Abstract
Background
Youth with family history of alcohol abuse have a greater risk of developing an alcohol use disorder (AUD). Brain and behavior differences may underlie this increased vulnerability. The current study examined delay discounting behavior and white matter microstructure in youth at high-risk for alcohol abuse, as determined by a family history of alcoholism (FH+), and youth without such family history (FH−).
Methods
Thirty-three healthy youth (FH+ = 15, FH− = 18), ages 11 to 15 years, completed a delay discounting task and underwent diffusion tensor imaging (DTI). Tract Based Spatial Statistics (Smith et al., 2006), as well as follow-up region-of-interest analyses, were performed in order to compare fractional anisotropy (FA) between FH+ and FH− youth.
Results
FH+ youth showed a trend toward increased discounting behavior and had significantly slower reaction times on the delay discounting paradigm compared to FH− youth. Group differences in FA were seen in several white matter tracts. Furthermore, lower FA in the left inferior longitudinal fasciculus and the right optic radiation statistically mediated the relationship between FH status and slower reaction times on the delay discounting task.
Conclusion
Youth with a family history of substance abuse have disrupted white matter microstructure, which likely contributes to less efficient cortical processing, and may act as an intrinsic risk-factor contributing to an increased susceptibility of developing AUD. In addition, FHP youth showed a trend toward greater impulsive decision making, possibly representing an inherent personal characteristic that may facilitate substance use onset and abuse in high-risk youth.
Keywords: adolescence, delay discounting, diffusion tensor imaging, alcohol abuse, family history
Introduction
A number of factors can increase one’s risk for developing an alcohol use disorder (AUD), including a family history of alcoholism (FH+). FH+ youth have a significantly greater risk for developing an AUD than their peers (Lieb et al., 2002; Merikangas et al., 2009). Adolescents with a family history of alcohol often begin using at younger ages (McGue et al., 2001), have greater severity of use (Chassin et al., 2004), and have a higher incidence of AUD than the general population (Milberger et al., 1999) Despite this emerging evidence supporting increased risk in FH+ youth, the neural mechanisms that underlie this vulnerability remain to be elucidated. One possibility is that premorbid neurobiological differences and behavioral phenotypes may exist in FH+ youth that contribute to the heritable aspects of AUD.
Ample evidence exists showing that prior to any substance use, FH+ youth exhibit behavioral phenotypes distinct from their peers. Family history of AUD has been associated with cognitive deficits in substance-naïve adolescents, as evidenced by poor performance on tests of executive function, attention, and spatial memory (Corral et al., 1999; Lovallo et al., 2006; Nigg et al., 2004; Tapert and Brown, 2000). In addition to cognitive deficits, FH+ individuals may display more impulsive behavior which could contribute to their increased risk for developing AUD. For example, self-reports of impulsivity have been shown to mediate the relationship between family history of alcohol abuse and increased alcohol problems (Deckel and Hesselbrock, 1996; Sher, 1991). Furthermore, the animal literature suggests that impulsive decision making, as measured by a preference for immediate rewards over larger, delayed rewards (delay discounting), may precede alcohol use and might increase one’s risk of developing an AUD. Specifically, inbred rats show strain-specific differences in discounting rates (Wilhelm and Mitchell, 2009), indicating a heritable component to discounting behavior. Furthermore, substance-naïve mice (Oberlin and Grahame, 2009; Wilhelm et al., 2007), selectively bred for high-alcohol intake preference, have higher rates of discounting delayed rewards compared to low-alcohol intake preferring lines. However, little research has been done to determine if FH+ individuals show similar behavior. In fact, only one study has examined impulsive decision making in FH+ adults, and found that FH+ females had higher discounting rates compared to controls (Petry et al., 2002); however, given that they examined only average drinking adults, their sample may have excluded the FH+ individuals who had already developed an abuse disorder. Nonetheless, these findings suggest that greater delay discounting may be an inheritable trait that predates drug and alcohol use in youth at high risk for alcoholism.
In addition to distinct behavioral phenotypes, a variety of structural and functional brain differences have been noted in FH+ populations that may directly contribute to an increased risk for AUD. During inhibition, individuals with high genetic risk display less frontal cortical activity (Schweinsburg et al., 2004) and reduced P300 amplitudes (Kamarajan et al., 2005), indicative of an overall decrease in cortical control. Furthermore, FH+ youth have been shown to have smaller amygdalae (Hill et al., 2001), and larger cerebellums (Hill et al., 2007) compared to aged matched controls. Moreover, Hill and colleagues (Hill et al., 2009) recently showed that FH+ adolescents had decreased right to left white matter volume in the orbital frontal cortex, which was related to greater impulsive tendencies on a multidimensional personality questionnaire. To date, however, no study has explored white matter microstructure in FH+ adolescents. It is possible that white matter microstructure may be an additional distinct neuroanatomical phenotype for increased risk for developing AUD. Furthermore, because white matter microstructure has been related to discounting behavior in youth (Olson et al., 2009), any microstructural differences in white matter may relate to greater impulsive choice in FH+ youth - ultimately contributing to increased risk for developing an AUD.
One way of examining white matter microstructure is by using diffusion tensor imaging (DTI). DTI is a non-invasive imaging technique that allows for the directional measurement of water diffusion in order to make indirect inferences about white matter fiber microstructure (Basser, 1995). A primary outcome variable from DTI is fractional anisotropy (FA), which is an assessment of restricted, or anisotropic, diffusion. Higher FA values are believed to represent an increase in axon fiber bundle diameter, an increase in myelination, and/or more coherent fiber organization (Beaulieu, 2002). Therefore, the current study examined white matter microstructure using DTI, impulsive decision making as measured by discounting behavior, as well as the relationship between the two, in FH+ and control (FH−) youth. Given previous findings that FH+ youth possess distinct structural and functional prefrontal cortex abnormalities (Hill et al., 2001; Hill et al., 2007; Hill et al., 2009; Kamarajan et al., 2005; Schweinsburg et al., 2004), we hypothesized that FH+ youths would show atypical white matter microstructure, as evidenced by decreases in FA, compared to FH− peers. Furthermore, based on the animal and adult human literature (Oberlin and Grahame, 2009; Petry et al., 2002; Wilhelm and Mitchell, 2009), we hypothesized that FH+ youth would show more impulsive decision making than FH− controls as measured by the delay discounting task. Lastly, because reduced FA has been shown to relate to increased discounting of delayed rewards (Olson et al., 2009), our third hypothesis was that more impulsive decision making in FH+ youth would be associated with lower values of fractional anisotropy in regions that show group differences in white matter microstructure.
Materials and Methods
Participants
Participants included 15 FH+ (7 females, 8 males) and 18 FH− (9 females, 9 males) substance naïve youth, ages 11 to 15 years. All participants were recruited and underwent comprehensive structured interviews as part of an ongoing study focused on adolescent neurodevelopment. Briefly, following written consent and assent from all youth and their parents in accordance with Oregon Health & Science University’s Institutional Review Board, separate structured telephone interviews were conducted with both the youth and one of their parents. Interviews consisted of the Diagnostic Interview Schedule for Children Predictive Scales (DISC-PS-4.32b)(Hoven et al., 2005; Lucas et al., 2001), the Family History Assessment Module (FHAM) (Rice et al., 1995), the Brief Lifetime version of the Customary Drinking and Drug Use Record (Brown et al., 1998), and the Structured Clinical Interview (SCI)(Brown et al., 1994). Exclusionary criteria for youth included the inability of a parent to provide family history information, lifetime history of a diagnosed DSM-IV psychiatric disorder, significant substance use (10 lifetime alcoholic drinks or 2 drinks/occasion, > 5 uses of marijuana, any other drug use, or > 4 cigarettes per day), neurological illness, significant head trauma (loss of consciousness > 2 minutes), serious medical problems, mental retardation or learning disability, prenatal exposure to drugs or alcohol, reported history of psychotic disorders in biological parents (i.e. bipolar 1 or schizophrenia), left-handedness (Edinburgh Handedness Inventory, (Oldfield, 1971)), irremovable metal, and pregnancy.
Classification of Family History Alcohol and Substance Use/Dependence
Dichotomizing individuals based on first, or first and second degree relatives with a AUD, has been shown to be a robust measure of substance abuse vulnerability, as it significantly relates to drug dependence, tolerance, and withdrawal (Stoltenberg et al., 1998). Thus, during the structured telephone interview with both the youth and their parent, the FHAM was used to assess DSM-IV criteria for substance abuse and dependence of first and second degree relatives. Based on the information provided by on the FHAM, youth were then categorized as either family history positive or negative. Youth were considered family history positive (FH+) if a history of alcohol abuse and/or dependence was reported for one, or both, biological parents, or two or more second degree relatives on either the maternal or paternal side of the family; a total absence of substance abuse/dependence among relatives was considered family history negative (FH−). In addition, a family history density (FHD) score was computed for each participant; parents and second degree relatives with a history of an AUD were given a score based on their familial relatedness to the participant. Parents received a .5, grandparents a .25, and maternal and paternal aunts and uncles received a weight ratio of .25 divided by the total number of siblings. Scores are summed across relatives, resulting in FHD scores that ranged from 0 to 1.50 in the current sample.
Participant Characteristics
In order to provide an estimate of overall intellectual functioning, youth were administered the 2-subtest version of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), as IQ has been shown to relate to discounting behavior (de Wit et al., 2007; Funder and Block, 1989; Olson et al., 2007), and some studies have shown lower IQ scores in individuals with a family history of abuse (Ozkaragoz et al., 1997). Pubertal maturation was assessed using the Pubertal Development Scale (Petersen et al., 1988), which has been shown to correlate significantly with other measures of pubertal status, including physician ratings (Petersen et al., 1988) and Tanner’s Sexual Maturation Scale self-ratings (Albertsson-Wikland et al., 1997). The Edinburgh Handedness Inventory was used to verify right-handedness (Oldfield, 1971). The Childhood Behavior Checklist was completed by parents to assess externalizing symptoms (Achenbach and Rescorla, 2001). Information was gathered on socioeconomic status by administering the Hollingshead Index of Social Position (ISP) to parents as part of the structured telephone interview. The Hollingshead ISP determines socioeconomic status based on occupation and educational attainment of each parent (Hollingshead, 1975).
Delay Discounting
A self-paced, computerized version of the delay discounting paradigm, as reported by Mitchell (Mitchell, 1999), was administered to subjects. Briefly, the task presented a series of choices between a standard monetary amount available after a delay and a variable monetary amount available immediately. The delay item was $10.00 available after a delay of 0, 7, 30, 90, 180, or 365 days. The immediate option was an amount of money ranging from $0–$10.50 available after 0 days (now). The standard delay and one variable immediate option were presented in random order to make up a total of 138 questions, and participants were asked to choose the option (the standard larger delayed option or the smaller immediate variable option) they preferred for each question. In order to enhance the saliency of the task, participants were informed that following the task, one of their choices would be reinforced (i.e. they would receive the respective amount of money chosen either immediately or at the given delay). Reaction time (RT, the time between presentation of the two choices and the subject’s response) was collected for each trial.
Participants usually prefer the smaller variable money immediately if the value is high (e.g., $10.50), but the standard delayed option when the variable option is low (e.g., $ 0.25). The point at which the subject’s preference switches from the variable immediate to the standard delayed option is termed the indifference point. Thus, indifference points were defined for each of the six delays as being the midway point between the lowest value of the smaller, immediate preferred, and the highest value of the immediate rejected. Trials falling between the lowest value of the preferred and the highest value of the rejected for the 7, 30, 90, 180, and 365 delays were considered hard choices, and all other trials outside of this indifference region were considered easy choices. Trials that offered an immediate amount of money and $10 at a 0 delay were used as control trials. The rate of discounting, also known as k, was determined by fitting the 6 indifference points to the hyperbolic equation: V =A/ (1+kD) for each group’s, as well as to each individual’s indifference points. In this equation, V, A, and D are known; V represents value of the $10 reward at a given delay, A represents amount of the delayed reward ($10), and D represents the delay associated with the $10 reward. Using these variables, the unknown value, k, can then be solved to determine the rate of reward devaluation.
Imaging
Subjects were scanned on a 3.0 Tesla Siemens Magnetom Tim Trio system (Siemens Medical Solutions, Erlangen, Germany) with a twelve channel head coil at OHSU’s Advanced Imaging Research Center (AIRC). Whole-brain, high-resolution structural anatomical images were acquired in the sagittal plane using a T1 weighted MPRAGE scanning sequence (TI = 900ms, Flip Angle = 10 degrees, TE = 3.58 ms, TR = 2300 ms, acquisition matrix = 256×240, slice thickness = 1.1mm). T2-weighted diffusion weighted images were acquired oblique to the AC-PC, using a high-angular resolution EPI sequence (TR = 9500ms, TE = 95 ms, FOV= 240 mm2, 72 slices, slice thickness = 2mm). Gradient encoding pulses were applied in 20 directions with a b-value of 1000s/mm2, 4 diffusion weighted runs were collected with 3 b0 (non-diffusion weighted) images per run. Previous research has shown that 20 diffusion gradient directions allow for calculating reliable FA measurements (Li et al., 2005; Ni et al., 2006).
Image Processing and Analysis
Eddy current effects, magnetic field inhomogeneities, and head motion were corrected using FMRIB’s Diffusion Toolbox and Utility for Geometrically Unwarping EPIs (Jenkinson, 2003). The 4 DWI runs were aligned using linear (affine) registration to one another using FMRIB’s Linear Image Registration Tool (Jenkinson et al., 2002), averaged, and brain-extracted using BET (Smith, 2002). Based on the 6 motion parameters established by the affine registration of the b0s from each of the four runs, an average root mean square was determined. This was used as an estimate of movement, and was used as a covariate during hypothesis testing. AFNI (Cox, 1996) was then used to calculate the diffusion tensor and identify the eigenvalues of the tensor (λ1, λ2, λ3) for each voxel. Fractional anisotropy (FA) was determined for each voxel using AFNI’s nonlinear computational algorithm (3dDWItoDT; (Cox, 1996)).
Voxelwise statistical analysis was performed on FA maps using Tract Based Spatial Statistics (TBSS) version 1.2 (see (Smith et al., 2006; Smith et al., 2007)). First, a common registration target image was identified out of all individual subjects’ FA maps and affine aligned to standard MNI152 space. Second, each subject’s FA map was nonlinearly registered to this common target using FMRIB’s Non-linear Image Registration Tool (Andersson et al., 2007a; Andersson et al., 2007b), and aligned FA images were averaged to create a group-wise mean FA map. Third, a white matter skeleton, representing only the major tracts that are common across all subjects, was created (see (Smith et al., 2006; Smith et al., 2007) for more detail). A mean FA threshold of 0.2 was applied to the white matter skeleton to reduce partial volume effects (Smith et al., 2006). Fourth, each subject’s aligned FA image was projected onto the white matter skeleton for subsequent voxelwise group level statistics.
Voxelwise independent sample t-tests were performed on the FA white matter skeleton to examine mean FA between FH+ and FH− youth (Cox, 1996). To correct for multiple comparisons, a Monte Carlo simulation (Cox, 1996) was performed. This method takes into account both individual voxel probability thresholding (p <.025) and cluster size to correct for inflated rates of type 1 error. The results indicated that 23 contiguous voxels exceeding a t-threshold of 2.75, p <.01, (cluster volume ≥ 23 µL, clusterwise α < .01) were necessary to consider a cluster significant using a 2.6 mm FWHM intrinsic smoothing. Mean FA values were extracted from the significant between-group FA clusters for each individual in order to examine the relationship between FA values and discounting behavior. White matter tracts were identified using Wakana and colleagues DTI fiber atlas (Wakana et al., 2004).
Region-of- Interest (ROI) Follow-up Analyses
To confirm group differences in FA, follow-up comparisons were performed using region-of-interest (ROI) analyses on the regions TBSS identified as different between groups. Specifically, using the center of mass coordinates from the clusters identified as significant by TBSS (see Table 2), 3.5 mm radius spherical ROIs were created. Each ROI was then projected onto individual subjects’ MNI-aligned FA images, and the mean FA for each ROI was extracted and imported to SPSS. Student’s t-tests were used to test for mean group differences in FA for each ROI. In addition, mean FA images were created for each group by separately averaging MNI-aligned FA images of FH+ and FH− youth. ROIs were then overlayed on each group mean FA map in order to qualitatively examine each ROI with respect to the underlying anatomy.
Table 2.
Group differences in FA. For each cluster, the location of significant voxels, the name(s) of the major fiber tract, the position of the center of the cluster in MNI space, the size, as well as the corresponding t-value and the effect size (Cohen’s d) are listed.
| Location | White Matter Tract | X | Y | Z | Voxels mm3 |
t-value | Cohen’s d | % FA Difference |
|---|---|---|---|---|---|---|---|---|
| FH− > FH+ | ||||||||
| Dorso-medial prefrontal | R anterior scr | 20 | 13 | 40 | 43 | 3.20 | 1.17 | 13.4 |
| R anterior scr | 20 | 32 | 25 | 30 | 3.26 | 1.19 | 14.5 | |
| Dorso-lateral prefrontal | L slf | −41 | −4 | 24 | 25 | 3.23 | 1.18 | 10.8 |
| Superior/Middle Temporal | R ec/ifo | 29 | 13 | −6 | 30 | 3.32 | 1.21 | 12.3 |
| Inferior Temporal | L ilf | −56 | −37 | −6 | 29 | 3.36 | 1.14 | 24.7 |
| Subcortical | L alic | −19 | 13 | 6 | 29 | 3.14 | 1.23 | 13.1 |
| Occipital lobe | R or | 27 | −76 | 7 | 26 | 3.23 | 1.18 | 14.5 |
| FH+ > FH− | ||||||||
| Parietal lobe | L posterior scr/cs | −38 | −61 | 29 | 82 | −3.28 | 1.20 | 50.7 |
| L posterior scr/cs | −43 | −63 | 27 | 25 | −3.15 | 1.15 | 40.5 |
Abbreviations: R = right; L = left; alic = anterior limb of the internal capsule; cs = centrum semiovale; ec = extreme capsule; inferior-frontal fasciculus; ilf = inferior longitudinal fasciculus; oc = optic radiation; scr = superior corona radiata; slf = superior longitudinal fasciculus.
Statistical Analysis
All statistical analyses were carried out using SPSS (Version 15; SPSS, Chicago, Illinois). Normality was verified using Shapiro-Wilk tests, and appropriate non-parametric tests or transformations were used when dealing with violations of normality. FH+ and FH− groups were compared on demographic variables using a 2-tailed Student’s t-test and χ2. Group differences on discounting behavior were analyzed using 2-tailed Student’s t-tests and Mann-Whitney U tests. Planned secondary analyses examined associations between delay discounting behavior and IQ and age using Pearson’s r correlations. 2-tailed Student’s t-tests were used to examine RT and control trial RT between the groups. Repeated measures ANOVA was performed to examine the effects of delay, trial type and group on delay discounting reaction times. Regression analysis was used to examine if FA predicted discounting behavior (log-transformed k values). Furthermore, separate regression analyses were performed to determine if FHD predicted discounting behavior and FA. Due to the preliminary nature of this study and the relatively small sample sizes, p < .05 was considered significant, and p < .10 a trend.
Results
Demographics
Participant demographic information is presented in Table 1. One subject did not have IQ data, due to a technical error. FH+ youth did not significantly differ from controls on age [t (31) = .001, p = 1], pubertal status [t (31) = −.17, p = .87], externalizing symptoms [t (31) = −1.30, P =.20], SES (Hollingshead) [t (31) = −1.32, p = .20], IQ [t (30) = .97, p = .34], or GPA [t (31) = 1.21, p = .23].
Table 1.
Demographic data of subjects by family history group. All values are means and standard deviations, unless otherwise noted.
| FH+ (n=15) | FH− (n=18) | |
|---|---|---|
| Female (n) | 7 | 9 |
| Age | 13.79 (1.45) | 13.79 (1.55) |
| Pubertal maturation a | 2.71 (.76) | 2.66 (.79) |
| Caucasian (%) | 86.7 | 94.4 |
| Family History Density | .63 (.30) | 0 |
| Full scale IQ b | 117.07 (9.19) | 120.41 (10.18)e |
| Externalizing symptoms T-score c | 46.3 (10.8) | 41.8 (9) |
| GPA | 3.44 (.42) | 3.61 (.38) |
| SES d | 28 (12.81) | 22.72 (10.73) |
Developmental Pubertal Scale,
WASI,
Childhood Behavioral Checklist,
Hollingshead Index of Social Position (ISP),
Denotes n = 17, due to data collection error.
Delay Discounting
Consistent and valid discounting behavior is reflected by a decrease in indifference points as the time to receive the reward increases. As put forth by Johnson and Bickel for identifying nonsystematic discounting behavior outliers, an increase in subjective value as delay increases is thought to reflect unreliable discounting and a failure to show minimal discounting may indicate invalid task performance (Johnson and Bickel, 2008). Based on these a priori criteria for defining outliers, 4 participants (1 FH+ and 3 FH− youth) failed to display reliable discounting for delayed rewards, and were thus excluded from the analyses. Specifically, three subjects repeatedly showed an increase in subjective value with an increase in delay, whereas one subject always picked the delayed choice.
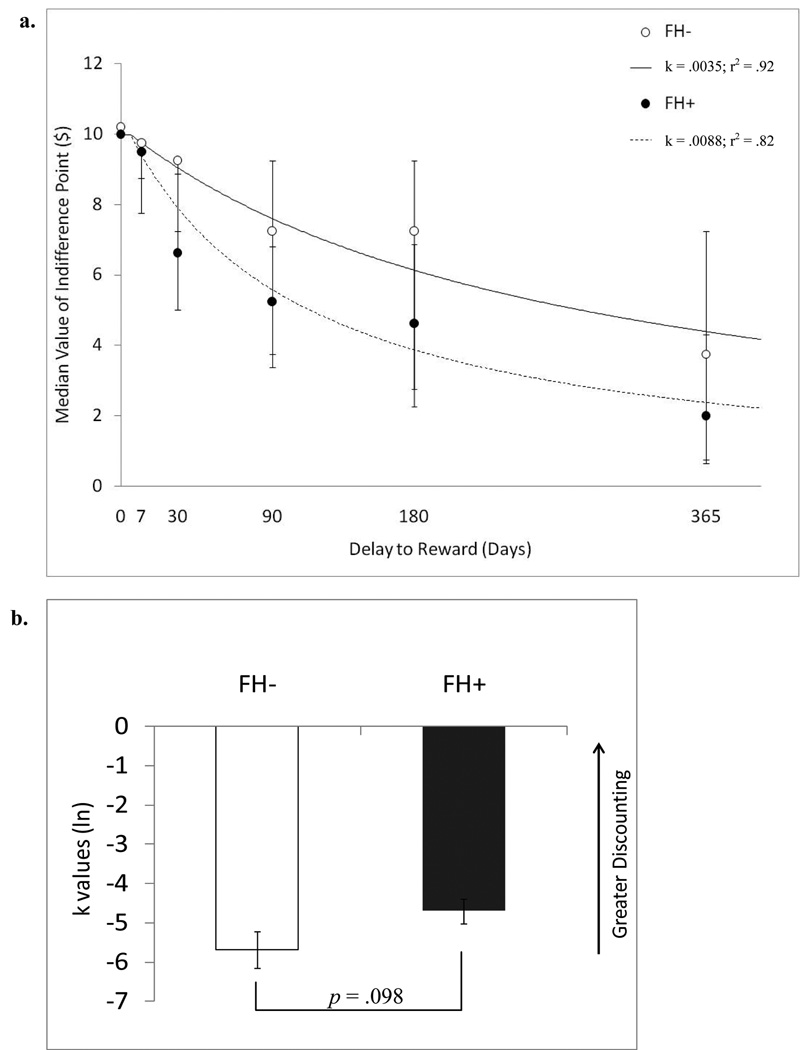
Figure 1a shows the median indifference points for FH+ and control youth. FH+ youth were lower on all five of the delays (7, 30, 90, 180, 365) compared to FH− youth. Mann-Whitney U tests revealed that indifference points were significantly different between the groups at the 30 day delay [z = −2.1, p = .04], however this did not pass multiple comparison correction. Furthermore, indifference points were not significantly different between the groups at 0 [z = 0, p = 1], 7 [z = −.47, p = .68], 90 [z = −1.5, p = .13], 180 [z = −1.2, p = .25], and 365 [z = −.85, p = .4] day delays. The distributions of k-values for individuals were skewed, so a natural logarithm of k was used in all further analyses. A Student’s t-test of log-transformed k-values between the groups revealed a trend for a larger rate of discounting (k-value) in the FH+ compared to FH− youth [t (27) = −1.76, p = .098, Cohen’s d = .68] (Figure 1b). However, FHD scores did not relate to discounting behavior [R2 = .108, p = .25]. While others have shown delay discounting rates to change across adolescence (Olson et al., 2009) and to relate to IQ (de Wit et al., 2007), secondary analyses showed no significant relationships between k-values and age [r(29) = −.1, p = .61], or k-values and estimated Full-scale IQ [r(29) = −.186, p = .34] in this group of adolescents.
Figure 1.
a.) Delay discounting in FH− and FH+ youth. Median subjective values of $10 reward delayed in time from 0 to 365 days. The group median indifference point for each of the delays is plotted, along with the inter-quartile range for each point (error bars). The lines reflect the fit of the hyperbolic function V =$10/ (1+kD) to the median group indifference points. The rate of discounting for each group (k-value) and the goodness of fit for each function (r2 × 100%) are also indicated. Note that the filled circles have been slightly offset on the x-axis for the 0 and 7 delay points to make them visible. b.) Delay discounting in FH− and FH+ youth. Mean (ln) k-values when the hyperbolic function is fitted to the data for each participant; smaller (ln) k-values indicate greater discounting. Error bars represent SEM.
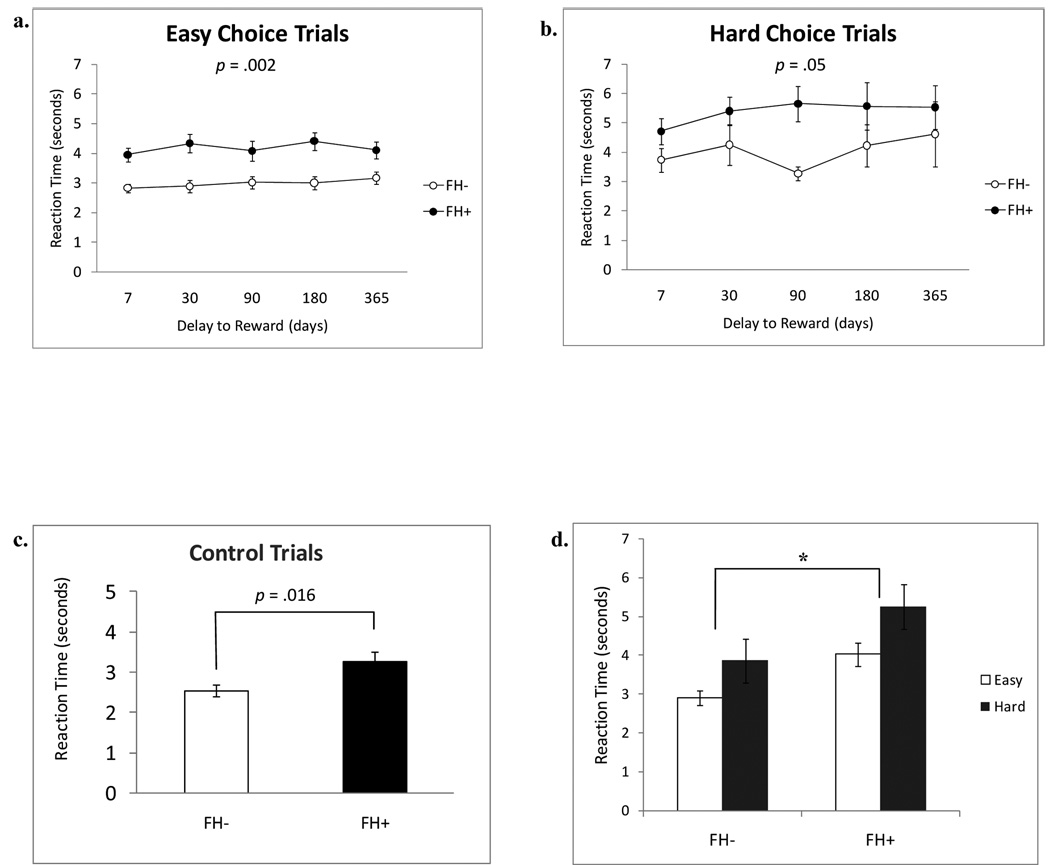
In addition to a trend in the rate of delay discounting (k-values) between the two groups, FH+ youth had significantly slower reaction times on delay choice trials [t(27) = −2.71, p < .02]. In order to clarify these findings, exploratory analyses were performed to examine reaction times between the groups during “easy”, “hard”, and “control” choice trials on the task. Choices made near an individual’s indifference point on any given delay require more contemplation (i.e. one should have longer reaction times) and have been described as “hard choices”, whereas choices made far from an individual’s indifference point are “easy choices” (Hoffman et al., 2008; Robles and Vargas, 2007; Robles and Vargas, 2008), and thus should be made more quickly. Control trials, however, ask between $10 now and a smaller variable amount of money now, and thus reaction times on these trials does not directly relate to discounting. Exploratory analyses revealed that one FH− youth was a consistent outlier on RT across multiple easy and hard choice trials, as defined by greater than 2.5 standard deviations from the mean, and thus was excluded from subsequent analyses. Figures 2a and 2b show average reaction times for easy and hard choice trials across the five delays (7, 30, 90, 180, 365) for both groups. After correcting for the violation of unequal variance (Levene’s Test F= 6.81, p = .015), a student’s t-test showed that FH+ youth had significantly slower RT on control trials compared to FH− youth [t(21.1) = −2.61, p = .016, Cohen’s d = 1.14] (Figure 2c). Furthermore, a two-within (delay, choice type i.e. easy or hard), one between (group), repeated measures ANOVA was performed to look at group differences in reaction time across delay and trial choice type. The sphericity assumption was not met [Mauchly’s W = .36, df = 9, p = .003], so a Greenhouse-Geisser correction was used to determine significance. There was a significant main effect of choice type [F (1, 26) = 19.41, p < .001, partial η2 = .43], with considerably longer reaction times for harder than easy choice trials. Furthermore, a between-subjects main effect of family history status was significant [F (1, 26) = 7.70, p = .01, partial η2 = .23]. Simple effects analyses showed that this group difference was seen for both easy and hard choice reaction times [easy choice RT: [F (1, 26) = 12.20, p = .002, partial η2 = .32; hard choice RT: F (1, 26) = 4.22, p = .05, partial η2 = .14]. FHD scores were not associated with overall RT [R2 = .05, p = .47].
Figure 2.
Reaction times (± SEM) plotted for each of the six delays for both groups on a.) easy and b.) hard choice trials. FH− (open circles) and FH+ (filled circles). Group differences were seen for both easy and hard choice reaction times [easy choice RT: F (1, 26) = 12.20, p = .002; hard choice RT: F (1, 26) = 4.22, p = .05]. c.) Reaction times (± SEM) on control trials for each group [t(21.1) = −2.61, p = .016]. d.) Summary graph showing easy and hard choices collapsed across the six delays for both groups. * denotes p = .01 between-subjects main effect on reaction time.
Given that FH+ youth showed significantly slower RT on control trials compared to FH− youth, further exploratory analyses were conducted to examine if these baseline differences in RT accounted for group differences on the delay discounting task. Thus, regression analyses were performed to examine group differences in rates of discounting (k-values), while controlling for control trial RT. Results showed that group differences in discounting rates were not significant when accounting for differences in control trial RT [Overall model R2 = .13; FH status: β = .20; F (2, 26) = 1.94, p = .16]. Furthermore, ANCOVA was performed to examine group differences in reaction time across delay and trial type while controlling for control trial RT. Similarly, once accounting for baseline control trial differences in RT, significant group differences in RT were no longer seen for easy and hard choice trials [F (1, 26) = .13, p = .72].
White Matter Microstructure
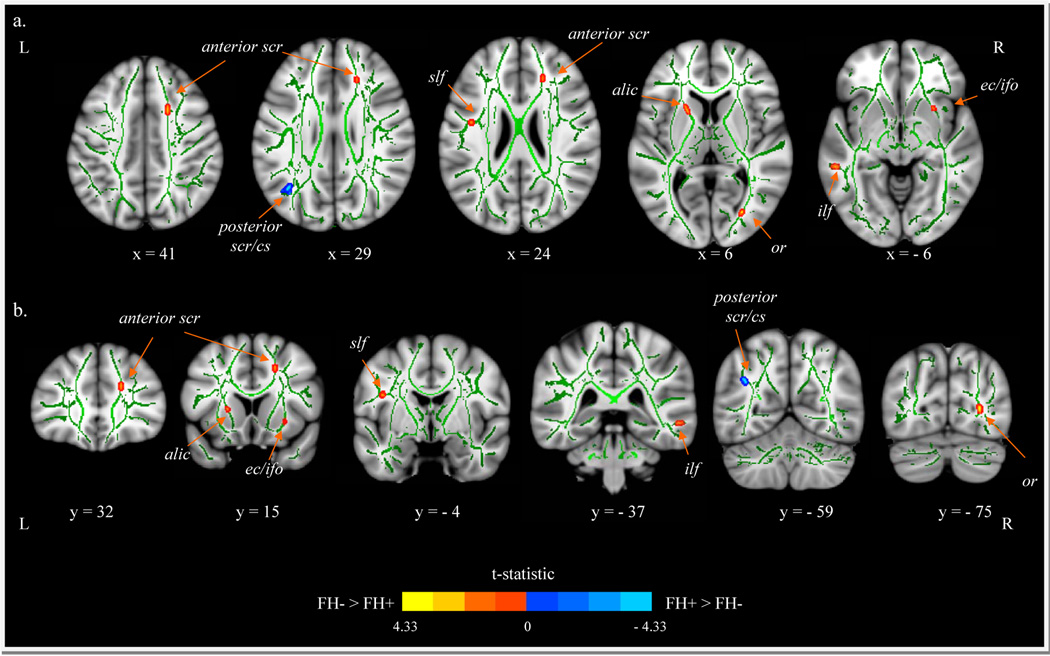
Due to equipment failure, DTI was not acquired for one FH+ subject, resulting in diffusion tensor data for 32 participants. The results of the independent sample t-tests are shown in Table 2. Listed are the locations of significant clusters, the names of the major fiber tract, the position of the center of the cluster in MNI space and its size, as well as the corresponding t-value, the effect size (Cohen’s d), and the % FA difference [ (|FH+ − FH−|)/(FH−)*100] for each cluster. FH+ youth had lower FA compared to FH− youth in the right anterior superior corona radiata (scr), left superior longitudinal fasciculus (slf), right extreme capsule (ec), left inferior longitudinal fasciculus (ilf), left anterior limb of the internal capsule (alic), and the right optic radiation (oc) (see Figure 3; red clusters). Furthermore, higher FA in FH+ group compared to controls was seen in the left posterior superior corona radiata (scr)/centrum semiovale (cs) (see Figure 3; blue clusters). FHD did not relate to FA in any of the regions in which significant differences in FA were seen between the groups. Lastly, movement was not significantly different between groups [t(30) = .63, p = .53], and all clusters remained significant (p < .001) after statistically controlling for movement.
Figure 3.
a.) axial and b.) coronal views of group differences in FA, represented by t-statistic maps overlaid on the mean FA skeleton (green) and a standardized brain. Red-yellow = FA is greater in FH− compared to FH+ youth. Blue-Light Blue = FA is greater in FH+ youth compared to FH− youth. Results have been dilated for viewing purposes. Abbreviations: R = right; L = left; alic = anterior limb of the internal capsule; cs = centrum semiovale; ec = extreme capsule; inferior-frontal fasciculus; ilf = inferior longitudinal fasciculus; oc = optic radiation; scr = superior corona radiata; slf = superior longitudinal fasciculus.
Region-of- Interest (ROI) Follow-up Analyses
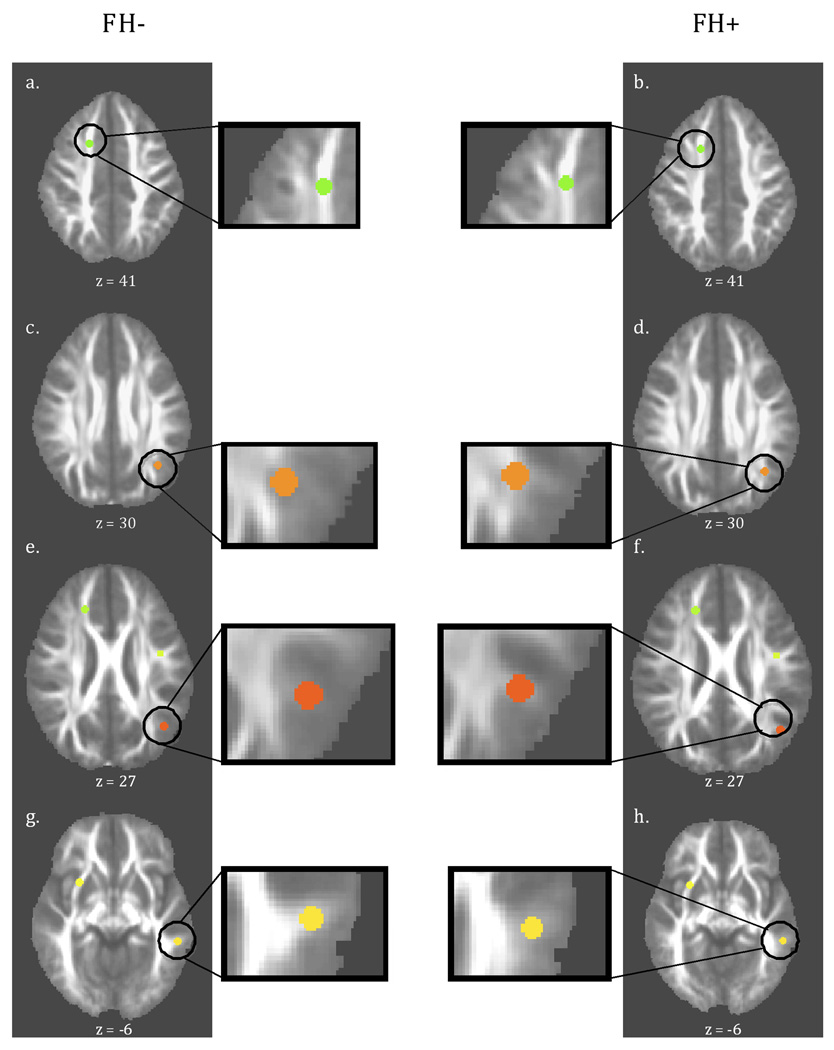
Follow-up ROI analyses, based on 3.5mm spheres grown from the center of mass from each significant TBSS cluster, confirmed that all 9 clusters (identified above) remained significantly different between FH+ and FH− youth (p < .002). Furthermore, scatterplots of mean FA values verified that group differences in each ROI and TBSS cluster were not due to outliers. However, qualitative examination of ROIs overlayed on group mean FA maps revealed that while 6 out of the 9 ROIs fell in white matter tracts (as deemed by high FA) in both groups, the two left parietal ROIs and the left inferior temporal ROI appeared to be in regions with a great deal of tissue heterogeneity. Specifically, both parietal ROIs were in the FH+ youth’s left posterior superior corona radiata (scr)/centrum semiovale (cs), while these ROIs were more susceptible to partial volume effects in FH− youth (see Figure 4). Similarly, the left temporal ROI clearly fell in a white matter tract in the inferior longitudinal fasciculus for FH− youth, but was less clearly in a major white matter region in FH+ youth (see Figure 4). In fact, for these three ROIs, a mean FA value of ≤0.2 was seen for one of the groups, confirming that these significant results may be influenced by differences in the amount of partial volume effects between the groups (Smith et al., 2006). Thus, while ROI analyses confirmed group differences for the majority of clusters, caution should be taken when interpreting the significant differences seen in the posterior parietal and the inferior temporal lobe between FH+ and FH− youth.
Figure 4.
Representative number of ROIs overlaid onto axial group mean FA images for FH− and FH+ youth. Most ROI analyses confirmed TBSS results and fell in areas of high FA representing white matter tracts in both groups (a & b). Inset pictures qualitatively show how partial volume effects are different between the groups and may contribute to significant group differences for the particular ROIs in the parietal and inferior temporal lobe (e, f, g, h).
Relationship Between Discounting Behavior and FA
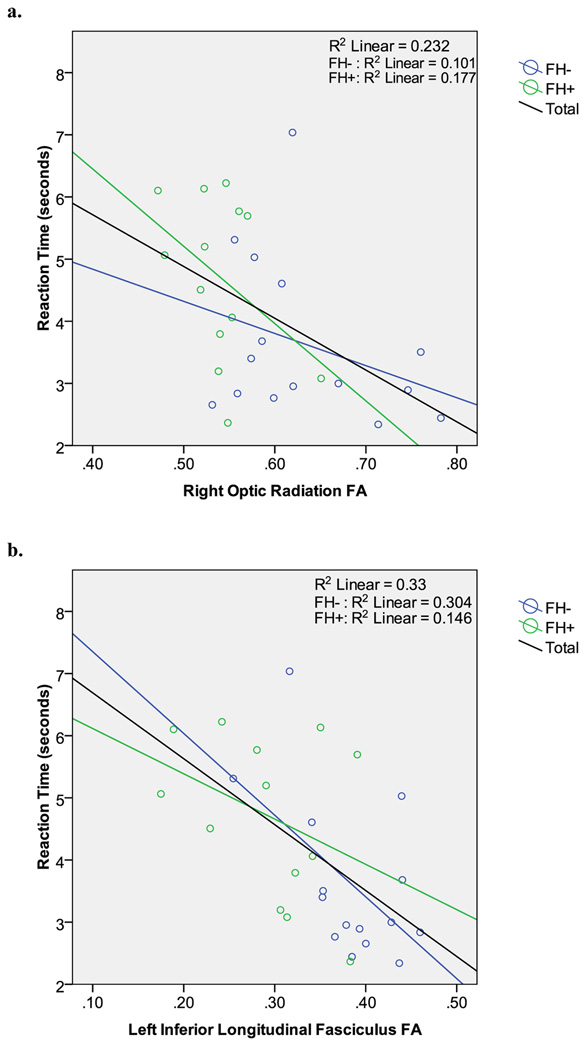
Regression analysis revealed that FA did not predict greater discounting in any of the 9 regions. Due to the unexpected findings that reaction time was significantly different between the groups, and that FA has been shown to be associated with RT (Gold et al., 2007; Gold et al., 2008; Salo et al., 2009; Silveri et al., 2006), we performed post-hoc analyses to examine the relationships between reaction times and FA. Median reaction times across all trials for each subject was computed and used for subsequent analyses. Regression analysis revealed higher FA in the left inferior longitudinal fasciculus tract of the temporal lobe [R2 = .33; β = −.58; F (1, 26) = −3.58, p = .001], and the right optic radiation [R2 = .23; β = −.48; F (1, 26) = −2.79, p = .01] were significant predictors of faster RT (Figure 5). Follow-up analyses showed that FA in the left inferior longitudinal fasciculus mediated the relationship between family history status and reaction time, as family history status no longer accounted for a significant proportion of the variance in reaction time after FA was entered into the model [Overall model R2 = .33; FA: β = −.53; F (2, 25) = −2.6, p = .02; FH status: β = .07; F (2, 25) = .35, p = .72]. In addition, analyses using Fairchild and MacKinnon’s model for examining mediation and moderation (Fairchild and MacKinnon, 2009), confirmed that FA was a mediating factor [Sobel’s test: t = 2.64, p = .008]. Similarly, FA in the right optic radiation also mediated the relationship between family history status and reaction time [Overall model R2 = .25; FA: β = −.38; F (2, 25) = −2.6, p = .09; FH status: β = .07; F (2, 25) = .16, p = .44; Sobel’s test: t = 2.24, p = .025]
Figure 5.
Scatter plot of reaction time and FA for the a.) right optic radiation [R2 = .23; β = −.48; F (1, 26) = −2.79, p <. 05] and the b.) left inferior longitudinal fasciculus [R2 = .33; β = −.58; F (1, 26) = −3.58, p < .001].
Discussion
The current study examined white matter microstructure and delay discounting in FH+ and FH− youth. Our results show that prior to any substance use, FH+ youth have differences in FA in segments of specific white matter tracts compared to FH− youth. Furthermore, FH+ youth tended to have increased rates of discounting behavior, and were significantly slower when choosing between immediate and delayed rewards compared to controls. FA in the right optic radiation and left inferior longitudinal fasciculus mediated the relationship between family history status and reaction time on this task.
Adult alcoholics have consistently been shown to discount delayed rewards more heavily than non-users (Petry, 2001). Likewise, similar results have been seen in the few studies that have examined discounting behavior in heavy drinking youth (Audrain-McGovern et al., 2004; Field et al., 2007). Furthermore, it has been proposed that drug addiction may result from an individual choosing the more immediate reward (drugs) over more rewarding delayed rewards (life goals, relationships, etc.) (Perry and Carroll, 2008). Thus, having an innate aversion to delay may make one more susceptible to the effects of alcohol dependence. Evidence for this theory includes previous studies showing higher rates of delay discounting to be linked with substance use severity in adolescents (Field et al., 2007), age of first use in college students (Kollins, 2003), and early onset (prior to age 25) alcoholism (Dom et al., 2006). In the current study, FH+ youth displayed a tendency to discount delayed rewards more heavily than FH− controls. This finding is consistent with the previous study by Petry and colleagues showing greater delay discounting in FH+ adult females (Petry et al., 2002), and lends further support to hypotheses that greater delay aversion and/or immediate reward saliency may be related to a positive family history for alcohol abuse and an increased risk for alcohol abuse. Furthermore, taken together with longitudinal studies linking impulsivity in youth with more frequent and heavier alcohol use and greater likelihood of developing a use disorder (Kirisci et al., 2007; Sher et al., 2000; Tarter et al., 2007), our findings may also attest to the role of trait impulsivity as a viable risk factor for future alcohol problems.
Similar to choice behavior, group differences were also seen in reaction time for making decisions on this task. Consistent with past research (Hoffman et al., 2008; Robles and Vargas, 2007; Robles and Vargas, 2008), both FH+ and FH− youth showed longer reaction times for hard compared to easy choice trials on the delay discounting task. However, FH+ youth had overall slower control reaction times. Interestingly, after covarying for these baseline differences in control trial RT, group differences in discounting behavior and RT on easy and hard decision trials were no longer significant. Thus, these findings suggest that overall slowed processing speed at least partially accounts for the longer RT on easy and hard choice trials and the more impulsive choices exhibited by FH+ teens. While these finding were somewhat unexpected, there are several possible explanations that may underlie these results. Differences in reaction times seen on this task have been suggested to reflect the amount of work or the level of difficulty in making and executing decisions (Robles and Vargas, 2007). Thus, one possibility is that decision making is more difficult for FH+ youth and that they require more time to comprehend and compare the two choices, even during relatively easy choices about delayed rewards. However, because group differences were seen in RT on control trials, and this difference significantly accounts for group differences in RT seen on easy and hard decisions, longer reaction times could reflect differences in vigilance, attention, or motor planning between the groups. Some studies have shown impaired attention in non-abusing FH+ children and adolescents compared to FH− youth (Corral et al., 1999; Tapert et al., 2002), which may suggest that these youth may have trouble focusing on the choice at hand, which could account for increased reaction time. In addition, it is possible that poor attention may explain the initially counterintuitive results that FH+ have slower RT but display greater impulsive decision-making on this task. As recently described by de Wit (de Wit, 2009), both an inability to sustain attention, or a momentary lapse in attention precipitated by an event, such as having to make a decision, may increase the difficulty of making an optimal decision. Thus, poor sustained attention may account for the fact that longer RT at least partially accounted for more impulsive decision making in FH+ youth. Furthermore, poor attention has been suggested as a mechanism to influence impulsive behavior that may be especially important when choosing to abstain from using alcohol or avoid relapse, as sustained attention is needed to suppress drug-seeking behaviors (de Wit, 2009). Thus, impaired attention may facilitate poor decision-making in FH+ youth, which could contribute to alcohol onset and an increased propensity toward alcohol abuse. More research is warranted to determine what factors underlie slower reaction times during the delay discounting task in FH+ teens, and how this may impact their overall decision making.
In addition to examining delay discounting behavior, the current study is the first to examine white matter microstructure in alcohol-naïve FH+ youth. This was accomplished using both TBSS and follow-up ROI analyses. While TBSS is superior to other measures in terms of aligning FA images of multiple subjects, a major limitation of this technique is that it is sensitive to outliers. Thus, ROI based analyses were performed to verify results. Notably, qualitative ROI analyses showed that the observed group FA differences in the parietal and inferior temporal lobe were subject to partial volume effects, and thus should be interpreted with caution. Both methods, however, confirmed a number of regions throughout the brain where FH+ youth had lower FA than FH− youth. This finding is striking in light of the fact that in both adolescent and adult studies of individuals with alcohol and substance dependence (Chung et al., 2007; Lim and Helpern, 2002; McQueeny et al., 2009; Pfefferbaum and Sullivan, 2002; Yeh et al., 2009), white matter microstructural differences have been noted and are thought to reflect demyelination, or axonal abnormalities that occur as a result of prolonged substance abuse (Pfefferbaum et al., 2006; Pfefferbaum and Sullivan, 2002). Specifically, Yeh and colleagues (Yeh et al., 2009) reported that alcoholics have lower FA compared to light drinkers in several white matter tracts, including the superior longitudinal fasciculus, superior corona radiata, extreme capsule, inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, anterior limb of the internal capsule, and the optic radiations. Importantly, however, the current study demonstrated that prior to any substance use, high-risk FH+ youth have significant FA abnormalities in many of these same white matter tracts, suggesting that premorbid white matter microstructural differences may precede alcohol abuse onset. Furthermore, FA in the right optic radiation and the left inferior longitudinal fasciculus was shown to mediate the relationship between family history status and reaction time, which is consistent with some of these inferred changes. In particular, decreased myelination is known to result in slower axonal conduction velocity (Felts et al., 1997), and thus could lead to slower or less efficient processing speed on this task.
Beyond white matter microstructural differences relating to slow processing speed, the present DTI findings, taken together with the abnormalities previously noted in FH+ adolescents (Corral et al., 1999; Hill et al., 2001; Hill et al., 2007; Hill et al., 2009; Lovallo et al., 2006; Nigg et al., 2004; Tapert and Brown, 2000), suggest atypical structure and function in FH+ youth. Importantly, these deficits may be related to increased susceptibility for alcohol abuse. The observed microstructural white matter abnormalities included prefrontal regions, as well as cortico-striatal and subcortical pathways (alic, slf, scr) that carry projection and association fibers to the prefrontal cortex (Wakana et al., 2004). Because the prefrontal cortex subserves high-order cognition by allowing for the ability to pay attention to, plan, and select specific actions in response to the external world and internal goals (Fuster, 2000; Fuster, 2002), disruption in these pathways may contribute to aberrant executive functioning, motivational behavior, and reward learning in FH+ youth. During adolescence, the brain is undergoing critical neurodevelopment including synaptic pruning, reorganization, and myelination in several brain regions, perhaps most notably the frontal cortex (Giedd et al., 1999; Giorgio et al., 2008; Luna and Sweeney, 2001; Sowell et al., 2004). The relatively late development of the prefrontal cortex, and its projections to reward-based subcortical and limbic regions, is thought to be responsible for overall poor decision making that is characteristic of the early adolescent years (Galvan et al., 2006; Yurgelun-Todd, 2007), and may make the adolescent brain especially vulnerable to drug induced neurotoxicity, and maladaptive drug reward learning (Crews and Boettiger, 2009). Thus, premorbid prefrontal abnormalities, such as atypical white matter microstructure, may prove to facilitate early onset of use through impaired cognitive control and aberrant motivational responses. Moreover, once initiated, the impact of substance use on preexisting fragile fiber connections may exacerbate impairments in executive functioning and cortical control processes in FH+ youth compared to individuals without these inherent deficits. As more research emerges on the topic of FH+ youth and the effects of substance abuse on the developing brain, we may begin to better understand the role of predisposing neural factors seen in high-risk adolescents, as well as the degree to which these preexisting differences may facilitate the development of addiction-related behaviors.
A few considerations should be taken into account when interpreting the current findings. First, our study was a cross-sectional design, and it is important to note that while FH+ youth have an increased risk of developing an AUD, a relatively large percentage of FH+ individuals do not develop substance related problems. A longitudinal design is necessary to determine a causal relationship between abnormal white matter microstructure, impulsive decision-making, and substance abuse onset and progression. In addition, the current sample was modest in size, predominantly Caucasian and of a high socioeconomic status. Although the sample size was large enough to detect robust significant differences in white matter integrity (all clusters had Cohen’s d > 1), only trends of heightened delay discounting were seen between the groups, and gender differences were not able to be evaluated. While, the effect size for discounting behavior was fairly large (Cohen’s d = .67), a sample size of 36 per group would be required to detected a significant difference with 80% power at a α = .05 (Erdfelder et al., 1996). It is also worthy to note that a number of the white matter tracts in which FH+ youth showed abnormalities carry fibers that project to more than one brain region (Wakana et al., 2004). Tractography, which uses information about the directional diffusion of water to generate a visual representation of white matter fiber tracts (for review see (Johansen-Berg and Rushworth, 2009)) will be an important next step for gaining a more comprehensive understanding of which fiber tracts are most affected in FH+ youth. Finally, in the current study, family history density did not relate to discounting behavior or fractional anisotropy, despite including youth with a combination of multiplex and simplex family history. While contributory, currently, the exact role genetic load plays in alcoholism is unclear, and thus our findings may speak to the fact that genetic effects may not be solely responsible for the differences reported here in white matter microstructure and discounting behavior in FH+ youth. Environmental (including in utero) factors, as well as gene-by-environment interactions, may also impact the brain and subsequent behavior, thus contributing to the current results. For example, having a family history of substance abuse has been shown to increase the likelihood of experiencing a range of childhood stressors (Sher et al., 1997), and these have been shown to partially mediate the effect of family history status on subsequent substance abuse (Sher et al., 1997). Furthermore, although our groups were free of externalizing disorders and were similar in rates of externalizing behavior, it’s important to note that externalizing disorders can increase an individual’s risk of alcohol onset and abuse (Elkins et al., 2007). Therefore, additional studies including a larger and more diverse sample of subjects are necessary to confirm our findings and support generalizability to a fully representative population of high-risk youths.
In summary, the results of the present study show that abnormal white matter structure, as well as a trend toward greater delay discounting, are apparent in high-risk FH+ youth. Further research on how temporal discounting behavior and white matter abnormalities more directly relate to increased susceptibility of alcohol abuse in these and other high-risk teens is warranted, and may provide valuable clues for better prevention strategies for these youths.
Acknowledgements
Emily Maxwell is thanked for her assistance in data collection and management, and Vanessa Wilson for her assistance with the implementation of the delay discounting task and analytic scheme.
Support: This research was supported by the Oregon Clinical and Translational Research Institute, the National Institute of Drug Abuse (T32 DA007262 – Herting), the National Institute of Alcohol Abuse (T32 AA007468 – Herting), pilot funds from the Portland Alcohol Research Center (P60 AA010760 – Nagel), and the National Institute of Neurological Disorders and Stroke (K08 NS52147 – Nagel).
References
- Achenbach T, Rescorla L. Manual for the ASEBA school-age forms and profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- Albertsson-Wikland K, Rosberg S, Lannering B, Dunkel L, Selstam G, Norjavaara E. Twenty-four-hour profiles of luteinizing hormone, follicle-stimulating hormone, testosterone, and estradiol levels: a semilongitudinal study throughout puberty in healthy boys. Journal of Clinical Endocrinology & Metabolism. 1997;82(2):541–549. doi: 10.1210/jcem.82.2.3778. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-linear optimisation. FMRIB technical report TR07JA1. 2007a from www.fmrib.ox.ac.uk/analysis/techrep.
- Andersson JLR, Jenkinson M, Smith S. Non-linear registration, aka Spatial normalisation. FMRIB technical report TR07JA2. 2007b from www.fmrib.ox.ac.uk/analysis/techrep.
- Audrain-McGovern J, Rodriguez D, Tercyak KP, Epstein LH, Goldman P, Wileyto EP. Applying a behavioral economic framework to understanding adolescent smoking. Psychol Addict Behav. 2004;18(1):64–73. doi: 10.1037/0893-164X.18.1.64. [DOI] [PubMed] [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8(7–8):333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59(4):427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Mott MA, Vik PW. Correlates of success following treatment for adolescent substance abuse. Applied & Preventive Psychology. 1994;3:61–73. [Google Scholar]
- Chassin L, Fora DB, King KM. Trajectories of alcohol and drug use and dependence from adolescence to adulthood: the effects of familial alcoholism and personality. J Abnorm Psychol. 2004;113(4):483–498. doi: 10.1037/0021-843X.113.4.483. [DOI] [PubMed] [Google Scholar]
- Chung A, Lyoo IK, Kim SJ, Hwang J, Bae SC, Sung YH, Sim ME, Song IC, Kim J, Chang KH, Renshaw PF. Decreased frontal white-matter integrity in abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2007;10(6):765–775. doi: 10.1017/S1461145706007395. [DOI] [PubMed] [Google Scholar]
- Corral MM, Holguin SR, Cadaveira F. Neuropsychological characteristics in children of alcoholics: familial density. J Stud Alcohol. 1999;60(4):509–513. doi: 10.15288/jsa.1999.60.509. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, an International Journal. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93(3):237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14(1):22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Flory J, Acheson A, McCloskey M, Manuck S. IQ and nonplanning impulsivity are independently associated with delay discounting in middle-aged adults. Personality & Individual Differences. 2007;42:111–121. [Google Scholar]
- Deckel AW, Hesselbrock V. Behavioral and cognitive measurements predict scores on the MAST: a 3-year prospective study. Alcohol Clin Exp Res. 1996;20(7):1173–1178. doi: 10.1111/j.1530-0277.1996.tb01107.x. [DOI] [PubMed] [Google Scholar]
- Dom G, D'Haene P, Hulstijn W, Sabbe B. Impulsivity in abstinent early- and late-onset alcoholics: differences in self-report measures and a discounting task. Addiction. 2006;101(1):50–59. doi: 10.1111/j.1360-0443.2005.01270.x. [DOI] [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Archives of General Psychiatry. 2007;64(10):1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- Erdfelder E, Faul F, Buchner A. GPOWER: A general power analysis program. Behavior Research Methods, Instruments, & Computers; 1996. pp. 28pp. 1–11. [DOI] [PubMed] [Google Scholar]
- Fairchild AJ, MacKinnon DP. A general model for testing mediation and moderation effects. Prev Sci. 2009;10(2):87–99. doi: 10.1007/s11121-008-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felts PA, Baker TA, Smith KJ. Conduction in segmentally demyelinated mammalian central axons. J Neurosci. 1997;17(19):7267–7277. doi: 10.1523/JNEUROSCI.17-19-07267.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Christiansen P, Cole J, Goudie A. Delay discounting and the alcohol Stroop in heavy drinking adolescents. Addiction. 2007;102:579–586. doi: 10.1111/j.1360-0443.2007.01743.x. [DOI] [PubMed] [Google Scholar]
- Funder DC, Block J. The role of ego-control, ego-resiliency, and IQ in delay of gratification in adolescence. J Pers Soc Psychol. 1989;57(6):1041–1050. doi: 10.1037//0022-3514.57.6.1041. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Prefrontal neurons in networks of executive memory. Brain Research Bulletin. 2000;52(5):331–336. doi: 10.1016/s0361-9230(99)00258-0. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Frontal lobe and cognitive development. Journal of Neurocytology. 2002;31(3–5):373–385. doi: 10.1023/a:1024190429920. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Watkins KE, Douaud G, James AC, James S, De Stefano N, Matthews PM, Smith SM, Johansen-Berg H. Changes in white matter microstructure during adolescence. Neuroimage. 2008;39(1):52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jiang Y, Hardy PA. Speed of lexical decision correlates with diffusion anisotropy in left parietal and frontal white matter: evidence from diffusion tensor imaging. Neuropsychologia. 2007;45(11):2439–2446. doi: 10.1016/j.neuropsychologia.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jicha GA, Smith CD. Age-related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiol Aging. 2008;31(3):512–522. doi: 10.1016/j.neurobiolaging.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J, Pitts T. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biol Psychiatry. 2001;49(11):894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- Hill SY, Muddasani S, Prasad K, Nutche J, Steinhauer SR, Scanlon J, McDermott M, Keshavan M. Cerebellar volume in offspring from multiplex alcohol dependence families. Biol Psychiatry. 2007;61(1):41–47. doi: 10.1016/j.biopsych.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Kostelnik B, Carter H, Holmes B, McDermott M, Zezza N, Stiffler S, Keshavan MS. Disruption of orbitofrontal cortex laterality in offspring from multiplex alcohol dependence families. Biol Psychiatry. 2009;65(2):129–136. doi: 10.1016/j.biopsych.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman WF, Schwartz DL, Huckans MS, McFarland BH, Meiri G, Stevens AA, Mitchell SH. Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology (Berl) 2008;201(2):183–193. doi: 10.1007/s00213-008-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University; 1975. [Google Scholar]
- Hoven CW, Duarte CS, Lucas CP, Wu P, Mandell DJ, Goodwin RD, Cohen M, Balaban V, Woodruff BA, Bin F, Musa GJ, Mei L, Cantor PA, Aber JL, Cohen P, Susser E. Psychopathology among New York city public school children 6 months after September 11. Arch Gen Psychiatry. 2005;62(5):545–552. doi: 10.1001/archpsyc.62.5.545. [DOI] [PubMed] [Google Scholar]
- Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med. 2003;49(1):193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MF. Using Diffusion Imaging to Study Human Connectional Anatomy. Annu Rev Neurosci. 2009 doi: 10.1146/annurev.neuro.051508.135735. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. An algorithm for identifying nonsystematic delay-discounting data. Exp Clin Psychopharmacol. 2008;16(3):264–274. doi: 10.1037/1064-1297.16.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H. Spatial-anatomical mapping of NoGo-P3 in the offspring of alcoholics: evidence of cognitive and neural disinhibition as a risk for alcoholism. Clin Neurophysiol. 2005;116(5):1049–1061. doi: 10.1016/j.clinph.2004.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirisci L, Tarter R, Mezzich A, Vanyukov M. Developmental trajectory classes in substance use disorder etiology. Psychol Addict Behav. 2007;21(3):287–296. doi: 10.1037/0893-164X.21.3.287. [DOI] [PubMed] [Google Scholar]
- Kollins SH. Delay discounting is associated with substance use in college students. Addict Behav. 2003;28(6):1167–1173. doi: 10.1016/s0306-4603(02)00220-4. [DOI] [PubMed] [Google Scholar]
- Li TQ, Mathews VP, Wang Y, Dunn D, Kronenberger W. Adolescents with disruptive behavior disorder investigated using an optimized MR diffusion tensor imaging protocol. Ann N Y Acad Sci. 2005;1064:184–192. doi: 10.1196/annals.1340.034. [DOI] [PubMed] [Google Scholar]
- Lieb R, Merikangas KR, Hofler M, Pfister H, Isensee B, Wittchen HU. Parental alcohol use disorders and alcohol use and disorders in offspring: a community study. Psychol Med. 2002;32(1):63–78. doi: 10.1017/s0033291701004883. [DOI] [PubMed] [Google Scholar]
- Lim KO, Helpern JA. Neuropsychiatric applications of DTI - a review. NMR in Biomedicine. 2002;15(7–8):587–593. doi: 10.1002/nbm.789. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Yechiam E, Sorocco KH, Vincent AS, Collins FL. Working memory and decision-making biases in young adults with a family history of alcoholism: studies from the Oklahoma family health patterns project. Alcoholism Clin Exp Res. 2006;30(5):763–773. doi: 10.1111/j.1530-0277.2006.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Bourdon K, Dulcan MK, Canino G, Rubio-Stipec M, Lahey BB, Friman P. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. J Am Acad Child Adolesc Psychiatry. 2001;40(4):443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. Studies of brain and cognitive maturation through childhood and adolescence: a strategy for testing neurodevelopmental hypotheses. Schizophrenia bulletin. 2001;27(3):443–455. doi: 10.1093/oxfordjournals.schbul.a006886. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink. I. Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol Clin Exp Res. 2001;25(8):1156–1165. [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, Tapert SF. Altered White Matter Integrity in Adolescent Binge Drinkers. Alcohol Clin Exp Res. 2009;33(7):1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Conway KP, Swendsen J, Febo V, Dierker L, Brunetto W, Stolar M, Canino G. Substance use and behaviour disorders in Puerto Rican youth: a migrant family study. J Epidemiol Community Health. 2009;63(4):310–316. doi: 10.1136/jech.2008.078048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milberger S, Faraone SV, Biederman J, Chu MP, Feighner JA. Substance use disorders in high-risk adolescent offspring. Am J Addict. 1999;8(3):211–219. doi: 10.1080/105504999305820. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl) 1999;164(4):455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Ni H, Kavcic V, Zhu T, Ekholm S, Zhong J. Effects of number of diffusion gradient directions on derived diffusion tensor imaging indices in human brain. AJNR Am J Neuroradiol. 2006;27(8):1776–1781. [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Glass JM, Wong MM, Poon E, Jester JM, Fitzgerald HE, Puttler LI, Adams KM, Zucker RA. Neuropsychological executive functioning in children at elevated risk for alcoholism: findings in early adolescence. J Abnorm Psychol. 2004;113(2):302–314. doi: 10.1037/0021-843X.113.2.302. [DOI] [PubMed] [Google Scholar]
- Oberlin BG, Grahame NJ. High-alcohol preferring mice are more impulsive than low-alcohol preferring mice as measured in the delay discounting task. Alcohol Clin Exp Res. 2009;33(7):1294–1303. doi: 10.1111/j.1530-0277.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olson EA, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. White matter integrity predicts delay discounting behavior in 9- to 23-year-olds: a diffusion tensor imaging study. J Cogn Neurosci. 2009;21(7):1406–1421. doi: 10.1162/jocn.2009.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EA, Hooper CJ, Collins P, Luciana M. Adolescents' performance on delay and probability discounting tasks: Contributions of age, intelligence, executive functioning, and self-reported externalizing behavior. Personality and Individual Differences. 2007;43:1886–1897. doi: 10.1016/j.paid.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkaragoz T, Satz P, Noble EP. Neuropsychological functioning in sons of active alcoholic, recovering alcoholic, and social drinking fathers. Alcohol. 1997;14(1):31–37. doi: 10.1016/s0741-8329(96)00084-5. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200(1):1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Petersen A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology (Berl) 2001;154(3):243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Petry NM, Kirby KN, Kranzler HR. Effects of gender and family history of alcohol dependence on a behavioral task of impulsivity in healthy subjects. J Stud Alcohol. 2002;63(1):83–90. [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiol Aging. 2006;27(7):994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. Neuroimage. 2002;15(3):708–718. doi: 10.1006/nimg.2001.1018. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism Clin Exp Res. 1995;19(4):1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Robles E, Vargas PA. Functional parameters of delay discounting assessment tasks: order of presentation. Behav Processes. 2007;75(2):237–241. doi: 10.1016/j.beproc.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Robles E, Vargas PA. Parameters of delay discounting assessment: number of trials, effort, and sequential effects. Behav Processes. 2008;78(2):285–290. doi: 10.1016/j.beproc.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Salo R, Ursu S, Buonocore MH, Leamon MH, Carter C. Impaired prefrontal cortical function and disrupted adaptive cognitive control in methamphetamine abusers: a functional magnetic resonance imaging study. Biol Psychiatry. 2009;65(8):706–709. doi: 10.1016/j.biopsych.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, Brown SA, Tapert SF. An FMRI study of response inhibition in youths with a family history of alcoholism. Ann N Y Acad Sci. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Sher KJ. Psychological characteristics of children of alcoholics. Overview of research methods and findings. Recent Dev Alcohol. 1991;9:301–326. [PubMed] [Google Scholar]
- Sher KJ, Bartholow BD, Wood MD. Personality and substance use disorders: a prospective study. J Consult Clin Psychol. 2000;68(5):818–829. [PubMed] [Google Scholar]
- Sher KJ, Gershuny BS, Peterson L, Raskin G. The role of childhood stressors in the intergenerational transmission of alcohol use disorders. J Stud Alcohol. 1997;58(4):414–427. doi: 10.15288/jsa.1997.58.414. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Rohan ML, Pimentel PJ, Gruber SA, Rosso IM, Yurgelun-Todd DA. Sex differences in the relationship between white matter microstructure and impulsivity in adolescents. Magn Reson Imaging. 2006;24(7):833–841. doi: 10.1016/j.mri.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, Robson MD, Jones DK, Klein JC, Bartsch AJ, Behrens TE. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protoc. 2007;2(3):499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenberg SF, Mudd SA, Blow FC, Hill EM. Evaluating measures of family history of alcoholism: density versus dichotomy. Addiction. 1998;93(10):1511–1520. doi: 10.1046/j.1360-0443.1998.931015117.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Substance dependence, family history of alcohol dependence and neuropsychological functioning in adolescence. Addiction. 2000;95(7):1043–1053. doi: 10.1046/j.1360-0443.2000.95710436.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychological Society. 2002;8(7):873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Feske U, Vanyukov M. Modeling the pathways linking childhood hyperactivity and substance use disorder in young adulthood. Psychol Addict Behav. 2007;21(2):266–271. doi: 10.1037/0893-164X.21.2.266. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230(1):77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corp.; 1999. [Google Scholar]
- Wilhelm CJ, Mitchell SH. Strain differences in delay discounting using inbred rats. Genes Brain Behav. 2009;8(4):426–434. doi: 10.1111/j.1601-183X.2009.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Reeves JM, Phillips TJ, Mitchell SH. Mouse lines selected for alcohol consumption differ on certain measures of impulsivity. Alcohol Clin Exp Res. 2007;31(11):1839–1845. doi: 10.1111/j.1530-0277.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- Yeh PH, Simpson K, Durazzo TC, Gazdzinski S, Meyerhoff DJ. Tract-based spatial statistics (TBSS) of diffusion tensor imaging data in alcohol dependence: Abnormalities of the motivational neurocircuitry. Psychiatry Res. 2009;173(1):22–30. doi: 10.1016/j.pscychresns.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Current Opinion in Neurobiology. 2007;17(2):251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]