Abstract
Fragile X Syndrome (FXS) is characterized by mental impairment and autism in humans, and it often features hyperactivity and repetitive behaviors. The mechanisms for the disease, however, remain poorly understood. Here we report that the dfmr1 mutant in the Drosophila model of FXS grooms excessively, which may be regulated differentially by two signaling pathways. Blocking metabotropic glutamate receptor signaling enhances grooming in dfmr1 mutant flies, whereas blocking the vesicular monoamine transporter (VMAT) suppresses excessive grooming. dfmr1 mutant flies also exhibit elevated levels of VMAT mRNA and protein. These results suggest that enhanced monoamine signaling correlates with repetitive behaviors and hyperactivity associated with FXS.
Introduction
Fragile X Syndrome (FXS) is the most common form of inheritable mental impairment and the leading identified cause of autism. It affects approximately 1/5000 males and roughly half as many females [1]. FXS is caused by the loss of the fragile X mental retardation protein (FMRP), largely due to transcriptional silencing that results from a tri-nucleotide (CGG) repeat expansion in the 5′ untranslated region of the fragile X mental retardation 1 (FMR1) gene [2]. In addition to cognitive impairment, individuals with FXS exhibit behavioral problems including hyperactivity, attentional deficits, and impulsivity [3], [4]. Autistic-like characteristics, such as anxiety and stereotypic, repetitive behavior, are also common features of FXS, and approximately 30% of patients meet the diagnostic criteria for autism [5], [6], [7], [8], [9]. Studying the role of FMRP in the nervous system is thus necessary to understand the pathogenesis of both mental impairment and autism and for developing new treatment strategies.
Mouse and fly models of FXS exhibit phenotypic defects remarkably similar to the human disorder. Fmr1 knockout (KO) mice, which lack expression of the mouse homolog of FMRP, exhibit morphological abnormalities, learning and memory defects, and behavioral problems including attentional dysfunction, impulsivity, anxiety, and excessive grooming [10], [11], [12], [13]. The Drosophila gene dfmr1 codes for the protein dFMRP [14], which contains the same functional domains as the mouse and human homologues [15]. dfmr1 mutant flies exhibit defects in neuronal morphology [15], [16], [17], [18], physiology [17], [19], circadian rhythm [15], [20], [21], [22], sleep [23], courtship [20], learning, memory [24], and locomotion [17], [19], [25].
Although there is currently no effective treatment for FXS, research in the past decade has significantly advanced the understanding of the disorder. A major finding indicates that synaptic plasticity is altered in KO mice due to hyperactive signaling via the metabotropic glutamate receptor (mGluR) [26]. Further, reduction of mGluR expression in mutant mice, as well as treatment with mGluR antagonists, remarkably improves a number of phenotypes, including learning and memory [27], [28], [29]. However, these measures do not correct maroorchidism, indicating that enhanced mGluR signaling cannot account for all FXS phenotypes [28]. This partial rescue in mice is consistent with findings in Drosophila where reducing expression of DmGluR, the Drosophila homolog of mGluR, rescues neuronal overbranching [30] and physiological defects, but only partially improves synaptic physiology [31]. Further, blocking DmGluR signaling with the antagonist MPEP improves courtship, learning, memory, and rescues morphology defects in dmfr1 mutant flies. MPEP, however, fails to rescue abnormal circadian rhythm and sleep [32]. These observations suggest that additional signaling pathways may be altered in both mouse and fly models of FXS. Given recent evidence suggesting that FMRP may regulate global translation, rather than just a subset of translation important for mGluR signaling, the inability of mGluR antagonists to correct all FXS defects is not surprising [33], [34]. While the evidence strongly suggests that cognitive impairment in FXS results from aberrant mGluR signaling, the neuronal mechanisms underlying hyperactivity, impulsivity, and autistic-like behaviors remain poorly understood.
Here we seek to determine other signaling pathways that may be affected in the absence of dFMRP. We first identify the novel behavioral phenotype of excessive grooming in dfmr1 mutant flies, which appears to reflect the hyperactive and autistic-like features of FXS seen in mice and humans and adds another aspect of the disorder that can be studied in Drosophila. We find that blocking DmGluRs with MPEP does not reduce the excessive grooming in dfmr1 mutant flies, supporting the idea that enhanced mGluR signaling underlies only a subset of FXS phenotypes. Instead, our results suggest that enhanced monoamine signaling correlates with the excessive repetitive behavior in dfmr1 mutant flies.
Results
Wild-type dfmr1 transgene rescues aberrant climbing in dfmr1 mutant flies
dfmr1 mutant flies are adult viable [17], but display a number of locomotion defects, including abnormal crawling as larvae [25] and impaired flight as adults [17]. We have shown that dfmr1 mutant flies fail to climb robustly and that climbing progressively worsens with age [19]. To verify that abnormal climbing is directly caused by the loss of dFMRP, we introduced a transgene containing the wild-type dfmr1 gene under the control of the endogenous promoter [20], into the dfmr1 mutant background. We then investigated the climbing activity of genetically rescued mutant flies (hereafter called control), dfmr1 mutant flies, and dfmr1 mutant flies containing a dfmr1 transgene with a frameshift (FS) in the open reading frame of the genomic rescue fragment (see Methods for further information on genotypes).
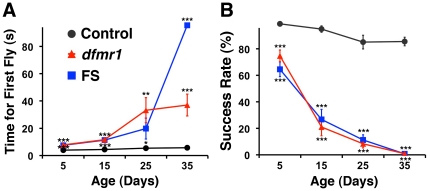
We monitored climbing performance at 5, 15, 25, and 35 days post-eclosion. We first measured the time for the first fly in a population of 10 flies to climb to a height of 17.5 cm (Movies S1 and S2). At 5 days old, the first fly in dfrm1 and FS populations took, on average, 7.8 s and 7.5 s, respectively, to climb 17.5 cm, significantly longer than the average of 3.9 s for control flies (P<0.001; Fig. 1A). As the flies aged, the top performer in mutant populations took progressively longer to reach the target height (dfmr1 averaged 37 s and FS averaged 96 s at 35 days old; Fig. 1A). In contrast, the climbing of top performers in control populations changed little with age, averaging 5.7 s at 35 days old.
Figure 1. Aberrant climbing is rescued by genomic expression of the dfrm1 gene.
(A). The time for the first fly to climb 17.5 cm. dfmr1 and FS (dfmr1 with a wild-type dfmr1 transgene that contains a frameshift mutation in the dfmr1 open reading frame) do not express functional dFMRP, and show a progressive change in climbing behavior over the course of 35 days. The abnormal climbing is rescued by a transgene containing the genomic wild-type dfmr1 locus (control). Data presented are the Mean +/- SEM (8 trials, total flies n = 80 for each genotype tested at each time point). (B). Total percentage of flies that successfully reach the 17.5 cm mark within 3 min. For all data, *p<0.05, **p<0.01, and ***p<0.001.
To better reflect the climbing activity of all flies in a population, we counted how many flies reached 17.5 cm after 3 min and determined the success rate (Fig. 1B). Similar to the data for the top performers, 5 day-old mutant flies had a significantly lower success rate than controls (P<0.001; Fig. 1B), and their success rate declined with age. By 35 days, mutant groups approached a 0% success rate, a dramatic reduction from rates of 75% and 65% for 5 day-old dfmr1 and FS flies, respectively (Fig. 1B). In contrast, control flies exhibited a noticeable, but much smaller, decline in success rate with age. We also measured the time for 50% of flies to reach 17.5 cm, as well as the failure rates for the first fly, or 50% of flies, in a population to complete the task successfully (Fig. S1). For all of these parameters we observed similar age-dependent declines in dfmr1 mutant flies. Hence, our climbing data demonstrate an age-dependent decrease in climbing activity in dfmr1 mutant flies, which is consistent with previous observations [19]. Further, adding a copy of the wild-type dfmr1 gene to dfmr1 mutant flies rescues the abnormal climbing behavior, indicating that this phenotype is caused specifically by the loss of dFMRP.
dfmr1 mutant flies groom excessively
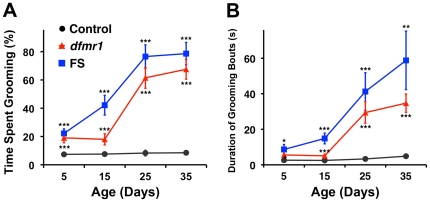
While performing the climbing tests we observed that dfmr1 mutant flies frequently stopped climbing and began grooming themselves. To study this behavior more directly we recorded the activity of individual flies in a small observation chamber (see Methods; Movies S3 and S4). At 5 days old, dfmr1 and FS flies groomed, on average, for 19% and 22% of the 5 min observation period, respectively (P<0.001; Fig. 2A). Control flies of the same age groomed significantly less, averaging 7% (Fig. 2A). Similar to the aberrant climbing, excessive grooming progressed with age in mutant flies, reaching as high as 79% in 35 day-old FS flies. Control flies, on average, spent 9% of the time grooming at 35 days, exhibiting little change in grooming activity with age.
Figure 2. dfmr1 mutant flies exhibit excessive grooming that increases with age.
(A). At 5 days old, dfmr1 and FS flies groom significantly more than control flies. Grooming increases with age in mutant flies; control flies show consistent levels of grooming at all ages tested. (B). The average duration of grooming bouts in mutant flies follows a similar trend to the total time spent grooming (A). In contrast, control flies show little change in the duration of grooming bouts from 5 to 35 days of age. Data are represented as the mean percentage of time single flies spend grooming during a 5 min period (Mean +/- SEM; n = 10–15 flies for each genotype at each time point). For all data, *p<0.05, **p<0.01, and ***p<0.001.
In addition, the duration of individual grooming bouts increased in dfmr1 mutant flies. At 5 days old, dfmr1 and FS flies had slightly longer grooming bouts than control flies, but a significant difference occurred only for FS flies (dfmr1: P>0.05, FS: P<0.05; Fig. 2B). Similar to the overall grooming time in figure 2A, the average grooming bout duration increased with age in mutant flies (Fig. 2B). At 35 days, dfmr1 and FS flies groomed 35 s and 59 s per bout on average, respectively, whereas control flies averaged 5 s per bout (dfmr1: P<0.001, FS: P<0.01; Fig. 2B).
The mGluR antagonist MPEP partially rescues courtship behavior, but enhances excessive grooming
To gain insight into the mechanisms underlying the excessive grooming phenotype, we investigated the role of mGluR signaling. We raised larvae and maintained adult flies on food containing 86 µM MPEP, a dosage previously shown to rescue courtship, learning, memory, and neuronal morphology defects in dfmr1 mutant flies [32]. We first tested the effects of MPEP on naïve courtship activity to ensure the drug's effectiveness. Courtship is an extensively studied, stereotypic behavior in Drosophila in which the male fly orients towards the female, tracks and follows her, produces wing songs, and attempts to lick her genitalia [35]. If the female is receptive, she then allows copulation. To quantify courtship activity, we used the standard ”Courtship Index” (CI), defined as the percentage of time a male fly spends performing any courtship behavior while in the presence of a female.
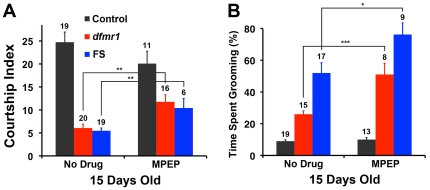
In agreement with the results of previous studies [20], [32], we observed reduced naïve courtship in dfmr1 mutant flies. Both 5 day-old dfmr1 and FS flies had an average CI of approximately 6, whereas control flies had a markedly higher CI of 25 (Fig. 3A). Mutant flies treated with MPEP showed a partial, but significant, improvement in naïve courtship (P<0.01; Fig. 3A). Control flies exhibited a small decrease in CI when treated with the drug, but the change was not significant. Our results suggest that we administered MPEP properly.
Figure 3. MPEP rescues courtship defects, but enhances excessive grooming in dfmr1 mutant flies.
(A). Treatment of dfmr1 mutant male flies with MPEP improves courtship of naïve females. Flies were grown as larvae and maintained as adults on either control food or food containing 86 µM MPEP. Data are presented as the mean courtship index (CI, +/- SEM) with sample sizes shown above each bar. dfmr1 and FS male flies treated with MPEP engage in courtship activity with wild-type virgin females significantly more than when treated with no drug. Control flies court less when treated with MPEP, but this difference is not significant. (B). 15 day-old dfmr1 and FS flies treated with 86 µM MPEP groom significantly more than when treated with no drug. MPEP does not affect grooming activity in control flies. Data are presented as the mean percentage of time single flies spend grooming during a 5 min period (Mean +/- SEM); sample sizes are displayed above each bar. For all data, *p<0.05 and ***p<0.001 (Two-tailed students t-test).
We proceeded to test the effect of MPEP on excessive grooming. Surprisingly, we found that 15 day-old dfmr1 and FS flies treated with MPEP showed a 2-fold and 1.5-fold increase in grooming activity, respectively, compared to those not treated with the drug (dfmr1: P<0.001, FS: P<0.05; Fig. 3B). In contrast, MPEP did not appear to affect the grooming activity of control flies (Fig. 3B).
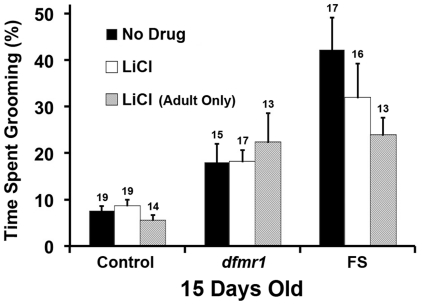
In addition to MPEP, lithium (LiCl, 5 mM) has also been shown to rescue courtship, learning, and memory defects in dfmr1 mutant flies [32]. In this study, 15 day-old dfmr1 and FS flies grown as larvae and maintained as adults on food containing 5 mM lithium showed no significant changes in grooming activity, nor did control flies given the same treatment (Fig. 4). We also treated flies with lithium only as adults (i.e. larvae were grown on food with no drug), but found no difference in the drug's effect (Fig. 4). We therefore conclude that lithium, at a dosage that rescues other dfmr1 behavioral defects, does not significantly affect the excessive grooming in dfmr1 mutant flies.
Figure 4. Lithium does not significantly affect grooming in dfmr1 mutant flies.
Grooming activity of 15 day-old flies grown as larvae and maintained as adults, or only maintained as adults on food containing 5 mM LiCl. Data are presented as the mean percentage of time single flies spend grooming during a 5 min period (Mean +/- SEM); sample sizes are displayed above each bar. For all genotypes, LiCl causes no significant change in grooming activity (Kruskal-Wallis one-way ANOVA with Dunn's post-hoc comparison).
Reserpine suppresses excessive grooming in dfmr1 mutant flies
As our results with MPEP suggested that excessive grooming in dfmr1 mutant flies results from changes outside of mGluR signaling, we searched for other disturbances caused by the loss of dFMRP. FMRP has been shown to regulate dopamine signaling in both mouse and fly models of FXS. In cultured neurons of Fmr1 KO mice, dopamine type 1 receptors are hyperphosphorylated and defective in signaling [36]. In dfmr1 mutant flies, dopamine, and to a lesser extent serotonin, is elevated in the brain [37]. Moreover, biogenic monoamines have been shown to play a positive role in grooming in Drosophila. Application of dopamine, octopamine, and serotonin to the ventral nerve cord of decapitated flies stimulates grooming [38]. It has also been demonstrated that overexpression of the Drosophila vesicular monoamine transporter (dVMAT), which loads monoamines into synaptic vesicles, increases grooming in flies [39]. Finally, blocking dVMAT with the drug reserpine suppresses the elevated grooming activity in flies that overexpress the transporter [39]. Hence, we next tested the ability of reserpine to suppress excessive grooming in dfmr1 mutant flies to examine if enhanced monoamine signaling might contribute to the behavior.
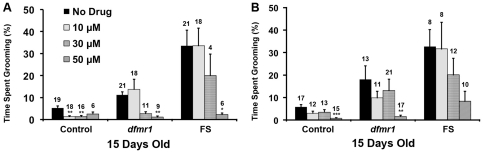
15 day-old mutant flies treated with varying concentrations of reserpine (10, 15, 20, 30, and 50 µM) as both larvae and adults exhibited a significant decrease in grooming at 50 µM (dfmr1: P<0.01, FS: P<0.05), but not at the lower concentrations (Fig. 5A). In contrast, control flies treated with reserpine as larvae and adults showed significantly suppressed grooming at 10 µM (P<0.01), 20 µM (P<0.001), and 30 µM (P<0.01) (Fig. 5A). To determine if reserpine is effective post-developmentally, we treated flies with the drug only after eclosion. Similar to figure 5A, 15 day-old dfmr1 flies treated only as adults groomed significantly less at 50 µM (P<0.01), but not at lower concentrations (Fig. 5B). FS flies treated with reserpine only as adults did not show a significant difference in grooming at any concentration, but it is worth noting that the reduction of grooming activity for 50 µM became significant upon exclusion of an outlier in the sample (data not shown). Like in figure 5A, post-developmental reserpine treatment significantly reduced grooming in controls at lower dosages than in mutants (Fig. 5B). Our results demonstrate that reserpine can effectively suppress excessive grooming in dfmr1 mutant flies, is effective when used only in adulthood, and that dfmr1 mutant flies are less sensitive to reserpine than control flies.
Figure 5. Dosage effects of reserpine on grooming.
(A). Grooming activity of 15 day-old flies grown as larvae and maintained as adults on food containing no drug, 10, 30, or 50 µM reserpine (15 and 20 µM are omitted from figure). Reserpine suppresses grooming in dfmr1 and FS flies, but only at 50 µM; control flies show significantly reduced grooming at the lowest concentration, 10 µM. (B). 15 day-old flies treated with reserpine only as adults (i.e. larvae were grown on control food), show a similar response. Suppressed grooming in mutant flies is only significant at 50 µM. In contrast, reserpine significantly decreases grooming activity in control flies at 15 µM. Data are presented as the mean percentage of time single flies spent grooming during a 5 min period (Mean +/- SEM); sample sizes are displayed above each bar. For all data, *p<0.05 and ***p<0.001 (Kruskal-Wallis one-way ANOVA with Dunn's post-hoc comparison).
dVMAT mRNA and protein levels are elevated in the absence of dFMRP
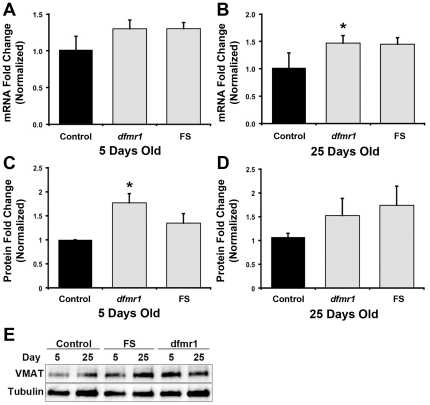
As suppression of excessive grooming in mutant flies required a higher dosage of reserpine, we hypothesized that dVMAT levels could be increased in dfmr1 mutant flies. Although dFMRP is primarily known to regulate translation, it has also been shown to influence transcript expression [40], [41], [42], [43]. To determine if dVMAT mRNA levels are affected by the loss of dFMRP, we used quantitative real-time polymerase chain reaction (qPCR) to quantify dVMAT transcript levels in dfmr1 mutant flies. At 5 days of age, dfmr1 mutant flies showed a 29% increase in dVMAT mRNA compared to control flies, but the change was not significant (P>0.05; Fig. 6A). At 25 days of age, we detected a 41% increase in dVMAT transcript levels in mutant flies compared to control flies. This increase was significant for dfmr1 flies, but not for FS flies (dfmr1: P<0.05, FS: P>0.05; Fig. 6B). Thus, dVMAT transcript levels increased in dfmr1 mutant flies over time, up to the age we tested.
Figure 6. dVMAT transcript and protein levels are upregulated in dfmr1 mutant flies.
Quantitative real-time PCR experiments indicate that dVMAT mRNA levels are increased in 5 day-old dfmr1 mutant flies (A) and in 25 day-old dfmr1 mutant flies (B) relative to control flies. Western blot analyses indicate that dfmr1 mutant flies also have increased levels of dVMAT protein at both (C) 5 days and (D) 25 days of age. (E) Representative blot showing increased dVMAT protein levels in dfmr1 and FS flies compared to control flies. Real-time PCR data are presented as the average of three biological replicates. Western blot data are shown as the average of three independent trials. *p<0.05 (One-way ANOVA with Dunnett's post-hoc comparison).
We next examined the effect of the absence of dFMRP on dVMAT protein levels. Consistent with the increase in mRNA, we detected a 77% and 35% elevation of dVMAT protein levels in 5 day-old dfmr1 and FS fly heads, respectively, compared to 5 day-old control fly heads. This represents a significant increase in dVMAT protein levels for dfmr1 but not for FS flies (dfmr1: P<0.05; FS: P>0.05; Fig. 6C). We also detected elevated dVMAT protein levels at 25 days in dfmr1 (52%) and FS (74%) flies, but neither increase was statistically significant compared to control flies at both 5 and 25 days (Fig. 6D). Hence, our results show a trend that dVMAT transcript and protein levels are both upregulated in dfmr1 mutant flies, and under certain conditions the increases reach statistically significant levels.
Discussion
There are four major findings from this study: 1) age-dependent abnormal climbing in dfmr1 mutant flies can be genetically rescued, 2) excessive grooming is identified as a new behavioral defect in dfmr1 mutant flies, 3) excessive grooming can be suppressed by reserpine, and 4) dVMAT mRNA and protein levels are increased in the absence of dFMRP.
In a previous study we revealed abnormal climbing activity in dfmr1 mutant flies that progresses with age. Our results in this study confirm this finding, and additionally show that introducing a wild-type dfmr1 transgene into the dfmr1 mutant background restores normal climbing behavior. Further, a frameshift mutation in the open reading frame of the transgene abolishes the rescue of climbing behavior. These results demonstrate that the abnormal climbing in dfmr1 mutant flies is directly caused by the loss of dFMRP.
In this study we have also identified excessive grooming as an important and novel behavioral defect in the fly model of FXS. Our results show that dfmr1 mutant flies groom significantly more than control flies, and that mutant flies also have significantly longer grooming bouts. Further, this excessive grooming intensifies with age in dfmr1 mutant flies, whereas control flies show essentially no change in grooming activity over time. A wild-type copy of the dfmr1 gene can rescue the excessive grooming defect in dfmr1 mutant flies. It is worth noting that FS mutants show more dramatic climbing and grooming defects compared to dfmr1 mutants. We do not know the exact underlying cause, but it may be due to the presence of either mRNA or a truncated peptide produced from the FS rescue fragment, having gain of function effects.
In video recordings, control flies mostly walk around the observation chamber and groom occasionally, but rarely stand motionless. In contrast, the mutant fly spends more time grooming. It is possible that grooming is a default activity that occurs whenever a fly is not walking. If this is the case, excessive grooming in dfmr1 mutant flies could result indirectly from problems in walking. This explanation would be consistent with our observation that dfmr1 mutant flies exhibit postural problems and uncoordinated movement. However, the dfmr1 flies are capable of climbing after a brief period of mechanical disturbance (i.e., knocking them down in the graduated cylinder), albeit at a slower speed compared to control flies. Further, reserpine suppresses grooming in dfmr1 mutant flies without improving walking (data not shown). These observations suggest that grooming is not simply a default behavior in the absence of walking and that dfmr1 mutations specifically cause excessive grooming. Notably, Fmr1 KO mice have also been reported to exhibit excessive grooming when presented with social stimuli [11], [13]. A study of self-injurious behavior in FXS patients reported a prevalence of harmful rubs and scratches [44]. Hence, heightened repetitive activity such as grooming is a common behavioral defect in FXS.
Although reducing mGluR signaling has been shown to rescue learning and memory defects in both mouse and fly FXS models, we find that the mGluR antagonist MPEP enhances excessive grooming in dfmr1 mutant flies. This is not completely surprising, as the absence of dFMRP likely alters numerous signaling pathways and developmental processes of the nervous system. MPEP also fails to rescue abnormal sleep [23] and circadian rhythm [32] in dfmr1 mutant flies, which may impact locomotor activity like grooming. It is worth noting that dfmr1 mutant flies did not groom more when treated with LiCl, suggesting that mGluR antagonists and LiCl may have different neuronal targets. An interesting question that arises from these results is whether an mGluR agonist might suppress grooming in dfmr1 mutant flies. Previous results have shown that glutamate at concentrations as low as 5 µM is toxic to dfmr1 mutant flies and significantly affects various behaviors in the fly [45]. This makes it difficult to assess the potential benefit of mGluR agonists on grooming.
Previous work shows that dopamine plays a role in FXS in both mice [36] and Drosophila [37], and that biogenic monoamines stimulate fly grooming [38], [39]. In our studies, blocking dVMAT with reserpine suppresses excessive grooming in dfmr1 mutant flies, but only significantly at 50 µM. Control flies groom significantly less when treated with just 10 µM. These results indicate that dfmr1 mutant flies are less sensitive to reserpine's effect on grooming. However, we cannot exclude the possibility that reserpine has additional targets and therefore generally sedates the fly. Both suppression of dVMAT as well as a non-specific target could slow down most motor activities including grooming. Alternatively, it is possible that basal monoamine activity is required for grooming, and therefore shutting down monoamine signaling may block the behavior.
In our study, we find elevated levels of dVMAT transcript and protein in dfmr1 mutant flies. Although these increases are not statistically significant in some instances, they are consistent in both mutant lines. However, it is not clear from our results how the loss of dFMRP leads to increased dVMAT expression. The transcription of dVMAT may be directly increased. Alternatively, degradation of dVMAT mRNA may decrease in the absence of dFMRP, a distinct possibility as FMRP has been previously indicated to regulate mRNA stability [46]. How dFMRP regulates dVMAT protein levels is also unclear. Elevated dVMAT protein levels may occur exclusively because of increased transcript levels, but could also result from increased translation or reduced degradation of the protein. Nonetheless, our observations are in agreement with the known function of FMRP as a regulator of transcription and translation [40], [42], [47].
Many factors may contribute to the excessive grooming in dfmr1 mutant flies, and our data do not resolve whether upregulation of dVMAT directly influences this behavior. Overexpression of dVMAT stimulates grooming in flies [39], and dopamine levels are increased in dfmr1 mutant brains [37]. Monoamines could directly or indirectly modulate multiple downstream signaling pathways involved in grooming. The hyposensitivity to reserpine seems to suggest that a greater number of dVMATs are present on mutant synaptic vesicles, as a higher concentration of the drug is required to reduce grooming. We note that overexpression of dVMAT in serotonergic and dopaminergic neurons leads to hypersensitivity to reserpine on grooming [39]. One likely explanation of these differences is that dfmr1 mutations affect not only monoamine cells but also other cells such as neurons in the mushroom bodies and neurons postsynaptic to monoamine cells. We are also are aware that dopamine signaling is reduced in the forebrain of Fmr1 KO mice [36]. Thus, while plausible given the effect of reserpine, we cannot establish a clear causal relationship between excessive grooming and dVMAT expression levels.
Understanding how FMRP functions in development and aging will be crucial for effective treatment of FXS [48], [49]. Studies in mouse and Drosophila indicate that FMRP is temporally regulated and that treatment requires proper timing [50], [51], [52], [53], [54]. Our results add to the growing evidence of the importance of FMRP in age-related processes, and also demonstrate that hyperactivity and repetitive behavior increase with age in the Drosophila model. Interestingly, the severity of autistic behavior and anxiety has been found to increase with age in studies of FXS patients [55], [56]. Our results indicating that reserpine is effective in adult dfmr1 mutant flies could help develop or improve treatment, as they suggest that hyperactive and repetitive behavior in older patients is potentially reversible.
Although we believe excessive grooming in dfmr1 mutant flies is a model of an impulsive and repetitive behavior, animal models can never completely recapitulate human disorders. The mechanisms underlying repetitive behaviors in FXS patients are likely much more complex. Nonetheless, we demonstrate a correlation between monoamine signaling and the excessive grooming phenotype in dfmr1 mutant flies and that VMAT is a protein that merits further study in FXS. Importantly, our study provides potentially useful information for improving the pharmaceutical treatment of FXS symptoms in human patients.
Materials and Methods
Fly stocks, Genetics, and Pharmacology
Flies were grown on a standard cornmeal-agar medium under a 12 h/12 h light/dark cycle. fragile X (dfmr1) mutant flies were generated by crossing w; dfmr183M/TM6B, Tb with w; dfmr13/TM6C, Sb flies and selecting w; dfmr183M/dfmr13 flies from the progeny [17], [20]. Control flies, which contain a transgene encoding the wild-type dfmr1 gene in the dfmr1 mutant background, were generated by crossing w; dfmr183M/TM6B, Tb with w; wild-type rescue (WT)/+; dfmr13/TM6C, Sb flies [20]. FS flies, which carry a frameshift in the open reading frame of the transgenic rescue fragment were generated by crossing w; dfmr183M/TM6B, Tb with w; frameshift rescue (FS)/+; dfmr13/TM6C, Sb flies [20]. For grooming assays, 1–3 day-old adult male flies were collected following brief anesthetization with CO2. Flies were stored in fresh food vials with 10–13 flies per vial (climbing) or 2–8 flies per vial (grooming). For MPEP and LiCl administration, an aqueous stock solution was mixed into recently cooked standard food after the food had cooled. As reserpine is insoluble in water, a stock solution in 1 M acetic acid was mixed into molten food in a 1∶9 ratio [39]. For control experiments, flies were raised on food containing the same amount of each vehicle (water or acetic acid). Flies were transferred to new food vials every 4–6 days.
Behavioral Assays
Climbing
For climbing trials, 10 male flies were transferred to a 250 mL glass graduated cylinder, which was sealed with parafilm to prevent escape. Next, the flies were knocked down to the bottom; and care was taken to use similar force for all trials. Measurements were taken for the (1) time for the first fly to cross the 150 mL line (17.5 cm from the bottom); (2) percentage of trials when a first fly did not cross the 17.5 cm line within 3 min; (3) time for 50% of the population to cross the 17.5 cm line; (4) percentage of trials when 50% of the population did not cross the 17.5 cm line within 3 min; and (5) the percentage of flies that crossed the 17.5 cm line within 3 min. Four trials were performed for each population and their average was taken for a sample value. A total of 8 samples were taken for each genotype. For data analysis we excluded events for (1) in which no fly, and for (3) in which 50% of a population, did not reach 17.5 cm within 3 min. Experiments were performed between 5–7 pm to minimize potential effects of circadian oscillation.
Courtship
Virgin male flies collected within 4 hours of eclosion were stored in individual food vials. Virgin wild-type (CS, Canton S) female flies, collected on the same day as the virgin males, were kept in groups of 10–20 per food vial. All courtship assays were performed with 5 day-old male and female flies between 3–6 pm. Male flies were first aspirated into an observation chamber of about 0.4 cm3, and after 1 min of acclimation, a virgin wild-type female was aspirated into the chamber and behavior was then monitored for 10 min. The courtship index (CI) was scored as the percentage of time that a male fly spent engaged in courtship activity while paired with a female [32], [57]. Courtship behavior was recorded on video and analyzed later using the iVideo program for Macintosh.
Grooming
Single male flies were aspirated into a 0.4 cm3 observation chamber, allowed to acclimate for 1 min, and then recorded for a 5 min observation period. Data were collected for (1) the percentage of time the fly spent grooming and (2) the duration of individual grooming bouts. Grooming bouts were recorded as ending when a fly either stopped grooming and remained motionless for 2 s, or stopped grooming and walked at least 4 steps. Grooming experiments were performed between 3–6 pm and were recorded and analyzed using video software.
Quantitative Real-time PCR
Flies were collected within 24 hours of eclosion, aged for 5 days or 25 days, and frozen in liquid nitrogen between 3–6 pm. Samples were stored at −80°C. Three biological replicates were used for each genotype at each age. RNA was extracted from 40 flies with TRIzol (Invitrogen) and purified using a Qiagen RNeasy Mini Kit (Qiagen) with on-column DNase I (Qiagen) treatment. RNA yield and purity was checked with a NanoDrop 2000 Specrophotometer (Thermo Scientific). To generate cDNA, 0.4 µg of RNA was used with a SuperScript III Reverse Transcriptase First-Strand Synthesis Kit (Invitrogen). Real-time PCR was performed using a Maxima SYBR Green/ROX qPCR Master Mix (Fermentas). Analyses were performed using an Applied Biosystems 7500 Real-time PCR System. Relative expression levels were determined with the 2−ddCt method [58], using rp49 as a reference gene. qPCR primer sequences for dVMAT were:
5′-AAAATTGGACGATGGTTTGC-3′ (forward) and
5′-ATTCGGGATGATCAGGTGAG-3′ (reverse);
primer sequences for rp49 were:
5′-CGGATCGATATGCTAAGCTGT-3′ (forward) and
5′-GCGCTTGTTCGATCCGTA-3′ (reverse).
Western blots
Flies were collected within 24 hours of eclosion, aged for 5 days or 25 days, and frozen in liquid nitrogen between 3–6 pm. Samples were stored at −80°C. An equal number of fly heads were isolated for each experiment (15–20 total/condition), homogenized in Buffer A (150 mM NaCl, 10 mM HEPES, pH 7.4, 1 mM EGTA, 0.1 mM MgCl2, 2 mM PMSF, and protease inhibitor cocktail) (Roche, Indianapolis, IN) using a plastic pestle. Protein lysates were cleared by centrifugation at 22,000 x g for 20 min. Total protein concentration was measured by a BCA Assay (Thermo Scientific, Rockford, IL), diluted with 2 x SDS Sample Buffer, boiled for 5 min, and equal concentrations of protein were separated on a 10% SDS-PAGE gel for each condition. Following transfer to nitrocellulose, the membranes were incubated overnight at 4°C at 1∶4000 in rabbit anti-dVMAT [59], washed in 0.5% TBS-Tween, and incubated for 1 h at 1∶4000 in HRP-conjugated rabbit secondary antibody. Protein was detected using the ECL method, normalized to tubulin (Sigma, St. Louis, MO), and quantified using Image J (NIH).
Statistical Analysis
Most statistical analyses were performed using GraphPad Prism 4. ANOVA with a Dunnett's multiple comparison test was used for statistical analysis, unless noted otherwise. For data that were not normally distributed, and for which transformation could not resolve this issue, a non-parametric test was used (Kruskal-Wallis one-way ANOVA with a Dunn's post-hoc test). All data are shown as mean +/- SEM. *p<0.05, **p<0.01, and ***p<0.001 are considered statistically significant.
Supporting Information
Additional measurements of climbing behavior in dfmr1 mutant flies. (A). Time for 50% of a population to climb 17.5 cm. Control flies contain a wild-type dfmr1 transgene under endogenous regulation in the dfmr1 mutant background. dfmr1 and FS (dfmr1 mutants that contain a wild-type dfmr1 transgene that has a frameshift mutation in the dfmr1 open reading frame) do not express functional dFMRP. By 35 days all dfmr1 and FS populations failed to have 50% reach 17.5 cm within 3 min. Data presented are the average of Mean +/- SEM (8 trials, total flies n = 80 for each genotype tested at each time point). (B). Percentage of failed attempts for populations to have a first fly reaching the 17.5 cm line. (C). Percentage of failed attempts for populations to have at least 50% of flies climb 17.5 cm. For all data, *p<0.05, **p<0.01, and ***p<0.001.
(TIF)
Sample video of control flies climbing. This video illustrates how climbing experiments were conducted. Ten control flies at 15 day-old were gently knocked to the bottom of a graduated cylinder and then observed to climb to the top.
(MOV)
Sample video of the dfmr1 mutant flies climbing. Ten dfmr1 mutant flies at 15 day-old were gently knocked to the bottom of a graduated cylinder. The flies then begin climbing, but some stop after a short period of time. Later analysis showed that these flies stopped to groom themselves.
(MOV)
Sample video of dfmr1 mutant grooming activity. A 15 day-old dfmr1 mutant fly initially explores the environment for 10 s, but then begins grooming excessively.
(MOV)
Sample video of grooming activity in a control fly. A 15 day-old control fly explores the environment, stopping only once to groom for 3 s.
(MOV)
Acknowledgments
We thank Drs. Yong Q. Zhang and Kendal Broadie for providing the w; dfmr183M/TM6B, Tb stock, and Dr. Thomas Jongens for providing the w; dfmr13/TM6C, Sb, wild-type rescue (Control) and frameshift rescue (FS) stocks. We also thank Dr. David Krantz for his advice on the use of acetic acid in dissolving reserpine and for providing the antibody to dVMAT, Drs. Michael Tranfaglia, Randy Hewes, Rosemary Knapp, and David McCauley for constructive comments on the manuscript, Dr. Lauren Ritterhouse for assistance in the statistical analysis of the data, Logan Cox for assistance on fly husbandry, and Emily Kumimoto, Rod Kumimoto, and Sunetra Das for their instruction and assistance with RNA isolation and real-time PCR. The MPEP drug was a generous gift of Dr. Michael Tranfaglia from the FRAXA Research Foundation. J.M.T. received the inaugural Vad Foundation Award for Outstanding Undergraduate Thesis for this research. We wish to thank Dr. Vijay Vad and his Foundation for his support.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by internal funds from the University of Oklahoma (to B.Z.) and in part by an NIH grant (RO1 NS060878 to B.Z.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Coffee B, Keith K, Albizua I, Malone T, Mowrey J, et al. Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA. Am J Hum Genet. 2009;85:503–514. doi: 10.1016/j.ajhg.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 3.Boyle L, Kaufmann WE. The behavioral phenotype of FMR1 mutations. Am J Med Genet C Semin Med Genet. 2010;154C:469–476. doi: 10.1002/ajmg.c.30277. [DOI] [PubMed] [Google Scholar]
- 4.Garber KB, Visootsak J, Warren ST. Fragile X syndrome. Eur J Hum Genet. 2008;16:666–672. doi: 10.1038/ejhg.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagerman R, Hoem G, Hagerman P. Fragile X and autism: intertwined at the molecular level leading to targeted treatments. Mol Autism. 2010;1:12. doi: 10.1186/2040-2392-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris SW, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, et al. Autism profiles of males with fragile X syndrome. Am J Ment Retard. 2008;113:427–438. doi: 10.1352/2008.113:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufmann WE, Cortell R, Kau ASM, Bukelis I, Tierney E, et al. Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. Am J Med Genet A. 2004;129A:225–234. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- 8.Reiss A, Freund L. Behavioral phenotype of fragile X syndrome: DSM–III–R autistic behavior in male children. Am J Med Genet. 1992;1:35–46. doi: 10.1002/ajmg.1320430106. [DOI] [PubMed] [Google Scholar]
- 9.Moss J, Oliver C, Arron K, Burbidge C, Berg K. The prevalence and phenomenology of repetitive behavior in genetic syndromes. J Autism Dev Disord. 2009;39:572–588. doi: 10.1007/s10803-008-0655-6. [DOI] [PubMed] [Google Scholar]
- 10.Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, et al. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci U S A. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNaughton CH, Moon J, Strawderman MS, Maclean KN, Evans J, et al. Evidence for social anxiety and impaired social cognition in a mouse model of fragile X syndrome. Behav Neurosci. 2008;122:293–300. doi: 10.1037/0735-7044.122.2.293. [DOI] [PubMed] [Google Scholar]
- 12.Moon J, Beaudin AE, Verosky S, Driscoll LL, Weiskopf M, et al. Attentional dysfunction, impulsivity, and resistance to change in a mouse model of fragile X syndrome. Behav Neurosci. 2006;120:1367–1379. doi: 10.1037/0735-7044.120.6.1367. [DOI] [PubMed] [Google Scholar]
- 13.Pietropaolo S, Guilleminot A, Martin B, D'Amato FR, Crusio WE. Genetic-background modulation of core and variable autistic-like symptoms in FMR1 knock-out mice. PloS One. 2011;6:e17073. doi: 10.1371/journal.pone.0017073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan L, Dockendorff TC, Jongens TA, Dreyfuss G. Characterization of dFMR1, a Drosophila melanogaster homolog of the fragile X mental retardation protein. Mol Cell Biol. 2000;20:8536–8547. doi: 10.1128/mcb.20.22.8536-8547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morales J, Hiesinger PR, Schroeder AJ, Kume K, Verstreken P, et al. Drosophila fragile X protein, DFXR, regulates neuronal morphology and function in the brain. Neuron. 2002;34:961–972. doi: 10.1016/s0896-6273(02)00731-6. [DOI] [PubMed] [Google Scholar]
- 16.Michel CI, Kraft R, Restifo LL. Defective neuronal development in the mushroom bodies of Drosophila fragile X mental retardation 1 mutants. J Neurosci. 2004;24:5798–5809. doi: 10.1523/JNEUROSCI.1102-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang YQ, Bailey AM, Matthies HJ, Renden RB, Smith MA, et al. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell. 2001;107:591–603. doi: 10.1016/s0092-8674(01)00589-x. [DOI] [PubMed] [Google Scholar]
- 18.Pan L, Zhang YQ, Woodruff E, Broadie K. The Drosophila fragile X gene negatively regulates neuronal elaboration and synaptic differentiation. Curr Biol. 2004;14:1863–1870. doi: 10.1016/j.cub.2004.09.085. [DOI] [PubMed] [Google Scholar]
- 19.Martinez VG, Javadi CS, Ngo E, Ngo L, Lagow RD, et al. Age-related changes in climbing behavior and neural circuit physiology in Drosophila. Dev Neurobiol. 2007;67:778–791. doi: 10.1002/dneu.20388. [DOI] [PubMed] [Google Scholar]
- 20.Dockendorff TC, Su HS, McBride SMJ, Yang Z, Choi CH, et al. Drosophila lacking dfmr1 activity show defects in circadian output and fail to maintain courtship interest. Neuron. 2002;34:973–984. doi: 10.1016/s0896-6273(02)00724-9. [DOI] [PubMed] [Google Scholar]
- 21.Inoue S, Shimoda M, Nishinokubi I, Siomi MC, Okamura M, et al. A role for the Drosophila fragile X-related gene in circadian output. Curr Biol. 2002;12:1331–1335. doi: 10.1016/s0960-9822(02)01036-9. [DOI] [PubMed] [Google Scholar]
- 22.Sofola O, Sundram V, Ng F, Kleyner Y, Morales J, et al. The Drosophila FMRP and LARK RNA-binding proteins function together to regulate eye development and circadian behavior. J Neurosci. 2008;28:10200–10205. doi: 10.1523/JNEUROSCI.2786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bushey D, Tononi G, Cirelli C. The Drosophila fragile X mental retardation gene regulates sleep need. J Neurosci. 2009;29:1948–1961. doi: 10.1523/JNEUROSCI.4830-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolduc FV, Bell K, Cox H, Broadie KS, Tully T. Excess protein synthesis in Drosophila fragile X mutants impairs long-term memory. Nat Neurosci. 2008;11:1143–1145. doi: 10.1038/nn.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu K, Bogert BA, Li W, Su K, Lee A, et al. The fragile X-related gene affects the crawling behavior of Drosophila larvae by regulating the mRNA level of the DEG/ENaC protein pickpocket1. Curr Biol. 2004;14:1025–1034. doi: 10.1016/j.cub.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 26.Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi CH, Schoenfeld BP, Bell AJ, Hinchey P, Kollaros M, et al. Pharmacological reversal of synaptic plasticity deficits in the mouse model of Fragile X syndrome by group II mGluR antagonist or lithium treatment. Brain Res. 2011;1380:106–119. doi: 10.1016/j.brainres.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dölen G, Osterweil E, Rao BSS, Smith GB, Auerbach BD, et al. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major fragile X syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Pan L, Woodruff E, Liang P, Broadie K. Mechanistic relationships between Drosophila fragile X mental retardation protein and metabotropic glutamate receptor A signaling. Mol Cell Neurosci. 2008;37:747–760. doi: 10.1016/j.mcn.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Repicky S, Broadie K. Metabotropic glutamate receptor-mediated use-dependent down-regulation of synaptic excitability involves the fragile X mental retardation protein. J Neurophysiol. 2008;101:672–687. doi: 10.1152/jn.90953.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McBride SM, Choi CH, Wang Y, Liebelt D, Braunstein E, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron. 2005;45:753–764. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 33.Sharma A, Hoeffer CA, Takayasu Y, Miyawaki T, McBride SM, et al. Dysregulation of mTOR signaling in fragile X syndrome. J Neurosci. 2010;30:694–702. doi: 10.1523/JNEUROSCI.3696-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cook D, Cameron SA, Jones EV. Fragile X mental retardation protein: regulator of specific mRNAs or master regulator of global translation? J Neurosci. 2010;30:7121–7123. doi: 10.1523/JNEUROSCI.1298-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall JC. The mating of a fly. Science. 1994;264:1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Wu L-J, Kim SS, Lee FJS, Gong B, et al. FMRP acts as a key messenger for dopamine modulation in the forebrain. Neuron. 2008;59:634–647. doi: 10.1016/j.neuron.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 37.Zhang YQ, Friedman DB, Wang Z, Woodruff E, Pan L, et al. Protein expression profiling of the drosophila fragile X mutant brain reveals up-regulation of monoamine synthesis. Mol Cell Proteomics. 2005;4:278–290. doi: 10.1074/mcp.M400174-MCP200. [DOI] [PubMed] [Google Scholar]
- 38.Yellman C, Tao H, He B, Hirsh J. Conserved and sexually dimorphic behavioral responses to biogenic amines in decapitated Drosophila. Proc Natl Acad Sci U S A. 1997;94:4131–4136. doi: 10.1073/pnas.94.8.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang HY, Grygoruk A, Brooks ES, Ackerson LC, Maidment NT, et al. Overexpression of the Drosophila vesicular monoamine transporter increases motor activity and courtship but decreases the behavioral response to cocaine. Mol Psychiatry. 2006;11:99–113. doi: 10.1038/sj.mp.4001742. [DOI] [PubMed] [Google Scholar]
- 40.Brown V, Jin P, Ceman S, Darnell JC, O'Donnell WT, et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- 41.Miyashiro KY, Beckel-Mitchener A, Purk TP, Becker KG, Barret T, et al. RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron. 2003;37:417–431. doi: 10.1016/s0896-6273(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 42.Zhong N, Ju W, Nelson D, Dobkin C, Brown WT. Reduced mRNA for G3BP in fragile X cells: evidence of FMR1 gene regulation. Am J Med Genet. 1999;84:268–271. doi: 10.1002/(sici)1096-8628(19990528)84:3<268::aid-ajmg20>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 43.Hong A, Zhang A, Ke Y, El Idrissi A, Shen C-H. Downregulation of GABA(A) β subunits is transcriptionally controlled by Fmr1p. J Mol Neurosci. 2011. [DOI] [PubMed]
- 44.Hall SS, Lightbody AA, Reiss AL. Compulsive, self-injurious, and autistic behavior in children and adolescents with fragile X syndrome. Am J Ment Retard. 2008;113:44–53. doi: 10.1352/0895-8017(2008)113[44:CSAABI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 45.Chang S, Bray SM, Li Z, Zarnescu DC, He C, et al. Identification of small molecules rescuing fragile X syndrome phenotypes in Drosophila. Nature Chemical Biology. 2008;4:256–263. doi: 10.1038/nchembio.78. [DOI] [PubMed] [Google Scholar]
- 46.Cordeiro L, Ballinger E, Hagerman R, Hessl D. Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: prevalence and characterization. J Neurodev Disord. 2011;3:57–67. doi: 10.1007/s11689-010-9067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bassell G, Warren S. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wright-Talamante C, Cheema A, Riddle JE, Luckey DW, Taylor AK, et al. A controlled study of longitudinal IQ changes in females and males with fragile X syndrome. Am J Med Genet. 1996;64:350–355. doi: 10.1002/(SICI)1096-8628(19960809)64:2<350::AID-AJMG23>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 49.Hagerman RJ, Schreiner RA, Kemper MB, Wittenberger MD, Zahn B, et al. Longitudinal IQ changes in fragile X males. Am J Med Genet. 1989;33:513–518. doi: 10.1002/ajmg.1320330422. [DOI] [PubMed] [Google Scholar]
- 50.Choi CH, Mcbride SMJ, Schoenfeld BP, Liebelt DA, Ferreiro D, et al. Age-dependent cognitive impairment in a Drosophila fragile X model and its pharmacological rescue. Biogerontology. 2010;11:347–362. doi: 10.1007/s10522-009-9259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galvez R, Greenough WT. Sequence of abnormal dendritic spine development in primary somatosensory cortex of a mouse model of the fragile X mental retardation syndrome. Am J Med Genet A. 2005;135A:155–160. doi: 10.1002/ajmg.a.30709. [DOI] [PubMed] [Google Scholar]
- 52.Gatto C, Broadie K. Temporal requirements of the fragile X mental retardation protein in the regulation of synaptic structure. Development. 2008;135:2637. doi: 10.1242/dev.022244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larson J, Jessen RE, Kim D, Fine A-KS, du Hoffmann J. Age-dependent and selective impairment of long-term potentiation in the anterior piriform cortex of mice lacking the fragile X mental retardation protein. J Neurosci. 2005;25:9460–9469. doi: 10.1523/JNEUROSCI.2638-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tessier CR, Broadie K. Drosophila fragile X mental retardation protein developmentally regulates activity-dependent axon pruning. Development. 2008;135:1547–1557. doi: 10.1242/dev.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hatton DD, Sideris J, Skinner M, Mankowski J, Bailey DB, et al. Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. Am J Med Genet A. 2006;140A:1804–1813. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- 56.Gabis LV, Kesner Baruch Y, Jokel A, Raz R. Psychiatric and autistic comorbidity in fragile X syndrome across ages. J Child Neurol. 2011. [DOI] [PubMed]
- 57.Siegel RW, Hall JC. Conditioned responses in courtship behavior of normal and mutant Drosophila. Proc Natl Acad Sci U S A. 1979;76:3430–3434. doi: 10.1073/pnas.76.7.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 59.Greer CL, Grygoruk A, Patton DE, Ley B, Romero-Calderon R, et al. A splice variant of the Drosophila vesicular monoamine transporter contains a conserved trafficking domain and functions in the storage of dopamine, serotonin, and octopamine. J Neurobiol. 2005;64:239–258. doi: 10.1002/neu.20146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional measurements of climbing behavior in dfmr1 mutant flies. (A). Time for 50% of a population to climb 17.5 cm. Control flies contain a wild-type dfmr1 transgene under endogenous regulation in the dfmr1 mutant background. dfmr1 and FS (dfmr1 mutants that contain a wild-type dfmr1 transgene that has a frameshift mutation in the dfmr1 open reading frame) do not express functional dFMRP. By 35 days all dfmr1 and FS populations failed to have 50% reach 17.5 cm within 3 min. Data presented are the average of Mean +/- SEM (8 trials, total flies n = 80 for each genotype tested at each time point). (B). Percentage of failed attempts for populations to have a first fly reaching the 17.5 cm line. (C). Percentage of failed attempts for populations to have at least 50% of flies climb 17.5 cm. For all data, *p<0.05, **p<0.01, and ***p<0.001.
(TIF)
Sample video of control flies climbing. This video illustrates how climbing experiments were conducted. Ten control flies at 15 day-old were gently knocked to the bottom of a graduated cylinder and then observed to climb to the top.
(MOV)
Sample video of the dfmr1 mutant flies climbing. Ten dfmr1 mutant flies at 15 day-old were gently knocked to the bottom of a graduated cylinder. The flies then begin climbing, but some stop after a short period of time. Later analysis showed that these flies stopped to groom themselves.
(MOV)
Sample video of dfmr1 mutant grooming activity. A 15 day-old dfmr1 mutant fly initially explores the environment for 10 s, but then begins grooming excessively.
(MOV)
Sample video of grooming activity in a control fly. A 15 day-old control fly explores the environment, stopping only once to groom for 3 s.
(MOV)