atherosclerosis Risk in Communities (ARIC) Study. These authors found that clinical measures of PD were not associated with coronary heart disease (CHD) while serum IgG antibodies to known periodontal pathogens were significantly associated with CHD.49
Our data herein support the growing literature that quantification of periodontopathogenic bacteria in subgingival biofilm50,51 is a more direct and specific measure of PD as an exposure contributing to CVD risk. Comparing our longitudinal findings in an HIV-infected cohort to the general population is limited at present because ongoing studies such as The Oral Infections and Vascular Disease Epidemiology Study (INVEST) have, to our knowledge, published only cross-sectional reports.50–53 These authors have linked the cumulative level of periodontopathogenic microbial DNA to increased IMT,50 hypertension, and blood pressure.51 Our findings support these earlier cross-sectional associations50,51 and extend them into an HIV-infected population examined longitudinally.
While it is plausible that P. gingivalis could have increased virulence in an HIV-infected host, our limited sample size did not permit us to analyze whether or not P. gingivalis is acting as an independent risk factor for IMT. Our present longitudinal study, “Immune and Inflammatory Consequences of Intensive Periodontal Disease Treatment in HIV-Infected Adults,” is designed to more effectively address the mechanisms of our finding linking PD to IMT in this population.
Our finding of an association between apparently worsening REC and improving FMD over time deserves further attention (see Supplementary Tables S2A and 2B for a time-adjusted model and the model adjusted for time and CVD-specific covariates; Supplementary Data are available online at www.liebertonline.com/aid). One possible explanation is that local gingival inflammation and swelling at baseline may have resolved during the study due to the initiation of HAART and resulting increase in CD4+ T cell count. This reduction in local swelling may have manifested itself clinically as an increase in gingival recession (REC), although it may not represent periodontal disease progression (i.e., tissue destruction) per se. Another explanation is that a process similar to immune reconstitution inflammatory syndrome (IRIS) may have hastened or promoted gingival REC at specific sites, although we expect that the impact of potential IRIS-like phenomena, which occur in a relatively small proportion of persons initiating HAART and are typically short-lived, would be small in this 24-month longitudinal follow-up study. Thus, in the context of HIV infection, periodontal REC may be influenced by multiple mechanisms.
Beck et al. proposed that when examining the connection between periodontal disease and systemic disease, components of PD (i.e., PPD, REC, and CAL) may individually influence outcome measures.54 This concept was clearly demonstrated in our earlier work wherein immune-related and metabolic independent variables were differentially associated with the dependent outcome variables PPD, REC, and CAL in a cross-sectional cohort of 112 HIV+-infected adults.31 Herein, our study findings that components of traditionally defined PD are differentially related to established markers of cardiovascular disease (i.e., IMT and FMD) further support this concept. Whether REC, a component of PD, is related to brachial artery FMD in HIV-uninfected cohorts is, to our knowledge, unknown.
This study revealed that defining PD more broadly (i.e., microbiologically and as several clinical constructs) uncovered associations linking PD to markers of cardiovascular disease. It is possible that the associations detected herein may be due to residual confounding; however, we identified and included a priori many critical confounding variables in our analytic model. Based on our results, including both CVD-specific and HIV-specific confounding covariates appears to be critical when examining markers of CVD in an HIV-infected cohort.
Our results showing a slight improvement in FMD over time in HIV-infected adults on HAART (Table 2) are in agreement with findings of improved FMD at 4 and 24 weeks after HAART initiation as reported by Torriani et al.,8 but differ from findings of worsened FMD over 1 year in a more heterogeneous cohort of HIV-infected adults as reported by Obueyungbo et al.17 We propose that our results are more similar to those of Torriani et al. because, like their cohort, ours had recently started HAART, had a relatively low baseline CD4+ T cell count, and most but not all of our subjects (59%) achieved plasma HIV RNA levels <50 copies/ml at the final study visit. In the context of other longitudinal studies, our findings suggest that the stage of HIV disease and the specific immune profile of an HIV-infected cohort may influence the trajectory of change in subclinical CVD markers across time.
Our finding that IMT worsened by 0.0035 mm per year (i.e., 0.007 mm/2 years) agree with four previous longitudinal studies of HIV-infected cohorts,14–17 but are closest to findings by Currier et al. indicating that IMT progressed a median of 0.0096 mm/year in a PI-treated group and 0.0058 mm/year in a non-PI-treated group.15 Among several pooled HIV-negative cohorts, the annual mean change in common carotid IMT is 0.015 mm55; thus, the institution of HAART and possibly other unmeasured effects (i.e., improved care, health education) might have played a role in putting our subjects at a risk profile similar to the general population. The fact that FMD improved but IMT worsened during our study may represent either that the time frame of change is longer for IMT than for FMD or that FMD and IMT may be influenced by different mechanisms in HIV-infected adults. Longitudinal findings in an HIV-infected cohort reported by Obueyungbo et al., wherein traditional risk factors for CVD (i.e., age, male gender, and smoking status) were significantly related to IMT but not FMD, would tend to support the latter interpretation.17
Our study had several strengths. We followed a relatively homogeneous cohort in terms of stage of HIV disease—as most of our cohort had recently started HAART. We measured PD thoroughly and often (a median of four times over 2 years) and were able to quantify this exposure as a continuous variable. We defined PD microbiologically as well as clinically—as a variable broken down into separate readouts (i.e., PPD, REC, and CAL) as advised by Beck et al.54,56 We measured both IMT and FMD longitudinally, and collected and quantified numerous potential confounding variables. We reported a thorough immunological profile of our cohort (including time on HAART, time since first seropositive, nadir CD4+ T cell count, and time since nadir CD4+ T cell count). Finally, this is the first report relating PD to IMT and FMD in an HIV-infected cohort. CVD is a leading cause of death in HIV-infected adults.1,57,58 We have previously found high levels of severe PD in a predominantly black urban male cohort of HIV-infected adults,31 and since black males are disproportionately affected with HIV/AIDS in the United States,59 these findings may be broadly generalizable. Finally, if PD represents a previously unrecognized modifiable risk factor for CVD in HIV-infected adults, this finding could have great public health importance.
Our study also had a number of limitations. Foremost, the sample size is small and, against the backdrop of significant immune reconstitution, may be underpowered to detect clinically significant cross-sectional and longitudinal associations between measures of PD and markers of CVD risk. Given the complexity of our statistical model, the necessity to include both cross-sectional and longitudinal effects of PD exposure on markers of CVD risk as well as our small sample size, the number of confounders that could be included in our final analytic model was limited; however, we included many previously identified confounding variables.4,5,10,13–17,40,41 Another limitation is that most subjects in our cohort had moderate to severe PD since a comparison group of HIV+ adults on HAART with low levels of PD was not readily available.31 Thus, our within-subjects study design controlling for time on HAART is a reasonable and practical approach to addressing this question.
This study suggests a potential link between PD and CVD risk in HIV-infected adults on HAART. Since PD is a chronic oral infection that can be reduced or eliminated, an intervention study designed to reduce PD in HIV-infected adults would help determine whether this association reflects a modifiable risk factor for CVD.
NCBI Accession Numbers
Porphyromonas gingivalis: NCBI accession number D64081.1; Treponema denticola: NCBI accession number U29399.1; Tannerella forsythia: NCBI accession number AY423857.1. 23S rRNA (Housekeeping gene; NCBI accession number not applicable).
Appendix
Probes and primers for select periodontal bacteria:
Note: Probe and primer sequences were as previously reported in Vernon et al.31:
Pg Probe: 5′-TTGCCCATTCTTTCCCGTTCTCTTGC-3′
Forward primer: 5′-TCTCGGAGAAAGGTACGCCTAT-3′
Reverse primer: 5′-TCATCGCACGTGTTTCAGAAA-3′
Td Probe: 5′-CCGGATTTGATCCTGCTGCAACATCT-3′
Forward primer: 5′-GGAAAGGCCGGTGTTCATG-3′
Reverse primer: 5′-CAATCCCATACCTAAATACGGCTTA-3′
23S ribosomal DNA:
Forward primer: 5′-AGCCCCAGTAAACGGCG-3′
Reverse primer: 5′-AATTTCGCTACCTTAGGACCGTTA-3′
Supplementary Material
Acknowledgments
We especially thank all of our research participants. We appreciate the professional suggestions, scientific input, and/or assistance from Drs. Christopher Whalen, Isaac Rodriguez-Chavez (R-21 Program Director, see below), Robert Kalayjian, Judith Currier, Robert Asaad, Yiping Han, Nabil Bissada, Wendy Armstrong, Mr. Alan Chiunda, Mr. Gene Doverspike, Ms. Leslie Anderson, and Ms. Katie Przepyszny. Supported by NIDCR, Grants K23 DE15746-01A1 and R21 DE21376-01, the Center for AIDS Research (CFAR), AI36219, the William T. Dahms, M.D. Clinical Research Unit (Dahms CRU) of the CTSC, UL1 RR024989, NIH, M01 RR000080, the General Clinical Research Center (GCRC), the CWRU Department of Biological Sciences, OPR892521 and the Case Western Reserve University/Cleveland Clinic CTSA Grant, UL1 RR024989 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health and NIH roadmap for Medical Research.
An abstract was submitted to the International Association of Dental Research (IADR) on 10/08/2010; 89th General Session and Exhibition of IADR, San Diego, California, meeting March 16–19, 2011. It was withdrawn prior to the meeting.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Friis-Moller N. Sabin CA. Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349(21):1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 2.Triant VA. Lee H. Hadigan C. Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bozzette SA. Ake CF. Tam HK. Chang SW. Louis TA. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med. 2003;348(8):702–710. doi: 10.1056/NEJMoa022048. [DOI] [PubMed] [Google Scholar]
- 4.Mangili A. Jacobson DL. Gerrior J. Polak JF. Gorbach SL. Wanke CA. Metabolic syndrome and subclinical atherosclerosis in patients infected with HIV. Clin Infect Dis. 2007;44(10):1368–1374. doi: 10.1086/516616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mercie P. Thiebaut R. Lavignolle V, et al. Evaluation of cardiovascular risk factors in HIV-1 infected patients using carotid intima-media thickness measurement. Ann Med. 2002;34(1):55–63. doi: 10.1080/078538902317338652. [DOI] [PubMed] [Google Scholar]
- 6.Nolan D. Watts GF. Herrmann SE. French MA. John M. Mallal S. Endothelial function in HIV-infected patients receiving protease inhibitor therapy: Does immune competence affect cardiovascular risk? QJM. 2003;96(11):825–832. doi: 10.1093/qjmed/hcg145. [DOI] [PubMed] [Google Scholar]
- 7.Stein JH. Klein MA. Bellehumeur JL, et al. Use of human immunodeficiency virus-1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction. Circulation. 2001;104(3):257–262. doi: 10.1161/01.cir.104.3.257. [DOI] [PubMed] [Google Scholar]
- 8.Torriani FJ. Komarow L. Parker RA, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008;52(7):569–576. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Wijk JP. de Koning EJ. Cabezas MC, et al. Functional and structural markers of atherosclerosis in human immunodeficiency virus-infected patients. J Am Coll Cardiol. 2006;47(6):1117–1123. doi: 10.1016/j.jacc.2005.09.073. [DOI] [PubMed] [Google Scholar]
- 10.Lekakis J. Tsiodras S. Ikonomidis I, et al. HIV-positive patients treated with protease inhibitors have vascular changes resembling those observed in atherosclerotic cardiovascular disease. Clin Sci (Lond) 2008;115(6):189–196. doi: 10.1042/CS20070353. [DOI] [PubMed] [Google Scholar]
- 11.Hsue PY. Hunt PW. Sinclair E, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS. 2006;20(18):2275–2283. doi: 10.1097/QAD.0b013e3280108704. [DOI] [PubMed] [Google Scholar]
- 12.Hsue PY. Hunt PW. Schnell A, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS. 2009;23(9):1059–1067. doi: 10.1097/QAD.0b013e32832b514b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Currier JS. Kendall MA. Zackin R, et al. Carotid artery intima-media thickness and HIV infection: traditional risk factors overshadow impact of protease inhibitor exposure. AIDS. 2005;19(9):927–933. doi: 10.1097/01.aids.0000171406.53737.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsue PY. Lo JC. Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109(13):1603–1608. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 15.Currier JS. Kendall MA. Henry WK, et al. Progression of carotid artery intima-media thickening in HIV-infected and uninfected adults. AIDS. 2007;21(9):1137–1145. doi: 10.1097/QAD.0b013e32811ebf79. [DOI] [PubMed] [Google Scholar]
- 16.Mercie P. Thiebaut R. Aurillac-Lavignolle V, et al. Carotid intima-media thickness is slightly increased over time in HIV-1-infected patients. HIV Med. 2005;6(6):380–387. doi: 10.1111/j.1468-1293.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- 17.Odueyungbo A. Smieja M. Thabane L, et al. Comparison of brachial and carotid artery ultrasound for assessing extent of subclinical atherosclerosis in HIV: A prospective cohort study. AIDS Res Ther. 2009;6:11. doi: 10.1186/1742-6405-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mujawar Z. Rose H. Morrow MP, et al. Human immunodeficiency virus impairs reverse cholesterol transport from macrophages. PLoS Biol. 2006;4(11):e365. doi: 10.1371/journal.pbio.0040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bussolino F. Mitola S. Serini G. Barillari G. Ensoli B. Interactions between endothelial cells and HIV-1. Int J Biochem Cell Biol. 2001;33(4):371–390. doi: 10.1016/s1357-2725(01)00024-3. [DOI] [PubMed] [Google Scholar]
- 20.Eugenin EA. Morgello S. Klotman ME, et al. Human immunodeficiency virus (HIV) infects human arterial smooth muscle cells in vivo and in vitro: Implications for the pathogenesis of HIV-mediated vascular disease. Am J Pathol. 2008;172(4):1100–1111. doi: 10.2353/ajpath.2008.070457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tebas P. Henry WK. Matining R, et al. Metabolic and immune activation effects of treatment interruption in chronic HIV-1 infection: Implications for cardiovascular risk. PLoS One. 2008;3(4):e2021. doi: 10.1371/journal.pone.0002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funderburg NT. Mayne E. Sieg SF, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: Relationship to in vivo coagulation and immune activation. Blood. 2010;115(2):161–167. doi: 10.1182/blood-2009-03-210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Vonderen MG. Smulders YM. Stehouwer CD, et al. Carotid intima-media thickness and arterial stiffness in HIV-infected patients: The role of HIV, antiretroviral therapy, and lipodystrophy. J Acquir Immune Defic Syndr. 2009;50(2):153–161. doi: 10.1097/QAI.0b013e31819367cd. [DOI] [PubMed] [Google Scholar]
- 24.Beck JD. Offenbacher S. Systemic effects of periodontitis: Epidemiology of periodontal disease and cardiovascular disease. J Periodontol. 2005;76(11 Suppl):2089–2100. doi: 10.1902/jop.2005.76.11-S.2089. [DOI] [PubMed] [Google Scholar]
- 25.Genco R. Offenbacher S. Beck J. Periodontal disease and cardiovascular disease: epidemiology and possible mechanisms. J Am Dent Assoc. 2002;133(Suppl):14S–22S. doi: 10.14219/jada.archive.2002.0375. [DOI] [PubMed] [Google Scholar]
- 26.Li L. Messas E. Batista EL., Jr. Levine RA. Amar S. Porphyromonas gingivalis infection accelerates the progression of atherosclerosis in a heterozygous apolipoprotein E-deficient murine model. Circulation. 2002;105(7):861–867. doi: 10.1161/hc0702.104178. [DOI] [PubMed] [Google Scholar]
- 27.Lalla E. Lamster IB. Hofmann MA, et al. Oral infection with a periodontal pathogen accelerates early atherosclerosis in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2003;23(8):1405–1411. doi: 10.1161/01.ATV.0000082462.26258.FE. [DOI] [PubMed] [Google Scholar]
- 28.Haraszthy VI. Zambon JJ. Trevisan M. Zeid M. Genco RJ. Identification of periodontal pathogens in atheromatous plaques. J Periodontol. 2000;71(10):1554–1560. doi: 10.1902/jop.2000.71.10.1554. [DOI] [PubMed] [Google Scholar]
- 29.Tonetti MS. D'Aiuto F. Nibali L, et al. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356(9):911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 30.Piconi S. Trabattoni D. Luraghi C, et al. Treatment of periodontal disease results in improvements in endothelial dysfunction and reduction of the carotid intima-media thickness. FASEB J. 2009;23(4):1196–1204. doi: 10.1096/fj.08-119578. [DOI] [PubMed] [Google Scholar]
- 31.Vernon LT. Demko CA. Whalen CC, et al. Characterizing traditionally defined periodontal disease in HIV+ adults. Community Dent Oral Epidemiol. 2009;37(5):427–437. doi: 10.1111/j.1600-0528.2009.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tong DRB. Antibiotic prophylaxis in dentistry: A review and practice recommendations. J Am Dent Assoc. 2000;131:366–374. doi: 10.14219/jada.archive.2000.0181. [DOI] [PubMed] [Google Scholar]
- 33.Advisory statement: Antibiotic prophylaxis for dental patients with total joint replacements. American Dental Association; American Academy of Orthopaedic Surgeons. J Am Dent Assoc. 1997;128(7):1004–1008. doi: 10.14219/jada.archive.1997.0307. [DOI] [PubMed] [Google Scholar]
- 34.Corretti MC. Anderson TJ. Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 35.Tien PC. Benson C. Zolopa AR. Sidney S. Osmond D. Grunfeld C. The study of fat redistribution and metabolic change in HIV infection (FRAM): Methods, design, and sample characteristics. Am J Epidemiol. 2006;163(9):860–869. doi: 10.1093/aje/kwj111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Leary DH. Polak JF. Wolfson SK, Jr., et al. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1991;22(9):1155–1163. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 37.Socransky SS. Haffajee AD. Cugini MA. Smith C. Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25(2):134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 38.Morillo JM. Lau L. Sanz M. Herrera D. Martin C. Silva A. Quantitative real-time polymerase chain reaction based on single copy gene sequence for detection of periodontal pathogens. J Clin Periodontol. 2004;31(12):1054–1060. doi: 10.1111/j.1600-051x.2004.00608.x. [DOI] [PubMed] [Google Scholar]
- 39.Matthews DR. Hosker JP. Rudenski AS. Naylor BA. Treacher DF. Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 40.McComsey GA. O'Riordan M. Hazen SL, et al. Increased carotid intima media thickness and cardiac biomarkers in HIV infected children. AIDS. 2007;21(8):921–927. doi: 10.1097/QAD.0b013e328133f29c. [DOI] [PubMed] [Google Scholar]
- 41.Lorenz MW. Markus HS. Bots ML. Rosvall M. Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation. 2007;115(4):459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 42.Gibson FC., 3rd Hong C. Chou HH, et al. Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109(22):2801–2806. doi: 10.1161/01.CIR.0000129769.17895.F0. [DOI] [PubMed] [Google Scholar]
- 43.Miyamoto T. Yumoto H. Takahashi Y. Davey M. Gibson FC., 3rd Genco CA. Pathogen-accelerated atherosclerosis occurs early after exposure and can be prevented via immunization. Infect Immun. 2006;74(2):1376–1380. doi: 10.1128/IAI.74.2.1376-1380.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayashi C. Gudino CV. Gibson FC., 3rd Genco CA. Review: Pathogen-induced inflammation at sites distant from oral infection: Bacterial persistence and induction of cell-specific innate immune inflammatory pathways. Mol Oral Microbiol. 2010;25(5):305–316. doi: 10.1111/j.2041-1014.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeituni AE. Jotwani R. Carrion J. Cutler CW. Targeting of DC-SIGN on human dendritic cells by minor fimbriated Porphyromonas gingivalis strains elicits a distinct effector T cell response. J Immunol. 2009;183(9):5694–5704. doi: 10.4049/jimmunol.0901030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pollreisz A. Hudson BI. Chang JS, et al. Receptor for advanced glycation endproducts mediates pro-atherogenic responses to periodontal infection in vascular endothelial cells. Atherosclerosis. 2010;212(2):451–456. doi: 10.1016/j.atherosclerosis.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Humphrey LL. Fu R. Buckley DI. Freeman M. Helfand M. Periodontal disease and coronary heart disease incidence: A systematic review and meta-analysis. J Gen Intern Med. 2008;23(12):2079–2086. doi: 10.1007/s11606-008-0787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Offenbacher S. Barros SP. Singer RE. Moss K. Williams RC. Beck JD. Periodontal disease at the biofilm-gingival interface. J Periodontol. 2007;78(10):1911–1925. doi: 10.1902/jop.2007.060465. [DOI] [PubMed] [Google Scholar]
- 49.Beck JD. Eke P. Lin D, et al. Associations between IgG antibody to oral organisms and carotid intima-medial thickness in community-dwelling adults. Atherosclerosis. 2005;183(2):342–348. doi: 10.1016/j.atherosclerosis.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 50.Desvarieux M. Demmer RT. Rundek T, et al. Periodontal microbiota and carotid intima-media thickness: The Oral Infections and Vascular Disease Epidemiology Study (INVEST) Circulation. 2005;111(5):576–582. doi: 10.1161/01.CIR.0000154582.37101.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Desvarieux M. Demmer RT. Jacobs DR, Jr, et al. Periodontal bacteria and hypertension: The oral infections and vascular disease epidemiology study (INVEST) J Hypertens. 2010;28(7):1413–1421. doi: 10.1097/HJH.0b013e328338cd36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Demmer RT. Papapanou PN. Jacobs DR., Jr Desvarieux M. Evaluating clinical periodontal measures as surrogates for bacterial exposure: The Oral Infections and Vascular Disease Epidemiology Study (INVEST) BMC Med Res Methodol. 2010;10:2. doi: 10.1186/1471-2288-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Demmer RT. Papapanou PN. Jacobs DR., Jr Desvarieux M. Bleeding on probing differentially relates to bacterial profiles: The Oral Infections and Vascular Disease Epidemiology Study. J Clin Periodontol. 2008;35(6):479–486. doi: 10.1111/j.1600-051X.2008.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beck JD. Offenbacher S. Relationships among clinical measures of periodontal disease and their associations with systemic markers. Ann Periodontol. 2002;7(1):79–89. doi: 10.1902/annals.2002.7.1.79. [DOI] [PubMed] [Google Scholar]
- 55.Bots ML. Evans GW. Riley WA. Grobbee DE. Carotid intima-media thickness measurements in intervention studies: Design options, progression rates, and sample size considerations: A point of view. Stroke. 2003;34(12):2985–2994. doi: 10.1161/01.STR.0000102044.27905.B5. [DOI] [PubMed] [Google Scholar]
- 56.Beck JD. Eke P. Heiss G, et al. Periodontal disease and coronary heart disease: A reappraisal of the exposure. Circulation. 2005;112(1):19–24. doi: 10.1161/CIRCULATIONAHA.104.511998. [DOI] [PubMed] [Google Scholar]
- 57.Sackoff JE. Hanna DB. Pfeiffer MR. Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145(6):397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 58.Crum NF. Riffenburgh RH. Wegner S, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: Analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr. 2006;41(2):194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- 59.Hall HI. Song R. Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300(5):520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.