Abstract
Weight-loss therapy to improve health in obese older adults is controversial because it causes further bone loss. Therefore, it is recommended that weight-loss therapy should include an intervention to minimize bone loss such as exercise training (ET). The purpose of this study was to determine the independent and combined effects of weight loss and ET on bone metabolism in relation to bone mineral density (BMD) in obese older adults. One-hundred-seven older (age >65 yrs) obese (BMI ≥30 kg/m2) adults were randomly assigned to a control group, diet group, exercise group, and diet-exercise group for 1 year. Body weight decreased in the diet (−9.6%) and diet-exercise (−9.4%) groups, not in the exercise (−1%) and control (−0.2%) groups (between-group P<.001). However, despite comparable weight loss, bone loss at the total hip was relatively less in the diet-exercise group (−1.1%) than in the diet group (−2.6%), whereas BMD increased in the exercise group (1.5%) (between-group P<.001) Serum C-terminal telopeptide (CTX) and osteocalcin concentrations increased in the diet group (31% and 24%) while they decreased in the exercise group (−13% and −15%) (between-group P<.001). In contrast, similar to the control group, serum CTX and osteocalcin concentrations did not change in the diet-exercise group. Serum procollagen propeptide concentrations decreased in the exercise group (−15%) compared with the diet group (9%) (P=.04). Serum leptin and estradiol concentrations decreased in the diet (−25% and −15%) and diet-exercise (−38% and −13%) groups, not in the exercise and control groups (between-group P=.001). Multivariate analyses revealed that changes in lean body mass (β=.33), serum osteocalcin (β= −.24), and 1-RM strength (β=.23) were independent predictors of changes in hip BMD (all P<.05). In conclusion, the addition of ET to weight-loss therapy among obese older adults prevents weight-loss-induced increase in bone turnover and attenuates weight-loss-induced reduction in hip BMD despite weight-loss-induced decrease in bone-active hormones.
INTRODUCTION
In older adults, obesity worsens the age-related decline in physical function leading to frailty, loss of independence, and increased nursing home admissions.(1,2) Therefore, the increasing number of obese older adults is a significant public health problem that challenges existing health-care delivery systems.(3) However, the appropriate treatment for obese older adults is controversial. One major concern is that inducing weight loss in obese older adults could lead to further loss of bone mass (on top of age-related bone loss) and increase the risk for osteoporotic fractures.(1) Accordingly, it is recommended that weight-loss therapy in older adults includes preventative measures against the loss of bone mass that occurs with weight loss.(1) We recently reported that exercise training (ET) added to diet-induced weight loss not only ameliorates frailty but also attenuates the weight loss-induced reduction in bone mineral density (BMD) and lean body mass, suggesting that a combination of weight loss and ET may be important therapy for obese older adults.(4) However, the mechanisms behind this osteoprotective effect of ET during weight loss therapy remain to be elucidated. Moreover, previous studies that examined the effect of weight loss alone or in combination with ET on bone metabolism have been conducted primarily in younger populations and have not directly compared the independent and combined effects of weight loss and ET on biochemical markers of bone turnover, bone-active hormones, and BMD.(5–8)
The purpose of this study was to determine the independent and combined effects of weight loss and ET on bone metabolism in relation to changes in BMD in obese older adults. We hypothesized that weight loss would increase while ET would decrease bone turnover; the addition of ET to weight loss would prevent the increase in bone turnover and attenuate the reduction in BMD. We also hypothesized that weight loss-induced reduction in BMD would be associated with decrease in bone-active hormones; the addition of ET to weight loss would protect against this bone loss despite decrease in bone-active hormones. The data reported in this article were obtained using the same subject group that participated in a randomized, controlled trial of the effect of weight loss and exercise on multiple health outcomes including BMD in obese older adults.(4)
METHODS
Subjects
This study was conducted at Washington University School of Medicine and was approved by the Institutional Review Board. Volunteers were recruited from the community through advertisements. All subjects gave informed consent before their participation. All potential subjects underwent a comprehensive medical examination, routine chemistries, and an exercise treadmill stress test. Eligibility criteria included older age ≥ 65 years, obese (body mass index ≥ 30 kg/m2), sedentary lifestyle (did not participatein regular exercise more than twice a week), stable body weight (± 2 kg) over the past year, and no changes in medications for at least 6 months before enrolling in the study. Subjects who were treated with bone-acting drugs (e.g. biphosphonates, glucocorticoids, sex-steroid compounds) during the previous year were excluded from participation. Additional inclusion and exclusion criteria have been previously described in detail.(4) The effects of weight loss and/or ET on measures of frailty, body composition, bone mineral density, specific physical functions, and quality of life on these subjects were reported previously.(4)
Design
Eligible subjects were randomized, with stratification for sex, for a 52 week period, to 1 of the following 4 groups: 1) a control group 2) diet-induced weight loss (diet group), 3) exercise training (exercise group), and 4) diet-induced weight loss and exercise training (diet-exercise group).
Participants randomized to the control group were instructed to maintain their usual diet and activities during the study period. Participants randomized to the diet group were prescribed a balanced diet to provide an energy deficit of 500–750 kcal/day.(1) Total calorie intake was adjusted to prevent > 1.5% loss of body weight per week. The goal was to achieve a 10% weight-loss at six months, followed by weight maintenance for an additional 6 months. Subjects met weekly as a group with a study dietitian. Standard behavioral strategies were used to modify eating habits.(9) Participants randomized to the exercise group were counseled on maintaining a weight-stable diet and participated in a multi-component ET program supervised by a physical therapist. The ET sessions were conducted as a group on three nonconsecutive days each week at our exercise facility. Each session lasted ~90 min: 15 min of flexibility exercises, 30 min of aerobic exercise,30 min of progressive resistance training, and15 min of balance exercises. Additional details about the interventions including exercise intensity have been described previously.4 Participants randomized to the diet-exercise group participated in both weight management and ET programs described above, which were conducted separately from the other groups. All subjects were provided supplements to ensure an intake of ~1500 mg of calcium/day and ~1000 IU vitamin D/day.(1) Additional details about the interventions including compliance data have been reported previously.(4)
Outcome assessments
Body weight and bone mineral density
Body weight was measured at baseline, 6 months, and 12 months in the morning after subjects fasted for 12 h. Bone mineral density (BMD) of the lumbar spine, proximal femur, and whole body were measured from dual-energy-xray absorptiometry (DXA) (Delphi 4500-W: Hologic Corporation, Waltham, Mass) at baseline, 6 months, and 12 months. The CV for this technique at our center is 1.1% for the lumbar spine and 1.2% for the proximal femur.(10)
Serum markers of bone metabolism
Venous blood samples were obtained in the morning after subjects fasted for at least 12-hours at baseline, 6-months, and 12-months. Enzyme-linked immunosorbent assay kits were used to measure C-terminal telopeptide of type I collagen (CTX) (Crosslaps; Nordic BioscienceDiagnostics, Herlev, Denmark; CV, 2.1%) as a marker of bone resorption and osteocalcin (Metra OC; Quidel Corporation, San Diego, Calif; CV, 4.4%) as a marker of bone formation. Radioimmunoassay kit was used to measure serum intact N-terminal propeptide of type I procollagen (PINP) (Orion Diagnostica, Espoo, Finland) as an additional marker of bone formation. Radioimmunoassay kits were also used to measure serum estradiol (Ultra-sensitive estradiol DSL-4800; Diagnostic Systems Laboratories Inc, Webster, Tex), leptin (Leptin HL-81K; Linco Research Inc, St Charles, Mo) and insulin-like growth factor 1 (IGF-1) (Diagnostic Products Group, Los Angeles, Calif), and 25-hydroxyvitaminD (25(OH)D (DiaSorin, Stilwater MN). Milliplex assay kit was used to measure serum parathyroid hormone (PTH) concentration. (Millipore, St. Charles, MO). The CV for these hormone measurements were <10%. Blood samples at 6- and 12- months were obtained ~40 h after the last bout of exercise.
Muscle strength testing
One-repetition maximums (1-RMs) for upper (biceps curl, bench press and seated row) and lower body exercises (knee extension, knee flexion and leg press)were determined and the sum was used to calculate the total 1-RM.(11)
Statistical Analysis
The primary outcome in this study was changes in total hip BMD. We estimated that with 26 to 28 participants in each group, the study would have more than 80% power to detect a clinically meaningful 2.5±2.4% difference in the change in total hip BMD (on the basis of preliminary data) among the groups, at an alpha level of 5%. Secondary outcomes included biochemical markers of bone turnover and bone-active hormones.
Intention-to-treat analyses were performed using SAS software, version 9.2 of the SAS System (SAS Institute Inc., Cary, NC), with inclusion of all participants who provided follow-up data at any time point. Baseline characteristics between groups were compared using analyses of variance or Fisher exact tests. Longitudinal changes between groups were tested with mixed model repeated-measures analyses of variance (ANOVA), adjusting for baseline values and sex. The primary focus of these analyses was on the significance of the interaction between group and time point. Within the framework of the mixed model, when the P value for an interaction was significant, the specific contrasts were used to test the null hypothesis that changes between 2 specific time points in 1 group were equal to corresponding changes in another group. Analyses testing for within-group changes also were performed using mixed-model repeated-measures ANOVA. Pearson’s correlation was first used to examine relationships among changes in selected variables and changes in BMD. Stepwise multiple linear regression analysis (forward elimination method) was then used to identify which among the selected variables were the important independent contributors to the changes in BMD. Data are presented in tables and text as mean ± SD, except in the figures where data are mean ± SE. All statistical tests were 2-tailed, and P<.05 was considered statistically significant.
RESULTS
The results of screening, randomization, and follow-up have been previously reported.(4) Briefly, ninety-three (87%) of the 107 volunteers who were randomized completed the study. Fourteen participants discontinued the intervention (e.g. due to personal or medical reasons) but were included in the intention-to-treat analyses.
The 4 groups did not significantly differ in baseline characteristics including age, sex, weight, BMI, BMC, BMD, T-score, Z-score, and serum concentration of bone turnover markers and bone-active hormones (Tables 1 and 2). The 4 groups had normal average T-scores and Z-scores.
TABLE 1.
Baseline characteristics of study participants
| Control (n = 27) | Diet (n = 26) | Exercise (n = 26) | Diet-exercise (n = 28) | P value | |
|---|---|---|---|---|---|
| Age (yr) | 69±4 | 70±4 | 70±4 | 70±4 | 0.85 |
| Female sex [n (%)] | 18 (67) | 17 (65) | 16 (61) | 16 (57) | 0.89 |
| Weight (kg) | 101±16 | 104±15 | 99±17 | 99±17 | 0.66 |
| BMI (kg/m2) | 37.3±4.7 | 37.2±4.5 | 36.9±5.4 | 37.2±5.4 | 0.93 |
| Whole Body BMC, g | 2624±660 | 2783±652 | 2536±542 | 2692±532 | 0.50 |
| BMD (g/cm2) | |||||
| Whole body | 1.207±0.177 | 1.252±0.178 | 1.163±0.119 | 1.259±0.173 | 0.29 |
| Lumbar spine | 1.096±0.152 | 1.123± 0.160 | 1.075±0.157 | 1.174±0.271 | 0.40 |
| Total hip | 0.962±0.132 | 1.021±0.139 | 0.958±0.151 | 1.014±0.151 | 0.25 |
| Femoral neck | 0.816±0.106 | 0.852±0.118 | 0.799±0.120 | 0.865±0.137 | 0.17 |
| Trochanter | 0.711±0.141 | 0.766±0.118 | 0.791±0.116 | 0.865±0.137 | 0.32 |
| T-score | |||||
| Lumbar spine | 0.2±1.4 | 0.5±1.4 | −0.2±1.3 | 0.9±2.4 | 0.21 |
| Total hip | −0.2±0.9 | 0.3±1.0 | −0.3±1.0 | 0.2±1.1 | 0.07 |
| Femoral neck | −0.6±0.8 | −0.3±0.9 | −0.8±0.9 | −0.5±1.0 | 0.08 |
| Z-score | |||||
| Lumbar spine | 1.8±1.3 | 2.2±1.5 | 1.5±1.4 | 2.5±2.3 | 0.20 |
| Total hip | 0.7±1.1 | 1.2±1.1 | 0.5±1.1 | 1.0±1.4 | 0.16 |
| Femoral neck | −0.6±0.9 | −0.3±1.2 | −0.9±1.1 | −0.4±1.4 | 0.14 |
| Bone turnover markers | |||||
| CTX (ng/ml) | 0.407± 0.197 | 0.309± 0.123 | 0.350±0.143 | 0.320±0.116 | 0.09 |
| OC (ng/l) | 12.4±4.4 | 11.6±36 | 13.2±4.4 | 12.2±4.4 | 0.64 |
| PINP (ug/l) | 52.2±26.3 | 41.5±12.2 | 45.2±14.1 | 43.1±13.1 | 0.13 |
Values are means ± SD. BMD, bone mineral density; BMI, = body mass index; CTX, C-terminal telopeptide of type 1 collagen; OC, Osteocalcin; PINP, Intact N-terminal propeptide of type 1 procollagen
Normal values: CTX (0.110–0.690 ng/mL), OC (3.7–10.0 ng/mL), PINP (19–96 ug/L).
TABLE 2.
Serum concentrations of bone-active hormones
| Control | Diet | Exercise | Diet-exercise | |
|---|---|---|---|---|
| 25(OH)vit D, ng/mL | ||||
| Baseline | 19.2±8.2 | 22.0±5.7 | 21.4±8.4 | 20.9±6.7 |
| 6 mo | 21.8±9.1* | 24.7±7.9* | 25.1±8.4* | 26.0±7.4* |
| 1 y | 22.4±8.3* | 24.5±7.1* | 26.3±7.0* | 25.6±7.0* |
| PTH, (pg/mL) | ||||
| Baseline | 32.0±5.0 | 31.8±4.4 | 31.0±6.4 | 31.1±5.0 |
| 6 mo | 31.5±3.5 | 31.8±4.9 | 29.1±6.8 | 31.2±6.9 |
| 1 y | 31.3±3.0 | 30.3±4.6 | 28.9±6.7 | 29.6±5.3 |
| Leptin (ug/L) | ||||
| Baseline | 33.2±17.7 | 36.6±17.8 | 34.4±21.9 | 31.3±22.5 |
| 6 mo | 30.1±18.4 | 24.9±15.0*† | 31.7±22.6‡ | 23.6±23.0*†§ |
| 1 y | 33.9±20.5 | 23.5±11.4*† | 33.9±24.1‡ | 20.3±19.0*†§ |
| Estradiol (pg/mL) | ||||
| All | ||||
| Baseline | 12.2±5.2 | 14.2±5.1 | 14.9±8.0 | 15.9±5.5 |
| 6 mo | 12.1±13.9 | 11.7±5.5*† | 14.9±10.5‡ | 11.9±4.5*†§ |
| 1 y | 13.9±5.3 | 12.0±4.5*† | 13.3±6.5 | 13.0±6.0*† |
| Women | ||||
| Baseline | 9.7±2.7 | 13.3±5.7 | 14.0-±9.5 | 14.7±6.9 |
| 6 mo | 9.5±3.7 | 11.0±4.0* | 14.4±13.6 | 10.7±4.8*†§ |
| 1 y | 11.7±3.6 | 11.3±5.1*† | 12.5±7.4† | 10.9±5.6*† |
| Men | ||||
| Baseline | 17.8±5.5 | 15.6±3.6 | 16.3±4.8 | 17.5±2.8 |
| 6 mo | 17.3±5.3 | 13.7±2.8* | 16.1±5.1 | 13.5±3.7*†§ |
| 1 y | 17.2±5.9 | 13.1±3.2* | 14.9±5.0 | 14.8±3.7* |
| IGF-1 (ng/mL) | ||||
| Baseline | 102.8±31.9 | 102.9±31.2 | 88.0±26.9 | 91.2±24.5 |
| 6 mo | 105.8±29.8 | 105.6±36.1 | 86.5±28.8 | 97.5±31.1 |
| 1 y | 104.6±31.8 | 109.4±36.8 | 88.8±28.9 | 99.3±31.9 |
Values are means ± SD.
P<0.05 for the comparison of the value from baseline, as calculated with the use of mixed-model repeated measures analyses of variance
P<0.05 for the comparisonof the value from control group,
P<0.05 for the comparison of the value from diet group,
P<0.05 for the comparison of the value from exercise group, as all calculated with the use of mixed-model repeated measures analyses of variance contrasts.
Normal values: 25 (OH)vit D (>30 ng/mL), PTH (10–60 pg/mL), Leptin (lean men [3.8±1.8 μg/L, lean women [7.4±3.7 μg/L]), Estradiol (untreated postmenopausal [5.7–102.8 pg/mL]), adult male [5.6–50.1 pg/mL], IGF-1 (25–39 yrs [90–360 ng/mL], 65–85 years [69 –166 ng/mL]).
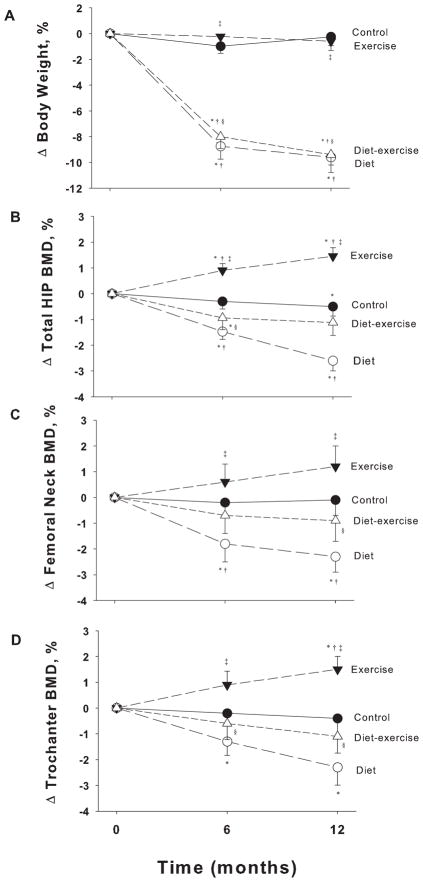
As previously reported,(4) body weight decreased significantly and similarly in the diet group (−9.6±1.2%) and the diet-exercise group (−9.4±0.8%) but not in the exercise group (−0.6±0.7%) and control group (−0.2±0.7%) (P<.001 for the between-group differences). However, despite comparable weight loss, bone loss at the total hip was relatively less in the diet-exercise group (−1.1±0.5%) than in the diet-group (−2.6±0.5%), whereas BMD increased in the exercise group (1.5±0.3%) (P<.001 for the between-group differences) (Figure 1). Similar trends were observed in BMD at the femoral neck and trochanter (Figure 1). There were no significant changes in BMD of the whole body: control (0.1±3.1%), diet: (0.6±2.7%), exercise (0.5±2.0%), diet-exercise (0.8±2.8%) or at the lumbar spine: (control (0.4±2.8%), diet: (1.1±3.0%), exercise (0.8±2.6%), diet-exercise (0.8±2.8%), as previously reported.(4) Likewise, there were no significant changes in BMC of the whole body: control (−0.3±4.4%), diet: (−0.1±3.1%), exercise (0.5±2.6%), diet-exercise (1.6±6.4%) (P=.59 for the between-group differences). Lean body mass decreased less in the diet-exercise group (3.2±3.1%) than in the diet group (−5.3±3.4%) while it increased in the exercise group (2.4±2.5%).(4) Moreover, in response to the exercise training, total 1-RM strength increased in the diet-exercise (34±30%) and exercise (35±36 %) group but not in the control (−3±26%) or diet (3±17%) group (P<.001 for the between-group differences).
Figure 1.
Changes from baseline in body weight (A) and bone mineral density at the total hip (B), femoral neck (C) and trochanter (D) in obese older adults during the 1-year interventions. Values are mean ± SE. *P<0.05 for the comparison of the value from baseline, as calculated with the use of mixed-model repeated measures analyses of variance. †P<0.05 for the comparison of the value from control group, ‡P<0.05 for the comparison of the value from diet group, §P<0.05 for the comparison of the value from exercise group, as all calculated with the use of mixed-model repeated measures analyses of variance contrasts.
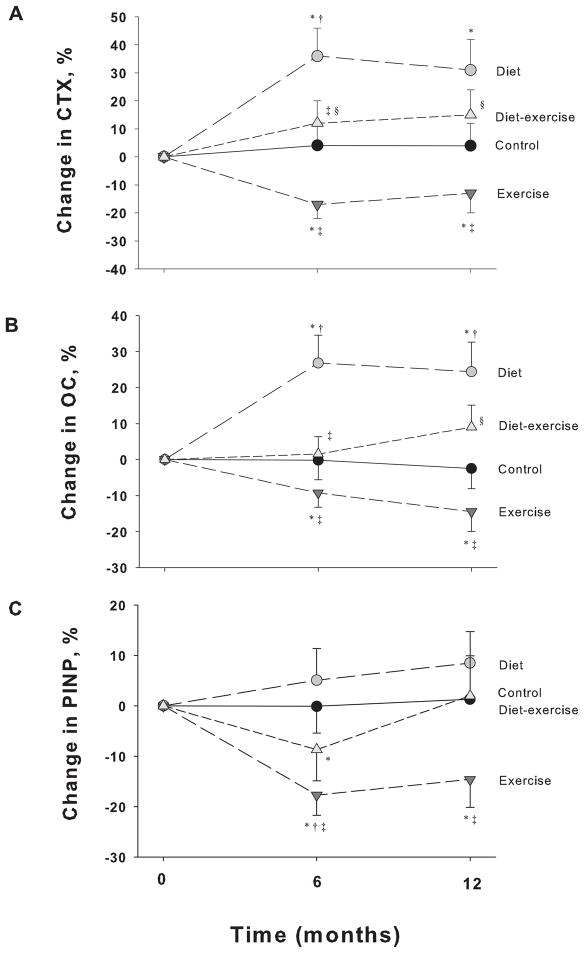
Serum CTX and osteocalcin concentrations increased in the diet group (31±11% and 24±8%, respectively) while they decreased in the exercise group (−13±7% and −15±6%, respectively) (P<.001 for the between-group differences) (Figure 2). In contrast, similar to the control group, serum CTX and osteocalcin concentrations did not significantly change in the diet-exercise group. Moreover, serum PINP concentrations decreased in the exercise group (−15±6%) compared with the diet group (9±6%) (P=.04 for the between-group differences).
Figure 2.
Changes from baseline in markers of bone turnover in obese older adults during the 1-year interventions. Values are given as mean ± SE. CTX, C-terminal telopeptide of type 1 collagen (A); OC, Osteocalcin (B); PINP, Intact N-terminal propeptide of type 1 collagen (C). *P<0.05 for the comparison of the value from baseline, as calculated with the use of mixed-model repeated measures analyses of variance. †P<0.05 for the comparison of the value from control group, ‡P<0.05 for the comparison of the value from diet group, §P<0.05 for the comparison of the value from exercise group, as all calculated with the use of mixed-model repeated measures analyses of variance contrasts.
Serum 25(OH)D concentrations increased in all groups (control; 20± 8%; diet; 16±6%, exercise; 30±9%; diet-exercise; 25±7%) (Table 2). Serum leptin concentrations decreased in the diet (−25±7%) and diet-exercise groups (−38±5%) but not in the exercise group or control group (P=.001 for the between-group differences). Likewise, serum estradiol concentrations decreased in the diet (−15±4%, respectively) and diet-exercise groups (−13±6%, respectively) but not in the exercise group or control group (P=.001 for the between-group differences). The decrease in both serum leptin and estradiol concentrations occurred at 6 months with no further decrease at 12 months. Stratification for gender showed similar decrease in serum estradiol between women and men. There were no significant changes in serum PTH and IGF-1 concentrations.
Changes in hip BMD correlated positively with changes in body weight (r=.46; P<.001), lean body mass (r=.49; P<.001), serum leptin concentrations (r=.29; P=.004), and strength (r=.23; P=.04), while it correlated negatively with changes in serum CTX (r=−.32; P=.004), osteocalcin (r=−.37; P=.001) and PINP (r=−.30; P=.005) concentrations. There was a trend for changes in hip BMD to correlate with changes in serum estradiol (r=.19: P=.06). In the stepwise multiple regression analyses of the variables affecting the changes in hip BMD, changes in lean body mass (β=.33), serum osteocalcin (β= −.24), and total 1-RM strength (β=.23) remained significant in the final model, explaining 29% of the variance in changes in hip BMD (multiple r=.56; P<.001) (Table 3).
TABLE 3.
Stepwise multiple regression analysis of the variables affecting changes in hip bone mineral density
| Final model
| |||
|---|---|---|---|
| Variable | Standardized Beta coefficient | T value | P value |
| Change in lean body mass | .329 | 2.791 | 0.01 |
| Change in serum osteocalcin | −.241 | −2.076 | 0.04 |
| Change in total 1-repetition strength | .230 | 2.076 | 0.04 |
Multiple r =.56; multiple r2 = .31; Candidate variables not included in the final model: change in body weight, change in serum estradiol, change in serum leptin, change in serum Intact N-terminal propeptide of type 1 procollagen, and change in serum C-terminal telopeptide of type 1 collagen
DISCUSSION
Obesity in older adults is a major public health problem.(3) However, weight-loss therapy to improve health in obese older adults is limited by concomitant bone loss, which could worsen age-related osteopenia.(12) The current study demonstrates that 1) weight loss increases bone turnover and decreases BMD, 2) ET decreases bone turnover and increases BMD and most importantly, 3) ET added to weight loss prevents the weight loss-induced increase in bone turnover and attenuates the weight loss-induced decrease in BMD. In addition, the ability of ET to offset the adverse effects of weight loss on bone turnover and BMD occurs despite weight-loss-induced decline in bone-active hormones (e.g. estradiol, leptin). This is the first study to directly compare the independent and combined effects of weight loss and ET on bone metabolism and BMD in obese older adults within a randomized-controlled trial (RCT). By demonstrating the importance of incorporating ET in a weight-loss program in order to minimize bone loss among obese older adults engaged in dietary caloric restriction, these findings have major clinical implications.
Bone turnover increases with advancing age leading to progressive bone loss and osteoporosis.(13,14) Weight loss further increases bone turnover and decreases BMD in older adults;(15–17) our results are consistent with these findings - the diet group lost ~10% body weight and had an increase in bone turnover associated with a ~3% reduction in hip BMD. Epidemiologic studies also suggest that the weight loss-induced bone loss increases the risk for osteoporotic fractures in older adults.(18,19) On the other hand, some studies have shown that ET increases BMD and decreases the risk for osteoporotic fractures.(20) Therefore ET is recommended to prevent and treat osteoporosis.(21) However, the effect of ET on bone turnover is unclear; previous studies reported that ET increased, (22–24) decreased, (25) or did not change markers of bone resorption and formation.(26,27) Such conflicting results may be related to differences in subject characteristics, type and quantity of exercises, and timing of the samples relative to the exercise. In the current study involving obese older adults, we used a multi-component, high-intensity, progressive ET protocol that included different types of exercise that stimulated major muscles attached to bone.(28) To avoid the acute effects of exercise,(29) we collected blood samples ~40 hours after the last bout of exercise. Our results clearly demonstrate for the first time that ET in older adults decreases bone turnover and increases BMD, effects that are the exact opposite of weight loss. Given these divergent effects, our 4-group study design directly assessed whether ET can effectively reverse the negative effects of weight loss on bone turnover and BMD. This issue is clinically relevant given that previous studies have shown inconsistent results regarding the efficacy of ET, likely related to limitations such as lack of a control group, non-randomization to groups, and/or lack of separate groups of weight loss alone and exercise alone to compare with weight loss plus exercise group.(30–32) Accordingly, the most important finding in the current study is that ET added to weight loss was able to offset the weight loss-induced increase in bone turnover and decrease in BMD. In contrast to the diet group, the diet-exercise group demonstrated no significant changes in biochemical markers of bone resorption (CTX) and formation (osteocalcin and PINP) associated with a ~60 % less reduction in hip BMD (−1.1% in the diet-exercise vs. −2.6% in the diet group).
The exact mechanisms underlying the weight loss-induced bone loss are currently unknown. One hypothesis is that it may be due to a reduced dietary calcium and vitamin D intake and/or efficiency of absorption.(33,34) In the current study, we supplemented all participants with calcium and vitamin D. As a result, serum 25(OH) vitamin D levels increased to levels considered optimum for bone health (>20 ng/ml)(35) and there were no significant changes in levels of serum PTH. Another hypothesis is that bone loss may be due to alterations in several bone-active hormones that occur with weight loss.(12) Indeed, we found significant declines in serum estradiol and leptin levels in the weight loss groups and the changes in both hormones tended to correlate with the changes in hip BMD. Thus, one possibility is that not only the further decline in estrogen levels but also the decline in leptin levels owing to weight loss could stimulate the RANKL/RANK pathway leading to enhanced bone resorption and bone loss in our subjects.(36,37) Contrary to previous studies,(38) we found no decline in serum IGF-1 levels in response to weight loss, probably because we used a diet protocol that ensured adequate protein intake to our participants.(4,39)
A common explanation given for the bone loss induced by weight loss is reduction in mechanical stress on the weight-bearing skeleton mediated by changes in local bone factors (e.g. prostaglandins) and the mechanostat.(6,40) Indeed, the important role of changes in mechanical stress in mediating the changes in BMD in our subjects is suggested by the positive correlations between the changes in 1) lean body mass and 2) total 1-RM strength with the changes in hip BMD. Lean body mass, which is predominantly skeletal muscle, is known to induce muscle and gravitational forces influencing bone mass via mechanical loading.(41) Further support for this hypothesis is provided by the fact that we found changes in BMD at the hip but not at the lumbar spine or whole body, which may be consistent with a site-specific skeletal effect of the changes in mechanical stress(28) that occurred during the interventions. This might suggest that the hip, an important weight and impact-bearing skeletal site, is the most sensitive site to respond to changes in bone unloading (e.g. weight loss) and loading (e.g. ET) in our obese older subjects. Given that hip fractures are an important cause of disability,(42) our findings that ET not only increases hip BMD but also attenuates the reduction in hip BMD that occurs with weight loss have important implications for the clinical care of this population.(1)
A more important role for changes in mechanical stress than bone-active hormones in mediating the changes in hip BMD was also suggested by the results from the stepwise multiple regression analyses, as only changes in lean body mass and total 1-RM strength along with osteocalcin were selected in the final model. Given that changes in estradiol and other hormones were excluded from the final model, our results suggest an indirect (e.g. modulatory) rather than direct role of hormones in the bone’s adaptive response to ET.(43) Although some studies have shown that HRT through the estrogen receptor may augment the osteogenic response to ET,(27,44) hormones do not appear essential for bone cells to be able to respond to mechanical strain.(43) Accordingly, the current study demonstrates that ET attenuates the weight loss-induced decrease in hip BMD despite weight-loss induced decline in bone-active hormones. In addition, it appears that the relative degree of changes in bone formation rather than bone resorption may be better predictors of the changes in hip BMD, as changes in serum osteocalcin remained in the final model. These data are consistent with the notion that a primary defect in osteoblastic function occurs during weight loss and is reversed by ET. Indeed caloric restriction has been shown to suppress bone formation(45) while exercise exerts anabolic effects on bone.(46) Further studies are needed to examine the molecular and signaling pathways by which weight loss and ET interact to induce adaptations of bone to changes in mechanical loading.
The strengths of our study include the RCT design, the 1-year duration, the good participants’ compliance, and the comprehensive assessments of bone metabolism. A limitation is that we did not measure bone quality (e.g. bone architecture) which is another determinant of bone fracture.(47) Although ET did not completely prevent the weight loss-induced decrease in hip BMD, it is possible that ET had a beneficial effect on bone quality. The relatively small number of subjects and duration of this study precluded an assessment of the intervention on falls and fractures. Whether the remaining modest loss of hip BMD increases the risk of fractures needs to be determined in future studies. It remains unclear whether the aggregate benefits of weight loss and ET on physical function (4,48) could lower the risk of falls and fractures despite the decline in hip BMD. Strategies to prevent further bone loss in future studies might include resistance training or endurance training alone (rather than combined resistance/endurance exercise(49)) due to the potential interference between the adaptations to the two types of exercise.(50) Our study was not powered to examine potential sex differences in BMD responses. We controlled for the effect of sex by including it as a covariate in the mixed model ANOVA.
In conclusion, the addition of ET to weight-loss therapy among obese older adults prevents the weight-loss-induced increase in bone turnover and attenuates the weight loss-induced reduction in hip BMD despite weight loss-induced decrease in bone-active hormones. Therefore, ET should be incorporated as part of a comprehensive weight loss program in obese older adults in order to minimize the adverse effects of weight loss on bone health.
Acknowledgments
Funded by the National Institutes of Aging; Clinical Trials.gov number, NCT00146107
This study was supported by grants RO1-AG025501, P30-DK56341 (Clinical Nutrition Research Unit), UL1-RR024992 (Clinical and Translational Science Award), and DK20579 (Diabetes Research and Training Center) from the National Institutes of Health. TH was supported by a New Investigator Fellowship Training Initiative from the Foundation for Physical Therapy and T32 HD007434-16
We thank the participants for their cooperation and the staff of the Intensive Research Unit of the Institute of Clinical and Translational Sciences for their skilled assistance in the performance of this study.
Footnotes
This work was presented in part to the 2010 annual meeting of the American Society for Bone and Mineral Research, Toronto, Canada
DISCLOSURE
All authors state that they have no conflicts of interest.
Authors’ roles: Study design: DTV and DRS. Study conduct: DTV, KS, TH, NP, NN, SC: Data analysis: DTV, KS, RA, NN, DS, CQ. Data interpretation: DTV, KS, RA, NN, DS, CQ. Revising manuscript content: DTV, KS, RA, DS, TH, NP, SH, CQ. Approving final version of manuscript: DTV, KS, RA, NP, SC, DRS, NN, CQ. DTV takes responsibility for the integrity of the data analysis.
REFERENCE LIST
- 1.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005;82:923–934. doi: 10.1093/ajcn/82.5.923. [also published in: Obes Res. 2005: 13:1849–1863] [DOI] [PubMed] [Google Scholar]
- 2.Jensen GL, Hsiao PY. Obesity in older adults: relationship to functional limitation. Curr Opin Clin Nutr Metab Care. 2010;13:46–51. doi: 10.1097/MCO.0b013e32833309cf. [DOI] [PubMed] [Google Scholar]
- 3.van Baak MA, Visscher TL. Public health success in recent decades may be in danger if lifestyles of the elderly are neglected. Am J Clin Nutr. 2006;84:1257–1258. doi: 10.1093/ajcn/84.6.1257. [DOI] [PubMed] [Google Scholar]
- 4.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen LB, Quaade F, Sorensen OH. Bone loss accompanying voluntary weight loss in obese humans. J Bone Miner Res. 1994;9:459–463. doi: 10.1002/jbmr.5650090404. [DOI] [PubMed] [Google Scholar]
- 6.Jensen LB, Kollerup G, Quaade F, Sorensen OH. Bone minerals changes in obese women during a moderate weight loss with and without calcium supplementation. J Bone Miner Res. 2001;16:141–147. doi: 10.1359/jbmr.2001.16.1.141. [DOI] [PubMed] [Google Scholar]
- 7.Pritchard JE, Nowson CA, Wark JD. Bone loss accompanying diet-induced or exercise-induced weight loss: a randomised controlled study. Int J Obes Relat Metab Disord. 1996;20:513–520. [PubMed] [Google Scholar]
- 8.Villareal DT, Fontana L, Weiss EP, et al. Bone Mineral Density Response to Caloric Restriction-Induced Weight Loss or Exercise-Induced Weight Loss: A Randomized Controlled Trial. Arch Intern Med. 2006;166:2502–2510. doi: 10.1001/archinte.166.22.2502. [DOI] [PubMed] [Google Scholar]
- 9.Klein S, Wadden T, Sugerman HJ. AGA technical review on obesity. Gastroenterology. 2002;123:882–932. doi: 10.1053/gast.2002.35514. [DOI] [PubMed] [Google Scholar]
- 10.Napoli N, Villareal DT, Mumm S, et al. Effect of CYP1A1 gene polymorphisms on estrogen metabolism and bone density. J Bone Miner Res. 2005;20:232–239. doi: 10.1359/JBMR.041110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villareal D, Holloszy JO. DHEA enhances effects of weight training on muscle mass and strength in elderly women and men. Am J Physiol Endocrinol Metab. 2006:00100. doi: 10.1152/ajpendo.00100.2006. [DOI] [PubMed] [Google Scholar]
- 12.Shapses SA, Riedt CS. Bone, body weight, and weight reduction: what are the concerns? J Nutr. 2006;136:1453–1456. doi: 10.1093/jn/136.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ensrud KE, Palermo L, Black DM, et al. Hip and calcaneal bone loss increase with advancing age: longitudinal results from the study of osteoporotic fractures. J Bone Miner Res. 1995;10:1778–1787. doi: 10.1002/jbmr.5650101122. [DOI] [PubMed] [Google Scholar]
- 14.Cawthon PM, Ewing SK, McCulloch CE, et al. Loss of hip BMD in older men: the osteoporotic fractures in men (MrOS) study. J Bone Miner Res. 2009;24:1728–1735. doi: 10.1359/JBMR.090419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricci TA, Heymsfield SB, Pierson RN, Jr, Stahl T, Chowdhury HA, Shapses SA. Moderate energy restriction increases bone resorption in obese postmenopausal women. Am J Clin Nutr. 2001;73:347–352. doi: 10.1093/ajcn/73.2.347. [DOI] [PubMed] [Google Scholar]
- 16.Chao D, Espeland MA, Farmer D, et al. Effect of voluntary weight loss on bone mineral density in older overweight women. J Am Geriatr Soc. 2000;48:753–759. doi: 10.1111/j.1532-5415.2000.tb04749.x. [DOI] [PubMed] [Google Scholar]
- 17.Riedt CS, Cifuentes M, Stahl T, Chowdhury HA, Schlussel Y, Shapses SA. Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/day calcium intake. J Bone Miner Res. 2005;20:455–463. doi: 10.1359/JBMR.041132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc. 2003;51:1740–1747. doi: 10.1046/j.1532-5415.2003.51558.x. [DOI] [PubMed] [Google Scholar]
- 19.Ensrud KE, Fullman RL, Barrett-Connor E, et al. Voluntary weight reduction in older men increases hip bone loss: the osteoporotic fractures in men study. J Clin Endocrinol Metab. 2005;90:1998–2004. doi: 10.1210/jc.2004-1805. [DOI] [PubMed] [Google Scholar]
- 20.Martyn-St JM, Carroll S. High-intensity resistance training and postmenopausal bone loss: a meta-analysis. Osteoporos Int. 2006;17:1225–1240. doi: 10.1007/s00198-006-0083-4. [DOI] [PubMed] [Google Scholar]
- 21.National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. 2011. [Google Scholar]
- 22.Yamazaki S, Ichimura S, Iwamoto J, Takeda T, Toyama Y. Effect of walking exercise on bone metabolism in postmenopausal women with osteopenia/osteoporosis. J Bone Miner Metab. 2004;22:500–508. doi: 10.1007/s00774-004-0514-2. [DOI] [PubMed] [Google Scholar]
- 23.Shibata Y, Ohsawa I, Watanabe T, Miura T, Sato Y. Effects of physical training on bone mineral density and bone metabolism. J Physiol Anthropol Appl Human Sci. 2003;22:203–208. doi: 10.2114/jpa.22.203. [DOI] [PubMed] [Google Scholar]
- 24.Vincent KR, Braith RW. Resistance exercise and bone turnover in elderly men and women. Med Sci Sports Exerc. 2002;34:17–23. doi: 10.1097/00005768-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Woitge HW, Friedmann B, Suttner S, et al. Changes in bone turnover induced by aerobic and anaerobic exercise in young males. J Bone Miner Res. 1998;13:1797–1804. doi: 10.1359/jbmr.1998.13.12.1797. [DOI] [PubMed] [Google Scholar]
- 26.Evans EM, Racette SB, Van Pelt RE, Peterson LR, Villareal DT. Effects of soy protein isolate and moderate exercise on bone turnover and bone mineral density in postmenopausal women. Menopause. 2007;14:481–488. doi: 10.1097/01.gme.0000243570.78570.f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohrt WM, Snead DB, Slatopolsky E, Birge SJ., Jr Additive effects of weight-bearing exercise and estrogen on bone mineral density in older women. J Bone Miner Res. 1995;10:1303–1311. doi: 10.1002/jbmr.5650100906. [DOI] [PubMed] [Google Scholar]
- 28.Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR. American College of Sports Medicine Position Stand: physical activity and bone health. Med Sci Sports Exerc. 2004;36:1985–1996. doi: 10.1249/01.mss.0000142662.21767.58. [DOI] [PubMed] [Google Scholar]
- 29.Delmas PD, Eastell R, Garnero P, Seibel MJ, Stepan J. The use of biochemical markers of bone turnover in osteoporosis. Committee of Scientific Advisors of the International Osteoporosis Foundation. Osteoporos Int. 2000;11 (Suppl 6):S2–17. doi: 10.1007/s001980070002. [DOI] [PubMed] [Google Scholar]
- 30.Ryan AS, Nicklas BJ, Dennis KE. Aerobic exercise maintains regional bone mineral density during weight loss in postmenopausal women. J Appl Physiol. 1998;84:1305–1310. doi: 10.1152/jappl.1998.84.4.1305. [DOI] [PubMed] [Google Scholar]
- 31.Svendsen OL, Hassager C, Christiansen C. Effect of an energy-restrictive diet, with or without exercise, on lean tissue mass, resting metabolic rate, cardiovascular risk factors, and bone in overweight postmenopausal women. Am J Med. 1993;95:131–140. doi: 10.1016/0002-9343(93)90253-l. [DOI] [PubMed] [Google Scholar]
- 32.Villareal DT, Shah K, Banks MR, Sinacore DR, Klein S. Effect of weight loss and exercise therapy on bone metabolism and mass in obese older adults: a one-year randomized controlled trial. J Clin Endocrinol Metab. 2008;93:2181–2187. doi: 10.1210/jc.2007-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ricci TA, Chowdhury HA, Heymsfield SB, Stahl T, Pierson RN, Jr, Shapses SA. Calcium supplementation suppresses bone turnover during weight reduction in postmenopausal women. J Bone Miner Res. 1998;13:1045–1050. doi: 10.1359/jbmr.1998.13.6.1045. [DOI] [PubMed] [Google Scholar]
- 34.Shapses SA, Von Thun NL, Heymsfield SB, et al. Bone turnover and density in obese premenopausal women during moderate weight loss and calcium supplementation. J Bone Miner Res. 2001;16:1329–1336. doi: 10.1359/jbmr.2001.16.7.1329. [DOI] [PubMed] [Google Scholar]
- 35.Gallagher JC, Sai AJ. Vitamin D insufficiency, deficiency, and bone health. J Clin Endocrinol Metab. 2010;95:2630–2633. doi: 10.1210/jc.2010-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burguera B, Hofbauer LC, Thomas T, et al. Leptin reduces ovariectomy-induced bone loss in rats. Endocrinology. 2001;142:3546–3553. doi: 10.1210/endo.142.8.8346. [DOI] [PubMed] [Google Scholar]
- 37.Thomas T, Burguera B. Is leptin the link between fat and bone mass? J Bone Miner Res. 2002;17:1563–1569. doi: 10.1359/jbmr.2002.17.9.1563. [DOI] [PubMed] [Google Scholar]
- 38.Smith WJ, Underwood LE, Clemmons DR. Effects of caloric or protein restriction on insulin-like growth factor-I (IGF-I) and IGF-binding proteins in children and adults. J Clin Endocrinol Metab. 1995;80:443–449. doi: 10.1210/jcem.80.2.7531712. [DOI] [PubMed] [Google Scholar]
- 39.Heaney RP, Layman DK. Amount and type of protein influences bone health. Am J Clin Nutr. 2008;87:1567S–1570S. doi: 10.1093/ajcn/87.5.1567S. [DOI] [PubMed] [Google Scholar]
- 40.Frost HM, Ferretti JL, Jee WS. Perspectives: some roles of mechanical usage, muscle strength, and the mechanostat in skeletal physiology, disease, and research. Calcif Tissue Int. 1998;62:1–7. doi: 10.1007/s002239900384. [DOI] [PubMed] [Google Scholar]
- 41.Reid IR. Relationships among body mass, its components, and bone. Bone. 2002;31:547–555. doi: 10.1016/s8756-3282(02)00864-5. [DOI] [PubMed] [Google Scholar]
- 42.Colon-Emeric C, Kuchibhatla M, Pieper C, et al. The contribution of hip fracture to risk of subsequent fractures: data from two longitudinal studies. Osteoporos Int. 2003;14:879–883. doi: 10.1007/s00198-003-1460-x. [DOI] [PubMed] [Google Scholar]
- 43.Price JS, Sugiyama T, Galea GL, Meakin LB, Sunters A, Lanyon LE. Role of Endocrine and Paracrine Factors in the Adaptation of Bone to Mechanical Loading. Curr Osteoporos Rep. 2011 doi: 10.1007/s11914-011-0050-7. [DOI] [PubMed] [Google Scholar]
- 44.Notelovitz M, Martin D, Tesar R, et al. Estrogen therapy and variable-resistance weight training increase bone mineral in surgically menopausal women. J Bone Miner Res. 1991;6:583–590. doi: 10.1002/jbmr.5650060609. [DOI] [PubMed] [Google Scholar]
- 45.Tatsumi S, Ito M, Asaba Y, Tsutsumi K, Ikeda K. Life-long caloric restriction reveals biphasic and dimorphic effects on bone metabolism in rodents. Endocrinology. 2008;149:634–641. doi: 10.1210/en.2007-1089. [DOI] [PubMed] [Google Scholar]
- 46.Turner CH, Robling AG. Exercise as an anabolic stimulus for bone. Curr Pharm Des. 2004;10:2629–2641. doi: 10.2174/1381612043383755. [DOI] [PubMed] [Google Scholar]
- 47.Seeman E, Delmas PD. Bone quality--the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 48.Villareal DT, Smith GI, Sinacore DR, Shah K, Mittendorfer B. Regular Multicomponent Exercise Increases Physical Fitness and Muscle Protein Anabolism in Frail, Obese, Older Adults. Obesity (Silver Spring) 2010 doi: 10.1038/oby.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1094–1105. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- 50.Nelson AG, Arnall DA, Loy SF, Silvester LJ, Conlee RK. Consequences of combining strength and endurance training regimens. Phys Ther. 1990;70:287–294. doi: 10.1093/ptj/70.5.287. [DOI] [PubMed] [Google Scholar]