Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a common disease characterized by airflow obstruction and lung hyperinflation leading to dyspnea and exercise capacity limitation.
Objectives
The present study was designed to evaluate whether an extra-fine combination of beclomethasone and formoterol (BDP/F) was effective in reducing air trapping in COPD patients with hyperinflation. Fluticasone salmeterol (FP/S) combination treatment was the active control.
Methods
COPD patients with forced expiratory volume in one second <65% and plethysmographic functional residual capacity ≥120% of predicted were randomized to a doubleblind, double-dummy, 12-week, parallel group, treatment with either BDP/F 400/24 μg/day or FP/S 500/100 μg/day. Lung volumes were measured with full body plethysmography, and dyspnea was measured with transition dyspnea index.
Results
Eighteen patients were evaluable for intention to treat. A significant reduction in air trapping and clinically meaningful improvement in transition dyspnea index total score was detected in the BDP/F group but not in the FP/S group. Functional residual capacity, residual volume (RV) and total lung capacity significantly improved from baseline in the BDP/F group only. With regard to group comparison, a significantly greater reduction in RV was observed with BDP/F versus FP/S.
Conclusion
BDP/F extra-fine combination is effective in reducing air trapping and dyspnea in COPD patients with lung hyperinflation.
Keywords: small airways, chronic obstructive pulmonary disease, airflow obstruction
Introduction
Chronic obstructive pulmonary disease (COPD) is a common disease with airflow limitation due to both small airways obstruction and parenchimal damage.1 Although the measure of airflow limitation is a key point for the diagnosis and staging of COPD, static lung volumes have a better correlation with individually perceived symptoms and exercise capacity.2,3 Moreover, parameters of hyperinflation show better correlation with patient-centered health outcomes than does forced expiratory volume in one second (FEV1).2 Indeed, the presence of hyperinflation in COPD, at rest or during exercise, can cause worsening of respiratory muscle function and gas exchange, and/or increase in work of breathing.4 Nonetheless, the degree of lung hyperinflation remains often undetected in the absence of detailed physiologic analysis.4 Daily physical activity of COPD patients is mainly associated with dynamic hyperinflation, regardless of severity classification5 and relief of exertional dyspnea following pharmacologic therapy shows good correlation with a reduction in dynamic hyperinflation.6 Hence, targeting hyperinflation can make a difference to the patient.
In COPD patients, an inhaled glucocorticosteroid (ICS) combined with a long-acting β2-agonist (LABA) has an important role in clinical exacerbations of disease,7 improving lung function and health status.1 This combination therapy is of particular relevance in patients with frequent exacerbations associated with a rapid decline in lung function and increased mortality.8,9
The fixed combination of beclomethasone (BDP) 100 μg plus formoterol (F) 6 μg in a solution formulation is now available in a hydrofluoralkane pressurized metered-dose inhaler (pMDI). This formulation delivers the two drugs with an extra-fine particle size, which results in high lung deposition with the potential to target inflammation and bronchoconstriction, throughout the entire bronchial tree.10
The aim of this study was to assess the role of beclomethasone and formoterol (BDP/F) extra-fine versus fluticasone/ salmeterol (FP/S) combination on lung function parameters and dyspnea in COPD patients with hyperinflation.
Methods
This trial was conducted in two Italian units of respiratory medicine (Parma and Pavullo n/F-Modena).
Patients
Patients were included if aged 40–70 years, current or past smokers (>20 pack years) with a confirmed diagnosis of COPD according to GOLD (Global initiative for chronic Obstructive Lung Disease) guidelines,1 a post-bronchodilator FEV1 <65%, plethysmographic functional residual capacity (FRC) ≥120% of the predicted normal values, and an increase from baseline in post-bronchodilator FEV1 value of at least 5% (but less than 12%). Exclusion criteria included: asthma or positive response to the reversibility test (FEV1 increase ≥12% and 200 mL after 400 μg salbutamol); COPD exacerbations and/or symptomatic infection of the airways in the previous 4 weeks requiring antibiotic therapy and or oral corticosteroid; history of clinically severe cardiovascular diseases, diabetes mellitus, and impaired hepatic and/or renal function; use of long-term oxygen therapy and patients undergoing a rehabilitation program. Moreover, patients treated with a higher daily dose of LABAs or ICS/LABA combination than that used in the study were excluded.
The trial was approved by the Ethics Review Board at each institution, and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. All patients gave written informed consent.
Study design
This was a double-blind, double dummy, randomized, parallel group study. During a 2-week run-in period, all previous medications with the exception of short-acting salbutamol for symptom relief were withdrawn and COPD patients were randomly assigned to a 12-week double-blind treatment period with either BDP/F 100/6 μg two inhalations twice daily or FP/S 250/50 μg one inhalation twice daily. No other bronchodilator, inhaled or systemic corticosteroid, or different combinations of these were permitted during the study.
To ensure the double-blind design, each patient was given a pMDI and a Diskus® (GlaxoSmithKline, London, UK) dry-powder inhaler (DPI), with either active drug or placebo (double dummy), in accordance with the randomization list. Inhalers containing drug or placebo were identical in shape and color. Randomization was in balanced-block design using a computerized list.
Morning dose started with two inhalations from the pMDI followed by one inhalation from the Diskus DPI, with a 30-second interval between inhalations; the same procedure was followed for the evening dose. Administration was performed according to the inhaler instruction leaflets provided by the sponsor. A total of five clinic visits were performed at screening, randomization, and after 4, 8, and 12 weeks of treatment. At each visit, the morning dose was administered onsite at the hospital clinics under the investigator’s supervision to ensure that the inhalation technique used by the patients was correct.
Measurements
Whole-body plethysmography was measured in accordance with a standard procedure11 at each visit, at least 12 hours following the previous evening dose, 8 hours after the last administration of short-acting bronchodilators, before study drug intake (pre-dose) and 30 minutes after drug administration (post-dose). Diffusion lung capacity for carbon monoxide was measured at each visit before drug administration. At the second visit, baseline dyspnea index (BDI) was recorded prior to the first administration of study medication to assess chronic activity-related dyspnea, whereas change in dyspnea over time was evaluated by the transition dyspnea index (TDI) prior to dosing at subsequent visits.12 To measure the endurance time (ET), in seconds, an incremental cardiopulmonary exercise test was also performed with cycle ergometer at the second visit, and constant-load tests were performed at 75% of the maximal work rate, as previously described,13 from the second visit onwards either pre-dose or 1 hour post dosing.
Statistics
Data are expressed as mean ± standard error of the mean (SEM), unless otherwise specified. Due to the explorative nature of the study and the lack of data with BDP/F in COPD, no formal sample size calculation was performed. The analysis of covariance, with treatment and center as factors and baseline value as a linear covariate, was applied. The values measured in the randomization visit before study drug intake (pre-dose) were considered for baseline values. The adjusted means for the two groups at each visit and the adjusted treatment difference between groups at each visit were recorded. All analyses were performed with SAS® System (SAS Institute Inc, Cary, NC), version 9.1.3, Service Pack 4. Statistical significance was set at 0.05 two tailed, and all analyses were performed on the intention-to-treat population (ITT). Imputation of missing data was completed following last observation carried forward method for post-baseline data.
Results
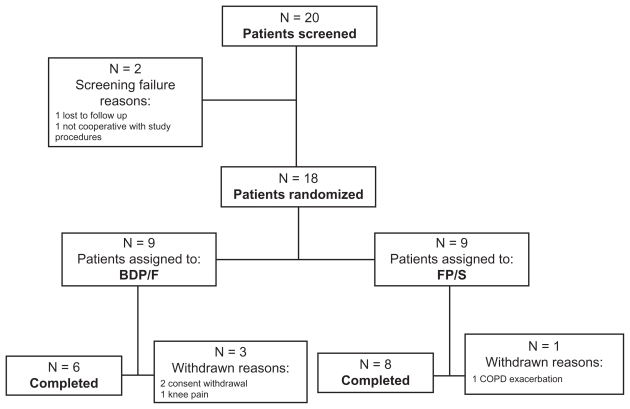
Twenty patients entered the run-in period between 2006 and 2009, eighteen (15 males, age range 57–72 years, body mass index range 22.7–30.9 kg/m2) were randomized, and 14 completed the study (Figure 1). During the run-in period, one patient was lost to follow-up and one was not compliant to study protocol and was not randomized. All randomized patients were treated and evaluated for ITT. Patient compliance to the scheduled doses of study drugs was 95% in both groups. Baseline lung function data of BDP/F and FP/S patient groups are presented in Table 1. Median (range) Medical Research Council and BDI scores were 2 (2–5) and 2.5 (2–5), and 8 (3–9) and 7 (3–9), in the BDP/F and FP/S groups, respectively. Groups were well balanced at baseline, and no difference between groups was detected in clinical functional and anthropometric parameters.
Figure 1.
A flowchart representing patient flow.
Abbreviations: BDP/F, beclomethasone dipropionate/formoterol; FP/S, fluticasone propionate/salmeterol.
Table 1.
Baseline lung function data of the patients
| Parameter | BDP/F (n = 9) | FP/S (n = 9) |
|---|---|---|
| FEV1, L | 1.27 ± 0.16 | 1.07 ± 0.11 |
| FEV1, % predicted | 45.1 ± 5.0 | 40.0 ± 3.0 |
| FVC, L | 2.80 ± 0.31 | 2.42 ± 0.27 |
| FVC, % predicted | 77 ± 7 | 72 ± 6 |
| FEV1/FVC, % | 45 ± 3 | 45 ± 3 |
| FRC, L | 5.90 ± 0.75 | 5.84 ± 0.38 |
| FRC, % predicted | 173.0 ± 21.0 | 186.3 ± 10.0 |
| RV, L | 4.73 ± 0.76 | 4.82 ± 0.28 |
| RV, % predicted | 201.4 ± 34.0 | 224.5 ± 13.0 |
| TLC, L | 7.90 ± 0.79 | 7.59 ± 0.49 |
| TLC, % predicted | 125.1 ± 10.0 | 131.4 ± 7.0 |
| RV/TLC, % | 58.22 ± 4.69 | 64.00 ± 2.29 |
| FRC/TLC, % | 73.65 ± 2.68 | 76.92 ± 1.33 |
| DLCO, mL/mmHg/min | 11.80 ± 3.06 | 11.66 ± 1.64 |
Notes: Data are mean ± standard error of the mean, measured in the randomization visit before study drug intake (pre-dose). No significant difference between groups was found in any parameter.
Abbreviations: BDP/F, beclomethasone dipropionate/formoterol; FP/S, fluticasone propionate/salmeterol; BMI, body mass index; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; FRC, functional residual capacity; RV, residual volume; TLC, total lung capacity; DLCO, diffusion lung capacity for carbon monoxide; DLCO/VA, diffusion lung capacity for carbon monoxide corrected for alveolar volume.
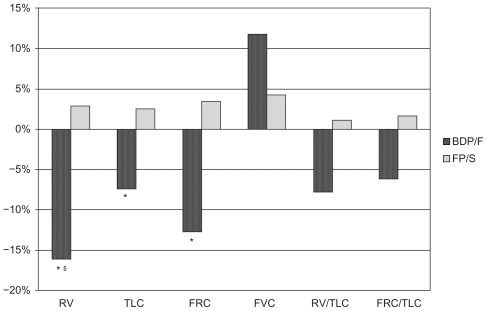
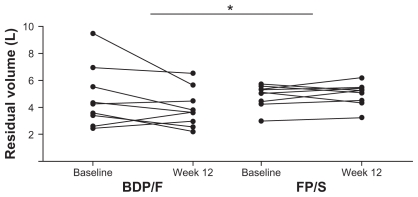
All parameters related to air trapping showed an improvement in pre-dose measurements, although not statistically significant, with BDP/F treatment but not with FP/S (Table 2). In post-dose measurements FRC, residual volume (RV) and total lung capacity (TLC) significantly improved from baseline to week 12 in the BDP/F group only (Table 3 and Figure 2). Similarly, a trend towards improvement in forced vital capacity (FVC) (P = 0.064), FRC/TLC (P = 0.059), and RV/TLC (P = 0.062) was detected in the BDP/F group but not in the FP/S group (Table 3 and Figure 2). With regard to group comparison, a significantly greater reduction in RV was observed with BDP/F versus FP/S (Table 3 and Figures 2 and 3).
Table 2.
Body plethysmography lung volumes (pre-dose)
| Parameter | BDP/F (n = 9) | FP/S (n = 9) | Difference | P-value |
|---|---|---|---|---|
| IRV, L | 0.10 ± 0.17 | −0.20 ± 0.24 | 0.31 | 0.338 |
| ERV, L | −0.08 ± 0.13 | 0.01 ± 0.13 | −0.09 | 0.646 |
| RV, L | −0.28 ± 0.23 | 0.19 ± 0.23 | −0.47 | 0.189 |
| TLC, L | −0.29 ± 0.21 | 0.10 ± 0.21 | −0.38 | 0.231 |
| FRC, L | −0.31 ± 0.25 | 0.23 ± 0.25 | −0.54 | 0.156 |
| FVC, L | 0.22 ± 0.20 | 0.02 ± 0.20 | 0.23 | 0.429 |
| IVC, L | 0.01 ± 0.17 | −0.10 ± 0.17 | 0.11 | 0.679 |
| RV/TLC, % | −0.38 ± 2.17 | 1.83 ± 2.17 | −2.21 | 0.495 |
| FRC/TLC, % | −1.62 ± 1.73 | 2.49 ± 1.73 | −4.12 | 0.127 |
Note: Data are adjusted mean ± standard error of the mean; changes from baseline to the last visit before study drug intake (pre-dose).
Abbreviations: BDP/F, beclomethasone dipropionate/formoterol; FP/S, fluticasone propionate/salmeterol; IRV, inspiratory reserve volume; ERV, expiratory reserve volume; RV, residual volume; TLC, total lung capacity; FRC, functional residual capacity; FVC, forced vital capacity; IVC, inspiratory vital capacity.
Table 3.
Body plethysmography lung volumes (post-dose)
| Parameter | BDP/F (n = 9) | FP/S (n = 9) | Difference | P-value |
|---|---|---|---|---|
| IRV, L | 0.42 ± 0.26 | −0.01 ± 0.36 | 0.43 | 0.372 |
| ERV, L | −0.05 ± 0.10 | −0.02 ± 0.10 | −0.03 | 0.862 |
| RV, L | −0.77 ± 0.29* | 0.14 ± 0.29 | −0.91 | 0.049 |
| TLC, L | −0.57 ± 0.26* | 0.20 ± 0.26 | −0.78 | 0.063 |
| FRC, L | −0.75 ± 0.32* | 0.23 ± 0.32 | −0.95 | 0.062 |
| FVC, L | 0.31 ± 0.15 | 0.11 ± 0.15 | 0.20 | 0.395 |
| IVC, L | 0.20 ± 0.13 | 0.06 ± 0.13 | 0.14 | 0.479 |
| RV/TLC, % | −4.76 ± 2.35 | 0.65 ± 2.35 | −5.40 | 0.137 |
| FRC/TLC, % | −4.62 ± 2.25 | 1.28 ± 2.25 | −5.90 | 0.096 |
Notes: Data are adjusted mean ± standard error of the mean; changes from baseline to the last visit after study drug intake (post-dose).
P < 0.05 versus baseline.
Abbreviations: BDP/F, beclomethasone dipropionate/formoterol; FP/S, fluticasone propionate/salmeterol; IRV, inspiratory reserve volume; ERV, expiratory reserve volume; RV, residual volume; TLC, total lung capacity; FRC, functional residual capacity; FVC, forced vital capacity; IVC, inspiratory vital capacity.
Figure 2.
Percentage changes from baseline in lung function parameters measured after drug intake (post-dose) in the last visit.
Notes: *Denotes P < 0.05 versus baseline; §denotes P < 0.05 versus FP/S.
Abbreviations: FRC, functional residual capacity; TLC, total lung capacity; FVC, forced vital capacity; RV, residual volume; BDP/F, beclomethasone dipropionate/formoterol; FP/S, fluticasone propionate/salmeterol.
Figure 3.
Individual patient changes from baseline to study end (post-dose) in residual volume.
Note: *Denotes P < 0.05 between groups.
Abbreviations: BDP/F, beclomethasone dipropionate/formoterol; FP/S, fluticasone propionate/salmeterol.
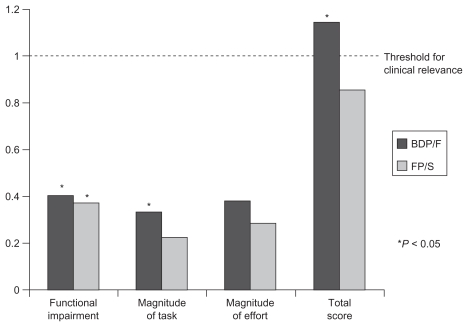
A significant improvement in functional impairment TDI mean (SEM) score from baseline of 0.40 (0.17) in the BDP/F group (P = 0.033) and of 0.37 (0.17) in the FP/S group (P = 0.046) was detected. A significant improvement in magnitude of task of 0.33 (0.13) in the BDP/F group (P = 0.024) was shown, whereas the change in the FP/S group was of 0.22 (0.13) and was not significant (P = 0.110). No significant change was detected in magnitude of effort, which showed a change from baseline of 0.38 (0.22) in the BDP/F group (P = 0.122) and of 0.29 (0.22) in the FP/S group (P = 0.222). The TDI total score showed a significant and clinically relevant improvement from baseline of 1.144 (0.46) in the BDP/F group (P = 0.026) but not in the FP/S group, for which the change was 0.86 (0.46) (P = 0.083) (Figure 4).
Figure 4.
Transition dyspnea index score.
Note: *Denotes P < 0.05 versus baseline.
Abbreviations: BDP/F, beclomethasone dipropionate/formoterol; FP/S, fluticasone propionate/salmeterol.
The mean ± SEM adjusted values of pre-dose and post-dose ET at end of treatment were not statistically different in the BDP/F group (477.2 ± 71.8 seconds and 513.6 ± 79.2 seconds), compared with the FP/S group (398.9 ± 71.8 seconds and 475.6 ± 79.2 seconds).
Six adverse events were reported in five patients (three in the FP/S group and two in the BDP/F group). All these adverse events were considered not related to the study drugs and mild in severity apart from a COPD exacerbation in the FP/S group that was considered moderate and led to study discontinuation. Vital signs (blood pressure and heart rate) did not show clinically relevant changes over time in either group.
Discussion
This is the first study comparing the effects of two different ICS/LABA combinations on lung hyperinflation and dyspnea in COPD. The two groups were well balanced in baseline characteristics including smoking habits, diffusion lung capacity, spirometry, and lung volumes. The FP/S combination was selected as active comparator instead of placebo for ethical reasons and to provide clinically relevant information as this is one of the most used drugs for COPD patients in clinical practice.
The main findings of this study are that BDP/F improves lung function parameters related to air trapping and dyspnea in COPD patients. Body plethysmography measurements showed significantly improved FRC, TLC, and RV in BDP/F-treated patients, with a significantly greater reduction in RV compared with FP/S. These findings on reduction of RV and FRC are important determinants of improvement of dyspnea in COPD.14 Since this is the first data provided with BDP/F combination on lung hyperinflation, we can assume that the extra-fine formulation of BDP/F is likely to be one of the reasons for this effect, as the small particles delivered easily reach the small airways, with a uniform anti-inflammatory and bronchodilating effect. Indeed, peripheral airways obstruction is known to cause progressive “air trapping” during expiration, worsening of perceived dyspnea, and limitation of exercise capacity in COPD patients.1
The greater efficacy of BDP/F compared with FP/S in reducing air trapping as observed in this study is in agreement with previous data. In asthmatics treated with the same daily doses of the same combinations, Papi and colleagues, in a randomized controlled trial, showed a significant increase in FVC with BDP/F when compared with FP/S.15 Moreover, Scichilone et al showed a reduction in closing capacity consistent with a reduction in air trapping with BDP/F but not with FP/S.16 A recently published large randomized controlled study showed a comparable efficacy of BDP/F extra-fine combination and budesonide/formoterol (BUD/F) combination in COPD patients.17 BDP/F treatment for 48 weeks improved pulmonary function and reduced symptoms compared with formoterol, improved the walking distance measured with the 6-minute walking test, improved quality of life, and was safe and well tolerated. Moreover, no significant difference was detected in any outcome between BDP/F extra-fine combination and BUD/F combination. It is noteworthy that a significant improvement in FVC was detected in COPD patients treated with BDP/F and not in those treated with BUD/F or F alone.17
As regards other lung function parameters measured in the present study, a trend towards improvement was shown in the BDP/F group in FVC, FRC/TLC, and RV/TLC, and a trend for difference between groups was detected in FRC and TLC favoring BDP/F. Nonetheless, the number of patients was not enough to draw definitive conclusions on the different effects of the two investigated combinations on lung hyperinflation. Indeed, the inclusion criteria of high FRC, as a sign of resting hyperinflation, limited the potential for recruitment.
Interestingly, the present study demonstrates that BDP/F reduced TDI-dyspnea in terms of functional impairment, magnitude of task, and total score, whereas FP/S improved only functional impairment. Moreover, the increase in TDI total score with BDP/F was greater than 1 unit, a change that is considered clinically relevant.18
The results of this study were achieved with a lower daily dose of corticosteroid in BDP/F extra-fine combination (400 μg) compared with FP/S (500 μg), due to the extra-fine formulation resulting in a greater efficacy per microgram of steroid, in agreement with previous studies.16,19,20 The use of a combination with a low corticosteroid dose can be of particular relevance in COPD patients as side effects of ICS are dose-dependent, with an increased risk of pneumonia recently highlighted in a study with high-dose FP/S.21
It could be argued that the greater efficacy of BDP/F observed in the present study could be related to the characteristics of F and S in terms of onset of action. Actually, the improvements in air trapping related parameters were more evident in post-dose measurements, even if the trend for a greater effect with BDP/F than with FP/S is numerically evident even in pre-dose measurements. Therefore, a contribution of the bronchodilator component of each combination to the effect on lung function is likely, and we cannot exclude that this contributes to the between-group differences detected. Anyway, it has been shown that both F and S reach a maximum increase in FEV1 and IC in COPD patients 30 minutes after dosing, with no further improvement at 60 and 120 minutes thereafter,22 thus making it unlikely that the major reason for the differences is the onset of action of the two LABAs. Nevertheless, it has been shown in human small airways that formoterol has a higher intrinsic efficacy in reversing the carbachol-induced contraction as compared with salmeterol.23 This could support the explanation of a greater efficacy due to pharmacological activity on small airways of the extra-fine BDP/F combination. It is noteworthy that the differences in favor of BDP/F in dyspnea improvements were detected in predose evaluation of dyspnea with the TDI score, thus suggesting that the difference between groups was evident even when the effect of the LABA was not present. We do not have all the data to conclude that the greater effect on air trapping of the BDP/F combination is due to formoterol, to BDP, or to the extra-fine formulation reaching the small airways.
In conclusion, this study shows the efficacy on lung air trapping and perceived dyspnea of extra-fine BDP/F combination – when compared with FP/S – in COPD patients. An improvement on patients’ daily performance with this combination drug could therefore be assumed. Larger studies will confirm the precise role of BDP/F and its potential advantages as compared with the other available treatments in the long-term management of COPD patients.
Footnotes
Note
This study was registered in the Italian Registry for Clinical Trials.24 The registration number is: 2005-005857-23.
Disclosure
The study was sponsored by Chiesi Farmaceutici SpA, Parma, Italy. PT and MA have no conflict of interest to declare. EC has undertaken research funded by Boehringer-Ingelheim, Chiesi Farmaceutici, Novartis, and Nycomed. GN is an employee of Chiesi Farmaceutici. AC and DO have undertaken research funded by Boehringer-Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Novartis, and Nycomed. EMC has undertaken research funded by Boehringer-Ingelheim, Italy, Medical Research Product, and Medinet.
References
- 1.GOLD Report Executive Summary. Global Strategy for Diagnosis, Management, and Prevention of COPD. GOLD – Global Initiative for Chronic Obstructive Lung Disease home page. Dec, 2010. http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html.
- 2.O’Donnell DE, Lam MIU, Webb KA. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:542–549. doi: 10.1164/ajrccm.160.2.9901038. [DOI] [PubMed] [Google Scholar]
- 3.O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:770–777. doi: 10.1164/ajrccm.164.5.2012122. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson GT. Why does the lung hyperinflate? Proc Am Thorac Soc. 2006;3(2):176–179. doi: 10.1513/pats.200508-094DO. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Rio F, Lores V, Mediano O, et al. Daily physical activity in patients with chronic obstructive pulmonary disease is mainly associated with dynamic hyperinflation. Am J Respir Crit Care Med. 2009;180(6):506–512. doi: 10.1164/rccm.200812-1873OC. [DOI] [PubMed] [Google Scholar]
- 6.Belman MJ, Botnick WC, Shin JW. Inhaled bronchodilators reduce dynamic hyperinflation during exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;153:967–975. doi: 10.1164/ajrccm.153.3.8630581. [DOI] [PubMed] [Google Scholar]
- 7.Wedzicha JA, Seemungal TA, MacCallum PK, et al. Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb Haemost. 2000;84(2):210–215. [PubMed] [Google Scholar]
- 8.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Backer W, Devolder A, Poli G, et al. Lung deposition of BDP/ formoterol HFA pMDI in healthy volunteers, asthmatic, and COPD patients. J Aerosol Med Pulm Drug Deliv. 2010;23(3):137–148. doi: 10.1089/jamp.2009.0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl. 1993;16:5–40. [PubMed] [Google Scholar]
- 12.Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85:751–758. doi: 10.1378/chest.85.6.751. [DOI] [PubMed] [Google Scholar]
- 13.O’Donnel DE, Fluge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23:832–840. doi: 10.1183/09031936.04.00116004. [DOI] [PubMed] [Google Scholar]
- 14.Taube C, Lehnigk B, Paasch K, et al. Factor analysis of changes in dyspnea and lung function parameters after bronchodilation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:216–220. doi: 10.1164/ajrccm.162.1.9909054. [DOI] [PubMed] [Google Scholar]
- 15.Papi A, Paggiaro P, Nicolini G, Vignola AM, Fabbri LM. Beclomethasone/ formoterol vs fluticasone/salmeterol inhaled combination in moderate to severe asthma. Allergy. 2007;62:1182–1188. doi: 10.1111/j.1398-9995.2007.01493.x. [DOI] [PubMed] [Google Scholar]
- 16.Scichilone N, Battaglia S, Sorino C, et al. Effects of extra-fine inhaled beclomethasone/formoterol on both large and small airways in asthma. Allergy. 2010;65(7):897–902. doi: 10.1111/j.1398-9995.2009.02306.x. [DOI] [PubMed] [Google Scholar]
- 17.Calverley PM, Kuna P, Monsó E, et al. Beclomethasone/formoterol in the management of COPD: a randomised controlled trial. Respir Med. 2010;104(12):1858–1868. doi: 10.1016/j.rmed.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Mahler DA, Witek TJ., Jr The MCID of the transition dyspnea index is a total score of one unit. COPD. 2005;2(1):99–103. doi: 10.1081/copd-200050666. [DOI] [PubMed] [Google Scholar]
- 19.Papi A, Paggiaro PL, Nicolini G, Vignola AM, Fabbri LM. Beclomethasone/formoterol versus budesonide/formoterol combination therapy in asthma. Eur Respir J. 2007;29:682–689. doi: 10.1183/09031936.00095906. [DOI] [PubMed] [Google Scholar]
- 20.Huchon G, Magnussen H, Chuchalin A, Dymek L, Gonod FB, Bousquet J. Lung function and asthma control with beclomethasone and formoterol in a single inhaler. Respir Med. 2009;103:41–49. doi: 10.1016/j.rmed.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Calverley PMA, Anderson JA, Celli B for the TORCH Investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 22.Di Marco F, Milic-Emili J, Boveri B, et al. Effect of inhaled bronchodilators on inspiratory capacity and dyspnoea at rest in COPD. Eur Respir J. 2003;21:86–94. doi: 10.1183/09031936.03.00020102. [DOI] [PubMed] [Google Scholar]
- 23.Sturton RG, Trifilieff A, Nicholson AG, Barnes PJ. Pharmacological characterization of indacaterol, a novel once daily inhaled 2 adrenoceptor agonist, on small airways in human and rat precision-cut lung slices. J Pharmacol Exp Ther. 2008;324(1):270–275. doi: 10.1124/jpet.107.129296. [DOI] [PubMed] [Google Scholar]
- 24.Italian Registry for Clinical Trials. [Accessed September 10, 2011]. http://ricerca-clinica.agenziafarmaco.it/en/node/22.