Abstract
Metabolic syndrome (MetS) is a collection of risk factors including obesity, dyslipidemia, insulin resistance/impaired glucose tolerance, and/or hypertension. The incidence of obesity has reached pandemic levels, as ~20–30% of adults in most developed countries can be classified as having MetS. This increased prevalence of MetS is critical as it is associated with a two-fold elevated risk for cardiovascular disease. Although the pathophysiology underlying this increase in disease has not been clearly defined, recent evidence indicates that alterations in the control of coronary blood flow could play an important role. The purpose of this review is to highlight current understanding of the effects of MetS on regulation of coronary blood flow and to outline the potential mechanisms involved. In particular, the role of neurohumoral modulation via sympathetic α-adrenoceptors and the renin-angiotensin-aldosterone system (RAAS) are explored. Alterations in the contribution of end-effector K+, Ca2+, and transient receptor potential (TRP) channels are also addressed. Finally, future perspectives and potential therapeutic targeting of the microcirculation in MetS are discussed.
Keywords: coronary circulation, neurohumoral factors, obesity, renin-angiotensin-aldosterone axis, sympathetic activation
1. Introduction
Metabolic syndrome (MetS) is a group of risk factors including obesity, dyslipidemia, insulin resistance/impaired glucose tolerance, and/or hypertension accompanied by pro-inflammatory and thrombotic states [1]. The incidence of obesity has reached pandemic levels, as ~20–30% of adults in most developed countries can be classified as having MetS [1;2]. The increased prevalence of MetS is important as it is associated with a 2-fold increased risk for cardiovascular disease, 5-fold increased risk for type 2 diabetes mellitus, and 1.5-fold increase in all-cause mortality [3–5]. Critically, the prevalence of coronary disease and heart failure is significantly elevated in patients with MetS [3;6–8]. Given that heart disease remains a leading cause of death around the world [1], elucidating mechanisms by which MetS increases cardiovascular risk is essential for developing future treatments and preventing this global epidemic.
Alterations in the control of coronary blood flow could underlie increased cardiovascular morbidity and mortality in the MetS. Regulation of myocardial oxygen delivery is critical to overall cardiac function as the heart has limited anaerobic capacity and maintains a very high rate of oxygen extraction at rest (70–80%) [9–12]. Thus, the myocardium is highly dependent on a continuous supply of oxygen to maintain normal cardiac output and blood pressure. MetS impairs the ability of the coronary circulation to regulate vascular resistance and balance myocardial oxygen supply and demand [13–15]. Coronary microvascular dysfunction in MetS is evidenced by reduced coronary venous PO2 [13–15], diminished vasodilation to endothelial-dependent and independent agonists (i.e. flow reserve) [16–21], and altered functional and reactive hyperemia [13–15;22;23]. Importantly, these changes occur prior to overt atherosclerotic disease and have been associated with left ventricular systolic and diastolic contractile dysfunction in humans [24–28] and animal models of MetS [13;29–31].
The purpose of the present review is to highlight current understanding of the effects of MetS on regulation of coronary blood flow and outline potential mechanisms involved. In particular, pathophysiologic roles of neurohumoral modulation via sympathetic α-adrenoceptors and the renin-angiotensin-aldosterone system (RAAS) are explored. In addition, the contribution of end-effector K+, Ca2+, and transient receptor potential (TRP) channels are addressed. Finally, future perspectives and potential therapeutic targeting of the microcirculation in MetS are discussed. Other recent reviews of microvascular dysfunction in MetS include those by Knudson et al. [32], Hodnett and Hester [33]; Frisbee [34]; Stepp et al [35;36]; Serne et al [37]; Bagi [38] and Krentz et al [39]; Liu and Gutterman [40].
2. Coronary blood flow in MetS
2.1 Resting flow and vasodilator responses
There is little change in baseline coronary blood flow in either animals [13–15;22;41–45] or humans [16–19;46] with MetS. While myocardial perfusion is equivalent, myocardial oxygen consumption (MVO2) is elevated in proportion to increases in stroke volume, cardiac output, and blood pressure; i.e. characteristic “hyperdynamic circulation” [13;30;31;46;47]. Basal coronary venous PO2 is reduced in MetS, indicating an imbalance between coronary blood flow and myocardial metabolism [13–15]. These findings suggest that the MetS forces the heart to utilize its limited oxygen extraction reserve by affecting one or more primary determinants of coronary flow, including: 1) myocardial metabolism; 2) arterial pressure; 3) neuro-humoral, paracrine and endocrine influences; and 4) myocardial extravascular compression [9;10]. As addressed below, MetS increases sympathetic output [48–51] and activates the RAAS [15;52–55], increasing blood pressure, myocardial oxygen demand, and coronary vascular resistance. The determinants of coronary flow in MetS are also influenced by diminished nitric oxide (NO) bioavailability [36;56–59] and augmented endothelial-dependent vasoconstriction [43;60–64]. However, despite these changes it is not surprising that basal coronary flow is largely unaffected by MetS, as it is well established that inhibition of NO synthesis [65–69] or endothelin-1 receptors [63;70–72] does not alter myocardial perfusion in normal, lean subjects. To date, no studies have specifically examined the effects of MetS on myocardial compressive forces.
MetS attenuates coronary flow responses to pharmacologic vasodilator compounds such as acetylcholine, adenosine, papaverine, and dipyridamole [16–20;45;73]. Decreases in coronary flow reserve directly correlate with waist-to-hip ratio [74], body mass index [17], blood pressure [20], degree of insulin resistance [16;20], and the clinical diagnosis of MetS [18]. Interestingly, our data indicate that specific receptor subtypes and downstream K+ channels involved in coronary microvascular dilation are altered in Ossabaw swine with early-stage MetS, prior to any absolute change in coronary flow reserve [44]. In contrast, decreased coronary flow reserve is evident in swine with later-stage MetS [41;73;75] and worsens with the onset of type 2 diabetes [16;17]. Exact mechanisms underlying impaired pharmacologic coronary vasodilation in MetS have not been clearly defined, but are likely related to altered functional expression of receptors and ion channels [41;44;45;73;76;77], endothelial and vascular smooth muscle function [36;56;77;78], paracrine and neuro-endocrine influences [32;48–50;54;79;80], structural remodeling of coronary arterioles [35;81;82], and/or microvascular rarefaction [83–85].
2.2 Coronary response to increases in cardiac metabolism
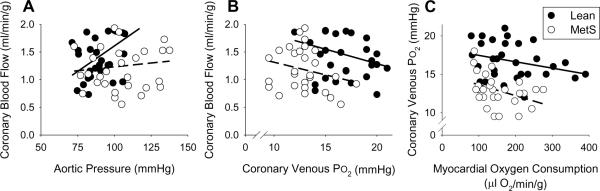
Energy production of the heart is almost entirely dependent on oxidative phosphorylation for contraction in relation to ventricular wall tension, myocyte shortening, heart rate, and contractility [9]. Since the heart maintains a very high rate of oxygen extraction at rest, increases in myocardial energy production must be met by parallel increases in myocardial oxygen delivery [9–11;86]. Exercise is the most important physiologic stimulus for increases in coronary blood flow, as many of the primary determinants of myocardial oxygen demand are elevated by β-adrenoceptor signaling [9;86]. Data from our laboratory indicate that MetS impairs the ability of the coronary circulation to adequately balance myocardial oxygen delivery with myocardial metabolism at rest and during exercise-induced increases in MVO2. In particular, coronary vasodilation in response to exercise is attenuated in Ossabaw swine with MetS. This effect is evidenced by reduction of the slope between coronary blood flow and aortic pressure, which supports that exercise-mediated increases in vascular conductance are attenuated in MetS (Fig. 1A). Diminished local metabolic control of the coronary circulation is also evidenced by decreased coronary blood flow at a given coronary venous PO2 (Fig. 1B), an index of myocardial tissue PO2 which is hypothesized to be a primary stimulus for metabolic coronary vasodilation [9–11]. Importantly, coronary venous PO2 is also depressed by MetS relative to alterations in MVO2 (the primary determinant of myocardial perfusion) both at rest and during exercise (Fig. 1C). Together, these findings demonstrate coronary microvascular dysfunction in MetS leads to an imbalance between coronary blood flow and myocardial metabolism that could contribute to the increased incidence of cardiac contractile dysfunction and the onset of myocardial ischemia in obese patients [1;3;7;8]. This point is supported by an ~25% reduction in baseline cardiac index (cardiac output normalized to body weight) and a marked increase in myocardial lactate production (onset of anaerobic glycolysis) in swine with the MetS [13].
Fig. 1.
Effects of metabolic syndrome on coronary blood flow at rest and during exercise. (A) Reduction of the slope between coronary blood flow and aortic pressure indicates that exercise-mediated increases in vascular conductance are significantly attenuated by the MetS. (B) Diminished local metabolic control of the coronary circulation is also evidenced by decreased coronary blood flow at a given coronary venous PO2, an index of myocardial tissue PO2 that is hypothesized to be a primary stimulus for metabolic coronary vasodilation. (C) Imbalance between myocardial oxygen supply and demand in MetS is evidenced by the reduction of coronary venous PO2 relative to alterations in MVO2 (the primary determinant of myocardial perfusion) both at rest and during exercise.
2.3 Coronary response to myocardial ischemia
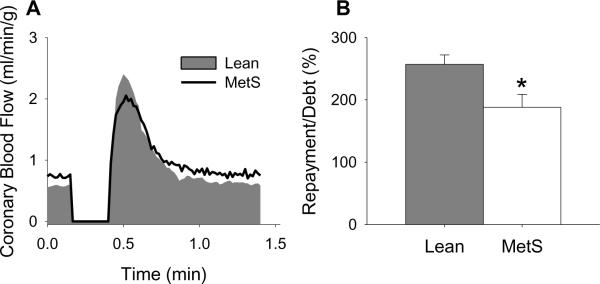
Coronary vasodilation in response to myocardial ischemia is a critical mechanism increasing oxygen delivery to the heart to mitigate ischemic injury and infarction [87;88]. To address the effects of MetS on ischemic vasodilation, we examined coronary flow responses to a 15 sec occlusion in anesthetized, open-chest lean and MetS Ossabaw swine [22]. Representative tracings illustrating reactive hyperemia in lean vs. MetS swine are shown in Fig. 2A. Because coronary reactive hyperemia varies directly with baseline blood flow, estimating overall repayment of incurred oxygen debt is critical for analyzing ischemic dilator responses [87;88]. Our finding that vasodilation in response to cardiac ischemia is impaired by MetS, relative to the deficit in coronary blood flow (i.e. repayment/debt ratio; Fig. 2B), is consistent with decreased reactive hyperemia of peripheral vascular beds in obese humans [89;90]. We propose that impaired ischemic dilation in MetS could exacerbate myocardial injury in patients with flow-limiting atherosclerotic lesions or acute coronary thrombosis.
Fig. 2.
Effect of the metabolic syndrome on coronary vasodilation in response to cardiac ischemia. (A) Representative tracings illustrating reactive hyperemic responses in lean and MetS swine. (B) Coronary vasodilation in response to cardiac ischemia is impaired by metabolic syndrome as evidenced by the significant reduction in percent repayment of incurred coronary flow debt (i.e. repayment/debt ratio). * P < 0.05 vs. lean-control.
In summary, microvascular dysfunction in MetS upsets the balance between coronary blood flow and myocardial metabolism as well as impairs blood flow responses to pharmacologic vasodilator compounds (coronary flow reserve), exercise-induced increases in MVO2 (physiologic stimuli), and cardiac ischemia (pathophysiologic stimuli). Potential mechanisms underlying these key alterations in the control of coronary blood flow are explored below.
3. Neurohumoral modulation of coronary flow in MetS
3.1 Sympathetic control of coronary blood flow
MetS is associated with sympathetic hyperactivity, as numerous studies have documented increased plasma and urinary catecholamines, sympathetic nerve activity, and cardiac autonomic activity [48–52;91;92]. Potential components of MetS that might contribute include increased plasma insulin, adipokines, nonesterified fatty acids, proinflammatory cytokines, as well as activation of RAAS, baroreflex impairment, and obstructive sleep apnea [48;50]. Sympathetic activation has important effects on the coronary circulation through direct actions on vascular α- and β-adrenoceptors and indirect initiation of local metabolic vasodilator mechanisms secondary to increases in contractility, heart rate, and arterial pressure [9;11;12;93]. It is well accepted that direct α-adrenoceptor mediated vasoconstriction limits myocardial flow in both normal and hypoperfused hearts [94–101] and that “feedforward” β-adrenoceptor vasodilation contributes to increased coronary blood flow when the sympathetic nervous system is activated, as during exercise [96;97;102;103]. Therefore, alterations in these opposing autonomic influences could play a key role in coronary microvascular dysfunction in MetS.
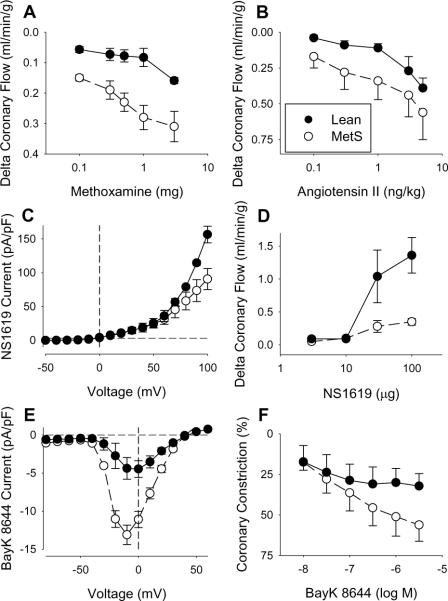
Vasoconstriction mediated by α-adrenoceptors is augmented by MetS in both coronary [42;104] and peripheral vascular beds [105–107]. Recent findings from Grisk et al. who documented that α1-adrenoceptor mediated vasoconstriction is enhanced in isolated coronaries from Wistar Ottawa Karlsburg W rats [104], are consistent with in vivo data from our laboratory which demonstrated increased coronary constriction to the α1-adrenoceptor agonist methoxamine in MetS dogs (Fig. 3A) [42]. Importantly, no differences in α2-adrenoceptor mediated coronary vasoconstriction or expression of α1B- or α1D-adrenoceptors were noted in lean vs. MetS canines. Thus, MetS is associated with increased coronary α1-adrenoceptor signaling that likely contributes to the imbalance between myocardial oxygen supply and demand, especially during heightened sympathetic activity. Given that α-adrenoceptor inhibition improves myocardial perfusion and cardiac contractile function clinically [108;108–111], therapeutic strategies to diminish coronary α1-adrenoceptor activation could improve cardiovascular outcomes in patients with MetS.
Fig. 3.
Effect of metabolic syndrome on neurohumoral-mediated coronary vasoconstriction and ion channel function. (A) Increased coronary constriction to the α1-adrenoceptor agonist methoxamine in obese dogs. Data from reference [42]. (B) Augmented angiotensin II type 1 (AT1) receptor-mediated coronary vasoconstriction in obese dogs with the MetS. Data from reference [15]. (C) Reductions in coronary vascular smooth muscle BKCa current in response to the BKCa channel agonist NS1619 directly correspond with (D) diminished coronary vasodilation to NS1619 in MetS swine. Data from reference [41]. (E) Increases in coronary vascular smooth muscle L-type Ca2+ channel current activation in response to Bay K 8644 are associated with (F) augmented coronary arteriolar vasoconstriction to Bay K 8644 in obese dogs. Data from reference [32].
Sympathetic vasodilation mediated by β-adrenoceptors is also diminished in MetS as D'Angelo et al. recently showed that an exaggerated blood pressure response to acute stress in obese Zucker rats is related, at least in part, to blunted β-adrenoceptor dilation [112]. Decreased vasodilation to the β-agonist isoproterenol has also been observed in isolated coronary arterioles [104]. However, the extent to which altered sympathetic β-adrenoceptor expression and/or signaling contribute to coronary vascular dysfunction in MetS in vivo has not been investigated.
3.2 RAAS and coronary blood flow
There is substantial evidence that MetS activates the RAAS and that effects of angiotensin II on vascular tone, inflammation, vascular remodeling, thrombosis, and plaque stability are central to the pathogenesis of cardiovascular disease [15;52–55]. Although angiotensin II has very modest effects on the coronary circulation in normal hearts [15;113;114], vasoconstriction to angiotensin II is enhanced in disease states associated with chronic RAAS activation [15;115;116]. Our data support this hypothesis, as increased circulating angiotensin II in our canine model of MetS was accompanied by increased angiotensin II type 1 (AT1) receptor-mediated coronary vasoconstriction (Fig. 3B) [15]. This augmented constriction directly corresponded with a ~40% increase in coronary AT1 receptor expression, while coronary AT2 receptor expression was unchanged. Importantly, inhibition of AT1 receptor-mediated coronary vasoconstriction with telmisartan significantly improved the balance between myocardial oxygen supply and demand in MetS animals at rest and during exercise-induced increases in MVO2.
Increases in aldosterone signaling have been linked with impaired vascular function, pro-atherosclerotic gene expression, vascular smooth muscle proliferation, and calcification, as well as diminished cardiac and renal function [117–120]. With regard to the coronary circulation, aldosterone produces dose-dependent vasoconstriction in vivo in open-chest dogs [121], in vitro in isolated perfused rat hearts [122], and in isolated coronary arterioles [123]. Interestingly, this non-genomic effect of aldosterone is blunted by inhibition of AT1 receptors [123;124] and endothelial denudation [123], but is unaffected by blockade of mineralocorticoid receptors with spironolactone [121;123]. Coronary effects of aldosterone also appear to be augmented in disease states such as hypertension [123] and to worsen contractile function during cardiac ischemia [121]. Although clinical trials have established a beneficial effect of aldosterone antagonism on cardiovascular morbidity and mortality in myocardial infarction and heart failure [55;125], pathophysiologic consequences of elevated coronary mineralocorticoid receptor stimulation in MetS have not been examined. We propose one mechanism by which increases in angiotensin II and aldosterone impair control of coronary blood flow in MetS is through alterations in the expression of microvascular ion channels and receptors. This hypothesis is discussed in detail below. Together, these findings implicate upregulation of the RAAS in MetS-induced coronary vascular dysfunction and provide strong rationale for future clinical studies with AT1 and mineralocorticoid receptor antagonists in patients with MetS.
4. Coronary ion channel dysfunction in MetS
4.1 Smooth muscle K+ channels in MetS
Coronary smooth muscle cells express a variety of K+ channels, which regulate membrane potential and vascular tone [77]. Major types include voltage-dependent (KV), large conductance, Ca2+-activated (BKCa), ATP-sensitive (KATP), and inwardly rectifying (Kir) K+ channels, but other channels such as IKCa and SKCa are functionally expressed in the coronary circulation [126].
KV channels are activated in the physiological range of membrane potential and thus have been implicated in the control of coronary blood flow [77]. In particular, our laboratory previously demonstrated that KV channels regulate coronary blood flow at rest, during ischemia, and with increasing MVO2 in normal-lean animals [127–131]. More recently, we documented that induction of the MetS markedly attenuates coronary vascular smooth muscle KV current and expression of KV1.5 channels [118], which is consistent with recent preliminary data indicating that metabolic coronary vasodilatation is reduced in KV1.5 knockout mice [132]. Importantly, these changes in functional expression of KV channels were directly associated with reductions in coronary blood flow, vascular conductance, and coronary venous PO2 in swine with the MetS [127]. Although the specific mechanisms underlying the impairment of coronary KV channels in MetS are unclear, there is evidence that dyslipidemia, hyperglycemia, hypertension, and/or oxidative stress may contribute [133–139]. Activation of the sympathetic nervous system, RAAS, and PLC-PKC signaling pathways could also be involved [77;140;141].
Compared to KV channels, BKCa channels activate at more depolarized membrane potentials [41], but also respond to local Ca2+ signaling [73]. Recently, we found that the MetS significantly attenuates coronary BKCa channel function, as evidenced by a reduction in vasodilation to the BKCa channel agonist NS1619 (Fig. 3D) [41]. This decrease in vasodilation corresponded with reductions in coronary vascular smooth muscle BKCa current (Fig. 3C) and a paradoxical increase in BKCa channel α and β1 subunit expression [41]. Decreases in total K+ current and spontaneous transient outward currents, which are elicited by Ca2+ sparks and indicative of BKCa channel activation, have also been reported in coronary microvessels of diabetic dyslipidemic swine [73;142]. Studies in obese, insulin resistant rat models also support these findings and suggest that the reductions in BKCa current are related to alterations in the regulatory β1 subunit [143]. Although diminished BKCa channel function in obesity/MetS is well established, data fail to support a significantly role for BKCa channels in the control of coronary blood flow at rest, during increases in MVO2 or during cardiac ischemia in lean or MetS animal models [22;41]. However, BKCa channels have been shown to modulate coronary endothelial-dependent vasodilation in normal-lean subjects [144;145]. Thus, we propose that decreases in BKCa channel function likely contribute to coronary endothelial dysfunction observed in the setting of the MetS (see recent review by Belin de Chantemele and Stepp [146]), but play little role in the overall impairment of coronary vascular function.
Evidence that coronary KATP channels are altered by MetS also exists as we recently documented that the functional contribution of KATP channels to coronary vasodilation in response to 2-chloroadenosine or a brief coronary artery occlusion (i.e. coronary reactive hyperemia) is significantly diminished in MetS vs. lean Ossabaw swine [22;44]. Decreases in KATP channel function have also been reported in the skeletal muscle microcirculation of obese Zucker rats [33] and in the coronary circulation of diabetic humans [147]. In contrast, other investigations have shown an increased role for coronary KATP channels in hypercholerestolemic swine [148] and type-1 diabetic dogs [149;150]. Overall, more studies are needed to understand the mechanisms by which the MetS affects coronary smooth muscle KATP channels. Particularly needed are direct measurements of KATP channel activity and subunit expression. The same is true of Kir channels, which are highly expressed in autoregulatory beds such as the coronary circulation and tend to increase in abundance with decreasing vessel diameter [151]. While studies indicate roles for Kir in the regulation coronary arteriole diameter and blood flow [152], the impact of MetS on Kir channel function is unknown.
4.2 Ca2+ channels in MetS
L-type (CaV1.2) Ca2+ channels are the predominant voltage-dependent Ca2+ channel expressed in coronary smooth muscle [153]. Ca2+ regulates contraction and gene expression; therefore, alterations in L-type channel function by MetS could have many consequences [154–156]. In particular, increased activation of vasoconstrictor pathways (e.g. α1 adrenoceptors, AT1 receptors) along with decreased function of smooth muscle K+ channels (e.g. BKCa channels, KV channels) would serve to augment L-type Ca2+ channel activity and vasoconstriction [77]. Data from our laboratory support this hypothesis as we previously demonstrated that the MetS increases intracellular Ca2+ concentration [41], L-type Ca2+ channel current (Fig. 3E) and arteriolar vasoconstriction to the L-type Ca2+ channel agonist Bay K 8644 [32] (Fig. 3F). We also found that coronary vasodilation in response to the L-type Ca2+ channel antagonist nicardipine is markedly elevated in obese dogs with the MetS [32]. These findings are in contrast with earlier studies which documented reductions in L-type Ca2+ channel current in hypercholesterolemic and/or diabetic dyslipidemic swine [157;158]. Taken together, these data indicate that the entire MetS milieu is critical in determining the overall functional expression of L-type Ca2+ channels in the coronary circulation. Whether increases in L-type Ca2+ channel activation contribute to the impaired control of coronary blood flow at rest or during increases in MVO2 in the MetS merits further investigation.
4.3 TRP channels in MetS
Transient receptor potential (TRP) channels are non-selective channels permeable to monovalent and divalent cations. Thus, changes in the function or expression of TRP channels could alter intracellular Ca2+ levels directly, indirectly or through membrane potential and the regulation of L-type Ca2+ channel activity. Several TRP subfamilies are expressed in vascular smooth muscle, including TRPC (canonical) and TRPV (vanilloid) channels in the coronary circulation.
TRPC channels are expressed throughout the vasculature and are activated by G protein-coupled receptors and receptor tyrosine kinases [159]. Selective TRPC subtypes regulate vascular tone in response to phenylephrine [160;161], hypoxia [162] and have been shown to be the predominant source of Ca2+ entry in response to endothelin-1 stimulation in rabbit coronary smooth muscle [163]. Although the exact role of TRPC channels in the control of coronary blood flow has not been extensively examined, data from the Sturek laboratory indicate that the MetS significantly augments TRPC1 expression and store operated Ca2+ entry in coronary smooth muscle [164;165]. TRPC1 is also upregulated following vascular injury [166;167] and inhibition of TRPC1 attenuates neointimal growth [168;169]. Thus, alterations in TRPC activity and expression have been implicated in smooth muscle Ca2+ dysregulation and proliferation in MetS.
TRPV channels are activated by various stimuli including capsaicin, lipids, acid, heat, shear stress, and hypoosmolarity [159]. To date, those best characterized are TRPV1 and TRPV4. TRPV1 is present in primary afferent capsaicin-sensitive neurons projecting to cardiac tissues [159]. Capsaicin causes vasodilation, but many studies fail to distinguish direct vascular effects of capsaicin from TRPV1-dependent release of CGRP and/or substance P. However, recent data from our laboratory indicate that TRPV1 channels are functionally expressed in the coronary circulation, and that the MetS significantly impairs endothelial-dependent responses to capsaicin administration [75]. This decrease in TRPV1-mediated dilation was directly associated with diminished coronary TRPV1 protein expression and capsaicin-induced divalent cation influx in endothelial cells. TRPV4 functions in flow-mediated dilation of coronary arterioles [170], a response that is impaired in MetS [171]. These findings indicate that TRP channel dysfunction could be an important mechanism underlying impaired vascular reactivity and disease that should be further explored.
5. Conclusions and future perspective
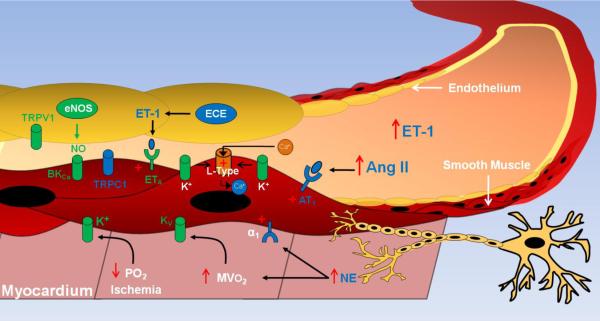
Investigations to date have demonstrated that the MetS has profound effects on the regulation of coronary blood flow (see schematic diagram in Fig. 4). We propose that therapies targeting of angiotensin/AT1 receptors, mineralocorticoid receptors, and/or sympathetic activation of α1-adrenoceptors are likely to be effective in attenuating cardiovascular complications associated with the MetS as such treatments would not only target vasoconstrictor pathways, but key signaling pathways that influence the functional expression of downstream K+ and Ca2+ channels. However, much research is needed to more clearly elucidate the mechanisms underlying coronary microvascular dysfunction in the MetS and to determine the efficacy and cardiovascular outcomes of targeted therapies in these patients.
Fig. 4.
Schematic diagram illustrating mechanisms by which the metabolic syndrome impairs control of coronary blood flow in response to increases in myocardial oxygen consumption (MVO2) or a brief coronary artery occlusion (i.e. decrease PO2/ischemia). Factors, receptors and ion channels that are downregulated in metabolic syndrome are depicted in green. Factors, receptors and pathways that are upregulated in metabolic syndrome are depicted in blue and/or with + symbol. ET-1 (endothelin-1); Ang II (angiotensin II); AT1 (angiotensin II type 1 receptor); α1 (α1 adrenoceptor); NE (norepinephrine); TRP (transient receptor potential channel); BKCa (large conductance, Ca2+ activated K+ channel); ETA (endothelin type A receptor). eNOS (endothelial nitric oxide synthase); ECE (endothelin converting enzyme).
RESEARCH HIGHLIGHTS
-
➣
Metabolic syndrome impairs the balance between myocardial oxygen supply and demand.
-
➣
Obesity impairs coronary response to exercise, ischemia, and vasodilator agonists.
-
➣
Increased neurohumoral and RAAS activation limits coronary blood flow.
-
➣
Alterations in functional expression of coronary ion channels are explored.
Acknowledgements
Funded by NIH HL-092245 (J.D. Tune). Thanks go to laboratory coworkers who helped with the research and our colleagues in the field, many of whom we were unable to cite.
Abbreviations
- MetS
metabolic syndrome
- TRP
transient receptor potential
- NO
nitric oxide
- RAAS
renin-angiotensin-aldosterone system
- MVO2
myocardial oxygen consumption
- TRP
Transient receptor potential
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures The authors have no conflicts of interest to disclose.
References
- 1.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–36. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119:812–9. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 4.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–32. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen NT, Nguyen XM, Wooldridge JB, Slone JA, Lane JS. Association of obesity with risk of coronary heart disease: findings from the National Health and Nutrition Examination Survey, 1999–2006. Surg Obes Relat Dis. 2010;6:465–9. doi: 10.1016/j.soard.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Curr Opin Cardiol. 2006;21:1–6. doi: 10.1097/01.hco.0000200416.65370.a0. [DOI] [PubMed] [Google Scholar]
- 7.Hunt KJ, Resendez RG, Williams K, Haffner SM, Stern MP. National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation. 2004;110:1251–7. doi: 10.1161/01.CIR.0000140762.04598.F9. [DOI] [PubMed] [Google Scholar]
- 8.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–16. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 9.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88:1009–86. doi: 10.1152/physrev.00045.2006. [DOI] [PubMed] [Google Scholar]
- 10.Feigl EO. Coronary physiology. Physiol Rev. 1983;63:1–205. doi: 10.1152/physrev.1983.63.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Tune JD, Gorman MW, Feigl EO. Matching coronary blood flow to myocardial oxygen consumption. J Appl Physiol. 2004;97:404–15. doi: 10.1152/japplphysiol.01345.2003. [DOI] [PubMed] [Google Scholar]
- 12.Bassenge E, Heusch G. Endothelial and neuro-humoral control of coronary blood flow in health and disease. Rev Physiol Biochem Pharmacol. 1990;116:77–165. doi: 10.1007/3540528806_4. [DOI] [PubMed] [Google Scholar]
- 13.Borbouse L, Dick GM, Payne GA, Payne BD, Svendsen MC, Neeb ZP, et al. Contribution of BKCa channels to local metabolic coronary vasodilation: Effects of metabolic syndrome. Am J Physiol Heart Circ Physiol. 2010;298:H966–H973. doi: 10.1152/ajpheart.00876.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Setty S, Sun W, Tune JD. Coronary blood flow regulation in the prediabetic metabolic syndrome. Basic Res Cardiol. 2003;98:416–23. doi: 10.1007/s00395-003-0418-7. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C, Knudson JD, Setty S, Araiza A, Dincer UD, Kuo L, et al. Coronary arteriolar vasoconstriction to angiotensin II is augmented in prediabetic metabolic syndrome via activation of AT1 receptors. Am J Physiol Heart Circ Physiol. 2005;288:H2154–H2162. doi: 10.1152/ajpheart.00987.2004. [DOI] [PubMed] [Google Scholar]
- 16.Kondo I, Mizushige K, Hirao K, Nozaki S, Tsuji T, Masugata H, et al. Ultrasonographic assessment of coronary flow reserve and abdominal fat in obesity. Ultrasound Med Biol. 2001;27:1199–205. doi: 10.1016/s0301-5629(01)00427-6. [DOI] [PubMed] [Google Scholar]
- 17.Schindler TH, Cardenas J, Prior JO, Facta AD, Kreissl MC, Zhang XL, et al. Relationship between increasing body weight, insulin resistance, inflammation, adipocytokine leptin, and coronary circulatory function. J Am Coll Cardiol. 2006;47:1188–95. doi: 10.1016/j.jacc.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 18.Pirat B, Bozbas H, Simsek V, Yildirir A, Sade LE, Gursoy Y, et al. Impaired coronary flow reserve in patients with metabolic syndrome. Atherosclerosis. 2008;201:112–6. doi: 10.1016/j.atherosclerosis.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 19.Teragawa H, Mitsuba N, Nishioka K, Ueda K, Kono S, Higashi Y, et al. Impaired coronary microvascular endothelial function in men with metabolic syndrome. World J Cardiol. 2010;2:205–10. doi: 10.4330/wjc.v2.i7.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teragawa H, Morita K, Shishido H, Otsuka N, Hirokawa Y, Chayama K, et al. Impaired myocardial blood flow reserve in subjects with metabolic syndrome analyzed using positron emission tomography and N-13 labeled ammonia. Eur J Nucl Med Mol Imaging. 2010;37:368–76. doi: 10.1007/s00259-009-1307-6. [DOI] [PubMed] [Google Scholar]
- 21.Belin De Chantemele EJ, Ali MI, Mintz J, Stepp DW. Obesity induced-insulin resistance causes endothelial dysfunction without reducing the vascular response to hindlimb ischemia. Basic Res Cardiol. 2009;104:707–17. doi: 10.1007/s00395-009-0042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borbouse L, Dick GM, Payne GA, Berwick ZC, Neeb ZP, Alloosh M, et al. Metabolic syndrome reduces the contribution of K+ channels to ischemic coronary vasodilation. Am J Physiol Heart Circ Physiol. 2010;298:H1182–H1189. doi: 10.1152/ajpheart.00888.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker BF, Chappell D, Jacob M. Endothelial glycocalyx and coronary vascular permeability: the fringe benefit. Basic Res Cardiol. 2010;105:687–701. doi: 10.1007/s00395-010-0118-z. [DOI] [PubMed] [Google Scholar]
- 24.Gong HP, Tan HW, Fang NN, Song T, Li SH, Zhong M, et al. Impaired left ventricular systolic and diastolic function in patients with metabolic syndrome as assessed by strain and strain rate imaging. Diabetes Res Clin Pract. 2009;83:300–7. doi: 10.1016/j.diabres.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, Barzilai B, et al. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol. 2004;43:1399–404. doi: 10.1016/j.jacc.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 26.Wong CY, O'Moore-Sullivan T, Fang ZY, Haluska B, Leano R, Marwick TH. Myocardial and vascular dysfunction and exercise capacity in the metabolic syndrome. Am J Cardiol. 2005;96:1686–91. doi: 10.1016/j.amjcard.2005.07.091. [DOI] [PubMed] [Google Scholar]
- 27.Volz HC, Seidel C, Laohachewin D, Kaya Z, Muller OJ, Pleger ST, et al. HMGB1: the missing link between diabetes mellitus and heart failure. Basic Res Cardiol. 2010;105:805–20. doi: 10.1007/s00395-010-0114-3. [DOI] [PubMed] [Google Scholar]
- 28.Falcao-Pires I, Palladini G, Goncalves N, van d V, Moreira-Goncalves D, Miranda-Silva D, et al. Distinct mechanisms for diastolic dysfunction in diabetes mellitus and chronic pressure-overload. Basic Res Cardiol. 2011 doi: 10.1007/s00395-011-0184-x. [DOI] [PubMed] [Google Scholar]
- 29.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321:225–36. doi: 10.1097/00000441-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Dincer UD, Araiza A, Knudson JD, Shao CH, Bidasee KR, Tune JD. Dysfunction of cardiac ryanodine receptors in the metabolic syndrome. J Mol Cell Cardiol. 2006;41:108–14. doi: 10.1016/j.yjmcc.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 31.Prakash R, Mintz JD, Stepp DW. Impact of obesity on coronary microvascular function in the Zucker rat. Microcirculation. 2006;13:389–96. doi: 10.1080/10739680600745919. [DOI] [PubMed] [Google Scholar]
- 32.Knudson JD, Dincer UD, Bratz IN, Sturek M, Dick GM, Tune JD. Mechanisms of coronary dysfunction in obesity and insulin resistance. Microcirculation. 2007;14:317–38. doi: 10.1080/10739680701282887. [DOI] [PubMed] [Google Scholar]
- 33.Hodnett BL, Hester RL. Regulation of muscle blood flow in obesity. Microcirculation. 2007;14:273–88. doi: 10.1080/10739680701282143. [DOI] [PubMed] [Google Scholar]
- 34.Frisbee JC. Vascular dysfunction in obesity and insulin resistance. Microcirculation. 2007;14:269–71. doi: 10.1080/10739680701296705. [DOI] [PubMed] [Google Scholar]
- 35.Stepp DW, Belin De Chantemele EJ. Structural remodeling in the limb circulation: impact of obesity and diabetes. Microcirculation. 2007;14:311–6. doi: 10.1080/10739680701285609. [DOI] [PubMed] [Google Scholar]
- 36.Stepp DW. Impact of obesity and insulin resistance on vasomotor tone: nitric oxide and beyond. Clin Exp Pharmacol Physiol. 2006;33:407–14. doi: 10.1111/j.1440-1681.2006.04381.x. [DOI] [PubMed] [Google Scholar]
- 37.Serne EH, de Jongh RT, Eringa EC, IJzerman RG, Stehouwer CD. Microvascular dysfunction: a potential pathophysiological role in the metabolic syndrome. Hypertension. 2007;50:204–11. doi: 10.1161/HYPERTENSIONAHA.107.089680. [DOI] [PubMed] [Google Scholar]
- 38.Bagi Z. Mechanisms of coronary microvascular adaptation to obesity. Am J Physiol Regul Integr Comp Physiol. 2009;297:R556–R567. doi: 10.1152/ajpregu.90817.2008. [DOI] [PubMed] [Google Scholar]
- 39.Krentz AJ, Clough G, Byrne CD. Vascular disease in the metabolic syndrome: do we need to target the microcirculation to treat large vessel disease? J Vasc Res. 2009;46:515–26. doi: 10.1159/000226220. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Gutterman DD. Vascular control in humans: focus on the coronary microcirculation. Basic Res Cardiol. 2009;104:211–27. doi: 10.1007/s00395-009-0775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borbouse L, Dick GM, Asano S, Bender SB, Dincer UD, Payne GA, et al. Impaired function of coronary BK(Ca) channels in metabolic syndrome. Am J Physiol Heart Circ Physiol. 2009;297:H1629–H1637. doi: 10.1152/ajpheart.00466.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dincer UD, Araiza AG, Knudson JD, Molina PE, Tune JD. Sensitization of coronary alpha-adrenoceptor vasoconstriction in the prediabetic metabolic syndrome. Microcirculation. 2006;13:587–95. doi: 10.1080/10739680600885228. [DOI] [PubMed] [Google Scholar]
- 43.Knudson JD, Rogers PA, Dincer UD, Bratz IN, Araiza AG, Dick GM, et al. Coronary vasomotor reactivity to endothelin-1 in the prediabetic metabolic syndrome. Microcirculation. 2006;13:209–18. doi: 10.1080/10739680600556894. [DOI] [PubMed] [Google Scholar]
- 44.Bender SB, Tune JD, Borbouse L, Long X, Sturek M, Laughlin MH. Altered mechanism of adenosine-induced coronary arteriolar dilation in early-stage metabolic syndrome. Exp Biol Med (Maywood ) 2009;234:683–92. doi: 10.3181/0812-RM-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Long X, Mokelke EA, Neeb ZP, Alloosh M, Edwards JM, Sturek M. Adenosine receptor regulation of coronary blood flow in Ossabaw miniature swine. J Pharmacol Exp Ther. 2010;335:781–7. doi: 10.1124/jpet.110.170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–6. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- 47.Cole MA, Murray AJ, Cochlin LE, Heather LC, McAleese S, Knight NS, et al. A high fat diet increases mitochondrial fatty acid oxidation and uncoupling to decrease efficiency in rat heart. Basic Res Cardiol. 2011;106:447–57. doi: 10.1007/s00395-011-0156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lambert GW, Straznicky NE, Lambert EA, Dixon JB, Schlaich MP. Sympathetic nervous activation in obesity and the metabolic syndrome--causes, consequences and therapeutic implications. Pharmacol Ther. 2010;126:159–72. doi: 10.1016/j.pharmthera.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Tentolouris N, Liatis S, Katsilambros N. Sympathetic system activity in obesity and metabolic syndrome. Ann N Y Acad Sci. 2006;1083:129–52. doi: 10.1196/annals.1367.010. [DOI] [PubMed] [Google Scholar]
- 50.Straznicky NE, Eikelis N, Lambert EA, Esler MD. Mediators of sympathetic activation in metabolic syndrome obesity. Curr Hypertens Rep. 2008;10:440–7. doi: 10.1007/s11906-008-0083-1. [DOI] [PubMed] [Google Scholar]
- 51.Mancia G, Bousquet P, Elghozi JL, Esler M, Grassi G, Julius S, et al. The sympathetic nervous system and the metabolic syndrome. J Hypertens. 2007;25:909–20. doi: 10.1097/HJH.0b013e328048d004. [DOI] [PubMed] [Google Scholar]
- 52.Aneja A, El-Atat F, McFarlane SI, Sowers JR. Hypertension and obesity. Recent Prog Horm Res. 2004;59:169–205. doi: 10.1210/rp.59.1.169. [DOI] [PubMed] [Google Scholar]
- 53.Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique C, et al. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol. 2007;293:H2009–H2023. doi: 10.1152/ajpheart.00522.2007. [DOI] [PubMed] [Google Scholar]
- 54.Whaley-Connell A, Pavey BS, Chaudhary K, Saab G, Sowers JR. Renin-angiotensinaldosterone system intervention in the cardiometabolic syndrome and cardio-renal protection. Ther Adv Cardiovasc Dis. 2007;1:27–35. doi: 10.1177/1753944707082697. [DOI] [PubMed] [Google Scholar]
- 55.Whaley-Connell A, Johnson MS, Sowers JR. Aldosterone: role in the cardiometabolic syndrome and resistant hypertension. Prog Cardiovasc Dis. 2010;52:401–9. doi: 10.1016/j.pcad.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bohlen HG. Mechanisms for early microvascular injury in obesity and type II diabetes. Curr Hypertens Rep. 2004;6:60–5. doi: 10.1007/s11906-004-0013-9. [DOI] [PubMed] [Google Scholar]
- 57.Busija DW, Miller AW, Katakam P, Erdos B. Adverse effects of reactive oxygen species on vascular reactivity in insulin resistance. Antioxid Redox Signal. 2006;8:1131–40. doi: 10.1089/ars.2006.8.1131. [DOI] [PubMed] [Google Scholar]
- 58.McVeigh GE, Cohn JN. Endothelial dysfunction and the metabolic syndrome. Curr Diab Rep. 2003;3:87–92. doi: 10.1007/s11892-003-0059-0. [DOI] [PubMed] [Google Scholar]
- 59.Han K, Patel Y, Lteif A, Chisholm R, Mather K. Contributions of dysglycemia, obesity and insulin resistance to impaired endothelium-dependent vasodilation in humans. Diabetes Metab Res Rev. 2011 doi: 10.1002/dmrr.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hopfner RL, Hasnadka RV, Wilson TW, McNeill JR, Gopalakrishnan V. Insulin increases endothelin-1-evoked intracellular free calcium responses by increased ET(A) receptor expression in rat aortic smooth muscle cells. Diabetes. 1998;47:937–44. doi: 10.2337/diabetes.47.6.937. [DOI] [PubMed] [Google Scholar]
- 61.Mather KJ, Mirzamohammadi B, Lteif A, Steinberg HO, Baron AD. Endothelin contributes to basal vascular tone and endothelial dysfunction in human obesity and type 2 diabetes. Diabetes. 2002;51:3517–23. doi: 10.2337/diabetes.51.12.3517. [DOI] [PubMed] [Google Scholar]
- 62.Mather KJ, Lteif A, Steinberg HO, Baron AD. Interactions between endothelin and nitric oxide in the regulation of vascular tone in obesity and diabetes. Diabetes. 2004;53:2060–6. doi: 10.2337/diabetes.53.8.2060. [DOI] [PubMed] [Google Scholar]
- 63.Mather KJ, Lteif AA, Veeneman E, Fain R, Giger S, Perry K, et al. Role of endogenous ET-1 in the regulation of myocardial blood flow in lean and obese humans. Obesity (Silver Spring) 2010;18:63–70. doi: 10.1038/oby.2009.196. [DOI] [PubMed] [Google Scholar]
- 64.Wu SQ, Hopfner RL, McNeill JR, Wilson TW, Gopalakrishnan V. Altered paracrine effect of endothelin in blood vessels of the hyperinsulinemic, insulin resistant obese Zucker rat. Cardiovasc Res. 2000;45:994–1000. doi: 10.1016/s0008-6363(99)00417-4. [DOI] [PubMed] [Google Scholar]
- 65.Altman JD, Kinn J, Duncker DJ, Bache RJ. Effect of inhibition of nitric oxide formation on coronary blood flow during exercise in the dog. Cardiovasc Res. 1994;28:119–24. doi: 10.1093/cvr/28.1.119. [DOI] [PubMed] [Google Scholar]
- 66.Bernstein RD, Ochoa FY, Xu X, Forfia P, Shen W, Thompson CI, et al. Function and production of nitric oxide in the coronary circulation of the conscious dog during exercise. Circ Res. 1996;79:840–8. doi: 10.1161/01.res.79.4.840. [DOI] [PubMed] [Google Scholar]
- 67.Canty JM, Jr., Schwartz JS. Nitric oxide mediates flow-dependent epicardial coronary vasodilation to changes in pulse frequency but not mean flow in conscious dogs. Circulation. 1994;89:375–84. doi: 10.1161/01.cir.89.1.375. [DOI] [PubMed] [Google Scholar]
- 68.Duncker DJ, Bache RJ. Inhibition of nitric oxide production aggravates myocardial hypoperfusion during exercise in the presence of a coronary artery stenosis. Circ Res. 1994;74:629–40. doi: 10.1161/01.res.74.4.629. [DOI] [PubMed] [Google Scholar]
- 69.Tune JD, Richmond KN, Gorman MW, Feigl EO. Role of nitric oxide and adenosine in control of coronary blood flow in exercising dogs. Circulation. 2000;101:2942–8. doi: 10.1161/01.cir.101.25.2942. [DOI] [PubMed] [Google Scholar]
- 70.Gorman MW, Farias M, III, Richmond KN, Tune JD, Feigl EO. Role of endothelin in alpha-adrenoceptor coronary vasoconstriction. Am J Physiol Heart Circ Physiol. 2005;288:H1937–H1942. doi: 10.1152/ajpheart.01076.2004. [DOI] [PubMed] [Google Scholar]
- 71.Merkus D, Duncker DJ, Chilian WM. Metabolic regulation of coronary vascular tone: role of endothelin-1. Am J Physiol Heart Circ Physiol. 2002;283:H1915–H1921. doi: 10.1152/ajpheart.00223.2002. [DOI] [PubMed] [Google Scholar]
- 72.Merkus D, Houweling B, Mirza A, Boomsma F, van den Meiracker AH, Duncker DJ. Contribution of endothelin and its receptors to the regulation of vascular tone during exercise is different in the systemic, coronary and pulmonary circulation. Cardiovasc Res. 2003;59:745–54. doi: 10.1016/s0008-6363(03)00479-6. [DOI] [PubMed] [Google Scholar]
- 73.Mokelke EA, Dietz NJ, Eckman DM, Nelson MT, Sturek M. Diabetic dyslipidemia and exercise affect coronary tone and differential regulation of conduit and microvessel K+ current. Am J Physiol Heart Circ Physiol. 2005;288:H1233–H1241. doi: 10.1152/ajpheart.00732.2004. [DOI] [PubMed] [Google Scholar]
- 74.Kiviniemi TO, Snapir A, Saraste M, Toikka JO, Raitakari OT, Ahotupa M, et al. Determinants of coronary flow velocity reserve in healthy young men. Am J Physiol Heart Circ Physiol. 2006;291:H564–H569. doi: 10.1152/ajpheart.00915.2005. [DOI] [PubMed] [Google Scholar]
- 75.Bratz IN, Dick GM, Tune JD, Edwards JM, Neeb ZP, Dincer UD, et al. Impaired capsaicin-induced relaxation of coronary arteries in a porcine model of the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2008;294:H2489–H2496. doi: 10.1152/ajpheart.01191.2007. [DOI] [PubMed] [Google Scholar]
- 76.Mokelke EA, Hu Q, Song M, Toro L, Reddy HK, Sturek M. Altered functional coupling of coronary K+ channels in diabetic dyslipidemic pigs is prevented by exercise. J Appl Physiol. 2003;95:1179–93. doi: 10.1152/japplphysiol.00972.2002. [DOI] [PubMed] [Google Scholar]
- 77.Dick GM, Tune JD. Role of potassium channels in coronary vasodilation. Exp Biol Med (Maywood) 2010;235:10–22. doi: 10.1258/ebm.2009.009201. [DOI] [PubMed] [Google Scholar]
- 78.Busija DW, Miller AW, Katakam P, Erdos B. Insulin resistance and associated dysfunction of resistance vessels and arterial hypertension. Minerva Med. 2005;96:223–32. [PubMed] [Google Scholar]
- 79.Knudson JD, Dick GM, Tune JD. Adipokines and coronary vasomotor dysfunction. Exp Biol Med (Maywood) 2007;232:727–36. [PubMed] [Google Scholar]
- 80.Payne GA, Borbouse L, Kumar S, Neeb Z, Alloosh M, Sturek M, et al. Epicardial Perivascular Adipose-Derived Leptin Exacerbates Coronary Endothelial Dysfunction in Metabolic Syndrome via a Protein Kinase C-{beta} Pathway. Arterioscler Thromb Vasc Biol. 2010;30:1711–7. doi: 10.1161/ATVBAHA.110.210070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frisbee JC. Remodeling of the skeletal muscle microcirculation increases resistance to perfusion in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2003;285:H104–H111. doi: 10.1152/ajpheart.00118.2003. [DOI] [PubMed] [Google Scholar]
- 82.Stepp DW, Pollock DM, Frisbee JC. Low-flow vascular remodeling in the metabolic syndrome X. Am J Physiol Heart Circ Physiol. 2004;286:H964–H970. doi: 10.1152/ajpheart.00836.2003. [DOI] [PubMed] [Google Scholar]
- 83.Stapleton PA, Goodwill AG, James ME, D'Audiffret AC, Frisbee JC. Differential impact of familial hypercholesterolemia and combined hyperlipidemia on vascular wall and network remodeling in mice. Microcirculation. 2010;17:47–58. doi: 10.1111/j.1549-8719.2009.00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Frisbee JC, Samora JB, Peterson J, Bryner R. Exercise training blunts microvascular rarefaction in the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2006;291:H2483–H2492. doi: 10.1152/ajpheart.00566.2006. [DOI] [PubMed] [Google Scholar]
- 85.Frisbee JC. Reduced nitric oxide bioavailability contributes to skeletal muscle microvessel rarefaction in the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol. 2005;289:R307–R316. doi: 10.1152/ajpregu.00114.2005. [DOI] [PubMed] [Google Scholar]
- 86.Tune JD, Richmond KN, Gorman MW, Feigl EO. Control of coronary blood flow during exercise. Exp Biol Med (Maywood) 2002;227:238–50. doi: 10.1177/153537020222700404. [DOI] [PubMed] [Google Scholar]
- 87.Olsson RA. Myocardial reactive hyperemia. Circ Res. 1975;37:263–70. doi: 10.1161/01.res.37.3.263. [DOI] [PubMed] [Google Scholar]
- 88.Ruiter JH, Spaan JA, Laird JD. Transient oxygen uptake during myocardial reactive hyperemia in the dog. Am J Physiol. 1978;235:H87–H94. doi: 10.1152/ajpheart.1978.235.1.H87. [DOI] [PubMed] [Google Scholar]
- 89.De FE, Cusi K, Ocampo G, Berria R, Buck S, Consoli A, et al. Exercise-induced improvement in vasodilatory function accompanies increased insulin sensitivity in obesity and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006;91:4903–10. doi: 10.1210/jc.2006-1142. [DOI] [PubMed] [Google Scholar]
- 90.Ishibashi Y, Takahashi N, Shimada T, Sugamori T, Sakane T, Umeno T, et al. Short duration of reactive hyperemia in the forearm of subjects with multiple cardiovascular risk factors. Circ J. 2006;70:115–23. doi: 10.1253/circj.70.115. [DOI] [PubMed] [Google Scholar]
- 91.Grassi G, Dell'oro R, Quarti-Trevano F, Scopelliti F, Seravalle G, Paleari F, et al. Neuroadrenergic and reflex abnormalities in patients with metabolic syndrome. Diabetologia. 2005 doi: 10.1007/s00125-005-1798-z. [DOI] [PubMed] [Google Scholar]
- 92.Huggett RJ, Burns J, Mackintosh AF, Mary DA. Sympathetic neural activation in nondiabetic metabolic syndrome and its further augmentation by hypertension. Hypertension. 2004;44:847–52. doi: 10.1161/01.HYP.0000147893.08533.d8. [DOI] [PubMed] [Google Scholar]
- 93.Feigl EO. Neural control of coronary blood flow. J Vasc Res. 1998;35:85–92. doi: 10.1159/000025569. [DOI] [PubMed] [Google Scholar]
- 94.Chilian WM. Adrenergic vasomotion in the coronary microcirculation. Basic Res Cardiol. 1990;85(Suppl 1):111–20. doi: 10.1007/978-3-662-11038-6_9. [DOI] [PubMed] [Google Scholar]
- 95.Chilian WM. Functional distribution of alpha 1- and alpha 2-adrenergic receptors in the coronary microcirculation. Circulation. 1991;84:2108–22. doi: 10.1161/01.cir.84.5.2108. [DOI] [PubMed] [Google Scholar]
- 96.Duncker DJ, Stubenitsky R, Verdouw PD. Autonomic control of vasomotion in the porcine coronary circulation during treadmill exercise: evidence for feed-forward beta-adrenergic control. Circ Res. 1998;82:1312–22. doi: 10.1161/01.res.82.12.1312. [DOI] [PubMed] [Google Scholar]
- 97.Gorman MW, Tune JD, Richmond KN, Feigl EO. Feedforward sympathetic coronary vasodilation in exercising dogs. J Appl Physiol. 2000;89:1892–902. doi: 10.1152/jappl.2000.89.5.1892. [DOI] [PubMed] [Google Scholar]
- 98.Heusch G. Control of coronary vasomotor tone in ischaemic myocardium by local metabolism and neurohumoral mechanisms. Eur Heart J. 1991;12(Suppl F):99–106. doi: 10.1093/eurheartj/12.suppl_f.99. [DOI] [PubMed] [Google Scholar]
- 99.Heusch G. The paradox of alpha-adrenergic coronary vasoconstriction revisited. J Mol Cell Cardiol. 2011;51:16–23. doi: 10.1016/j.yjmcc.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 100.Huang AH, Feigl EO. Adrenergic coronary vasoconstriction helps maintain uniform transmural blood flow distribution during exercise. Circ Res. 1988;62:286–98. doi: 10.1161/01.res.62.2.286. [DOI] [PubMed] [Google Scholar]
- 101.Heusch G. Alpha-adrenergic mechanisms in myocardial ischemia. Circulation. 1990;81:1–13. doi: 10.1161/01.cir.81.1.1. [DOI] [PubMed] [Google Scholar]
- 102.Gorman MW, Tune JD, Richmond KN, Feigl EO. Quantitative analysis of feedforward sympathetic coronary vasodilation in exercising dogs. J Appl Physiol. 2000;89:1903–11. doi: 10.1152/jappl.2000.89.5.1903. [DOI] [PubMed] [Google Scholar]
- 103.Miyashiro JK, Feigl EO. Feedforward control of coronary blood flow via coronary beta-receptor stimulation. Circ Res. 1993;73:252–63. doi: 10.1161/01.res.73.2.252. [DOI] [PubMed] [Google Scholar]
- 104.Grisk O, Frauendorf T, Schluter T, Kloting I, Kuttler B, Krebs A, et al. Impaired coronary function in Wistar Ottawa Karlsburg W rats-a new model of the metabolic syndrome. Pflugers Arch. 2007;454:1011–21. doi: 10.1007/s00424-007-0267-6. [DOI] [PubMed] [Google Scholar]
- 105.Frisbee JC. Enhanced arteriolar alpha-adrenergic constriction impairs dilator responses and skeletal muscle perfusion in obese Zucker rats. J Appl Physiol. 2004;97:764–72. doi: 10.1152/japplphysiol.01216.2003. [DOI] [PubMed] [Google Scholar]
- 106.Naik JS, Xiang L, Hodnett BL, Hester RL. Alpha-adrenoceptor-mediated vasoconstriction is not involved in impaired functional vasodilation in the obese Zucker rat. Clin Exp Pharmacol Physiol. 2008;35:611–6. doi: 10.1111/j.1440-1681.2007.04849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stepp DW, Frisbee JC. Augmented adrenergic vasoconstriction in hypertensive diabetic obese Zucker rats. Am J Physiol Heart Circ Physiol. 2002;282:H816–H820. doi: 10.1152/ajpheart.00695.2001. [DOI] [PubMed] [Google Scholar]
- 108.Gregorini L, Marco J, Kozakova M, Palombo C, Anguissola GB, Marco I, et al. Alpha-adrenergic blockade improves recovery of myocardial perfusion and function after coronary stenting in patients with acute myocardial infarction. Circulation. 1999;99:482–90. doi: 10.1161/01.cir.99.4.482. [DOI] [PubMed] [Google Scholar]
- 109.Gregorini L, Marco J, Palombo C, Kozakova M, Anguissola GB, Cassagneau B, et al. Postischemic left ventricular dysfunction is abolished by alpha-adrenergic blocking agents. J Am Coll Cardiol. 1998;31:992–1001. doi: 10.1016/s0735-1097(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 110.Gregorini L, Marco J, Farah B, Bernies M, Palombo C, Kozakova M, et al. Effects of selective alpha1- and alpha2-adrenergic blockade on coronary flow reserve after coronary stenting. Circulation. 2002;106:2901–7. doi: 10.1161/01.cir.0000040998.88272.a7. [DOI] [PubMed] [Google Scholar]
- 111.Heusch G, Baumgart D, Camici P, Chilian W, Gregorini L, Hess O, et al. alpha-adrenergic coronary vasoconstriction and myocardial ischemia in humans. Circulation. 2000;101:689–94. doi: 10.1161/01.cir.101.6.689. [DOI] [PubMed] [Google Scholar]
- 112.D'Angelo G, Mintz JD, Tidwell JE, Schreihofer AM, Pollock DM, Stepp DW. Exaggerated cardiovascular stress responses and impaired beta-adrenergic-mediated pressor recovery in obese Zucker rats. Hypertension. 2006;48:1109–15. doi: 10.1161/01.HYP.0000247306.53547.d4. [DOI] [PubMed] [Google Scholar]
- 113.Myers PR, Katwa LC, Tanner M, Morrow C, Guarda E, Parker JL. Effects of angiotensin II on canine and porcine coronary epicardial and resistance arteries. J Vasc Res. 1994;31:338–46. doi: 10.1159/000159062. [DOI] [PubMed] [Google Scholar]
- 114.Zhang C, Hein TW, Wang W, Kuo L. Divergent roles of angiotensin II AT1 and AT2 receptors in modulating coronary microvascular function. Circ Res. 2003;92:322–9. doi: 10.1161/01.res.0000056759.53828.2c. [DOI] [PubMed] [Google Scholar]
- 115.Ertl G, Hu K, Bauer WR, Bauer B. The renin-angiotensin system and coronary vasomotion. Heart. 1996;76:45–52. doi: 10.1136/hrt.76.3_suppl_3.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Magrini F, Reggiani P, Paliotti R, Bonagura F, Ciulla M, Vandoni P. Coronary hemodynamics and the renin angiotensin system. Clin Exp Hypertens. 1993;15(Suppl 1):139–55. [PubMed] [Google Scholar]
- 117.Caprio M, Newfell BG, la SA, Baur W, Fabbri A, Rosano G, et al. Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ Res. 2008;102:1359–67. doi: 10.1161/CIRCRESAHA.108.174235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jaffe IZ, Mendelsohn ME. Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circ Res. 2005;96:643–50. doi: 10.1161/01.RES.0000159937.05502.d1. [DOI] [PubMed] [Google Scholar]
- 119.Jaffe IZ, Tintut Y, Newfell BG, Demer LL, Mendelsohn ME. Mineralocorticoid receptor activation promotes vascular cell calcification. Arterioscler Thromb Vasc Biol. 2007;27:799–805. doi: 10.1161/01.ATV.0000258414.59393.89. [DOI] [PubMed] [Google Scholar]
- 120.Xiao F, Puddefoot JR, Barker S, Vinson GP. Mechanism for aldosterone potentiation of angiotensin II-stimulated rat arterial smooth muscle cell proliferation. Hypertension. 2004;44:340–5. doi: 10.1161/01.HYP.0000140771.21243.ed. [DOI] [PubMed] [Google Scholar]
- 121.Fujita M, Minamino T, Asanuma H, Sanada S, Hirata A, Wakeno M, et al. Aldosterone nongenomically worsens ischemia via protein kinase C-dependent pathways in hypoperfused canine hearts. Hypertension. 2005;46:113–7. doi: 10.1161/01.HYP.0000171184.84077.80. [DOI] [PubMed] [Google Scholar]
- 122.Moreau D, Chardigny JM, Rochette L. Effects of aldosterone and spironolactone on the isolated perfused rat heart. Pharmacology. 1996;53:28–36. doi: 10.1159/000139412. [DOI] [PubMed] [Google Scholar]
- 123.Kushibiki M, Yamada M, Oikawa K, Tomita H, Osanai T, Okumura K. Aldosterone causes vasoconstriction in coronary arterioles of rats via angiotensin II type-1 receptor: influence of hypertension. Eur J Pharmacol. 2007;572:182–8. doi: 10.1016/j.ejphar.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 124.Yamada M, Kushibiki M, Osanai T, Tomita H, Okumura K. Vasoconstrictor effect of aldosterone via angiotensin II type 1 (AT1) receptor: possible role of AT1 receptor dimerization. Cardiovasc Res. 2008;79:169–78. doi: 10.1093/cvr/cvn064. [DOI] [PubMed] [Google Scholar]
- 125.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 126.Kurian MM, Berwick ZC, Tune JD. Contribution of IKCa channels to the control of coronary blood flow. Exp Biol Med (Maywood) 2011;236:621–7. doi: 10.1258/ebm.2011.010351. [DOI] [PubMed] [Google Scholar]
- 127.Berwick ZC, Dick GM, Moberly SP, Kohr MC, Sturek M, Tune JD. Contribution of voltage-dependent K+ channels to metabolic control of coronary blood flow. J Mol Cell Cardiol. 2011 doi: 10.1016/j.yjmcc.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dick GM, Bratz IN, Borbouse L, Payne GA, Dincer UD, Knudson JD, et al. Voltage-dependent K+ channels regulate the duration of reactive hyperemia in the canine coronary circulation. Am J Physiol Heart Circ Physiol. 2008;294:H2371–H2381. doi: 10.1152/ajpheart.01279.2007. [DOI] [PubMed] [Google Scholar]
- 129.Rogers PA, Dick GM, Knudson JD, Focardi M, Bratz IN, Swafford AN, Jr., et al. H2O2-induced redox-sensitive coronary vasodilation is mediated by 4-aminopyridine-sensitive K+ channels. Am J Physiol Heart Circ Physiol. 2006;291:H2473–H2482. doi: 10.1152/ajpheart.00172.2006. [DOI] [PubMed] [Google Scholar]
- 130.Rogers PA, Chilian WM, Bratz IN, Bryan RM, Jr., Dick GM. H2O2 activates redox- and 4-aminopyridine-sensitive Kv channels in coronary vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2007;292:H1404–H1411. doi: 10.1152/ajpheart.00696.2006. [DOI] [PubMed] [Google Scholar]
- 131.Saitoh S, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, et al. Hydrogen peroxide: a feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol. 2006;26:2614–21. doi: 10.1161/01.ATV.0000249408.55796.da. [DOI] [PubMed] [Google Scholar]
- 132.Ohanyan VA, Bratz IN, Guarini G, Yin L, Chilian WM. Kv1.5 channels play a critical role in coronary metabolic dilation. Circulation. 2010;122:A16997. [Google Scholar]
- 133.Bratz IN, Swafford AN, Jr., Kanagy NL, Dick GM. Reduced functional expression of K(+) channels in vascular smooth muscle cells from rats made hypertensive with N{omega}-nitro-L-arginine. Am J Physiol Heart Circ Physiol. 2005;289:H1284–H1290. doi: 10.1152/ajpheart.01053.2004. [DOI] [PubMed] [Google Scholar]
- 134.Bratz IN, Dick GM, Partridge LD, Kanagy NL. Reduced molecular expression of K(+) channel proteins in vascular smooth muscle from rats made hypertensive with N{omega}-nitro-L-arginine. Am J Physiol Heart Circ Physiol. 2005;289:H1277–H1283. doi: 10.1152/ajpheart.01052.2004. [DOI] [PubMed] [Google Scholar]
- 135.Heaps CL, Tharp DL, Bowles DK. Hypercholesterolemia abolishes voltage-dependent K+ channel contribution to adenosine-mediated relaxation in porcine coronary arterioles. Am J Physiol Heart Circ Physiol. 2005;288:H568–H576. doi: 10.1152/ajpheart.00157.2004. [DOI] [PubMed] [Google Scholar]
- 136.Heaps CL, Jeffery EC, Laine GA, Price EM, Bowles DK. Effects of exercise training and hypercholesterolemia on adenosine activation of voltage-dependent K+ channels in coronary arterioles. J Appl Physiol. 2008;105:1761–71. doi: 10.1152/japplphysiol.90958.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li H, Chai Q, Gutterman DD, Liu Y. Elevated glucose impairs cAMP-mediated dilation by reducing Kv channel activity in rat small coronary smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003;285:H1213–H1219. doi: 10.1152/ajpheart.00226.2003. [DOI] [PubMed] [Google Scholar]
- 138.Liu Y, Gutterman DD. The coronary circulation in diabetes: influence of reactive oxygen species on K+ channel-mediated vasodilation. Vascul Pharmacol. 2002;38:43–9. doi: 10.1016/s1537-1891(02)00125-8. [DOI] [PubMed] [Google Scholar]
- 139.Liu Y, Gutterman DD. Oxidative stress and potassium channel function. Clin Exp Pharmacol Physiol. 2002;29:305–11. doi: 10.1046/j.1440-1681.2002.03649.x. [DOI] [PubMed] [Google Scholar]
- 140.Berg T. The vascular response to the K+ channel inhibitor 4-aminopyridine in hypertensive rats. Eur J Pharmacol. 2003;466:301–10. doi: 10.1016/s0014-2999(03)01555-3. [DOI] [PubMed] [Google Scholar]
- 141.Cox RH. Molecular determinants of voltage-gated potassium currents in vascular smooth muscle. Cell Biochem Biophys. 2005;42:167–95. doi: 10.1385/CBB:42:2:167. [DOI] [PubMed] [Google Scholar]
- 142.Yang Y, JONES AW, Thomas TR, Rubin LJ. Influence of sex, high-fat diet, and exercise training on potassium currents of swine coronary smooth muscle. Am J Physiol Heart Circ Physiol. 2007;293:H1553–H1563. doi: 10.1152/ajpheart.00151.2007. [DOI] [PubMed] [Google Scholar]
- 143.Lu T, Ye D, He T, Wang XL, Wang HL, Lee HC. Impaired Ca2+-dependent activation of large-conductance Ca2+-activated K+ channels in the coronary artery smooth muscle cells of Zucker Diabetic Fatty rats. Biophys J. 2008;95:5165–77. doi: 10.1529/biophysj.108.138339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Miura H, Liu Y, Gutterman DD. Human coronary arteriolar dilation to bradykinin depends on membrane hyperpolarization: contribution of nitric oxide and Ca2+-activated K+ channels. Circulation. 1999;99:3132–8. doi: 10.1161/01.cir.99.24.3132. [DOI] [PubMed] [Google Scholar]
- 145.Miura H, Wachtel RE, Liu Y, Loberiza FR, Jr., Saito T, Miura M, et al. Flow-induced dilation of human coronary arterioles: important role of Ca(2+)-activated K(+) channels. Circulation. 2001;103:1992–8. doi: 10.1161/01.cir.103.15.1992. [DOI] [PubMed] [Google Scholar]
- 146.Belin De Chantemele EJ, Stepp DW. Influence of obesity and metabolic dysfunction on the endothelial control in the coronary circulation. J Mol Cell Cardiol. 2011 doi: 10.1016/j.yjmcc.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Miura H, Wachtel RE, Loberiza FR, Jr., Saito T, Miura M, Nicolosi AC, et al. Diabetes mellitus impairs vasodilation to hypoxia in human coronary arterioles: reduced activity of ATP-sensitive potassium channels. Circ Res. 2003;92:151–8. doi: 10.1161/01.res.0000052671.53256.49. [DOI] [PubMed] [Google Scholar]
- 148.Mathew V, Lerman A. Altered effects of potassium channel modulation in the coronary circulation in experimental hypercholesterolemia. Atherosclerosis. 2001;154:329–35. doi: 10.1016/s0021-9150(00)00493-7. [DOI] [PubMed] [Google Scholar]
- 149.Kersten JR, Brooks LA, Dellsperger KC. Impaired microvascular response to graded coronary occlusion in diabetic and hyperglycemic dogs. Am J Physiol. 1995;268:H1667–H1674. doi: 10.1152/ajpheart.1995.268.4.H1667. [DOI] [PubMed] [Google Scholar]
- 150.Tune JD, Yeh C, Setty S, Downey HF. ATP-dependent K(+) channels contribute to local metabolic coronary vasodilation in experimental diabetes. Diabetes. 2002;51:1201–7. doi: 10.2337/diabetes.51.4.1201. [DOI] [PubMed] [Google Scholar]
- 151.Haddy FJ, Vanhoutte PM, Feletou M. Role of potassium in regulating blood flow and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R546–R552. doi: 10.1152/ajpregu.00491.2005. [DOI] [PubMed] [Google Scholar]
- 152.Ko EA, Han J, Jung ID, Park WS. Physiological roles of K+ channels in vascular smooth muscle cells. J Smooth Muscle Res. 2008;44:65–81. doi: 10.1540/jsmr.44.65. [DOI] [PubMed] [Google Scholar]
- 153.Keef KD, Hume JR, Zhong J. Regulation of cardiac and smooth muscle Ca(2+) channels (Ca(V)1.2a,b) by protein kinases. Am J Physiol Cell Physiol. 2001;281:C1743–C1756. doi: 10.1152/ajpcell.2001.281.6.C1743. [DOI] [PubMed] [Google Scholar]
- 154.Gollasch M, Nelson MT. Voltage-dependent Ca2+ channels in arterial smooth muscle cells. Kidney Blood Press Res. 1997;20:355–71. doi: 10.1159/000174250. [DOI] [PubMed] [Google Scholar]
- 155.Wamhoff BR, Bowles DK, Owens GK. Excitation-transcription coupling in arterial smooth muscle. Circ Res. 2006;98:868–78. doi: 10.1161/01.RES.0000216596.73005.3c. [DOI] [PubMed] [Google Scholar]
- 156.Sonkusare S, Palade PT, Marsh JD, Telemaque S, Pesic A, Rusch NJ. Vascular calcium channels and high blood pressure: pathophysiology and therapeutic implications. Vascul Pharmacol. 2006;44:131–42. doi: 10.1016/j.vph.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bowles DK, Heaps CL, Turk JR, Maddali KK, Price EM. Hypercholesterolemia inhibits L-type calcium current in coronary macro-, not microcirculation. J Appl Physiol. 2004;96:2240–8. doi: 10.1152/japplphysiol.01229.2003. [DOI] [PubMed] [Google Scholar]
- 158.Witczak CA, Wamhoff BR, Sturek M. Exercise training prevents Ca2+ dysregulation in coronary smooth muscle from diabetic dyslipidemic yucatan swine. J Appl Physiol. 2006;101:752–62. doi: 10.1152/japplphysiol.00235.2006. [DOI] [PubMed] [Google Scholar]
- 159.Firth AL, Remillard CV, Yuan JX. TRP channels in hypertension. Biochim Biophys Acta. 2007;1772:895–906. doi: 10.1016/j.bbadis.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dietrich A, Chubanov V, Kalwa H, Rost BR, Gudermann T. Cation channels of the transient receptor potential superfamily: their role in physiological and pathophysiological processes of smooth muscle cells. Pharmacol Ther. 2006;112:744–60. doi: 10.1016/j.pharmthera.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 161.Dietrich A, Mederos YS, Gollasch M, Gross V, Storch U, Dubrovska G, et al. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol. 2005;25:6980–9. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Weissmann N, Dietrich A, Fuchs B, Kalwa H, Ay M, Dumitrascu R, et al. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci U S A. 2006;103:19093–8. doi: 10.1073/pnas.0606728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Saleh SN, Albert AP, Large WA. Activation of native TRPC1/C5/C6 channels by endothelin-1 is mediated by both PIP3 and PIP2 in rabbit coronary artery myocytes. J Physiol. 2009;587:5361–75. doi: 10.1113/jphysiol.2009.180331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Edwards JM, Neeb ZP, Alloosh MA, Long X, Bratz IN, Peller CR, et al. Exercise training decreases store-operated Ca2+entry associated with metabolic syndrome and coronary atherosclerosis. Cardiovasc Res. 2010;85:631–40. doi: 10.1093/cvr/cvp308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hu G, Oboukhova S, Kumar S, Sturek M, Obukhov AG. Expression level of canonical transient receptor potential channels is elevated in the adrenal medulla of Ossabaw miniature pigs exhibiting metabolic syndrome. Mol Endocrinol. 2008 doi: 10.1210/me.2008-0350. Ref Type: Generic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kumar B, Dreja K, Shah SS, Cheong A, Xu SZ, Sukumar P, et al. Upregulated TRPC1 channel in vascular injury in vivo and its role in human neointimal hyperplasia. Circ Res. 2006;98:557–63. doi: 10.1161/01.RES.0000204724.29685.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhu Z, Luo Z, Ma S, Liu D. TRP channels and their implications in metabolic diseases. Pflugers Arch. 2011;461:211–23. doi: 10.1007/s00424-010-0902-5. [DOI] [PubMed] [Google Scholar]
- 168.Watanabe H, Murakami M, Ohba T, Takahashi Y, Ito H. TRP channel and cardiovascular disease. Pharmacol Ther. 2008;118:337–51. doi: 10.1016/j.pharmthera.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 169.Takahashi Y, Watanabe H, Murakami M, Ohba T, Radovanovic M, Ono K, et al. Involvement of transient receptor potential canonical 1 (TRPC1) in angiotensin II-induced vascular smooth muscle cell hypertrophy. Atherosclerosis. 2007;195:287–96. doi: 10.1016/j.atherosclerosis.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 170.Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, et al. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol. 2010;298:H466–H476. doi: 10.1152/ajpheart.00854.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Focardi M, Dick GM, Picchi A, Zhang C, Chilian WM. Restoration of coronary endothelial function in obese Zucker rats by a low-carbohydrate diet. Am J Physiol Heart Circ Physiol. 2007;292:H2093–H2099. doi: 10.1152/ajpheart.01202.2006. [DOI] [PubMed] [Google Scholar]