Abstract
The Drosophila melanogaster TRPA family member painless, expressed in a subset of multidendritic neurons embeding in the larval epidermis, is necessary for larval nociception of noxious heat or mechanical stimuli. However, the function of painless in adult flies remains largely unknown. Here we report that mutation of painless leads to a defect in male–male courtship behavior and alteration in olfaction sensitivity in adult flies. Specific downregulation of the expression of the Painless protein in the olfactory projection neurons (PNs) of the antennal lobes (ALs) resulted in a phenotype resembling that found in painless mutant flies, whereas overexpression of Painless in PNs of painless mutant males suppressed male–male courtship behavior. The downregulation of Painless exclusively during adulthood also resulted in male–male courtship behavior. In addition, mutation of the painless gene in flies caused changes in olfaction, suggesting a role for this gene in olfactory processing. These results indicate that functions of painless in the adult central nervous system of Drosophila include modulation of olfactory processing and inhibition of male–male courtship behavior.
Introduction
Transient receptor potential (TRP) channels play important roles in a variety of sensory systems [1]. In mammals, TRPA1 is critical for nociception and may contribute to cold sensation [2]–[4]. As a Drosophila TRPA channel [5], the painless gene was first identified as essential for the sensation of high temperature (above ∼39°C) and for noxious mechanical stimulation in the larvae [6], [7]. In addition, painless has been reported to be involved in sugar-stimulated neural excitation and avoidance in larvae [8], in wasabi sensation and noxious heat sensation in adult flies [9], [10], as well as in negative gravity sensation [11]. The expression pattern of the Gal4 enhancer-trap allele painless-Gal4 (painGal4) suggests that painless may be endogenously expressed in the adult fly brain [9], [10], but likely functional consequences of this expression are largely unknown.
The Drosophila olfactory system is critical for its detection of volatile chemicals in the external environment [12]–[14]. These chemical cues are first detected and converted into electric signals by the primary olfactory receptor neurons (ORNs). After further processing by secondary projection neurons (PNs) and local interneurons (LNs) of the antennal lobes (ALs), this information is then conveyed to higher brain regions to direct appropriate behaviors, including foraging, fighting and courtship [15]. Pheromones act as important cues for gender identification in flies [16]. Thus, the Drosophila olfactory system, which detects volatile pheromone cues, plays an important role in courtship behavior. Dysfunction of the primary pheromone-sensing ORNs impairs pheromone detection and results in aberrant courtship behavior [17]. Additionally, activity of higher-order olfactory neurons, where information from ORNs is processed and modified, may also influence Drosophila courtship behavior.
In this study, we first examined the endogenous expression pattern of the painless gene in the adult Drosophila brain. Analysis of the painless mutant flies led to the observation that their olfactory sensitivity was decreased. In addition, we identified abnormal male-male courtship behavior. Manipulating of painless expression in specific brain regions revealed that its expression in the PNs of the ALs was critical for preventing the male–male courtship behavior. Further experiments using time-specific knockdown of painless under the regulation of the temperature-sensitive Gal80 protein (Gal80ts) showed that painless expression in the adult brain was essential for normal courtship behavior. Thus, painless may function in PNs to regulate male courtship behavior.
Materials and Methods
Drosophila Stocks and Culture
Flies were cultured on standard cornmeal–agar–molasses medium at 25°C with a relative humidity of 50–70%. The painless1, painless-Gal4, and UAS-painlessAR9 lines generated in the w1118 background were a generous gift from Dr. D. Tracey (Duke University, Durham, NC, USA). The CS strain was used as a WT control. The painless3 and painless4 lines in a w1118 background were obtained from the Szeged Drosophila Stock Center (Szeged, Hungary). The GH146-Gal4, Poxn-Gal4-13-1, c507-Gal4, and UAS-shibirets lines were kindly provided by Drs. R.F. Stocker (University of Fribourg, Fribourg, Switzerland), M. Noll (University of Zurich, Zurich, Switzerland), D. Armstrong (University of Edinburgh, Edinburgh, UK), and T. Kitamoto (University of Iowa, Iowa City, USA), respectively. The UAS-pain-RNAi lineS were obtained from the Vienna Drosophila RNAi center (stock No. v39477 and v39478). Other strains were from the Bloomington Drosophila Stock Center (stock No. 854, 8769, 7018, 5130, and 23129). All flies used in behavior experiments have been back-crossed to CS flies for at least four generations.
Behavioral Assays
All behavioral assays were carried out at 25±1°C with 40–60% relative humility at zeitgeber time ZT1-ZT8, except where otherwise indicated. Flies used in behavioral assays were collected by gentle aspiration or under light CO2 anesthesia shortly after eclosion (within 6 h), and aged 4–6 days in all the experiment except that of RNAi experiment and Gal80ts experiment. Males were reared in groups of 8–10 animals or individually [18], [19], while females were kept in groups of 10 animals.
Chaining Assay. Eight males reared in groups were introduced into a 3.5 cm plastic dish by gentle aspiration or after immobilization by anesthetics. The courtship behavior between these males was video recorded and analyzed manually. The chaining behavior was defined as the display of courtship behavior among at least three males, with formation of a chain. The chaining index (ChI) is the percentage of time during which chaining behavior was observed [20]. For the antenna ablation experiment, the antenna (antennae) of males was (were) carefully removed by using fine forceps under light CO2 anesthesia in the first day after eclosion. Males were left to recover for 4 days before the chaining assay.
Courtship Pairing Assay. One individually reared tester male and one target female were sequentially introduced into a courtship chamber (diameter, 1 cm; height, 0.3 cm) by gentle aspiration, and the courtship behavior between the pair was video recorded and analyzed manually. The courtship index (CI) is the percentage of time in a given observation period during which the tester exhibits courtship behavior towards the target, which includes chasing, wing vibration, tapping, and attempted copulation [20].
Courtship Preference Assay. One individually reared tester male was introduced into a courtship chamber by gentle aspiration or by light CO2 anesthesia, and was left alone for 5 min. Two decapitated target flies, i.e., a WT male and a WT female, were then presented to the tester. The CI values towards the female and the male during a 10 min period were simultaneously measured and were subsequently compared [21].
Olfactory Sensitivity. Olfactory sensitivity was measured using a T-maze test [22], [23]. Briefly, flies were collected shortly after eclosion without anesthesia and were reared in groups until they were 2–3 days old. After being food deprived and kept in darkness for 0.5 h, ∼100 flies were introduced into the elevator of the T-maze and were left to choose between the two tubes, one filled with air and the other with MCH (Fluka, USA), for 2 min. The flies in each tube were then counted and the PI was calculated. All flies were reared on food without propionic acid and yeast, and the experiments were performed at 25±1°C with 75–90% relative humility in exclusive darkness with a red LED as a light source.
RT–PCR
Adult males aged 2–3 days were lightly anesthetized by CO2 and dissected in cold (0°C) extracellular solution (ECS) containing the following: 103 mM NaCl, 3 mM KCl, 5 mM N-tris(hydroxymethyl) methyl-2-aminoethane-sulfonic acid, 10 mM trehalose, 10 mM glucose, 26 mM NaHCO3, 1 mM NaH2PO4, 1.5 mM CaCl2, and 4 mM MgCl2 (osmolarity was adjusted to 270–275 mOsm). About fifty proboscises and brains were separately collected in TRIzol reagent (Invitrogen, USA), and RNA was extracted according to the manufacturer's instructions. cDNAs from proboscises and brains were synthesized using standard techniques. The primers used to detect the presence of the painless cDNA were: 5′–GGAAACCTGGCGGCTCTCC–3′ and 5′– CAGCGGTGCCCTGGCCGA–3′.
Immunostaining and Confocal Microscopy of Adult Brains
Two rabbit antisera against Painless were obtained from Novus Biologicals (NB100-98736 and NB100-98737) and were used at a dilution of 1∶200. Other antibodies (and dilutions) were as follows: rabbit anti-GFP (Invitrogen, 1∶1,000), mouse nc82 (Developmental Studies Hybridoma Bank, 1∶50), biotinylated goat antirabbit (GAR) IgG (Vector Labs, 1∶800), rhodamine avidin D (Vector Labs, 1∶500), and Alexa-fluor-conjugated secondary antibodies (Invitrogen, 1∶200). Flies aged 3–5 days were lightly anesthetized by CO2, fixed in PBS with 4% paraformaldehyde on ice for 1 h, washed and dissected in PBS with 0.2% Triton X-100 (PBST), and blocked for 1 h at 25°C in PBST containing 10% heat inactivated normal goat serum. For experiments using anti-Painless antibodies, brains were first incubated with anti-Painless antiserum for 8 h at 4°C. After washing in PBST (4×15 min), brains were incubated with biotinylated GAR IgG and nc82 for 8 h at 4°C. The brains were then washed in PBST (4×15 min) and incubated with rhodamine avidin D and Alexa-fluor-633-conjugated goat anti-mouse (GAM) IgG. For other stainings, brains were first incubated with anti-GFP and nc82 antibodies for 8 h at 4°C. After washing in PBST (4×15 min), brains were incubated with Alexa-fluor-488-conjugated GAR and Alexa-fluor-546-conjugated GAM IgGs. After final washes in PBST (3×5 min), brains were mounted in VECTASHIELD (Vector Labs). Confocal images were captured using a Carl Zeiss LSM510 microscope equipped with a Plan-Apochromat 20× objective or a 63× oil-immersion objective.
Blocking Neurotransmission using shibirets
For the experiments that required the use of UAS-shibirets, flies were kept at 19°C until the experiment. To shift the environment temperature, flies were housed in 3.5 cm dishes made of aluminum and placed on a custom-built heating plate that can shift between 19 and 30°C within 1 min.
Data analysis
Student's t-test was used to compare the intensity of courtship behaviour, after testing the normality of the data distribution with the Kolmogorov-Smirnov test. Otherwise, the Kruskal-Wallis test was used.
Results
Painless Is Expressed in Adult Fly Brain
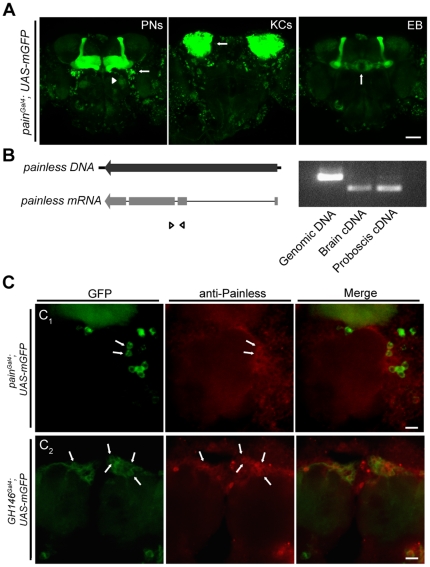
The Gal4 expression pattern in the Gal4 enhancer-trap line painGal4, which contains Gal4 inserted within the painless transcriptional unit [6], revealed that this Gal4 is broadly expressed in the adult brain [10]. Using the same Gal4 line, we found that painless is expressed in several clusters of PNs of the ALs, in Kenyon cells (KCs) of mushroom bodies (MBs), and in the neurons that form the ellipsoid body (Figure 1A ). To confirm the endogenous expression of painless in the adult fly brain, we performed RT–PCR to examine the presence of the painless mRNA. As shown in Figure 1B , painless mRNA was detected in dissected brains of WT Canton-S (CS) adult flies, indicating the endogenous expression of the painless gene in the adult brain. We further confirmed the endogenous expression of painless by immunostaining. The specificity of the Painless antibody was first validated by specific staining of the Painless-myc fusion protein in cell lines overexpressing the protein, and detection of overexpression Painless protein in adult brains (Figure S1). We next examined the expression pattern of endogenous painless in adult brains using GFP expressed in PNs of the ALs (painGal4; UAS-mGFP and GH146Gal4; UAS-mGFP). As shown in Figures 1C 1 and 1C 2 , Painless signal was detected in the circumference of the ALs, where the somata of olfactory PNs and LNs are located, and co-staining experiments showed that Painless was expressed in certain PNs. Also, we observed that some glomeruli, which receive the dendrites of PNs, were labeled by the antibody (Figure S1), suggesting a function of Painless in the dendrites. Although strong Painless signal was not detected in other brain regions, such as MBs, we could not exclude the possibility that Painless was also expressed in KCs and other neurons at relatively low levels.
Figure 1. Expression of painless in the central nervous system.
(A) Confocal images of the brains of painGal4; UAS-mCD8-GFP flies. Cells in green were painGal4-positive. White arrows indicate the projection neurons (PNs), the Kenyon cells (KCs), and the ellipsoid body (EB), respectively. White arrowhead in (A) shows one glomerulus innervated by neurites of painGal4-positive PNs (Scale bar, 50 µm.) (B) Left, schematic structure of the painless gene locus and mRNA. Small triangles represent the PCR primers used. Right, RT–PCR products of mRNAs from brains and proboscis, with PCR product from genomic DNA as a control. (C) Confocal images of fly brains stained with the antibody against Painless. The genotype of each brain is indicated. White arrows show the PNs expressing both Painless and GFP. White arrowhead in (C1) indicates the glomerulus formed by the neurites of GFP–positive PNs. (Scale bar, 10 µm.)
Olfaction Is Affected in painless Mutant Flies
As expression of endogenous Painless was detected in the PNs, we speculated that painless may function in the olfactory pathway, and contribute to the olfactory behavior. In order to test this possibility, we examined the olfactory sensitivity of painless mutant flies using the T-maze test [22], [23]. As shown in Figure S2, when provided with the odorant 4-methyl-cyclohexanol (MCH), WT flies preferred the tube perfused with air to that perfused with MCH. And as the concentration of MCH increased, the MCH avoidance behavior became stronger. Interestingly, this MCH avoidance behavior was significantly weakened in painless mutant flies (painGal4 and pain1), suggesting that olfactory sensitivity of these flies was affected. When MCH was provided at lower concentrations (5.0×10−6 and 5×10−7, v/v dilution), painless mutant flies still displayed a preference behavior, suggesting an alteration in the responses towards this odor. To further test whether this olfactory defect was due to mutation of painless, we examined the avoidance behavior of painless mutant flies overexpressing Painless in painGal4 positive neurons. We found that the avoidance behavior could be partially restored to a level resembling that of WT flies at high concentrations of MCH, but not at low concentrations (Figure S2). These results indicate that painless is involved in olfactory processing in flies.
Male–male Courtship Behavior in painGal4 Flies
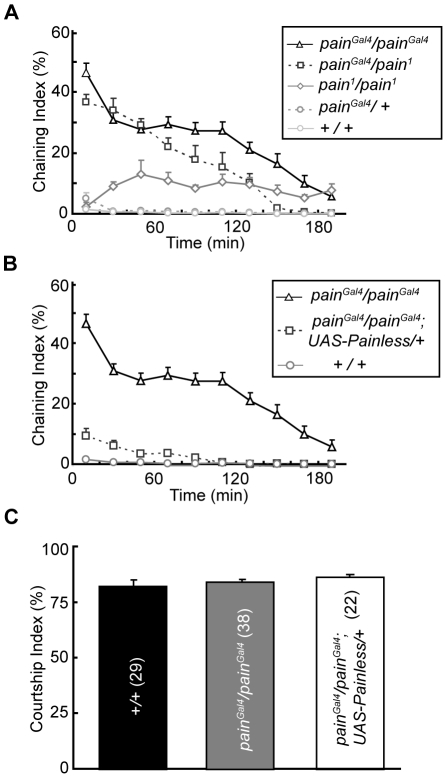
In addition to the olfactory defects, we observed that male painGal4 flies, in which the expression of painless was interfered by the Gal4 insertion, spontaneously displayed a male–male courtship behavior in the morning (zeitgeber time: ZT0.5–ZT3) (Figure 2A ). We measured the chaining index (ChI) of the males, i.e., the percentage of time spent courting one another and forming chains. We found that the ChI of male painGal4 flies was ∼10%, significantly higher than that of WT males (∼0%). We fortuitously observed that, after recovery from a mild anesthesia (brief treatments with CO2, nitrogen, or −20°C chilling), the intensity of this male–male courtship behavior was greatly enhanced over a long period (up to ∼3 h after CO2 treatment, Figure 2B ). Interestingly, this anesthesia-induced enhancement of the male–male courtship behavior was not specific to painless mutant flies, as a brief anesthesia with either CO2 or nitrogen also induced a partial male–male courtship behavior lasting a very short period (0.3–0.5 s) in WT flies, i.e., an increase in the occurrence of courtship initiation (Figure 2C ), which included the orientation, chasing, and wing vibration steps, but not followed by other courtship steps as in the painless mutant males. These observations suggest that male-male courtship behavior could be triggered among flies after recovery from a treatment of mild anesthesia, and WT males are able to inhibit this abnormal courtship behavior whereas painless mutant males cannot. Thus, in the following experiments, we treated male flies with brief CO2 anesthesia (10 s or 1 min) before the chaining assay to examine their ability to inhibit male-male courtship behavior.
Figure 2. painless mutant males exhibited a male–male courtship behavior.
(A) The average Chaining index (ChI) of 8–10 males that displayed spontaneous chaining behavior during a 10 min period in the morning (ZT0.5–ZT1.5). For both WT and painGal4males, more than six groups of males were analyzed. P<0.01 vs. the WT (Kruskal-Wallis test). (B) Average ChI of males recovered from the indicated anesthesia (top). Three different anesthetics were used in four different doses (indicated in the boxes above the traces). For each trace, 12 groups of males were observed every 5 min with a 15 min interval. (C) Average time spent by eight WT males on courting (left) and average time of courtship initiation (middle), before and after the indicated anesthesia treatments (grey arrows). Average time spent by the WT males on each courtship bout after anesthesia treatment (right). Values shown are means ± SEM.
Male–male Courtship Behavior Is Caused by painless Mutation
To confirm that the male–male courtship behavior of painGal4 flies was caused by mutation of the painless gene, we examined the behavior of other mutant alleles of painless. We found that painless1 (pain1) males exhibited long-lasting male–male courtship behavior after recovery from mild CO2 anesthesia, with a ChI lower than that of painGal4 males, but significantly higher than that of WT males (Figure 3A ). In addition, we performed complementation experiments and found strong male–male courtship behavior in painGal4/pain1 double-heterozygous flies, with an average ChI as high as that observed in painGal4 homozygous males. In contrast, painGal4/+ and pain1/+ heterozygous male flies did not display obvious male-male courtship behavior. In addition to painGal4/pain1 males, we also examined the behavior of pain1/pain3, painGal4/pain3, and painGal4/pain4 heterozygous males and found that the intensity of their male–male courtship behavior was significantly higher than that of WT animals (Figure S3), but shorter and weaker as compared with painGal4/pain1 males.
Figure 3. Mutation of painless caused a male–male courtship behavior.
(A) and (B) Average ChI of males of indicated genotypes during the 3 h session. For each trace, more than ten groups of males was analyzed. Note that the experiments in (A) and (B) were performed simultaneously, and some data (ChI of WT and painGal4) was used in both panels. (C) Average courtship index of males of indicated genotypes towards WT virgin females. The number of males examined is shown in parenthesis. Histograms represent the mean ± SEM.
Further rescue experiments showed that overexpression of Painless suppressed the male–male courtship behavior of painGal4; UAS-Painless/+ flies (Figure 3B ). Moreover, the courting ability of these Painless-overexpressing males towards WT virgin females was similar to that of WT males (Figure 3C ). These results demonstrate that the abnormal courtship behavior found in painGal4 mutant males was due to the painless mutation.
Courtship Preference of painGal4 Males towards Females
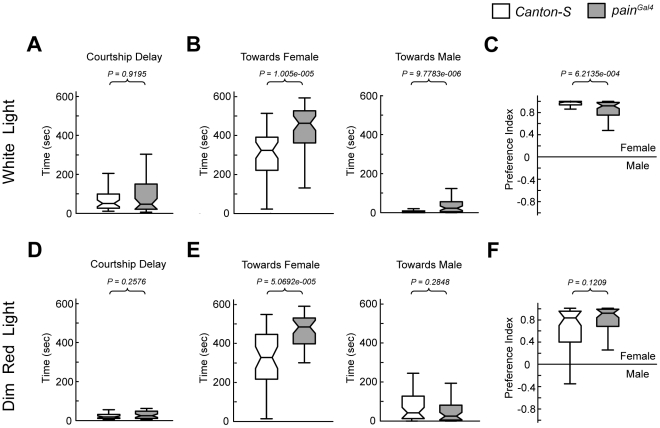
As the olfactory sensitivity to MCH was decreased in painless mutant flies, we next assessed whether the sensitivity of these flies to specific pheromones was also altered. If so, this could affect the ability of males to distinguish females from males and, thus be a cause for abnormal courtship behavior. We performed a courtship preference assay by measuring the preference index (PI) of painless mutant males towards decapitated male and female flies. As shown in Figure 4A , when placed with two decapitated WT flies of opposite genders, painGal4 males and WT males displayed a similar delay in courtship initiation, suggesting that painGal4 males were capable of sensing the targets and of initiating courtship behavior. We then examined the time spent by the painGal4 male and WT males courting the two decapitated targets and found that painGal4 males were able to distinguish female from male flies, although the PI was slightly lower as compared with that of WT flies (Figure 4B and 4C ). These results suggest that the male–male courtship behavior of painless mutant flies was unlikely to be caused exclusively by their inability to differentiate between female and male flies. In addition, we noticed that the total time spent by painGal4 males on courting was significantly longer than that observed for WT males (Figure 4B ).
Figure 4. painless mutant males were capable of distinguishing female from male flies.
(A) Box plots of courtship delay, which represents the latency before males initiated courtship behavior. (B) Total time spent by each individual male on courting decapitated females (left) and decapitated males (right). (C) Average PI of males of indicated genotypes towards females vs. males. Error bars represent SEM. (D–F) are similar as (A–C) except that these experiments were performed under dim red light instead of under white light as in (A–C). P values were determined by the Kruskal-Wallis test, and 43–48 males were examined in each group.
We also performed the experiment under dim red light to remove effects of the visual cues. We found that under such condition, both WT males and painGal4 mutant males showed a shorter courtship delay (Figure 4D ). In addition, WT males spent more time in courting the male target (Figure 4E ), suggesting that visual input is important for the WT males to distinguish males from females. In contrast, painGal4 mutant males showed similar sex-discriminating ability as that under white light (Figure 4B and 4F ). Thus, it is possible that painGal4 males are incapable of sensing inhibitory visual cues, or painGal4 males have additional deficiencies which mask the vision-deprivation effect.
Painless Expression in PNs Inhibits Male–male Courtship Behavior
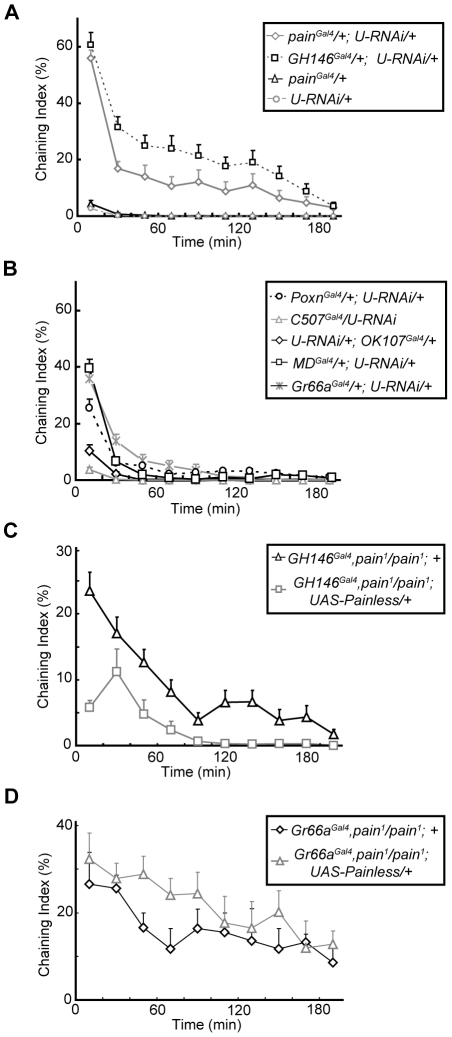
The above-described results showed that disruption of the painless gene led to decreased olfactory sensitivity and male–male courtship behavior. However, the brain regions in which Painless expression was required for the prevention of this aberrant behavior are unknown. The expression pattern of painless suggested a role for painless in olfactory processing. Therefore, we manipulated Painless expression in the olfactory PNs of the ALs. We first used two RNAi transgenic lines targeting painless [24] (UAS-pain-RNAi-1 and UAS-pain-RNAi-2) to downregulate Painless expression globally under the control of painGal4. As shown in Figures 5A and Figure S4, global downregulation of Painless expression in painGal4-positive neurons using either RNAi line induced male–male courtship behavior, while painGal4/+ heterozygous flies and the two UAS-pain-RNAi-alone flies did not exhibit this behavior. These results suggest that these two RNAi lines effectively downregulated Painless expression and induced the male–male courtship behavior.
Figure 5. The expression of Painless in PNs inhibited the male–male courtship behavior.
(A and B) Male–male courtship behavior caused by down-regulation of painless expression in different brain regions. (C and D) Overexpression of painless in GH146Gal4-positive neurons, but not in Gr66aGal4-positive neurons, suppressed the male–male courtship behavior in painless mutant males. Average ChI of males of indicated genotypes during the 3 h observing session are shown. For each trace, more than eight groups of males were analyzed. Error bars represent SEM.
We next used different Gal4 lines to knockdown Painless expression preferentially in selective subsets of neurons in an attempt to localize the site of action of Painless during the regulation of courtship behavior. We found that preferential knockdown of Painless expression in about two thirds of the olfactory PNs [25] using a GH146Gal4-driven RNAi caused severe male–male courtship behavior (Figure 5A ). Downregulation of Painless expression in certain gustatory receptor neurons (GRNs) using RNAi driven by Gr66aGal4, PoxnGal4, or MDGal4 resulted in weak male–male courtship behavior (Figure 5B ). As KCs in MBs and the neurons in the central complex were painGal4-positive, we further downregulated Painless expression in these two clusters of neurons by driving RNAi expression under the control of OK107Gal4 and C507Gal4, respectively. No significant male–male courtship behavior was observed in these two fly genotypes (Figure 5B ).
In parallel, we conducted rescue experiments to examine whether preferential overexpression of Painless in PNs or GRNs prevented the male–male courtship behavior caused by the painless mutation. As shown in Figures 5C and 5D , the intensity and duration of the male–male courtship behavior in pain1 flies were significantly reduced following preferential expression of Painless in the GH146Gal4 labeled PNs. However, overexpression of Painless in Gr66aGal4-positive neurons did not prevent male–male courtship behavior. These results of the cell-type-specific knockdown and rescue experiments suggest that the expression of Painless in PNs is necessary and sufficient for the suppression of male–male courtship behavior.
Male–male Courtship Behavior in painGal4 Flies Is Not Caused by Developmental Defects
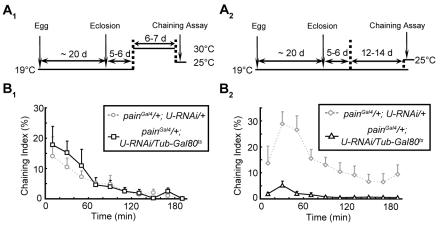
To determine whether the male–male courtship behavior is caused by developmental defects associated with painless mutation, we used the temporal and regional gene-expression targeting (TARGET) system to switch on RNAi expression in adult flies in a time-specific manner [26]. We used the temperature-sensitive Gal80 protein (Gal80ts) to manipulate the activity of Gal4. The effectiveness of Gal80ts was shown by the following experiments. The maintenance of flies carrying a copy of Gal80ts at a permissive temperature (19°C) led to negligible detection of GFP signal in the brain of painGal4;UAS-mGFP/TubP-Gal80ts animals. However, the transfer of the flies to the restrictive temperature (30°C) for 5–6 additional days restored GFP expression (Figure S5). We then used the TARGET system to switch on RNAi expression in adult flies. As shown schematically in Figure 6A , we inhibited the expression of UAS-pain-RNAi during early development by maintaining the flies at 19°C until 5–6 days after eclosion to ensure their proper development, and then relieved the inhibiting activity of Gal80 to restore the RNAi expression by elevating the temperature to 30°C for additional 5–6 days. Male–male courtship behavior was observed in these flies, with intensity and duration similar to those observed for the RNAi flies not expressing Gal80ts (Figure 6B ). This result indicates that downregulation of painless in adult flies was sufficient to induce male–male courtship behavior.
Figure 6. Downregulation of the expression levels of painless exclusively in adulthood resulted in male–male courtship behavior.
(A1 and A2) Schematic representations of the experimental design used to manipulate the expression levels of painless. Flies were raised and maintained at different temperatures, as indicated. Average ChI of males of indicated genotypes reared according to the strategies shown in A1 (B1) or A2 (B2). Error bars represent SEM, and n>8 in each trace.
Discussion
To study the physiological role of Painless in the central nervous system of adult fruit flies, we examined the behavior of painless mutant flies. We found that the painless mutation significantly increased male–male courtship behavior (Figures 2 and 3). By altering the expression level of Painless in a subpopulation of PNs in ALs, we were able to induce this male-male courtship behavior, suggesting that Painless expression in the olfactory system was necessary for inhibition of this abnormal courtship behavior (Figure 5). Furthermore, temporally specific Painless knockdown in the adult fly was sufficient to induce the male-male courtship phenotype (Figure 6). Moreover, mutation of the painless gene reduced olfactory sensitivity (Figure S2). Together, our results suggest that the expression of Drosophila Painless in a subset of PNs is essential for normal male courtship behavior. Because PNs are the principal relay neurons of the Drosophila olfactory system, Painless may play an important role in gating olfactory information; thus, mutation of this protein may lead to deficits in odor/pheromone perception and aberrant courtship behaviors.
Effects of Manipulating Painless Expression
In this study, the behaviors of several painless mutants were examined, and the expression level and patterns of painless were manipulated by using UAS-pain-RNAi and UAS-Painless driven by different Gal4 strains. We found that painless mutant males exhibited male-male courtship behavior (Figure 2 and Figure S3), at various intensities depending on the genotype. The painless mutants we used (pain1, pain3 and painGal4) have been reported to have P-element insertions immediate upstream of the first non-coding exon of painless gene, resulting in the expression of mutant Painless proteins [6]. Thus, it is possible that the male-male courtship behavior observed in painless mutants is due to loss of WT Painless protein function, or the expression of mutant Painless proteins, or both. Our results showing that overexpression of WT Painless in the mutant background significantly inhibited male-male courtship behavior (Figure 3B and Figure 5C ), and that knockdown of WT Painless expression induced male-male courtship behavior (Figure 5 and Figure S4) indicate that the loss of WT Painelss function is likely the major cause of this abnormal courtship behavior. However, we cannot exclude the possibility that the mutant Painless proteins resulting from the P-element insertion also contributes to the male-male courtship behavior, as the intensity of this behavior differed between painless mutant alleles.
By preferentially down-regulating Painless expression in different subsets of neurons, we found that the expression of painless in PNs was essential for the inhibiting courtship behavior among males. However, the contribution of painless expression in other neurons cannot be excluded. As shown in Figure 5C , preferential overexpression of painless in PNs of painless mutants did not fully suppress the abnormal courtship behavior. In addition, downregulation of painless expression in neurons (Gr66aGal4, PoxnGal4, and MDGal4) other than PNs resulted in a mild male–male courtship behavior (Figure 5B ). As Painless is expressed in a subset of GRNs that overlaps with the Gr66a-expressing neurons [10], involved in the detection of bitter substances and certain cuticular pheromones [27]–[29], we speculate that painless may also contribute to the sensation of these non-volatile pheromones by the Drosophila gustatory system [30]–[32].
Role of Painless in Gender Preference in flies
Male flies use multiple sensory modalities to discriminate female from males. When confronting with two targets of opposite genders, WT males spent almost all their time courting the female target, while painless mutant males also courted the male target (Figure 4). This observation suggests that painless mutant males have deficency in sensing the inhibitory cues on the male targets. Combined with results obtained from down- or up-regulating Painless expression in subsets of neurons (Figure 5), we surmise that painless expression in the olfactory system is involved in sensing cues. Meanwhile, the gender-preference assay revealed that painless mutants exhibited a longer courtship period towards both females and males as compared with WT flies (Figure 4), suggesting a general enhancement of courtship activity. This alteration is also suggested by the finding that painless mutant females exhibit enhanced sexual receptivity [33]. Thus, painless may play a role both in perception of inhibitory cues from males and in gating the intensity of the courtship behavior.
Effects of Anesthesia on Courtship Behavior
It is known that anesthesia affects the courtship behavior of individual males towards females [34]. The copulation latency of WT flies becomes longer after recovery from CO2 or chilling anesthesia [34]. Consistently, we found that brief anesthesia led to a significant decrease in the intensity of the courtship behavior of individual males (both WT males and painless mutants) towards decapitated females (Figure 4). However, the courtship behavior of an individual male towards another decapitated male was unchanged, while this behavior among a group of males was markedly enhanced. One explanation for these differential effects of anesthesia on male–male and male–female courtship behaviors is that anesthesia could weaken the sensation of attractive or repulsive pheromonal cues from females or males, respectively.
Distinct parts of the nervous system have differential sensitivities to anesthetics [35]. We speculate that in males there are neural circuits for sensing cues from other males to inhibit the male-male courtship behavior, and these circuits are highly sensitive to anesthetics. In addition, there are neural components which are responsible for the exhibition of courtship behavior, which are less sensitive than the inhibitory circuits. In the presence of mild anesthetics, e.g., brief CO2, nitrogen, and chilling used in the present study, the inhibitory neural circuits may loss their function more easily than the neural circuits responsible for courting, causing the animals to exhibit male-male courting. As the dose of anesthetics increased, the neural circuits responsible for courting were also disabled, resulting in decreases and eventually abolishment of courtship behavior. (Figure 2B ).
We also noticed that after recovery from an anesthesia of same dose, two painless mutants with the same P-element insertion (pain1 and painGal4) exhibited male-male courtship behavior of distinct intensities and temporal patterns (Figure 3). This is probably due to differences between the natures of these two painless mutant alleles (e.g. motility, vision). Furthermore, this might be due to the different sensitivity to anesthesia in pain1 and painGal4 mutants, as the recovery times from anesthesia in pain1 was longer than that in painGal4 males (Figure S6).
We also tested the idea whether anesthesia-sensitive neural circuits have some overlap with the painless circuits. By time-specific blockade of the neural transmission of painGal4-expressing neurons with a temperature-sensitive Shibire protein [36], a male–male courtship behavior could be triggered in the absence of anesthetics (Figure S7), suggesting that Painless might function in some courtship-inhibiting neural circuits which are also anesthesia-sensitive. Therefore, the enhanced courtship behavior observed among the painless mutant males after recovery from anesthesia should comprise integrated effects of both the impairment of painless function and anesthesia.
The mating behavior of painless mutant male flies towards other male flies may be caused by removal of the inhibition mechanism that prevents a male fly from pursuing other male flies. Removal of the inhibition mechanism in painless mutant flies is probably caused by their inability to sense the signal from other male flies correctly. Our results showing that the loss of function of the painless gene in PNs was essential for male–male courtship behavior, and that olfactory sensitivity was decreased in painless mutant flies, suggest that olfactory perception in painless mutant flies is impaired and may result in their inability to perceive inhibitory chemical cues (e.g. cis-Vaccenyl acetate) from other male flies effectively [30], [37], [38]. As one of the members of the TrpA family of ion channels, the expression of Painless in PNs may contribute to the electrophysiological properties of these neurons (e.g., the excitability and firing pattern of PNs). Thus, we speculate that painless may be involved in the coding of the olfactory/pheromone signals in PNs, which are important for the inhibition of male–male mating.
Supporting Information
(A) COS cells overexpressing Painless-myc fusion protein (left) were labeled by anti-Painless and anti-myc antibodies, while COS cells transfected with control plasmid showed no detectable signals (right) (Scale bar, 50 µm). (B) Confocal images of different peripheral organs of male flies of painGal4; UAS-mGFP, with green signal indicating GFP. Note that GFP was not detected in the third segment of antennae and the maxillary palps. (C) Confocal images of fly brains stained with the antibody against Painless. White arrows show the PNs expressing both Painless and GFP. White arrowhead indicates the glomerulus formed by the neurites of GFP–positive PNs. Some GFP-negative but Painless-positive neurons could be observed, suggesting that painGal4 might not label all Painless-expressing neurons. (Scale bar, 10 µm.)
(TIF)
Olfactory sensitivity was affected by painless mutation. The average preference indices (PI) to different concentrations of MCH were examined using a T-maze assay (A), and the olfactory sensitivity of flies of indicated genotypes was shown in (B). For each point, 13–35 groups of flies were examined. *, P<0.05, **, P<0.01 vs. the wild-type group (Kruskal-Wallis test).
(TIF)
Male-male courtship behavior in three painless mutant flies. Average ChI of males of indicated genotypes during the 3 h observation session. For each trace, more than eight groups of males were analyzed. Error bars mean SEM.
(TIF)
Expression of RNAi targeting painless in painGal4-positive neurons resulted in the male-male courtship behavior. The traces show the average ChI of males of indicated genotypes. Error bars represent SEM. For each trace, more than eight groups were analyzed.
(TIF)
Effectiveness of Gal80ts in suppressing the transcriptional activity of Gal4. The confocal images of brains of indicated genotype were shown. After maintained the flies at the restrictive temperature (30°C) for 6–7 days, GFP signal could be detected in painGal4-positive neurons. In contrast, maintenance of the flies at the permissive temperature (19°C) could effectively suppress the expression of GFP.
(TIF)
Recovery time of males of indicated genotypes from a 15 s CO2 anesthesia. Histograms represent the means, and error bars are SEM. No significant difference was found between the WT males and pain1 males, whereas painGal4 males have a shorter recovery time. P values were analyzed by Student's t test. The numbers of males examined are shown in parenthesis.
(TIF)
Blockade of the neurotransmission of the painGal4-positive neurons resulted in the male-male courtship behavior. The temperature was firstly shifted from 19°C to 30°C, and after maintaining for a period, was shifted back to 19°C. The behavior between eight males of indicated genotypes at either 19°C or 30°C were analyzed. Histograms show the average ChI, and error bars mean SEM. For each genotype, more than eight groups were analyzed.
(TIF)
Acknowledgments
We thank the following individuals for their contribution to this work: D. Tracey, R. F. Stocker, M. Noll, D. Armstrong, and T. Kitamoto for fly strains; Feng Wang for cell cultures; X. Yu, C. F. Wu and M. M. Poo for critical reading of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the China 973 project (2011CBA00404) awarded to ZW. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Damann N, Voets T, Nilius B. TRPs in our senses. Curr Biol. 2008;18:R880–889. doi: 10.1016/j.cub.2008.07.063. [DOI] [PubMed] [Google Scholar]
- 2.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 3.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 4.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 5.Viswanath V, Story GM, Peier AM, Petrus MJ, Lee VM, et al. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423:822–823. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- 6.Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 7.Rosenzweig M, Kang K, Garrity PA. Distinct TRP channels are required for warm and cool avoidance in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2008;105:14668–14673. doi: 10.1073/pnas.0805041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu J, Sornborger AT, Lee JK, Shen P. Drosophila TRPA channel modulates sugar-stimulated neural excitation, avoidance and social response. Nat Neurosci. 2008;11:676–682. doi: 10.1038/nn.2119. [DOI] [PubMed] [Google Scholar]
- 9.Xu SY, Cang CL, Liu XF, Peng YQ, Ye YZ, et al. Thermal nociception in adult Drosophila: behavioral characterization and the role of the painless gene. Genes Brain Behav. 2006;5:602–613. doi: 10.1111/j.1601-183X.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- 10.Al-Anzi B, Tracey WD, Jr, Benzer S. Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr Biol. 2006;16:1034–1040. doi: 10.1016/j.cub.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Sun Y, Liu L, Ben-Shahar Y, Jacobs JS, Eberl DF, et al. TRPA channels distinguish gravity sensing from hearing in Johnston's organ. Proc Natl Acad Sci U S A. 2009;106:13606–13611. doi: 10.1073/pnas.0906377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- 13.Wilson RI, Mainen ZF. Early events in olfactory processing. Annu Rev Neurosci. 2006;29:163–201. doi: 10.1146/annurev.neuro.29.051605.112950. [DOI] [PubMed] [Google Scholar]
- 14.Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15:1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 15.Masse NY, Turner GC, Jefferis GS. Olfactory information processing in Drosophila. Curr Biol. 2009;19:R700–713. doi: 10.1016/j.cub.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 16.Ferveur JF, Savarit F, O'Kane CJ, Sureau G, Greenspan RJ, et al. Genetic feminization of pheromones and its behavioral consequences in Drosophila males. Science. 1997;276:1555–1558. doi: 10.1126/science.276.5318.1555. [DOI] [PubMed] [Google Scholar]
- 17.Datta SR, Vasconcelos ML, Ruta V, Luo S, Wong A, et al. The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature. 2008;452:473–477. doi: 10.1038/nature06808. [DOI] [PubMed] [Google Scholar]
- 18.Svetec N, Houot B, Ferveur JF. Effect of genes, social experience, and their interaction on the courtship behaviour of transgenic Drosophila males. Genet Res. 2005;85:183–193. doi: 10.1017/S0016672305007536. [DOI] [PubMed] [Google Scholar]
- 19.Svetec N, Ferveur JF. Social experience and pheromonal perception can change male-male interactions in Drosophila melanogaster. J Exp Biol. 2005;208:891–898. doi: 10.1242/jeb.01454. [DOI] [PubMed] [Google Scholar]
- 20.Villella A, Gailey DA, Berwald B, Ohshima S, Barnes PT, et al. Extended reproductive roles of the fruitless gene in Drosophila melanogaster revealed by behavioral analysis of new fru mutants. Genetics. 1997;147:1107–1130. doi: 10.1093/genetics/147.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu T, Dartevelle L, Yuan C, Wei H, Wang Y, et al. Increased dopamine level enhances male-male courtship in Drosophila. J Neurosci. 2008;28:5539–5546. doi: 10.1523/JNEUROSCI.5290-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou X, Yuan C, Guo A. Drosophila olfactory response rhythms require clock genes but not pigment dispersing factor or lateral neurons. J Biol Rhythms. 2005;20:237–244. doi: 10.1177/0748730405274451. [DOI] [PubMed] [Google Scholar]
- 23.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 24.Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 25.Stocker RF, Heimbeck G, Gendre N, de Belle JS. Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J Neurobiol. 1997;32:443–456. doi: 10.1002/(sici)1097-4695(199705)32:5<443::aid-neu1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 26.McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- 27.Lacaille F, Everaerts C, Ferveur JF. Feminization and alteration of Drosophila taste neurons induce reciprocal effects on male avoidance behavior. Behav Genet. 2009;39:554–563. doi: 10.1007/s10519-009-9286-8. [DOI] [PubMed] [Google Scholar]
- 28.Lacaille F, Hiroi M, Twele R, Inoshita T, Umemoto D, et al. An inhibitory sex pheromone tastes bitter for Drosophila males. PLoS One. 2007;2:e661. doi: 10.1371/journal.pone.0000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Yew JY, Dreisewerd K, Luftmann H, Muthing J, Pohlentz G, et al. A new male sex pheromone and novel cuticular cues for chemical communication in Drosophila. Curr Biol. 2009;19:1245–1254. doi: 10.1016/j.cub.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoshita T, Martin JR, Marion-Poll F, Ferveur JF. Peripheral, Central and Behavioral Responses to the Cuticular Pheromone Bouquet in Drosophila melanogaster Males. PLoS One. 2011;6:e19770. doi: 10.1371/journal.pone.0019770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grillet M, Dartevelle L, Ferveur JF. A Drosophila male pheromone affects female sexual receptivity. Proc Biol Sci. 2006;273:315–323. doi: 10.1098/rspb.2005.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakai T, Kasuya J, Kitamoto T, Aigaki T. The Drosophila TRPA channel, Painless, regulates sexual receptivity in virgin females. Genes Brain Behav. 2009;8:546–557. doi: 10.1111/j.1601-183X.2009.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barron AB. Anaesthetising Drosophila for behavioural studies. J Insect Physiol. 2000;46:439–442. doi: 10.1016/s0022-1910(99)00129-8. [DOI] [PubMed] [Google Scholar]
- 35.Lin M, Nash HA. Influence of general anesthetics on a specific neural pathway in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1996;93:10446–10451. doi: 10.1073/pnas.93.19.10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitamoto T. Conditional disruption of synaptic transmission induces male-male courtship behavior in Drosophila. Proc Natl Acad Sci U S A. 2002;99:13232–13237. doi: 10.1073/pnas.202489099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferveur JF, Cobb M, Jallon JM. Complex Chemical Messages in Drosophila. . In: Singh R, Strausfeld N, editors. Neurobiology of Sensory System, 1989, New York: Plenum; 2007. pp. 397–409. [Google Scholar]
- 38.Bartelt R, Schaner A, Jackson L. cis-Vaccenyl acetate as an aggregation pheromone in Drosophila melanogaster. J Chem Ecol. 1985;11:1747–1756. doi: 10.1007/BF01012124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) COS cells overexpressing Painless-myc fusion protein (left) were labeled by anti-Painless and anti-myc antibodies, while COS cells transfected with control plasmid showed no detectable signals (right) (Scale bar, 50 µm). (B) Confocal images of different peripheral organs of male flies of painGal4; UAS-mGFP, with green signal indicating GFP. Note that GFP was not detected in the third segment of antennae and the maxillary palps. (C) Confocal images of fly brains stained with the antibody against Painless. White arrows show the PNs expressing both Painless and GFP. White arrowhead indicates the glomerulus formed by the neurites of GFP–positive PNs. Some GFP-negative but Painless-positive neurons could be observed, suggesting that painGal4 might not label all Painless-expressing neurons. (Scale bar, 10 µm.)
(TIF)
Olfactory sensitivity was affected by painless mutation. The average preference indices (PI) to different concentrations of MCH were examined using a T-maze assay (A), and the olfactory sensitivity of flies of indicated genotypes was shown in (B). For each point, 13–35 groups of flies were examined. *, P<0.05, **, P<0.01 vs. the wild-type group (Kruskal-Wallis test).
(TIF)
Male-male courtship behavior in three painless mutant flies. Average ChI of males of indicated genotypes during the 3 h observation session. For each trace, more than eight groups of males were analyzed. Error bars mean SEM.
(TIF)
Expression of RNAi targeting painless in painGal4-positive neurons resulted in the male-male courtship behavior. The traces show the average ChI of males of indicated genotypes. Error bars represent SEM. For each trace, more than eight groups were analyzed.
(TIF)
Effectiveness of Gal80ts in suppressing the transcriptional activity of Gal4. The confocal images of brains of indicated genotype were shown. After maintained the flies at the restrictive temperature (30°C) for 6–7 days, GFP signal could be detected in painGal4-positive neurons. In contrast, maintenance of the flies at the permissive temperature (19°C) could effectively suppress the expression of GFP.
(TIF)
Recovery time of males of indicated genotypes from a 15 s CO2 anesthesia. Histograms represent the means, and error bars are SEM. No significant difference was found between the WT males and pain1 males, whereas painGal4 males have a shorter recovery time. P values were analyzed by Student's t test. The numbers of males examined are shown in parenthesis.
(TIF)
Blockade of the neurotransmission of the painGal4-positive neurons resulted in the male-male courtship behavior. The temperature was firstly shifted from 19°C to 30°C, and after maintaining for a period, was shifted back to 19°C. The behavior between eight males of indicated genotypes at either 19°C or 30°C were analyzed. Histograms show the average ChI, and error bars mean SEM. For each genotype, more than eight groups were analyzed.
(TIF)