Abstract
Alzheimer’s disease (AD) is the most common cause of progressive cognitive decline and dementia in adults. While the amyloid cascade hypothesis of AD posits an initiating role for the β-amyloid (Aβ) protein, there is limited understanding of why Aβ is deposited. A growing body of evidence based on in vitro, animal studies and human imaging work suggests that synaptic activity increases Aβ, which is deposited preferentially in multimodal brain regions that show continuous levels of heightened activation and plasticity across the lifespan. Imaging studies of people with genetic predispositions to AD are consistent with these findings, suggesting a mechanism whereby neural efficiency or cognitive reserve may diminish Aβ deposition. The aggregated findings unify observations from cellular and molecular studies with human cognitive neuroscience to reveal potential mechanisms of AD development.
What causes amyloid deposition?
Although controversial, the amyloid cascade hypothesis (see Glossary) has been the dominant etiologic theory of Alzheimer’s disease (AD) for two decades (Box 1). The hypothesis holds that deposition of the β-amyloid (Aβ) protein is the initiating event in a pathological process, eventually leading to alterations in neural function and cognitive decline. In this article, we do not seek to challenge the amyloid hypothesis but rather to address a key feature of the model: why does Aβ deposition occur? Although the rare forms of autosomal dominant AD are associated with biochemical lesions that affect Aβ processing, the reasons why some individuals and not others develop Aβ deposits is unclear. Here we propose a model that integrates recent cellular and molecular data with findings from the cognitive and imaging literature that suggest that life-long patterns of neural activity lead to Aβ deposition. Although AD is a complex disorder that likely has multiple etiologies and pathways to decline, the observations presented in this article are integrated to outline potential mechanisms that mediate the pathogenesis of the disease.
Box 1. The amyloid cascade hypothesis.
The amyloid cascade hypothesis holds that aberrant processing of the β-amyloid (Aβ) protein initiates a series of downstream events that include synaptic dysfunction and damage, leading to cognitive decline and dementia [48]. After cleavage from the transmembrane amyloid precursor protein, resulting Aβ peptides oligomerize to form different species that are collectively referred to as soluble Aβ, likely the most toxic Aβ species. Soluble Aβ oligomers form fibrils, which are the main component of Aβ plaques. Given the multiple forms of Aβ aggregation, it is important to keep in mind that amyloid-imaging agents specifically bind to fibrillar Aβ plaques and do not directly measure the damaging oligomeric form of Aβ, although oligomers are likely in equilibrium with plaques.
Both the basic tenets of this model and many of the steps in the cascade leading to dementia are contentious [49]. As one example, the amyloid hypothesis fails to adequately account for the other major pathological aspect of AD, neurofibrillary tangles (NFTS), which are composed of a different protein, tau. Indeed, theories of AD causation based on the failure of neuroplasticity may better explain simultaneous abnormalities in tau and Aβ [50]. Nevertheless, a wealth of data stemming from human genetics, transgenic animal models, and clinical studies continues to support a crucial role of Aβ in causing AD. The evidence has been reviewed in many recent papers [51,52] and includes data showing that the overproduction of the 42 amino acid form of the protein (Aβ42) is sufficient to cause AD in human autosomal dominant disease and that soluble forms of the protein produce detrimental synaptic alterations that are closely related to cognitive decline [53]. One major challenge to the amyloid hypothesis is the lack of a relationship between Aβ and cognitive function (plaques, for example, are seen in cognitively normal individuals) but these findings can be reconciled through the observation that Aβ initiates downstream functional and structural changes that are more closely related to cognition [54] and which may ultimately become decoupled from the initiating Aβ [55]. Another major challenge involves the medial temporal lobe (MTL), which shows early dysfunction but no Aβ plaques until late stages [56]. Explanations posit that the MTL may become disconnected from Aβ-harboring regions, which leads to degeneration; that the MTL is exposed to only oligomeric forms of Aβ; or that MTL damage is non-amyloid in nature. Finally, the amyloid cascade hypothesis does not address the origin or selective localization of aberrant accumulation of Aβ, which is the focus of this article.
Neural activity and Aβ secretion
A major finding in the past decade is that neural activity regulates the production and secretion of Aβ, such that greater activity is associated with more Aβ release. Initial research found that neural activity modulates the release of cleavage products of the amyloid precursor protein [1], and that Aβ secretion could be affected by manipulation of neural activity [2] through a process that involves synaptic exocytosis (the process whereby cells release substances through the cell membrane into the synapse) [3]. Activity-dependent Aβ release has been further explored in transgenic mice, in which microdialysis-measured interstitial fluid Aβ levels have been related to regional neural activity and to later fibrillar amyloid plaques [4]. Interstitial release of Aβ could be increased through vibrissal stimulation or reduced via denervation effects on barrel cortex. In other studies, greater Aβ release is seen during wakefulness than sleep in mice, and has similar diurnal fluctuation in humans [5]. These results parallel known fluctuations in synaptic strength during sleep/wake cycles that may reflect synaptic activity [6]. In comatose humans, interstitial Aβ is associated with the level of neuronal function, such that higher levels of consciousness are associated with more Aβ secretion, further consistent with its regulation by neural activity [7]. Taken together, these data provide strong molecular evidence linking neural activity and Aβ.
In vivo studies of Aβ deposition in humans
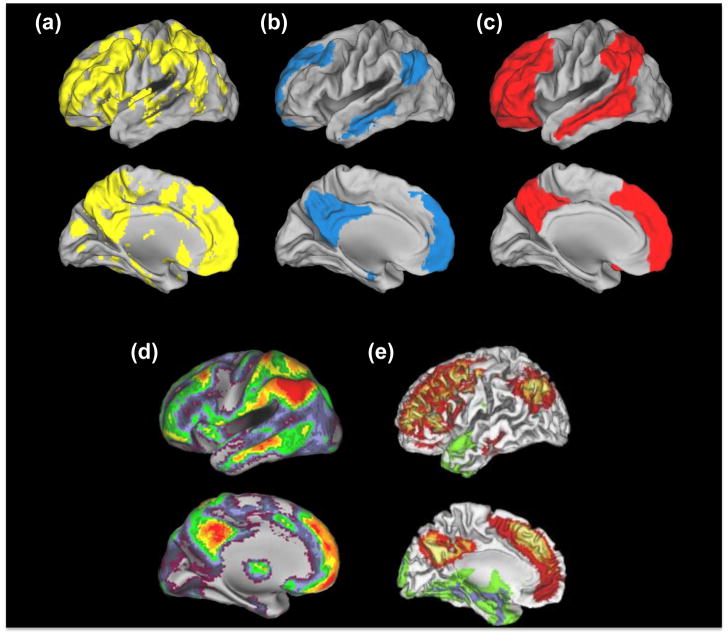
The recent advent of positron emission tomography (PET) methods for imaging Aβ has provided a noninvasive way of visualizing the distribution of the protein in the human brain in vivo. The most widely used PET amyloid imaging tracer is [11C]PIB (Pittsburgh compound B), a thioflavin derivative whose distribution reflects fibrillar, aggregated plaques [8,9]. PIB-PET studies have enabled the investigation of relationships between neural activity, Aβ, and cognition. These relationships are linked to the concept of large-scale networks, including the ‘default mode network’ (DMN), a group of regions highly metabolically active in a baseline resting state and deactivated during externally-driven cognition [10]. This network has received considerable attention using both resting state functional magnetic resonance imaging (fMRI) techniques and studies of deactivation during task-related fMRI, since it has become clear that both patients with AD and cognitively normal older individuals with Aβ deposition show reductions in the strength of connectivity and deactivation in this network [11–14]. Although PIB-PET studies demonstrate that the pattern of Aβ deposition overlaps with the major nodes of the network [15], in actuality, the topography of amyloid deposition reveals a pattern far more promiscuous than just the DMN (compare Figure 1a with 1b).
Figure 1. Spatial convergence between Aβ, DMN, heteromodal cortex and cortical hubs.
Examination across these panels reveals a high degree of convergence between amyloid distribution, multimodal cortex, cortical hubs and regions utilizing aerobic glycolysis, which is more consistent than the overlap between PIB uptake and the DMN. (a) PIB. Patterns of amyloid deposition in normal elderly controls with slightly elevated PIB values (N=15) are contrasted to low PIB elderly control subjects (N=49; thresholded using clusters at z>1.64 with a cluster significance threshold of p=0.05, corrected; statistical maps were binarized and displayed in Caret v5.6). These slightly elevated subjects have PIB levels below AD but higher than presumably Aβ-free young subjects (N=11; age 20–30), and likely represent the earliest signs of Aβ accumulation (see [44]). (b) DMN. 1 sample t-test of functional connectivity maps of the DMN (using a seed in the posterior cingulate at MNI coordinates 0, −54, 26; N=51; thresholded using clusters at z>3.09 with a cluster significance threshold of p=0.05, corrected; statistical maps were binarized and displayed in Caret v5.6). (c) Heteromodal Cortex. Cortical labeling of heteromodal cortex, adapted from [73]. (d) Hubs. Distribution of cortical hubs. Warmer colors reflect a greater degree of connectivity with other voxels (assessed by counting the number of voxels with a correlation coefficient greater than 0.25 with that specific voxel). Reproduced, with permission, from[16]. (e) Aerobic Glycolysis. Distribution of aerobic glycolysis. Red/yellow colors reflect greater aerobic glycolysis whereas green/blue colors reflect less aerobic glycolysis. Reproduced, with permission, from [18].
The DMN is only one of several strongly connected networks that integrates multimodal information and, therefore, has a continuous demand for high neuronal activation and plasticity. Brain regions that interact with multiple networks can be considered ‘cortical hubs’ or nodes that are highly connected to other brain regions and are involved in linking segregated processing streams and networks. These hubs are highly congruent with regions denoted as ‘multimodal cortex’ (compare Figure 1c and 1d). Furthermore, resting state functional connectivity analyses have confirmed the existence of hubs outside the DMN (compare Figure 1b with 1d). The distribution of PIB uptake, in fact, recapitulates the spatial pattern of hubs and multimodal cortex with more topographic fidelity than the DMN itself [16]. This is particularly obvious in lateral prefrontal and superior parietal cortices, which have hub properties and Aβ deposition but do not participate in the DMN; rather, these regions are consistently associated with networks distinct from the DMN in resting state fMRI studies [17].
Interestingly, these highly connected multimodal regions have recently been noted by Vaishnavi et al. [18] to share common metabolic properties in relying upon aerobic glycolysis for energy generation, a process that refers to glucose metabolism via glycolytic (and not oxidative) pathways, despite oxygen availability (Figure 1e). These same regions, as noted by Vlassenko et al. [19], are subject to Aβ deposition. The link between aerobic glycolysis and Aβ deposition is unclear but well discussed in these papers that note that aerobic glycolysis rapidly produces adenosine-5′-triphosphate (ATP), the molecule which is the major source of energy in all cells. These multimodal regions play a central role in cognitive processes, which could result in both high levels of synaptic activity over the lifespan and the need for rapid energy generation. In addition, aerobic glycolysis can support alternate biosynthetic pathways involved in receptor turnover and other aspects of neural plasticity, which are likely vital processes in multimodal cortex.
In a nutshell, and as demonstrated in Figure 1, it appears that regions vulnerable to Aβ deposition do not simply involve the DMN, but rather comprise multimodal brain regions that are highly interconnected, plastic, and capable of rapid ATP generation, suggesting that high levels of neural activity may predispose these brain regions to amyloid deposition. While relationships between neural activity and Aβ secretion are difficult to test in humans, studies of Aβ deposition with PIB and brain activation using fMRI in people with genetic risks for sporadic AD and in older individuals provide supportive evidence that lifelong structural and functional brain characteristics lead to increased neural activity that we hypothesize may deposit Aβ; these data are discussed in the following sections. These relationships, however, are complex because Aβ itself has effects on neural function, necessitating the separation of relationships between Aβ and brain activity into ‘pre-amyloid’ and ‘post-amyloid’ plaque stages (Box 2).
Box 2. Phase-dependent relationships with brain activation.
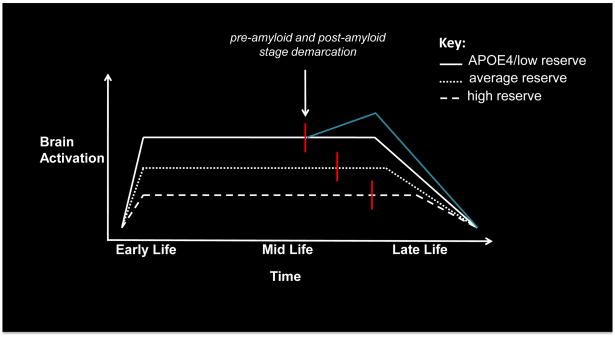
By the time individuals demonstrate cognitive impairment it is likely that the process of depositing Aβ has been active for 10 or more years [57] and ‘downstream’ processes that entail alterations to synaptic structure and function [53] are well underway. Thus, the relationship between neural activity and Aβ is complex and must be evaluated in terms of a pre-amyloid plaque stage, when synaptic activity is independent of Aβ deposition and a post-amyloid plaque stage, when deposition of Aβ has begun to affect neural function (Figure 2).
Distinction between these amyloid stages is relevant when examining cross-sectional imaging data of subjects in the post-amyloid phase (e.g., mild cognitive impairment (MCI) and AD), as well as analyses that directly contrast (presumably) pre- and post-amyloid plaque stage individuals. For instance, studies of MCI have revealed heightened activation during episodic memory (EM) processes that is associated with subsequent cognitive decline [58], whereas AD subjects typically show reduced activation [59]. This pattern suggests that heightened activation may exist during the initial portion of the post-amyloid stage, which may reflect a secondary compensatory response to amyloid, or continuation of heightened activation from the pre-amyloid plaque stage. Activation reduction in AD is confirmation that eventually these synapses become compromised and hypoactive late in the post-amyloid stage.
A handful of studies have provided evidence for an effect of stage on brain activation within the same experiment (see main article for a discussion of APOE effects). O’Brien and colleagues showed that heightened baseline activation predicted subsequent cognitive decline as well as lower activation during follow-up fMRI scanning [60]. Similar evidence has also been observed in the context of autosomal dominant familial AD (FAD): increased activation was seen during EM processes in a young carrier (~30 years before expected AD onset) and reduced activation was seen in an older carrier (within years of expected AD onset) [61]. Although amyloid was not measured in these studies, we can surmise that younger subjects (or ‘baseline’ subjects in O’Brien et al.[60]) are more likely within the pre-amyloid plaque phase, whereas older/follow-up subjects are more likely to be in the post-amyloid phase. Overall, this pattern supports the claim that initial activation increases precede activation decreases.
Cognitive reserve, ApoE genotype, brain efficiency and Aβ
Individual differences in brain activity during cognitive tasks are partly driven by factors, such as aging and genetics. One of the key constructs that may explain age-related response to pathology is cognitive reserve (CR). This construct invokes both static and dynamic responses to pathological change, and implies varying levels of cognitive efficiency in the performance of tasks (Box 3). The concept of CR may explain variability in the relationship between AD pathology and cognitive function, as individuals with high CR may tolerate substantial pathology before showing cognitive loss, whereas those with low CR may decline earlier. We postulate that one model for low CR is the presence of the Apolipoprotein E4 allele.
Box 3. Cognitive reserve.
The concept of cognitive reserve (CR) invokes both static and dynamic processes that permit some individuals to remain cognitively intact despite substantial pathology [62]. For example, individuals with larger temporal lobe volumes may better withstand the effects of Aβ deposition [63]. Reserve, however, is likely a complex construct: in epidemiological studies it is related to education, occupation, socioeconomic status, social networks and lifelong participation in cognitive and physical activity [64–66]. Such factors are difficult to disentangle and, taken together, are likely to have profound effects on the brain across the lifespan that affect cognition through multiple pathways.
We consider reserve to reflect lifelong patterns of behaviors, endogenous factors (including genetics) and exposure to environmental factors that have consequences for how the brain processes information. Furthermore, cognitive reserve may play different roles during pre- and post-amyloid plaque stages (Box 2). Once Aβ deposition begins, cognitive reserve may mitigate detrimental effects by allowing high CR individuals to cope with more downstream neuronal dysfunction and loss than individuals with low CR. However, in pre-amyloid plaque stages CR could act to diminish Aβ production through better ‘neural efficiency’, a concept that is widely applied in interpreting fMRI data. For example, a polymorphism in the catechol-O-methyltransferase (COMT) gene affects the rate of dopamine metabolism: individuals with the less active enzyme show greater neural efficiency, demonstrated as less brain activation required for executive tasks [67]. Similar mechanisms of neural efficiency have been invoked to explain age-related differences in behavioral performance and fMRI activation on cognitive tasks [40]. Even during development, low CR (in this case, linked to socioeconomic status) is associated with greater bilateral prefrontal recruitment during phonological processing [68].
Evidence linking increased neuronal efficiency to reduced Aβ comes from a recent study demonstrating that individuals who participated in more physical exercise (which has been independently associated with improvements in network efficiency [69]) showed less evidence of brain Aβ [70]. The Nun study revealed that adolescent women with more complex autobiographical essays (and thus presumably high CR) showed less cognitive decline and Aβ deposition in old age [71]. Animal data showing that transgenic mice reared in enriched environments deposit less Aβ [72] are also consistent with this idea. Thus cognitive reserve could act differently in pre- and post-amyloid plaque stages reducing Aβ deposition early and mitigating its effects later.
Whereas high cognitive reserve may result in life-long patterns of efficient neural activation that might mitigate Aβ deposition, genetic factors may underlie neural differences that promote Aβ deposition. The most important known genetic polymorphism in Alzheimer’s disease is the apolipoprotein E gene (APOE), which plays a role in the transport of cholesterol and other lipids, and has 3 polymorphisms (APOE 2/3/4). The APOE4 allele has a dose-dependent effect on risk for AD and decreases the age of AD onset by more than 10 years [20]. This polymorphism also has effects on neural activity that may ultimately lead to Aβ accumulation through mechanisms involving diminished neural efficiency and reserve.
Animal and human studies have converged to reveal lifelong neuronal deficiencies associated with APOE4. Specifically, mice expressing APOE4 show reduced synaptic plasticity and spine density [21,22] and older cognitively normal human carriers show reduced glucose metabolism [23] as well as impaired DMN deactivation during episodic memory (EM) encoding [24]. DMN dysfunction has also been shown amongst normal elderly APOE4 carriers confirmed to have low levels of amyloid, suggesting an effect of APOE4 that is independent of fibrillar Aβ [25].
Although there is evidence that suggests an impact of APOE4 on AD via Aβ and non-Aβ pathways [26], it is difficult to separate these effects in human studies when Aβ measurements are unavailable. However, elevated Aβ deposition is uncommon prior to age 50 (in 3 studies, only 6 out of 60 carriers younger than 50 had evidence for detectable quantities of elevated Aβ [27–29], while elevated Aβ amongst APOE4 carriers after age 50 is present across multiple studies [27,28,30]). Based on this pattern, a reasonable demarcation into pre-amyloid and post-amyloid plaque stages is possible around age 50 in carriers.
Examination of APOE4 carriers in the pre-amyloid plaque stage reveals evidence for substantial detrimental neuronal effects. For instance, carriers under age 40 have reduced glucose metabolism (measured with fluorodeoxyglucose [FDG] PET) in the multimodal brain regions characteristic of Aβ deposition discussed above [31] and also show impaired mitochondrial activity (measured in postmortem tissue) [29]. Furthermore, structural MRI studies have revealed reduced cortical thickness in child carriers [32] and smaller hippocampal volume in young adult carriers [33]. Although this primarily occurs in the medial temporal lobe, thinning is also seen in more widespread cortical areas [32]. These findings require more study as they have not been uniformly confirmed [34] and their predominant presence in the medial temporal lobe, an area with minimal Aβ, is unexplained. Overall, however, these studies provide human evidence for an early detrimental effect of APOE4 that is unrelated to and likely to precede abnormal Aβ accumulation, suggesting that carriers may demonstrate alterations in brain structure and function consistent with low reserve.
These neural deficits are accompanied by alterations in brain activation during EM processes in cognitively normal APOE4 carriers [35]. While these results fail to reveal a consistent pattern, a major confound is the difference in cohort age, which results in different proportions of individuals in pre- and post-amyloid plaque stages. Thus, there are little existing data on how brain activation differs between carriers and noncarriers over the lifespan.
In our proposed model, individuals in the pre-amyloid plaque stage would show increased brain activation, whereas individuals in the post-amyloid plaque phase may show additional increases (a secondary compensatory response) and ultimately decreased activation (Figure 2). To our knowledge, there are 4 fMRI studies of EM encoding in APOE4 carriers safely within the pre-amyloid plaque stage. Although these studies differ in task design, 3/4 studies revealed increased activation in APOE4 carriers [36–38] whereas 1 study did not (however, the design and analysis in this study was remarkably different from the 3 other studies, complicating direct comparisons [39]). In one study that examined APOE4 carriers at different ages, there was an interaction between age and genotype on activation. Specifically, activation during memory encoding across multiple brain regions was increased in young APOE4 carriers (20’s) whereas activation in these same regions was decreased in elderly carriers (70’s) [37]. This pattern is consistent with the trajectory presented in Figure 2, suggesting that older APOE4 carriers have lost the ability to maintain high levels of activation and consequently show reduced brain activation. Together with studies using transgenic models, FDG-PET, and structural MRI, these results suggest that synaptic and structural alterations underlie the greater neural responses in APOE4 carriers during the pre-amyloid phase that, we hypothesize, in turn, precipitate Aβ deposition.
Figure 2. Amyloid and brain activity across the lifespan.
Hypothetical trajectories of brain activation across the life span as a function of cognitive reserve (CR) and APOE4. In this model, reserve is associated with increased neuronal efficiency (i.e., lower activation). Consequently, variability in lifelong patterns of brain activation results in different starting points for amyloid accumulation (red line). The blue line represents a ‘secondary’ compensatory response that may be induced by the additional burden imposed by amyloid accumulation, which eventually also fails during a period of decline. This alternative end is possible in all 3 scenarios, but for simplicity is shown only for the low reserve trajectory. These trajectories also describe proposed patterns across different brain regions that vary in their propensity for Aβ deposition. For instance, the high CR trajectory, which displays low levels of activation across the lifespan, is indicative of unimodal regions, whereas the low CR trajectory, which displays high levels of activation across the lifespan, is indicative of multimodal regions.
Aging may also be associated with heightened activation
Similar to the activation pattern revealed by APOE4 carriers, normal elderly adults often show higher activation compared to young adults, as well as recruitment of non-typical regions across a variety of cognitive tasks [40], suggesting a compensatory response to underlying age-related brain changes [40,41]. Longitudinal data have suggested that, over time, aging is associated with decreased activation [42], consistent with our model, whereby neural failure ultimately supervenes (see Figure 2). Although a substantial percentage of normal individuals show evidence for Aβ accumulation [30, 43, 44], age-related, non-Aβ dependent synapse reduction also occurs [45]. Thus, it is possible that increased activation that serves as compensation for synaptic loss in aging has a causal role in aberrant amyloid accumulation. Further investigation of this phenomenon, however, will require studies pairing amyloid imaging with fMRI in the same individuals.
Initial studies measuring both amyloid and brain activation have revealed heightened activation during EM processes amongst high Aβ normal individuals [13,46,47]. However, cross-sectional designs do not allow the direction of causality to be inferred, so it is possible that the greater levels of activation in fact cause the Aβ deposition rather than the reverse.
Concluding remarks
Amyloid imaging is transforming research in the field of cognitive aging. The ability to differentiate normal individuals into those with evidence of brain β-amyloid deposition from those without permits the investigation of stage-dependent relationships between neural activity and Aβ. While there is likely more than one mechanism that leads to Aβ accumulation, we hypothesize that lifetime brain activation is one possible pathway that may increase Aβ. This model leads to a number of interesting and testable hypotheses (Box 4). Within this framework, different stages of Aβ deposition will be characterized by different relationships between Aβ and activity. Early on, those at risk for AD will show heightened activity, while later there will be evidence of further compensatory increases followed by declines. Investigations of these relationships will necessarily involve complex multimodal longitudinal studies, but may provide insights into cognitive mechanisms associated with AD. In this context, AD is not only a disease of aging, but a disease that develops throughout the lifetime.
Box 4. Predictions and questions for future research.
Longitudinal studies of brain activity and Aβ deposition will be crucial for testing many of the hypotheses outlined in this article. For example, how is brain activation related to subsequent deposition of Aβ? Are rates of Aβ deposition different in people who show more or less brain activation? Do individuals with greater cognitive reserve or enhanced neural efficiency show less Aβ deposition than individuals without these beneficial factors?
How are individual differences in brain activation related to neural efficiency and reserve?
What are the effects of ApoE on brain function in pre-amyloid stages and how does this change over time?
Does Aβ deposition begin and progress most rapidly in multimodal cortex?
What is the relationship between Aβ pathology in neocortex and neurofibrillary pathology in the medial temporal lobe/hippocampus?
Why do regions of the brain with similar amounts of Aβ deposition (such as posteromedial parietal cortex and prefrontal cortex) differ so greatly in the amount of neurodegeneration seen?
Do people with sleep disorders show earlier and more severe Aβ deposition than control subjects?
Are epileptic brain foci particularly susceptible to deposition of Aβ?
If Aβ reduces synaptic activity in late disease stages, will the rate of Aβ deposition slow in later stages of AD?
Acknowledgments
The authors would like to thank Michael Greicius, Susan Landau and Gil Rabinovici for helpful discussion and feedback during the drafting of this opinion. This work was supported by NIH grants AG034570 and AG032814 and the Alzheimer’s Association ZEN-08-87090.
Glossary
- Adenosine-5′-triphosphate (ATP)
the molecule that provides the basic source of energy in all living cells
- Aerobic glycolysis
in the presence of oxygen, cells metabolize glucose to produce adenosine-5′-tri-phospate through oxidative phosporylation. Glycolysis is a less efficient metabolic pathway that can metabolize glucose in the absence of oxygen. Aerobic glycolysis occurs when cells utilize glycolysis despite the presence of ample oxygen
- Alzheimer’s disease (AD)
the most common cause of progressive cognitive loss and dementia in adults. AD occurs in familial forms (which can also be autosomal dominant) and sporadic forms
- β-Amyloid (Aβ)
a 40 or 42-amino acid protein that is deposited in AD. β-amyloid aggregates to form soluble forms and fibrils. Soluble Aβ occurs as small aggregates (oligomers) and are likely the most neurotoxic forms. Larger aggregates are fibrils, deposited in the amyloid plaques (also known as senile plaques or neuritic plaques) that constitute a pathological hallmark of AD
- Apolipoprotein E
a gene involved in lipid transport and processing that is also a major risk factor for AD. The gene occurs in 3 forms (or polymorphic alleles); 2, 3, and 4. The APOE4 allele confers an increased risk of developing AD
- Cognitive reserve
a characteristic of individuals typically associated with a person’s ability to withstand the effects of brain pathology. An individual with greater cognitive reserve might have either pre-existing neural resources or the dynamic ability to utilize new resources online, which may preserve cognition in the face of AD pathology
- Default mode network
a group of brain regions that are more active in the resting state than during externally-driven cognitive activity. This network, therefore, shows deactivation during most cognitive tasks. It can also be seen with functional MRI during a resting state in the absence of an externally driven cognitive task
- Dementia
progressive and eventually global loss of cognitive abilities, leading to loss of daily function
- Mild cognitive impairment (MCI)
a condition of mild cognitive loss, often marked by episodic memory failure. A substantial proportion of individuals with MCI represent early-stage AD
- Neurofibrillary tangles (NFTs)
the other major pathological hallmark of AD (besides Aβ), NFTs are composed of the protein tau, a component of the neuronal cytoskeleton. The relationship between Aβ and tau pathology is unclear
- [11C]-PIB (Pittsburgh compound B)
a radiopharmaceutical that, when injected into humans and mapped using positron emission tomography, detects the presence of fibrillar forms of the β-amyloid protein
- Positron emission tomography (PET)
a nuclear medicine technique that can be used to map the distribution of radiolabeled molecules. The most commonly used PET ligand is [18F]Fluorodeoxyglucose (FDG), which provides a measure of glucose metabolism
- Resting state functional connectivity
acquisition of functional MRI data in the absence of a cognitive task reveals patterns of correlated activity that can be grouped into networks. These networks are highly congruent with activation patterns observed during task-related fMRI
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nitsch RM, et al. Release of amyloid-beta protein precursor derivatives by electrical depolarization of rat hippocampal slices. Proc Natl Acad Sci U S A. 1993;90:5191–5193. doi: 10.1073/pnas.90.11.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamenetz F, et al. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 3.Cirrito JR, et al. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 4.Bero AW, et al. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci. 2011 doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang JE, et al. Amyloid-beta dynamics are regulated by orexin and the sleep–wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilestro GF, et al. Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science. 2009;324:109–112. doi: 10.1126/science.1166673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brody DL, et al. Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science. 2008;321:1221–1224. doi: 10.1126/science.1161591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikonomovic MD, et al. Post–mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131:1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klunk WE, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 10.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greicius MD, et al. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedden T, et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29:12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sperling RA, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mormino EC, et al. Relationships between beta-amyloid and functional connectivity in different components of the default mode network in aging. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buckner RL, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buckner RL, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith SM, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaishnavi SN, et al. Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci U S A. 2010;107:17757–17762. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vlassenko AG, et al. Spatial correlation between brain aerobic glycolysis and amyloid-beta (Abeta ) deposition. Proc Natl Acad Sci U S A. 2010;107:17763–17767. doi: 10.1073/pnas.1010461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corder EH, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 21.Ji Y, et al. Apolipoprotein E isoform-specific regulation of dendritic spine morphology in apolipoprotein E transgenic mice and Alzheimer’s disease patients. Neuroscience. 2003;122:305–315. doi: 10.1016/j.neuroscience.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Levi O, et al. ApoE4 impairs hippocampal plasticity isoform–specifically and blocks the environmental stimulation of synaptogenesis and memory. Neurobiol Dis. 2003;13:273–282. doi: 10.1016/s0969-9961(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 23.Reiman EM, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 24.Persson J, et al. Altered deactivation in individuals with genetic risk for Alzheimer’s disease. Neuropsychologia. 2008;46:1679–1687. doi: 10.1016/j.neuropsychologia.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 25.Sheline YI, et al. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Abeta42. J Neurosci. 2010;30:17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verghese PB, et al. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kok E, et al. Apolipoprotein E–dependent accumulation of Alzheimer disease-related lesions begins in middle age. Ann Neurol. 2009;65:650–657. doi: 10.1002/ana.21696. [DOI] [PubMed] [Google Scholar]
- 28.Morishima–Kawashima M, et al. Effect of apolipoprotein E allele epsilon4 on the initial phase of amyloid beta-protein accumulation in the human brain. Am J Pathol. 2000;157:2093–2099. doi: 10.1016/s0002-9440(10)64847-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valla J, et al. Reduced posterior cingulate mitochondrial activity in expired young adult carriers of the APOE epsilon4 allele, the major late-onset Alzheimer’s susceptibility gene. J Alzheimers Dis. 2010;22:307–313. doi: 10.3233/JAD-2010-100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris JC, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiman EM, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw P, et al. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurol. 2007;6:494–500. doi: 10.1016/S1474-4422(07)70106-0. [DOI] [PubMed] [Google Scholar]
- 33.Alexopoulos P, et al. Hippocampal volume differences between healthy young apolipoprotein E epsilon2 and epsilon4 carriers. J Alzheimers Dis. 2011 doi: 10.3233/JAD–2011–110356. [DOI] [PubMed] [Google Scholar]
- 34.Richter–Schmidinger T, et al. Influence of brain-derived neurotrophic-factor and apolipoprotein E genetic variants on hippocampal volume and memory performance in healthy young adults. J Neural Transm. 2010;118:249–257. doi: 10.1007/s00702-010-0539-8. [DOI] [PubMed] [Google Scholar]
- 35.Trachtenberg AJ, et al. The effects of APOE-epsilon4 on the BOLD response. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Dennis NA, et al. Temporal lobe functional activity and connectivity in young adult APOE varepsilon4 carriers. Alzheimers Dement. 2010;6:303–311. doi: 10.1016/j.jalz.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Filippini N, et al. Differential effects of the APOE genotype on brain function across the lifespan. Neuroimage. 2011;54:602–610. doi: 10.1016/j.neuroimage.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Filippini N, et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mondadori CR, et al. Better memory and neural efficiency in young apolipoprotein E epsilon4 carriers. Cereb Cortex. 2007;17:1934–1947. doi: 10.1093/cercor/bhl103. [DOI] [PubMed] [Google Scholar]
- 40.Park DC, Reuter–Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cabeza R, et al. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- 42.Nyberg L, et al. Longitudinal evidence for diminished frontal cortex function in aging. Proc Natl Acad Sci U S A. 2010;107:22682–22686. doi: 10.1073/pnas.1012651108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 44.Mormino EC, et al. Not quite PIB-positive, not quite PIB-negative: slight PIB elevations in elderly normal control subjects are biologically relevant. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masliah E, et al. Quantitative synaptic alterations in the human neocortex during normal aging. Neurology. 1993;43:192–197. doi: 10.1212/wnl.43.1_part_1.192. [DOI] [PubMed] [Google Scholar]
- 46.Vannini P, et al. Age and amyloid-related alterations in default network habituation to stimulus repetition. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mormino E, et al. Aβ deposition in aging is associated with increases in brain activation during successful memory encoding. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 49.Herrup K. Reimagining Alzheimer’s disease–an age-based hypothesis. J Neurosci. 2010;30:16755–16762. doi: 10.1523/JNEUROSCI.4521-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mesulam MM. Neuroplasticity failure in Alzheimer’s disease: bridging the gap between plaques and tangles. Neuron. 1999;24:521–529. doi: 10.1016/s0896-6273(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 51.Mucke L. Neuroscience: Alzheimer’s disease. Nature. 2009;461:895–897. doi: 10.1038/461895a. [DOI] [PubMed] [Google Scholar]
- 52.Golde TE, et al. Anti-abeta therapeutics in Alzheimer’s disease: the need for a paradigm shift. Neuron. 2011;69:203–213. doi: 10.1016/j.neuron.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lacor PN, et al. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jack CR, Jr, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hyman BT. Amyloid-dependent and amyloid-independent stages of Alzheimer disease. Arch Neurol. 2011 doi: 10.1001/archneurol.2011.70. [DOI] [PubMed] [Google Scholar]
- 56.Thal DR, et al. Phases of Aβ deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 57.Sperling RA, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimers Dement. 2011 doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dickerson BC, et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwindt GC, Black SE. Functional imaging studies of episodic memory in Alzheimer’s disease: a quantitative meta-analysis. Neuroimage. 2009;45:181–190. doi: 10.1016/j.neuroimage.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 60.O’Brien JL, et al. Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology. 2010;74:1969–1976. doi: 10.1212/WNL.0b013e3181e3966e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mondadori CR, et al. Enhanced brain activity may precede the diagnosis of Alzheimer’s disease by 30 years. Brain. 2006;129:2908–2922. doi: 10.1093/brain/awl266. [DOI] [PubMed] [Google Scholar]
- 62.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsych Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- 63.Chetelat G, et al. Larger temporal volume in elderly with high versus low beta–amyloid deposition. Brain. 2010;133:3349–3358. doi: 10.1093/brain/awq187. [DOI] [PubMed] [Google Scholar]
- 64.Wilson RS, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA -J Am Med Assoc. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 65.Fratiglioni L, et al. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 66.Middleton LE, Yaffe K. Targets for the prevention of dementia. J Alzheimers Dis. 2010;20:915–924. doi: 10.3233/JAD-2010-091657. [DOI] [PubMed] [Google Scholar]
- 67.Egan MF, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raizada RD, et al. Socioeconomic status predicts hemispheric specialisation of the left inferior frontal gyrus in young children. Neuroimage. 2008;40:1392–1401. doi: 10.1016/j.neuroimage.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Voss MW, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Ag Neurosci. 2010 doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liang KY, et al. Exercise and Alzheimer’s disease biomarkers in cognitively normal older adults. Ann Neurol. 2010;68:311–318. doi: 10.1002/ana.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Snowdon DA, et al. Linguistic ability in early life and cognitive function and Azheimer’s disease in late life: findings from the Nun study. JAMA -J Am Med Assoc. 1996;275:528–532. [PubMed] [Google Scholar]
- 72.Lazarov O, et al. Evironmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 73.Mesulam M. Principles of behavioral and cognitive neurology. Oxford University Press; 2000. [Google Scholar]