Abstract
It is more than 20 years since the first cord blood transplant (CBT) was performed, following the realisation that cord blood (CB), which is normally wasted, is rich in progenitor cells and capable of rescuing haematopoiesis. Since then it has been appreciated that CB is rich in stem cells, and has many other features not the least of which is its ability to rescue the transplanted patient without a rigid need for full human lymphocyte antigen (HLA) compatibility. Also it is easily accessible, relatively free from infections and poses no medical risk to the donor. However, the quantity of the stem cells is rather small, thus predominantly restricting its use to children or adults requiring double units. In Oman, we have taken a keen interest in stem cell research and also CBT. We see such activities as an avenue for our patients, for whom a compatible bone marrow (BM) or a peripheral blood donor cannot be found, to have an alternative in the form of CBT. This has encouraged us to establish a national voluntary cord blood bank (CBB) which is a valuable option open to a selected group of patients, as compared to the controversial private CBB. This national CBB will have a better representation of HLA-types common in the region, an improvement on relying on banks in other countries. Considering the need for stem cell transplant/therapy in this country, it is only appropriate that this sort of bank is established to cater for some of these requirements.
Keywords: Umbilical cord blood, Cord blood transplantation, Cord blood stem cell, Cord blood banking, Private cord blood bank, National cord blood bank
It is about 20 years since the first cord blood transplant (CBT) was performed in a young patient with Fanconi's anaemia. Since then, due to technological advances, there has being a tremendous progress in the utilisation and application of CBT, both in terms of quantity and quality.1
The use of CBT has risen following the understanding that cord blood (CB), which is normally wasted following delivery of a baby, contains large quantities of pluripotential stem cells that could be utilised to regenerate or rescue haematopoietic stem cells (HSC), following their exposure to accidental or therapeutic ablative therapy. Pleuripotent stem cells are undifferentiated cells that are capable of dividing, self-renewal, and generating progeny of highly specialised cells, upon exposure to optimal environmental stimuli in what is termed a bone marrow microenvironment.2,3 This microenvironment is under the influence of various biomolecules, with interplay between adhesions molecules, cytokines and their receptors.3
The stem cells found in CB are also similar to those found in the bone marrow and are responsible for the regenerative capacity of the bone marrow’s haematopoietic activities. The initial experiments centered on a severe combined immune deficiency (SCID) mouse model that could be rescued by giving these stem cells. The progress that was made in this area was paralleled by progress in the ability to define these stem cells through the process of antibody labelling using flowcytometry, combining both functional and phenotypic assays.4 More recently, the focus has also been on the stromal support cells as they are found to play a crucial role in maintaining the integrity of the haematopoietic cellular milieu.5 These cells originate from mesenchymal stem cells that are not only necessary for the integrity of other marrow stem cells, but are capable of giving rise to many tissue types including cartilage, fat, muscle and bones.5,6 Subsequently, it has been demonstrated that cord blood units (CBU) have a number of features which makes them a highly favourable source of stem cells in the ever expanding range of stem cell therapy. Having realised the potential uses of CBUs, it was deemed necessary to establish cord blood banks (CBBs) in order to collect and store these units until the need for them arose.
Advantages of Cord Blood Transplants
A CBU contains a large number of progenitor cells that can be used to transplant most paediatric and some adult patients who require this form of therapy. These units have a low risk of viral infection (although this is not completely eliminated as Epstein-Barr virus [EBV] cases has been reported), in particular from cytomegalovirus [CMV ].7 Moreover, CBUs are accessible and secured, so that they could be used at any time. This means that CBU units are ready for use when the recipient is ready for them, and so the transplant can be scheduled at the convenience of the recipient (patient) and not that of the donor. Furthermore, it is also known that the incidence of graft-versus-host disease (GVHD) is lower compared with bone marrow or peripheral blood transplantation. This may be related to the immaturity of the immune system at birth and the decreased potential of alloreactive lymphocytes.8 As a result, it is not strictly necessary to have a complete HLA match in CBU selection compared to peripheral blood or bone marrow selection. At the same time, the graft-versus-leukaemia (GVL) effect is maintained, fulfilling one of the main purposes of any allogeneic stem cell transplant procedure. Additional advantages, include zero risk to the donor since this blood is normally not used and wasted [Table 1].
Table 1:
Advantages of cord blood transplantation
| Advantage | Comments |
|---|---|
| Rich in progenitor cells | Capable of durable engraftment and long-term haematopoiesis in adults and children |
| Relatively free from infections | Translates to reduced transplant mortality/morbidity |
| CBUs are accessible | Transplantation scheduled to recipient convenience |
| GVHD is less | Improved survival |
| GVL is maintained | Achieves transplantation purposes |
| Zero risk to the donor | Positive utilisation of CBU |
| Less stringent HLA matching requirements with easy access to rare haplotypes | Transplant possible for many recipients |
Legend: CBU = cord blood unit; GVHD = graft-versus-host disease; GVL = graft-versus-leukaemia; HLA = human lymphocyte antigen
Disadvantages of Cord Blood Transplants
There are, however, some obstacles to the widespread use of CBU [Table 2], including the sometimes small number of stem cells in each unit, which leads to a delayed engraftment, and may be reflected in the increased transplant related mortality/morbidity and reduced survival. This is probably the most important limitation in CBT.9 Furthermore, since these units are small, (with reduced volume and quantity of stem cells), they may not be sufficient for large patients, although this could be augmented by using two human lymphocyte antigen (HLA) matched units at the same time. Efforts are being made to enhance the number, potency and engrafting capabilities of the collected stem cells through ex-vivo and in vivo manoeuvres, by modulating the microenvironment, cell receptors, and signalling pathways that control them.2
Table 2:
Disadvantages of cord blood transplantation
| Disadvantages | Consequences |
|---|---|
| Reduced cell dose | May delay engraftment/reduce survival |
| Units may be small | Large adult recipients (>60 Kg) require two units |
| Haematologic and immunologic disorders may not be apparent at birth | Requires careful donor screening and testing for common hereditary blood and immune disorders |
There are also efforts to enhance engraftment, including enhancing homing of the transfused cells as well as direct intra-bone marrow injections.10,11 The stored unit is not known for any severe haematologic or immune disorders, which may be transferred by the transplant process, and only for this reason, the donor is examined carefully after 6 months and the unit is also tested for haematologic and immune deficiency syndromes which may manifest only in the adult life. Extensive testing for common hereditary disorders, although very relevant to a country such as Oman, would be quite expensive, and the predictive value of such tests could be improved by taking a detailed family history from the parents.
Despite all of the above difficulties, CBT is growing, and CBU storage is rising worldwide. The increasing interest in CBU has therefore necessitated the need for CBBs of which there are currently two types.
National Voluntary Cord Blood Banks
National voluntary CBBs generally cater for mothers who have children with a disease that is cured by bone marrow transplant (BMT) such as thalassaemia, sickle cell disease or leukaemia. In these cases, mothers are encouraged to collect their babies' CB for possible future related umbilical CBT. These CBUs are collected and a process of HLA matching is done. It is also now possible in some centres to make a selection of disease-free offspring and HLA-matched babies as part of preimplantation genetic determination (PGD).12
Parents also can elect to donate the CBU of their baby to the national CBB to be used for patients where donors could not be found from their own family, allowing many matched but unrelated CBTs to be performed. With the average family size declining in the Western world, it is estimated that only about 25% of Caucasians will have a fully matched unrelated donor, and CBT has become in this regard an attractive source of stem cells.13 Recent statistics suggest that more than 400,000 CBU units are stored in banks in about 40 countries worldwide for use in unrelated transplants; of these, around 20,000 CBUs have been used for transplant so far worldwide, contributing to the cure of many children with haematological malignancies and bone marrow failure disorders who do not have matched family donors.14 Interestingly in fact, CBT has become more popular, particularly in children, and in 2009 more unrelated CBTs were performed than unrelated BMTs worldwide.15,13 Public banks store CBUs free of charge, samples are used to treat any patient without a donor and these banks are financed by public money.16
Private Cord Blood Banking
Upon requests from interested parents, CBUs are also collected for personal use of that newborn in the future and, although the odds ratio for their utilisation has risen with time, it remains between 1 in 25,000 to 1 in 200,000 in the first 20 years of life.17 This has resulted in the proliferation of private (for-profit) CBBs that store these units. Their number is rising worldwide, capitalising on the idea that CB usage is expanding daily, beyond the currently available indications. These banks charge a fee to store CBUs exclusively for that neonate’s family only and only this family may access them. There are about 900,000 CBUs stored in private banks and more than 100 reports of successful autologous CBTs done worldwide by this method. Although the use of such CBUs for non-malignant conditions is reasonable, their use for the treatment of malignant conditions such as leukaemia will be limited by the lack of GVL—one of the main reasons for having allogeneic transplants. Caution has to be exercised in unrestrictedly advocating the widespread use of such banks, one of the many reasons being the limited future clinical utility of CBUs; for example, the European Group for Blood and Marrow Transplantation (EMBT) reported 544 cord blood transplants in 2006, but none of them was autologous.18
Furthermore, the professional reluctance to accept private CBBs stems from the fact that the amount of total nucleated cells (TNC) in these units is small allowing at most only paediatric transplants. It is also possible that these CBUs are contaminated with leukaemogenic cells prohibiting the most important indications for cord transplant in childhood leukaemia.19 The yield of CBUs as well as their utilisation for mesenchymal stem cell therapy remains controversial.20 Arguments for lack of support for private (for profit) banks from academic and scientific community, rest on the lack of clinical justifications for autologous banking, poor quality standards regarding collection and storage, no guarantees in case of bankruptcy and misleading advertising from CB companies.20 Recent studies from European countries suggest a strong preference for public CBB as opposed to private blood banking.16,21 Hybrid banks are also gaining popularity, where companies are collecting units for a fee for a private family use, and at the same time collect units for national use. This enables local health authorities to cover some of the costs of storing these public units.19
What are the Indications for Cord Blood Transplantation?
CBUs contain a large number of stem cells that could be used in transplanting many diseases including acute and chronic leukaemia, other malignancies such as lymphoma, hereditary blood disorders such as sickle cell disease (SCD), thalassaemia and many bone marrow failure syndromes as well as immune deficiency states. The reports of disease free survival in various conditions range from 40–80% in paediatric unrelated transplants and 20–57% in adults transplants with varying times of follow-up.12,22–24 According to the most recent data from multicentre registries, there are several factors that determine the outcome of transplants including the cell dose, age, gender, CMV status and stage of the disease at the time of the transplant.24–25 In the presence of in vitro expansion, there may be a role for CB in the treatment of degenerative diseases such as cardiac, neurological and rheumatic diseases in what is known as cellular regenerative therapy, an argument used by the advocates for private CBB groups.26
Umbilical Cord Blood Banking and Stem Cell Transplantation in Oman
Stem cell transplantation (bone marrow [BM], peripheral blood [PB] and CB), has been carried out at Sultan Qaboos University Hospital since 1995. Although the start was modest in terms of numbers, over the years more has been achieved, and results are now comparable with the best transplant centres worldwide. Currently, having done over 200 transplants of BM, PB, and CBT for leukaemia, myeloma, lymphoma, hereditary blood disorders and immune deficiency syndromes, the programme is gaining strength. Diversification was achieved in the programme by the use of BM, PB, and CB both in related and, more recently, unrelated donors, as well autologous transplantations. The programme aims to address the unique disease distributions seen in our country by catering for transplants in haemoglobin disorders such as thalassaemia and sickle cell disease, as well as immune deficiency syndromes, and malignant disorders such as leukaemia, lymphoma and myeloma. Furthermore, for successful stem cell transplantation, traditionally a full myeloablative conditioning therapy using chemotherapy, radiotherapy or both has been used. This helps to create a less hostile BM environment, eradicates any residual abnormal clone and suppresses the recipient’s immune response. However, more recently we have started reduced intensity conditioning transplantations (RIC) allowing us to include older patients with malignant conditions as well as more complicated patients with non-malignant disease such as SCD patients. RIC, particularly in SCD, has been used following the understanding of improved survival of healthy donor erythrocytes as compared to the recipient abnormal cells. Furthermore, as ineffective erythropoiesis lends a competitive advantage to donor erythroid progenitors, this creates a mixed donor/recipient chimera to co-habilitate together first. Later the donor clone eventually dominates and replaces the recipient’s original clone. RIC has also allowed many patients who could not tolerate a fully myeloablative therapy to have a stem cell transplant, allowing appropriate disease control with acceptable transplant related morbidity/mortality.27
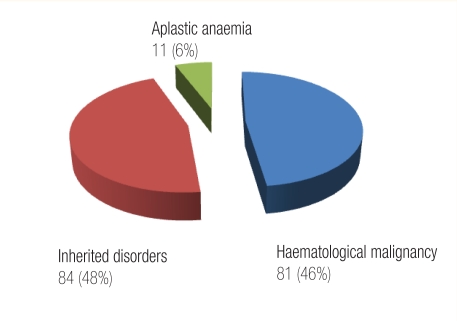
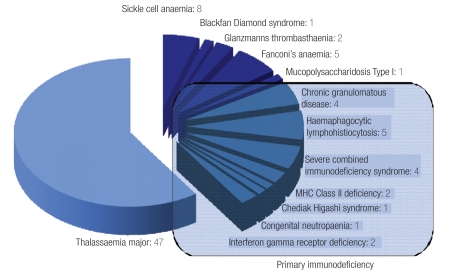
The spectrum of the conditions that we have treated in our centre is shown in Figure 1, whereas Figure 2 outlines in detail the transplantations for hereditary blood disorders and is reflective of pattern of diseases that are prevalent in Oman. Our CBB was established as the first national CBB in the country to cater for parents who have a child suffering from one of the disorders that are cured by BMT and who wanted their newborn baby’s CBU to be collected and processed for possible matched related transplant for their offspring. We have also created a voluntary CB banking system for those who want to donate their baby’s CB to our national CBB. Having obtained local ethical approval, our counsellors approach expectant mothers and their partners in the antenatal clinic for consent to collect cord blood. This is then collected by trained midwifes in the delivery ward, and undergoes red cell depletion at our stem cell processing laboratory. Samples are taken for HLA typing, serology for hepatitis and HIV as well as microbial cultures. Also the sample undergoes cell counts and, in related (sibling) cord samples, any count is acceptable; however, in voluntary cord blood samples, only units with total nucleated cells above 6 × 108/kg are cryopreserved, other units being discarded.28 So far we have collected cord blood from 81 siblings and >40 unrelated CB units. The number of the frozen CBUs is increasing and our aim is to establish a fully fledged CBB according to the international regulations of the Foundation for Accreditation on Cell Therapy (FACT). This foundation edits the NETCORD-FACT international standards for CB collection, processing, testing and banking selection.29
Figure 1:
Indications for bone marrow transplantation, Sultan Qaboos University Hospital, Oman, June 1995 to Oct 2010.
Figure 2:
Bone marrow transplantation for hereditary blood disorders at Sultan Qaboos University Hospital, Oman, June 1995 to Oct 2010.
Conclusion
Stem cells derived from CB, BM, and PB are contributing significantly to the curative treatment potential available for many acquired and congenital disorders in Oman and worldwide. National CBBs remain the preferred option, on economic, scientific and ethical grounds, until there are further advances in knowledge on the use of autologous CBT. Our strategy is to make stem cell therapy accessible to our patients, employing well established procedures for stem cell collection, storage, in vitro expansion and manipulation in order to achieve a successful national, culturally accepted CBB.
References
- 1.Brunstein CG, Setubal DC, Wagner JE. Expanding the role of umbilical cord blood transplantation. Br J Haematol. 2007;137:20–35. doi: 10.1111/j.1365-2141.2007.06521.x. [DOI] [PubMed] [Google Scholar]
- 2.Broxymeyer HE. Stem Cells (internet) Cambdrige (MA): Harvard Stem Cell Institute; Cord blood hematopoietic stem cell transplantation. Update 2008–2010, 26 May 2010. [PubMed] [Google Scholar]
- 3.Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: The paradigmatic tissue-specific stem cell. Am J Pathol. 2006;169:338–46. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006:1075–79. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia M, Bonnet D, Murdoch B, Gan OI, Dick JE. A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nat Med. 1998;4:1038–45. doi: 10.1038/2023. [DOI] [PubMed] [Google Scholar]
- 6.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multi-lineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 7.Kogler G, Calleias J, Hakenbern P, Enczmann J, Adams O, Daubener W, et al. Hematopoietic transplant potential of unrelated cord blood: Critical issues. J Hematother. 1996;5:105–16. doi: 10.1089/scd.1.1996.5.105. [DOI] [PubMed] [Google Scholar]
- 8.Eapen M. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukemia: A comparison study. Lancet. 2007;369:1947–54. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 9.Gluckman E, Rocha V. Donor selection for unrelated cord blood transplants. Curr Opin Immunol. 2006;18:565–70. doi: 10.1016/j.coi.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Campbell TB, Hangoc G, Liu Y, Pollok K, Broxmeyer HE. Inhibition of CD 26 in human cord blood CD34+ cells enhance their engraftment of non obese-diabetic/severe combined immunodeficiency mice. Stem cell Dev. 2007;16:347–54. doi: 10.1089/scd.2007.9995. [DOI] [PubMed] [Google Scholar]
- 11.Frassoni F, Gualandi F, Podestà M, Raiola AM, Ibatici A, Piaggio G, et al. Direct intrabone transplant of umbilical cord-blood cells in acute leukaemia phase I/II study. Lancet Oncol. 2008;9:831–9. doi: 10.1016/S1470-2045(08)70180-3. [DOI] [PubMed] [Google Scholar]
- 12.Locatelli F, Rocha V, Reed W, Bernauudin F, Ertem M, Grafakos S, et al. Related umbilical cord blood transplantation in patients with thalassaemia and sickle cell disease. Blood. 2003;101:2137–43. doi: 10.1182/blood-2002-07-2090. [DOI] [PubMed] [Google Scholar]
- 13.Rocha V, Lacatelli F. Searching for alternative hematopoietic stem cell donors for pediatric patients. Bone Marrow Transplantat. 2008;41:207–14. doi: 10.1038/sj.bmt.1705963. [DOI] [PubMed] [Google Scholar]
- 14.Ballen K. Challenges in umbilical cord blood stem cell banking for stem cell reviewers and reports. Stem Cell Rev. 2010;6:8–14. doi: 10.1007/s12015-009-9105-x. [DOI] [PubMed] [Google Scholar]
- 15.Broxmeyer HE. Umbilical cord transplantation epilogue. Semin Hematol. 2010;47:97–103. doi: 10.1053/j.seminhematol.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz G, Mills A, Garcia J, Hooper K, McGuckin C, Platz A, Rebulla P, et al. Banking cord blood stem cells: attitude and knowledge of pregnant women in five European countries. Transfusion. 2011;51:578–86. doi: 10.1111/j.1537-2995.2010.02954.x. [DOI] [PubMed] [Google Scholar]
- 17.Ballen K, Barker JN, Stewart SK, Greene MF, Lane TA. Collection and preservation of cord blood for personal use. Biol Blood Marrow Transplant. 2008;14:356–63. doi: 10.1016/j.bbmt.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Gratwohl A, Baldomero H, Frauendorfer K, Rocha V, Apperley J, Niederwieser D. The EMBT activity survey 2006 on haematopoietic stem cells transplantation: Focus on the use of cord blood products. Bone Marrow Transplant. 2008;41:687–705. doi: 10.1038/sj.bmt.1705956. [DOI] [PubMed] [Google Scholar]
- 19.Fisk NM, Atun R. Public-private partnerships in cord blood banking. BMJ. 2008;336:642–4. doi: 10.1136/bmj.39489.454699.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisk NM, Roberts IA, Markwald R, Mironov V. Can routine commercial cord blood banking be scientifically and ethically justified? PloS Med. 2005;2:e44. doi: 10.1371/journal.pmed.0020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edozien LC. NHS maternity units should not encourage commercial banking of umbilical cord blood. BMJ. 2006;333:801–4. doi: 10.1136/bmj.38950.628519.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtzberg J, Laughlin M, Graham ML, Smith C, Olson JF, Halperin EC, et al. Placental blood as a source of hematopoetic stem cells for transplantation into unrelated recipients. New Engl J Med. 1996;335:157–66. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 23.Laughlin MJ, Barker J, Bambach B, Koc ON, Rizzieri DA, Wagner JE, et al. Hematopeitic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med. 2001;344:1815–22. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 24.Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Charnplin RE, et al. Outcome after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–75. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 25.Cohen YC, Scaradayou A, Stevens CE, Rubinstein P, Gluckman E, Rocha V, et al. Factors affecting mortality following myeloablative cord blood transplantation in adults: A pooled analysis of three international registries. Bone Marrow Transplant. 2011;46:70–6. doi: 10.1038/bmt.2010.83. Epub 3 May 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arminan A, Gandia C, Garcia-Verduqo JM, Liedo E, Triqueros C, Ruiz-Sauri A, et al. Mesenchymal stem cells provide better results than hematopieetic processors for the treatment of myocardial infarction. J Am Coll Cardiol. 2010;55:2244–53. doi: 10.1016/j.jacc.2009.08.092. [DOI] [PubMed] [Google Scholar]
- 27.Diaconescu R, Flowers CR, Storer B, Sorror ML, Maris MB, Maloney DG, et al. Mortality and morbidity with nonmyeloablative compared to myeloablative conditioning before haematopoietic cell transplantations from HLA matched related donors. Blood. 2004;104:1550–8. doi: 10.1182/blood-2004-03-0804. [DOI] [PubMed] [Google Scholar]
- 28.Barker JN, Byan C, Scaradavou A. How I treat: The selection and acquisition of unrelated cord blood grafts. Blood. 2011;117:2332–9. doi: 10.1182/blood-2010-04-280966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wall DA. Regulatory issues in cord blood banking and transplantation. Best Pract Res Clin Haematol. 2010;23:171–7. doi: 10.1016/j.beha.2010.05.006. [DOI] [PubMed] [Google Scholar]