Abstract
Background
Observational studies have demonstrated an association between ERCC1 expression level and health outcomes in patients with advanced non-small cell lung cancer (NSCLC) treated with platinum-based regimens. This analysis presents pooled estimates of association from these studies to better elucidate the prognostic role of ERCC1 in advanced NSCLC.
Methods
A systematic literature search was conducted using the MEDLINE, EMBASE, and ASCO annual meeting databases from June, 1995 to December, 2010. Included studies were evaluated for clinical, methodological, and statistical heterogeneity. Pooled analyses were conducted using fixed and random effects models.
Results
In high ERCC1 expression vs. low ERCC1 expression patients, pooled analysis results demonstrated a significantly lower response (RR: 0.80, 0.66–0.98) and significantly higher risk of death (HR: 2.03, 1.49–2.78), respectively. Sub-group analyses demonstrated significant heterogeneity in outcomes by ERCC1 measurement method (I2: 90.7%, p=0.001) and patient population ethnicity (I2: 66%, p=0.003).
Conclusion
This study’s findings support the hypothesis that ERCC1 expression is associated with response rate and overall survival in advanced NSCLC patients treated with platinum-based chemotherapy. Heterogeneity in sub-group analyses demonstrates the need for standardized methods to classify ERCC1 expression level, studies evaluating the association between ERCC1 expression and overall survival in non-Asian populations, and studies evaluating interaction between ERCC1 and other known prognostic factors in advanced NSCLC.
Keywords: ERCC1, advanced lung cancer, non-small cell lung cancer, prognostic, meta-analysis
Background
Lung cancer is the currently the most common cancer in the United States, with an estimated 222,000 new cases diagnosed in 2010. (1) The disease’s high incidence and mortality rates make it the leading cause of cancer-related death in the United States, with an estimated 157,000 deaths in 2010. (1) Lung cancer consists of two primary types, non-small cell lung cancer (NSCLC) and small cell lung cancer, with NSCLC comprising approximately 80% of cases.(2) Because of the asymptomatic nature of the disease and lack of standard screening modalities to detect NSCLC at early stages, approximately 65% of cases are diagnosed at an advanced stage.(3)
Patients diagnosed with advanced non-small lung cancer (NSCLC) have poor life expectancy and few effective treatment options. (4,5) Potentially curative surgery is not a treatment option in this patient population, and so standard treatment involves platinum-based chemotherapeutic regimens with cisplatin or carboplatin.(6,7) While this treatment approach has been demonstrated to extend overall and progression free survival, these increases in survival are, on average, no more than several weeks to several months. (4,5) Additionally, research has demonstrated that a substantial proportion of advanced NSCLC patients do not respond to standard platinum-based regimens. (4) Because of the limited effectiveness of standard regimens, substantial research efforts have been undertaken to target specific agents to patient sub-groups that derive maximum benefit. (4,5) One strategy of this type is targeting platinum-based regimens based on expression levels of various DNA repair mechanisms.
DNA repair mechanisms are hypothesized to play an important role in the treatment of patients with advanced NSCLC because the presence of these factors in tumors is associated with resistance to platinum agents like cisplatin, carboplatin, and oxaliplatin. (8–10) Platinum agents exert their anti-tumor activity by binding to DNA and creating platinum–DNA adducts that can lead to cell destruction. However, this process can be inhibited when DNA repair mechanisms, such as excision repair cross-complementation group 1 (ERCC1), recognize and remove platinum-induced DNA adducts. (8–10) A prognostic association between high ERCC1 expression level and low response rates and overall survival has been established by a number of small observational studies examining advanced NSCLC patients treated with platinum-based chemotherapy. (11–21) Additionally, ERCC1 expression level has been demonstrated to have similar associations in bladder, biliary tract, pancreatic, colorectal, and ovarian cancer treated with platinum-based regimens. (22–26) To our knowledge, there has been only one prospective trial that has examined the predictive ability of an ERCC1-based strategy to select either platinum based or non-platinum based regimens in advanced NSCLC, and this study demonstrated significant increases in response in an ERCC1-guided arm versus an arm where all patients received platinum-based therapy. (27)
There has been varied use of the terms “prognostic” and “predictive” in the ERCC1 literature. We define the term “prognostic” to mean the impact of ERCC1 status on survival among patients receiving the same treatment. Therefore, a prognostic association would relate to differences in survival due to ERCC1 status, rather than differences in treatment. We define the term “predictive” to mean information about a differential treatment effect (i.e. the relative survival in the treated vs. control groups) based on ERCC1 status. Therefore, a predictive association would relate to differences in survival due to an interaction between ERCC1 status and treatment. These definitions are consistent with a number of previous reviews and analyses that have discussed the role of prognostic and predictive biomarkers in cancer outcomes. (28–34)
While ERCC1-guided treatment strategies hold great promise for enhancing the ability of physicians to “personalize” advanced NSCLC regimens, the translation of these findings into clinical practice has been limited by several factors. First, there has been only one large randomized trial examining an ERCC1-based strategy in advanced NSCLC, and many stakeholders expect more robust prospective evidence before clinical implementation. (27) Second, because most studies to date have involved small sample sizes, many have lacked power to detect small to moderate differences in response rates or overall survival based on ERCC1 status. (11,15–18, 20–21) Third, the ERCC1 studies that have been conducted have utilized varied methods to ascertain ERCC1 levels, with some using real-time polymerase chain reaction (RT-PCR), and others using immunohistochemistry (IHC). As a result of this variation in methods, there is uncertainty about whether the ERCC1 expression level classifications of each approach are associated with equivalent health outcomes. Lastly, the majority of ERCC1 studies have been conducted in Asian populations. These populations have fundamentally different prognosis relative to alternative racial/ethnic populations, and this can limit the generalizability of the findings to other patient populations. For example, in Japanese and U.S. NSCLC patient populations, Gandara and colleagues have demonstrated different distributions of ERCC1 and other markers, as well as different health outcomes in patients treated with the same chemotherapy regimen. (35) As a result of all of these factors, considerable uncertainty remains about the association between ERCC1 status and treatment response and overall survival. To help address this uncertainty, we performed a systematic review and meta-analysis to evaluate the scientific evidence for the prognostic association between ERCC1 expression level and treatment outcomes in advanced NSCLC patients.
Materials & Methods
Literature Search Strategy
MEDLINE and EMBASE electronic databases were searched using the broad search terms: “ERCC1” and “Lung” to identify potential studies for inclusion in the analysis. Additionally, a computerized search of abstracts presented at the Annual Meetings of the American Society of Clinical Oncology (ASCO) was performed. The references in all reviewed articles were screened to identify additional articles that were not identified in the literature search described above. The time frame for all searches was June, 1995 to December, 2010. Only publications reporting results in English were evaluated for inclusion in the analysis.
Selection Criteria
This meta-analysis includes publications from studies meeting the following criteria: 1) patients had a diagnosis of advanced NSCLC, 2) all patients received platinum-based chemotherapy, 3) results are presented stratified by ERCC1 expression level, 4) the results are part of an original analysis.
Several of the studies identified using the inclusion criteria above were updates of earlier publications, and involved the same patient population. In such cases, only the most recent publication results were included in the analysis.
Data Extraction
Data were manually extracted from each publication using a data extraction form developed in Microsoft Excel (Redmond, WA, 2008). The following information was recorded for each publication: first author’s name, publication date, country of study, total number of patients in the study sample, proportion male, number of ERCC1 “high” expression patients, number of ERCC1 “low” expression patients, method used to ascertain ERCC1 expression level, covariates utilized in multivariate analyses of overall survival, proportion chemotherapy naïve at baseline, proportion with Stage IV disease, and proportion with ECOG performance status of 0. In cases where information was not presented, the article’s corresponding author was contacted to obtain the information. In the event that the given information was still not made available, it was classified as “not reported”.
Statistical Methods
The endpoints considered in the pooled analyses were response rate and overall survival. RECIST criteria were utilized to define response, with “complete response” or “partial response” classified as “response”, and “stable” or “progressive” disease classified as “non-response”. The risk ratio (RR) was abstracted or calculated to quantitatively evaluate the association between ERCC1 expression level and response rate. The association between ERCC1 level and overall survival was evaluated using the hazard ratio (HR) and 95% confidence interval from multivariate Cox proportional hazards models. In instances where the HR 95% confidence interval was not reported, the interval was derived using the reported HR and p value.
The pooled RR, HR, and 95% confidence intervals (CI) were calculated. Analyses were weighted by inverse variance. In the analysis of the association between ERCC1 level and response rate, an RR of 1 indicates a lack of association, an RR greater than 1 indicates greater response in high ERCC1 patients, and an RR less than 1 indicates greater response in low ERCC1 patients. In the analysis of the association between ERCC1 level and overall survival, a HR of 1 indicates a lack of association between ERCC1 level and risk of death, a HR of greater than 1 indicates a greater risk of death in high ERCC1 patients, and a HR less than 1 indicates a greater risk of death in low ERCC1 level patients.
The pooled RR and HR estimates were initially calculated using a fixed effects model. If the fixed effects p value for the I2 statistic was less than 0.10, indicating significant heterogeneity across studies, the pooled estimate was calculated using a random effects model. Additionally, in instances where there was qualitative evidence of methodological heterogeneity across studies (e.g. different ERCC1 expression ascertainment methods), a random effects model was utilized.
In the sub-group analysis of ERCC1 expression ascertainment method, studies were classified as either using RT-PCR or IHC, as reported in the given publication. In the sub-group analysis of patient population type, studies conducted in Korea, China, and Japan were classified as “Asian population” and studies conducted in Spain, Denmark, Germany, Switzerland, and England were classified as “European population”.
All statistical analyses were carried out using RevMan 5.0 software (Copenhagen, Denmark, 2008).
Results
Study Characteristics
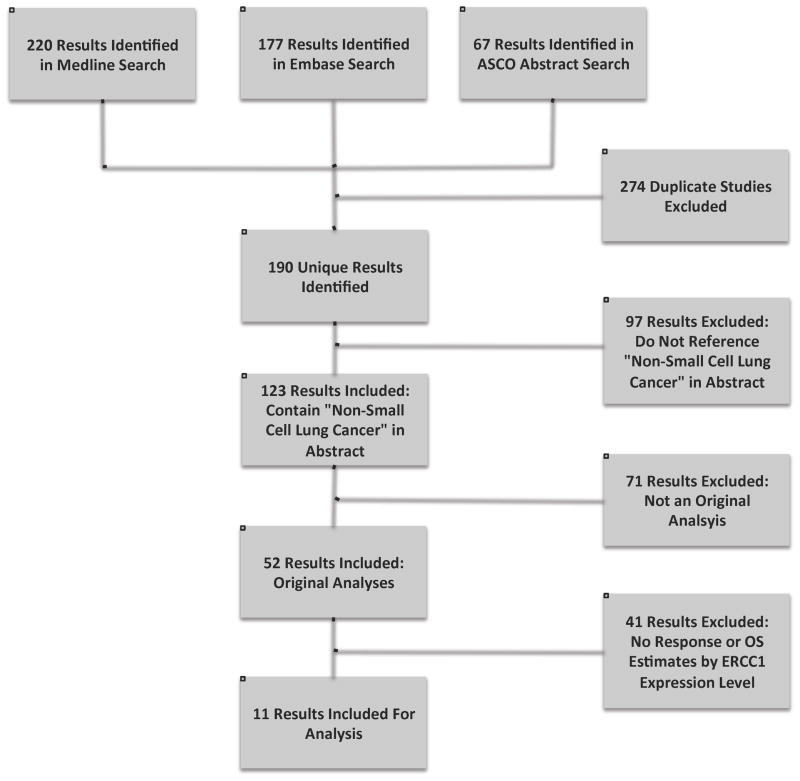
The MEDLINE electronic database search using the search terms “ERCC1” and “lung” identified a total of 220 studies. An EMBASE search did not reveal any additional studies. Searches of the ASCO meeting abstracts database (1995–2010) identified 15 abstracts, but all were early reports of studies identified through the MEDLINE and EMBASE searches. After exclusion of the studies that did not meet the inclusion criteria, 11 studies remained for analysis. Additional information about this search strategy is illustrated in flowchart form in Figure 1.
Figure 1. Electronic Search Flow Chart.
Of the 11 total studies identified through the search strategy, 9 were included in the pooled analyses of the association between ERCC1 level and response rate, and 8 were included in the analysis of association between ERCC1 level and overall survival. Table 1 lists the studies identified, their critical characteristics, and the specific analyses in which they were included. Sample sizes in the included studies ranged from 40 to 264.
Table 1. Studies Included in Pooled Analyses.
This table provides the key characteristics of the studies included in this meta-analysis, as well as the specific analyses to which each study contributed.
| First Author | Year | Population | Patients | ERCC1 Measurement Method | Response Rate Pooled Analysis | Overall Surviva Pooled Analysis |
|---|---|---|---|---|---|---|
| Ren11 | 2010 | Asian | 100 | RT-PCR | X | X |
| Su12 | 2010 | Asian | 85 | RT-PCR | X | |
| Wang13 | 2010 | Asian | 124 | IHC | X | X |
| Ikeda14 | 2009 | Asian | 40 | IHC | X | |
| Lee15 | 2009 | Asian | 50 | IHC | X | X |
| Lord16 | 2002 | European | 56 | RT-PCR | X | X |
| Ota17 | 2009 | Asian | 156 | IHC | X | X |
| Booton18 | 2007 | European | 66 | RT-PCR | X | |
| Holm19 | 2009 | European | 163 | IHC | X | |
| Li20 | 2010 | Asian | 115 | RT-PCR | X | X |
| Vilmar21 | 2010 | European | 264 | IHC | X |
Clinical and Methodological heterogeneity
The included studies varied in their approaches, with some utilizing retrospective observational designs, and other utilizing prospective observational designs. The included studies also varied in ways that could affect response rate or overall survival, including: racial/ethnic composition of the study sample, proportion with previous exposure to therapy, proportion of patients with stage IV disease, proportion of patients with different performance status levels, and proportion male. In addition, studies varied in terms of the testing methods and cutoff criteria employed to characterize ERCC1 expression levels as either “high” or “low”, with 6 utilizing RT-PCR and 5 utilizing IHC. Due to these factors, there was considerable clinical and methodological heterogeneity between studies. Consequently, random effects models were utilized to pool study results in all analyses reported below.
Statistical Pooling
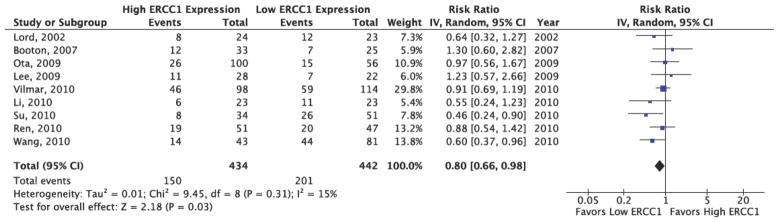
The results of the pooled analysis of the association between ERCC1 level and response rate are provided in Figure 2. Though the I2 statistic in the fixed effects model did not demonstrate significant heterogeneity in the results (I2: 13%, p=0.33), a random effects model was utilized to pool the risk ratios due to evidence of methodological heterogeneity across studies. As Figure 2 demonstrates, patients with high ERCC1 expression levels were 20% less likely to experience response relative to patients with low ERCC1 expression levels (RR: 0.80, 95% CI: 0.66–0.98). These results indicated a statistically significant difference in response between high and low ERCC1 expression patients.
Figure 2. ERCC1 Expression and Response Rate Pooled Analysis Results.
This figure presents the study-level results and pooled analysis results for the association between ERCC1 status and response. The pooled analysis demonstrates a RR=0.80 (95% CI: 0.66, 0.98), significantly favoring low ERCC1 patients.
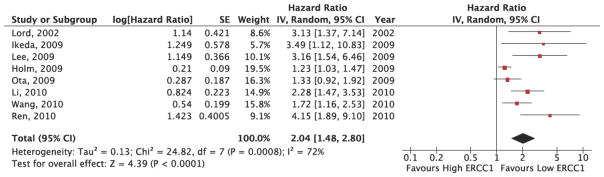
The results of the pooled analysis of the association between ERCC1 level and overall survival are provided in Figure 3. Because the I2 statistic in the fixed effects model demonstrated statistically significant heterogeneity in the results (I2: 70%, p=0.02), a random effects model was utilized to pool the hazard ratios for the included studies. As Figure 3 demonstrates, patients with high ERCC1 expression levels had a risk of death 2.03 times greater than patients with low ERCC1 expression levels (HR: 2.03, 95% CI: 1.49, 2.78). These results indicated a statistically significant difference in overall survival between high and low ERCC1 expression patients. The pooled hazard ratios were derived from multivariate Cox proportional hazards models that adjusted for potential confounders. While many of the covariates included in these models were the same across studies (e.g. age, gender, performance status, stage, histology), some studies adjusted for factors that were not included in other analyses (e.g. IIIβ tubulin expression, pleural effusion, BCRP expression). This finding also supports use of random effects models to pool study results.
Figure 3. ERCC1 Expression and Overall Survival Pooled Analysis Results.
This figure presents the study-level results and pooled analysis results for the association between ERCC1 status and overall survival. The pooled analysis demonstrates a HR=2.04 (95% CI: 1.48, 2.80), significantly favoring low ERCC1 patients.
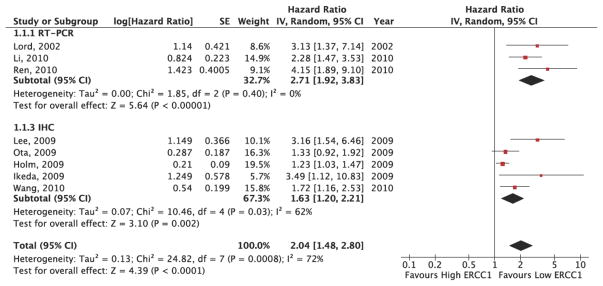
The results of the ERCC1 ascertainment method sub-group analysis are provided in Figure 4. Because the overall I2 statistic in the fixed effects model demonstrated statistically significant heterogeneity in the results (I2: 70%, p=0.02), a random effects model was utilized to pool the sub-group hazard ratios. As Figure 3 demonstrates, in the RT-PCR sub-group, patients with high ERCC1 expression levels had a risk of death 3.02 times greater than patients with low ERCC1 expression levels (HR: 3.02, 95% CI: 1.96, 4.63). These results indicated a statistically significant difference in overall survival between high and low ERCC1 expression patients. Within the RT-PCR sub-group, there was very little heterogeneity in outcome (I2: 0%, p=0.55). In the IHC sub-group, patients with high ERCC1 expression levels had a risk of death 1.63 times greater than patients with low ERCC1 expression levels (HR: 1.63, 95% CI: 1.20, 2.21). These results indicated a statistically significant difference in overall survival between high and low ERCC1 expression patients. Within the IHC sub-group, there was significant heterogeneity in outcome (I2: 59%, p=0.04). Overall, the sub-group analysis demonstrated significant heterogeneity between the outcomes of the RT-PCR and IHC sub-groups (I2: 90.7%, p=0.001).
Figure 4. ERCC1 Expression and Overall Survival Ascertainment Method SubGroup Pooled Analysis Results.
This figure presents the study-level results and pooled analysis results for the association between ERCC1 status and overall survival by ascertainment method sub-group. The RT-PCR sub-group pooled analysis demonstrates a HR=2.71 (95% CI: 1.92, 3.83), significantly favoring low ERCC1 patients. The IHC subgroup pooled analysis demonstrates a HR=1.63 (95% CI: 1.20, 2.21), and also significantly favors low ERCC1 patients. There is evidence of significant heterogeneity within the IHC sub-group (I2=62%, p=0.03), as well as between sub-groups (I2=72%, p=0.0008).
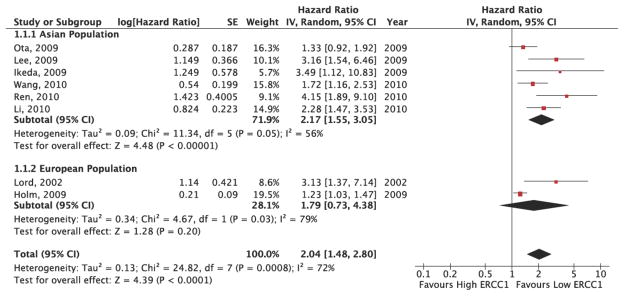
The results of the patient population sub-group analysis are provided in Figure 5. Because the overall I2 statistic in the fixed effects model demonstrated statistically significant heterogeneity in the results (I2: 70%, p=0.02), a random effects model was utilized to pool the sub-group hazard ratios. As Figure 5 demonstrates, in the Asian population sub-group, patients with high ERCC1 expression levels had a risk of death 2.16 times greater than patients with low ERCC1 expression levels (HR: 2.16, 95% CI: 1.59, 2.95). These results indicated a statistically significant difference in overall survival between high and low ERCC1 expression patients. Within the Asian population subgroup, there was significant heterogeneity in outcome (I2: 48%, p=0.07). In the European population sub-group, patients with high ERCC1 expression levels had a risk of death 1.8 times greater than patients with low ERCC1 expression levels (HR: 1.8, 95% CI: 0.75, 4.35). These results did not indicate a statistically significant difference in overall survival between high and low ERCC1 expression patients. Within the European population sub-group, there was significant heterogeneity in outcome (I2: 78%, p=0.03). Overall, the sub-group analysis demonstrated significant heterogeneity between the outcomes of the Asian and European population sub-groups (I2: 66%, p=0.003).
Figure 5. ERCC1 Expression and Overall Survival Population Sub-Group Pooled Analysis Results.
This figure presents the study-level results and pooled analysis results for the association between ERCC1 status and overall survival by population sub-group. The Asian population sub-group pooled analysis demonstrates a HR=2.17 (95% CI: 1.55, 3.05), significantly favoring low ERCC1 patients. The European population sub-group pooled analysis demonstrates a HR=1.79 (95% CI: 0.73, 4.38), favoring low ERCC1 patients, but with non-significant results. There is evidence of significant heterogeneity within the Asian (I2=56%, p=0.05) and European (I2=79%, p=0.03) sub-groups, as well as between sub-groups (I2=72%, p=0.0008).
Publication Bias
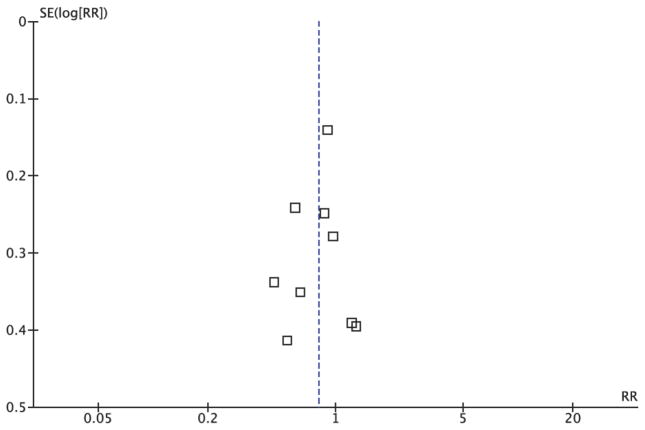
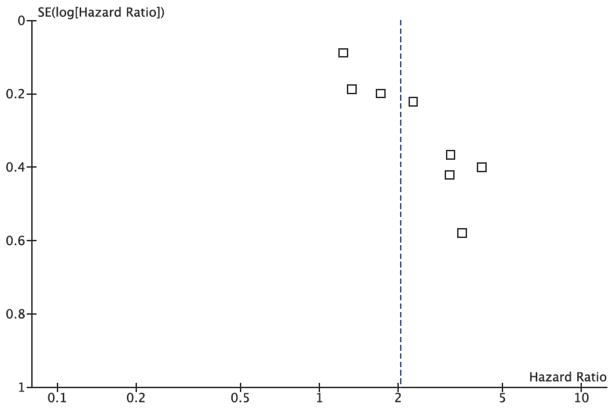
The funnel plot for the overall pooled analysis of the association between ERCC1 level and response (Figure 6) revealed little evidence of publication bias, with a symmetrical distribution of study results around the pooled measurement of effect. Alternatively, the funnel plot for the overall pooled analysis of the association between ERCC1 level and overall survival (Figure 7) revealed an absence of studies in the lower left quadrant of the graph, where we would expect to find studies with smaller effect sizes and wider 95% confidence intervals. This finding indicated potential publication bias in favor of more positive studies. The evaluation of publication bias using the funnel plot approach was somewhat limited by the small number of studies identified for inclusion in the pooled analyses.
Figure 6. Publication Bias Funnel Plot for ERCC1 Expression and Response Rate Random Effects Pooled Analysis.
This figure demonstrates little evidence of publication bias based on its symmetrical distribution of study results around the pooled effect estimate.
Figure 7. Publication Bias Funnel Plot for ERCC1 Expression and Overall Survival Random Effects Pooled Analysis.
This figure demonstrates evidence of publication bias based on its lack of studies in its lower left quadrant, where we would expect to find studies with smaller effect sizes and greater levels of variability.
Discussion
The results of this systematic review and meta-analysis demonstrate the prognostic significance of ERCC1 expression level in advanced NSCLC patients with platinum-based chemotherapy. In the overall pooled analysis of the association between ERCC1 level and response, the results indicated a statistically significant increase in response in patients with low ERCC1 expression relative to patients with high ERCC1 expression. Additionally, the overall pooled analysis of the association between ERCC1 level and overall survival demonstrated statistically significant increases in overall survival in patients with low ERCC1 expression relative to patients with high ERCC1 expression. Collectively, these findings support the hypothesis that ERCC1 plays an important prognostic role in the health outcomes of advanced NSCLC patients receiving platinum-based chemotherapy. (27)
Sub-group analysis quantitatively supported the hypothesis that the strength of association between ERCC1 level and overall survival can vary based on the method utilized to ascertain ERCC1 status. While both the RT-PCR and IHC sub-groups demonstrated statistically significant associations between ERCC1 status and overall survival, the test of heterogeneity in outcome between sub-groups was statistically significant. Additionally, there was evidence of significant heterogeneity between studies in the IHC group (I2=62%, p=0.03), but results were very consistent in the RT-PCR subgroup (I2=0% p=0.40). This finding suggests that the methods and cut-points in studies utilizing RT-PCR may be more consistent than those in studies utilizing IHC. Issues surrounding the comparability of RT-PCR and IHC results have been discussed in greater detail by Vilmar and Sorenson. (36) Overall, the heterogeneity demonstrated in this subgroup analysis emphasizes the need for standardized methods and cut points to classify ERCC1 expression level as “high” or “low”.
Sub-group analyses also demonstrated that the strength of association between ERCC1 status and overall survival varies between populations of Asian and European descent. This effect was demonstrated by the significant heterogeneity in outcome between Asian and European sub-groups. It is unclear if this heterogeneity is the result of a true underlying biological effect, or merely the result of the small number of studies that have been conducted in European populations. While several studies have demonstrated favorable NSCLC prognostic factors in Asian patients relative to Caucasian patients (e.g. never-smokers, EGFR status, adenocarcinoma histology), it remains unclear if these factors impact ERCC1-based health outcomes. (35,37,38) Our study-level data did not allow us to evaluate these types of relationships, but this is an interesting area for future research. For example, Gandara and colleagues recently demonstrated an association between ERCC1 status and EGFR status, however this study was unable to evaluate the impact of this association on health outcomes due to data limitations. (39) Overall, the findings of this sub-group analysis emphasize the need for additional study of the association between ERCC1 status and overall survival in non-Asian populations, as well as interactions between prognostic factors in the general NSCLC population.
While the response rate pooled analysis showed little evidence of publication bias, the overall survival analysis did demonstrate some evidence of publication bias. The overall survival funnel plot lacked studies in the lower left quadrant, demonstrating a lack of studies with smaller effect sizes and greater variability, and indicating bias in favor of more positive outcomes. Inclusion of studies in the lower left quadrant would be expected to decrease the strength of association between low ERCC1 status and prolonged survival. However, because these studies would be expected to have very small sample sizes and high variability, they would contribute very little weight to the pooled analyses. Consequently, the association between ERCC1 status and overall survival would be likely to remain statistically significant, and relatively strong, even if such studies were included in the overall survival analysis. This suggests that the association between ERCC1 status and overall survival demonstrated by this study should be robust to any potential publication bias.
This analysis only addresses the prognostic significance of ERCC1 expression because there is a lack of studies examining the predictive role of this biomarker. No studies to date have investigated ERCC1 expression testing as a predictive means to select advanced NSCLC patients for platinum-based chemotherapy with the intention of improving survival. Cobo et al. conducted the only predictive ERCC1 study identified by the search strategy outlined above, but this study was not included in the pooled analyses because high ERCC1 patients did not receive platinum-based chemotherapy. In that study, patients were randomized to either a standard care arm that received platinum-doublet therapy (cisplatin/docetaxel), or an ERCC1-guided arm where patients with high ERCC1 received non-platinum therapy (gemcitabine/docetaxel) and patients with low ERCC1 received platinum-doublet therapy (cisplatin/docetaxel). (27) The primary outcome was objective response rate. The results of this study demonstrated statistically significant increases in response in the ERCC1-guided arm relative to the standard care arm (59.2% vs. 39.3%, p=0.03). (18) The study also examined secondary endpoints for progression-free survival (PFS) and overall survival (OS), but demonstrated a non-significant protective effect in the ERCC1-guided arm (PFS HR: 0.9, 0.7 to 1.1; OS HR: 0.9, 0.7 to 1.2). (27) This study serves as an example of a prospective design that could be used to investigate the role of an ERCC1-guided treatment strategy in advanced NSCLC. However, future studies of this type should focus on overall survival, as this is the outcome that is likely most relevant to patients and medical decision-makers. It should also be noted that studies in early-stage NSCLC, as well as bladder, biliary tract, pancreatic, colorectal, and ovarian cancer, suggest there may be a prognostic and/or predictive role for ERCC1 status and treatment with platinum-based chemotherapy. (22–26,40)
There has been only one other meta-analysis of the association between ERCC1 expression and health outcomes in advanced NSCLC patients undergoing platinum-based chemotherapy. This study was conducted by Chen et al. in 2010, and demonstrated an association between low ERCC1 expression levels and increased odds of response (OR: 0.48, 0.35 to 0.64), as well as a more favorable median overall survival ratio (MR: 0.77, 0.47 to 1.07). (41) Additionally, Chen et al. conducted sub-group analyses by ERCC1 ascertainment method, and found that there was not statistically significant differences between RT-PCR and IHC subgroups in measuring the strength of association between ERCC1 status and response rate (I2: 0%, p=0.57). (41) While many of the findings of the Chen et al. study are concordant with the findings of this meta-analysis, there are several methodological factors that this study seeks to improve upon. Chiefly, in the pooled analysis of overall survival, the Chen et al. study utilized median overall survival ratios, which have been demonstrated to be problematic in pooled analyses. (42) Michiels et al. illustrated the problems with using median ratios as a surrogate marker for time-to-event outcomes in a 2005 publication comparing the ability of median ratios, odds ratios, and hazard ratios to represent patient-level outcomes. (42) Additionally, Chen et al. did not clearly define their methods for pooling the median survival ratios, and did not assess heterogeneity in that analysis. Also, in the Chen et al. pooled analysis of objective response rate, a pooled odds ratio was presented even though response fails to meet the rare disease assumption, and is not likely to approximate the risk ratio. For example, in this analysis, we calculated a pooled risk ratio of 0.80 (0.66, 0.98), whereas a pooled analysis of the same studies yields an odds ratio of 0.69 (0.51, 0.91). Additionally, the Chen et al. analysis of response rate utilized a fixed effects model despite evidence of significant heterogeneity between studies according to commonly accepted meta-analysis practices (I2=45%, p=0.05). (43) Lastly, the Chen et al. analysis did not include a number of studies that have been recently published. This analysis seeks to overcome these issues by pooling hazard ratios to evaluate the association between ERCC1 status and overall survival, utilizing random effects models to pool outcomes in the presence of significant heterogeneity, and including studies published through December of 2010.
This study had a number of limitations that are worth noting. First, this analysis was performed at the study level, which limited ability to explore the potential for confounding by various demographic and clinical factors (e.g. ethnicity, gender, smoking status, and histology). Second, this study was predominately based on the findings of observational studies, which inherently contain greater potential for confounding than randomized controlled trials. Lastly, because the vast majority of studies included in the pooled analyses of overall survival were carried out in Asian populations, it is possible that the results of these analyses are not readily generalizable to other populations.
Collectively, this study’s overall findings support the hypothesis that ERCC1 expression level is associated with both response and overall survival in advanced NSCLC treated with platinum-based regimens. Future studies should seek to extend these findings to include prediction of platinum-based chemotherapy benefit in terms of both progression-free and overall survival. Additional studies are required in this area before ERCC1 testing can move toward routine clinical application as a predictive and prognostic tool in advanced non-small cell lung cancer.
Table 2. Studies Included in the ERCC1 Expression and Response Pooled Analysis.
This table provides response rates by ERCC1 status for each study included in the pooled analysis of response.
| First Author | Year | % Response Low ERCC1 | % Response High ERCC1 |
|---|---|---|---|
| Ren11 | 2010 | 42.6% | 37.3% |
| Su12 | 2010 | 51.9% | 23.5% |
| Wang13 | 2010 | 54.3% | 32.6% |
| Lee15 | 2009 | 32.0% | 39.0% |
| Lord16 | 2002 | 52.0% | 36.4% |
| Ota17 | 2009 | 27.0% | 26.0% |
| Booton18 | 2007 | 28.0% | 36.4% |
| Li20 | 2010 | 47.8% | 26.1% |
| Vilmar21 | 2010 | 51.8% | 46.9% |
Response by RECIST criteria
Table 3. Studies Included in the ERCC1 Expression and Overall Survival Pooled Analysis.
This table provides the covariates included in the cox proportional hazards models for each study included in the overall survival pooled analyses and sub-group analyses.
| First Author | Year | Cox Proportional Hazard Model Covariates |
|---|---|---|
| Ren11 | 2010 | Age, Gender, Stage, Histology, PS, Smoking, Response |
| Wang13 | 2010 | Age, Gender, Histology, PS, Pleural Effusion, Metastatic Sites |
| Ikeda14 | 2009 | Age, Gender, Stage, Histology, IIIB Tubulin |
| Lee15 | 2009 | Smoking, Histology, PS, Response |
| Lord16 | 2002 | Age, Gender, Stage, Histology, PS, Weight Loss |
| Ota17 | 2009 | Age, Gender, Histology, PS, Smoking, BCRP |
| Holm19 | 2009 | Age, Gender, Histology |
| Li20 | 2010 | Response, MRP1, LRP |
PS=Performance Status
Acknowledgments
Supported in part by the Center for Comparative Effectiveness Research in Cancer Genomics – CANCERGEN (5RC2CA148570-01, Ramsey S, PI)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Cancer Facts and Figures 2010. Atlanta, GA: American Cancer Society; 2010. [Google Scholar]
- 2.NICE. ERG report: erlotinib for the treatment of relapsed non-small cell lung cancer. Liverpool, England: The National Institute for Health and Clinical Excellence; 2006. [Google Scholar]
- 3.William WN, Lin HY, Lee J, Lippman SM, Roth JA, Kim ES. Revisiting stage IIIB and IV non-small cell lung cancer: Analysis of the surveillance, epidemiology, and end results data. Chest. 2009;136:701–709. doi: 10.1378/chest.08-2968. [DOI] [PubMed] [Google Scholar]
- 4.Burris HA. Shortcomings of current therapies for non–small-cell lung cancer: unmet medical needs. Oncogene. 2009;28:S4–S13. doi: 10.1038/onc.2009.196. [DOI] [PubMed] [Google Scholar]
- 5.Pallis AG, Serfass L, Dziadziuszko R, van Meerbeeck JP, Fennell D, Lacombe D, Welch J, Gridelli C. Targeted therapies in the treatment of advanced/metastatic NSCLC. European Journal of Cancer. 2009;45:2473–2487. doi: 10.1016/j.ejca.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Azzoli CG, Baker S, Temin S, Pao W, Aliff T, Brahmer J, Johnson DH, Laskin JL, Masters G, Milton D, Nordquist L, Pfister DG, Pianadosi S, Schiller JH, Smith R, Smith TJ, Strawn JR, Trent D, Giaccone G. American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for Stage IV Non–Small-Cell Lung Cancer. 2009 doi: 10.1200/JCO.2009.23.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. V.1. 2011. [Google Scholar]
- 8.Mu D, Hsu DS, Sancar A. Reaction mechanism of human DNA repair excision nuclease. J Biol Chem. 1996;271:8285–8294. doi: 10.1074/jbc.271.14.8285. [DOI] [PubMed] [Google Scholar]
- 9.Sancar A. Mechanisms of DNA excision repair. Science. 1994;266:1954–6. doi: 10.1126/science.7801120. [DOI] [PubMed] [Google Scholar]
- 10.Zamble DB, Mu D, Reardon JT, Sancar A, Lippard SJ. Repair of cisplatin–DNA adducts by the mammalian excision nuclease. Biochemistry. 1996;35:10004–10013. doi: 10.1021/bi960453+. [DOI] [PubMed] [Google Scholar]
- 11.Ren S, Zhou S, Zhang L, Xu J, Lv M, Zhang J, Zhou C, Zhang J. High-Level mRNA of Excision Repair Cross-Complementation Group 1 Gene Is Associated With Poor Outcome of Platinum-Based Doublet Chemotherapy of Advanced Non-Small Cell Lung Cancer Patients. Cancer Investigation. 2010 doi: 10.3109/07357901003735659. [DOI] [PubMed] [Google Scholar]
- 12.Su C, Zhou S, Zhang L, Ren S, Xu J, Zhang J, Lv M, Zhou C. ERCC1, RRM1 and BRCA1 mRNA expression levels and clinical outcome of advanced non-small cell lung cancer. Medical Oncology. 2010 doi: 10.1007/s1203201095539. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Zhao J, Yang L, Mao L, An T, Bai H, Wang S, Liu X, Feng G, Wang J. Positive expression of ERCC1 predicts a poorer platinum-based treatment outcome in Chinese patients with advanced non–small-cell lung cancer. Medical Oncology. 2010;27:484–490. doi: 10.1007/s12032-009-9239-3. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda S, Takabe K, Suzuki K. Expression of ERCC1 and class IIIb tubulin for predicting effect of carboplatin/paclitaxel in patients with advanced inoperable non-small cell lung cancer. Pathology International. 2009;59:863–867. doi: 10.1111/j.1440-1827.2009.02463.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee HW, Choi YW, Han JH, Kim JH, Jung JH, Jeong SH, Kang SY, Choi JH, Oh YT, Park KJ, Hwang SC, Sheen SS. Expression of excision repair cross-complementation group 1 protein predicts poor outcome in advanced non-small cell lung cancer patients treated with platinum-based doublet chemotherapy. Lung Cancer. 2009;65:377–382. doi: 10.1016/j.lungcan.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Lord RVN, Brabender J, Gandara D, Alberola V, Camps C, Domine M, Cardenal F, Sanchez JM, Gumerlock PH, Taron M, Sanchez JJ, Danenberg KD, Danenberg PV, Rosell R. Low ERCC1 Expression Correlates with Prolonged Survival after Cisplatin plus Gemcitabine Chemotherapy in Non-Small Cell Lung Cancer. Clinical Cancer Research. 2002;8:2286–2291. [PubMed] [Google Scholar]
- 17.Ota S, Ishii G, Goto K, Kubota K, Km YH, Kojika M, Murata Y, Yamazaki M, Nishiwaki Y, Eguchi K, Ochiai A. Immunohistochemical expression of BCRP and ERCC1 in biopsy specimen predicts survival in advanced non-small-cell lung cancer treated with cisplatin-based chemotherapy. Lung Cancer. 2009;64:98–104. doi: 10.1016/j.lungcan.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Booton R, Ward T, Ashcroft L, Morris J, Heighway J, Thatcher N. ERCC1 mRNA Expression Is Not Associated with Response and Survival after Platinum-Based Chemotherapy Regimens in Advanced Non-Small Cell Lung Cancer. Journal of Thoracic Oncology. 2007;2(10):902–906. doi: 10.1097/JTO.0b013e318155a637. [DOI] [PubMed] [Google Scholar]
- 19.Holm B, Mellemgaard A, Skov T, Skov BG. Different impact of excision repair cross-complementation group 1 on survival in male and female patients with inoperable non-small-cell lung cancer treated with carboplatin and gemcitabine. Journal of Clinical Oncology. 2009;27(26):4254–4259. doi: 10.1200/JCO.2008.18.8631. [DOI] [PubMed] [Google Scholar]
- 20.Li XQ, Li J, Shi SB, Chen P, Yu LC, Bao QL. Expression of MRP1, BCRP, LRP and ERCC1 as prognostic factors in non-small cell lung cancer patients receiving postoperative cisplatin-based chemotherapy. International Journal of Biological Markers. 2009;24(4):230–237. doi: 10.1177/172460080902400403. [DOI] [PubMed] [Google Scholar]
- 21.Vilmar AC, Santoni-Ruglu E, Sorensen JB. ERCC1 and histopathology in advanced NSCLC patients randomized in a large multicenter phase III trial. Annals of Oncology. 2010;21(9):1817–1824. doi: 10.1093/annonc/mdq053. [DOI] [PubMed] [Google Scholar]
- 22.Bellmunt J, Paz-Ares L, Ceullo M, Cecere FL, Albiol S, Guillem V, Gallardo E, Carles J, Mendez P, de la Cruz JJ, Taron M, Rosell R, Baselga J. Gene expression of ERCC1 as a novel prognostic marker in advanced bladder cancer patients receiving cisplatin-based chemotherapy. Annals of Oncology. 2007;18:522–528. doi: 10.1093/annonc/mdl435. [DOI] [PubMed] [Google Scholar]
- 23.Hwang IG, Jang JS, Do JH, Kang JH, Lee GW, Oh SY, Kwon HC, Jun HJ, Lim HY, Lee S, Chi KC, Lee SJ. Different relation between ERCC1 overexpression and treatment outcomes of two platinum agents in advanced biliary tract adenocarcinoma patients. Cancer Chemother Pharmacol. 2011 doi: 10.1007/s00280-011-1558-3. [DOI] [PubMed] [Google Scholar]
- 24.Maithel SK, Coban I, Kneuertz PJ, Kooby DA, El Rayes BF, Kauh JS, Sarmiento J, Staley CA, Adsay NV. Differential Expression of ERCC1 in Pancreas Adenocarcinoma: High Tumor Expression is Associated with Earlier Recurrence and Shortened Survival after Resection. Ann Surg Oncol. 2011 doi: 10.1245/s10434-011-1610-x. [DOI] [PubMed] [Google Scholar]
- 25.Kim SH, Kwon HC, Oh SY, Lee DM, Lee S, Lee JH, Roh MS, Kim DC, Park KJ, Choi HJ, Kim HJ. Prognostic Value of ERCC1, Thymidylate Synthase, and Glutathione S-Transferase for 5-FU/Oxaliplatin Chemotherapy in Advanced Colorectal Cancer. American Journal of Clinical Oncology. 2009;32(1):38–43. doi: 10.1097/COC.0b013e31817be58e. [DOI] [PubMed] [Google Scholar]
- 26.Steffenson KD, Waldstrom M, Jakobsen A. The Relationship of Platinum Resistance and ERCC1 Protein Expression in Epithelial Ovarian Cancer. Int J Gynecol Cancer. 2009;19:820–825. doi: 10.1111/IGC.0b013e3181a12e09. [DOI] [PubMed] [Google Scholar]
- 27.Cobo M, Isla D, Massuti B, Montes A, Sanchez JM, Provencio M, Olas NV, Paz-Ares L, Lopez-Vivanco G, Munoz MA, Alberola EFV, Camps C, Domine M, Sanchez JJ, Sanchez-Ronco M, Danenberg K, Taron M, Gandara D, Rosell R. Customizing Cisplatin Based on Quantitative Excision Repair Cross-Complementing 1 mRNA Expression: A Phase III Trial in Non–Small-Cell Lung Cancer. Journal of Clinical Oncology. 2007;25(19):2747–2754. doi: 10.1200/JCO.2006.09.7915. [DOI] [PubMed] [Google Scholar]
- 28.Bunn PA, Dziadziuszko R, Varella-Garcia M, Franklin WA, Witta SE, Kelly K, Hirsch FR. BiologicalMarkers for Non^Small Cell Lung Cancer Patient Selection for Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Therapy. Clin Cancer Res. 2006;12(12):3652–3656. doi: 10.1158/1078-0432.CCR-06-0261. [DOI] [PubMed] [Google Scholar]
- 29.Carlson JJ, Garrison LP, Ramsey SD, Veenstra DL. Epidermal growth factor receptor genomic variation in NSCLC patients receiving tyrosine kinase inhibitor therapy: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2009;135:1483–1493. doi: 10.1007/s00432-009-0595-3. [DOI] [PubMed] [Google Scholar]
- 30.Oldenhuis CNAM, Oosting SF, Gietema JA, de Vries EGE. Prognostic versus predictive value of biomarkers in oncology. European Journal of Cancer. 2008;44:946–953. doi: 10.1016/j.ejca.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: can tumor gene expression profiling improve outcomes in patients with breast cancer? Genetics in Medicine. 2009;11(1):66–73. doi: 10.1097/GIM.0b013e3181928f56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buyse M, Michiels S, Sargen DJ, Grothey A, Matheson A, de Gamont A. Integrating Biomarkers in Clinical Trials. Expert Review of Molecular Diagnostics. 2011;11(2):171–182. doi: 10.1586/erm.10.120. [DOI] [PubMed] [Google Scholar]
- 33.Van Schaeybroeck S, Allen WL, Turkington RC, Johnston PG. Implementing prognostic and predictive biomarkers in CRC clinical trials. Nature Reviews in Clinical Oncology. 2011 doi: 10.1038/nrclinonc.2011.15. [DOI] [PubMed] [Google Scholar]
- 34.Rizzo S, Bronte G, Fanale D, Corsini L, Silvestrisb N, Santinic D, Gulottad G, Bazana V, Gebbiaa N, Fulfaroa F, Russoa A. Prognostic vs predictive molecular biomarkers in colorectal cancer: is KRAS and BRAF wild type status required for anti-EGFR therapy? Cancer Treatment Reviews. 2010;36S3:S56–S61. doi: 10.1016/S0305-7372(10)70021-9. [DOI] [PubMed]
- 35.Gandara DR, Kawaguchi T, Crowley J, Moon J, Furuse K, Kawahara M, Teramukai S, Ohe Y, Kubota K, Williamson SK, Gautschi O, Lenz HJ, McLeod HL, Lara PN, Coltman CA, Fukuoka M, Saijo N, Fukushima M, Mack PC. Japanese-US Common-Arm Analysis of Paclitaxel Plus Carboplatin in Advanced Non–Small-Cell Lung Cancer: A Model for Assessing Population-Related Pharmacogenomics. Journal of Clinical Oncology. 2009;27(21):3540–3546. doi: 10.1200/JCO.2008.20.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vilmar A, Sorenesen JB. Excision repair cross-complementation group 1 (ERCC1) in platinum-based treatment of non-small cell lung cancer with special emphasis on carboplatin: A review of current literature. Lung Cancer. 2009;64:131–139. doi: 10.1016/j.lungcan.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Ahn MJ, Lee J, Park YH, Ahn JS, Ziogas A, Zell JA, Park K, Ou SHI. Korean Ethnicity as Compared with White Ethnicity Is an Independent Favorable Prognostic Factor for Overall Survival in Non-small Cell Lung Cancer before and after the Oral Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Era. Journal of Thoracic Oncology. 2010;5(8):1185–1196. doi: 10.1097/JTO.0b013e3181e2f624. [DOI] [PubMed] [Google Scholar]
- 38.Ou SHI, Ziogas A, Zell JA. Asian Ethnicity Is a Favorable Prognostic Factor for Overall Survival in Non-small Cell Lung Cancer (NSCLC) and Is Independent of Smoking Status. Journal of Thoracic Oncology. 2009;4(9):1083–1093. doi: 10.1097/JTO.0b013e3181b27b15. [DOI] [PubMed] [Google Scholar]
- 39.Gandara DR, Grimminger P, Mack PC, Lara PN, Li T, Danenberg PV, Danenberg KD. Association of Epidermal Growth Factor Receptor Activating Mutations with Low ERCC1 Gene Expression in Non-small Cell Lung Cancer. Journal of Thoracic Oncology. 2010;5(12):1933–1938. doi: 10.1097/JTO.0b013e3181fd418d. [DOI] [PubMed] [Google Scholar]
- 40.Olaussen KA, Dunant A, Fouret P, Brambilla E, Andre F, Haddad V, Taranchon E, Filipits M, Pirker R, Popper HH, Stahel R, Sabatier L, Pignon JP, Tursz T, Le Chevalier TL, Soria JC. DNA Repair by ERCC1 in Non–Small-Cell Lung Cancer and Cisplatin-Based Adjuvant Chemotherapy. NEJM. 2006;355(10):983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 41.Chen S, Zhang J, Wang R, Luo W, Chen H. The platinum-based treatments for advanced non-small cell lung cancer, is low/negative ERCC1 expression better than high/positive ERCC1 expression: A meta-analysis. Lung Cancer. 2010;70(1):63–70. doi: 10.1016/j.lungcan.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Michiels S, Piedbois P, Burdett S, Syz N, Stewart L, Pignon JP. Meta-analysis when only the median survival times are known: A comparison with individual patient data results. International Journal of Technology Assessment in Health Care. 2005;21:119–125. doi: 10.1017/s0266462305050154. [DOI] [PubMed] [Google Scholar]
- 43.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]