Abstract
Drug abuse and dependence present significant health burdens for our society, affecting roughly 10% of the population. Stress likely contributes to the development and persistence of drug use; for example, rates of substance dependence are elevated among individuals diagnosed with post-traumatic stress disorder (PTSD). Thus, understanding the interaction between stress and drug use, and associated neuroadaptations, is key for developing therapies to combat substance use disorders. For this purpose, many rodent models of the effects of stress exposure on substance use have been developed; the models can be classified according to three categories of stress exposure: developmental, adult nonsocial, and adult social. The present review addresses preclinical findings on the effect of each type of trauma on responses to and self-administration of drugs of abuse by focusing on a key exemplar for each category. In addition, the potential efficacy of targeting neuropeptide systems that have been implicated in stress responses and stress system neuroadaptation in order to treat comorbid PTSD and substance abuse will be discussed.
1. Introduction
Substance abuse and addiction cause significant health care burdens on society, with current estimates suggesting 10% of the population suffers from some form of substance use disorder (Hall et al., 1999; Ross, 1995; Stinson et al., 2005). The progression of drug abuse has been depicted as a downward spiral comprised of three stages: binge/intoxication, preoccupation/anticipation, and withdrawal/negative affect (Koob and Le Moal, 1997). During the acquisition phase, characterized by episodes of intoxication, drug taking produces positive reinforcement. With the development of drug dependence, withdrawal leads to a negative emotional state; as a result, drugs are taken to alleviate or avoid withdrawal symptoms (i.e., negative reinforcement). Periods of abstinence are characterized by pervasive thoughts about the addictive drug, yielding a high rate of relapse (Koob et al., 2004). Brain stress systems are thought to play a significant role in generating the negative emotional state characteristic of drug dependence, with dysregulation of stress systems also underlying the persistence of drug-seeking and relapse (Koob, 2008). The recruitment of brain stress systems during the progression to drug dependence suggests that anxiety disorders, characterized by heightened stress responses, may predispose individuals to develop addictive disorders and/or perpetuate and worsen addictive disorders once established.
One anxiety-related disorder receiving increased attention as a possible contributing factor to the development of addictive disorders in humans is post-traumatic stress disorder (PTSD). Triggered by exposure to a traumatic experience, PTSD is characterized by persistent maladaptive symptoms related to the trauma, including blunted emotional responses, hyperarousal, and flashbacks. Among individuals diagnosed with PTSD, the incidence of drug abuse and addiction is markedly elevated, with the highest comorbidity observed for alcohol dependence, followed by other depressants, such as opioids and cannabinoids, although stimulants like cocaine are also abused by some, possibly dependent on the sequelae of symptoms experienced by the individual (Jacobsen et al., 2001). Multiple studies have shown three- to five-fold increases in the development of substance abuse among PTSD patients, yielding substance abuse comorbidity in nearly half of all PTSD patients (Breslau et al., 2003; Mills et al., 2006; Perkonigg et al., 2000). Conversely, upwards of 25% of the substance abusing population may suffer from some form of PTSD (Driessen et al., 2008). Even in the absence of PTSD, traumatic experiences can precipitate relapse in recovering addicts (Dewart et al., 2006; Zywiak et al., 2003). Individuals with substance abuse disorders with comorbid PTSD face worse treatment outcomes (Brown et al., 1995; Brown and Wolfe, 1994), indicating a need for improved therapies to address the dual diagnosis. Thus, understanding the neural mechanisms that may jointly subserve PTSD and substance abuse presents an important target for therapeutic development.
Several animal models have been established that resemble key phenotypes of PTSD, such as long-term persistence of conditioned fear responses and heightened sensitivity to novel stressful stimuli. Such paradigms include delivery of electric shocks to tail or paws, social stress from exposure to predators or aggressive conspecifics, or multiple stressors experienced one after the other, termed single prolonged stress (Stam, 2007). Lack of predictability and controllability of the stressors are central features of paradigms that generate persistent post-traumatic effects (Foa et al., 1992; Koolhaas et al., 2011). While not fully validated as rodent models of PTSD, early life traumas, such as maternal separation, can have long-lasting effects on adult stress responsiveness, including heightened startle and novelty responses, hypothesized to be key elements of rodent PTSD models (Kalinichev et al., 2002a; Kalinichev et al., 2002b). Exposure to social and nonsocial stressors in adulthood has been used to model how adult traumatic stress alters behavior, both acutely and following extended post-stress intervals. Social stressors, such as isolated housing and defeat by a dominant animal, rely on the innate sociability and social structures of the animals in question. Numerous paradigms exist to generate nonsocial stress, including restraint, forced swimming, tail pinch, and electric shock. Chronic variable stress may utilize both varieties of adult stressors, as it involves sequential exposure to multiple stressors over time. Although published studies have not explicitly investigated the interaction between a PTSD-like state and drug self-administration, many have utilized stress delivery paradigms in the context of drug self-administration that have otherwise been verified to generate PTSD-like symptoms in rodents.
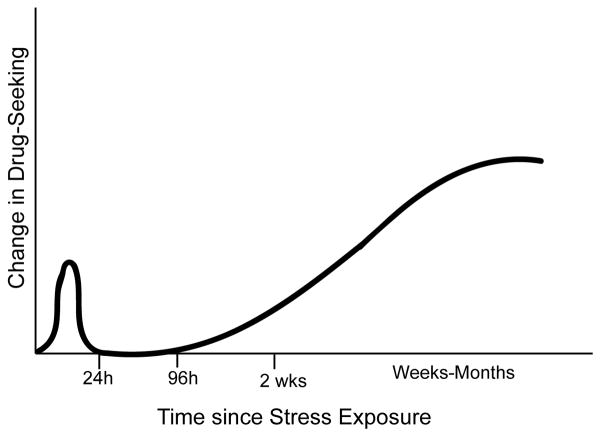
This review focuses on rodent models designed to test the effects of stressors on motivational properties of alcohol and drugs of abuse. The interaction between stressful life events and drug addiction will be analyzed by focusing on one prototypical stressor from each category – maternal separation, footshock and social defeat as models of early life, adult nonsocial and adult social stress – and their ability to modulate rodent behavioral responses to and self-administration of drugs of abuse. The studies discussed below highlight the dual temporal impact of stressors, which can both acutely precipitate behavioral changes and exert effects following an extended post-stress interval, as is characteristic of PTSD (Figure 1). It should be noted, however, that none of the studies has directly addressed the generation of a PTSD-like state in the rodent on drug self-administration, and thus the intention is not to validate the existing paradigms as rodent models of comorbid PTSD and drug abuse. Instead, synthesis of the current knowledge regarding stress modulation of rodent drug self-administration, as well as pharmacological targets showing promise for decreasing drug self-administration in these models, may provide a foundation for the future development of behavioral paradigms that more directly address the role of trauma in modulating drug use, as well as the efficacy of new pharmacotherapies to treat comorbid PTSD and drug abuse.
Figure 1.
Temporal modulation of stress effects on drug-seeking in rodents. Stress exposure acutely elevates behavioral responses to drugs of abuse and drug-seeking at all phases of drug self-administration. Post-stress increases in drug-seeking are usually short-lived, dissipating within 24h after cessation of the stressor. The effects of traumatic stress history on drug-seeking begin to emerge again after an interval, postulated to be on the order of days to several weeks depending on the nature and severity of the stressor. These distal stress history-dependent elevations in drug-seeking behavior may persist for weeks or even months, akin to the effects of post-traumatic stress on human drug-seeking.
2. Effects of early life trauma: maternal separation
Stress experienced early in life can profoundly affect adult behavior, with childhood trauma associated with the severity of drug dependence (Enoch et al., 2010; Triffleman et al., 1995). One common model of early life traumatic stress in rodents is maternal separation, a paradigm involving repeated separation of pre-weaning pups from their mothers (Lehmann and Feldon, 2000; Plotsky and Meaney, 1993). The effects of maternal separation depend on the length of separation, as brief (15-minute) separation may be protective from, and extended (>180 minute) separation predisposing to, heightened stress responses (Plotsky and Meaney, 1993). Extended maternal separation results in increased anxiety-like behavior in adult rats (Huot et al., 2001; Kalinichev et al., 2002b; Romeo et al., 2003), as well as elevated startle responses (Caldji et al., 2000), indicative of the face validity of maternal separation as a model for early life trauma yielding PTSD-like symptoms in adulthood. In humans, newborns that required isolation from their mothers during the first few months of life for medical reasons had increased risk of developing drug dependence (Veijola et al., 2008). Thus maternal separation provides a plausible model for studying the protracted effects of childhood trauma on drug self-administration in rodents.
2.1 Alcohol
A history of maternal separation dose-dependently alters alcohol self-administration in adulthood; that is, the duration of the maternal separation directly impacts its modulation of alcohol drinking. Brief, 15-minute daily separations of the litter from the dam, often referred to as “handling,” reduced two-bottle choice alcohol intake, as compared with non-handled controls, in male rats from multiple strains (Hilakivi-Clarke et al., 1991; Jaworski et al., 2005; Ploj et al., 2003), including the alcohol-preferring Alko alcohol (AA) rats (Roman et al., 2003). Handling also reduced the acquisition rate of two-bottle drinking in AA rats, suggesting that brief intervals of maternal separation may protect against the development of high alcohol intake in rats otherwise prone to excessive alcohol consumption.
Unlike brief exposure, extended maternal separation increased alcohol intake in adulthood. Moderate, 3-hour neonatal separation elevated sweetened 10% alcohol intake in adult mice (Cruz et al., 2008). Rats with similar extended separation histories also drank significantly more sweetened alcohol in adulthood than handled rats, although results varied in comparison to control rats, with maternally separated rats showing either significant increases (Huot et al., 2001) or no significant difference (Jaworski et al., 2005) in alcohol intake. Increasing the duration of separation to 6 hours yielded elevated adult alcohol intake compared with both handled and control rats (Ploj et al., 2003). This effect was less pronounced in AA rats, which displayed higher alcohol consumption than handled, but not control, rats despite having experienced 6-hour intervals of maternal separation (Roman et al., 2003). Interestingly, while males show altered alcohol intake following maternal separation, most data suggest lesser effects of maternal separation in female rats (Roman et al., 2005; Roman et al., 2004). Taken together, the data indicate that, in rodents, brief maternal separation may generate resilience against the development of alcohol addiction, while prolonged neonatal separations may dose-dependently contribute to elevated alcohol intake in adulthood. To date, little data exist on the role neonatal stress history may play in relapse to alcohol drinking; however, given that maternal stress promotes an anxiety-like state (Huot et al., 2001; Kalinichev et al., 2002b; Romeo et al., 2003) and heightened alcohol consumption in the adult offspring (Cruz et al., 2008; Huot et al., 2001; Ploj et al., 2003; Roman et al., 2005; Roman et al., 2003), elevation of both reinstatement and relapse-like responding might be predicted.
2.2 Psychostimulants
Maternal separation also differentially modulates adult responses to psychostimulants, in relation to the severity of the maternal separation history. Neonatal handling blunted male rats’ locomotor responses to acute doses of cocaine (Brake et al., 2004), and handling decreased adult cocaine self-administration under a fixed ratio schedule to saline-like levels across all doses tested (Moffett et al., 2006). The rats still showed preference for the cocaine-paired (vs. inactive) lever, and responses during initial food training were not different from other groups, suggesting that the low response was not due to impaired task acquisition (Moffett et al., 2006). Handling also reduced male rats’ sensitivity to the rewarding effects of amphetamine in adulthood, resulting in blunted preference for the previously drug-paired chamber in a conditioned place preference (CPP) test (Campbell and Spear, 1999). Together, the results indicate that, in rodents, brief maternal separation may confer protection against the development of psychostimulant dependence by reducing the drugs’ rewarding and activating potency.
In contrast to brief separation, longer maternal separation periods enhance the activating and reinforcing properties of psychostimulants in adulthood. Mice with a history of extended maternal separation displayed elevated locomotor sensitization to cocaine (Kikusui et al., 2005), although this effect was not observed after amphetamine treatment in rats (Weiss et al., 2001). Extended maternal separation also facilitated the acquisition of cocaine self-administration at a low per-infusion unit dose – one that did not otherwise support self-administration in non-handled or briefly handled rats. The results suggest a heightened sensitivity to the reinforcing effects of low doses of cocaine results from extended maternal separation (Moffett et al., 2006). Extended maternal separation also enabled rats to discriminate between the cocaine-paired and inactive levers, a behavioral selectivity not observed in control groups (Moffett et al., 2006). Additional research demonstrated that acute amphetamine treatment reduced threshold currents for intracranial self-stimulation (ICSS) of the lateral hypothalamus to a greater degree in rats with a history of maternal separation than in controls (Der-Avakian and Markou, 2010), indicative of enhancement of the facilitatory effect of psychostimulants on brain reward function following a history of extended maternal separation. It should be noted that Robbins and colleagues showed reductions in both amphetamine-induced hyperactivity (Matthews et al., 1996) and cocaine self-administration on the first day of acquisition (Matthews et al., 1999) in rats with a history of maternal separation. The rats also showed no change in amphetamine-induced reductions in lateral hypothalamic ICSS thresholds (Matthews and Robbins, 2003). However, the Matthews and Robbins study involved more sporadic delivery and later onset of maternal separation than most other studies cited, with separation occurring on 10 randomly selected days between postnatal days 5 and 20, rather than daily for the first 2–3 weeks of life. Thus the discrepancy between results may arise from reduced severity of the maternal separation when delivered more infrequently or outside a critical period of development. Regardless, the balance of the data supports the hypothesis that lengthy maternal separation elevates responsiveness to and propensity to self-administer psychostimulants in adulthood.
Reinstatement of psychostimulant-seeking may also be modulated by a history of maternal separation. Zhang and colleagues (2005) found no significant alteration of cocaine-induced reinstatement in adult rats that had experienced 1-hour maternal isolation, a paradigm involving not only maternal separation but also isolation from littermates. However, the same isolation paradigm yielded increased cue-induced reinstatement (Lynch et al., 2005). Additional studies using maternal separation and brief handling are required to fully understand the impact of neonatal handling on reinstatement responding; nonetheless, the existing data suggest that early postnatal maternal separation in rodents may have a lasting impact not only on drug response and intake in adulthood but also on the magnitude of relapse to drug-seeking after periods of abstinence.
2.3 Opiates
While fewer studies have investigated the effects of maternal separation on the activating and reinforcing properties of opiates, the available data suggest similarities to the results described for alcohol (Section 2.1) and psychostimulants (Section 2.2). Three-hour neonatal maternal separation sessions resulted in increased locomotor sensitization to morphine as well as elevated morphine CPP in adult male rats (Kalinichev et al., 2002a; Michaels and Holtzman, 2008). Maternal separation also increased rats’ sensitivity to the acute modulation of lateral hypothalamus ICSS thresholds by heroin, with threshold elevations, normally observed only after high–dose exposure, occurring at moderate doses in rats with a history of maternal separation (Matthews and Robbins, 2003). In addition, when the rats were made dependent on morphine, those with a history of maternal separation showed elevated somatic withdrawal scores (Kalinichev et al., 2001). Together the data suggest that maternal separation not only enhances the acute activating and reinforcing properties of opiates, but may increase sensitivity to the drugs’ dependence-associated hypohedonic effects.
2.4 Summary
As detailed in Sections 2.1–2.3, maternal separation enhances sensitivity to and self-administration of multiple drugs of abuse in adulthood. These data are consistent with human studies indicating a relationship between childhood trauma and drug dependence in adulthood (Enoch et al., 2010; Hyman et al., 2008; Triffleman et al., 1995; Veijola et al., 2008). Maternal separation therefore may provide a valid model for investigation of therapeutic interventions for treatment of drug addiction in patients with a history of childhood trauma.
3. Effects of adult nonsocial stress: footshock
Many paradigms that involve delivery of mild physical stressors to adult rodents trigger anxiety-like behavior suggestive of a stress-like state. One of the most commonly used methods of stress delivery in rodents is footshock, the primary stimulus for aversive Pavlovian conditioning in rodents. Footshock provides a discretely manipulatable stressor, as duration, frequency, intensity, predictability, and controllability of the stress presentation can all be regulated. As discussed elsewhere, several paradigms of inescapable shock delivery in unpredictable patterns have been shown to create a PTSD-like state in rodents (Foa et al., 1992; Garrick et al., 1997; Maier, 2001; Pynoos et al., 1996; Rau et al., 2005; Siegmund and Wotjak, 2007), supporting the face validity of this model for providing useful information regarding the effects of traumatic stress on drug self-administration. Unlike maternal separation, which is by nature temporally removed from adult drug self-administration, footshock stress delivered to the adult animal allows for determination of the temporal relationship between stress exposure and alterations in drug self-administration, as both precipitant (immediate) and post-traumatic (delayed) effects of footshock exposure can be investigated.
3.1 Alcohol
Footshock has varying effects on alcohol intake, having been found to increase (Caplan and Puglisi, 1986; Casey, 1960; Chester et al., 2008; Funk et al., 2004; Matthews et al., 2008; Mills et al., 1977; Opsahl and Hatton, 1972; Vengeliene et al., 2003; Volpicelli et al., 1986; Volpicelli and Ulm, 1990; Volpicelli et al., 1990), have no effect (Brunell and Spear, 2005; Chester et al., 2008; Fidler and LoLordo, 1996; Myers and Holman, 1967) and decrease (Brunell and Spear, 2005; Caplan and Puglisi, 1986; Dayas et al., 2004) alcohol intake. The discrepancies between these studies may stem from key differences in stress delivery (Table 2), alcohol access, and housing conditions, demonstrating the importance not only of the stress delivery but also of stress cues in modulating alcohol intake. In particular, living continuously in the shock delivery chambers tended to suppress the rats’ consumption of all fluids, including alcohol, during the shock delivery period (Caplan and Puglisi, 1986; Casey, 1960; Opsahl and Hatton, 1972), except in cases where cues signaled the approach of uncontrollable shock delivery (Cicero et al., 1968; Opsahl and Hatton, 1972). For rats returned to live in a safe environment after the completion of footshock exposure, alcohol intake increased over the period following the termination of shock, generally reaching significantly elevated levels within the first 24–48 hours, an effect observed both in mice (Matthews et al., 2008; Racz et al., 2003) and rats (Caplan and Puglisi, 1986; Vengeliene et al., 2003) (but see also Dayas et al., 2004). Elevations in alcohol preference tended to be more delayed for rats living in the shock environment, with increased intake commencing anywhere from 24 hours (Volpicelli and Ulm, 1990) to 1 week (Casey, 1960) after termination of footshock. Even in the absence of heightened cumulative alcohol intake, analysis of drinking patterns during shock presentations indicated that alcohol intake clustered in the immediate post-shock interval for rats living in the shock chambers (Mills et al., 1977). Casey (1960) observed a prolonged increase in alcohol intake that peaked 2 weeks after the final footshock, suggestive of protracted-onset, long-term alterations in alcohol intake due to a history of footshock exposure. Together, these data indicate that footshock stress may not only increase alcohol consumption in the immediate post-stress interval but also have longer lasting effects.
Table 2.
Footshock delivery parameters used in cited studies
| Intensity & Duration | Frequency | Subjects | Drug & References |
|---|---|---|---|
| 0.2 mA, 0.5 sec | FT2min, 15 shocks, 10 days | adult & adolescent male & female mice | Alcohol: Chester et al. (2008) |
| 0.2 mA, 0.2 sec | 10 shocks | adult male rats | Cocaine: Sanchez and Sorg (2001) |
| 0.6 mA, 0.1 sec | RR15, mean 50 shocks 25–35 days | adult male rats | Cocaine: Goeders and Guerin (1994) |
| 0.23–0.42 mA, 0.5 sec | VI40sec, 15 min session | adult male mice | Alcohol: Matthews et al. (2008) |
| 0.25 mA, 1 sec | 10 shocks in 10 min | adult male rats | Cocaine: Ramsey and Van Ree (1993) |
| 0.25, 0.5, 0.75 mA, 0.5 sec | VI40sec, 15 min session | adult male rats | Cocaine: Buffalari and See (2009) |
| 0.5 mA, 0.1 sec | 55–60s ITI, 5 shocks | adult male mice | Alcohol: Racz et al. (2003) |
| 0.5 mA, 0.5 sec | VI40sec, 10 min session | adult male rats | Alcohol: Dayas et al. (2004), Liu and Weiss (2002), Liu and Weiss (2003); Cocaine: Erb et al. (1996); Heroin: Shaham and Stewart (1994), Shaham et al. (1996, 1998) |
| 0.6 mA, 0.1 sec | VI74sec, 1 h, >35 days | adult male rats | Methamphetamine: Moffett and Goeders (2005) |
| 0.6 mA, train of 3 × 0.1 sec, 1 sec apart | VI45sec, 5 min session | adult male rats | Cocaine: Mantsch and Katz (2007) |
| 0.7 mA, max 0.8/8 seca | FT1min, 2 h, 20 days | adult male rats | Alcohol: Cicero et al. (1968) |
| 0.7 mA, max 3/15 seca | FT1min, 30 min, 38days | adult male rats | Alcohol: Opsahl and Hatton (1972) |
| 0.7 mA, 2.5 sec | 1/h or 6/h, 14 days | adult male rats | Alcohol: Myers and Holman (1967) |
| 0.8 mA, 0.5 sec | VI40sec, 5–20 min session | adult male rats or mice | Alcohol: Le et al. (1998, 2002)rats; Cocaine: Brown and Erb (2007) rats, Redila and Chav in (2008) mice |
| 0.8 mA, 1 sec | VI33sec, 10 min, 5 days | adult male rats | Alcohol: Funk et al. (2004) |
| 0.8 mA, 2 sec | FT1min, 1 h; 4 or 10 days | adult male rats | Alcohol: Fidler and LoLordo (1996), Volpicelli et al. (1986) |
| 0.8 mA, 5–15 sec | 5 min shock/10 min, 3 days | adult male rats | Alcohol: Vengeliene et al. (2003) |
| 0.8 mA, up to 30 sec | VI60sec, 50 trials, 3 days | adult male rats | Alcohol: Volpicelli and Ulm (1990) |
| 0.86 mA, 0.5 sec | VI40sec, 15 min | adult male rats | Cocaine: Ahmed and Koob (1997) |
| 1 mA, 0.5 sec | VI40sec, 15 min | adult male rats | Heroin: Ahmed et al. (2000) |
| 1 mA, 1 sec | VI30sec, 15 min | adult & adolescent male & female rats | Alcohol: Brunell and Spear (2005) |
| 1 mA, 2 sec | FT1min, 1 h, 5 days | adult male & female rats | Alcohol: Caplan and Puglisi (1986) |
| 1 mA, 5 sec | FT1min, 1 h | adult male rats | Alcohol: Volpicelli et al. (1990) |
| 1.5 mA, 1 sec | 12 min/h for 24 h, 15 days | adult male rats | Alcohol: Mills et al. (1977) Alcohol: Myers and Holman (1967) |
| 2.5 mA, up to 5 sec | 180/day, 3 days | adult male rats | Cocaine: MacLennan and Maier (1983) |
| 35 V, 1.5 sec | VI11.3min for 16 days | adult male rats | Alcohol: Casey (1960) |
| 40 V, 1 sec | 15 shocks in 15 min | adult male mice | Morphine: Kuzmin et al. (1996) |
Rats could terminate shock train by lever pressing, trains involved 0.2 sec shock on, 2 sec shock off for 8 or 15 sec total if shock not terminated by lever press.
FT, fixed time between shock presentations
RR, random ratio indicating average number of lever presses resulting in shock delivery
VI, variable interval between shock presentations, with time reported as mean inter-shock interval
In addition to modulating maintenance drinking, footshock can also acutely alter alcohol-related behaviors following a period of abstinence, as seen in stress-induced reinstatement models. Footshock reinstated operant responding on a previously alcohol-paired lever in the absence of reinforcement when delivered to rats in the 5- to 15-minute period prior to the operant session (Le et al., 2002; Le et al., 1998). Delivery of cues previously paired with alcohol during the reinstatement session further enhanced the alcohol-seeking triggered by footshock stress (Liu and Weiss, 2002). The reviewed experiments demonstrate that stressors may acutely trigger elevated alcohol seeking; however, they do not address the ability of stressful situations to create a long-lasting propensity to relapse. One series of studies beginning to address that issue utilized cues previously paired with footshock and alcohol delivery to successfully reinstate alcohol-seeking more than a month after the last alcohol-cue pairing and 5 days after the final footshock conditioning session (Liu and Weiss, 2003). Neither cue independently reinstated operant responding in nondependent rats, but co-presentation of the cues significantly elevated responding on the previously alcohol-paired lever. These effects were heightened in rats with a history of alcohol dependence, in which both the footshock and alcohol cues elevated operant responding independently and to an even greater degree when presented together (Liu and Weiss, 2003). These data demonstrate that stressful experiences or situational reminders can precipitate alcohol-seeking, and these effects are more prominent in subjects with a history of alcohol dependence.
3.2 Psychostimulants
Uncontrollable footshock enhanced the locomotor effects of acute amphetamine and cocaine injections delivered the following day, as demonstrated by enhanced stereotypic behaviors in rats with a history of footshock stress (MacLennan and Maier, 1983). Given its ability to modulate the acute response to psychostimulants, footshock might be predicted to modify acquisition of psychostimulant self-administration. Acquisition of cocaine self-administration was enhanced by noncontingent footshock presentations delivered during 1-hour food self-administration sessions immediately preceding cocaine self-administration, with rats demonstrating task learning at lower doses and attaining higher overall cocaine intake than controls (Goeders and Guerin, 1994). The authors also demonstrated the importance of uncontrollability of the footshocks, as rats with the ability to terminate their footshocks did not escalate their self-administration. Because footshocks were delivered for at least 9 days prior to the onset of cocaine self-administration training and throughout the acquisition period, it cannot be determined whether the observed effects were due to acute effects of footshock exposure or to the extensive history of stress exposure. Using different training procedures, Ramsey and Van Ree (1993) found that exposure to footshock just prior (15 min) to self-administration elevated responding for cocaine only on the first day of acquisition, an effect not observed for amphetamine self-administration (Moffett and Goeders, 2005). Rats forced to observe footshock delivery to conspecifics displayed an increased rate of acquiring cocaine self-administration across the entire acquisition period (Ramsey and Van Ree, 1993). Thus, either footshock stress itself or the psychosocial experience of witnessing footshock of a conspecific may elevate acquisition of psychostimulant self-administration. The effects of footshock are not confined to acquisition, however. Daily exposure to 4 alternating blocks of 5-minute footshock stress and 30-minute cocaine self-administration triggered a significant escalation in cocaine intake within three days of the first footshock exposure and lasted for the remainder of the 14-day experimental period, during which time rats continued to experience alternating footshock/self-administration sessions (Mantsch and Katz, 2007). These studies indicate that exposure to footshock can elevate both acquisition and maintenance levels of cocaine intake, although further investigation is required to differentiate between the short- versus long-term effects of footshock stress on psychostimulant self-administration.
Like acquisition and maintenance, reinstatement of operant responding or CPP for psychostimulants can be precipitated by footshock stress (stress-induced reinstatement). Intermittent footshock exposure prior to operant sessions elevated responding on the lever previously paired with cocaine in rats (Erb et al., 1996), an effect that did not generalize to responding for food (Ahmed and Koob, 1997) or sucrose (Le et al., 1998). These effects were often observed immediately after the footshock conditioning session, although the reinstating effects of acute footshock were shown to persist for up to 40 minutes after a single session of intermittent footshock (Brown and Erb, 2007). As previously described for alcohol (Section 3.1), footshock and cocaine-paired cues function additively in reinstatement of cocaine-seeking in rats (Buffalari and See, 2009). The reinstating effect of footshock stress is not restricted to operant drug-seeking, however, but also applies to preference for a drug-paired environment. Administration of 15-minute footshock sessions prior to CPP testing reinstated preference for the previously cocaine-paired chamber in mice (Redila and Chavkin, 2008) and rats (Wang et al., 2000). Discrete footshock-conditioned cues also reinstated cocaine CPP in rats, despite several weeks’ delay between fear conditioning and reinstatement testing (Sanchez and Sorg, 2001). Together these data suggest that traumatic stressors such as footshock, or even conditioned reminders of the stress, can trigger psychostimulant-seeking in otherwise abstinent individuals.
3.3 Opiates
Fewer studies have investigated the effects of footshock stress on opiate self-administration. However, the existing literature resembles the findings discussed above for alcohol (3.1) and psychostimulants (3.2). Footshock immediately prior to heroin self-administration sessions resulted in enhanced responding for high doses of heroin on a progressive ratio schedule (Shaham and Stewart, 1994); that is, footshock increased the amount of work rats would perform for each additional dose of heroin. As with cocaine, the emotional stress of observing footshock heightened morphine self-administration in mice, including in mice that demonstrated no difference in self-administration of morphine and saline prior to stress exposure (Kuzmin et al., 1996). These data support a role for footshock stress in acutely upregulating opiate intake.
Footshock also acutely reinstated preference for a chamber formerly paired with morphine (Wang et al., 2000) as well as operant responding on a lever that previously delivered heroin (Shaham et al., 1996) in rats. Reinstatement of self-administration was observed not only under standard drug-free test conditions but even when heroin levels were maintained throughout extinction and reinstatement via a minipump delivering constant infusions of heroin (Shaham et al., 1996). This finding suggests that acute stress-induced elevations in heroin-seeking are not driven solely by the desire to regain the pre-extinction pharmacological effects of heroin. However, that does not mean that reinstatement following footshock is independent of pre-extinction self-administration levels. Rats provided extended daily access to heroin self-administration during training, which results in escalation of heroin self-administration, showed significantly greater footshock-induced reinstatement than did rats that received brief daily access and whose heroin self-administration had not escalated (Ahmed et al., 2000). Together, the data indicate that acute footshock stress can precipitate relapse to heroin self-administration, and that the severity of the relapse may directly correspond to the amount of drug taken prior to abstinence.
3.4 Summary
Footshock has been used as a stressor in several rodent models of PTSD (Foa et al., 1992; Garrick et al., 1997; Maier, 2001; Pynoos et al., 1996; Wakizono et al., 2007) and thus provides a plausible model for the effects of adult traumatic experiences on drug self-administration. All phases of drug self-administration – acquisition, maintenance, and relapse – were acutely elevated in rats recently exposed to footshock stress, and these effects were observed across all drugs of abuse tested. However, most stress delivery paradigms addressed the acute, rather than protracted, consequences of footshock exposure, and thus these data fail to address PTSD-like delayed effects of traumatic stress. As existing data suggest that behavioral responses to and self-administration of drugs of abuse are elevated in the weeks following exposure to multiple psychological stressors (Table 1), additional research into the role of stress history, rather than stress delivery, on drug self-administration is warranted. Nonetheless, acute footshock-induced modulation of drug-taking presents a useful model for investigating pharmacologic tools for treatment of comorbid drug and anxiety disorders.
Table 1.
Selected protracted effects of adult psychological stressors on drug responsiveness and self-administration
| Stressor | Drug | Effect | Reference |
|---|---|---|---|
| Chronic variable stress | |||
| Psychostimulants | Enhanced acute amphetamine reduction in ICSS thresholds | (Lin et al., 2002) | |
| Elevated acute locomotor response to cocaine | (Haile et al., 2001) | ||
| Blocked low-dose amphetamine CPP | (Papp et al., 1991) | ||
| Opiates | Elevated locomotor response to low -dose morphine | (Molina et al., 1994) | |
| Attenuated morphine CPP | (Valverde et al., 1997) | ||
| Footshock | |||
| Alcohol | Alcohol-preferring rat lines HAD and P escalate alcohol drinking 1–3 weeks after shock cessation | (Vengeliene et al., 2003) | |
| Increased alcohol preference 24h after completion of shock exposure | (Volpicelli and Ulm, 1990) | ||
| Elevated alcohol intake beginning 1 week after shock exposure | (Casey, 1960) | ||
| Forced swim | |||
| Alcohol | Increased intake 3 weeks after cessation of stress | (Lowery et al., 2008) | |
| Psychostimulants | Enhanced cocaine CPP | (Kreibich et al., 2009) | |
| Restraint | |||
| Alcohol | Increased moderate drinkers’ intake and decreased high drinkers’ intake during first 5 days post-stress | (Rockman et al., 1986) | |
| Transiently elevated preferring P rats’ intake on the first day post-stress; prolonged elevation in non-preferring NP rats’ intake from the third day post-stress | (Chester et al., 2004) | ||
| Elevated alcohol intake over 3 weeks post-stress | (Nash and Maickel, 1985) | ||
| Psychostimulants | Increased acute locomotor response but not sensitization to amphetamine | (Deroche et al., 1992) | |
| Opiates | Elevated acute locomotor response but not sensitization to morphine | (Deroche et al., 1992) | |
| Tail pinch | |||
| Psychostimulants | Increased locomotor sensitization and acquisition of amphetamine self-administration | (Piazza et al., 1990) | |
| Tail shock | |||
| Psychostimulants | No change in locomotor response to amphetamine | (Will et al., 2002) | |
| Opiates | Elevated acute locomotor response to morphine | (Will et al., 2002) | |
| Increased morphine CPP | (Will et al., 1998) | ||
4. Effects of adult social psychological stress: social defeat
Anxiety-provoking social encounters can precipitate increased alcohol intake in both dependent (Miller et al., 1974) and nondependent (de Wit et al., 2003) human subjects, and social phobia is a significant predictor of future alcohol dependence (Kessler et al., 1997). One rodent model of social stress is the social defeat paradigm, in which a territorial resident rat or mouse confronts and dominates an intruder (Miczek et al., 2008). Aggressive social interactions, such as muggings, rape, and physical abuse, can trigger the development of PTSD in humans, suggesting reasonable face validity for the social defeat model. Thus, alterations in behavior, including modulation of drug intake, following one or more experiences of subordination provide insight into the persistent effects of socially stressful experiences as well as a mechanism for testing possible therapies.
4.1 Alcohol
In rodents, alcohol consumption is regulated by social hierarchies in group-housing situations, with submissive rats (Blanchard et al., 1987) and mice (Hilakivi-Clarke and Lister, 1992) consuming higher quantities of alcohol than their dominant cage mates. Thus, it would be predicted that the experience of defeat, which engenders subordination in the intruder rat, should elevate alcohol intake. However, as with footshock exposure (Section 3.1), the modulation of alcohol consumption by social defeat stress varies under different experimental paradigms. Studies by van Erp and colleagues demonstrated decreased home cage 10% alcohol intake on all days that rats experienced social defeat immediately preceding or coincident with alcohol access (van Erp and Miczek, 2001; van Erp et al., 2001), regardless of whether alcohol access was brief (15 min) or continuous. Similarly, under operant access conditions, rats self-administered less alcohol on defeat days when the defeat session immediately preceded the operant session, although intake levels recovered within 1–2 days of the final defeat exposure (Funk et al., 2005; van Erp and Miczek, 2001). The reduction in operant alcohol self-administration following social defeat abates as the temporal delay between defeat and self-administration sessions increases, with no significant change in 15% alcohol self-administration observed 4 or 24 hours after defeat exposure (van Erp and Miczek, 2001). However, on the day after the final defeat exposure, rats trained with a 4-hour interval between defeat and operant sessions showed a significant elevation in alcohol intake, suggesting persistent effects of a history of social stress (van Erp and Miczek, 2001). Milder, 5-minute defeat exposure paradigms have yielded different results, with modest elevation of home cage 6% alcohol consumption observed both the week of and the week after stress exposure when alcohol access was provided 2 hours after the defeat session (Caldwell and Riccio, 2010). Repeated, but not single, mild social defeat encounters did not alter home cage 8% alcohol intake on defeat days but rather yielded increased alcohol intake in the second week after defeat exposure (Croft et al., 2005). Together, these data suggest a temporal delay in the upregulation of alcohol intake following social stress exposure.
Relapse-like alcohol intake and reinstatement of alcohol-seeking behavior, similar to maintenance alcohol consumption, respond variably to social defeat stress. Repeated episodes of defeat exposure, delivered over a 2-week alcohol deprivation period, elevated alcohol drinking in rats upon renewed access (Funk et al., 2004). However, unlike footshock stress, which can trigger reinstatement of alcohol-seeking when delivered immediately prior to the operant session (Section 3.1), acute social defeat did not significantly alter rats’ activity at the previously alcohol-paired lever (Funk et al., 2005). Nonetheless, when rats were conditioned to associate social defeat with an odor cue, the presentation of the odor cue alone reinstated operant responding (Funk et al., 2005). Together, these data demonstrate that while social stress may acutely suppress both ongoing intake and relapse to alcohol self-administration, a history of social defeat stress and situational reminders of the stress may, over time, result in elevated alcohol intake.
4.2 Psychostimulants
Similar to other stressors applied in adulthood, social defeat enhances locomotor responses to amphetamine and cocaine (Covington et al., 2005; Covington and Miczek, 2001; Miczek et al., 1999; Nikulina et al., 1998; Yap and Miczek, 2007). Perhaps due to this heightened response, social defeat stress occurring 24 hours (Tidey and Miczek, 1997) or 1 week (Haney et al., 1995) prior increased the rate of acquisition of cocaine self-administration. Defeat immediately prior to operant sessions also increased cocaine self-administration (Miczek and Mutschler, 1996). With increased delays between social defeat exposure and cocaine self-administration training, effects of stress history on acquisition dissipated (Covington et al., 2005; Covington and Miczek, 2001); instead, elevated self-administration was observed during later 24-hour binge sessions (Covington et al., 2005; Covington and Miczek, 2001, 2005; Cruz et al., 2011). Certain subpopulations may be more susceptible to social defeat’s effects on cocaine self-administration. For example, rats that exhibit reduced locomotor activity in novel environments self-administer low levels of cocaine initially. Social defeat subsequently elevated their self-administration to the level of high novelty response rats, whose high baseline cocaine self-administration levels, in contrast, were insensitive to stress history (Kabbaj et al., 2001). Interestingly, acute defeat, administered immediately prior to lateral hypothalamic ICSS sessions, elevated response thresholds (Der-Avakian and Markou, 2010). This increase in thresholds suggests that social defeat impairs brain reward function. Together, these data demonstrate that social defeat stress heightens behavioral activation by psychostimulants and self-administration of psychostimulants, with effects dependent on the temporal contingency between the defeat and psychostimulant experiences. In addition, social stress may differentially escalate drug intake in individuals that would otherwise be less prone to psychostimulant dependence, possibly by altering the drugs’ reinforcing efficacy.
Little research has investigated the role of social defeat in the reinstatement of psychostimulant-seeking behaviors. One study investigated the effect of acute social defeat on reinstatement of CPP in the presence of a low dose of cocaine (Ribeiro Do Couto et al., 2009). The authors found greater reinstatement of preference for the previously cocaine-paired chamber in recently defeated mice than in controls. This effect may depend on both the defeat experience and the cocaine prime, as the lowest unit cocaine doses administered did not significantly reinstate cocaine CPP. Nonetheless, in light of the upregulation of psychostimulant self-administration following social stress, the results warrant further investigation into the effects of social stress on relapse to psychostimulant-seeking.
4.3 Opiates
Investigation of the impact of social stress on opiate-related behaviors has lagged behind that for alcohol and psychostimulants. Most studies thus far have focused on the rewarding effects of opiates as determined by conditioned preference for an opiate-paired environment. Exposure to defeat prior to place conditioning demonstrated an interaction between social defeat exposure and pre-existing social status in socially housed mice (Coventry et al., 1997). Under control conditions, subordinate mice failed to show preference for a morphine-paired environment, while dominant rats spent significantly more time in the drug-paired chamber. However, a history of defeat reversed these effects, with defeated subordinate mice displaying significant morphine CPP, while dominant mice rendered subordinate by their defeat experiences no longer expressed conditioned preference. In a separate study, social defeat reinstated extinguished morphine CPP in mice tested either immediately or 15 minutes after the defeat experience (Ribeiro Do Couto et al., 2006). Together, these studies suggest that acute social defeat stress may interact with the rewarding properties of morphine, and that perceived social status may modulate the effect of social stressors on opiate response. However, a recent study involving operant self-administration of heroin, with operant training beginning nearly 2 weeks after social defeat, showed no difference in either acquisition or binge-like 24-hour self-administration of heroin (Cruz et al., 2011). In light of the correlation between CPP and self-administration of opiates following footshock stress (Section 3.3), further investigation is required to determine whether, under different conditions, social stress may trigger elevated opiate self-administration.
4.4 Summary
Social stress, which can increase drug intake in humans, has been modeled in rodents as defeat in a resident-intruder paradigm. Similar to social stress effects in humans, both acute and repeated social defeat exposure enhanced ongoing drug-taking and relapse to drug-seeking across multiple drugs of abuse, although studies indicate a complex temporal relationship between exposure to the social stressor and elevation of drug intake, including temporary suppression of proximal drug-taking in the case of alcohol. An important line of future investigation should address the long-term impact of social stress history on drug self-administration, because existing data for alcohol suggest that protracted effects of defeat history may be more pronounced than the acute post-defeat effects that comprise the majority of existing data. Nonetheless, the comparability of social stress effects in rodents and humans affirms the use of social defeat as a paradigm for investigating putative pharmacotherapies developed to ameliorate the impact of social stress on drug consumption.
5. Pharmacological targets
Patients with comorbid PTSD and substance abuse often receive separate treatments for their disorders, rather than having the comorbid diagnosis addressed as a single illness with a common pathophysiology. Thus determining the efficacy of existing treatment options in models of stress/drug interactions may be useful for improving therapy options. In addition, exploring new options suggested by molecular targets with success in reducing stress-induced increases in drug self-administration in rodents has promise for providing better treatment options for individuals suffering from both PTSD and substance use disorders.
Neuropeptides that both positively and negatively regulate stress responses also modulate the negative emotional state generated by drug dependence (reviewed in Koob, 2008). One brain system that may underlie the interaction between stress and drug use is the extended amygdala, a neural circuit that includes the central nucleus of the amygdala, the bed nucleus of the stria terminalis and the shell of the nucleus accumbens (Alheid and Heimer, 1988). As the extended amygdala lies at the interface of the stress and drug reward systems, it is uniquely positioned to coordinate stress and drug responses. The amygdala has consistently been suggested as a brain region central to PTSD, as multiple fMRI studies have demonstrated elevated amygdala reactivity in PTSD patients (reviewed in Liberzon and Sripada, 2008). The extended amygdala is also rich in expression of several peptides, as well as their cognate receptors, involved in modulating stress responses. Anxiogenic neural signaling systems, including norepinephrine, corticotropin-releasing factor (CRF), dynorphin, vasopressin, and orexin (also known as hypocretin), increase stress-like behavior and relapse to drug-seeking in rodents, while neuropeptides that reduce anxiety-like behavior in rodents, such as serotonin, neuropeptide Y (NPY) and nociceptin, can inhibit stress-induced reinstatement. This section presents an overview of pharmacological data from rodent models of stress-induced drug seeking which suggests several new targets that deserve consideration as possible treatments for comorbid PTSD and substance use disorders.
5.1 Serotonin
The drugs most commonly prescribed to patients diagnosed with PTSD are the selective serotonin reuptake inhibitors (SSRIs), a class of drugs that block the serotonin transporter, thereby enhancing serotonergic signaling. A polymorphism in the serotonin transporter has been associated, in conjunction with trauma history, with elevated risk for developing PTSD (Xie et al., 2009) (but see also (Grabe et al., 2009)), supporting the focus on the serotonin system for PTSD medication. However, few investigations have explored the efficacy of SSRIs to reduce stress effects on drug self-administration in rodents. The existing data suggest some ability for SSRIs to reduce stress-induced drug intake. Paroxetine treatment significantly reduced both anxiety-like behavior and alcohol intake in adult rats with a history of extended maternal separation (Huot et al., 2001), with 20-day paroxetine treatment prior to testing indicating good predictive validity for the amelioration of protracted effects of human childhood trauma by SSRIs. Fluoxetine was shown to decrease both maintenance operant alcohol self-administration and footshock-induced reinstatement of alcohol-seeking (Le et al., 1999). This inhibition of footshock-induced reinstatement was later confirmed with dexfenfluramine, a serotonin releaser and reuptake inhibitor, as well as the serotonin 3 receptor antagonists ondansetron and tropisetron, all of which are hypothesized to increase overall activity of the serotonin system (Le et al., 2006). It should be noted that Le and colleagues administered SSRIs acutely prior to the reinstatement sessions, a dosing regimen unlike that required for efficacy in humans. Regardless, these data suggest that the existing first treatment of choice for PTSD may also reduce stress-heightened drug intake or relapse.
5.2 Norepinephrine
Norepinephrine (noradrenaline), a neurotransmitter that binds to adrenergic receptors (ARs) to modulate physiologic processes involved in attention and arousal, has been implicated as one possible mediator of symptoms associated with PTSD (reviewed in Southwick et al., 1999). Dysregulation of the system has been observed in human PTSD sufferers, who show elevated norepinephrine levels in cerebrospinal fluid (CSF) (Geracioti et al., 2001; Geracioti et al., 2008), indicating enhanced activity of the central adrenergic system. The system presents an attractive target for the interaction between PTSD and drug abuse, as it may regulate elevated drug intake in dependent individuals and stress-induced reinstatement. Antagonism of the α1-AR by prazosin reduced heroin (Greenwell et al., 2009), cocaine (Wee et al., 2008), and alcohol (Walker et al., 2008) self-administration in rat models of dependence-heightened drug taking. Prazosin also attenuated the hypohedonic-like effects of nicotine withdrawal as determined by elevated ICSS thresholds (Bruijnzeel et al., 2010). Similar to α1-AR antagonists, inhibition of the β-AR reduced nicotine withdrawal symptoms, although its effects centered on the somatic rather than the affective aspects of withdrawal (Bruijnzeel et al., 2010). The β-AR antagonist propranolol also reduced dependence-heightened alcohol self-administration (Gilpin and Koob, 2010) as well as stress-induced cocaine (Mantsch et al., 2010) and cue-induced nicotine (Chiamulera et al., 2010) reinstatement in rats. Unlike the α1- and β-ARs, the α2-AR functionally inhibits adrenergic system activity. Thus, systemic administration of α2-AR agonists reduced somatic withdrawal signs for multiple drugs of abuse (Bruijnzeel et al., 2010; Riihioja et al., 1997; Tierney et al., 1988). α2-AR agonists also prevented stress-induced reinstatement of alcohol (Le et al., 2005), cocaine (Erb et al., 2000), heroin (Shaham et al., 2000), and nicotine (Zislis et al., 2007) self-administration. Because α2-AR agonists block stress-induced reinstatement, blockade of α2-ARs was hypothesized to mimic the effects of stressors on drug self-administration. As predicted, the α2-AR antagonist yohimbine, an anxiogenic-like compound, has been shown to substitute for stress exposure in the reinstatement of alcohol- (Le et al., 2005), psychostimulant- (Feltenstein and See, 2006; Shepard et al., 2004), and opiate-seeking (Banna et al., 2010). These results suggest inhibition of the norepinephrine system, via antagonism of α1- or β-ARs or agonism of α2-ARs, as a potential therapeutic target in individuals suffering from substance abuse secondary to stress disorders.
5.3 CRF
Among the stress-generating neuropeptide systems, the greatest wealth of evidence exists for regulation of drug-seeking by CRF, the neuropeptide responsible for initiating the canonical neuroendocrine stress response as well as behavioral responses to stressors. Elevated CRF levels have been demonstrated in the CSF of PTSD patients (Bremner et al., 1997), supporting the proposal that altered central CRF levels may regulate the interaction between PTSD and drug abuse in humans. Involvement of extrahypothalamic CRF systems in stress-induced reinstatement of drug-seeking has been demonstrated for alcohol (Le et al., 2000), cocaine (Erb et al., 1998), heroin (Shaham et al., 1997), and nicotine (Zislis et al., 2007). Most data implicate the CRF1 receptor in CRF modulation of elevated self-administration and reinstatement triggered by footshock stress (Bruijnzeel et al., 2009; Gehlert et al., 2007; Shaham et al., 1998) or yohimbine injection (Marinelli et al., 2007). Recently a CRF1 antagonist has also been shown to inhibit social defeat stress-induced locomotor sensitization to and escalated “binge” operant self-administration of cocaine (Boyson et al., 2011). As CRF1 antagonists also reduce drug-taking in substance-dependent animals (Koob, 2010), they present attractive targets for continued drug development to combat drug addiction and its interaction with stress.
5.4 KOR/dynorphin, vasopressin and orexin
Like CRF, the kappa opioid receptor (KOR) and its endogenous ligand, dynorphin, as well as vasopressin and orexin have all been shown to modulate stress-induced reinstatement. Multiple studies have demonstrated KOR antagonist inhibition of both footshock- and swim stress-induced reinstatement of cocaine- (Beardsley et al., 2005; Carey et al., 2007; Redila and Chavkin, 2008) and alcohol-seeking (Sperling et al., 2010). Fewer data exist for vasopressin and orexin, although human PTSD data showing upregulation of plasma vasopressin (de Kloet et al., 2008) and downregulation of plasma and CSF orexin-A (Strawn et al., 2010) support a role for these peptides in the neuroadaptations underlying the disorder. Studies in rodents suggest similar effects for inhibition of vasopressin and orexin as observed for norepinephrine, CRF and dynorphin. Antagonism of the vasopressin V1b receptor blocked stress-induced reinstatement of heroin-seeking (Zhou et al., 2008) and withdrawal-induced elevated alcohol self-administration in dependent rats (Edwards et al., 2008). Blockade of the orexin-A receptor inhibited yohimbine-induced reinstatement of alcohol-seeking (Richards et al., 2008), as well as stress-induced reinstatement of cocaine self-administration (Boutrel et al., 2005). Together, these data suggest that dynorphin, vasopressin and orexin, like CRF and norepinephrine, potentiate stress-induced reinstatement of drug-seeking. However, the efficacy of these systems in modulating protracted stress effects on drug self-administration remains to be determined.
5.5 Anxiolytic neuropeptide systems: NPY and nociceptin
Most extant data addressing the role of anxiolytic neuropeptide systems in regulating drug intake has focused on alcohol. Activation of the NPY system decreased alcohol intake in female rats following a deprivation period (Bertholomey et al., 2011; Gilpin et al., 2008; Gilpin et al., 2003), as well as blocking dependence-induced escalation of alcohol self-administration in male rats (Gilpin et al., 2011). NPY also reduced stress-induced reinstatement of alcohol-seeking in male rats (Cippitelli et al., 2010). Further research is required to determine NPY efficacy following a history of, rather than acute exposure to, stress. Data from human PTSD patients, who show reduced CSF NPY levels (Sah et al., 2009), support the proposed upregulation of NPY signaling as a means of treating PTSD and comorbid substance abuse. However, determination of the applicability of these findings to other drugs of abuse is vital, as NPY itself has been shown to elevate cocaine self-administration (Maric et al., 2009) and to reinstate heroin-seeking (Maric et al., 2008).
Like NPY, the bulk of nociceptin studies have focused on alcohol. Activation of the nociceptin receptor (NOP) blocked drug-induced reinstatement of alcohol CPP (Kuzmin et al., 2003) and stress- (Martin-Fardon et al., 2000) and cue-induced (Ciccocioppo et al., 2004) reinstatement of alcohol self-administration. Additionally, deprivation-induced elevated alcohol self-administration was inhibited by NOP agonism and prolonged by NOP antagonism (Kuzmin et al., 2007), suggesting a role for nociceptin/NOP in dampening alcohol intake, although these effects may not be stress-specific. Applicability to other drugs of abuse is debatable, as NOP agonist treatment inhibited drug-primed reinstatement of morphine CPP (Shoblock et al., 2005), but was ineffective in blocking stress-induced reinstatement of cocaine-seeking (Martin-Fardon et al., 2000). Thus although potential promising targets for future treatments for alcohol use disorders, more investigation is required into the generality of therapeutic-like efficacy of activating anxiolytic neuropeptide systems.
5.6 Summary
Given the high prevalence of comorbidity between anxiety disorders and substance abuse (Grant et al., 2005) and the reduced efficacy of existing treatments in these comorbid patients as compared to those suffering only from substance use disorders (Brown et al., 1995; Brown and Wolfe, 1994), priority should be given to developing new therapies for the comorbid disorders. Systems involved in regulating stress responses show promise for medication development to treat drug problems in PTSD patients.
6. Conclusions
Comorbid stress and substance use disorders, often observed in patients with PTSD, present a major health care burden without sufficient effective treatment strategies. Existing rodent models demonstrate that a variety of stressors elevate the acute response to, intake of, and relapse to drugs of abuse in the wake of stress exposure, although additional efforts should be directed towards determining the protracted effects of traumatic stress on drug self-administration. As prototypic models of early life trauma and adult stressors, maternal separation, footshock, and social defeat may provide key tools for the development of further models and therapies to treat co-occurring PTSD and addiction.
HIGHLIGHTS.
We review literature on traumatic stress effects on rodent drug self-administration.
Early life stress and social and nonsocial stress in adulthood impact drug use.
Stress exposure acutely elevates all phases of drug self-administration.
Traumatic stress may also have long-term impact on drug taking and relapse.
We discuss several therapeutic targets for post-traumatic and drug use disorders.
Acknowledgments
The authors would like to thank Michael Arends for editorial assistance in preparation of this manuscript. Financial support was received from the Pearson Center for Alcoholism and Addiction Research and National Institutes of Health grant DK26741 from the National Institute of Diabetes and Digestive and Kidney Diseases, AA06420, AA08459, and AA018914 from the National Institute on Alcohol Abuse and Alcoholism, and DA04043, DA04398, and DA023957 from the National Institute on Drug Abuse. This is publication number 21009 from The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Koob GF. Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology (Berl) 1997;132:289–295. doi: 10.1007/s002130050347. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Banna KM, Back SE, Do P, See RE. Yohimbine stress potentiates conditioned cue-induced reinstatement of heroin-seeking in rats. Behav Brain Res. 2010;208:144–148. doi: 10.1016/j.bbr.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Bertholomey ML, Henderson AN, Badia-Elder NE, Stewart RB. Neuropeptide Y (NPY)-induced reductions in alcohol intake during continuous access and following alcohol deprivation are not altered by restraint stress in alcohol-preferring (P) rats. Pharmacol Biochem Behav. 2011;97:453–461. doi: 10.1016/j.pbb.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Hori K, Tom P, Blanchard DC. Social structure and ethanol consumption in the laboratory rat. Pharmacol Biochem Behav. 1987;28:437–442. doi: 10.1016/0091-3057(87)90502-8. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyson CO, Miguel TT, Quadros IM, Debold JF, Miczek KA. Prevention of social stress-escalated cocaine self-administration by CRF-R1 antagonist in the rat VTA. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci. 2004;19:1863–1874. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Schultz LR. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Arch Gen Psychiatry. 2003;60:289–294. doi: 10.1001/archpsyc.60.3.289. [DOI] [PubMed] [Google Scholar]
- Brown PJ, Recupero PR, Stout R. PTSD substance abuse comorbidity and treatment utilization. Addict Behav. 1995;20:251–254. doi: 10.1016/0306-4603(94)00060-3. [DOI] [PubMed] [Google Scholar]
- Brown PJ, Wolfe J. Substance abuse and post-traumatic stress disorder comorbidity. Drug Alcohol Depend. 1994;35:51–59. doi: 10.1016/0376-8716(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Brown ZJ, Erb S. Footshock stress reinstates cocaine seeking in rats after extended post-stress delays. Psychopharmacology (Berl) 2007;195:61–70. doi: 10.1007/s00213-007-0846-4. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Bishnoi M, van Tuijl IA, Keijzers KF, Yavarovich KR, Pasek TM, Ford J, Alexander JC, Yamada H. Effects of prazosin, clonidine, and propranolol on the elevations in brain reward thresholds and somatic signs associated with nicotine withdrawal in rats. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-010-1970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Prado M, Isaac S. Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biol Psychiatry. 2009;66:110–117. doi: 10.1016/j.biopsych.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, See RE. Footshock stress potentiates cue-induced cocaine-seeking in an animal model of relapse. Physiol Behav. 2009;98:614–617. doi: 10.1016/j.physbeh.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Caldwell EE, Riccio DC. Alcohol self-administration in rats: Modulation by temporal parameters related to repeated mild social defeat stress. Alcohol. 2010;44:265–274. doi: 10.1016/j.alcohol.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Campbell J, Spear LP. Effects of early handling on amphetamine-induced locomotor activation and conditioned place preference in the adult rat. Psychopharmacology (Berl) 1999;143:183–189. doi: 10.1007/s002130050934. [DOI] [PubMed] [Google Scholar]
- Caplan MA, Puglisi K. Stress and conflict conditions leading to and maintaining voluntary alcohol consumption in rats. Pharmacol Biochem Behav. 1986;24:271–280. doi: 10.1016/0091-3057(86)90350-3. [DOI] [PubMed] [Google Scholar]
- Carey AN, Borozny K, Aldrich JV, McLaughlin JP. Reinstatement of cocaine place-conditioning prevented by the peptide kappa-opioid receptor antagonist arodyn. Eur J Pharmacol. 2007;569:84–89. doi: 10.1016/j.ejphar.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey A. The effect of stress on the consumption of alcohol and reserpine. Q J Stud Alcohol. 1960;21:208–216. [PubMed] [Google Scholar]
- Chester JA, Barrenha GD, Hughes ML, Keuneke KJ. Age- and sex-dependent effects of footshock stress on subsequent alcohol drinking and acoustic startle behavior in mice selectively bred for high-alcohol preference. Alcohol Clin Exp Res. 2008;32:1782–1794. doi: 10.1111/j.1530-0277.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Zweifel M, Froehlich JC. Effects of stress on alcohol consumption in rats selectively bred for high or low alcohol drinking. Alcohol Clin Exp Res. 2004;28:385–393. doi: 10.1097/01.alc.0000117830.54371.7a. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Tedesco V, Zangrandi L, Giuliano C, Fumagalli G. Propranolol transiently inhibits reinstatement of nicotine-seeking behaviour in rats. J Psychopharmacol. 2010;24:389–395. doi: 10.1177/0269881108097718. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Fedeli A, Angeletti S, Weiss F, Heilig M, Massi M. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology (Berl) 2004;172:170–178. doi: 10.1007/s00213-003-1645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Myers RD, Black WC. Increase in volitional ethanol consumption following interference with a learned avoidance response. Physiol Behav. 1968;3:657–660. [Google Scholar]
- Cippitelli A, Damadzic R, Hansson AC, Singley E, Sommer WH, Eskay R, Thorsell A, Heilig M. Neuropeptide Y (NPY) suppresses yohimbine-induced reinstatement of alcohol seeking. Psychopharmacology (Berl) 2010;208:417–426. doi: 10.1007/s00213-009-1741-y. [DOI] [PubMed] [Google Scholar]
- Coventry TL, D’Aquila PS, Brain P, Willner P. Social influences on morphine conditioned place preference. Behav Pharmacol. 1997;8:575–584. doi: 10.1097/00008877-199711000-00015. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Jr, Miczek KA. Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30:310–321. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology (Berl) 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology (Berl) 2005;183:331–340. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Croft AP, Brooks SP, Cole J, Little HJ. Social defeat increases alcohol preference of C57BL/10 strain mice; effect prevented by a CCKB antagonist. Psychopharmacology (Berl) 2005;183:163–170. doi: 10.1007/s00213-005-0165-6. [DOI] [PubMed] [Google Scholar]
- Cruz FC, Quadros IM, Hogenelst K, Planeta CS, Miczek KA. Social defeat stress in rats: escalation of cocaine and “speedball” binge self-administration, but not heroin. Psychopharmacology (Berl) 2011;215:165–175. doi: 10.1007/s00213-010-2139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Quadros IM, Planeta Cda S, Miczek KA. Maternal separation stress in male mice: long-term increases in alcohol intake. Psychopharmacology (Berl) 2008;201:459–468. doi: 10.1007/s00213-008-1307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Martin-Fardon R, Thorsell A, Weiss F. Chronic footshock, but not a physiological stressor, suppresses the alcohol deprivation effect in dependent rats. Alcohol Alcohol. 2004;39:190–196. doi: 10.1093/alcalc/agh046. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Wiegant VM, Westenberg HG. Elevated plasma arginine vasopressin levels in veterans with posttraumatic stress disorder. J Psychiatr Res. 2008;42:192–198. doi: 10.1016/j.jpsychires.2006.11.009. [DOI] [PubMed] [Google Scholar]
- de Wit H, Soderpalm AH, Nikolayev L, Young E. Effects of acute social stress on alcohol consumption in healthy subjects. Alcohol Clin Exp Res. 2003;27:1270–1277. doi: 10.1097/01.ALC.0000081617.37539.D6. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. Neonatal maternal separation exacerbates the reward-enhancing effect of acute amphetamine administration and the anhedonic effect of repeated social defeat in adult rats. Neuroscience. 2010;170:1189–1198. doi: 10.1016/j.neuroscience.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Casolini P, Maccari S, Le Moal M, Simon H. Stress-induced sensitization to amphetamine and morphine psychomotor effects depend on stress-induced corticosterone secretion. Brain Res. 1992;598:343–348. doi: 10.1016/0006-8993(92)90205-n. [DOI] [PubMed] [Google Scholar]
- Dewart T, Frank B, Schmeidler J. The impact of 9/11 on patients in New York City’s substance abuse treatment programs. Am J Drug Alcohol Abuse. 2006;32:665–672. doi: 10.1080/00952990600919435. [DOI] [PubMed] [Google Scholar]
- Driessen M, Schulte S, Luedecke C, Schaefer I, Sutmann F, Ohlmeier M, Kemper U, Koesters G, Chodzinski C, Schneider U, Broese T, Dette C, Havemann-Reinicke U. Trauma and PTSD in patients with alcohol, drug, or dual dependence: a multi-center study. Alcohol Clin Exp Res. 2008;32:481–488. doi: 10.1111/j.1530-0277.2007.00591.x. [DOI] [PubMed] [Google Scholar]
- Edwards S, Guerrero M, Ghoneim O, Roberts E, Koob GF. V1b vasopressin receptor antagonism reduces ethanol self-administration in ethanol-dependent rats. Soc Neurosci Abstr 2008 [Google Scholar]
- Enoch MA, Hodgkinson CA, Yuan Q, Shen PH, Goldman D, Roy A. The influence of GABRA2, childhood trauma, and their interaction on alcohol, heroin, and cocaine dependence. Biol Psychiatry. 2010;67:20–27. doi: 10.1016/j.biopsych.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology (Berl) 1996;128:408–412. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Fidler TL, LoLordo VM. Failure to find postshock increases in ethanol preference. Alcohol Clin Exp Res. 1996;20:110–121. doi: 10.1111/j.1530-0277.1996.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Foa EB, Zinbarg R, Rothbaum BO. Uncontrollability and unpredictability in post-traumatic stress disorder: an animal model. Psychol Bull. 1992;112:218–238. doi: 10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- Funk D, Harding S, Juzytsch W, Le AD. Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2005;183:341–349. doi: 10.1007/s00213-005-0194-1. [DOI] [PubMed] [Google Scholar]
- Funk D, Vohra S, Le AD. Influence of stressors on the rewarding effects of alcohol in Wistar rats: studies with alcohol deprivation and place conditioning. Psychopharmacology (Berl) 2004;176:82–87. doi: 10.1007/s00213-004-1859-x. [DOI] [PubMed] [Google Scholar]
- Garrick T, Morrow N, Eth S, Marciano D, Shalev A. Psychophysiologic parameters of traumatic stress disorder in rats. Ann N Y Acad Sci. 1997;821:533–537. doi: 10.1111/j.1749-6632.1997.tb48323.x. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Ciccocioppo R, Heilig M. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl- imidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geracioti TD, Jr, Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, Schmidt D, Rounds-Kugler B, Yehuda R, Keck PE, Jr, Kasckow JW. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158:1227–1230. doi: 10.1176/appi.ajp.158.8.1227. [DOI] [PubMed] [Google Scholar]
- Geracioti TD, Jr, Baker DG, Kasckow JW, Strawn JR, Jeffrey Mulchahey J, Dashevsky BA, Horn PS, Ekhator NN. Effects of trauma-related audiovisual stimulation on cerebrospinal fluid norepinephrine and corticotropin-releasing hormone concentrations in post-traumatic stress disorder. Psychoneuroendocrinology. 2008;33:416–424. doi: 10.1016/j.psyneuen.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Koob GF. Effects of beta-adrenoceptor antagonists on alcohol drinking by alcohol-dependent rats. Psychopharmacology (Berl) 2010;212:431–439. doi: 10.1007/s00213-010-1967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Herman MA, Cruz MT, Koob GF, Roberto M. Neuropeptide y opposes alcohol effects on gamma-aminobutyric Acid release in amygdala and blocks the transition to alcohol dependence. Biol Psychiatry. 2011;69:1091–1099. doi: 10.1016/j.biopsych.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Badia-Elder NE. Neuropeptide Y suppresses ethanol drinking in ethanol-abstinent, but not non-ethanol-abstinent, Wistar rats. Alcohol. 2008;42:541–551. doi: 10.1016/j.alcohol.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, Li TK, Badia-Elder NE. Neuropeptide Y reduces oral ethanol intake in alcohol-preferring (P) rats following a period of imposed ethanol abstinence. Alcohol Clin Exp Res. 2003;27:787–794. doi: 10.1097/01.ALC.0000065723.93234.1D. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Non-contingent electric footshock facilitates the acquisition of intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1994;114:63–70. doi: 10.1007/BF02245445. [DOI] [PubMed] [Google Scholar]
- Grabe HJ, Spitzer C, Schwahn C, Marcinek A, Frahnow A, Barnow S, Lucht M, Freyberger HJ, John U, Wallaschofski H, Volzke H, Rosskopf D. Serotonin transporter gene (SLC6A4) promoter polymorphisms and the susceptibility to posttraumatic stress disorder in the general population. Am J Psychiatry. 2009;166:926–933. doi: 10.1176/appi.ajp.2009.08101542. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Stinson FS, Dawson DA, Patricia Chou S, June Ruan W, Huang B. Co-occurrence of 12-month mood and anxiety disorders and personality disorders in the US: results from the national epidemiologic survey on alcohol and related conditions. J Psychiatr Res. 2005;39:1–9. doi: 10.1016/j.jpsychires.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Greenwell TN, Walker BM, Cottone P, Zorrilla EP, Koob GF. The alpha1 adrenergic receptor antagonist prazosin reduces heroin self-administration in rats with extended access to heroin administration. Pharmacol Biochem Behav. 2009;91:295–302. doi: 10.1016/j.pbb.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile CN, GrandPre T, Kosten TA. Chronic unpredictable stress, but not chronic predictable stress, enhances the sensitivity to the behavioral effects of cocaine in rats. Psychopharmacology (Berl) 2001;154:213–220. doi: 10.1007/s002130000650. [DOI] [PubMed] [Google Scholar]
- Hall W, Teesson M, Lynskey M, Degenhardt L. The 12-month prevalence of substance use and ICD-10 substance use disorders in Australian adults: findings from the National Survey of Mental Health and Well-Being. Addiction. 1999;94:1541–1550. [PubMed] [Google Scholar]
- Haney M, Maccari S, Le Moal M, Simon H, Piazza PV. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res. 1995;698:46–52. doi: 10.1016/0006-8993(95)00788-r. [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke L, Lister RG. Social status and voluntary alcohol consumption in mice: interaction with stress. Psychopharmacology (Berl) 1992;108:276–282. doi: 10.1007/BF02245112. [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke LA, Turkka J, Lister RG, Linnoila M. Effects of early postnatal handling on brain beta-adrenoceptors and behavior in tests related to stress. Brain Res. 1991;542:286–292. doi: 10.1016/0006-8993(91)91580-t. [DOI] [PubMed] [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 2001;158:366–373. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Paliwal P, Chaplin TM, Mazure CM, Rounsaville BJ, Sinha R. Severity of childhood trauma is predictive of cocaine relapse outcomes in women but not men. Drug Alcohol Depend. 2008;92:208–216. doi: 10.1016/j.drugalcdep.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Southwick SM, Kosten TR. Substance use disorders in patients with posttraumatic stress disorder: a review of the literature. Am J Psychiatry. 2001;158:1184–1190. doi: 10.1176/appi.ajp.158.8.1184. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Francis DD, Brommer CL, Morgan ET, Kuhar MJ. Effects of early maternal separation on ethanol intake, GABA receptors and metabolizing enzymes in adult rats. Psychopharmacology (Berl) 2005;181:8–15. doi: 10.1007/s00213-005-2232-4. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Norton CS, Kollack-Walker S, Watson SJ, Robinson TE, Akil H. Social defeat alters the acquisition of cocaine self-administration in rats: role of individual differences in cocaine-taking behavior. Psychopharmacology (Berl) 2001;158:382–387. doi: 10.1007/s002130100918. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Easterling KW, Holtzman SG. Early neonatal experience of Long-Evans rats results in long-lasting changes in morphine tolerance and dependence. Psychopharmacology (Berl) 2001;157:305–312. doi: 10.1007/s002130100806. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Easterling KW, Holtzman SG. Early neonatal experience of Long-Evans rats results in long-lasting changes in reactivity to a novel environment and morphine-induced sensitization and tolerance. Neuropsychopharmacology. 2002a;27:518–533. doi: 10.1016/S0893-133X(02)00326-3. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol Biochem Behav. 2002b;73:131–140. doi: 10.1016/s0091-3057(02)00781-5. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Faccidomo S, Miczek KA. Repeated maternal separation: differences in cocaine-induced behavioral sensitization in adult male and female mice. Psychopharmacology (Berl) 2005;178:202–210. doi: 10.1007/s00213-004-1989-1. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O’Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flugge G, Korte SM, Meerlo P, Murison R, Olivier B, Palanza P, Richter-Levin G, Sgoifo A, Steimer T, Stiedl O, van Dijk G, Wohr M, Fuchs E. Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev. 2011;35:1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Kreibich AS, Briand L, Cleck JN, Ecke L, Rice KC, Blendy JA. Stress-induced potentiation of cocaine reward: a role for CRF R1 and CREB. Neuropsychopharmacology. 2009;34:2609–2617. doi: 10.1038/npp.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin A, Kreek MJ, Bakalkin G, Liljequist S. The nociceptin/orphanin FQ receptor agonist Ro 64–6198 reduces alcohol self-administration and prevents relapse-like alcohol drinking. Neuropsychopharmacology. 2007;32:902–910. doi: 10.1038/sj.npp.1301169. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Sandin J, Terenius L, Ogren SO. Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of opioid receptor-like 1 receptor agonists and naloxone. J Pharmacol Exp Ther. 2003;304:310–318. doi: 10.1124/jpet.102.041350. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Semenova S, Zvartau EE, Van Ree JM. Enhancement of morphine self-administration in drug naive, inbred strains of mice by acute emotional stress. Eur Neuropsychopharmacol. 1996;6:63–68. doi: 10.1016/0924-977x(95)00066-x. [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Harding S, Juzytsch W, Fletcher PJ, Shaham Y. Effects of dexfenfluramine and 5-HT3 receptor antagonists on stress-induced reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2006;186:82–92. doi: 10.1007/s00213-006-0346-y. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Fletcher PJ, Shaham Y. The role of corticotropin-releasing factor in the median raphe nucleus in relapse to alcohol. J Neurosci. 2002;22:7844–7849. doi: 10.1523/JNEUROSCI.22-18-07844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Harding S, Watchus J, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- Le AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology (Berl) 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Rev Neurosci. 2000;11:383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Lin D, Bruijnzeel AW, Schmidt P, Markou A. Exposure to chronic mild stress alters thresholds for lateral hypothalamic stimulation reward and subsequent responsiveness to amphetamine. Neuroscience. 2002;114:925–933. doi: 10.1016/s0306-4522(02)00366-4. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F. Stimulus conditioned to foot-shock stress reinstates alcohol-seeking behavior in an animal model of relapse. Psychopharmacology (Berl) 2003;168:184–191. doi: 10.1007/s00213-002-1267-z. [DOI] [PubMed] [Google Scholar]
- Lowery EG, Sparrow AM, Breese GR, Knapp DJ, Thiele TE. The CRF-1 receptor antagonist, CP-154,526, attenuates stress-induced increases in ethanol consumption by BALB/cJ mice. Alcohol Clin Exp Res. 2008;32:240–248. doi: 10.1111/j.1530-0277.2007.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Mangini LD, Taylor JR. Neonatal isolation stress potentiates cocaine seeking behavior in adult male and female rats. Neuropsychopharmacology. 2005;30:322–329. doi: 10.1038/sj.npp.1300594. [DOI] [PubMed] [Google Scholar]
- MacLennan AJ, Maier SF. Coping and the stress-induced potentiation of stimulant stereotypy in the rat. Science. 1983;219:1091–1093. doi: 10.1126/science.6681679. [DOI] [PubMed] [Google Scholar]
- Maier SF. Exposure to the stressor environment prevents the temporal dissipation of behavioral depression/learned helplessness. Biol Psychiatry. 2001;49:763–773. doi: 10.1016/s0006-3223(00)01095-7. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Katz ES. Elevation of glucocorticoids is necessary but not sufficient for the escalation of cocaine self-administration by chronic electric footshock stress in rats. Neuropsychopharmacology. 2007;32:367–376. doi: 10.1038/sj.npp.1301077. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H. Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for beta-2 adrenergic receptors. Neuropsychopharmacology. 2010;35:2165–2178. doi: 10.1038/npp.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maric T, Cantor A, Cuccioletta H, Tobin S, Shalev U. Neuropeptide Y augments cocaine self-administration and cocaine-induced hyperlocomotion in rats. Peptides. 2009;30:721–726. doi: 10.1016/j.peptides.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Maric T, Tobin S, Quinn T, Shalev U. Food deprivation-like effects of neuropeptide Y on heroin self-administration and reinstatement of heroin seeking in rats. Behav Brain Res. 2008;194:39–43. doi: 10.1016/j.bbr.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Le AD. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2007;195:345–355. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Ciccocioppo R, Massi M, Weiss F. Nociceptin prevents stress-induced ethanol- but not cocaine-seeking behavior in rats. Neuroreport. 2000;11:1939–1943. doi: 10.1097/00001756-200006260-00026. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Morrow AL, O’Buckley T, Flanigan TJ, Berry RB, Cook MN, Mittleman G, Goldowitz D, Tokunaga S, Silvers JM. Acute mild footshock alters ethanol drinking and plasma corticosterone levels in C57BL/6J male mice, but not DBA/2J or A/J male mice. Alcohol. 2008;42:469–476. doi: 10.1016/j.alcohol.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K, Hall FS, Wilkinson LS, Robbins TW. Retarded acquisition and reduced expression of conditioned locomotor activity in adult rats following repeated early maternal separation: effects of prefeeding, d-amphetamine, dopamine antagonists and clonidine. Psychopharmacology (Berl) 1996;126:75–84. doi: 10.1007/BF02246414. [DOI] [PubMed] [Google Scholar]
- Matthews K, Robbins TW. Early experience as a determinant of adult behavioural responses to reward: the effects of repeated maternal separation in the rat. Neurosci Biobehav Rev. 2003;27:45–55. doi: 10.1016/s0149-7634(03)00008-3. [DOI] [PubMed] [Google Scholar]
- Matthews K, Robbins TW, Everitt BJ, Caine SB. Repeated neonatal maternal separation alters intravenous cocaine self-administration in adult rats. Psychopharmacology (Berl) 1999;141:123–134. doi: 10.1007/s002130050816. [DOI] [PubMed] [Google Scholar]
- Michaels CC, Holtzman SG. Early postnatal stress alters place conditioning to both mu- and kappa-opioid agonists. J Pharmacol Exp Ther. 2008;325:313–318. doi: 10.1124/jpet.107.129908. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Mutschler NH. Activational effects of social stress on IV cocaine self-administration in rats. Psychopharmacology (Berl) 1996;128:256–264. doi: 10.1007/s002130050133. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Nikulina E, Kream RM, Carter G, Espejo EF. Behavioral sensitization to cocaine after a brief social defeat stress: c-fos expression in the PAG. Psychopharmacology (Berl) 1999;141:225–234. doi: 10.1007/s002130050829. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE., 3rd Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PM, Hersen M, Eisler RM, Hilsman G. Effects of social stress on operant drinking of alcoholics and social drinkers. Behav Res Ther. 1974;12:67–72. doi: 10.1016/0005-7967(74)90094-1. [DOI] [PubMed] [Google Scholar]
- Mills KC, Bean JW, Hutcheson JS. Shock induced ethanol consumption in rats. Pharmacol Biochem Behav. 1977;6:107–115. doi: 10.1016/0091-3057(77)90166-6. [DOI] [PubMed] [Google Scholar]
- Mills KL, Teesson M, Ross J, Peters L. Trauma, PTSD, and substance use disorders: findings from the Australian National Survey of Mental Health and Well-Being. Am J Psychiatry. 2006;163:652–658. doi: 10.1176/ajp.2006.163.4.652. [DOI] [PubMed] [Google Scholar]
- Moffett MC, Goeders NE. Neither non-contingent electric footshock nor administered corticosterone facilitate the acquisition of methamphetamine self-administration. Pharmacol Biochem Behav. 2005;80:333–339. doi: 10.1016/j.pbb.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Moffett MC, Harley J, Francis D, Sanghani SP, Davis WI, Kuhar MJ. Maternal separation and handling affects cocaine self-administration in both the treated pups as adults and the dams. J Pharmacol Exp Ther. 2006;317:1210–1218. doi: 10.1124/jpet.106.101139. [DOI] [PubMed] [Google Scholar]
- Molina VA, Heyser CJ, Spear LP. Chronic variable stress enhances the stimulatory action of a low dose of morphine: reversal by desipramine. Eur J Pharmacol. 1994;260:57–64. doi: 10.1016/0014-2999(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Myers RD, Holman RB. Failure of stress of electric shock to increase ethanol intake in rats. Q J Stud Alcohol. 1967;28:132–137. [PubMed] [Google Scholar]
- Nash JF, Jr, Maickel RP. Stress-induced consumption of ethanol by rats. Life Sci. 1985;37:757–765. doi: 10.1016/0024-3205(85)90546-6. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Marchand JE, Kream RM, Miczek KA. Behavioral sensitization to cocaine after a brief social stress is accompanied by changes in fos expression in the murine brainstem. Brain Res. 1998;810:200–210. doi: 10.1016/s0006-8993(98)00925-1. [DOI] [PubMed] [Google Scholar]
- Opsahl CA, Hatton GI. Volitional ethanol increases during acquisition and extinction of avoidance responding. Physiol Behav. 1972;8:87–93. doi: 10.1016/0031-9384(72)90133-3. [DOI] [PubMed] [Google Scholar]
- Papp M, Willner P, Muscat R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl) 1991;104:255–259. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- Perkonigg A, Kessler RC, Storz S, Wittchen HU. Traumatic events and post-traumatic stress disorder in the community: prevalence, risk factors and comorbidity. Acta Psychiatr Scand. 2000;101:46–59. doi: 10.1034/j.1600-0447.2000.101001046.x. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, le Moal M, Simon H. Stress- and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Res. 1990;514:22–26. doi: 10.1016/0006-8993(90)90431-a. [DOI] [PubMed] [Google Scholar]
- Ploj K, Roman E, Nylander I. Long-term effects of maternal separation on ethanol intake and brain opioid and dopamine receptors in male Wistar rats. Neuroscience. 2003;121:787–799. doi: 10.1016/s0306-4522(03)00499-8. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Pynoos RS, Ritzmann RF, Steinberg AM, Goenjian A, Prisecaru I. A behavioral animal model of posttraumatic stress disorder featuring repeated exposure to situational reminders. Biol Psychiatry. 1996;39:129–134. doi: 10.1016/0006-3223(95)00088-7. [DOI] [PubMed] [Google Scholar]
- Racz I, Bilkei-Gorzo A, Toth ZE, Michel K, Palkovits M, Zimmer A. A critical role for the cannabinoid CB1 receptors in alcohol dependence and stress-stimulated ethanol drinking. J Neurosci. 2003;23:2453–2458. doi: 10.1523/JNEUROSCI.23-06-02453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey NF, Van Ree JM. Emotional but not physical stress enhances intravenous cocaine self-administration in drug-naive rats. Brain Res. 1993;608:216–222. doi: 10.1016/0006-8993(93)91461-z. [DOI] [PubMed] [Google Scholar]
- Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 2005;29:1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Redila VA, Chavkin C. Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology (Berl) 2008;200:59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Do Couto B, Aguilar MA, Lluch J, Rodriguez-Arias M, Minarro J. Social experiences affect reinstatement of cocaine-induced place preference in mice. Psychopharmacology (Berl) 2009;207:485–498. doi: 10.1007/s00213-009-1678-1. [DOI] [PubMed] [Google Scholar]
- Ribeiro Do Couto B, Aguilar MA, Manzanedo C, Rodriguez-Arias M, Armario A, Minarro J. Social stress is as effective as physical stress in reinstating morphine-induced place preference in mice. Psychopharmacology (Berl) 2006;185:459–470. doi: 10.1007/s00213-006-0345-z. [DOI] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riihioja P, Jaatinen P, Oksanen H, Haapalinna A, Heinonen E, Hervonen A. Dexmedetomidine alleviates ethanol withdrawal symptoms in the rat. Alcohol. 1997;14:537–544. doi: 10.1016/s0741-8329(97)00044-x. [DOI] [PubMed] [Google Scholar]
- Rockman GE, Hall A, Glavin GB. Effects of restraint stress on voluntary ethanol intake and ulcer proliferation in rats. Pharmacol Biochem Behav. 1986;25:1083–1087. doi: 10.1016/0091-3057(86)90089-4. [DOI] [PubMed] [Google Scholar]
- Roman E, Gustafsson L, Hyytia P, Nylander I. Short and prolonged periods of maternal separation and voluntary ethanol intake in male and female ethanol-preferring AA and ethanol-avoiding ANA rats. Alcohol Clin Exp Res. 2005;29:591–601. doi: 10.1097/01.alc.0000158933.70242.fc. [DOI] [PubMed] [Google Scholar]
- Roman E, Hyytia P, Nylander I. Maternal separation alters acquisition of ethanol intake in male ethanol-preferring AA rats. Alcohol Clin Exp Res. 2003;27:31–37. doi: 10.1097/01.ALC.0000047352.88145.80. [DOI] [PubMed] [Google Scholar]
- Roman E, Ploj K, Nylander I. Maternal separation has no effect on voluntary ethanol intake in female Wistar rats. Alcohol. 2004;33:31–39. doi: 10.1016/j.alcohol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Mueller A, Sisti HM, Ogawa S, McEwen BS, Brake WG. Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Horm Behav. 2003;43:561–567. doi: 10.1016/s0018-506x(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Ross HE. DSM-III-R alcohol abuse and dependence and psychiatric comorbidity in Ontario: results from the Mental Health Supplement to the Ontario Health Survey. Drug Alcohol Depend. 1995;39:111–128. doi: 10.1016/0376-8716(95)01150-w. [DOI] [PubMed] [Google Scholar]
- Sah R, Ekhator NN, Strawn JR, Sallee FR, Baker DG, Horn PS, Geracioti TD., Jr Low cerebrospinal fluid neuropeptide Y concentrations in posttraumatic stress disorder. Biol Psychiatry. 2009;66:705–707. doi: 10.1016/j.biopsych.2009.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez CJ, Sorg BA. Conditioned fear stimuli reinstate cocaine-induced conditioned place preference. Brain Res. 2001;908:86–92. doi: 10.1016/s0006-8993(01)02638-5. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Leung S, Buczek Y, Stewart J. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berl) 1998;137:184–190. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Highfield D, Delfs J, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur J Neurosci. 2000;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Rajabi H, Stewart J. Relapse to heroin-seeking in rats under opioid maintenance: the effects of stress, heroin priming, and withdrawal. J Neurosci. 1996;16:1957–1963. doi: 10.1523/JNEUROSCI.16-05-01957.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Exposure to mild stress enhances the reinforcing efficacy of intravenous heroin self-administration in rats. Psychopharmacology (Berl) 1994;114:523–527. doi: 10.1007/BF02249346. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Wichmann J, Maidment NT. The effect of a systemically active ORL-1 agonist, Ro 64–6198, on the acquisition, expression, extinction, and reinstatement of morphine conditioned place preference. Neuropharmacology. 2005;49:439–446. doi: 10.1016/j.neuropharm.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Siegmund A, Wotjak CT. A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. J Psychiatr Res. 2007;41:848–860. doi: 10.1016/j.jpsychires.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Bremner JD, Rasmusson A, Morgan CA, 3rd, Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;46:1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- Sperling RE, Gomes SM, Sypek EI, Carey AN, McLaughlin JP. Endogenous kappa-opioid mediation of stress-induced potentiation of ethanol-conditioned place preference and self-administration. Psychopharmacology (Berl) 2010;210:199–209. doi: 10.1007/s00213-010-1844-5. [DOI] [PubMed] [Google Scholar]
- Stam R. PTSD and stress sensitisation: a tale of brain and body Part 2: animal models. Neurosci Biobehav Rev. 2007;31:558–584. doi: 10.1016/j.neubiorev.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, Saha T. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2005;80:105–116. doi: 10.1016/j.drugalcdep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Strawn JR, Pyne-Geithman GJ, Ekhator NN, Horn PS, Uhde TW, Shutter LA, Baker DG, Geracioti TD., Jr Low cerebrospinal fluid and plasma orexin-A (hypocretin-1) concentrations in combat-related posttraumatic stress disorder. Psychoneuroendocrinology. 2010;35:1001–1007. doi: 10.1016/j.psyneuen.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology (Berl) 1997;130:203–212. doi: 10.1007/s002130050230. [DOI] [PubMed] [Google Scholar]
- Tierney C, Nadaud D, Koenig-Berard E, Stinus L. Effects of two alpha 2 agonists, rilmenidine and clonidine, on the morphine withdrawal syndrome and their potential addictive properties in rats. Am J Cardiol. 1988;61:35D–38D. doi: 10.1016/0002-9149(88)90462-6. [DOI] [PubMed] [Google Scholar]
- Triffleman EG, Marmar CR, Delucchi KL, Ronfeldt H. Childhood trauma and posttraumatic stress disorder in substance abuse inpatients. J Nerv Ment Dis. 1995;183:172–176. doi: 10.1097/00005053-199503000-00008. [DOI] [PubMed] [Google Scholar]
- Valverde O, Smadja C, Roques BP, Maldonado R. The attenuation of morphine-conditioned place preference following chronic mild stress is reversed by a CCKB receptor antagonist. Psychopharmacology (Berl) 1997;131:79–85. doi: 10.1007/s002130050268. [DOI] [PubMed] [Google Scholar]
- van Erp AM, Miczek KA. Persistent suppression of ethanol self-administration by brief social stress in rats and increased startle response as index of withdrawal. Physiol Behav. 2001;73:301–311. doi: 10.1016/s0031-9384(01)00458-9. [DOI] [PubMed] [Google Scholar]
- van Erp AM, Tachi N, Miczek KA. Short or continuous social stress: suppression of continuously available ethanol intake in subordinate rats. Behav Pharmacol. 2001;12:335–342. doi: 10.1097/00008877-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Veijola J, Laara E, Joukamaa M, Isohanni M, Hakko H, Haapea M, Pirkola S, Maki P. Temporary parental separation at birth and substance use disorder in adulthood. A long-term follow-up of the Finnish Christmas Seal Home Children. Soc Psychiatry Psychiatr Epidemiol. 2008;43:11–17. doi: 10.1007/s00127-007-0268-y. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Siegmund S, Singer MV, Sinclair JD, Li TK, Spanagel R. A comparative study on alcohol-preferring rat lines: effects of deprivation and stress phases on voluntary alcohol intake. Alcohol Clin Exp Res. 2003;27:1048–1054. doi: 10.1097/01.ALC.0000075829.81211.0C. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Davis MA, Olgin JE. Naltrexone blocks the post-shock increase of ethanol consumption. Life Sci. 1986;38:841–847. doi: 10.1016/0024-3205(86)90601-6. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Ulm RR. The influence of control over appetitive and aversive events on alcohol preference in rats. Alcohol. 1990;7:133–136. doi: 10.1016/0741-8329(90)90074-m. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Ulm RR, Hopson N. The bidirectional effects of shock on alcohol preference in rats. Alcohol Clin Exp Res. 1990;14:913–916. doi: 10.1111/j.1530-0277.1990.tb01837.x. [DOI] [PubMed] [Google Scholar]
- Wakizono T, Sawamura T, Shimizu K, Nibuya M, Suzuki G, Toda H, Hirano J, Kikuchi A, Takahashi Y, Nomura S. Stress vulnerabilities in an animal model of post-traumatic stress disorder. Physiol Behav. 2007;90:687–695. doi: 10.1016/j.physbeh.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Walker BM, Rasmussen DD, Raskind MA, Koob GF. alpha1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008;42:91–97. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Luo F, Zhang WT, Han JS. Stress or drug priming induces reinstatement of extinguished conditioned place preference. Neuroreport. 2000;11:2781–2784. doi: 10.1097/00001756-200008210-00034. [DOI] [PubMed] [Google Scholar]
- Wee S, Mandyam CD, Lekic DM, Koob GF. Alpha 1-noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur Neuropsychopharmacol. 2008;18:303–311. doi: 10.1016/j.euroneuro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss IC, Domeney AM, Heidbreder CA, Moreau JL, Feldon J. Early social isolation, but not maternal separation, affects behavioral sensitization to amphetamine in male and female adult rats. Pharmacol Biochem Behav. 2001;70:397–409. doi: 10.1016/s0091-3057(01)00626-8. [DOI] [PubMed] [Google Scholar]
- Will MJ, Der-Avakian A, Pepin JL, Durkan BT, Watkins LR, Maier SF. Modulation of the locomotor properties of morphine and amphetamine by uncontrollable stress. Pharmacol Biochem Behav. 2002;71:345–351. doi: 10.1016/s0091-3057(01)00719-5. [DOI] [PubMed] [Google Scholar]
- Will MJ, Watkins LR, Maier SF. Uncontrollable stress potentiates morphine’s rewarding properties. Pharmacol Biochem Behav. 1998;60:655–664. doi: 10.1016/s0091-3057(98)00027-6. [DOI] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Brady K, Weiss RD, Farrer L, Gelernter J. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Arch Gen Psychiatry. 2009;66:1201–1209. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap JJ, Miczek KA. Social defeat stress, sensitization, and intravenous cocaine self-administration in mice. Psychopharmacology (Berl) 2007;192:261–273. doi: 10.1007/s00213-007-0712-4. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Sanchez H, Kehoe P, Kosten TA. Neonatal isolation enhances maintenance but not reinstatement of cocaine self-administration in adult male rats. Psychopharmacology (Berl) 2005;177:391–399. doi: 10.1007/s00213-004-1963-y. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Leri F, Cummins E, Hoeschele M, Kreek MJ. Involvement of arginine vasopressin and V1b receptor in heroin withdrawal and heroin seeking precipitated by stress and by heroin. Neuropsychopharmacology. 2008;33:226–236. doi: 10.1038/sj.npp.1301419. [DOI] [PubMed] [Google Scholar]
- Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW. Effects of the CRF receptor antagonist D-Phe CRF(12–41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007;53:958–966. doi: 10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zywiak WH, Stout RL, Trefry WB, LaGrutta JE, Lawson CC, Khan N, Swift RM, Schneider RJ. Alcohol relapses associated with September 11, 2001: a case report. Subst Abus. 2003;24:123–128. doi: 10.1080/08897070309511540. [DOI] [PubMed] [Google Scholar]