Abstract
Purpose
The therapeutic effect of trastuzumab monoclonal antibody (mAb) therapy has been shown to be partially dependent on functional NK cells. Novel agents that enhance NK cell function could potentially improve the anti-tumor effect of trastuzumab. We recently identified polysaccharide krestin (PSK), a natural product extracted from medicinal mushroom Trametes Versicolor, as a potent TLR2 agonist. The current study was undertaken to evaluate the effect of PSK on human NK cells and the potential of using PSK to enhance HER2-targeted mAb therapy.
Experimental Design
Human PBMC were stimulated with PSK to evaluate the effect of PSK on NK cell activation, IFN-γ production, cytotoxicity, and trastuzumab-mediated ADCC. Whether the effect of PSK on NK cells is direct or indirect was also investigated. Then in vivo experiment in neu transgenic mice was carried out to determine the potential of using PSK to augment the anti-tumor effect of HER2-targeted mAb therapy.
Results
PSK activated human NK cells to produce IFN-γ and to lyse K562 target cells. PSK also enhanced trastuzumab-mediated ADCC against SKBR3 and MDA-MB-231 breast cancer cells. Both direct and IL-12-dependent indirect effects seem to be involved in the effect of PSK on NK cells. Oral administration of PSK significantly potentiated the anti-tumor effect of anti-HER2/neu mAb therapy in neu-transgenic mice.
Conclusion
These results demonstrated that PSK activates human NK cells and potentiates trastuzumab-mediated ADCC. Concurrent treatment of PSK and trastuzumab may be a novel way to augment the anti-tumor effect of trastuzumab.
Keywords: PSK, TLR2, NK, ADCC, breast cancer, trastuzumab
Introduction
Trastuzumab is a humanized anti-HER2 monoclonal antibody (mAb), and is the first HER2-targeted therapy approved by FDA. Trastuzumab has significantly advanced the clinical management of patients with HER2+ breast cancer by prolonging disease-free and overall survival in early stage breast cancer patients, and progression-free and overall survival in patients with metastatic breast cancer (1, 2). Trastuzumab inhibits tumor cell growth through multiple mechanisms including signaling blockade and downregulating the HER2/neu receptor. One of the major mechanisms is believed to be antibody-dependent cell-mediated cytotoxicity (ADCC), in which the tumor cells are coated with trastuzumab and then lysed by immune cells via binding of Fc gamma receptor (FcγR) to the Fc portion of the mAb (3). Increase in tumor infiltrating NK cells after trastuzumab therapy has been found in human breast cancer biopsy samples (4, 5), and FcγR gene polymorphism can impact the clinical response to trastuzumab (6). NK cells constitutively express FcγRIIIA (CD16) and are the major effectors of ADCC (7). Therefore the function of NK cells may impact the efficacy of ADCC and clinical response to trastuzumab (8). Unfortunately, NK cell function is frequently impaired in cancer patients as compared to healthy donors (9-11), and lytic function of NK cells in patients with advanced disease (stages II, III, and IV) is even lower than in those with limited disease (stage I) (12). Therefore novel approaches that can enhance NK cell function and improve ADCC would potentially benefit many cancer patients receiving mAb therapy.
The activation of NK cells is determined by the coordination of inhibitory and activating receptors on the surface of NK cells. The inhibitory receptors include killer Ig-like receptor (KIR) and CD94 (NKG2A/B), which prevent NK cell activation upon encounter of normal MHC Class I. The activating receptors include CD16 that is involved in ADCC, NKG2D that recognizes stress-induced ligand MICA/B and UL16 binding proteins (ULBPs) on malignant cells, and natural cytotoxicity receptors (NKp30, NKp44, and NKp46) whose ligands remain unclear (13). Two subsets of NK cells have been identified in humans according to their phenotype (CD56 expression) and function (regulatory versus effector cells) (14). The CD56brightCD16-/low NK cells, which account for approximately 10% of NK cells in peripheral blood, are the major producer of IFN-γ. The CD56dimCD16+ NK cells that account for approximately 90% of NK cells are cytotoxic effector cells in mediating ADCC (14). Both types of NK cells have been found to express toll-like receptors (TLRs) (15). TLR agonists, especially the agonist of TLR3 (poly I:C), TLR7/8 (imiquimod, resiquimod, and 3M-002), and TLR9 (CpG), have been shown to activate NK cells either directly or indirectly via stimulation of accessory cells (15-19). A Phase II clinical trial that combines rituximab and 1018 ISS (CpG) has been conducted in patients with relapsed or refractory follicular lymphoma, and the results showed that biologically relevant increase in ADCC was observed in 35% of patients (20), demonstrating the potential of using TLR agonists to improve mAb therapy.
The effect of TLR2 agonist on NK cell function and ADCC is relatively less well known, although there is some evidence that NK cells can be activated via TLR2 either directly or indirectly via DC (21-23). A recent publication by Moreno et al compared the effects of TLR-2, 3, 4, 5, 8, and 9 agonists on NK cells and ADCC and found that only TLR2 agonist peptidoglycan, TLR8 agonist CL075, and TLR9 agonist CpG ODN A showed enhanced anti-MUC1 mAb-mediated ADCC (24), indicating that TLR2 ligation could be as potent as TLR8 or TLR9 ligation in augmenting NK cell function. We recently identified polysaccharide krestin (PSK), a mushroom extract from Trametes versicolor, as a selective and potent TLR2 agonist and revealed the potential of using a natural product to enhance NK cell function (25).
The major component of PSK is protein-bound polysaccharide with an approximate molecular weight of 90-100kDa. PSK was approved as a prescription drug for the treatment of cancer in Japan in 1977 (26). Clinical trials in Japan have shown that oral intake of PSK significantly extended survival at five years or beyond in patients with different types of cancer, especially stomach and colorectal cancer (27-29). Using HEK293 cells transfected with different TLRs, we demonstrated that PSK is a selective and potent TLR2 agonist (25). We further showed that the anti-tumor effect of PSK in a mouse model of breast cancer is dependent on both CD8 T cells and NK cells (25). Expanding from our previous findings in mice, the current study was undertaken to investigate the effect of PSK on human NK cells and trastuzumab-mediated ADCC and the potential of using this natural product with TLR2 agonist activity to augment the anti-tumor effect of trastuzumab.
Materials and Methods
Animals
A colony of neu-transgenic (neu-T) mice [strain name, FVB/N-TgN (MMTVneu)-202Mul] was established in our animal facilities from breeding pairs obtained from the Jackson Laboratory (Bar Harbor, ME) and maintained as previously described (30). Mice were maintained under strict inbreeding conditions. All of the procedures were performed in compliance with the University of Washington Institutional Animal Care and Use Committee guidelines.
Human PBMC and Cell lines
Human PBMC were isolated from whole blood or leukapheresis products by centrifugation through a Ficoll-hypaque gradient (Amersham Biosciences, Uppsala, Sweden). Blood or leukapheresis samples were collected from healthy volunteer donors with informed consent using a protocol approved by the Institutional Review Board (IRB) of University of Washington. NK cells were purified from PBMC by magnetic negative selection using Miltenyi NK cell Isolation kit II (Auburn, CA). NK-92, a cell line that has the characteristics of human NK cells (31), were obtained from American Type Culture Collection (ATCC, Manassas, VA) and maintained in Alpha MEM medium without ribonucleosides and deoxyribonucleosides but with 2 mM L-glutmine, 0.2 mM inosital, 0.1 mM 2-mercaptoethanol, 0.02 mM folic acid, 100 U/mL IL-2, 12.5% fetal bovine serum (FBS) and 12.5% horse serum. The breast cancer cell lines, SKBR3 and MDA-MB-231, were obtained from ATCC and maintained in DMEM (Cellgro, Herndon, VA) supplemented with 10% FBS at 37 °C in a 5% CO2 atmosphere. The K562 leukemia cell line was also obtained from ATCC and maintained in RPMI (Cellgro) with 10% FBS (Gemini Bioproducts, Woodland, CA).
Antibodies and other Reagents
The HER2-specific mAb trastuzumab (Herceptin™) was manufactured by Genentech (San Francisco, CA) and purchased from the University of Washington Pharmacy. Fluorochrome-conjugated monoclonal antibodies against CD3, CD56, CD25, CD69, and CD107a were from eBiosciences (San Diego, CA). Fluorochrome-conjugated mAbs against CD16 and IFN-γ was from Biolegend (San Diego, CA). Recombinant human IL-12 and anti-human IL-12 neutralizing antibody were purchased from Peprotech (Rocky Hill, NJ). Phosphate-buffered saline (PBS), penicillin-streptomycin, and L-glutamine were obtained from Invitrogen. PSK was purchased from Kureha Corporation (Tokyo, Japan). PSK was dissolved in PBS at a stock concentration of 10 mg/ml. Aliquots of 100 μl were stored at −80 °C. The frozen aliquots were thawed immediately before use. Anti-rat neu mAb (clone 7.16.4) was produced from 7.16.4 hybridoma cells (kindly provided by Dr. Mark Green) by the UCSF monoclonal antibody core.
Measurement of human NK cell activation and production of IFN-γ by FACS
PBMC or purified NK cells were cultured in RPMI in the presence of PSK (100 μg/ml) or control PBS for 24 or 48 hr. Brefeldin-A (BFA, 5 μg/mL, Sigma-Aldrich), a secretion inhibitor, was included for the last 6 hr of the incubation. At the end of activation period, the cells were first stained with fluorophore-conjugated antibodies to surface markers (anti-CD3, CD56, CD25, and CD69). After subsequent fixation and permeabilization, the cells were stained with anti-IFN-γ-PE. In some experiments with PBMC, the cells were co-incubated with anti-IL12 to determine whether the production of IFN-γ by NK cells is dependent on this cytokine. In experiments with purified NK cells, a suboptimal dose of IL-12 (1 ng/ml) or PSK plus IL-12 were also included. Samples were acquired on FACS Canto II. List mode file was analyzed using FlowJo (Treestar, OR).
Measurement of CD107a degranulation in NK cells
The degranulation of NK cells was measured by the expression of CD107a, lysosome-associated membrane protein-1 (LAMP-1). In brief, PBMC treated with PSK (100 μg/ml, 24 hr) or medium alone were incubated with K562 target cells at effector:target (E:T) ratio of 2:1 for 6 hr. Anti-CD107a-PE antibody was added directly to the co-cultures. After 1 hr incubation, brefeldin A was included to the culture and incubated for another 5 hr. Cells were then stained with CD3 and CD56 and analyzed on FACS Canto II.
Cytotoxicity assay
A non-radioactive, fluorometric cytotoxicity assay with calcein-acetoxymethyl (Calcein AM) (32) was used to measure the lysis of K562 and trastuzumab-mediated ADCC. PBMC were stimulated with PSK (10 μg/ml) or control PBS for 48 hr before co-incubation with target cells. The K562 tumor target cells were loaded with Calcein AM (10 μg/ml, from Invitrogen) for 1 hour and washed. Labeled target cells were mixed with PBMC at different E:T ratios (100:1, 50:1, 25:1, and 12.5:1) and plated on 96 well culture plates. After 4 hr incubation at 37 °C, the release of Calcein into culture medium was measured by a Victor 3 fluorescent plate reader (Perkin Elmer). The percentages of specific lysis were calculated according to the formula: (experimental release – spontaneous release) / (maximal release – spontaneous release) x100, where experimental release represents the mean fluorescence for target cells incubated in the presence of effector cells, and spontaneous release represents the mean fluorescence for target cells incubated without effector cells, and maximal release represents the mean fluorescence for target cells incubated with Triton X-100. The measurement of trastuzumab-mediated ADCC was done similarly as described above for the K562 lysis assay except that the target breast cancer cells, SKBR3 and MDA-MB-231, were coated with trastuzumab (5 μg/ml) or control IgG1 for 30 minutes before labeling with Calcein AM. The percentages of specific lysis were calculated as above. Triplicate wells were set up for each E:T ratio. Results were expressed at mean±SD of triplicate wells at each E:T ratio.
Analysis of TLR2 mRNA expression using real time RT-PCR
To analyze TLR2 expression on purified CD56bright and CD56dim NK cells, NK cells were first enriched from PBMC by magnetic negative selection using Miltenyi NK cell Isolation kit II. Then the enriched NK cells were stained with anti-CD3, CD56, and CD16 to sort for CD56brightCD16-/low and CD56dimCD16+ NK cells using BD FACS Aria sorter. The sorted populations had more than 99% purity. CD3+ T cells, CD19+ B cells, and CD11c+ DC were also sorted from PBMC as control. RNA was isolated from FACS-sorted cells or whole PBMC using RNAqeous4PCR kit (Ambion, TX). cDNAs were prepared using Superscript III reverse transcriptase (Invitrogen). Quantitative PCR was performed using Taqman primer and probe from Applied Biosystems (Foster City, CA) in 384-well plates using an ABI 7900 (Applied Biosystems). Cycling conditions were similar as previously described (30). The expression of TLR2 mRNA was normalized to hypoxanthine ribosyltransferase (HPRT) using the delta Ct method (30).
Measurement of cytokine and chemokine secretion from PSK-stimulated PBMC or purified NK cells using Luminex analysis
PBMC or MACS-purified NK cells (200,000 per well) were plated in 96-well round bottom culture plates and treated with serial dilutions of PSK (25-400 μg/ml) for 24 or 48 hours. The supernatants were harvested and levels of various cytokines and chemokines (IL-12p40, IL-12p70, TNF-α, IL-6, IL-8, MIP-1α, MIP-1β, IL-1α, and IL-1β) were measured using a Luminex kit purchased from Millipore (Pittsburgh, PA) following the manufacturer’s instruction.
Treatment of tumor-bearing mice with PSK and anti-HER2/neu mAb (7.16.4)
Neu transgenic mice received subcutaneous implant of 1 million MMC cells, a cell line derived from a syngeneic spontaneous breast cancer in these mice (33). At two weeks after implantation (average tumor size = 50 mm3), mice were randomly assigned to receive treatment with 7.16.4 alone (15 mg/kg, tail vein injection, 3 times per week), PSK alone (100 mg/kg, oral gavage, 3 times per week), 7.16.4 plus PSK, or PSK plus a control irrelevant IgG of the same isotype. Mice in the 7.16.4 alone group received oral gavage of PBS of the same volume. To determine the role of immune cells in the anti-tumor effect of PSK and 7.16.4, some mice received depletion of CD4, CD8 T cells, or NK cells using the monoclonal antibodies (clone GK1.5 for CD4, clone 2.43 for CD8, and clone PK136 for NK) at 1 week prior to and during PSK and 7.16.4 treatment, using similar protocol as previously described (34). Tumors were measured every other day with vernier calipers and tumor volume was calculated as the product of length x width x height x 0.5236. In vivo data are presented as mean±SD of each treatment group.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software, San Diego, CA). Data were analyzed using Student’s t test, ANOVA, or Mann Whitney test when Guassian distribution can not be assumed. A value of p<0.05 was considered statistically significant.
Results
PSK stimulates human CD56bright NK cells to produce IFN-γ
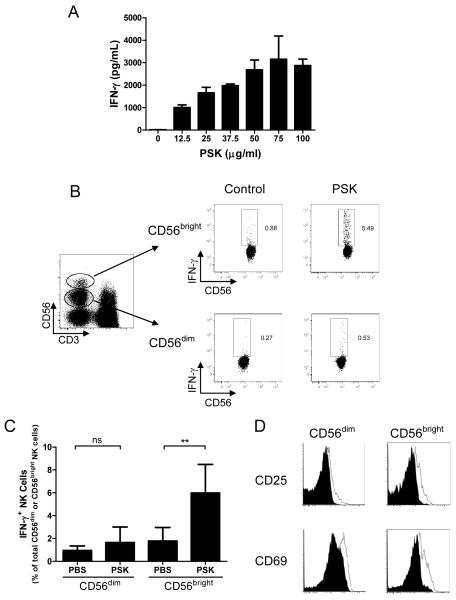
PSK induces IFN-γ secretion from PBMC in a dose-dependent manner (Fig. 1A). Intracellular staining showed that IFN-γ is mainly produced by CD56bright NK cells although there is a slight induction of IFN-γ in CD56dim NK cells (Fig. 1B). The percentage of cells that are positive for IFN-γ are 1.0±0.2% in control CD56dim cells and 1.6±0.6% in PSK-treated CD56dim NK cells (p=0.3), and are 1.8±0.5% in control CD56bright cells and 5.9±1.1% in PSK-stimulated CD56bright NK cells (p=0.009, Fig. 1C). To determine if both CD56dim and CD56bright cells are activated, we measured the expression of activation markers, CD25 and CD69, on NK cells after PSK treatment. As shown in Figure 1D, PSK upregulates the expression of CD25 and CD69 in both CD56dim and CD56bright NK cells.
Figure 1.
PSK stimulates IFN-γ production from CD56bright NK cells. (A) Dose-dependent induction of IFN-γ secretion by PSK. Shown are IFN-γ concentrations (mean±SD) in culture supernatant from duplicate culture wells of PBMC stimulated with different concentrations of PSK for 24 hours. Similar results were obtained from three different donors. (B) Representative dot plots showing the gating of CD56dim and CD56bright NK cells and IFN-γ production in PBS control or PSK-stimulated CD56dim or CD56bright NK cells. (C) Summary graph showing the mean±SD of the percentages of IFN-γ positive cells among total CD56dim and CD56bright NK cells in PBMC from 5 different donors. ns, not significant; **, p<0.01 using two-tailed Student t test. (D) Overlay histogram showing CD25 and CD69 expression on CD56dim and CD56bright NK cells. Filled histogram: NK cells from Control PBS group; Unfilled histogram: NK cells from PBMC treated with PSK (100 μg/ml). Results are representative of 3 independent experiments.
PSK stimulates the cytolytic function of human NK cells and augments trastuzumab-mediated ADCC
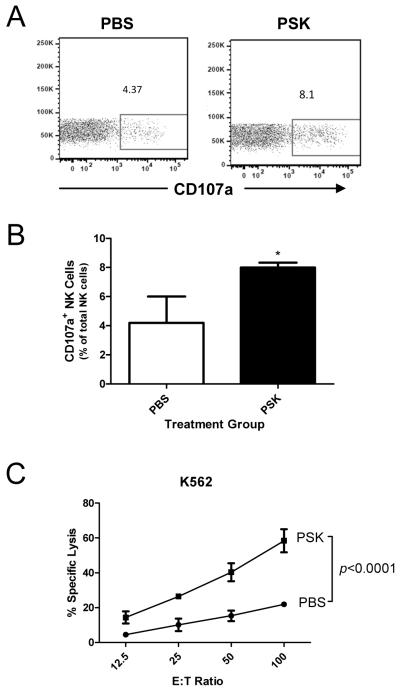
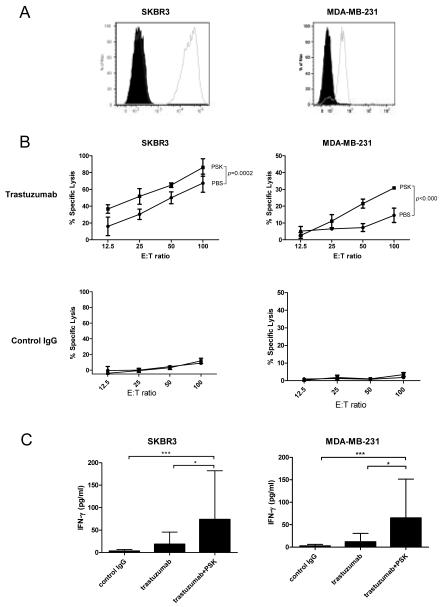
Expression of CD107a in the presence of K562 cells, a MHCI devoid leukemia cell line, has been used as a marker of NK cell cytotoxicity (35). FACS analysis showed that NK cells in PSK-treated PBMC have higher expression of CD107a (8.0±0.2%) than control group (4.2±1.0%, p=0.02 between PBS and PSK, Fig. 2A and B). The specific lysis of K562, as measured by Calcein AM release assay, was also significantly enhanced in PSK-stimulated PBMC. As shown in Fig. 2C, the percentage of specific lysis was approximately 2 fold higher in PSK-stimulated PBMC as compared to unstimulated PBMC at different E:T ratios (p<0.0001). We next measured the potential of PSK to augment trastuzumab-mediated ADCC against two breast cancer cell lines, SKBR3 and MDA-MB-231. As shown in Figure 3A, SKBR3 expresses high levels of HER2, and MDA-MB-231 expresses low levels of HER2. Pretreatment of PBMC with PSK (10 μg/ml, 72hr) resulted in significantly enhanced ADCC against both cancer cell lines (Figure 3B). Similar results were obtained using PBMC from 5 different donors as summarized in Supplemental Table. Measurement of IFN-γ in ADCC supernatant showed that pre-treatment with PSK results in significantly enhanced IFN-γ production in response to trastuzumab-coated cancer cells (Figure 3C).
Figure 2.
PSK stimulates CD107a mobilization and enhances the lysis of K562 tumor cells. (A) Representative dot plots showing expression of CD107a in NK cells in control and PSK-treated PBMC. The PBMC was stimulated with PSK (100 μg/ml) or control PBS for 24 hr. Then the cells were mixed with target K562 cells at 2:1 ratio. Anti-CD107a was added to the culture and incubated for 6 hr. (B) Summary graph showing the percentage (mean±SD) of CD107a positive NK cells from 3 different donors and treated with or without PSK stimulation. *, p<0.05 using two-tailed Student t test. (C) The lysis of K562 target tumor cells by PSK-stimulated or unstimulated PBMC. Shown are the percentages of specific lysis (mean±SD in triplicate wells) at the indicated E:T ratios. PBMC were stimulated with PSK (or control PBS) for 48 hr before the initiation of cytolytic assay. Difference between PBS and PSK group at different E:T ratio were analyzed using 2 way ANOVA. Similar results were obtained from three independent experiments using PBMC from 3 different donors.
Figure 3.
PSK enhances trastuzumab-mediated ADCC against SKBR3 and MDA-MB-231 breast cancer cells. (A) Expression of HER2 on SKBR3 and MDA-MB-231 cells. The cells were stained with PE conjugated anti-human HER2 (empty histogram) or isotype control (filled histogram). (B) Percentages of specific lysis of trastuzumab- or control IgG-coated SKBR3 and MDA-MB-231 target cells. Shown are mean±SD of triplicate wells at different E:T ratios. ■ indicates PSK-stimulated effector PBMC; ● indicates control PBS-treated effector cells. PBMC were treated with PSK (10 μg/ml) in RPMI for 72 hr before the initiation of cytolytic assay. Difference between PBS and PSK group at different E:T ratio were analyzed using ANOVA. Similar results were obtained using PBMC from 5 different donors as summarized in Supplemental Table. (C) IFN-γ levels (mean±SD) in culture supernatant from the 4 hr ADCC incubation. Control IgG: tumor targets were coated with control irrelevant IgG and incubated with PBMC with no prior PSK stimulation; trastuzumab: tumor cells were coated with trastuzumab and then incubated with PBMC with no prior PSK; trastuzumab+PSK: tumor cells were coated with trastuzumab and incubated with PSK (10 μg/ml, 72 hr)-stimulated PBMC. *, p<0.05; ***, p<0.001 by Mann-Whitney test. Results are representative of three independent experiments.
PSK has both direct and IL-12-dependent indirect effects on NK cells
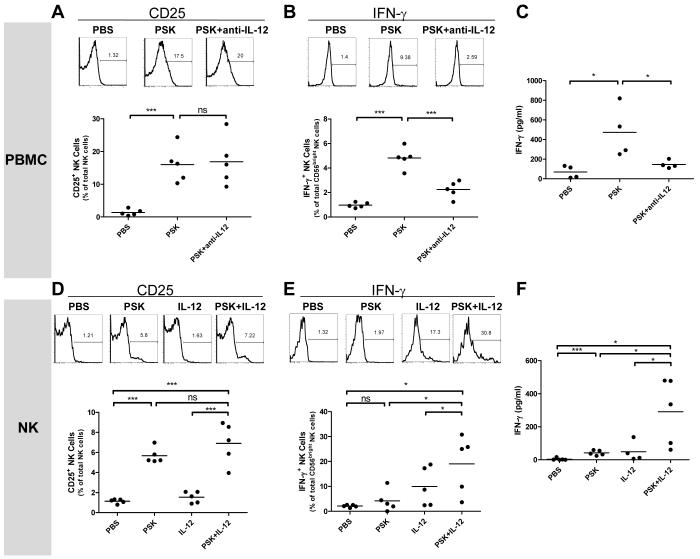
There are controversial reports as to whether NK cells are activated by TLR2 agonists directly or indirectly via accessory cells (21-23). To determine whether the effect of PSK on NK cells is direct or indirect, we first used IL-12 blockade by including anti-IL12 antibody during PSK treatment of PBMC. As shown in Figure 4A-C, IL-12 blockade did not affect PSK-induced upregulation of CD25 on NK cells, but significantly decreased PSK-induced IFN-γ production, as shown by decreased levels of IFN-γ+ CD56bright NK cells (Figure 4B) and decreased levels of IFN-γ in culture supernatant from PBMC (Figure 4C). It is noted that there is residual amount of IFN-γ production even when IL-12 is blocked, suggesting that IL-12 independent induction of IFN-γ by PSK may also exist (Figure 4B and C). In contrast, PSK-induced TNF-α production by PBMC was not decreased in the presence of anti-IL12 antibody (Supplemental Figure 1). This could be due to that fact that monocytes and DC can produce large amounts of TNF-α in addition to NK cells. It also suggests that TNF-α production by NK cells could be regulated differently from IFN-γ production by NK, and is independent of IL-12. To confirm that PSK-stimulated IFN-γ production by NK cells is dependent on IL-12, we treated MACS-purified NK cells with PSK, a suboptimal dose of IL-12 (1 ng/mL, as determined by dose titration experiment shown in Supplemental Figure 2), or PSK plus IL-12. Results showed that PSK by itself significantly induced the expression of CD25 on purified NK cells (Figure 4D), but the effect on IFN-γ production is moderate (not significant by intracellular staining but significant by ELISA measurement, Figure 4E and F). In the presence of IL-12, PSK resulted in enhanced IFN-γ production, which is significantly higher than either PSK or IL-12 single treatment (Figure 4E and F). These data suggest that PSK-induced IFN-γ production but not CD25 unpregulation is dependent on IL-12. Experiments using NK-92 cells yielded results consistent to those from purified NK cells showing that the production of IFN-γ in response to PSK is dependent on IL-12 (Supplemental Figure 3). We also measured the expression of TLR2 on NK cells by FACS analysis and real-time PCR. FACS analysis showed that TLR2 is detectable in CD56bright NK cells, but the expression level is much lower than that on B cells or DC (Supplemental Figure 4A). RT-PCR analysis using FACS-sorted cells confirmed that CD56bright NK cells express more TLR2 mRNA than CD56dim NK cells and T cells, although there is inconsistency between mRNA and FACS data on the relative expression levels of TLR2 on CD56bright NK cells as compared to that on B cell or DC (Supplemental Figure 4B). TLR2 expression was not induced upon PSK treatment (data not shown).
Figure 4.
PSK has both direct and IL-12-dependent effects on NK cells. Results shown in A-C were generated using PBMC, and results in D-F were generated using purified NK cells. (A) Expression of CD25 on NK cells in PBMC treated with PBS, PSK (100 μg/ml), or PSK plus anti-IL-12 Ab (10 μg/ml) for 24 hr. (B) Percentage of IFN-γ positive NK cells among total CD56bright NK cells in PBMC treated with PBS, PSK, or PSK plus anti-IL-12 Ab. (C) The level of IFN-γ in culture supernatant from PBMC treated with PBS, PSK, or PSK plus anti-IL-12 Ab. (D) CD25 expression in purified NK cells stimulated with PBS, PSK (100 μg/ml), IL-12 (1 ng/ml), or PSK+IL-12 for 24 hr. (E) Percentage of IFN-γ positive NK cells among total CD56bright NK cells in purified NK cells stimulated with PBS, PSK, IL-12, or PSK+IL-12. (F) The level of IFN-γ in culture supernatant of purified NK cells stimulated with PBS, PSK, IL-12, or PSK+IL-12. The FACS graphs show response from a representative donor. The summary graphs show data from 4-5 different donors analyzed in independent experiments. Each dot represents an individual donor. The horizontal line represents the group average. *, p<0.05; **, p<0.01; ***, p<0.001 between two treatment groups using Student t test.
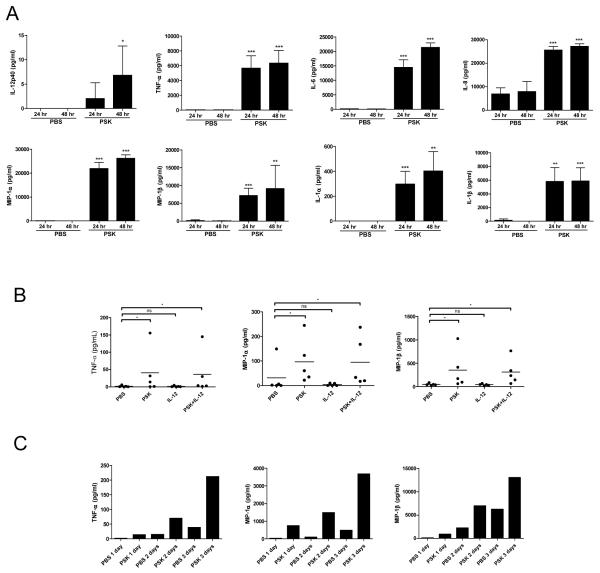
PSK stimulates the production of IL-12 and other proinflammatory cytokines and chemokines from PBMC
Culture supernatant from PSK-treated PBMC was collected for Luminex analysis of IL-12 and other cytokines and chemokines. As shown in Figure 5A, PSK significantly induced the production of IL-12p40. PSK also significantly induced other proinflammatory cytokines and chemokines (TNF-α, IL-6, IL-8, MIP-1α, MIP-1β, IL-1α, and IL-1β from PBMC. The level of cytokine/chemokine induction is similar to our previous observation on the effect of PSK on mouse splenocytes (25). Interestingly, this panel of cytokines and chemokines (except IL-12) were also induced in the cytolytic assay when PSK-stimulated PBMC were co-incubated with K562 (Supplemental Figure 5), indicating that PSK stimulates the cytokine-secreting and cytolytic activity of NK cells simultaneously.
Figure 5.
PSK induces the secretion of proinflammatory cytokines and chemokines by PBMC and NK cells. (A) The levels of IL-12p40, TNF-α, IL-6, IL-8, MIP-1α, MIP-1β, IL-1α, and IL-1β in culture supernatant from PBMC treated with PSK (100 μg/ml) or control PBS for 24 or 48 hr, as determined in Luminex analysis. *, p<0.05; **, p<0.01; ***, p<0.001 between PSK and PBS group at the same time point using two-tailed Student t test. Shown are mean±SD of results from 3 independent donors. (B) Shown are levels of TNF-α, MIP-1α, and MIP-1β in culture supernatant from purified NK cells treated with PSK (100 μg/ml), IL-12 (1 ng/ml), or PSK+IL-12. Each data point represents response from an individual donor (N=5). (C) Time course of TNF-α, MIP-1α, and MIP-1β induction by PSK in one donor.
Luminex analysis was also performed to measure the potential secretion of cytokine/chemokines by PSK-treated purified NK cells. As shown in Figure 5B, PSK induced the production of TNF-α, MIP-1α, and MIP-1β by NK cells. Interestingly, the pattern of induction for these cytokine/chemokines seems to be different from that for IFN-γ (Figure 4F) and appears to be independent of IL-12 (Figure 5B). The time course of cytokine/chemokine induction in one of the 5 donors tested in Figure 5B was shown in Figure 5C.
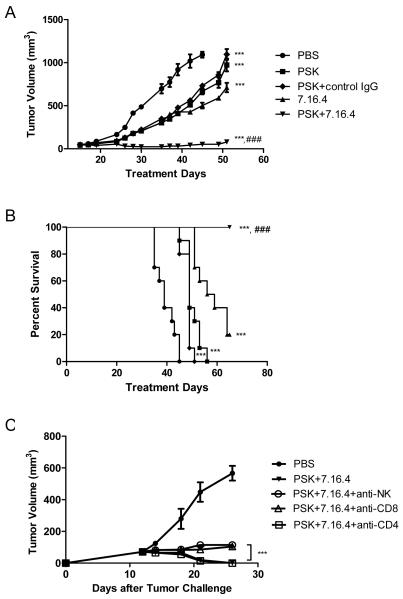
Combination of PSK and anti-HER2/neu mAb (7.16.4) has enhanced anti-tumor effect in a mouse model of breast cancer
To evaluate the potential synergistic anti-tumor effect between PSK and anti-HER2 mAb therapy, we treated neu transgenic mice bearing HER2/neu+ breast tumors with 7.16.4, an anti-ErbB2 mAb, alone or in combination with oral PSK. The mechanisms of action of 7.16.4 remain unclear, and both direct inhibition of tumor cell growth (36) and immune cell-mediated anti-tumor effect (37) have been reported. As shown in Figure 6A, 7.16.4 mAb by itself inhibits tumor growth by 58±2%. PSK by itself inhibits tumor growth by 50±3%. The combination of the two treatments inhibits tumor growth by 96±2% (p<0.0001 compared to either treatment alone), demonstrating the potential of PSK to augment the anti-tumor effect of trastuzumab. The overall survival was also significantly improved in the group of mice that received both PSK and 7.16.4 mAb (p=0.0003 between 7.16.4 alone and 7.16.4 plus PSK, Fig. 6B). Selective depletion of CD4, CD8 T cells or NK cells during 7.16.4 plus PSK treatment showed that the anti-tumor effect is partially dependent on CD8 T cells and NK cells, but not CD4 T cells (Figure 6C).
Figure 6.
PSK enhances the anti-tumor effect of 7.16.4 in neu transgenic mice, and the anti-tumor effect of combinatorial PSK and 7.16.4 is partially dependent on NK cells and CD8 T cells. In (A) and (B), shown are tumor growth curve (A) and overall survival (B) in mice receiving PBS (●), PSK (■), control IgG+PSK (◇;), 7.16.4 (▲), 7.16.4 plus PSK (▼). Each data point in (A) represents mean±SD in the treatment group, n=10 per group. Similar results were obtained from two independent experiments. ***, p<0.001 from control PBS group using ANOVA analysis. ###, p<0.001 between 7.16.4 and PSK+7.16.4 using ANOVA analysis. (C) shown are tumor growth curves in mice receiving PBS (●), PSK+7.16.4 (▼), PSK+7.16.4 with NK cell depletion (○), PSK+7.16.4 with CD8 T cell depletion (△), or PSK+7.16.4 with CD4 T cell depletion (□). The Treatment with PSK+7.16.4 mAb or control PBS was initiated on Day 12. The depletion antibodies were administered 3 times on the week before PSK treatment and then 2 times a week during PSK treatment. Each data point represents the average tumor volume in each group (mean±SD, n=5 per group). The difference between no depletion group (PSK+7.16.4) and depletion groups was calculated using two tailed Student t test. ***, p<0.001 between CD8 T cell or NK cell depletion group and no depletion group (PSK+7.16.4). There is no difference between CD4 T cell depletion group and no depletion group.
Discussion
Enhancing NK cell function is important to improve the clinical response to trastuzumab and other mAb therapy. In this study, we have demonstrated that in vitro treatment with TLR2 agonist PSK can activate human NK cells and augment trastuzumab-mediated ADCC. In a mouse model of HER2+ breast cancer, orally administered PSK augments the anti-tumor effect of anti-HER2/neu mAb therapy. These findings indicate the potential of using a natural product as an adjuvant to improve the clinical response to trastuzumab.
NK cells are the major mediator of ADCC and the function of NK cells has been shown to impact the treatment outcome of trastuzumab, rituximab, and cetuximab (8). NK cell function is frequently impaired in cancer patients and improving NK cell function via cytokines or TLR agonists has shown promise to augment ADCC (38-42). For example, IL-2 ex vivo treatment of NK cells was shown to be able to restore the impairment of trastuzumab-mediated ADCC in the patients with gastric cancer (11). IL-12 has also been shown to augment trastuzumab-mediated ADCC and enhance the anti-tumor actions of trastuzumab via NK IFN-γ secretion (41, 42). Multiple TLR agonists, especially the agonists of TLR9 and 7/8 have shown potential to augment NK cell function (24, 40, 43, 44). For example, CpG ODN has been reported to increase IFN-γ production by NK cells and enhance trastuzumab-mediated lysis of breast cancer cells (17). TLR7 and 8 agonists have also been shown to induce IFN-γ production by NK cells (15, 18). Our study demonstrates the potential of using a natural product with TLR2 agonist activity to augment NK cell function. The concentration of PSK used in our study (10-100 μg/mL) has been reported to be achievable in the blood of cancer patients who received standard oral administration of the drug (3g daily) (45).
Whether TLR2-mediated activation of NK cells is direct or indirect remains controversial in the literature (21-23). Our results showed that TLR2 is expressed on NK cells, although the level is significantly lower than that on B cells or DC. Our finding that CD56bright NK cells expresses more TLR2 than CD56dim cells is consistent with the results by Gorski et al showing that CD56bright NK cells express more TLR2 mRNA than CD56dim NK cells (15). Similar to our observation that PSK induces CD25 expression on NK cells independent of IL-12, Gorski et al observed upregulation of CD69 in both CD56dim and CD56bright NK cells when purified NK cells were stimulated with TLR2 agonist MALP2 (15). It is interesting that although CD56bright cells, the major producer of IFN-γ, express more TLR2 than CD56dim NK cells, they still seem to require the help from IL-12-producing dendritic cells to produce IFN-γ. This might be explained by the concept that CD56bright NK cells generally need 2 signals to produce IFN-γ, and one of these almost always includes IL-12 (46). Previously we have reported the effect of PSK on DC maturation and induction of both IL-12p40 and p70 using mouse bone marrow derived DC. In the current study, we found significant induction of IL-12p40 in PSK-treated human PBMC. IL-12p70 was detected in some PSK-treated PBMC and not in untreated samples. However, the levels were very low and didn’t reach statistical significance (data not shown).
In addition to IFN-γ and IL-12, PSK induced the secretion of other proinflammatory cytokines and chemokines (TNF-α, MIP-1α, and MIP-1β) by PBMC and purified NK cells which could potentially promote chemotaxis. A previous study by Roda et al has shown that IL-8, MIP-1α and RANTES secreted by IL-21-stimulated NK cells in the presence of mAb-coated tumor cells resulted in enhanced migration of T cells (38, 39). Although we didn’t evaluate the potential chemotactic effect of culture supernatant from PSK-stimulated NK cells, our previous study in mouse using selective depletion during PSK treatment showed that the anti-tumor effect of PSK is dependent on both NK cells and CD8 T cells (25). Selective depletion of CD4, CD8 T cells, or NK cells during PSK and 7.16.4 combination therapy also showed the involvement of both NK and CD8 T cells (Figure 6C). This indicates that chemokines released by NK cells could have led to recruitment of T cells that contributed to the anti-tumor effect of PSK. It is noted that NK or CD8 T cell depletion only partially abrogated the anti-tumor effects of PSK and 7.16.4 therapy. This could be explained by the potential of 7.16.4 to directly inhibit tumor cell growth, as suggested in publication (36). It is also noted that in about 50% of the mice that received both PSK and 7.16.4 mAb, the tumor will relapse at a later date, indicating that long-term immunologic memory has not been established (data not shown). Whether the tumor-free mice can reject a second tumor challenge remains to be tested.
In summary, our study indicates the potential of using PSK, a natural product with potent TLR2 agonist activity, to augment the function of NK cells and enhance ADCC. The major advantage of PSK as compared to other TLR agonists that are currently evaluated in clinical trials, such as CpG, imiquimod, or poly I:C, is its known safety profile. PSK is a mushroom extract that has been widely used in Asian countries for its immune potentiating and anti-tumor effects. A meta-analysis of data from three randomized clinical trials in Japan in 1,094 patients with colorectal cancer showed that PSK significantly increased both overall survival and disease-free survival of patients with curatively resected colorectal cancer (29). The anti-tumor effect of PSK has also been demonstrated in other types of cancer, including stomach cancer (27, 47) and lung cancer (48). To our knowledge, our study represents the first report on the potential of PSK to augment trastuzumab-mediated ADCC and the synergistic anti-tumor effect between PSK and HER2-targeted mAb therapy in a preclinical model. This provides rationale for future clinical trials testing the adjuvant effect of PSK when administered concurrently with trastuzumab. While the study reported in this manuscript only used PBMC from normal healthy donors, a recently finished clinical study in breast cancer patients conducted by Dr. Leanna Standish et al. also showed a trend toward increased NK cell cytolytic activity after oral administration of Turkey tail mushroom extract, the same species of mushroom from which PSK is extracted (manuscript under preparation by Standish et al). Whether PSK can enhance the therapeutic effect of trastuzumab and a HER2-targeted vaccine in breast cancer patients will be tested in our group. Since our previous studies have shown that the potential of PSK to stimulate NK cells is dependent on TLR2 (25), we speculate that the clinical response to combination therapy with PSK and trastuzumab may also be dependent on TLR2 and may be impacted by functional TLR2 gene polymorphism that have been reported (49). Hopefully these questions can be addressed in clinical trials in the future. NK cell function not only impacts the clinical response to trastuzumab, but to other monoclonal antibody therapies as well, such as rituximab for lymphoma and cetuximab for head and neck cancer (8). Thus, results from the current study could potentially be expanded to other types of cancer. Our study highlights the potential of combining CAM therapy to mainstream cancer therapy for enhanced therapeutic effect.
Supplementary Material
Translational Relevance.
Trastuzumab has become the standard of care for patients with HER2+ breast cancer. Although the mechanism of the anti-tumor actions of trastuzumab is multifaceted, antibody-dependent cell-mediated cytotoxicity (ADCC) is believed to be one of the major mechanisms. The clinical response to trastuzumab therapy has been shown to be partially associated with the function of NK cells, which are the main mediators of ADCC. Immune response modifiers that can enhance NK cell function and ADCC could potentially improve the clinical response to trastuzumab. In this study, we demonstrated that polysaccharide krestin (PSK), a mushroom extract and a toll-like receptor 2 (TLR2) agonist, can activate human NK cells to secrete IFN-γ and exert enhanced cytolytic activity in lysing K562 target cells and trastuzumab-coated breast cancer cells. Using a mouse model of HER2+ breast cancer, we further demonstrated that concurrent administration of PSK can augment the anti-tumor effect of anti-HER2/neu mAb therapy. These findings suggest the potential of using PSK, a natural product with potent TLR2 agonist activity, as an adjuvant therapy for breast cancer patients to improve the therapeutic effect of trastuzumab.
Acknowledgments
This work was supported by NIH grants R01CA138547 (H.L.), R01AT004314 (M.L.D.), U19AT006028 (L.J.S.), and a gift to the Tumor Vaccine Group.
Abbreviations
- ADCC
antibody-dependent cell-mediated cytotoxicity
- CAM
complementary and alternative medicine
- CTL
cytotoxic T lymphocyte
- DC
dendritic cells
- E:T
effector: target ratio
- ELISA
enzyme-linked immunosorbant assay
- FBS
fetal bovine serum
- FcγR
immunoglobulin G Fc receptor
- HER2
human epidermal growth factor receptor 2
- HPRT
hypoxanthine ribosyltransferase
- IFN-γ
interferon gamma IL interleukin
- KIR
killer Ig-like receptor
- mAb
monoclonal antibody
- NK
natural killer
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate buffered saline
- PSK
polysaccharide krestin
- TGF-β
transforming growth factor beta
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor alpha
References
- 1.Hudis CA. Drug therapy: Trastuzumab - Mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 2.Baselga J, Perez EA, Pienkowski T, Bell R. Adjuvant trastuzumab: a milestone in the treatment of HER-2-positive early breast cancer. Oncologist. 2006;11(Suppl 1):4–12. doi: 10.1634/theoncologist.11-90001-4. [DOI] [PubMed] [Google Scholar]
- 3.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 4.Varchetta S, Gibelli N, Oliviero B, Nardini E, Gennari R, Gatti G, et al. Elements related to heterogeneity of antibody-dependent cell cytotoxicity in patients under trastuzumab therapy for primary operable breast cancer overexpressing Her2. Cancer Res. 2007;67:11991–9. doi: 10.1158/0008-5472.CAN-07-2068. [DOI] [PubMed] [Google Scholar]
- 5.Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006;94:259–67. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–96. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 7.Zamai L, Ponti C, Mirandola P, Gobbi G, Papa S, Galeotti L, et al. NK cells and cancer. J Immunol. 2007;178:4011–6. doi: 10.4049/jimmunol.178.7.4011. [DOI] [PubMed] [Google Scholar]
- 8.Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J Clin Oncol. 2010;28:4390–9. doi: 10.1200/JCO.2009.27.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol. 2004;172:7335–40. doi: 10.4049/jimmunol.172.12.7335. [DOI] [PubMed] [Google Scholar]
- 10.Varker KA, Terrell CE, Welt M, Suleiman S, Thornton L, Andersen BL, et al. Impaired natural killer cell lysis in breast cancer patients with high levels of psychological stress is associated with altered expression of killer immunoglobin-like receptors. J Surg Res. 2007;139:36–44. doi: 10.1016/j.jss.2006.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kono K, Takahashi A, Ichihara F, Sugai H, Fujii H, Matsumoto Y. Impaired antibody-dependent cellular cytotoxicity mediated by herceptin in patients with gastric cancer. Cancer Res. 2002;62:5813–7. [PubMed] [Google Scholar]
- 12.Garner WL, Minton JP, James AG, Hoffmann CC. Human breast cancer and impaired NK cell function. J Surg Oncol. 1983;24:64–6. doi: 10.1002/jso.2930240115. [DOI] [PubMed] [Google Scholar]
- 13.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 14.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–51. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 15.Gorski KS, Waller EL, Bjornton-Severson J, Hanten JA, Riter CL, Kieper WC, et al. Distinct indirect pathways govern human NK-cell activation by TLR-7 and TLR-8 agonists. Int Immunol. 2006;18:1115–26. doi: 10.1093/intimm/dxl046. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt KN, Leung B, Kwong M, Zarember KA, Satyal S, Navas TA, et al. APC-independent activation of NK cells by the Toll-like receptor 3 agonist double-stranded RNA. J Immunol. 2004;172:138–43. doi: 10.4049/jimmunol.172.1.138. [DOI] [PubMed] [Google Scholar]
- 17.Roda JM, Parihar R, Carson WE., 3rd. CpG-containing oligodeoxynucleotides act through TLR9 to enhance the NK cell cytokine response to antibody-coated tumor cells. J Immunol. 2005;175:1619–27. doi: 10.4049/jimmunol.175.3.1619. [DOI] [PubMed] [Google Scholar]
- 18.Hart OM, Athie-Morales V, O’Connor GM, Gardiner CM. TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J Immunol. 2005;175:1636–42. doi: 10.4049/jimmunol.175.3.1636. [DOI] [PubMed] [Google Scholar]
- 19.Ebihara T, Azuma M, Oshiumi H, Kasamatsu J, Iwabuchi K, Matsumoto K, et al. Identification of a polyI:C-inducible membrane protein that participates in dendritic cell-mediated natural killer cell activation. J Exp Med. 2010;207:2675–87. doi: 10.1084/jem.20091573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedberg JW, Kelly JL, Neuberg D, Peterson DR, Kutok JL, Salloum R, et al. Phase II study of a TLR-9 agonist (1018 ISS) with rituximab in patients with relapsed or refractory follicular lymphoma. Br J Haematol. 2009;146:282–91. doi: 10.1111/j.1365-2141.2009.07773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez J, Huang X, Yang Y. Direct TLR2 signaling is critical for NK cell activation and function in response to vaccinia viral infection. PLoS Pathog. 2010;6:e1000811. doi: 10.1371/journal.ppat.1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcenaro E, Ferranti B, Falco M, Moretta L, Moretta A. Human NK cells directly recognize Mycobacterium bovis via TLR2 and acquire the ability to kill monocyte-derived DC. Int Immunol. 2008;20:1155–67. doi: 10.1093/intimm/dxn073. [DOI] [PubMed] [Google Scholar]
- 23.Azuma M, Sawahata R, Akao Y, Ebihara T, Yamazaki S, Matsumoto M, et al. The peptide sequence of diacyl lipopeptides determines dendritic cell TLR2-mediated NK activation. PLoS One. 2010:5. doi: 10.1371/journal.pone.0012550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreno M, Mol BM, von Mensdorff-Pouilly S, Verheijen RH, von Blomberg BM, van den Eertwegh AJ, et al. Toll-like receptor agonists and invariant natural killer T-cells enhance antibody-dependent cell-mediated cytotoxicity (ADCC) Cancer Lett. 2008;272:70–6. doi: 10.1016/j.canlet.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 25.Lu H, Yang Y, Gad E, Wenner CA, Chang A, Larson ER, et al. Polysaccharide Krestin Is a Novel TLR2 Agonist that Mediates Inhibition of Tumor Growth via Stimulation of CD8 T Cells and NK Cells. Clin Cancer Res. 2011;17:67–76. doi: 10.1158/1078-0432.CCR-10-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher M, Yang LX. Anticancer effects and mechanisms of polysaccharide-K (PSK): implications of cancer immunotherapy. Anticancer Res. 2002;22:1737–54. [PubMed] [Google Scholar]
- 27.Oba K, Teramukai S, Kobayashi M, Matsui T, Kodera Y, Sakamoto J. Efficacy of adjuvant immunochemotherapy with polysaccharide K for patients with curative resections of gastric cancer. Cancer Immunol Immunother. 2007;56:905–11. doi: 10.1007/s00262-006-0248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohwada S, Ikeya T, Yokomori T, Kusaba T, Roppongi T, Takahashi T, et al. Adjuvant immunochemotherapy with oral Tegafur/Uracil plus PSK in patients with stage II or III colorectal cancer: a randomised controlled study. Br J Cancer. 2004;90:1003–10. doi: 10.1038/sj.bjc.6601619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakamoto J, Morita S, Oba K, Matsui T, Kobayashi M, Nakazato H, et al. Efficacy of adjuvant immunochemotherapy with polysaccharide K for patients with curatively resected colorectal cancer: a meta-analysis of centrally randomized controlled clinical trials. Cancer Immunol Immunother. 2006;55:404–11. doi: 10.1007/s00262-005-0054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu H, Knutson KL, Gad E, Disis ML. The tumor antigen repertoire identified in tumor-bearing Neu transgenic mice predicts human tumor antigens. Cancer Res. 2006;66:9754–61. doi: 10.1158/0008-5472.CAN-06-1083. [DOI] [PubMed] [Google Scholar]
- 31.Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8:652–8. [PubMed] [Google Scholar]
- 32.Papadopoulos NG, Dedoussis GV, Spanakos G, Gritzapis AD, Baxevanis CN, Papamichail M. An improved fluorescence assay for the determination of lymphocyte-mediated cytotoxicity using flow cytometry. J Immunol Methods. 1994;177:101–11. doi: 10.1016/0022-1759(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 33.Knutson KL, Lu H, Stone B, Reiman JM, Behrens MD, Prosperi CM, et al. Immunoediting of cancers may lead to epithelial to mesenchymal transition. J Immunol. 2006;177:1526–33. doi: 10.4049/jimmunol.177.3.1526. [DOI] [PubMed] [Google Scholar]
- 34.Lu H, Wagner WM, Gad E, Yang Y, Duan H, Amon LM, et al. Treatment failure of a TLR-7 agonist occurs due to self-regulation of acute inflammation and can be overcome by IL-10 blockade. J Immunol. 2010;184:5360–7. doi: 10.4049/jimmunol.0902997. [DOI] [PubMed] [Google Scholar]
- 35.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Knutson KL, Almand B, Dang Y, Disis ML. Neu antigen-negative variants can be generated after neu-specific antibody therapy in neu transgenic mice. Cancer Res. 2004;64:1146–51. doi: 10.1158/0008-5472.can-03-0173. [DOI] [PubMed] [Google Scholar]
- 37.Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H, et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci U S A. 2011;108:7142–7. doi: 10.1073/pnas.1016569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roda JM, Joshi T, Butchar JP, McAlees JW, Lehman A, Tridandapani S, et al. The activation of natural killer cell effector functions by cetuximab-coated, epidermal growth factor receptor positive tumor cells is enhanced by cytokines. Clin Cancer Res. 2007;13:6419–28. doi: 10.1158/1078-0432.CCR-07-0865. [DOI] [PubMed] [Google Scholar]
- 39.Roda JM, Parihar R, Lehman A, Mani A, Tridandapani S, Carson WE., 3rd. Interleukin-21 enhances NK cell activation in response to antibody-coated targets. J Immunol. 2006;177:120–9. doi: 10.4049/jimmunol.177.1.120. [DOI] [PubMed] [Google Scholar]
- 40.Moga E, Alvarez E, Canto E, Vidal S, Rodriguez-Sanchez JL, Sierra J, et al. NK cells stimulated with IL-15 or CpG ODN enhance rituximab-dependent cellular cytotoxicity against B-cell lymphoma. Exp Hematol. 2008;36:69–77. doi: 10.1016/j.exphem.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Parihar R, Dierksheide J, Hu Y, Carson WE. IL-12 enhances the natural killer cell cytokine response to Ab-coated tumor cells. J Clin Invest. 2002;110:983–92. doi: 10.1172/JCI15950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaime-Ramirez AC, Mundy-Bosse BL, Kondadasula S, Jones NB, Roda JM, Mani A, et al. IL-12 Enhances the Antitumor Actions of Trastuzumab via NK Cell IFN-{gamma} Production. J Immunol. 2011 doi: 10.4049/jimmunol.1000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sivori S, Falco M, Della Chiesa M, Carlomagno S, Vitale M, Moretta L, et al. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci U S A. 2004;101:10116–21. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Butchar JP, Mehta P, Justiniano SE, Guenterberg KD, Kondadasula SV, Mo X, et al. Reciprocal regulation of activating and inhibitory Fc{gamma} receptors by TLR7/8 activation: implications for tumor immunotherapy. Clin Cancer Res. 2010;16:2065–75. doi: 10.1158/1078-0432.CCR-09-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kariya Y, Inoue N, Kihara T, Okamoto N, Sugie K, Mori T, et al. Activation of human natural killer cells by the protein-bound polysaccharide PSK independently of interferon and interleukin 2. Immunol Lett. 1992;31:241–5. doi: 10.1016/0165-2478(92)90121-4. [DOI] [PubMed] [Google Scholar]
- 46.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–9. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakazato H, Koike A, Saji S, Ogawa N, Sakamoto J. Efficacy of immunochemotherapy as adjuvant treatment after curative resection of gastric cancer. Study Group of Immunochemotherapy with PSK for Gastric Cancer. Lancet. 1994;343:1122–6. doi: 10.1016/s0140-6736(94)90233-x. [DOI] [PubMed] [Google Scholar]
- 48.Hayakawa K, Mitsuhashi N, Saito Y, Takahashi M, Katano S, Shiojima K, et al. Effect of krestin (PSK) as adjuvant treatment on the prognosis after radical radiotherapy in patients with non-small cell lung cancer. Anticancer Res. 1993;13:1815–20. [PubMed] [Google Scholar]
- 49.Misch EA, Hawn TR. Toll-like receptor polymorphisms and susceptibility to human disease. Clin Sci (Lond) 2008;114:347–60. doi: 10.1042/CS20070214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.