Abstract
Objectives:
During 2010, there was an increase in dengue virus infections in New Delhi, India compared to 2009. This study was conducted at Sant Parmanand Hospital during this outbreak to determine the utility of a ‘Dengue Package’, comprising simultaneous detection of dengue non-structural protein 1 (NS1), anti-dengue IgM, anti-dengue IgG and platelet enumeration for early diagnosis, better case management and faster public health response.
Methods:
Blood samples were tested for Dengue NS1, IgM and IgG using the single-step immunochromatigrahic One-step dengue NS1 Ag and IgG/IgM test, while platelets were enumerated with automatic analysers yielding results within 1–2 hours.
Results:
Of the 1,886 patients screened with the ‘Dengue Package’, 678 and 1208 were NS1-positive and -negative respectively, in different combinations. In 394 cases, NS1 was exclusively positive while 29 were also IgG positive. In 942 cases NS1, IgM and IgG were negative (triple negative). The platelet counts in the NS1 positives were lower than the NS1 negatives, mean and standard deviation (SD) 116.8±70.2 × 109/L: 95% confidence interval (CI) 66.6–74.1 and 167.2±94.0 × 109/L, P<0.0001. Platelet counts were <20 × 109/L in 20 NS1 antigen-positives and 42 NS1 antigen-negatives.
Conclusion:
During the 2010 outbreak, swift availability of the ‘Dengue Package’ assisted patient management, platelet transfusions, implementation of anti-vector measures and public health notifications. Testing for NS1 assisted the diagnosis of an additional 22.4% cases; of these 394 had evidence of primary infection and 29 of secondary infection. The ‘Dengue Package’ was useful in tackling the rise in suspected cases.
Keywords: Dengue virus, Viral nonstructural protein NS1, Neutralizing antibody, Viral antigens, Blood platelets, Platelet count
Advances in Knowledge
Concurrent assays for dengue virus non-structural protein 1 (NS1), anti-dengue IgM, anti-dengue IgG along with platelet enumeration in the ‘Dengue Package’ is immensely beneficial for patients, clinicians and public health officials.
Cases with a primary or secondary dengue virus infection in the initial febrile phase of illness will not escape detection with the ‘Dengue Package’.
Notifications to public health officials based on the results obtained by using the ‘Dengue Package’ would help in prompt initiation of anti-vector measures.
Application to patient care
The ‘Dengue Package’ produces an earlier differential diagnosis in suspected cases enabling appropriate intervention and prevention of any death from shock.
Patients with severe thrombocytopenia, when diagnosed as positive by use of the ‘Dengue Package’, can be offered platelet infusions immediately.
Clinicians can discontinue the empirical use of antibiotics in patients who test positive for dengue virus infection by use of the ‘Dengue Package’.
It should be possible to implement anti-vector measures around hospitalised patients found positive by use of the ‘Dengue Package’. Such measures will protect against any cross-infection on hospital premises.
During the late incubation period, or initial phase of dengue virus infection, laboratory disease confirmation is through viral isolation in cell culture and/or molecular investigations, or immunofluorescence, or immunohistochemistry.1 The dengue virus non-structural antigen non-structural protein 1 (NS1) that develops right at the beginning of the feverish period and before the appearance of dengue IgM and/or IgG is emerging as a suitable option for dengue diagnosis.2 Consequent to a multi-country evaluation of two commercially available NS1 enzyme-linked immunoabsorbent assay (ELISA) assays, a combination of NS1 and IgM detection in samples during the first few days of illness was recommended to increase overall dengue diagnostic sensitivity.3
Platelet therapy is a standard clinical practice for dengue patients with severe thrombocytopenia.4 However, during introductory screening, a platelet count is not done in many cases. This results in delays in staring platelet therapy. During the 2010 spurt in the incidence of dengue in New Delhi,5 simultaneous screening for NS1, IgM and IgG and platelet enumeration was launched at the Sant Parmanand Hospital, a 140-bed tertiary care, multi-disciplinary, private hospital in Delhi which caters to populations in the national capital and adjoining townships. The study was aimed to assess the utility of a concurrent identification of NS1, IgM, IgG, and platelet counting offered as the ‘Dengue Package’ for patients, clinicians and community.
Methods
During the period August to November 2010, samples from 1,886 suspected dengue patients were taken for testing by the combined ‘Dengue Package’ that included assays for NS1, IgM, IgG and automated platelet enumeration. Blood samples were collected by venipuncture in the hospital laboratory. They were drawn into ethylene diamine tetraacetic acid (EDTA) coated tubes and tested for dengue components employing the single-step immunochromatigrahic One-step dengue NS1 Ag and IgG/IgM test, Dengue Duo, in accordance with the manufacturer’s instructions (Standard Diagnostics, Inc., St. Ingbert, Germany). The platelet enumeration was in the 5- or 3-differential analyzers, Coulter®AC. T5diff AL (Beckman Coulter, Fullerton, CA, USA) or BC-3000Plus (Mindary, Shenzhen, China). The individual reports were authenticated by two technologists and were available within 1–2 hours. Apart from the in-built controls in the One-step dengue NS1 Ag and IgG/IgM test, Dengue Duo, no independent controls from NS1 antigen, IgM and IgG were used. Data were entered and analysed using the Statistical Package for Social Sciences (SPSS Inc, Chicago, IL, USA, Version 11.5). Non-parametric testing was carried out. P <0.05 was considered significant. The performance of this study was approved by the Director of our hospital.
Results
The patients tested by the ‘Dengue Package” were aged between 2 and 92 years, mean age 31.2 years: standard deviation (SI) 15.5 years, 95% confidence interval (CI) 2–62 years, 5th percentile 9 years and 95th percentile 62 years. In 678 patients, the NS1 antigen was detected with IgM and/or IgG while 1,208 were likewise NS1-negative. Among the 678 NS1-positive, 394 were exclusively positive for NS1, 145 were also positive for IgM, and 29 for IgG and 110 were positive for IgM and IgG (triple positive). The 1,208 NS1-negatives included 48 IgM-positives and 96 IgG-positives, while 122 were IgM-positive and IgG-positive and 942 were IgM and IgG negatives [Tables 1 and 2].
Table 1:
Platelet counts as 109/L in 678 cases screened NS1 positive by ‘Dengue package’ testing at Sant Parmanand Hospital Delhi during the 2010 dengue outbreak
| Category | Cases | Mean ± standard deviation | Standard error | Minimum platelet count | Maximum platelet count | Cases with counts <20 × 109/L |
|---|---|---|---|---|---|---|
| IgM negative IgG negative | 394 | 139.9±72.6 | 3.66 | 10 | 481 | 4 |
| IgM positive IgG negative | 145 | 100.0±52.9 | 4.39 | 11 | 2.74 | 1 |
| IgM negative IgG positive | 29 | 66.8±48.6 | 9.04 | 10 | 210 | 3 |
| IgM positive IgG positive | 110 | 69.6±45.1 | 4.3 | 10 | 206 | 12 |
Table 2:
Platelet counts as 109/L in 1208 cases screened NS1 negative by ‘Dengue package’ testing at Sant Parmanand Hospital during the 2010 dengue outbreak
| Category | Cases | Mean ± standard deviation | Standard error | Minimum Platelet count | Maximum Platelet count | Cases with counts <20 × 109/L |
|---|---|---|---|---|---|---|
| IgM negative IgG positive | 96 | 114.3±84.7 | 8.65 | 9 | 364 | 12 |
| IgM positive IgG negative | 48 | 136.5±63.5 | 9.71 | 13 | 302 | 3 |
| IgM positive IgG positive | 122 | 86.1±77.7 | 7.03 | 7 | 339 | 15 |
| IgM negative IgG negative | 942 | 184.7±90.2 | 29.4 | 5 | 693 | 12 |
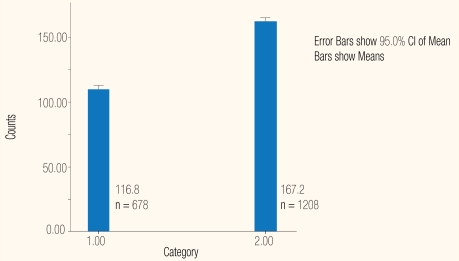
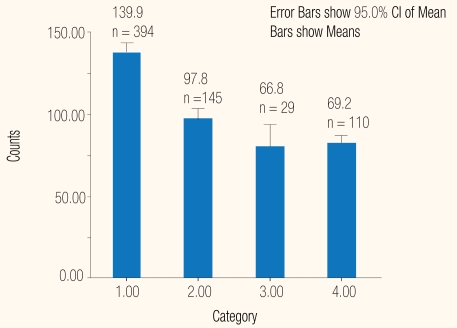
The platelet counts among the 678 NS1-positives were lower than the 1,208 NS1-negatives: the respective mean and SD were 116.8±70.2 × 109/L; 95% CI 66.6–74.1 and 167.2±94.0 × 109/L 95% CI 90.7–98.2 [Figure 1]; the Mann-Whitney, 2-tailed test gave P = 0.002. The average platelet counts were highest among 394 who were exclusively positive for NS1, 140.0±72.6 × 109/L. They were lowest among 29 who were co-positive for IgG, 66.8±48.6 × 109/l; the Mann-Whitney, 2-tailed test P < 0.0001 [Figures 2 and 3]. There was no significant difference in platelet counts in cases who were positive for all three markers, 69.2±40 × 109/L,or those positive for NS1 and IgG: and 66.8±48 × 109/L. The counts in 145 IgM co-positives, 100±52 × 109/L were higher than 110 triple-positives, 69.2±45 × 109/L; the Mann-Whitney, 2-tailed test gave P < 0.0001.
Figure 1:
Platelet counts as 109/L in NS 1-positives and N 1-negatives tested by the ‘Dengue Package’ at Sant Parmanand Hospital, Delhi.
Legend: 1 = NS 1 positive; 2 = NS 1 negative
Figure 2:
Platelet counts as 109/L in NS1-positive as tested by the ‘Dengue Package’ at Sant Parmanand Hospital, Delhi.
Legend: 1 = NS 1 positive; 2 = IgM positive; 3 = IgG positive; 4 = Triple positive.
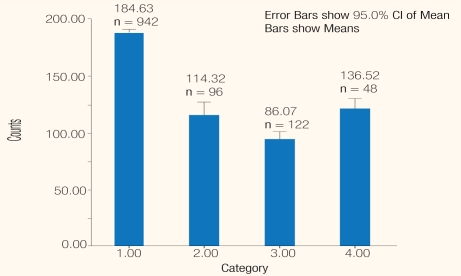
Figure 3:
Platelet counts as 109/L in NS1-negtives as tested by the ‘Dengue Package’ at Sant Parmanand Hospital, Delhi.
Legend: 1 = Triple negatives; 2 = IgG positives; 3 = IgG and IgM positive; 4 = IgM positive.
Among 1,208 NS1-negative cases, the average platelet counts were highest among 942 triple-negatives, mean 84.7±90.25 × 109/L and lowest among 122 positives for IgM and IgG, mean 86.1±77.7 × 109/L; the Mann-Whitney 2-tailed test gave P < 0.0001. The counts among 48 positive for IgM, 136.5±63.5 × 109/L were higher than for the 96 co-positives for IgG and IgM, 114.3±84.7 × 109/L; the Mann-Whitney 2-tailed test P = 0.028 [Figure 3].
Discussion
We initiated this feasibility study in August 2010 to ascertain the utility of concurrent testing of dengue virus NS1, IgM, IgG and platelet enumeration. The response was encouraging. Initial data on 175 suspected cases of dengue showed that just a single laboratory visit was sufficient both for serology and platelet enumeration;6 consequently, the ‘Dengue Package’ was chosen by clinicians for patients with febrile illness.
While working with the One step dengue NS1 Ag and IgG/IgM test, Dengue Duo, the in-built controls were used. For enumeration of platelets using different analysers, both internal quality control and external quality assessment were implemented. The performance of the BC-3000Plus (Mindary, Shenzhen, China) matched the results obtained on Coulter®Ac. TTm 5diff Autoloader (Beckman Coulter, Fullerton, CA, USA).7 The laboratory has participated in the Randox International Quality Assessment (RIQAS) programme for biochemistry for over three years;8 consequently, laboratory technologists are well trained in constant quality control.
The platelet counts among the 678 NS1-positives were lower than the 1,208 NS1-negatives: the respective mean and SD were 116.8±70.2 × 109/L, 95%, CI 66.6–74.1 and 167.2±94.0 × 109/L, 95% CI 90.7–98.2 [Figure 1]; Mann Whitney, 2-tailed test gave P = 0.002. On average, the counts in exclusive NS1-positive cases were the highest, and lowest in those who were also IgG positives: 139.8±3.6 X109/L and 66.8±9.04 × 109/L [Table 1], P < 0.0001. There was no significant difference in the counts in cases that were positive for all three markers and those positive for NS1 and IgG: 69.2±4 × 109/L and 66.8±9 × 109/L.
Among 1,208 NS1-negative cases, the average platelet counts were highest among those who were negative for IgG and IgM, mean 184.7±90.3 × 109/L and lowest among 122 positives for IgM and IgG, mean 86.1±77.7 × 109/L. Mann-Whitney, 2-tailed test gave P = 0.0001. The counts among 48 positives for IgM 136.5±63.5 × 109/L were higher than the 96 co-positives for IgG and IgM, 114.3±84.7 × 109/L; Mann-Whitney, 2-tailed, P = 0.0228 [Figure 3].
Among the 678 NS1-positive patients there were 394 who were negative for IgM [Table 1] and these would have otherwise been missed. They were suffering from a primary infection in the early phase of illness and were also viremic, i.e. they could transmit the virus if bitten by a mosquito. The 29 patients who were exclusively IgG positives, with a secondary viral infection, would have also been overlooked. Without NS1 screening they would have been labelled as “dengue negative”. They could have been infectious for mosquitoes during the earlier phase of illness. The concurrent NS1-positive and IgM-positive status of 145 patients [Table 1] reinforced the utility of antigen detection during the earlier phase of illness. Furthermore, 110 patients who were positive for NS1, IgM and IgG (triple-positives) [Table 1] were in the late stage of either a primary or a secondary infection and might have been infectious for mosquitoes. The NS1 test in the ‘Dengue Package’ helped in diagnosing an additional 423 (394 NS1 and 35 IgG positives) otherwise IgM negative cases. Those would have been labelled negative if the dengue IgM alone had been used. The NS1 negatives included 48 IgM positives [Table 2] who had a primary infection presenting a later phase of illness. They were most likely IgG negative. It is unlikely that dengue IgG antibody levels became undetectable in the convalescent phase of illness. Furthermore, there were 96 cases that were positive only for IgG [Table 2] who might have presented themselves for ‘Dengue Package’ fairly early during a secondary infection. A cell culture or reverse transcription polyermerase chain reaction (RT-PCR) would be required to exclude dengue virus replication. Furthermore, 122 cases were positive for IgM and IgG [Table 2]. Such patients with a primary or secondary infection had presented in a later stage of illness. Dengue viruses are generally extruded from the host when IgM antibodies are present.
Among the 1,208 cases who were NS1-negative there were 942 triple-negative cases. It would not be possible to rule out dengue infection in the negative cases without testing for viral replication in cell culture and/or molecular investigations or immunofluorescence, or immunohistochemistry;1 facilities for these are not available in our hospital. Such patients should be investigated for acute febrile illnesses including malaria, urinary tract infection, enteric fever, Chikungunya virus and the influenza virus H1N1 infection.
Thrombocytopenia is observable in several patients with dengue virus infection. Cases with severe thrombocytopenia, platelet counts ≤30 × 109/L (normal range = platelet counts of 150 to 400 × 109/L), would need hospitalisation and/or platelet infusions.4 With the ‘Dengue Package’, it was possible to recognise 19 cases, four NS1 and IgM positive and three IgG positives [Table 1], and 12 triple-negatives [Table 2]. They would have missed detection during any one-tier combined screening for NS1, IgG and IgM.
Platelet counts were generally higher in NS1-positives than the NS1-negatives [Figure 1]. The average platelet counts in triple-negatives were highest. The counts were lowest among those with a secondary infection who were positive for NS1 and IgG [Figure 2]. In both NS1 positive and negatives, they declined further upon production of IgM and IgG antibodies. There was a progressive decline in counts in cases presenting in later stages of illness. The platelet count in 12 NS1, IgM and IgG-negatives was <20 × 109/L. It would not be possible to rule out dengue infection in such cases without testing for viral replication in cell culture and/or molecular investigations or immunofluorescence, or immunohistochemistry.1 This would help to differentiate between patients with dengue-associated thrombocytopenia and those with severe bleeding episodes associated with trauma, invasive intensive care procedures or emergency surgery. Moreover, in patients with platelet count <20 × 109/L, investigation for decreased megakaryocytic production, splenic sequestration, non-immune or immune destruction of platelets would need to be carried out.
The wide variation in the platelet counts of the patients [Tables 1 and 2] is natural since the mechanism of dengue related thrombocytopenia and coagulopathy is complex. It would involve platelet activation, pro-coagulant and anticoagulant arms of the coagulation system, complement, cytokines, and endothelial cells.7 Moreover, symptomatic thrombocytopenia would require platelet transfusion though platelet counts might not correlate well with clinical bleeding.
Public health officials were not notified about the 394 NS1-positive cases that were viremic and infectious to mosquitoes. Notifications are based on clinical and laboratory categorisation of a probable or confirmed case. As a rule rather than exception, WHO guidelines are applicable in dengue-endemic countries, including India.8 Nevertheless, IgM and/or IgG positive patients were notified as probable cases. A mandate for NS1-based notification is awaited. That would be ideal for future comprehensive reporting of every probable case of dengue.
The ‘Dengue Package’ was beneficial during the outbreak since a single laboratory visit for serology and platelet enumeration was an asset for clinicians and patients. Based on results obtained with the ‘Dengue Package’, platelet transfusion could be started straightaway. Those who were exclusively NS1-positives could be offered appropriate supportive therapy, thus avoiding any irrational usage of antibiotics. The patients and their attendants could be briefed regarding the basics of vector biology and provided with mosquito nets during hospitalisation.11 Moreover, with concurrent NS1 testing in the ‘Dengue Package’, one could even distinguish between a secondary or past infection in a patient positive only for IgG antibody; the demonstrable NS1 will indicate a secondary infection.
There were limitations in the present study, conducted during an outbreak of dengue with a three-fold higher than normal workload. There was no gold standard obtainable for authentication. Apart from the in-built controls in the One-step Dengue NS1 Ag and IgG/IgM test, Dengue Duo, no independent third-party controls were employed during the outbreak. Facilities for molecular testing or cell culture were not available. Some of the NS1-negative, but IgG and IgM positive cases, might have evolved from a NS1-positive status to a NS1-negative stage when examined by the ‘Dengue Package’. Alternatively, there might have been other patients who had previously suffered from dengue virus infection. Unfortunately, none of them was available later to evaluate any seroconversion. Furthermore, precise details about the duration of fever and allied clinical presentations were not made available prior to the individual’s ‘Dengue Package’ testing in the hospital.
The ‘Dengue Package’ has substantiated the earlier utility of enhanced diagnostic sensitivity of the combined antigen-antibody testing protocols. To our knowledge, there are no reports that contradict the simultaneous use of the dengue NS1 antigen and antibody testing for disease diagnosis.
A combined antibody and NS1 diagnostic protocol was reported during the 2007 dengue outbreak in Puerto Rico. Employing a 90% plaque-reduction neutralisation test with dengue virus IgG depletion and NS1 antigen ELISA, it was possible to diagnose 85% of the 43 samples which could not be diagnosed using the standard diagnostic methods.12
During the multi-country evaluation of two commercially available NS1 ELISA assays, a combination of NS1 and IgM detection in samples during the first few days was recommended to increase the overall dengue diagnostic sensitivity.3 Evaluation of the Dengue Duo rapid test kit for detection of the NS1 antigen, IgM/IgG antibody at the University of Malaysia on 320 dengue acute or convalescent sera in comparison with an in-house IgM capture ELISA, haemagglutination-inhibition test, or to real-time reverse transcription-polymerase chain reaction (RT-PCR) was more affirmative. The rapid test kit was highly sensitive (88.65%) and highly specific (98.7%). NS1 and IgM detection by a rapid combined kit gave detection rates which were comparable to testing either by serology or RT-PCR.13
The circulation of dengue NS1, IgM and IgG antibodies was examined by using an in-house dengue type 1 (DENV1)-specific NS1 capture ELISA and the commercial Panbio Dengue IgM and IgG capture ELISA in Guangzhou in China. In a panel of 313 acute and early convalescent-phase sera from 140 DENV1 primary infected patients, NS1 detection was established in 81.8–91.1% samples during the first 7 days. The anti-dengue IgM antibody was detectable on the third day of onset with the positive rate of 42.9%, and rapidly increasing to 100% by the 8th day of illness. The anti-dengue IgG antibody was detectable on the fifth day of onset, with a low level in the first week of onset, and slowly increasing to 100% by the 15th day of illness. Combining the results of NS1 and IgM antibody detection allowed positive diagnosis in 96.9–100% of samples taken after day 3 of onset. A combination of dengue NS1 antigen and IgM antibody testing enhanced the rates of the disease diagnosis.14
The performance of the Standard Diagnostics (SD, South Korea) dengue virus SD dengue NS1 Ag ELISA for dengue diagnosis on 320 dengue sera in Malaysia was compared with parallel real-time PCR, IgM capture ELISA and a haemagglutination-inhibition assay. The NS1 antigen detection had a sensitivity and specificity of 76.76% and 98.31%, respectively. The assay was able to detect NS1 antigen in the convalescent phase sera till the 14th day of illness. A combined assay with IgM and/or IgG was speculated to increase the NS1 sensitivity of detection still further.15
Conclusion
Utilisation of the ‘Dengue Package’ can be important in the diagnosis of dengue virus infection in health care centres lacking sophisticated laboratory facilities as it means that one laboratory session would rule out dengue virus infection and also identify cases with severe thrombocytopenia who might have otherwise escaped detection. The ‘Dengue Package’ would enable physicians to offer rational therapy to their patients. They could ask appropriate government agencies to initiate vector control measures including active disease surveillance. Last but not least, the one-stage ‘Dengue Package’ should emerge as the ideal option for the management of patients with dengue since it would be economical during dengue outbreaks even at secondary or tertiary care health centres. Dengue outbreaks overpower outpatient and inpatient facilities in hospitals and both medical and health care personnel are rapidly exhausted.
Acknowledgments
The technical assistance of Mr. John D Kutty, Ms CG Sherin, Mr. Hawa Singh and Mr. Jaldeep Singh is acknowledged.
Footnotes
CONFLICT OF INTEREST
The authors reported no conflict of interest.
References
- 1.Centers for Disease Prevention and Control. Dengue: Laboratory criteria for diagnosis for case definitions. From http://www.cdc.gov/dengue/clinicalLab/caseDef.html Accessed Sep 10.
- 2.Thomas L, Najioullah F, Verlaeten O, Martial J, Brichler S, Kaidomar S, et al. Relationship between nonstructural protein 1 detection and plasma virus load in Dengue patients. Am J Trop Med Hyg. 2010;83:696–9. doi: 10.4269/ajtmh.2010.10-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzman MG, Jaenisch T, Gaczkowski R. Multi-country evaluation of the sensitivity and specificity of two commercially-available NS1 ELISA assays for dengue diagnosis. PLoS Negl Trop Dis. 2010;4:e811. doi: 10.1371/journal.pntd.0000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas L, Kaidomar S, Kerob-Bauchet B, Moravie V, Brouste Y, King JP, et al. Prospective observational study of low thresholds for platelet transfusion in adult dengue patients. Transfusion. 2009;49:1400–11. doi: 10.1111/j.1537-2995.2009.02132.x. Epub 20 Mar 2009. [DOI] [PubMed] [Google Scholar]
- 5.BBC News South Asia. Disease threat at Delhi Commonwealth Games site. From: http://www.bbc.co.uk/news/world-south-asia-11241218. Accessed: Sep 2010.
- 6.Arya SC, Agarwal N, Parikh SC. Usefulness of detection of dengue NS1 antigen alongside IgM plus IgG, and concurrent platelet enumeration during an outbreak. Trans R Soc Trop Med Hyg. 2011;105:384–5. doi: 10.1016/j.trstmh.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Arya SC, Agarwal N. Evaluation of automated blood count analyzers for utility in resource poor laboratories. Clin Chim Acta. 2009;401:187. doi: 10.1016/j.cca.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Arya SC, Agarwal N, Agarwal S, Michael B. External quality assessment of clinical biochemical assays in a medium non-academic, non-research hospital laboratory. Saudi M J. 2011;32:87–8. [PubMed] [Google Scholar]
- 9.Schexneider KI, Reedy EA. Thrombocytopenia in dengue fever. Curr Hematol Rep. 2005;4:145–8. [PubMed] [Google Scholar]
- 10.World Health Organization. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. 2nd ed. Geneva: World Health Organization; 1997. [Google Scholar]
- 11.Arya SC, Rajagopal S, Agarwal N, Kaushik M, Maheshwari P, Agarwal S, et al. Private sector hospital response to 2003 dengue outbreak in Indian capital metropolis. Am J Infect Control. 2004;32:489–92. doi: 10.1016/j.ajic.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Hunsperger E, Beltran M, Acosta LN, et al. Serological evaluation of suspected West Nile virus human cases following its introduction during a dengue outbreak in Puerto Rico in 2007. Clin Vaccine Immunol. 2011;18:978–83. doi: 10.1128/CVI.00040-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang SM, Sekaran SD. Early diagnosis of Dengue infection using a commercial Dengue Duo rapid test kit for the detection of NS1, IgM, and IgG. Am J Trop Med Hyg. 2010;83:690–5. doi: 10.4269/ajtmh.2010.10-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu D, Di B, Ding X, Wang Y, Chen Y, Pan Y, et al. Kinetics of non-structural protein 1, IgM and IgG antibodies in dengue type 1 primary infection. Virol J. 2011;8:47. doi: 10.1186/1743-422X-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang SM, Sekaran SD. Evaluation of a commercial SD dengue virus NS1 antigen capture enzyme-linked immunosorbent assay kit for early diagnosis of dengue virus infection. J Clin Microbiol. 2010;48:2793–7. doi: 10.1128/JCM.02142-09. Epub 23 Jun 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]