Several factors accounted for the initial explanations of common infant pain outcomes espoused during the 1950s to 1970s. However, recent research evidence has supported the anatomical and functional capacity of infants to perceive and respond to insults interpretable as pain. Although acute pain and distress during medical procedures are commonplace for young children, they remain undermanaged or unmanaged. The exhaustive version of this abridged review was prompted by the absence of comprehensive meta-analyses and significant gaps in the literature pertaining to the broad range of nonpharmacological interventions for managing acute pain and distress in young children from zero to three years of age. These types of analyses are essential for the development of management strategies to mitigate distress in young children undergoing acutely painful procedures.
Keywords: Acute pain, Caregiver, Infant, Pain management
Abstract
BACKGROUND:
Acute pain and distress during medical procedures are commonplace for young children.
OBJECTIVE:
To assess the efficacy of nonpharmacological interventions for acute procedural pain in children up to three years of age.
METHODS:
Study inclusion criteria were: participants <3 years of age, involved in a randomized controlled or crossover trial, and use of a ‘no-treatment’ control group (51 studies; n=3396). Additional studies meeting all criteria except for study design (eg, use of active control group) were qualitatively described (n=20).
RESULTS:
For every intervention, data were analyzed separately according to age group (preterm-born, term-born neonate and older infant/young child) and type of pain response (pain reactivity, immediate pain-related regulation). The largest standardized mean differences (SMD) for pain reactivity were as follows: sucking-related interventions (preterm: −0.42 [95% CI −0.68 to −0.15]; neonate −1.45 [CI −2.34 to −0.57]), kangaroo care (preterm −1.12 [95% CI −2.04 to −0.21]), and swaddling/facilitated tucking (preterm −0.97 [95% CI −1.63 to −0.31]). For immediate pain-related regulation, the largest SMDs were: sucking-related interventions (preterm −0.38 [95% CI −0.59 to −0.17]; neonate −0.90 [CI −1.54 to −0.25]), kangaroo care 0.77 (95% CI −1.50 to −0.03]), swaddling/facilitated tucking (preterm −0.75 [95% CI −1.14 to −0.36]), and rocking/holding (neonate −0.75 [95% CI −1.20 to −0.30]). The presence of significant heterogeneity limited confidence in nonsignificant findings for certain other analyses.
CONCLUSIONS:
Although a number of nonpharmacological treatments have sufficient evidence supporting their efficacy with preterm infants and healthy neonates, no treatments had sufficient evidence to support efficacy with healthy older infants/young children.
Abstract
HISTORIQUE :
Il est courant que les jeunes enfants ressentent une douleur aiguë et de la détresse pendant des interventions médicales.
OBJECTIF :
Évaluer l’efficacité de mesures non pharmacologiques pour sou-lager une douleur aiguë causée par une intervention chez des enfants de moins de trois ans.
MÉTHODOLOGIE :
Les critères d’inclusion dans l’étude s’établissaient comme suit : participants de moins de trois ans faisant partie d’un essai aléatoire et contrôlé ou transversal et utilisation d’un groupe témoin « sans traitement » (51 études; n=3 396). Des études supplémentaires respectant tous les critères sauf la méthodologie (p. ex., recours à un groupe témoin actif) ont fait l’objet d’une description qualitative (n=20).
RÉSULTATS :
À chaque intervention, les chercheurs ont analysé les données séparément compte tenu du groupe d’âge (nouveau-né prématuré ou à terme et nourrisson plus âgé ou jeune enfant) et du type de réponse à la douleur (réactivité à la douleur, régulation immédiate liée à la douleur). Les plus grandes différences moyennes standardisées (DMS) de réactivité à la douleur s’établissaient comme suit : interventions liées à la succion (prématuré : −0,42 [95 % IC −0,68 à −0,15]; nouveau-né : −1,45 [IC −2,34 à −0,57]), technique kangourou (prématuré : −1,12 [95 % IC −2,04 à −0,21]) et emmaillotement ou enroulement facilité (prématuré : −0,97 [95 % IC −1,63 à −0,31]). En cas de régulation immédiate liée à la douleur, les plus grandes DMS s’établissaient comme suit : interventions liées à la succion (prématuré : −0,38 [95 % IC −0,59 à −0,17]; nouveau-né : −0,90 [IC −1,54 à −0,25]), technique kangourou : 0,77 (95 % IC −1,50 à −0,03]), emmaillotement ou enroulement facilité (prématuré : −0,75 [95 % IC −1,14 à −0,36]), et fait de bercer ou de prendre dans les bras (nouveau-né : −0,75 [95 %IC −1,20 à −0,30]). Une hétérogénéité importante limitait l’intervalle de confiance des résultats non significatifs de certaines autres analyses.
CONCLUSIONS :
Même si les données sont suffisantes pour appuyer l’efficacité d’un certain nombre de traitements chez les prématurés et les nouveau-nés en santé, aucun traitement ne s’associe à des données probantes suffisantes pour en étayer l’efficacité chez les nourrissons plus âgés et les jeunes enfants.
Initial misinterpretations of common infant pain outcomes, such as the lack of declarative memory for painful experiences during infancy (1), the muted responses of premature infants after a barrage of painful procedures (2), and unacceptable rates of serious adverse events due to poor knowledge of infant responses to analgesics and anesthetics during the 1950s to 1970s (3), perpetuated widespread neglect of infant pain treatment.
Established research supports infants’ anatomical and functional capacity to perceive pain (4,5) and respond to tissue insult in a manner interpretable as pain (6). However, despite significant advocacy work, infant acute pain is still undermanaged or unmanaged (7). Comprehensive meta-analytic reviews of nonpharmacological pain management strategies are essential to the ethical and humane treatment of infants and young children during acutely painful procedures.
While several reviews summarize certain acute pain management techniques for painful procedures in infants and young children (8–10), no comprehensive meta-analyses were attempted. Moreover, to our knowledge, there have been no meta-analyses conducted on the broad range of nonpharmacological interventions for managing acute pain and distress in young children from zero to three years of age. Given the frequency of acutely painful procedures for healthy children in early childhood (eg, immunizations) and for hospitalized infants (11), this significant gap in the literature deserves a higher priority.
The full review (12) also takes a novel and in-depth look at the nonpharmacological pain management literature by controlling for age and type of pain response. Age was considered crucial due to the steep trajectory of infant development, both psychologically and physiologically. Moreover, given the different physiological and psychological mechanisms subsuming the initial reaction to a painful stimulus (more automatic and/or reflexive reactivity such as initial distress cry) and during the period of recovery from the painful insult (more under voluntary control; such as whimpering cry minutes after a painful stimuli), timing of pain response was also considered important to explore (see Hadistavropolis and Craig [13] for in-depth discussion regarding observational measures of reflexive and voluntary pain reactions during infancy and childhood).
METHODS
Search strategy
Only randomized controlled trials (RCTs) and randomized crossover trials (RCrTs) using a no-treatment control group that involved the nonpharmacological management of acute procedural pain in infants and children zero to three years of age were included. No language restrictions were used during the search. Due to the existence of meta-analyses specifically pertaining to circumcision surgery (14,15), sucrose (16), breastfeeding (17) and music (18), these types of studies were excluded from the review.
A unique search strategy for MEDLINE (1966 to April 2011), PsycINFO (1967 to April 2011), EMBASE (1980 to April 2011), and CINAHL (1982 to April 2011) was created in collaboration with three librarians affiliated with the Cochrane Collaboration (online Appendix 1). Completed unpublished trials were located through Dissertation Abstracts International (1980 to 2010), the Cochrane Central Register of Controlled Trials (CENTRAL; Issue 3 2010) on The Cochrane Library and www.clinicaltrials.gov (2010). Appeals were made to pediatric electronic mailing lists (Pain in Child Health [PICH; pich-l@lists.dal.ca], Pediatric Pain [Pediatric-pain@lists.dal.ca], American Psychological Association Division 54 [Pediatric Psychology; div54-members@lists.apa.org]). Finally, the reference lists of recently published reviews were also consulted.
Study selection
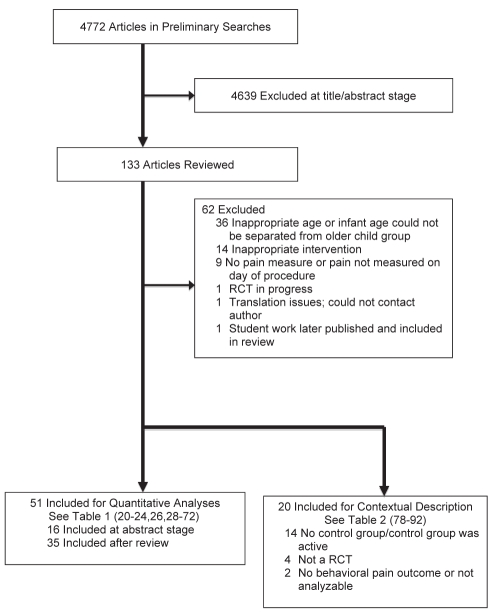
Review authors were not blinded to authors, institutions, journals or results. Using the full-text articles, authors reviewed articles for inclusion (see Acknowledgements). Figure 1 displays the summary of the literature search.
Figure 1).
Literature search results. RCT Randomized controlled trial
Data extraction
Data extraction was conducted using a form designed for the present review. Each form also included a risk of bias/quality questionnaire (online Appendix 1). Every included article was extracted independently by at least two authors and compared. When two authors disagreed, the original article and/or the lead author was consulted to achieve consensus.
Qualitative, quantitative, and study quality data were compiled in Excel 2007 (Microsoft Corporation, USA), RevMan 5 or SPSS version 19.0 (SPSS Inc, USA), respectively. A random sampling of 25% (RevMan5) to 30% (Excel and SPSS) of all data entered across all three programs was double checked by a subteam of review authors.
Only objectively measured behaviorally-based responses to pain were analyzed (see full review (12) for detailed list of measures included). When studies had more than one unidimensional behavioral pain measure, the most specific measure available was used. For example, pain facial expression was used over cry duration. If multimodal measures were used (ie, measures that provided a total score based on behavioural and physiological measurement or multiple behavioural scales), the total score was used.
Quality and treatment integrity assessment
Every study included in the present review was scored for quality and treatment integrity by at least two review authors. The Yates Quality of Study Design and Methods Scale (19) was used to develop the study quality rating form for this review, as this scale was specifically validated for nonpharmacological treatments.
Three minor modifications were made to the Yates scale (12). The maximum score on the revised Yates scale was 23 points, with higher scores indicating higher quality (Table 3 for risk of bias scores). See online appendix for the modified measure.
TABLE 3.
Summary of meta-analyses
| Treatment | Age group | Pain type | Total, n | Effect size (95% CI) | Heterogeneity analysis (95% CI) | Risk of bias analysis (95% CI) |
|---|---|---|---|---|---|---|
| Kangaroo care | Preterm | Reactivity | 177 | −1.12 (−2.04 to −0.21) | −0.38 (−0.65 to −0.12) | – |
| I2 = 89% | I2 = 0% | |||||
| Kangaroo care | Preterm | Immediate regulation | 163 | −0.77 (−1.50 to −0.03) | −0.45 (−0.69 to −0.20) | – |
| I2 = 82% | I2 = 0% | |||||
| Kangaroo care | Neonate | Reactivity | 420 | −0.89 (−2.89 to 1.10) | − | – |
| I2 = 98% | ||||||
| Kangaroo care | Neonate | Immediate regulation | 343 | −0.66 (−1.73 to 0.42) | − | – |
| I2 = 82% | ||||||
| Swaddling/tucking | Preterm | Reactivity | 261 | −0.97 (−1.63 to −0.31) | −0.90 (−1.22 to −0.59) | – |
| I2 = 88% | I2 = 0% | |||||
| Swaddling/tucking | Preterm | Immediate regulation | 65 | −0.75 (−1.14 to −0.36) | − | −0.61 (−1.12 to −0.11) |
| I2 = 0% | I2 = 0% | |||||
| Swaddling/tucking | Neonate | Reactivity | 42 | −1.26 (−1.92 to −0.60) | − | – |
| Non nutritive sucking | Preterm | Reactivity | 305 | −0.42 (−0.68 to −0.15) | −0.32 (−0.05 to −0.15) | – |
| I2 = 48% | I2 = 0% | |||||
| Non nutritive sucking | Preterm | Immediate regulation | 226 | −0.38 (−0.59 to −0.17) | − | −0.36 (−0.59 to −0.13) |
| I2 = 0% | I2 = 0% | |||||
| Non nutritive sucking | Neonate | Reactivity | 220 | −1.45 (−2.34 to −0.57) | −1.88 (−2.25 to −1.50) | – |
| I2 = 88% | I2 = 0% | |||||
| Non nutritive sucking | Neonate | Immediate regulation | 325 | −0.90 (−1.54 to −0.25) | − | −0.51 (−0.91 to −0.29) |
| I2 = 84% | I2 = 11% | |||||
| Non nutritive sucking | Older infants | Immediate regulation | 41 | −0.89 (−1.53 to −0.25) | − | – |
| Swallowing water | Preterm | Reactivity | 36 | −0.24 (−0.71 to 0.23) | − | – |
| Swallowing water | Preterm | Immediate regulation | 36 | −0.23 (−0.70 to 0.24) | − | – |
| Swallowing water | Neonate | Reactivity | 50 | 0.10 (−0.45 to 0.66) | − | – |
| Swallowing water | Neonate | Immediate regulation | 34 | −0.53 (−1.21 to 0.16) | − | – |
| Swallowing water | Older infants | Immediate regulation | 30 | 0.00 (−0.72 to 0.72) | − | – |
| Rocking/holding | Neonate | Reactivity | 131 | −0.33 (−1.05 to 0.39) | − | – |
| I2 = 73% | ||||||
| Rocking/holding | Neonate | Immediate regulation | 81 | −0.75 (−1.20 to −0.30) | − | – |
| I2 = 0% | ||||||
| Rocking/holding | Older infants | Reactivity | 106 | 0.23 (−0.15 to 0.62) | − | – |
| Simulated rocking + water | Preterm | Reactivity | 44 | 0.00 (−0.59 to 0.59) | − | – |
| Touch or massage | Preterm | Immediate regulation | 34 | −0.71 (−2.33 to 0.90) | − | – |
| I2 = 86% | ||||||
| Touch or massage | Neonate | Reactivity | 40 | −0.30 (−0.92 to 0.32) | − | – |
| Touch or massage | Neonate | Immediate regulation | 66 | −0.24 (−0.73 to 0.24) | − | – |
| Touch or massage | Older infants | Reactivity | 20 | −0.21 (−0.84 to 0.41) | − | – |
| Environment modification | Preterm | Reactivity | 64 | −6.44 (−17.13 to 4.26) | − | – |
| I2 = 97% | ||||||
| Environment modification | Preterm | Immediate regulation | 45 | −4.01 (−5.26 to −2.77) | − | – |
| Toy distraction | Older infants | Reactivity | 259 | −0.10 (−0.35 to 0.14) | − | – |
| I2 = 0% | ||||||
| Toy distraction | Older infants | Immediate regulation | 133 | −0.08 (−0.50 to 0.33) | − | – |
| I2 = 0% | ||||||
| Video distraction | Older infants | Reactivity | 90 | −0.70 (−1.13 to −0.27) | − | – |
| Video distraction | Older infants | Immediate regulation | 126 | −0.84 (−1.20 to −0.47) | − | – |
| Structured parent involvement | Older infants | Reactivity | 209 | −0.26 (−0.70 to 0.17) | −0.49 (−0.83 to −0.14) | – |
| I2 = 60% | I2 = 0% | |||||
| Structured parent involvement | Older infants | Immediate regulation | 288 | 0.02 (−0.21 to 0.25) | − | – |
| Mother’s voice | Preterm | Reactivity | 19 | −0.29 (−0.94 to 0.35) | − | – |
| Parent present | Older infants | Immediate regulation | 278 | 0.00 (−0.24 to 0.23) | − | – |
Dash indicates no research performed for that treatment, age and pain response combination
While the majority of studies were deemed of satisfactory-marginal quality, five studies were classified as lower/unknown quality due to receiving a score of 13 or less (20–24).
Treatment integrity was also assessed because almost one-half of the analyzed studies were crossover designs. Two raters independently judged the treatment integrity on five dimensions: treatment adherence, treatment exposure, quality of delivery, participant responsiveness, and program differentiation (25) to arrive at an overall judgment. Only one study had lower/unknown treatment integrity (26).
Data synthesis
Overall strategy:
Three main principles guided the meta-analysis of the data collected for the present review:
Certain types of methods had to be excluded to validly pool results. Accordingly, for a treatment study to be included in the meta-analysis, it had to have at least one trial that was an RCT or RCrT that used a no-treatment control group and included a measure that included a behavioural pain outcome. Trials that studied a relevant nonpharmacological treatment that did not meet these requirements were qualitatively described within the review to further contextualize the findings.
- Studies within the 13 different treatments (Table 3) were first separated into one of three age groups:
- Preterm born: infants born at 36 weeks gestation or less.
- Neonate full-term: infants born at 37 weeks until one month of age.
- Older infant/young child: infants older than one month to 36 months of age. For brevity, this group will be referred to as ‘Older infant’.
- Studies within each age group were then further divided into one of two pain response types:
- Pain reactivity: measured within the first 30 s after the painful stimulus was discontinued.
- Immediate pain-related regulation: measured after the first 30 s post acutely painful stimulus. If multiple measurements were taken after the first 30 s elapsed, the measurement closest to the 30s-time point was used.
Statistical analyses
Primary analyses:
The results from individual studies were pooled using the generic inverse method for a random-effects model in RevMan 5. Using this statistical methodology, an index of the variability of the sample (standard error) and sample size was used to determine how influential each study would be to the final meta-analytic statistic (standardized mean difference [SMD]). A 95% CI was also reported, which incorporated the standard error of the pooled treatment effect for the treatment effect (ie, SMD). As RCTs and RCrTs were included, different procedures, obtained from a Cochrane statistical consultant, were used. Details of this procedure are presented elsewhere (12). When data were missing, study authors were contacted or recommended techniques for interpolation/estimation from P values, t scores and F scores were used (25).
Secondary analyses:
When two or more trials were meta-analyzed in the primary analysis and heterogeneity and/or quality was an issue, secondary sensitivity analyses were conducted. The I2 statistic (27) determined the impact of heterogeneity. When heterogeneity was an issue due to highly variable SMD(s), analyses were re-run without these studies to examine the impact of the pooled findings. When analyses included a study of lower/unknown quality or treatment integrity, analyses were also re-run without these studies to examine the impact on the pooled findings. Authors’ conclusions were based on a synthesis of all three potential analytic steps. Qualitative information from excluded studies were only provided as additional contextual information.
RESULTS
For the final analysis, 51 trials (3396 participants) were included quantitatively (Table 1) (20–24,26,28–72) and 20 trials (Table 2) (73–92) were qualitatively mentioned. Thirty-five separate primary analyses were run among the 13 interventions, three age groups and two pain response types. Table 3 presents the primary meta-analytic results from this review (SMD, 95% CI and I2) and, when applicable, the secondary statistics re-run with studies removed due to heterogeneity and/or study quality.
TABLE 1.
Quantitative studies Included in analyses
| First author (reference) | Year | Between/crossover | Age | Sample size | Intervention | Reactivity findings | Regulation findings | Risk of bias score |
|---|---|---|---|---|---|---|---|---|
| de Sousa (28) | 2008 | Between-groups trial | Preterm | 64 | Kangaroo care | Supports efficacy | – | 19 |
| Akcan (29) | 2009 | Between-groups trial | Preterm | 50 | Kangaroo care | – | Supports efficacy | 19 |
| Castral (30) | 2008 | Between-groups trial | Preterm | 59 | Kangaroo care | Supports efficacy | Does not support efficacy | 15 |
| Ferber (31) | 2008 | Crossover trial | Preterm | 29 | Kangaroo care | Supports efficacy | Supports efficacy | 19 |
| Johnston (32) | 2003 | Crossover trial | Preterm | 74 | Kangaroo care | Supports efficacy | Supports efficacy | 19 |
| Kostandy (33) | 2008 | Crossover trial | Preterm | 10 | Kangaroo care | Supports efficacy | Supports efficacy | 16 |
| Kashaninia (34) | 2008 | Between-groups trial | Neonate | 100 | Kangaroo care | Supports efficacy | – | 16 |
| Gray (35) | 2000 | Between-groups trial | Neonate | 23 | Kangaroo care | – | Supports efficacy | 18 |
| Chermont (36) | 2009 | Between-groups trial | Neonate | 320 | Kangaroo care | Does not support efficacy | Does not support efficacy | 20 |
| Bellieni (37) | 2001 | Crossover trial | Preterm | 17 | Non-nutritive sucking-related | Supports efficacy | – | 15 |
| Liaw (38) | 2010 | Between-groups trial | Preterm | 104 | Non-nutritive sucking-related | Does not support efficacy | Supports efficacy | 22 |
| Corbo (39) | 2000 | Crossover trial | Preterm | 26 | Non-nutritive sucking-related | Supports efficacy | Supports efficacy | 15 |
| Bo (40) | 2000 | Crossover trial | Neonate | 27 | Non-nutritive sucking-related | – | Supports efficacy | 17 |
| Blass (41) | 1999 | Between-groups trial | Neonate | 20 | Non-nutritive sucking-related | – | Supports efficacy | 14 |
| Greenberg (20) | 2002 | Between-groups trial | Neonate | 42 | Non-nutritive sucking-related | – | Does not support efficacy | 11 |
| Yilmaz (42) | 2010 | Between-groups trial | Neonate | 60 | Non-nutritive sucking-related | Does not support efficacy | Supports efficacy | 17 |
| Liu (43) | 2010 | Between-groups trial | Neonate | 70 | Non-nutritive sucking-related | Supports efficacy | Supports efficacy | 18 |
| Curtis (44) | 2007 | Between-groups trial | Older infant | 41 | Non-nutritive sucking-related | – | Supports efficacy | 21 |
| Axelin (45) | 2009 | Crossover trial | Preterm | 20 | Swaddling or tucking | Supports efficacy | – | 21 |
| Comaru (46) | 2009 | Crossover trial | Preterm | 47 | Swaddling or tucking | Supports efficacy | – | 21 |
| Hill (47) | 2005 | Crossover trial | Preterm | 12 | Swaddling or tucking | Supports efficacy | – | 21 |
| Ward-Larson (48) | 2004 | Crossover trial | Preterm | 40 | Swaddling or tucking | Supports efficacy | – | 17 |
| Corff (26) | 1995 | Crossover trial | Preterm | 30 | Swaddling or tucking | – | Supports efficacy | 14 |
| Fearon (49) | 1997 | Crossover trial | Preterm | 15 | Swaddling or tucking | – | Supports efficacy | 20 |
| Axelin (50) | 2006 | Crossover trial | Preterm | 20 | Swaddling or tucking | Supports efficacy | Does not support efficacy | 16 |
| Morrow (51) | 2010 | Between-groups trial | Neonate | 42 | Swaddling or tucking | Supports efficacy | – | 18 |
| Herrington (52) | 2007 | Crossover trial | Preterm | 11 | Touch or massage | – | Does not support efficacy (touch) | 20 |
| Jain (53) | 2006 | Crossover trial | Preterm | 23 | Touch or massage | – | Supports efficacy (massage) | 19 |
| Kozub (54) | 2001 | Crossover trial | Older Infant | 20 | Touch or massage | Does not support efficacy | – | 22 |
| Sizun (55) | 2002 | Crossover trial | Preterm | 19 | Environmental modification | Supports efficacy | – | 14 |
| Catelin (56) | 2005 | Crossover trial | Preterm | 45 | Environmental modification | Supports efficacy | Supports efficacy | 16 |
| Johnston (57) | 1997 | Between-groups trial | Preterm | 44 | Simulated rocking | Does not support efficacy | – | 16 |
| Johnston (58) | 2007 | Crossover trial | Preterm | 19 | Maternal voice | Does not support efficacy | – | 17 |
| Carbajal (59) | 2003 | Between-groups trial | Neonate | 90 | Rocking and/or holding | Does not support efficacy | – | 20 |
| Gormally (60) | 2001 | Between-groups trial | Neonate | 41 | Rocking and/or holding | Supports efficacy | Supports efficacy | 14 |
| Ipp (61) | 2004 | Between-groups trial | Older Infant | 106 | Rocking and/or holding | Does not support efficacy | – | 19 |
| Cohen (21) | 2002 | Between-groups trial | Older Infant | 90 | Video distraction | Supports efficacy | – | 10 |
| Cohen (62) | 2006 | Between-groups trial | Older Infant | 126 | Video distraction | - | Supports efficacy | 17 |
| Bustos (63) | 2008 | Between-groups trial | Older Infant | 50 | Structured parent involvement | Does not support efficacy | – | 18 |
| Stevens (64) | 1999 | Crossover trial | Preterm | 122 | Non-nutritive sucking-related | Supports efficacy | – | 17 |
| Swaddling or tucking | Does not support efficacy | |||||||
| Whipple (22) | 2004 | Between-groups trial | Preterm | 60 | Non-nutritive sucking-related (pacifier & lullaby) | – | Supports efficacy | 8 |
| Non-nutritive sucking-related (pacifier only) | – | Supports efficacy | ||||||
| Elserafy (65) | 2009 | Crossover trial | Preterm | 36 | Non-nutritive sucking-related-pacifier | Does not support efficacy | Does not support efficacy | 18 |
| Does not support efficacy | Does not support efficacy | |||||||
| Swallowing water | Does not support efficacy | Does not support efficacy | ||||||
| Non-nutritive sucking-related-pacifier with water | ||||||||
| Carbajal (66) | 1999 | Between-groups trial | Neonate | 75 | Swallowing water | Does not support efficacy | – | 22 |
| Non-nutritive sucking-related | Supports efficacy | – | ||||||
| Bellieni (67) | 2002 | Between-groups trial | Neonate | 60 | Non-nutritive sucking-related | Supports efficacy | – | 20 |
| Touch or massage | Does not support efficacy | – | ||||||
| Campos (23) | 1994 | Between-groups trial | Neonate | 60 | Non-nutritive sucking-related | – | Supports efficacy | 13 |
| Rocking and/or holding | – | Supports efficacy | ||||||
| Im (68) | 2008 | Between-groups trial | Neonate | 99 | Non-nutritive sucking-related | – | Does not support efficacy | 13 |
| Touch or massage | – | Does not support efficacy | ||||||
| Allen (24) | 1996 | Between-groups trial | Neonate | 34 | Swallowing water | – | Supports efficacy | 7 |
| Older Infant | 30 | – | Does not support efficacy | |||||
| Cramer-Berness (69) | 2005 | Between-groups trial | Older Infant | 123 | Toy distraction | Does not support efficacy | – | 18 |
| Structured parent involvement | Supports efficacy | – | ||||||
| Cramer-Berness (70) | 2005b | Between-groups trial | Older Infant | 117 | Toy distraction | Does not support efficacy | – | 19 |
| Structured parent involvement | Does not support efficacy | – | ||||||
| Bauchner (71) | 1996 | Between-groups trial | Older Infant | 435 | Parent presence | – | Does not support efficacy | 17 |
| Structured parent involvement | – | Does not support efficacy | ||||||
| Hillgrove Stuart (72) | 2008 | Between-groups trial | Older Infant | 99 | Toy distraction - RA | Does not support efficacy | Does not support efficacy | 20 |
| Toy distraction - Parent | Does not support efficacy | Does not support efficacy |
Dash indicates no research performed for that treatment, age and pain response combination. RA Research assistant
TABLE 2.
Studies included for further contextual information
| First author (reference) | Year | Age | Intervention | Pain reactivity findings | Pain regulation findings | Reason for exclusion from quantitative analyses |
|---|---|---|---|---|---|---|
| Cong (73) | 2009 | Preterm | Facilitated tucking vs non-nutritive sucking | Supports efficacy | – | No behavioural pain measure reported |
| Johnston (74) | 2008 | Preterm | Kangaroo care | Does not support efficacy | Supports efficacy | Control group was active |
| Johnston (75) | 2009 | Preterm | Kangaroo care | No difference between kangaroo care and enhanced kangaroo care | No difference between kangaroo care and enhanced kangaroo care | Control group was active |
| Ludington-Hoe (76) | 2005 | Preterm | Kangaroo care | Supports efficacy | Supports efficacy | Control group was active |
| Huang (77) | 2004 | Preterm | Kangaroo care | No difference between swaddling and containment | – | Control group was active |
| Cignacco (78) | 2008 | Preterm | Kangaroo care | No difference between multisensorial stimulation and facilitated tucking | No difference between multisensorial stimulation and facilitated tucking | Control group was active |
| Diego (79) | 2009 | Preterm | Kangaroo care | – | Supports efficacy | Control group was active |
| Goubet (80) | 2003 | Preterm | Smell (familiar vs unfamiliar) | No difference between familiar and unfamiliar odour | Familiar odour more efficacious than unfamiliar odour | Control group was active |
| Grunau (81) | 2004 | Preterm | Multisensorial stimulation vs facilitated tucking | No difference between prone and supine positioning | – | No control group |
| Vivancos (82) | 2010 | Neonate | Non-nutritive sucking | Does not support efficacy | Does not support efficacy | Not an RCT |
| Okan (83) | 2010 | Neonate | Non-nutritive sucking | Does not support efficacy | Supports efficacy | No means or SDs reported; could not contact author |
| Aguirre (84) | 2008 | Neonate | Non-nutritive sucking vs non-nutritive sucking & swaddling | Non-nutritive sucking more efficacious than facilitated tucking | – | Control group was active |
| Bueno (85) | 2010 | Neonate | Pacifier vs swaddling | No difference between non-nutritive sucking and non-nutritive sucking with swaddling | No difference between non-nutritive sucking and non-nutritive sucking with swaddling | Control group was active |
| Campos (86) | 1989 | Neonate | Positioning (prone vs supine) | No difference between pacifier and swaddling | Pacifier more efficacious than swaddling | Control group was active |
| Goubet (87) | 2007 | Neonate | Rocking and/or holding | – | Familiar odour more efficacious than unfamiliar odour | Control group was active |
| Rattaz (88) | 2005 | Neonate | Smell (familiar vs unfamiliar) | No difference between familiar and unfamiliar odour | Familiar odour more efficacious than unfamiliar odour | No control group |
| Weissman (89) | 2009 | Neonate | Smell (familiar vs unfamiliar) | Supports efficacy | – | Not an RCT |
| Smell (familiar vs unfamiliar) | Supports efficacy | – | ||||
| Felt (90) | 2000 | Older Infant | Structured parental intervention | – | Supports Efficacy | Not an RCT |
| Morelius (91) | 2009 | Older Infant | Swaddling or tucking | – | Does Not Support Efficacy | Not an RCT |
| Swallowing water | – | Does Not Support Efficacy | ||||
| Ipp (92) | 2009 | Older Infant | Vaccination order | Supports injecting DPTAP-Hib vaccine before PCV | – | No control group |
Dash indicates no research done for that treatment, age and pain response combination. DPTAP-Hib Diphtheria and tetanus toxoids and acellular pertussis and Haemophilus influenza type b vaccine; PCV Pneumococcal conjugated vaccine; RCT Randomized controlled trial
Of the 3396 participants, 1581 were in treatment conditions only, 1153 were in control conditions and 662 were in a crossover condition. Of the 51 studies, 21 used a cross-over design and 30 used a between-groups design.
The following painful procedures (determined by respective study authors rather than review authors) were included in this review: 29 studies examined treatments for heelstick, 10 studies examined needle-injection procedures, six studies assessed venipuncture, two examined NICU diaper changes, two studies investigated endotracheal suctioning and two studied a neonatal intensive care unit (NICU) weighing procedure.
DISCUSSION
The summary interpretation of the primary meta-analytic findings, contextualized by secondary heterogeneity and quality/treatment integrity analyses, are presented in Table 4. Based on these results, treatments were assigned a number from 1 to 4, for each age and pain response type. As will be discussed below, the ratings reflect whether, as the literature currently stands, evidence supported the specific treatment for pain management (efficacy) or did not support the specific treatment for pain management (inefficacy). Each treatment’s efficacy or inefficacy was further qualified by the level of support (sufficient versus limited).
TABLE 4.
Summary conclusions
|
Preterm infants |
Neonates |
Older infants |
||||
|---|---|---|---|---|---|---|
| Treatment arm | Reactivity | Immediate regulation | Reactivity | Immediate regulation | Reactivity | Immediate regulation |
| Kangaroo care | 1 | 1 | 3 | 3 | – | – |
| Non-nutritive sucking-related | 1 | 1 | 1 | 1 | – | 2 |
| Swaddling/tucking-related | 1 | 1 | 2 | – | – | – |
| Touch or massage-related | – | 3 | 3 | 3 | 3 | – |
| Environment modification | 3 | 2 | – | – | – | – |
| Simulated rocking and water | 3 | – | – | – | – | – |
| Simulated mother’s voice | 3 | – | – | – | – | – |
| Swallowing water | 3 | 3 | 3 | 3 | – | 3 |
| Rocking or holding | – | – | 3 | 1 | 3 | – |
| Toy distraction | – | – | – | – | 4 | 3 |
| Video distraction | – | – | – | – | 2 | 2 |
| Parent present | – | – | – | – | – | 3 |
| Structured parent involvement | – | – | – | – | 3 | 3 |
Sufficient evidence supports efficacy for reducing pain-related behaviours (support of two or more trials);
Limited evidence suggests efficacy for reducing pain-related behaviours (eg, support of one trial or heterogeneity among trials);
Limited evidence suggests inefficacy for reducing pain-related behaviours (eg, support of one trial or heterogeneity among trials);
Sufficient evidence supports inefficacy for reducing pain-related behaviours (support of one or more trials). Dash indicates no research performed for that treatment, age and pain response combination
Treatment efficacy was denoted by either a 1 (sufficient evidence, ie, two or more quality trials supporting efficacy) or 2 (limited evidence, ie, either due to quality, quantity or heterogeneity of trials, supporting efficacy). Treatment inefficacy was denoted by either a 3 (limited evidence [ie, either due to quality, quantity or trial heterogeneity]) or a 4 (sufficient evidence [ie, two or more quality trials supporting inefficacy]). Blank cells indicate no applicable research for that combination of treatment, age and pain response. A discussion of each of the findings follows.
Kangaroo care (also known as skin-to-skin contact)
An infant is placed on their caregiver’s bare chest before, during and after a painful procedure.
Preterm infants:
Sufficient evidence suggests kangaroo care is efficacious in reducing pain reactivity and improving immediate pain-related regulation. While there was substantial heterogeneity, secondary analyses confirmed this finding.
Four studies that were excluded from the statistical analyses (73–76) also indirectly support kangaroo care as efficacious in improving pain reactivity and immediate pain-related regulation in preterm infants.
Neonates:
Limited evidence suggests that kangaroo care is not efficacious as an intervention for pain reactivity or immediate pain-related regulation. However, heterogeneity undermines our confidence in the pooled results. Given the exposure times in the premature infant literature, future research should explore whether using a longer exposure time in kangaroo care for neonates prior to the painful procedure (ie, 10 min or longer akin to preterm techniques), could lead to a significant treatment effect.
Swaddling/facilitated tucking
A swaddled infant is securely wrapped in a blanket to prevent excessive movement. Facilitated tucking is a hand-swaddling technique that holds the infant’s extremities flexed and contained.
Preterm infants:
There was sufficient evidence to support the use of swaddling/tucking as an efficacious intervention for reducing pain-related distress reactivity and immediate pain-related regulation in pre-term infants. Two studies (74,77), that were not included in the analysis due to use of an active control group, suggested that swaddling was as efficacious as containment but not as efficacious as kangaroo care.
Neonates:
Limited evidence supports the efficaciousness of swaddling/tucking related interventions for the healthy neonate.
Non-nutritive sucking-related strategies
An object (eg, pacifier, nonlactating nipple) is placed into an infant’s mouth to stimulate orotactile or sucking behaviours during a painful event.
Preterm infants:
There is sufficient evidence that sucking is efficacious in reducing pain-related distress reactivity and improving immediate pain-related regulation. Pain relief may be maximized if sucking begins at least 3 min before the painful stimuli. Two studies that were not included in the analyses due to the use of an active control group (85,89) also suggest that sucking helps diminish pain reactivity.
Neonates:
The results show sufficient evidence for sucking to reduce pain reactivity and immediate pain-related regulation. Four studies that were not included due to the exclusion criteria (84–86,91), also lend support to the efficacy of sucking to improve immediate pain-related regulation.
Older infants:
Limited evidence suggests that sucking may be an efficacious intervention to improve pain reactivity.
Swallowing water
Water is administered for ingestion without inciting extensive sucking (eg, water administered by a dropper).
Preterm infants:
There was limited evidence that water is an inefficacious intervention for pain reactivity or immediate pain-related regulation for preterm infants.
Neonates:
There was limited evidence that water is an inefficacious intervention for pain reactivity or immediate pain-related regulation.
Older infants:
There was limited evidence that water is an inefficacious intervention for immediate pain-related regulation.
The above studies used ‘water’ as a treatment arm (comparing them to a ‘no-treatment’ control), while most other studies in the literature used water as the ‘no-treatment’ control group. Given the more common use of water in the literature and the limited evidence at every age group of its inefficacy, it is not recommended that further research use water as a treatment arm for young child procedural pain studies.
Rocking and/or holding
An infant is held and/or gently moved up and down or side-to-side by a caregiver.
Neonates:
In terms of pain reactivity, rocking/holding was not efficacious in reducing pain reactivity but substantial heterogeneity reduces our confidence. One study, not included due to lack of randomization (89), suggested a significant difference in pain reactivity between infants who were held and control infants. However, there was sufficient evidence to support the efficaciousness of rocking/holding interventions for immediate pain-related regulation.
Older infants:
There was limited evidence suggesting rocking/holding is not an efficacious intervention for pain-related distress reactivity in older infants.
Artificial rocking and water
An infant is placed in a bassinet-type machine that provides a swaying motion. Water is administered via a dropper.
Preterm infants:
Limited evidence indicates that simulated rocking and water is not an efficacious intervention for reducing pain-related distress pain reactivity for preterm infants.
Touch/massage/therapeutic touch
An infant’s body (i.e. touch, massage) or energy field (therapeutic touch) is ‘stroked’ or rubbed to provide some type of counter-stimulation to the nociceptive input.
Preterm infants:
Current evidence does not support touch/massage-related interventions as efficacious in improving the immediate pain-related regulation but caution is warranted given the presence of substantial heterogeneity. One study not included in the analysis due to exclusion criteria (79) demonstrated that massage was more efficacious at reducing preterm infant’s heart rate than light pressure or no massage therapy.
Neonates:
Limited evidence suggests touch/massage related interventions are not efficacious to reduce pain reactivity or immediate pain-related regulation. One study that was not included in the analysis due to exclusion criteria (78) provides further support to these findings.
Older infants:
Limited evidence suggests that therapeutic touch is not efficacious in reducing pain reactivity in older infants.
Environmental modification
Interventions involved modifying the environment in which an infant experiences painful procedures (ie, low noise and lighting, clustering procedures to avoid over handling, soothing smells).
Preterm infants:
While the pooled result from two studies suggest that environmental modification was not efficacious for pain reactivity, this must be interpreted with caution due to substantial heterogeneity. However, there is limited evidence to suggest that environmental modification is efficacious for immediate pain-related regulation.
Toy distraction
Toy distraction is defined as the use of a toy to divert attention from the painful stimulus.
Older infants:
Sufficient evidence suggests that toy distraction is not efficacious for reducing pain-related distress reactivity in older infants. Limited evidence suggests that it is also not efficacious for improving immediate pain-related regulation.
Video distraction
An audio-visual screen displaying two-dimensional moving images with coordinated audio is used to divert the infants attention from the painful stimulus
Older infants:
Limited evidence suggests that video distraction is efficacious in reducing pain-related reactivity. Limited evidence also supports efficacy for improving immediate pain-related regulation.
Structured parental involvement
Parents are instructed about strategies that are accepted as pain-reducing but are not given any materials to aid them (eg, rocking, holding, shushing, talking, rubbing, tickling, and distracting attention without toy or video).
Older infants:
Structured parent involvement was not found to be efficacious for pain reactivity. However, caution should be applied to this finding as post-hoc heterogeneity analyses contradicted this finding when one outlying study was removed. In addition, one excluded study also suggested that structured parental involvement significantly improved time to regulate post-immunization (90).
Simulated maternal voice
An infant is exposed to a reproduction of the mother’s voice to help simulate the fetal environment.
Preterm infants:
Results from one study indicated that mother’s voice was not more efficacious than a no-treatment control for reducing pain-related distress reactivity for preterm infants.
Parent presence
The parent is present during a painful procedure but not interacting extensively with the child in a manner thought to be pain reducing.
Older infants:
Limited evidence indicates that parent presence is not efficacious for improving immediate pain-related regulation.
CONCLUSIONS
Implications for practice
For preterm infants, there was sufficient evidence to recommend kangaroo care, sucking-related interventions, and swaddling/facilitated tucking interventions for both pain reactivity and immediate pain-related regulation. For neonates, there was sufficient evidence to recommend sucking-related interventions as an efficacious treatment for pain reactivity and immediate pain-related regulation. Rocking/holding was also found to be efficacious for neonatal immediate pain-related regulation. For older infants, there were no treatments reviewed that demonstrated sufficient evidence. Overall, due to heterogeneity, some analyses that found a lack of treatment effect need to be interpreted with caution. Finally, while more rigorous research is needed to confirm these findings, environmental modification (preterms), sucking (neonates) and video distraction (older infant/child) have limited evidence supporting efficacy.
Implications for research
Significant gaps in the existing treatment literature on non pharmacological management of acute pain in infancy have been discerned. Based on established patterns of efficacy in other age groups/pain response types, it would seem especially productive to the field of infant pain management to investigate:
Kangaroo care for older infants’ pain reactivity and immediate pain-related regulation (eg, for the two-month immunization)
Sucking-related interventions for older infants’/young children’s pain reactivity
Swaddling or tucking-related interventions for older infants pain reactivity and immediate pain-related regulation (eg, two-month immunization)
Rocking/holding for older infants’ immediate pain-related regulation
In addition, preliminary work from other studies (excluded from our overall quantitative analyses for methodological reasons) suggests that more research is needed to explore: exposing an infant to a familiar odor (80,87,88), feeding an infant formula (89) and administering the least painful immunization first (92) as potential nonpharmacological interventions for acute pain.
It is also important to note that certain treatments were grouped together based on similar mechanisms of action despite not being the same treatment (eg, swaddling and tucking, non-nutritive sucking related strategies), which was supported by our heterogeneity analyses. However, with more research, future revisions may be able to report SMDs and CIs separately for treatments encompassed within these groups.
Moreover, given the frequency of immunization during the first years of life, it was disheartening that there were no efficacious nonpharmacological treatments for the older infant/young child. Although there is a substantial evidence base for pharmacological strategies such as sucrose and topical anaesthetics (93), from an economic and pragmatic perspective, it would behoove researchers to spend more resources in investigating efficacious nonpharmacological pain management for older infants.
The lack of developmentally-informed work on parent-mediated interventions was also a cause for a concern. Over the first years of life, it has been argued (94) that the caregiver is the most important context for the infant in pain. Currently, studies that have attempted to formally structure parent behaviour have been limited and, thus, shown to be ineffective. More work on improved parent interventions, especially ones that capitalize on an infant’s primary need for proximity to the parent during periods of distress (95), is needed. Teaching a parent how to soothe more efficaciously their infant or young child in acute pain is a simple, low-cost intervention that is not being used to its potential in today’s acute pain context.
In conclusion, while a number of non pharmacological treatments have a sufficient body of evidence supporting their use with preterm infants and to a lesser extent, healthy neonates, more research is needed to create a sound repertoire of empirically-supported nonpharmacological treatments for procedural pain in older infants/young children.
Acknowledgments
This work is a brief summary of a review created for the Cochrane Collaboration. The full review can be found at Cochrane Database of Systematic Reviews 2011, Issue 10. Art. No.: CD006275. DOI:10.1002/146517858.CD006275.pub2. Dr Pillai Riddell and Ms Racine had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
No authors have potential conflicts of interest, including financial, activities, relationships, and affiliations. Funding sources, in the form of salary support to one or more authors, included Canadian Institutes of Health Research, Social Sciences and Humanities Research Council, Ontario Graduate Scholarship Program, Lillian Wright Foundation and the Signy Hildur Chair in Pediatric Nursing. None of these organizations had input regarding the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, nor final approval of the manuscript.
Ms Alanna Gerwitz-Stern contributed to the initial stages of the full Cochrane review and was listed as an author on that work. Ms Jennifer Lyons entered data into data management software and was paid by Dr Pillai Riddell. Ms Grace Lee and Ms Janet Yamada extracted data from three articles and were financially compensated by Dr Stevens.
Rebecca Pillai Riddell PhD: Study selection, article extraction, data analysis, data interpretation, manuscript preparation, and manuscript review Nicole Racine MA: Study selection, article extraction, data checking, data analysis, data interpretation, and manuscript review. Kara Turcotte BA: Study selection, article extraction, data checking, data interpretation, and manuscript review. Lindsay Uman PhD: Study selection, article extraction, data interpretation, and manuscript review. Rachel Horton MA: Article extraction, data interpretation, and manuscript review. Laila Din Osmun MA: Article extraction, data interpretation, and manuscript review. Sara Ahola Kohut MA: Article extraction, data interpretation, and manuscript review. Jessica Hillgrove Stuart MA: Article extraction, data interpretation, and manuscript review. Bonnie Stevens RN, PhD, FCAHS: Data interpretation, manuscript review. Diana Lisi BA: Study selection, data checking, data interpretation, manuscript review. Source of support: Discretionary funds to Dr Pillai Riddell from York University.
Co-publication with the Cochrane Collaboration: Pillai Riddell RR, Racine NM, Turcotte K, Uman LS, Horton RE, Din Osmun L, Ahola Kohut S, Hillgrove Stuart J, Stevens B, Gerwitz-Stern A. Non-pharmacological management of infant and young child procedural pain. Cochrane Database of Systematic Reviews 2011, Issue 10. Art. No.: CD006275. DOI:10.1002/146517858.CD006275.pub2
FUNDING SOURCES: York University, Canadian Institutes of Health Research, Social Sciences and Humanities Research Council, Ontario Graduate Scholarship Program, Lillian Wright Foundation and the Signy Hildur Chair in Pediatric Nursing.
REFERENCES
- 1.Field T. Infancy is not without pain. Vasta R, editor. Annals of Child Development: A research annual. 1995;10:1–26. [Google Scholar]
- 2.Johnston CC, Stevens B, Craig KD, Grunau RV. Developmental changes in pain expression in premature, full-term, two and four-month-old infants. Pain. 1993;52:201–208. doi: 10.1016/0304-3959(93)90132-9. [DOI] [PubMed] [Google Scholar]
- 3.Berde CB, Jaksic T, Lynn AM, Maxwell LG, Soriano SG, Tibboel D. Anaesthesia and analgesia during and after surgery in neonates. Clin Ther. 2005;27:900–21. doi: 10.1016/j.clinthera.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–20. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- 5.Slater R, Cornelissen L, Fabrizi L, Patten D, Yoxen J, Worley A. Oral sucrose as an analgesic drug for procedural pain in newborn infants: A randomized controlled trial. Lancet. 2010;376:1225–32. doi: 10.1016/S0140-6736(10)61303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grunau R, Craig KD. Pain expression in neonates: Facial action and cry. Pain. 1987;28:395–410. doi: 10.1016/0304-3959(87)90073-X. [DOI] [PubMed] [Google Scholar]
- 7.Taddio A, Appleton M, Bortolussi R, et al. Reducing the pain of childhood vaccination: An evidence-based clinical practice guidelines (summary) CMAJ. 2010;182:1–7. doi: 10.1503/cmaj.092048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cignacco E, Hamers JPH, Stoffel L, et al. The efficacy of non-pharmacological interventions in the management of procedural pain in preterm and term neonates. A systematic literature review. Eur J Pain. 2007;11:139–52. doi: 10.1016/j.ejpain.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Schechter NL, Zempsky WT, Cohen LL, McGrath PJ, McMurtry CM, Bright NS. Pain reduction during pediatric immunizations: Evidence-based review and recommendations. Pediatrics. 2007;119:184–98. doi: 10.1542/peds.2006-1107. [DOI] [PubMed] [Google Scholar]
- 10.Johnston CC, Fernandes AM, Campbell-Yeo M. Pain in neonates is different. Pain. 2011;152(3 Suppl):S65–S73. doi: 10.1016/j.pain.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Stevens BJ, Abbott LK, Yamada J, et al. Epidemiology and management of painful procedures in children in Canadian hospitals. CMAJ. 2011;183:E403–10. doi: 10.1503/cmaj.101341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pillai Riddell RR, Racine NM, Turcotte K, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD006275.pub2. (In press) [DOI] [PubMed] [Google Scholar]
- 13.Hadistavropolis T, Craig K. A theoretical framework for understanding self-report and observational measures of pain: A communications model. Beh Res Ther. 2002;40:551–70. doi: 10.1016/s0005-7967(01)00072-9. [DOI] [PubMed] [Google Scholar]
- 14.Brady-Fryer B, Wiebe N, Lander JA. Pain relief for neonatal circumcision [Review] Cochrane Database Syst Rev. 2009;(1):CD004217. doi: 10.1002/14651858.CD004217.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cyna AM, Middleton P. Caudal epidural block versus other methods of postoperative pain relief for circumcision in boys. Cochrane Database Syst Rev. 2010;(11):CD003005. doi: 10.1002/14651858.CD003005.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevens B, Yamada J, Ohlsson A. Sucrose for analgesia in newborn infants undergoing painful procedures (Updated Cochrane Review) Cochrane Database Syst Rev. 2010;(1):CD001069. doi: 10.1002/14651858.CD001069.pub3. [DOI] [PubMed] [Google Scholar]
- 17.Shah PS, Aliwalas LL, Shah V. Breastfeeding or breast milk for procedural pain in neonates. Cochrane Database Syst Rev. 2009;(1):CD004950. doi: 10.1002/14651858.CD004950.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Cepeda MS, Carr DB, Lau J, Alvarez H. Music for pain relief. Cochrane Database Syst Rev. 2006;(2):CD004843. doi: 10.1002/14651858.CD004843.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Yates SL, Morley S, Eccleston C, de C Williams AC. A scale for rating the quality of psychological trials for pain. Pain. 2005;117:314–25. doi: 10.1016/j.pain.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg CS. A sugar-coated pacifier reduces procedural pain in newborns. Pediatr Nurs. 2002;28:271–7. [PubMed] [Google Scholar]
- 21.Cohen LL. Reducing infant immunization distress through distraction. Health Psychol. 2002;21:207–11. [PubMed] [Google Scholar]
- 22.Whipple J. The effect of music-reinforced nonnutritive sucking on state of preterm, low birthweight infants experiencing heelstick (unpublished doctoral dissertation) Tallahassee: The Florida State University; Nov, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Campos RG. Rocking and pacifiers: Two comforting interventions for heelstick pain. Res Nurs Health. 1994;17:321–31. doi: 10.1002/nur.4770170503. [DOI] [PubMed] [Google Scholar]
- 24.Allen KD, White DD, Walburn JN. Sucrose as an analgesic agent for infants during immunization injections. Arch Pediatr Adolesc Med. 1996;150:270–4. doi: 10.1001/archpedi.1996.02170280040007. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [updated March 2011]. Cochrane Collaboration; 2011. < www.cochrane-handbook.org> (Accessed on April 29, 2011).
- 26.Corff KE, Seideman R, Venkataraman PS, Lutes L, Yates B. Facilitated tucking: A nonpharmacologic comfort measure for pain in preterm neonates. J Obstet Gynecol Neonatal Nurs. 1995;24:143–7. doi: 10.1111/j.1552-6909.1995.tb02456.x. [DOI] [PubMed] [Google Scholar]
- 27.Deeks JJ, Higgins JPT, Altman DG. Analysing and presenting results. In: Higgins JPT, Green S, editors. The Cochrane Library. Chichester: John Wiley & Sons; 2005. pp. 97–165. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5. [updated May 2005]; section 8. [Google Scholar]
- 28.de Sousa Freire NB, Santos Garcia JB, Carvahlo Lamy Z. Evaluation of analgesic effect of skin-to-skin contact compared to oral glucose in preterm neonates. Pain. 2008;139:28–33. doi: 10.1016/j.pain.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 29.Akcan E, Yigit R, Atici A. The effect of kangaroo care on pain in premature infants during invasive procedures. Turk J Pediatr. 2009;51:14–18. [PubMed] [Google Scholar]
- 30.Castral TC, Warnock F, Leite AM, Haas VJ, Scochi CGS. The effects of skin-to-skin contact during acute pain in preterm newborns. Eur J Pain. 2008;12:464–71. doi: 10.1016/j.ejpain.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Ferber SG, Makhoul IR. Neurobehavioral assessment of skin-to-skin effects on reaction to pain in preterm infants: A randomized, controlled within-subject trial. Acta Paediatr. 2008;97:171–6. doi: 10.1111/j.1651-2227.2007.00607.x. [DOI] [PubMed] [Google Scholar]
- 32.Johnston CC, Stevens B, Pinelli J, et al. Kangaroo care is effective in diminishing pain response in preterm neonates. Arch Pediatr Adolesc Med. 2003;157:1084–8. doi: 10.1001/archpedi.157.11.1084. [DOI] [PubMed] [Google Scholar]
- 33.Kostandy RR, Ludington-Hoe SM, Cong X, et al. Kangaroo care (skin contact) reduces crying response to pain in preterm neonates: Pilot results. Pain Manag Nurs. 2008;9:55–65. doi: 10.1016/j.pmn.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kashaninia Z, Sajedi F, Rahgozar M, Noghabi FA. The effect of kangaroo care on behavioral responses to pain of an intramuscular injection in neonates. J Spec Pediatr Nurs. 2008;13:275–80. doi: 10.1111/j.1744-6155.2008.00165.x. [DOI] [PubMed] [Google Scholar]
- 35.Gray L, Watt L, Blass EM. Skin-to-skin contact is analgesic in healthy newborns. Pediatrics. January. 2000;105:e14. doi: 10.1542/peds.105.1.e14. [DOI] [PubMed] [Google Scholar]
- 36.Chermont AG, Falcao LFM, de Souza Silva EHL, de Cassia Xavier Balda R, Guinsburg R. Skin-to-skin contact and/or oral 25% dextrose for procedural pain relief for term newborn infants. Pediatrics. 2009;124:e1101–7. doi: 10.1542/peds.2009-0993. [DOI] [PubMed] [Google Scholar]
- 37.Bellieni CV, Buonocore G, Nenci A, Franci N, Cordelli DM, Bagnoli F. Sensorial saturation: An effective analgesic tool for heel-prick in preterm infants. Biol Neonate. 2001;80:15–8. doi: 10.1159/000047113. [DOI] [PubMed] [Google Scholar]
- 38.Liaw J, Yang L, Ti Y, Tucker Blackburn S, Chang Y, Sun L. Non-nutritive sucking relieves pain for preterm infants during heel stick procedures in Taiwan. J Clin Nurs. 2010;19:2741–51. doi: 10.1111/j.1365-2702.2010.03300.x. [DOI] [PubMed] [Google Scholar]
- 39.Corbo MG, Mansi G, Stagni A, et al. Nonnutritive sucking during heelstick procedures decreases behavioral distress in the newborn infant. Biol Neonate. 2000;77:162–7. doi: 10.1159/000014211. [DOI] [PubMed] [Google Scholar]
- 40.Bo KL, Callaghan P. Soothing pain-elicited distress in Chinese neonates. Pediatrics. 2000;105:E49. doi: 10.1542/peds.105.4.e49. [DOI] [PubMed] [Google Scholar]
- 41.Blass EM, Watt LB. Suckling- and sucrose-induced analgesia in human newborns. Pain. 1999;83:611–23. doi: 10.1016/S0304-3959(99)00166-9. [DOI] [PubMed] [Google Scholar]
- 42.Yilmaz F, Arikan D. The effects of various interventions to newborns on pain and duration of crying. J Clin Nurs. 2011;20:1008–17. doi: 10.1111/j.1365-2702.2010.03356.x. [DOI] [PubMed] [Google Scholar]
- 43.Liu MF, Lin KC, Chou YH, Lee TY. Using non-nutritive sucking and oral glucose solution with neonates to relieve pain: A randomised controlled trial. J Clin Nurs June. 2010;19:1604–11. doi: 10.1111/j.1365-2702.2009.03014.x. [DOI] [PubMed] [Google Scholar]
- 44.Curtis SJ, Jou H, Ali S, Vandermeer B, Klassen T. A randomized controlled trial of sucrose and/or pacifier as analgesia for infants receiving venipuncture in a pediatric emergency department. BMC Pediatr. 2007;7:27. doi: 10.1186/1471-2431-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Axelin A, Salantera S, Kirjavainen J, Lehtonen L. Oral glucose and parental holding preferable to opioid in pain management in preterm infants. Clin J Pain. 2009;25:138–45. doi: 10.1097/AJP.0b013e318181ad81. [DOI] [PubMed] [Google Scholar]
- 46.Comaru T, Miura E. Postural support improves distress and pain during diaper change in preterm infants. J Perinatol. 2009;29:504–7. doi: 10.1038/jp.2009.13. [DOI] [PubMed] [Google Scholar]
- 47.Hill S, Engle S, Jorgensen J, Kralik A, Whitman K. Effects of facilitated tucking during routine care of infants born preterm. Pediatr Phys Ther. 2005;17:158–63. doi: 10.1097/01.pep.0000163097.38957.ec. [DOI] [PubMed] [Google Scholar]
- 48.Ward-Larson C, Horn RA, Gosnell F. The efficacy of facilitated tucking for relieving procedural pain of endotracheal suctioning in very low birthweight infants. MCN Am J Matern Child Nurs. 2004;29:151–6. doi: 10.1097/00005721-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 49.Fearon I, Kisilevsky BS, Hains SM, Muir DW, Tranmer J. Swaddling after heel lance: Age-specific effects on behavioral recovery in preterm infants. J Dec Behav Pediatr. 1997;18:222–32. [PubMed] [Google Scholar]
- 50.Axelin A, Salantera S, Lehtonen L. Facilitated tucking by parents in pain management of preterm infants – a randomized crossover trial. Early Hum Dev. 2006;82:241–7. doi: 10.1016/j.earlhumdev.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 51.Morrow C, Hidinger A, Wilkinson-Faulk D. Reducing neonatal pain during routine heel lance procedures. MCN Am J Matern Child Nurs. 2010;35:346–54. doi: 10.1097/NMC.0b013e3181f4fc53. [DOI] [PubMed] [Google Scholar]
- 52.Herrington CJ. Reducing pain of heelstick in premature infants with gentle human touch (unpublished doctoral dissertation) Wayne State University; Jan, 2007. p. AAI3295977. ETD Collection: [Google Scholar]
- 53.Jain S, Kumar P, McMillan DD. Prior leg massage decreases pain responses to heel stick in preterm babies. J Pediatr Child Health. 2006;42:505–8. doi: 10.1111/j.1440-1754.2006.00912.x. [DOI] [PubMed] [Google Scholar]
- 54.Kozub ML. The effect of therapeutic touch on pain response in infants receiving injections (unpublished MSN dissertation) Ohio: Medical College of Ohio at Toledo; Aug, 2001. [Google Scholar]
- 55.Sizun J, Ansquer H, Browne J, Tordjman S, Morin JF. Developmental care decreases physiologic and behavioral pain expression in preterm neonates. J Pain. 2002;3:446–50. doi: 10.1054/jpai.2002.128066. [DOI] [PubMed] [Google Scholar]
- 56.Catelin C, Tordjman S, Morin V, Oger E, Sizun J. Clinical, physiologic, and biologic impact of environmental and behavioral interventions in neonates during a routine nursing procedure. J Pain. 2005;6:791–7. doi: 10.1016/j.jpain.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 57.Johnston CC, Stremler RL, Stevens BJ, Horton LJ. Effectiveness of oral sucrose and simulated rocking on pain response in preterm neonates. Pain. 1997;72:193–9. doi: 10.1016/s0304-3959(97)00033-x. [DOI] [PubMed] [Google Scholar]
- 58.Johnston CC, Filion F, Nuyt AM. Recorded maternal voice for preterm neonates undergoing heel lance. Adv Neonatal Care. 2007;7:258–66. doi: 10.1097/01.ANC.0000296634.26669.13. [DOI] [PubMed] [Google Scholar]
- 59.Carbajal R, Veerapan S, Couderc S, Jugie M, Ville Y. Analgesic effect of breast feeding in term neonates: randomised controlled trial. BMJ. 2003;326:13. doi: 10.1136/bmj.326.7379.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gormally S, Barr RG, Wertheim L, Alkawaf R, Calinoiu N, Young SN. Contact and nutrient caregiving effects on newborn infant pain responses. Dev Med Child Neurol. 2001;43:28–38. doi: 10.1017/s0012162201000056. [DOI] [PubMed] [Google Scholar]
- 61.Ipp M, Taddio A, Goldbach M, Ben David S, Stevens B, Koren G. Effects of age, gender and holding on pain response during infant immunization. Can J Clin Pharmacol. 2004;11:e2–7. [PubMed] [Google Scholar]
- 62.Cohen LL, MacLaren JE, Fortson BL, et al. Randomized clinical trial of distraction for infant immunization pain. Pain. 2006;125:165–71. doi: 10.1016/j.pain.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 63.Bustos T, Jaaniste T, Salmon K, Champion GD. Evaluation of a brief parent intervention teaching coping-promoting behavior for the infant immunization context: A randomized controlled trial. Behav Modif. 2008;32:450–67. doi: 10.1177/0145445507309031. [DOI] [PubMed] [Google Scholar]
- 64.Stevens B, Johnston C, Franck L, Petryshen P, Jack A, Foster G. The efficacy of developmentally sensitive interventions and sucrose for relieving procedural pain in very low birth weight neonates. Nurs Res. 1999;48:35–43. doi: 10.1097/00006199-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 65.Elserafy FA, Alsaedi SA, Louwrens J, Bin Sadiq B, Mersal AY. Oral sucrose and a pacifier for pain relief during simple procedures in preterm infants: a randomized controlled trial. Ann Saudi Med. 2009;29:184–8. doi: 10.4103/0256-4947.52821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carbajal R, Chauvey X, Couderc S, Olivier-Martin M. Randomised trial of analgesic effects of sucrose, glucose, and pacifiers in term neonates. BMJ. 1999;319:1393–7. doi: 10.1136/bmj.319.7222.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bellieni CV, Bagnoli F, Perrone S, et al. Effect of multisensory stimulation on analgesia in term neonates: a randomized controlled trial. Pediatr Res. 2002;51:460–3. doi: 10.1203/00006450-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 68.Im H, Kim E, Park E, Sung K, Oh W. Pain reduction of heel stick in neonates: Yakson compared to non-nutritive sucking. J Trip Pediatr. 2008;54:31–5. doi: 10.1093/tropej/fmm083. [DOI] [PubMed] [Google Scholar]
- 69.Cramer-Berness LJ, Friedman AG. Behavioral interventions for infant immunizations. Child Health Care. 2005;34:95–111. [Google Scholar]
- 70.Cramer-Berness LJ. A comparison of behavioral interventions for infant immunizations (Doctoral dissertation) New York: State University of New York at Binghamton; 2005. p. AAT3203883. [Google Scholar]
- 71.Bauchner H, Vinci R, Bak S, Pearson C, Corwin MJ. Parents and procedures: A randomized controlled trial. Pediatrics. 1996;98:861–7. [PubMed] [Google Scholar]
- 72.Hillgrove Stuart J, Pillai Riddell R. Distraction as a pain management strategy for infants. 2008. (Unpublished master’s thesis)
- 73.Cong X, Ludington-Hoe SM, McCain G, Fu P. Kangaroo care modifies preterm infant heart rate variability in response to heel stick pain: Pilot study. Early Hum Dev. 2009;85:561–7. doi: 10.1016/j.earlhumdev.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnston CC, Filion F, Campbell-Yeo M, et al. Kangaroo mother care diminishes pain from heel lance in very preterm neonates. A crossover trial. BMC Pediatr. 2008;8:13. doi: 10.1186/1471-2431-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnston CC, Filion F, Campbell-Yeo M, et al. Enhanced kangaroo mother care for heel lance in preterm neonates: A crossover trial. J Perinatol. 2009;29:51–6. doi: 10.1038/jp.2008.113. [DOI] [PubMed] [Google Scholar]
- 76.Ludington-Hoe SM, Hosseini R, Torowicz DL. Skin-to-skin contact (kangaroo care) analgesia for preterm infant heel stick. AACN Clin Issues. 2005;16:373–87. doi: 10.1097/00044067-200507000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang CM, Tung WS, Kuo LL, Ying-Ju C. Comparison of pain responses of premature infants to the heelstick between containment and swaddling. J Nurs Res. 2004;12:31–40. doi: 10.1097/01.jnr.0000387486.78685.c5. [DOI] [PubMed] [Google Scholar]
- 78.Cignacco E, Hamers JP, Van Lingen RA, et al. Pain relief in ventilated preterm infants during endotracheal suctioning: A randomized controlled trial. Swiss Med Wkly. 2008;138:635–45. doi: 10.4414/smw.2008.12288. [DOI] [PubMed] [Google Scholar]
- 79.Diego MA, Field T, Hernandez-Reif M. Procedural pain heart rate responses in massaged preterm infants. Infant Behav Dev. 2009;32:226–9. doi: 10.1016/j.infbeh.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goubet N, Rattaz C, Pierrat V, Bullinger A, Lequien P. Olfactory experience mediates response to pain in preterm newborns. Dev Psychobiol. 2003;42:171–80. doi: 10.1002/dev.10085. [DOI] [PubMed] [Google Scholar]
- 81.Grunau RE, Linhares MB, Holsti L, Oberlander TF, Whitfield MF. Does prone or supine position influence pain responses in preterm infants at 32 weeks gestational age? Clin J Pain. 2004;20:76–82. doi: 10.1097/00002508-200403000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vivancos RBZ, Leite AM, Scochi CGS, dos Santos CB. The skin to skin contact at birth and newborn crying during vaccination against hepatitis B. [O cantata pele a pele ao nascimento e o choro de recem-nascidos durante vacinacao contra Hepatite B] Acta Paul Enferm. 2010;23:461–5. [Google Scholar]
- 83.Okan F, Ozdil A, Bulbul A, Yapici Z, Nuhoglu A. Analgesic effects of skin-to-skin contact and breastfeeding in procedural pain in healthy term neonates. Ann Trop Paediatr. 2010;30:119–28. doi: 10.1179/146532810X12703902516121. [DOI] [PubMed] [Google Scholar]
- 84.Aguirre Unceta-Barrenechea A, Saitua Iturriaga G, Sainz de Rozas Aparicio I, Riveira Fernandez D. Analgesia when taking heel-lance blood in the newborn. Anales de Pediatria. 2008;69:544–47. doi: 10.1016/s1695-4033(08)75237-8. [DOI] [PubMed] [Google Scholar]
- 85.Bueno M, Bussotti E, DaSilva A, Leao E. Non nutritive sucking and swaddling for pain relief in term neonates. Abstract from the 8th International Symposium on Pediatric Pain; Acapulco, Mexico. March 7 to 11, 2010. [Google Scholar]
- 86.Campos RG. Soothing pain-elicited distress with swaddling and pacifiers in early infancy. Child Dev. 1989;60:781–92. [PubMed] [Google Scholar]
- 87.Goubet N, Strasbaugh K, Chesney J. Familiarity breeds content? Soothing effect of a familiar odor on full-term newborns. J Dev Behav Pediatr June. 2007;28:189–94. doi: 10.1097/dbp.0b013e31802d0b8d. [DOI] [PubMed] [Google Scholar]
- 88.Rattaz C, Goubet N, Bullinger A. The calming effect of a familiar odor on full-term newborns. J Dev Behav Pediatr. 2005;26:86–92. doi: 10.1097/00004703-200504000-00003. [DOI] [PubMed] [Google Scholar]
- 89.Weissman A, Aranovitch M, Blazer S, Zimmer EZ. Heel-lancing in newborns: Behavioral and spectral analysis assessment of pain control methods. Pediatrics. 2009;124:921–6. doi: 10.1542/peds.2009-0598. [DOI] [PubMed] [Google Scholar]
- 90.Felt BT, Mollen E, Diaz S, et al. Behavioral interventions reduce infant distress at immunization. Arch Pediatr Adolesc Med. 2000;154:719–24. doi: 10.1001/archpedi.154.7.719. [DOI] [PubMed] [Google Scholar]
- 91.Morelius E, Theodorsson E, Nelson N. Stress at three-month immunization: parents’ and infants’ salivary cortisol response in relation to the use of pacifier and oral glucose. Eur J Pain. 2009;13:202–8. doi: 10.1016/j.ejpain.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 92.Ipp M, Parkin PC, Lear N, Goldbach M, Taddio A. Order of vaccine injection and infant pain response. Arch Pediatr Adolesc Med. 2009;163:469–72. doi: 10.1001/archpediatrics.2009.35. [DOI] [PubMed] [Google Scholar]
- 93.Taddio A, Illersich AL, Ipp M, Kikuta A, Shah V, HELPinKIDS Team Physical interventions and injection techniques for reducing injection pain during routine childhood immunizations: Systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin Ther. 2009;31(Suppl 2):S48–S76. doi: 10.1016/j.clinthera.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 94.Pillai Riddell R, Racine N. Assessing pain in infancy: The caregiver context. Pain Res Manag. 2009;14:27–32. doi: 10.1155/2009/410725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bowlby J. Attachment. 2nd edn. USA: Tavistock Institute of Human Relations; 1969/1982. [Google Scholar]