Abstract
Background
The natural history of Crohn’s disease follows a path of progression from an inflammatory to a fibrostenosing disease, with most patients requiring surgical resection of fibrotic strictures. Potent anti-inflammatory therapies reduce inflammation but do not appear to alter the natural history of intestinal fibrosis. The aim of this study was to determine the relationship between intestinal inflammation and fibrogenesis and the impact of a very early “top-down” interventional approach on fibrosis in vivo.
Methods
In this study, we removed the inflammatory stimulus from the Salmonella typhimurium mouse model of intestinal fibrosis by eradicating the S. typhimurium infection with levofloxacin at sequential time points during the infection. We evaluated the effect of this elimination of the inflammatory stimulus on the natural history of inflammation and fibrosis as determined by gross pathology, histopathology, mRNA expression, and protein expression.
Results
Fibrogenesis is preceded by inflammation. Delayed eradication of the inflammatory stimulus by antibiotic treatment represses inflammation without preventing fibrosis. Early intervention significantly ameliorates but does not completely prevent subsequent fibrosis.
Conclusions
This study demonstrates that intestinal fibrosis develops despite removal of an inflammatory stimulus and elimination of inflammation. Early intervention ameliorates but does not abolish subsequent fibrosis, suggesting that fibrosis, once initiated, is self-propagating, suggesting that a very early “top-down” interventional approach may have the most impact on fibrostenosing disease.
INTRODUCTION
Crohn’s disease (CD) is characterized by repeated cycles of intestinal inflammation (flares) and healing. Despite the increasing use of potent anti-inflammatory therapies which effectively treat inflammatory flares, a majority of CD patients ultimately develop fibrostenotic disease, necessitating surgical intervention at substantial personal and economic costs (1–4). Indeed, some anti-inflammatory therapies are associated with an increased risk of stenosis, stricture, or obstruction (5). The persistence of intestinal fibrosis, and the failure of effective control of inflammation to alter the natural history of CD, suggests that intestinal fibrosis can pass a “point of no return”, after which the fibrotic process becomes auto-propagative and fails to respond to anti-inflammatory interventions (6).
In this study, we used an experimental mouse model of fibrosing colitis to examine the impact of eliminating the inflammatory stimulus on the development of fibrosis. In the S. typhimurium model, inflammation is driven by persistent S. typhimurium colonization of the cecum. As described by Grassl, chronic Salmonella typhimurium infection, which can persist up to 70 days, induces colitis and severe tissue fibrosis mainly in the cecum, characterized by markedly reduced cecal size, transmural tissue fibrosis, and a Th1/Th17 cytokine profile which recapitulates human Crohn’s disease (7, 8). In addition, the S. typhimurium model reproduces the bacterial antigen stimulus of Crohn’s disease while readily allowing for the rapid removal of the inflammatory stimulus with antibiotic treatment. Thus, the model affords the opportunity to evaluate how removal of inflammation at different time points impacts the natural history of intestinal fibrosis.
We describe the first reported manipulation of the S. typhimurium colitis model by intervention with levofloxacin, a fluoroquinolone antibiotic with bactericidal activity against intracellular bacteria. Very early antibiotic intervention (a “top-down” approach) with levofloxacin in S. typhimurium-infected mice repressed S. typhimurium-induced inflammation and subsequent fibrosis. However late (day 8) eradication of S. typhimurium did not alter the development of intestinal fibrosis, suggesting that fibrosis, once initiated, is self-propagative.
MATERIALS AND METHODS
Reagents
Levofloxacin (levaquin in 5% dextrose, Hospira, Austin, TX) was obtained from the University of Michigan Hospital pharmacy.
Mice
Female 8–12 week old CBA/J mice (Jackson Laboratories, Bar Harbor, ME) received 20 mg streptomycin in 0.1 M HBSS to eradicate the commensal microbiota 24 hours prior to infection with 3×106 cfu S. typhimurium strain SL1344 in 100 λ 0.1M HEPES buffer (pH = 8.0) by oral gavage. Control mice received 100 λ 0.1M HEPES by oral gavage.
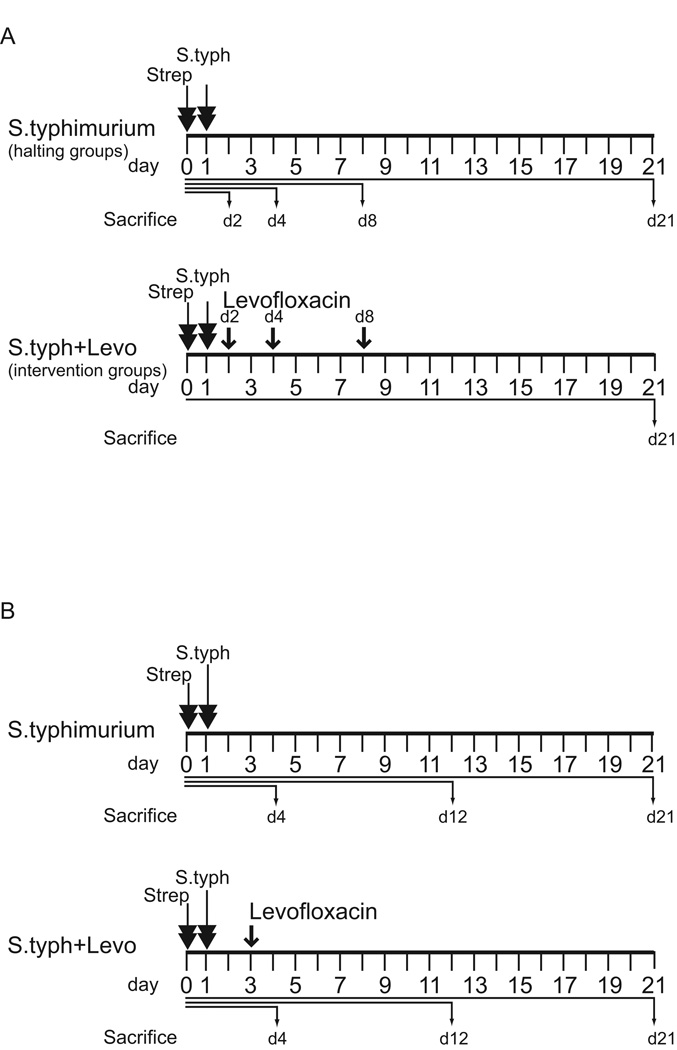
In the first endpoint analysis experiment, we examined the effect of eradication of S. typhimurium at different time points after the infection. (Figure 1A) Mice were infected with S. typhimurium as described above. To determine the natural progression of inflammation and fibrogenesis in this model, S. typhimurium-infected mice (halting groups) were euthanized at day 2, 4, 8, and 21 post-infection. Mice in the S. typhimurium-infected levofloxacin treatment groups (intervention groups) received 0.5 mg/ml levofloxacin ad libitum in drinking water starting at day 2, 4, or 8 post-infection and continuing until euthanasia at day 21 post-infection.
Figure 1. Treatment schematic of levofloxacin endpoint analysis and time course comparison experiments.
(A) Treatment schematic of the endpoint analysis experiment. Mice in the S. typhimurium infection halting groups (upper panel) were treated with streptomycin (strep, double arrows) 1 day prior to infection with S. typhimurium (S. typh, double arrows) and sacrificed at day 2, 4, 8 and 21 post-infection. In the levofloxacin intervention groups (lower panel), mice received streptomycin and S. typhimurium on the same schedule. Levofloxacin intervention (single arrows) began day 2, 4, or 8 post-S. typhimurium infection and continued until sacrifice at day 21 post-infection. (B) Treatment schematic of the time course comparison experiments. Mice in the S. typhimurium infection groups (upper panel) were treated with streptomycin (strep, double arrows) 1 day prior to infection with S. typhimurium (S.typh, double arrows) and sacrificed day 4, 12 and 21 post-infection. In the levofloxacin intervention time course (lower panel) mice received streptomycin and S. typhimurium on the same schedule. Levofloxacin intervention (arrow) began day 3 post-S. typhimurium infection and continued until sacrifice at day 4, 12, and 21 post-infection. In all experiments, 5 animals were used per experimental group.
In the second time course comparison experiment, which was designed to determine how early eradication of S. typhimurium impacted inflammation and fibrosis at early and late time points (Figure 1B); mice were infected with S. typhimurium as described above. Half the infected animals then received 0.5 mg/ml levofloxacin starting at day 3 post-infection through day 21. Animals in the S. typhimurium/levofloxacin group and matched S. typhimurium-infected animals were euthanized at day 4, 12, and 21 post-infection. Uninfected control animals were divided into two groups of which half received 0.5 mg/ml levofloxacin in drinking water through day 21. Uninfected control animals (treated with or without levofloxacin) were euthanized at day 21 of the study. All animal experiments were conducted with the approval and oversight of the University of Michigan UCUCA (University Committee on Use and Care of Animals).
Bacterial Strains
Salmonella typhimurium strain SL1344 (a gift from M. O’Riordan, University of Michigan, Ann Arbor, MI), which is naturally resistant to streptomycin, was grown in LB broth containing 100 µg/ml streptomycin at 37°C.
Gross Pathology and Tissue Collection
Cecum and distal colon were collected at sequential time points post S. typhimurium infection, photographed, measured, and weighed. Cecal area was determined from digital photographs using ImageJ ROI to delineate the cecum. To control for differences in focal distance, cecal area was normalized against a 1 cm marker in the photographic image.
Tissues were snap-frozen in liquid nitrogen and stored at −80°C prior to molecular analysis. Cecal contents were collected and serially diluted before plating onto LB-streptomycin plates to determine bacterial titers.
Stool Cultures
A fresh stool was collected, suspended in 500 ul sterile PBS, streaked onto LB plates containing 10 µg/ml streptomycin, and cultured overnight at 37°C.
Histology
Formalin-fixed and paraffin-embedded tissues were stained with Masson’s trichrome by the University of Michigan CCGC Research Histology and Immunoperoxidase Laboratory (Ann Arbor, MI). Digital photomicrographs of tissue sections were taken with an Olympus BX microscope (University of Michigan Microscopy and Image Analysis Laboratory). Tissue measurements were quantitated with ImageJ analysis software (NIH, Bethesda, MD).
Histological Scoring
Histological scoring was performed in a blinded manner by a pathologist (D.M.). Cecal inflammation was determined using a Wirtz Scale 0 to 4 point scoring system as previously described (9, 10). Cecal fibrosis was scored separately as follows: (0) no fibrosis; (1) mild fibrosis (focal mucosal/submucosal collagen deposition without architectural distortion); (2) moderate fibrosis (significant mucosal/submucosal collagen deposition with modest distortion of mucosal/submucosal architecture but without obscuring of the mucosal/submucosal border); and (3) severe fibrosis (extensive mucosal submucosal collagen deposition with marked architectural distortion obscuring the mucosal/submucosal border).
Immunoblotting
Immunoblotting was utilized for the detection of α-smooth muscle actin. Whole tissue was pulverized under liquid nitrogen and lysed in ice-cold RIPA buffer with a cocktail of proteinase inhibitors (Roche). Protein content was determined using a modified Bradford assay kit (BioRad, Hercules, CA). Total protein was separated by SDS polyacrylamide gel electrophoresis and transferred to PVDF membranes (Amersham Biosciences, Piscataway, NJ). Membranes were blocked in 5% milk solution for one hour at room temperature or overnight at 4°C. α-smooth muscle actin was detected by incubating the membrane overnight at 4°C with mouse anti-human monoclonal antibody (Sigma, St. Louis, MO) at 1: 5000 dilution in 5% milk/TBST. As a loading control, a mouse antibody for GAPDH (Chemicon, Temecula, CA) was used after a stripping procedure. Secondary antibody anti-mouse IgG+ HRP (Amersham, Piscataway, NJ) was incubated for one hour at room temperature and the signal was detected by the Pierce detection system (Pierce, Rockford, IL). Autoradiographs were scanned and quantitated using Image J analysis software (NIH, Bethesda, MD).
Real-time Quantitative PCR
RNA was extracted from the cecum using the RNeasy kit (Qiagen, Valencia, CA). Reverse transcription of 2 µg of total RNA was performed with the Superscript First Strand RT kit (Invitrogen). Quantitative real-time PCR (qRT-PCR) of IL-1β (Mm01336189_m1), TNFα (Mm00443258_m1), IL-17 (Mm00439619_m1), IL-6 (Mm99999064_m1), IGF-1 (Mm00439560_m1), TGFβ (Mm00441726_m1), IL-12p40 (Mm01288993_m1), CTGF (Mm01192933_g1) and GAPDH (4352932E) was performed with the TaqMan assays-on-demand primer/probe gene expression assays (ABI, Foster City, CA). qRT-PCR was performed using a Stratagene Mx3000P real-time PCR system (Stratagene, La Jolla, CA). Cycling conditions were 95°C 10 minutes, followed by 40 cycles of 95°C 15 seconds and 62°C 60 seconds. ΔΔCt values were calculated from 18s expression.
Statistical Analysis
Comparisons between several groups of mice and associated tissues or RNA expression were analyzed with ANOVA, while pairwise comparisons of two groups were performed with Student’s t test.
Results
Inflammation precedes fibrosis in the S. typhimurium mouse model of intestinal fibrosis
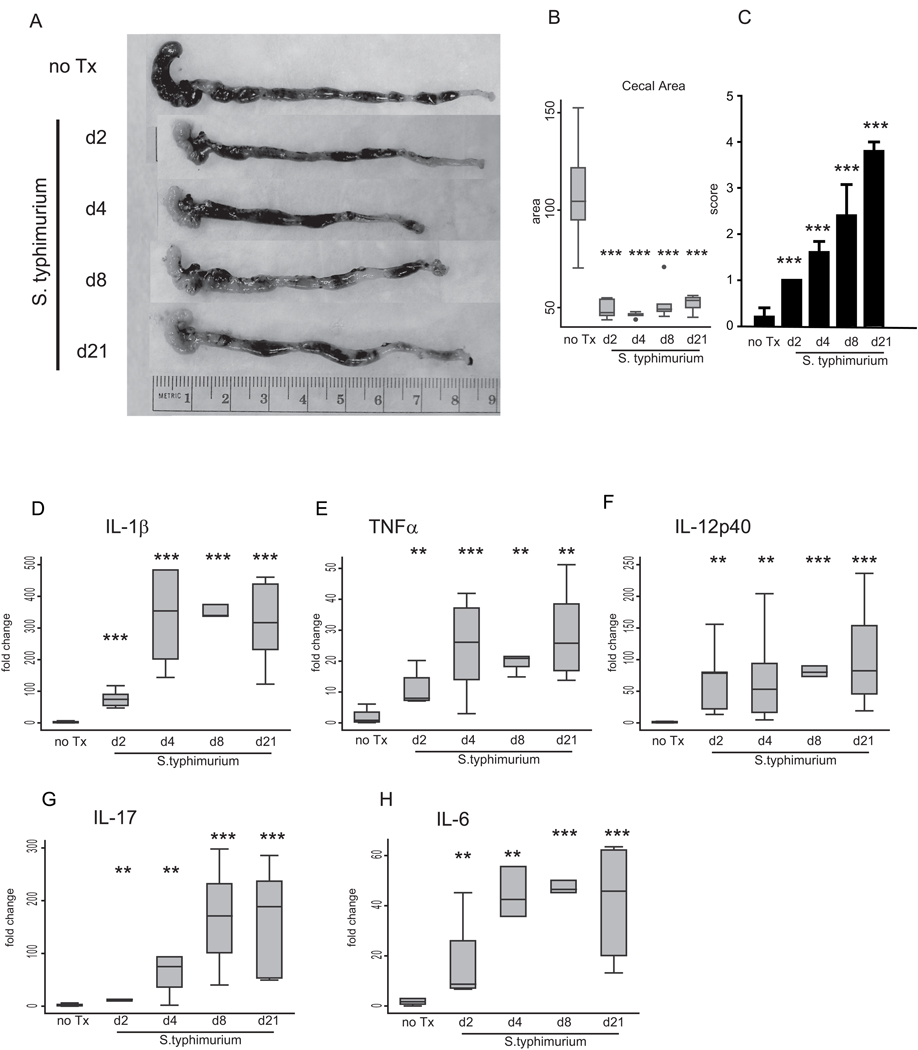
To determine the relationship between inflammation and fibrosis, mice were infected with S. typhimurium and sacrificed at days 2, 4, 8, and 21 post-infection (halting groups). Disease activity was assessed by gross pathology, histopathology, inflammatory and pro-fibrotic cytokine expression, and αSMA protein expression. S. typhimurium heavily colonized the mouse cecum at 1.7×107 cfu/ml by day 2 post-infection and persisted through day 21 (3.4×107 cfu/ml, data not shown). By day 2 post-infection, the gross cecal pathology demonstrated a striking reduction in cecal size that persisted and was virtually indistinguishable from the gross pathology following a chronic 21 day infection (Figure 2A). The gross pathology was consistent with quantification of cecal area, which showed a significant 2-fold reduction at day 2 which was indistinguishable from the chronic day 21 group (Figure 2B).
Figure 2. Inflammation precedes fibrosis in the S. typhimurium mouse model of intestinal fibrosis.
(A) Representative gross appearance of the cecum and distal colon of S. typhimurium-infected mice day 2 (d2), day 4 (d4), day 8 (d8) and day 21 (d21) post-infection compared to uninfected (no Tx) against a 1-cm reference ruler. (B) Cecal area calculated from uninfected mice (no Tx) compared to S. typhimurium-infected mice day 2, 4, 8, and 21 post-infection. (C) Blinded inflammatory scoring of cecal sections using the 0–4 point Wirtz scale. (D–H) qRT-PCR gene expression of Th1 and inflammatory cytokines in the cecum of uninfected (no Tx) compared to S. typhimurium-infected mice day 2, 4, 8, and 21 post-infection. Gene expression was normalized to GAPDH expression. Statistical comparisons between uninfected controls (no Tx) and the infected groups are denoted with asterisks. * p <0.05, ** p<0.01, *** p<0.001
To determine the onset of intestinal inflammation, cecal tissue sections were scored for inflammation on the Wirtz scale (0 to 4) (9, 10). Early inflammatory changes were seen at day 2 post-infection with a significant increase in histological inflammatory score compared to uninfected animals (1.0 vs. 0.0, p<0.001) (Figure 2 C). Inflammation progressed during the course of infection through day 21. The early gross phenotypic and inflammatory changes in the cecum were associated with a rapid and dramatic 12 to 120-fold induction of pro-inflammatory Th1 cytokines IL-1β and TNFα by day 2 post-infection which peaked by day 4 and persisted through day 21 (Figure 2 D–E). Similarly, IL-12p40, a cytokine involved in both the Th1 and Th17 responses in the pathogenesis of Crohn’s disease (11), was markedly induced by day 2 post-infection (Figure 2 F). S. typhimurium infection also elicited an early Th17 response, characterized by 17 to 19-fold induction of IL-17 and IL-6 cytokines by day 2 post-infection which increased over the time course of infection (Figure 2 G–H).
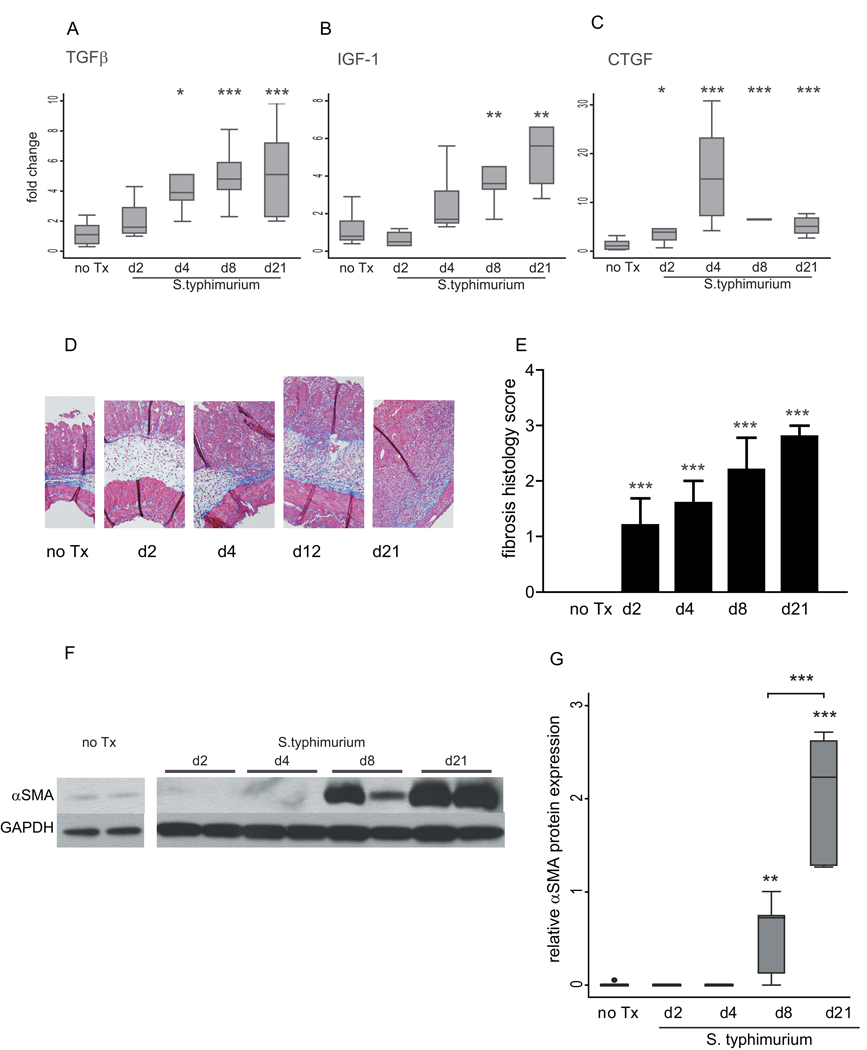
The induction of fibrosis followed the inflammatory phase, with significant increases in TGFβ, IGF-1, and CTGF expression observed at days 4–8 (Figure 3 A–C). In contrast to the inflammatory cytokines, which peaked at day 4, the fibrogenic markers showed a progressive increase through day 21 (Figure 3 A–C). Histopathological analysis of trichrome-stained cecal tissue demonstrated mild but significant fibrotic changes as early as day 2 post-infection (1.2 vs. 0, p=0.04), which progressed to extensive tissue fibrosis with architectural distortion by day 21. (Figure 3 D, E). An independent quantification of trichrome staining for collagen revealed a similar trend, with a modest but significant increase in collagen staining at day 2 post-infection with maximal collagen deposition by day 21 (data not shown). αSMA protein expression, a marker of myofibroblast activation, was not observed until day 8 and continued to increase with a significant 4-fold increase in protein expression between day 8 and day 21 (Figure 3 F–G).
Figure 3. Development of fibrosis in the S. typhimurium mouse model of fibrosis.
(A–C) qRT-PCR expression of fibrotic genes in cecum from uninfected mice (no Tx) compared to S. typhimurium-infected mice day 2, 4, 8, and 21 post-infection. Gene expression was normalized to GAPDH expression. (D) Representative trichrome histological sections (100× magnification). (E) Blinded fibrosis scoring of cecal sections was determined from trichrome stained cecal sections. (F) Representative Western blot of αSMA protein expression in S. typhimurium mice days 2, 4, 8, and 21 post-infection compared to uninfected controls. The samples shown were run on the same protein gel. The image has been cut to rearrange the loading order for clarity. GAPDH expression was used as a loading control. (G) Quantitation of αSMA protein expression in cecum from uninfected mice (no Tx) compared to S. typhimurium infected mice day 2, 4, 8, and 21 post-infection. Western blots were digitally scanned and quantitated using ImageJ. Relative expression was normalized to GAPDH protein expression. Statistical comparisons between uninfected controls (no Tx) and the infected groups are denoted with asterisks. Brackets represent statistical comparisons between S. typhimurium-infected groups. * p <0.05, ** p<0.01, *** p<0.001
Intervention reduces the S. typhimurium-induced inflammatory (Th1) response
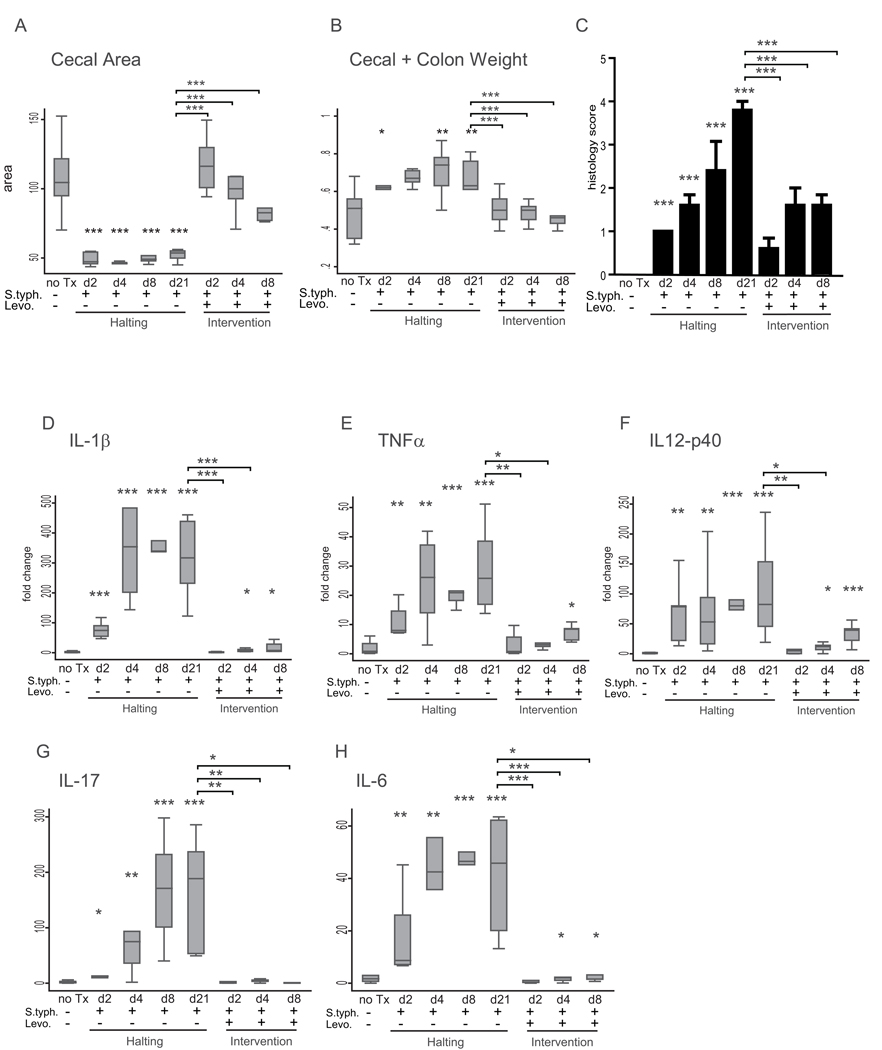
To determine whether the inflammatory response to S. typhimurium infection persists without continued inflammatory stimulus (bacterial colonization); an endpoint analysis experiment was performed. Mice were infected with S. typhimurium and treated with oral doses of levofloxacin beginning at day 2, 4, or 8 post-infection and sacrificed at day 21 (Figure 1A). Within 24 hours of levofloxacin administration, 100% of the animals were negative for S. typhimurium as determined by stool cultures (data not shown). Gross pathology, histopathology, cytokine expression and protein expression were compared between animals in the levofloxacin intervention groups, uninfected animals, and animals infected with S. typhimurium. Both early (day 2) and later (day 4, 8) intervention reduced cecal contraction and tissue weight at day 21 (Figure 4 A, B).
Figure 4. Intervention represses the inflammatory response.
(A) Cecal area from uninfected mice (no Tx) compared to S. typhimurium-infected mice day 2, 4, 8, and 21 post-infection, and S. typhimurium-infected mice treated with levofloxacin day 2, 4, and 8 post-infection and sacrificed day 21 post-infection. (B) Combined cecal + colon weight. (C) Blinded inflammatory scoring of cecal sections using the 0–4 point Wirtz scale. (D–H) qRT-PCR expression of IL-1β (D), TNFα (E), IL-12p40 (F), IL-17 (G), and IL-6 (H) in mice infected with S. typhimurium-infected mice day 2, 4, 8, and 21 post-infection, and S. typhimurium-infected mice treated with levofloxacin day 2, 4, and 8 post-infection and sacrificed day 21 post-infection compared to uninfected mice (no Tx). Statistical comparisons between the infection groups or intervention groups and the uninfected controls are denoted with asterisks. Statistically significant comparisons between the S. typhimurium infected and levofloxacin intervention groups at day 21 post-infection are denoted in brackets. * p <0.05, ** p<0.01, *** p<0.001
Histological analysis of inflammation in the cecal tissue sections determined that early intervention reduced tissue inflammation. Inflammation was significantly repressed in the day 2, 4, and 8 intervention groups at day 21 compared to the infected group (0.6, 1.6, 1.6 vs. 3.8, p <0.0001) (Figure 4 C). However, inflammation scores were still significantly higher at day 21 in the day 2, 4, and 8 levofloxacin intervention groups compared to uninfected animals (0.6, 1.6, 1.6 vs. 0.0, p=0.04, p=0.004, p<0.001).
Molecular analysis of the cecal tissue revealed a more profound anti-inflammatory effect of early intervention. Early (day 2) intervention repressed the induction of pro-inflammatory cytokines IL-1β, TNFα, and IL12p40 at day 21 to levels indistinguishable from uninfected controls (Figure 4 D–F). Although intervention at later time points failed to completely abolish the induction of IL-1β, TNFα, IL12p40, and IL-6, the expression of these cytokines was still markedly suppressed in comparison to the untreated/S. typhimurium-infected animals. In contrast, both early and late intervention repressed IL-17 (Figure 4 G).
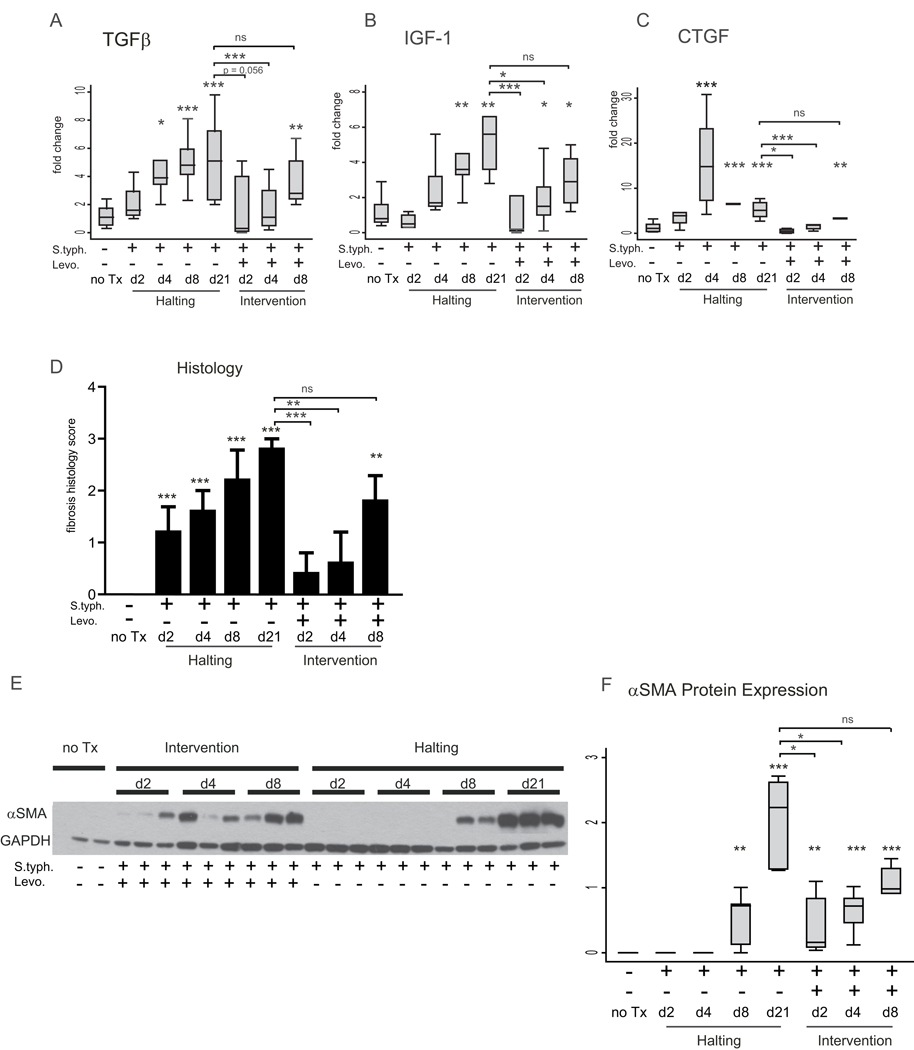
Early intervention ameliorates but does not completely prevent fibrosis
Given that fibrosis develops in response to inflammation, removal of the inflammatory stimulus would be expected to limit fibrosis. In mice treated with levofloxacin at day 2 or day 4 post-infection, expression of TGFβ and CTGF cytokines in the cecum at day 21 was indistinguishable from the expression in uninfected animals at day 21 post-infection. In contrast, in the day 4 levofloxacin group, IGF-1 was still significantly elevated, reflecting a heterogeneous fibrogenic response to day 4 intervention (Figure 5 A–C). However, day 8 levofloxacin intervention did not alter the expression of the fibrotic markers at day 21 post-infection. TGFβ, IGF-1, and CTGF expression were significantly induced compared to uninfected animals and did not differ from S. typhimurium infected animals at day 21 post-infection (Figure 5 A–C).
Figure 5. Early intervention ameliorates but does not completely prevent fibrosis.
(A–C) qRT-PCR expression of fibrotic markers TGFβ (A), IGF-1 (B), and CTGF (C) in mice infected with S. typhimurium day 2, 4, 8, and 21 post-infection, and S. typhimurium-infected mice treated with levofloxacin day 2, 4, and 8 post-infection and sacrificed day 21 post-infection compared to uninfected mice (no Tx). (D) Blinded fibrosis scoring of cecal sections was determined from trichrome stained cecal sections. (E) Representative Western blot illustrating αSMA protein expression in the cecum of uninfected mice (no Tx) compared to mice infected with S. typhimurium and treated with levofloxacin 2, 4, and 8 post-infection and sacrificed day 21 post-infection and mice infected with S. typhimurium-infected mice day 2, 4, 8, and 21 post-infection. GAPDH protein expression was used as a loading control. (F) Quantitation of αSMA protein expression in the cecum. Western blots were digitally scanned and quantitated using ImageJ. Relative expression was normalized to GAPDH protein expression. Statistical comparisons between the infection groups or intervention groups and the uninfected controls are denoted with asterisks. Statistically significant comparisons between the S. typhimurium-infected and levofloxacin intervention groups at day 21 post-infection are denoted in brackets. ns = not statistically significant, * p <0.05, ** p<0.01, *** p<0.001
Similarly, histological scoring of fibrosis revealed that day 2 or 4 levofloxacin intervention reduced tissue fibrosis at day 21 to levels indistinguishable from uninfected animals (0.4, 0.6 vs. 0.0, p=0.35, p=0.35 respectively) (Figure 5 D). The later (day 8) levofloxacin intervention group had significant tissue fibrosis at day 21 that was similar to the infected group at day 21 (1.8 vs. 2.8, p=0.36) and significantly greater than the fibrosis in uninfected controls (1.8 vs. 0.0, p <0.001).
In contrast, αSMA protein expression at day 21 post-infection was significantly induced despite early intervention with levofloxacin (Figure 5 E, F). Though αSMA protein expression in the early (day 2 and day 4) levofloxacin groups was 3 to 5-fold lower than the day 21 S. typhimurium-infected mice, αSMA expression in all three intervention groups was increased compared to uninfected controls at day 21. During the course of infection, αSMA protein expression was not induced until day 8 post-infection. However, the persistence of αSMA protein expression at day 21 in the infected/levofloxacin-treated groups indicates the fibrogenic process and myofibroblast activation is initiated early and persists despite removal of the inflammatory stimulus and eradication of the inflammatory response.
Early intervention represses gross pathological changes and inflammation
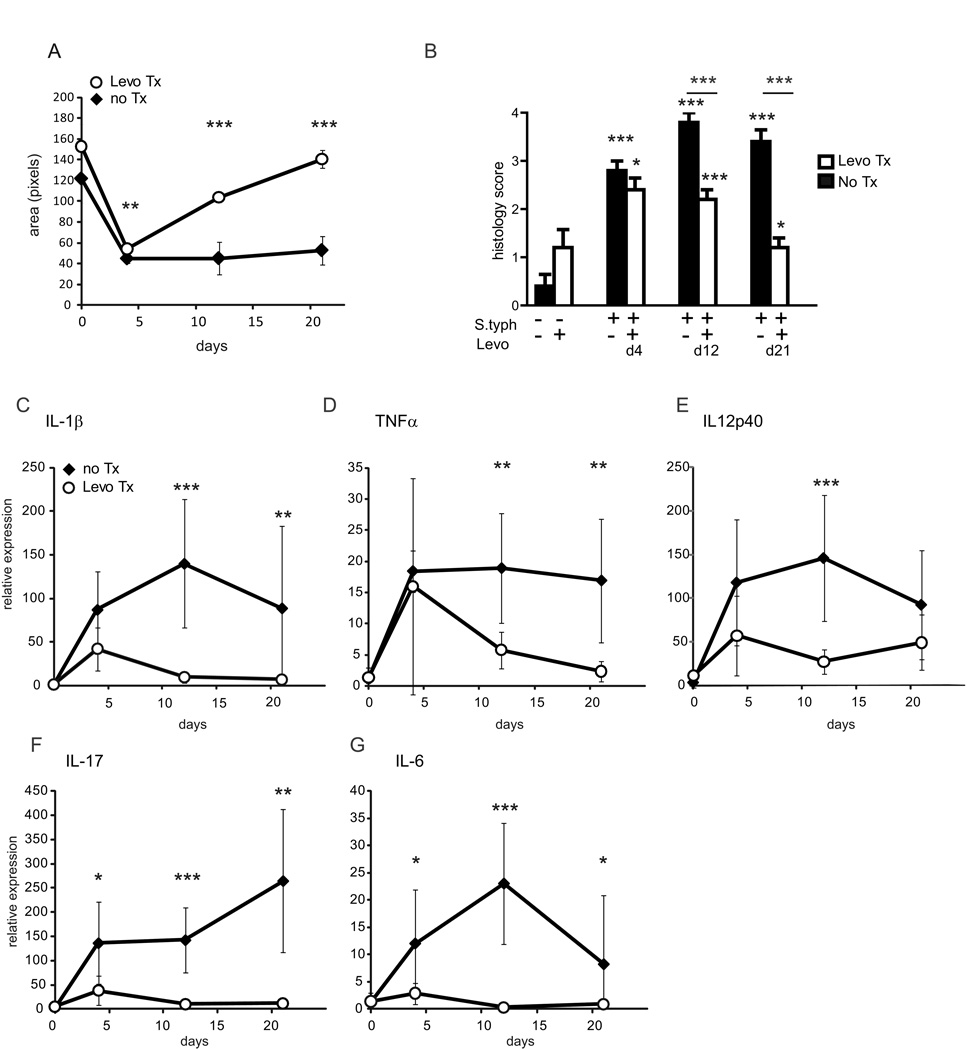
As demonstrated above, the fibrogenic process is initiated between day 4 and day 8 with maximum fibrosis at day 21 post-infection. Therefore, in a second time course comparison experiment, we investigated the effect of early removal of the inflammatory stimulus by treatment with levofloxacin at day 3 upon the development of fibrosis over the next 18 days (Figure 1B). Following infection, S. typhimurium was eradicated in half the animals by treatment with levofloxacin day 3 post-infection (during the inflammatory phase of infection but before the fibrogenic phase). Mice from both the untreated and levofloxacin intervention groups were sacrificed at day 4, 12, and 21 post-infection to determine the effect of eradication upon inflammation and fibrosis and compared to uninfected animals which received either levofloxacin treatment or no intervention.
Twenty-four hours after initiating levofloxacin therapy, all animals in the intervention group had cleared the S. typhimurium as determined by stool culture (data not shown). In the day 4 post-infection group, this clearance was associated with a concurrent change in gross pathology with a small (17%) but significant increase in cecal area compared to matched day 4 S. typhimurium-infected animals. (Figure 6 A) Similar improvement in cecal + colon weight and length were also observed (data not shown). By day 12 post-infection, the gross pathology of the levofloxacin intervention group was indistinguishable from uninfected controls.
Figure 6. Early intervention represses inflammation.
(A) Levofloxacin intervention reverses cecal contraction in response to S. typhimurium infection. Cecal area of mice infected with S. typhimurium (solid diamonds) compared to S. typhimurium-infected animals treated with levofloxacin day 3 post-infection (open circles) at day 4, 12, and 21 post-infection. (B) Blinded inflammatory scoring of cecal sections using the 0–4 point Wirtz scale. (C–G) qRT-PCR expression of IL-1β, TNFα IL-12p40, IL-17, and IL-6 in the cecum day 4, 12, and 21 post-infection. Statistical comparisons between the infection and intervention groups are denoted with asterisks. *p <0.05, ** p<0.01, *** p<0.001
Histological analysis of cecal tissue sections identified significant inflammation at both day 4 and day 12 in the levofloxacin-treated group compared to uninfected controls (inflammation score 2.4 vs. 0.4 at day 4, p= 0.035, 2.2 vs. 0.4 at day 12, p<0.001) (Figure 6 B). Compared to the matched S. typhimurium/untreated animals, inflammatory scores were indistinguishable between the day 4 groups (2.4 vs. 2.8, p=0.25). Early Levofloxacin intervention did significantly repress inflammation by day 12 and day 21 (2.2 vs. 3.8, p<0.001 and 1.2 vs. 3.4, p<0.001), though the inflammatory score in the levofloxacin intervention group at day 12 remained significantly higher than uninfected controls (1.2 vs. 0.4, p=0.035).
The histological inflammatory response was reflected by a similar pattern of change in pro-inflammatory cytokine gene expression. Cecal expression of pro-inflammatory cytokines IL-1β, TNFα, and IL12p40 were significantly induced and did not differ in between the S. typhimurium infected and levofloxacin-treated mice at day 4 post-infection compared to uninfected controls (Figure 6 C–E). However, by day 12 post-infection IL-1β, TNFα, and IL12p40 expression was significantly reduced in levofloxacin-treated compared to day 12 infected and untreated mice.
Early intervention with levofloxacin at day 3 exerted a profound and immediate effect upon the Th17 cytokine response. At day 4 post-infection, IL-17 and IL-6 were repressed 4-fold in the levofloxacin intervention group compared to the S. typhimurium group (Figure 6 F, G).
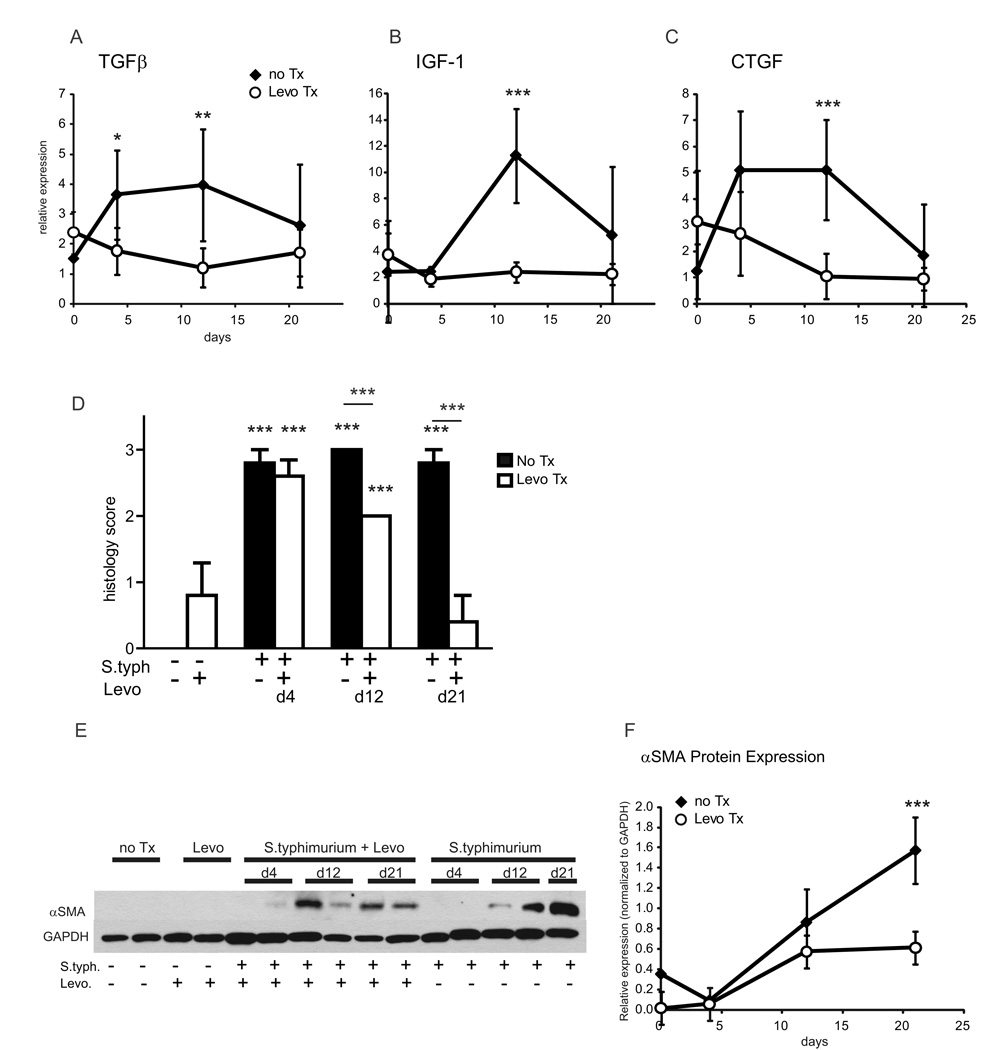
Early intervention represses inflammation but does not prevent fibrosis
Early intervention with levofloxacin at day 3 post-infection repressed the induction of pro-fibrotic genes TGFβ, IGF-1, and CTGF. Though infection induced TGFβ >3-fold at day 4 post-infection, treatment with levofloxacin at day 3 repressed TGFβ to levels indistinguishable from uninfected controls (Figure 7 A). A similar trend occurred for CTGF (Figure 7 C). Though IGF-1 was not induced until later in the time course, levofloxacin treatment significantly repressed IGF-1 induction (Figure 7 B).
Figure 7. Early intervention represses inflammation but does not prevent fibrosis.
(A–C) qRT-PCR expression of fibrotic markers TGFβ (A), IGF-1 (B), and CTGF (C) in mice infected with S. typhimurium (solid diamonds) compared to S. typhimurium-infected mice treated with levofloxacin day 3 post-infection (open circles) day 4, 12, and 21 post-infection. (D) Blinded fibrosis scoring of cecal sections was determined from trichrome stained cecal sections. (E) Representative Western blot of cecal αSMA protein expression in mice infected with S. typhimurium compared to mice infected with S. typhimurium and treated with levofloxacin, uninfected mice (no Tx), and levofloxacin treated uninfected mice (Levo). GAPDH was used as a control for protein loading. (F) αSMA protein expression in the cecum in response to S. typhimurium infection or S. typhimurium infection and levofloxacin intervention. Relative αSMA expression was normalized to GAPDH expression. Statistically significant comparisons between the infection and intervention groups are denoted with asterisks. * p <0.05, ** p<0.01, *** p<0.001
Histologic scoring of cecal fibrosis identified a significant reduction in fibrosis in the levofloxacin intervention groups by day 12 and day 21. At day 12, the fibrosis score was significantly lower in the intervention group compared to the untreated group (2.0 vs. 3.0, p<0.001) (Figure 7D). By day 21, fibrosis had resolved in the levofloxacin intervention group to levels similar to uninfected controls (1.0 vs. 0.6, p=0.35).
αSMA protein expression was significantly increased at day 12 post-infection in both the levofloxacin intervention and S. typhimurium groups compared to uninfected controls (Figure 7 E, F). While overall cecal αSMA protein expression was lower in the levofloxacin intervention group, this was not statistically significant at day 12 compared to those without levofloxacin treatment. By day 21 post-infection, αSMA protein expression was significantly repressed by 2.6-fold in the levofloxacin intervention (p<0.0001 vs. S. typhimurium-infected mice). Though αSMA protein expression was attenuated at day 21 in the levofloxacin intervention group compared to S. typhimurium-infected mice, αSMA protein was still significantly higher compared to uninfected controls; suggesting early intervention attenuated but did not completely resolve myofibroblast activation and fibrogenesis.
Discussion
Crohn’s disease, despite available potent anti-inflammatory therapies, largely progresses to a complicated fibrostenosing phenotype. Current therapies induce and maintain remission of inflammation but have a poor record in preventing fibrostenotic complications with a majority of Crohn’s patients inexorably progressing towards fibrostenotic disease and subsequent surgical intervention (1–3, 12, 13). In a small but recent study, ~20% of CD patients with fibrostenotic disease treated with biologics required surgical intervention within 2 to 5 years (14). Current therapies which target inflammation do not alter the natural history of CD, suggesting that intestinal fibrosis, once initiated, becomes irreversible (15).
To better understand why anti-inflammatory therapy fails to alter the natural history of intestinal fibrosis, we examined the temporal relationship between inflammation and fibrosis and the consequences of removal of inflammation from the S. typhimurium murine model of fibrosis. Though other murine colitis models (PG-PS, TNBS, DSS) are commonly used to study IBD, these have limitations for elucidating the pathobiology of fibrosis due to inherent murine resistance to fibrotic disease, incomplete disease penetrance, and high mortality (8). In addition, none are particularly amenable to experimental manipulation of inflammation. Our study, the first systematic manipulation inflammation in a rodent colitis model, demonstrates that eradication of the inflammatory stimulus is not sufficient to prevent progression of fibrosis. As we demonstrated in the infection time course comparison experiments, inflammatory changes occur early (day 2) and precede fibrogenesis (day 4 – day 8). Treatment of S. typhimurium-infected mice with levofloxacin at day 2, 4, and 8 post-infection reversed inflammatory changes in gross pathology, histopathology, and repressed the Th1/Th17 inflammatory cytokine gene expression at day 21 post-infection. However, levofloxacin intervention at day 4 or day 8 failed to prevent fibrogenesis at day 21 post-infection as evidenced by increased pro-fibrotic gene expression, increased tissue fibrosis, and increased αSMA protein expression. Strikingly, fibrosis not only persisted, but actually progressed even after eradication of the inflammatory stimulus, suggesting that established fibrosis is not readily reversible, even with eradication of the inciting inflammatory stimulus. Further, this suggests that established intestinal fibrogenesis is, to some extent, a self-sustaining process.
Current CD treatment paradigms follow a “step-up” approach in which increasing disease severity (flares) or treatment failure drives therapeutic escalation (16). However, recent clinical studies suggest an early, aggressive or “top-down” approach may improve patient outcomes (17, 18). To determine whether a “top-down” approach alters the natural history of intestinal fibrosis, we treated S. typhimurium infected mice with levofloxacin at day 3 post-infection and compared the impact on progression of fibrosis with infected mice at days 4, 12, and 21 post-infection. Consistent with a potential benefit to the “top-down” approach, early intervention improved tissue pathology as determined by gross pathology and histopathology, and repressed the induction of inflammatory and pro-fibrogenic genes. In addition, early intervention suppressed, yet did not completely abrogate, induction of αSMA protein expression, suggesting partial myofibroblast activation. In contrast, late eradication of the inflammatory stimulus did not prevent fibrotic progression.
In summary, our study demonstrates that by manipulating a rodent model of fibrosing colitis we can examine biologically and clinically important questions concerning the interaction between inflammation and fibrosis. Late removal of the inflammatory stimulus does not prevent subsequent tissue fibrosis, suggesting a “point of no return” at which fibrogenesis becomes autopropagative. This finding is similar to studies demonstrating that liver fibrosis is reversible if the activating stimulus is suppressed at an early time point (19). While no human study has yet addressed the connection between very early use of biologics (anti-inflammatory therapy within a week of diagnosis) and the development of fibrostenotic disease, we demonstrate here that very early therapeutic intervention can ameliorate the late endpoint of fibrosis. These findings support the potential benefits of a very early “top-down” treatment approach (17), and suggest that the benefits may wane as the time between the start of symptoms and therapeutic intervention increases.
Crohn’s disease is chronic and progressive, with a natural history filled with complications. Currently, we cannot reliably stratify patients by their risk of fibrostenotic disease (20). Given that current therapies target inflammation, no effective anti-fibrotics exist, and that once initiated, and that fibrosis can autopropagate even in the absence of inflammation, very early intervention or a “top-down” approach may have the greatest impact on the natural history of Crohn’s disease.
Acknowledgements
This study was funded by NIH K08DK080172-01 to P.D.R.H. and a grant from the University of Michigan Peptide Center. We thank Eva Rodansky of the University of Michigan for critical review of the manuscript.
References
- 1.Andres PG, Friedman LS. Epidemiology and the natural course of inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:255–281. doi: 10.1016/s0889-8553(05)70056-x. vii. [DOI] [PubMed] [Google Scholar]
- 2.Sands BE, Arsenault JE, Rosen MJ, et al. Risk of early surgery for Crohn's disease: implications for early treatment strategies. Am J Gastroenterol. 2003;98:2712–2718. doi: 10.1111/j.1572-0241.2003.08674.x. [DOI] [PubMed] [Google Scholar]
- 3.Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis. 2002;8:244–250. doi: 10.1097/00054725-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Feagan BG, Vreeland MG, Larson LR, et al. Annual cost of care for Crohn's disease: a payor perspective. Am J Gastroenterol. 2000;95:1955–1960. doi: 10.1111/j.1572-0241.2000.02261.x. [DOI] [PubMed] [Google Scholar]
- 5.Lichtenstein GR, Olson A, Travers S, et al. Factors associated with the development of intestinal strictures or obstructions in patients with Crohn's disease. Am J Gastroenterol. 2006;101:1030–1038. doi: 10.1111/j.1572-0241.2006.00463.x. [DOI] [PubMed] [Google Scholar]
- 6.Louis E, Collard A, Oger AF, et al. Behaviour of Crohn's disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777–782. doi: 10.1136/gut.49.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grassl GA, Valdez Y, Bergstrom KS, et al. Chronic enteric salmonella infection in mice leads to severe and persistent intestinal fibrosis. Gastroenterology. 2008;134:768–780. doi: 10.1053/j.gastro.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 8.Brand S. Crohn's disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn's disease. Gut. 2009;58:1152–1167. doi: 10.1136/gut.2008.163667. [DOI] [PubMed] [Google Scholar]
- 9.Wirtz S, Neufert C, Weigmann B, et al. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 10.Higgins PD, Johnson LA, Sauder K, et al. Transient or persistent norovirus infection does not alter the pathology of Salmonella typhimurium induced intestinal inflammation and fibrosis in mice. Comp Immunol Microbiol Infect Dis. 2011 doi: 10.1016/j.cimid.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strober W, Zhang F, Kitani A, et al. Proinflammatory cytokines underlying the inflammation of Crohn's disease. Curr Opin Gastroenterol. 2010;26:310–317. doi: 10.1097/MOG.0b013e328339d099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cosnes J. Can we modulate the clinical course of inflammatory bowel diseases by our current treatment strategies? Dig Dis. 2009;27:516–521. doi: 10.1159/000233291. [DOI] [PubMed] [Google Scholar]
- 13.Jones DW, Finlayson SR. Trends in surgery for Crohn's disease in the era of infliximab. Ann Surg. 2010;252:307–312. doi: 10.1097/SLA.0b013e3181e61df5. [DOI] [PubMed] [Google Scholar]
- 14.Peyrin-Biroulet L, Lemann M. Review article: remission rates achievable by current therapies for inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:870–879. doi: 10.1111/j.1365-2036.2011.04599.x. [DOI] [PubMed] [Google Scholar]
- 15.Vermeire S, van Assche G, Rutgeerts P. Review article: Altering the natural history of Crohn's disease--evidence for and against current therapies. Aliment Pharmacol Ther. 2007;25:3–12. doi: 10.1111/j.1365-2036.2006.03134.x. [DOI] [PubMed] [Google Scholar]
- 16.Hanauer SB. Medical management of Crohn's disease: treatment algorithms 2009. Dig Dis. 2009;27:536–541. doi: 10.1159/000233294. [DOI] [PubMed] [Google Scholar]
- 17.Domenech E, Manosa M, Cabre E. Top-down therapy: is the evidence strong enough? Dig Dis. 2009;27:306–311. doi: 10.1159/000228565. [DOI] [PubMed] [Google Scholar]
- 18.Lin MV, Blonski W, Lichtenstein GR. What is the optimal therapy for Crohn's disease: step-up or top-down? Expert Rev Gastroenterol Hepatol. 2010;4:167–180. doi: 10.1586/egh.10.4. [DOI] [PubMed] [Google Scholar]
- 19.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 20.Rieder F, Lawrance IC, Leite A, et al. Predictors of fibrostenotic Crohn's disease. Inflamm Bowel Dis. 2011 doi: 10.1002/ibd.21627. [DOI] [PubMed] [Google Scholar]