Abstract
An anticipatory contrast effect (ACE) occurs when, across daily trials, an animal comes to respond less than normally to a first stimulus when it is followed shortly by a second, more preferred solution. Classically, ACE is studied using a low (L) concentration of saccharin or sucrose, followed by access to a higher (H) concentration of sucrose. Subjects in the control condition have two bouts of access to the weaker solution presented on the same schedule. The ACE is measured by the difference in intake of the first bout low solution between subjects in the low-low (L-L) vs. the low-high (L-H) conditions. Here we used this paradigm with sham feeding rats and determined that nutritional feedback was unnecessary for the development of ACE with two concentrations of sucrose or with two concentrations of corn oil. Next we showed that ibotenic acid lesions centered in the orosensory thalamus spared ACEs for both sucrose and corn oil. In contrast, lesions of the pontine parabrachial nuclei (PBN), the second central relay for taste in the rat, disrupted ACEs for both sucrose and corn oil. Although the sensory modalities needed for the oral detection of fats remain controversial, it appears that the PBN is involved in processing the comparison of disparate concentrations of sucrose and oil reward.
Keywords: anticipatory contrast effect, orosensory stimulation, parabrachial nucleus, sham feeding, thalamic orosensory area
1. Introduction
In the previous two articles in this series, the results showed a dissociation in the role of the gustatory system in orosensory processing of corn oil. Rats with lesions of the parabrachial nuclei (PBN) exhibited weaker than normal operant responding for corn oil emulsions [1, 2], but learned a condition aversion to corn oil [3]. Similar PBN damage disrupted responding for sucrose in both tasks. Rats with lesions of the thalamic orosensory area (TOA), on the other hand, showed no deficits in responding for sucrose or corn oil during fixed or progressive ratio tasks and they acquired a conditioned aversion to both stimuli. These results did not fully support our initial hypothesis that the gustatory PBN is important for orosensory processing of sucrose but not corn oil, and, conversely, that the TOA is necessary for processing oil but not for sucrose reward.
In the present study, we focused on reward comparison for orosensory sucrose and corn oil using the anticipatory contrast effect (ACE). The same hypothesis was tested, but with respect to relative, rather than absolute, reward value. Again, ACE previously was demonstrated only with real feeding. In order to focus on the orosensory effects of fluid rewards, Experiment 1 first demonstrated that intact sham feeding rats can exhibit ACE for sucrose and corn oil, the latter of which has never been tested. Experiment 2 tested whether PBN lesions block an ACE for sucrose and TOA damage interferes with the parallel effect for oil. A preliminary report of these results was presented at the annual meeting of the Society for the Study of Ingestive Behavior in 2009.
2. Experiment 1: Anticipatory Contrast Effects in Sham Feeding Rats
Ingestion of one preferred sapid stimulus is affected by the relative value of another such stimulus presented closely in time. This change in responding as a function of experience is referred to as a contrast effect [4]. An anticipatory contrast effect develops when rats suppress intake of a weak stimulus, e.g., 0.15% saccharin or 0.06 M sucrose, as it comes to predict the future availability of a stronger, more preferred, stimulus, e.g., 1.0 M sucrose. The comparison is with intake by rats that only experience two bouts of the lower concentration [5–8]. Previous studies demonstrate that this contrast effect is due to anticipation of access to the more rewarding solution, not to the memory of having received the preferred 1.0M sucrose solution on the previous day [ [9, 10], but see ref. [11]]. Thus, in a Pavlovian conditioning context, the first solution is considered as a conditioned stimulus (CS) and the second, more preferred solution, as an unconditioned stimulus (US) [9, 12].
The caloric value of the CS plays an important role in the development of an ACE. When the interstimulus interval (ISI) is a matter of seconds, similar ACEs occur with both sucrose-sucrose and saccharin-sucrose parings. As the ISI increases from seconds to minutes (e.g., 5 or 10 minutes), the ACE diminishes in sucrose-sucrose pairings but not when saccharin serves ass the CS. The difference between sucrose and saccharin pairing is not due to differences in taste, but to the caloric load of the CS. Food deprived rats are, apparently, unwilling to forgo the available calories in the first bottle while they wait for access to the more preferred stimulus. When not deprived, however, anticipatory contrast is evident, i.e. rats avoid intake of a weaker sucrose solution when waiting for access to the more preferred second solution [12].
An ACE also occurs when neither the CS nor the US contains a caloric load. Using saccharin-saccharin pairings, Flaherty and Rowan (1986) produced an ACE using 0.05% – 0.15% concentrations of saccharin as the first and second solution [13]. These results suggest that an ACE could be based solely upon the relative taste intensity of the CS and the US. Accordingly, our study was designed using sham feeding to determine whether an ACE can be obtained in rats when the CS and US provide limited or no postingestive consequences. Furthermore, previous studies have always used a sweet stimulus in the ACE paradigm. This study, therefore, investigated whether disparate concentration pairs of two rewarding orosensory stimuli, sucrose and corn oil [14–16] can support an ACE in intact rats.
2.1. Materials and Methods
2.1.1. Subjects
The subjects were 36 naïve male Sprague-Dawley rats, 18 for each experiment (Charles River, Wilmington, MA), weighing 275–300g at the start of testing. They were individually housed on a 12:12 h light:dark schedule with ad libitum access to tap water and standard laboratory diet [Rodent diet (W) 2018; Harlan Teklad, Madison, WI]. Once the experiment began, the rats were maintained on a food deprivation regimen as described in more detail below. Distilled water was available at all times, except when the rats were in the test chamber. Normal pelleted chow was weighed and provided at least one hour after the daily session.
2.1.2. Surgery
For experiment 1A and 1B, the rats were divided into low-low (L-L) and low-high (L-H) groups (n=9/ each). They were treated with atropine sulfate (0.15 mg/kg ip) and, 20 min later, anesthetized with pentobarbital sodium (50 mg/kg ip) for the gastric fistula surgery. Details for the design and implantation of the gastric fistulas are described elsewhere [17]. The rats had at least two weeks to recover before starting the experiment.
2.1.3. Apparatus
Testing occurred in 6 identical modular operant chambers measuring 30.5 cm × 24.1 cm × 29.2 cm. Each chamber was equipped with a house light, a white noise generator, and 3 sipper tubes that could be programmed to advance and retract depending on the testing schedule; only 2 tubes were used for this experiment. These sipper tubes could enter the chamber through 1.3-cm holes, spaced 16.4-cm apart from left to right of one aluminum wall. The house light and white noise generator were located on the wall opposite to the sipper tubes. The white noise generator provided a background noise level of 75 dB. Three chambers served as L-L chambers where only low concentration pairs were presented. The other three served as L-H chambers where both the low and the high concentration pairs were presented. Spout licking was recorded using a triple lickometer circuit. Each test chamber was located in a sound attenuating cubicle that was fitted with a ventilation fan. This set up for ACE tasks and on-line data collection was operated by a PC computer and an interface (MedPC; MED Associates Inc. St. Albans, VT).
2.1.4. Procedure
The rats were run in squads of 6, with 3 rats placed in the L-L chambers and the other 3 placed in the L-H chambers. Before each rat was placed in a chamber, its stomach was flushed with lukewarm water as described in the companion articles. Testing was preceded by one 5-min habituation trial, in which the rats were placed in the chamber with the house light and white noise on. Food was removed from the home cage the day before the habituation trial. Thereafter, normal pelleted chow was weighed (20–25 g) and given to the rats in their home cage at least one hour after they finished their daily trial. The body weight was maintained at 90% of free feeding. During testing, the rats were given 3 min access to 0.06M sucrose in bottle 1 (B1). Immediately after that, B1 retracted and bottle 2 (B2) advanced. Rats in the L-L condition were then given 3 min access to the same 0.06M sucrose solution in B2. Rats in the L-H condition, on the other hand, were given 3 min access to the 1.0M sucrose solution in B2. There was one such pairing a day for 14 days in succession.
After a week off, the L-L and L-H groups were reversed and tested for ACE using corn oil concentration pairs. During the first 7 trials, 1% corn oil served as the L concentration and 25% corn oil served as the H concentration. Thereafter, the L concentration was increased to 2.5% corn oil for another 8 trials. This design failed to support the development of an oil ACE in rats with open fistulas and a history of experience with sucrose. Experiment 1B addressed the same question, but with rats that were naïve to sucrose.
In Experiment 1B, 18 new rats were first trained for 14 days using 1.5% corn oil followed by a second 1.5% corn oil as the L-L condition or 1.5% followed by 25% corn oil as the L-H condition. After 14 trials, it became clear that even sucrose naïve rats did not lick the 1.5% corn oil emulsions consistently when tested at 90% of free feeding body weight. Given the low intake of the L concentration by rats in the L-L control group, it was not possible to assess contrast (i.e., suppressed intake of the L concentration when paired with the future availability of the H concentration) due to a floor effect. Consequently, the rats were placed back in a free feeding condition for two weeks and then began training with the 2.5% vs. 25% condition using the same L-L and L-H groups. The rats licked 2.5% corn oil consistently after 10 trials. After the 10th trial, more pellet chow (3–5g) was given to the rats for the rest of 8 trials in order to reduce the deprivation level from 90% to 95% of free feeding body weight. Although the fistula was open and post-ingestive feedback should have been nil, it was thought that rats may be more likely to forego intake of the lesser corn oil cue in anticipation of the more concentrated emulsion if they were less food deprived. At 95% of free feeding body weight, the rats did develop an ACE with corn oil using 2.5% as the L stimulus and 25% as the H stimulus. The rats were then given two weeks of free feeding without training and placed back on the food deprivation regimen with a target of 95% of their free feeding body weight. The L-L and L-H groups were reversed and the L concentration was increased to 5% corn oil. There were 8 more such trials.
2.1.5. Solutions
The sucrose solutions were made with distilled water and the corn oil emulsions were blended with distilled water and Tween-80 [100 ml corn oil-water mixture with 0.75 ml Tween-80 (Sigma-Aldrich, St. Louis, MO)]. All procedures in this experiment were approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University College of Medicine.
2.1.6. Statistical Analysis
It took the rats 6–8 days to begin licking consistently. Only rats that licked consistently thereafter contributed data to the analysis. In Experiment 1A, all rats contributed data to the analyses. In experiment 1B, data from three rats were omitted. One rat died after surgery and one rat from each L-L and L-H group did not lick throughout training. The data included daily 3-min sham licks on B1 and B2 and the latency to start licking each bottle. The lick and latency data were averaged into 2-day blocks and were analyzed by mixed factorial ANOVAs varying fluids and blocks. Post hoc Newman-Keuls tests were conducted where appropriate.
2.2. Results
2.2.1. Experiment 1A. Sucrose ACE
Bottle 1 and Bottle 2 licks (0.06M – 1.0M sucrose)
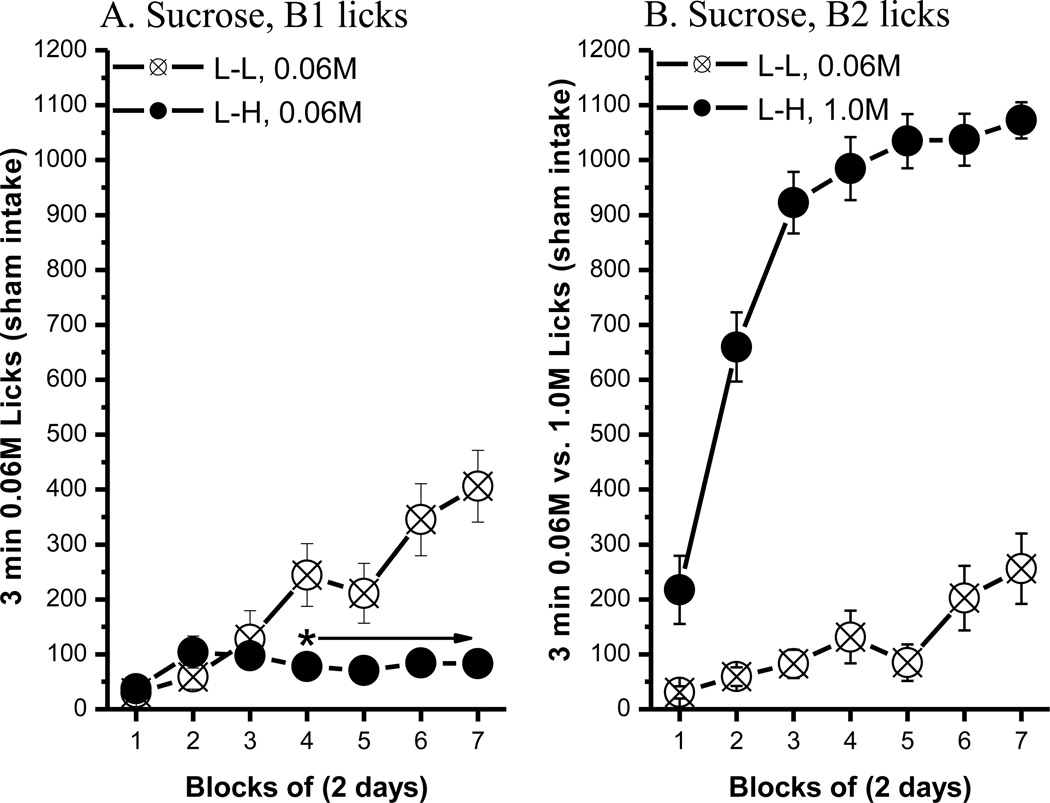
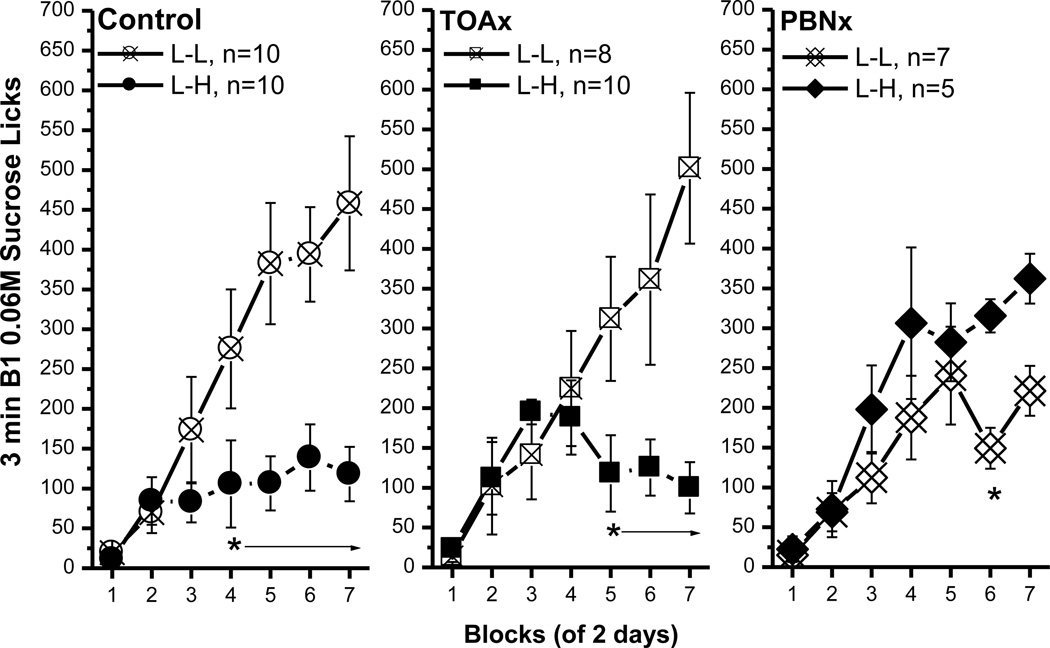
After 14 trials, rats in the L-L (0.06M-0.06M) and L-H (0.06M–1.0M) condition showed differential intake on B1 0.06M sucrose. Comparisons of B1 licks (Fig. 1A) between the L-L and L-H groups revealed that the rats developed a significant ACE. Specifically, post hoc tests of a significant group × block interaction, F(6, 96)=12.45, p<0.0001, revealed that the average B1 licks for the L-H group were significantly lower than those made by rats in the L-L group, beginning with block 4, ps<0.05. The main effect of group, F(1, 16)=8.87, p<0.009, and block, F(6, 96)=14.02, p<0.0001, also attained statistical significance. The number of licks made on B1 vs. B2 did not differ for rats in the L-L group, but the number of licks increased across blocks, F(6, 96)=19.66, p<0.0001. The L-H group, in contrast, licked significantly more of the high concentration of sucrose in B2 than the low concentration in B1, beginning with the first block [bottle: F(1, 16)=313.16; block: F(6, 96)=61.76; bottle × block: F(6, 96)=52.72; p<0.0001 for all effects]. The results of B2 licks in the L-L and L-H groups are shown in Fig. 1B.
Fig. 1.
The anticipatory contrast effect for 0.06M vs. 1.0M sucrose comparison. (A) An ACE for sucrose was shown in bottle 1 licks. (B) Comparisons of bottle 2 licks between the L-L and L-H groups. Mean (± SEM) number of 3 min licks (sham intake) made for the bottle 1 (B1: 0.06M sucrose) or bottle 2 (B2: 0.06M or 1.0 M sucrose) over 7 2-day blocks for rats in the Low-Low (L-L, n=9) or Low-High (L-H, n=9) condition. (* indicates a significant difference in 0.06M sucrose lick between the L-L and L-H condition)
Latency to lick (0.06M – 1.0M sucrose)
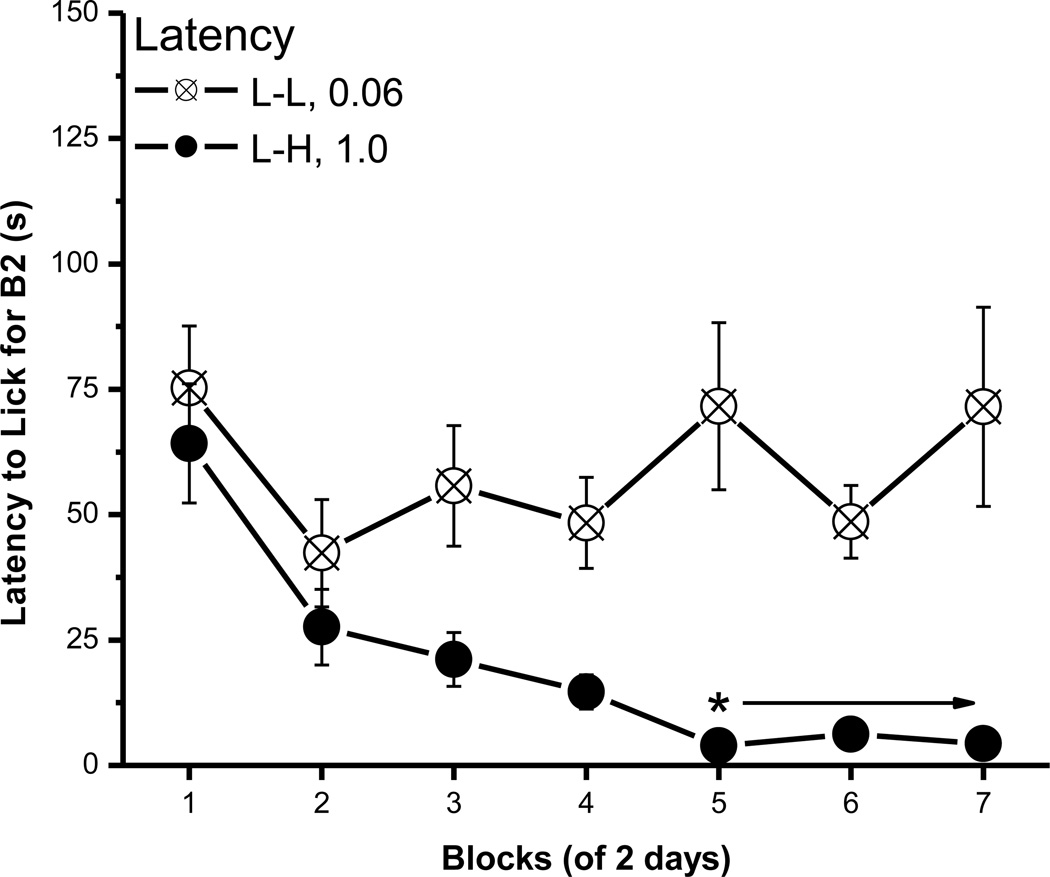
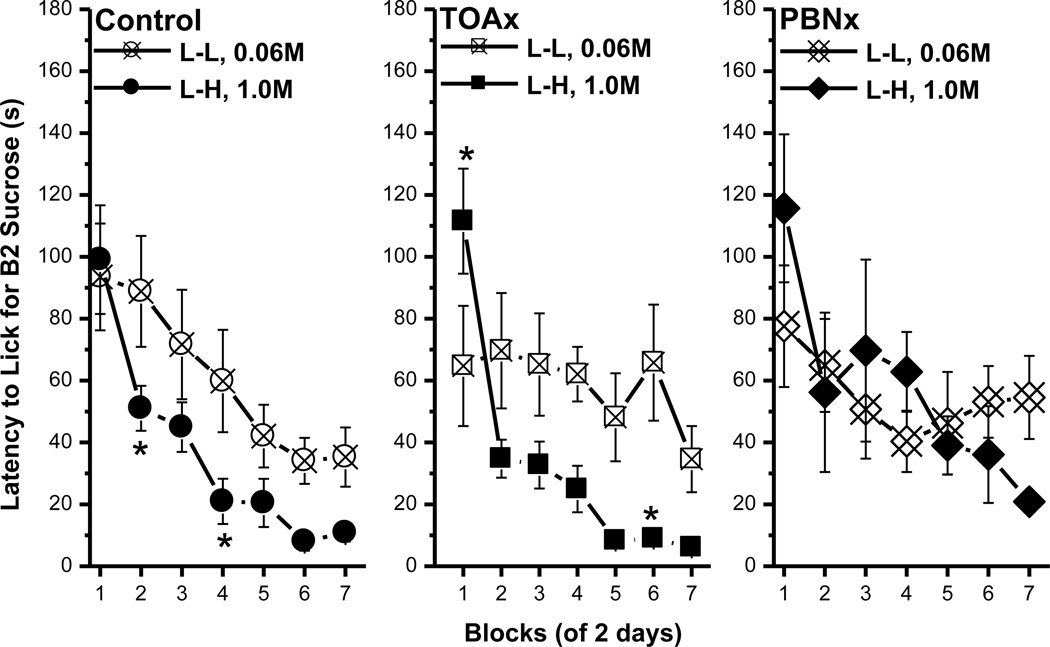
Unlike the B1 lick data, an ACE was not evident in the latency to make the first lick on B1. Thus, rats in the L-L and L-H condition did not differ in their latency to initiate licking B1 0.06M sucrose [Fs < 1, data now shown], but their latency to lick the L vs. H concentration in B2 did differ (Fig. 2). Post hoc tests of a significant group × block interaction, F(6, 96)=2.91, p<0.02, revealed that rats were faster to initiate licking B2 1.0M than B2 0.06M sucrose beginning with block 5. The main effect of group, F(1, 16)=25.61, p<0.0002, and block, F(6, 96)=4.41, p<0.0006, also were significant.
Fig. 2.
The latency to lick for B2 sucrose. Mean (± SEM) second of latency to lick for the B2 over 7 2-day blocks for rats in the L-L (0.06M) and L-H (1.0M) conditions. (* indicates a significant difference between the L-L and L-H condition)
Bottle 1 and 2 licks (1% – 25% corn oil → 2.5% – 25% corn oil)
As described in the methods for phase II testing in Experiment 1A, the rats in L-L and L-H conditions for sucrose ACE testing were reversed for corn oil ACE training. The data from this manipulation are not presented, but the results can be summarized as follows. First, rats at 90% of free feeding body weight did not ingest 1% corn oil emulsion. When the concentration was increased to 2.5%, they made 473 licks at the highest within 3 minutes. At this level of deprivation, however, the L-L and L-H groups showed no difference in B1 licks (i.e., no ACE). The failure of rats to display an oil ACE may have been due to the history with sucrose. Experiment 1B was conducted in sucrose naïve rats to determine the parameters required to obtain an ACE with corn oil in sham feeding rats.
2.2.2. Experiment 1B. Corn oil ACE
Bottle 1 and B2 licks (2.5% vs. 25%)
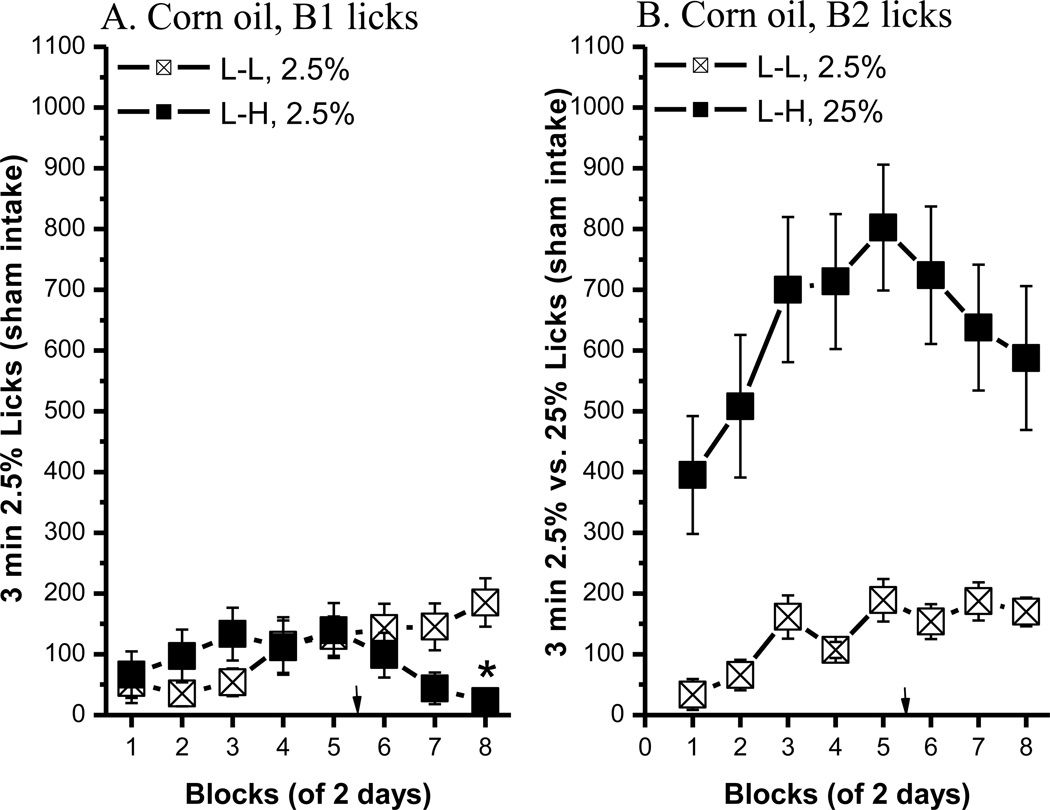
Across successive trials, three low concentrations of corn oil (1.5%, 2.5% and 5%) in Bottle 1 were paired with a 25% corn oil emulsion in Bottle 2. Testing initially began with a body weight at 90% of the free feeding. At this deprivation level, the rats did not lick 1.5% corn oil voluntarily; at best they licked less than 50 times in 3 min. When the concentration was increased to 2.5%, they began licking (Fig. 3, 2.5% – 25%; Fig. 4, 5% – 25%). The statistical analyses include comparisons of B1 licks between the L-L and L-H groups (for ACE), and comparisons of B1 and B2 licks for both groups. When the L concentration was 2.5% corn oil, the contrast effect was significant in block 8 only [Fig. 3A; group × block interaction, F(7, 91)=4.27, p<0.0005; post hoc p<0.008]. Thus, after increasing the food ration at block 5, it took 3 trials to develop a contrast effect. In the L-L condition, the mean number of licks increased on both B1 and B2 across blocks (both 2.5% oil) [block, F(7, 84)=6.92, p<0.0001], but they did not differ significantly from one another [bottle, F(1, 12)=1, p=0.34]. Rats in the L-H group preferred 25% to 2.5% corn oil emulsion. Across blocks, the number of licks on B2 was significantly higher than those obtained on B1 [bottle: F(1, 14)=29.39, p<0.0001; block: F(7, 98)=6.16, p<0.0001; bottle × block: F(7, 98)=3.2 p<0.005].
Fig. 3.
The anticipatory contrast effect for 2.5% vs. 25% corn oil comparison. (A) An ACE for corn oil was shown in B1 licks. (B) Bottle 2 licks in the L-L (n=7) and L-H (n=8) groups. Mean (± SEM) number of 3 min licks (sham intake) made for the B1 (2.5% corn oil) or B2 (2.5% or 25% corn oil) over 7 2-day blocks for rats in the L-L or L-H condition. The arrows indicate the point when the rats began to receive more food post training. (* indicates a significant difference in 2.5% oil lick between the L-L and L-H condition)
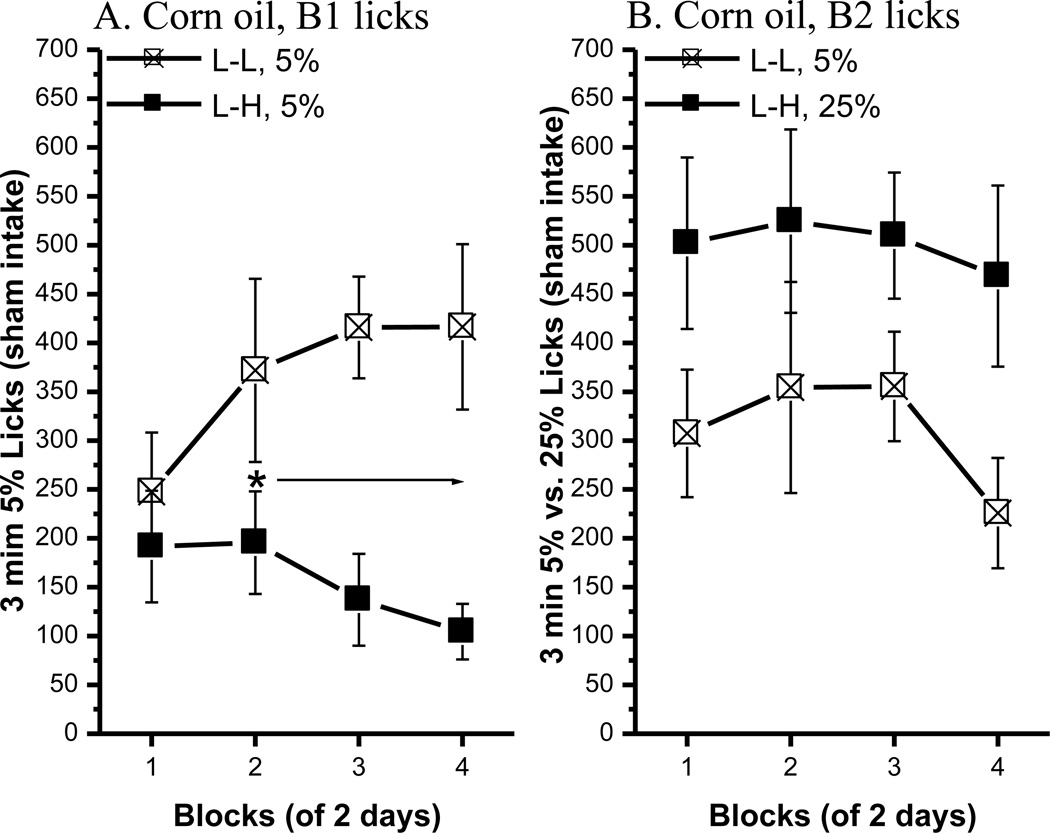
Fig. 4.
The anticipatory contrast effect for 5% vs. 25% corn oil comparison. (A) An ACE for corn oil was shown in B1 licks. (B) Bottle 2 licks in the L-L (n=8) and L-H (n=7) groups. Mean (± SEM) number of 3 min licks (sham intake) made for the B1 (5% corn oil) or B2 (5% or 25% corn oil) over 4 2-day blocks for rats in the L-L or L-H condition. (* indicates a significant difference in 5% oil lick between the L-L and L-H condition.
Bottle 1 and 2 licks (5% vs. 25%)
As described, the L-L rats were reassigned to the L-H condition and vice versa for the L-H group and the L concentration was increased to 5%. At 95% of their free-feeding body weight, a significant ACE developed rapidly (Fig. 4, left panel). Post hoc tests of a significant group × block interaction, F(3, 39)=3.81, p<0.02, showed that B1 licks of 5% corn oil in the L-H group were significantly lower than the number emitted by rats in the L-L group beginning with block 2, ps < 0.05. The main effect of group also was significant [F(1, 13)=7.32, p<0.02]. Thus, an ACE occurred for 5% corn oil. There was no significant difference in B1 and B2 licks in the L-L condition [bottle, F<1; block, F(3, 42)=2.31, p=0.09; bottle × block, F(3, 42)=2.78, p=0.053]. The L-H group licked significantly more B2 25% oil than B1 5% oil [F(1, 12)=17.63, p<0.002], but the main effect of block was not significant [F(3, 36)=1.09, P=0.36] nor was the bottle × block interaction [F<1]. Overall, the results of this experiment indicate that rats can exhibit an ACE for oil when they are mildly food deprived and when the gastric fistula is open.
Latency to lick
During the 2.5% vs. 25% phase of the experiment, there was no significant difference between the L-L and L-H groups in the latency to lick B1 [group, F<1, p=0.51]. By block 8, however, the rats in the L-L condition did begin to lick B1 sooner than the L-H rats [group × block, F(7, 91)=4.19, p<0.0005; post hoc, p<0.05]. This suggests that a significant contrast effect in B1 latency may develop more slowly than when it is measured in licks. When comparing the latency to lick B2, the L-H rats made contact with B2 sooner than the L-L rats overall [group, F(1, 13)=6.49, p<0.03; block, F(7, 91)=3.9, p<0.001]. The group × block interaction, however, did not attain statistical significance [F(7, 91)=1.41, p=0.21]. During the 5% vs. 25% experiment, there was no significant difference between the L-L and L-H groups in the latency to lick for either B1 or B2 (data not shown).
2.3. Discussion
This is the first study to demonstrate that an ACE can be obtained using orosensory stimulation with either sucrose or corn oil during sham feeding. The concentrations of sucrose were chosen based on previous studies of ACE with real feeding [12]. The same concentrations produced ACE while sham feeding. The results of this study are consistent with the previous report that used saccharin-saccharin parings and suggested that differences in the concentration of a taste cues alone are sufficient to suppress intake of the initial, weaker solution [13]. When the stimuli were oil-oil pairings, reliable contrast effects were obtained with 2.5 or 5% vs. 25% emulsions. Because this was the first study to test whether animals develop an ACE with corn oil emulsions, the concentrations were based on pilot data collected in our laboratory. In a sham feeding experiment, rats that were overnight food and water deprived had daily 30-min access to corn oil emulsions. This experiment demonstrated that sham intake of 2.5% corn oil (8.9 ml) was about a third of the intake of the 25% corn oil emulsion (28.7 ml). Thus, the first few concentrations chosen for the contrast paradigm were lower than 2.5%. The results showed that food restricted rats did not consume the 1 and 1.5% corn oil emulsions reliably.
Developing an ACE depends on the reward value, rather than the caloric value, of the low vs. the high concentration of the stimulus. Nevertheless, animals can associate the sensory properties of oral stimuli with their nutritional value based on previous feeding experience [18]. During sham feeding, some leakage into the duodenum is possible, even when the recovery of drained fluid was more than what is ingested [19]. In the present study, the rats had never consumed either sucrose or corn oil before sham feeding. The stimuli were novel and, consequently, the rats never had the opportunity to associate the orosensory cue with its caloric value. If leakage into the duodenum was a factor in forming an ACE, then from the calorie point of view, 1 or 1.5% corn oil and 0.06M sucrose should be equally potent as CSs. In fact, rats developed an ACE with a 0.06 M sucrose CS but, in these experiments, not with 1% or 1.5% corn oil. Thus, the caloric value of the stimulus does not appear to be important in developing an ACE. Further support comes from the fact that 2.5% corn oil has a higher caloric value than 0.06M sucrose, but a 2.5% oil CS supported a smaller ACE than did 0.06M sucrose. If caloric value is excluded, the other obvious explanation for the greater ACE with 0.06M sucrose vs. 2.5% corn oil is a difference in reward value. Specifically, the ACE may have been greater with 0.06 M sucrose because it elicits greater baseline licking than 2.5% corn oil. In fact, up to a point greater ACE effects occur as the CS increases spontaneous licking [8]. Such a conclusion, however, must await two-bottle tests to determine the relative preference for different concentrations of sucrose vs. corn oil in sham feeding rats.
Although calories do not seem to be a contributing factor in these studies, it is clear that deprivation state plays an important role in the formation of an ACE for corn oil in the sham feeding condition. In both Experiment 1A and 1B, when deprived to 90% of free feeding weight (FFW), rats failed to develop an ACE when given 2.5% vs. 25% oil. When permitted to rise to 95% of their FFW, the rats did exhibit an ACE with the same stimulus concentrations. When saccharin is the first solution, deprivation state does not alter contrast effects. With sucrose, however, the delay becomes important, i.e. with a nutritive CS and a long ISI, food deprivation diminishes ACE [12]. In other words, deprivation state is not a factor for contrast effects when the CS produces no caloric consequences. In the current experiment, however, sham feeding should have excluded the caloric effects of corn oil. If so, deprivation state should not have influenced an oil ACE, but it apparently did. Given its relatively high caloric value, even minor leakage of the 2.5% oil into the duodenum might explain this [19], but the small change in deprivation needed to maintain a 5% difference in body weight makes this seem less likely.
This study is the first to demonstrate an ACE for corn oil, and an ACE for either corn oil or sucrose during sham feeding. In our laboratory, we have shown that rodents can learn operant tasks [1], conditioned taste aversions [3] and anticipatory contrast effects when postingestive feedback was excluded or limited by sham feeding. The results of those studies suggest that sucrose and corn oil provide different orosensory stimulation. They drive behavior differently, i.e. the deprivation level influences the degree of contrast effects for corn oil but not for sucrose.
3. Experiment 2: Anticipatory Contrast Effects in Lesioned Rats
The original hypothesis is that sensory processing of corn oil requires the intraoral trigeminal somatosensory system that bypasses the PBN and projects directly to the thalamus [20]. This hypothesis has been tested after lesions of the PBN and TOA using operant tasks and conditioned taste aversion (CTA). The results provide some support for the hypothesis in that PBN lesions eliminated learning a conditioned aversion to sucrose but not to 100% corn oil and they eliminated operant responding for sucrose but only depressed it for corn oil. Thus the results are inconclusive on the role of the PBN in processing the sensory activity produced by ingesting corn oil. Lesions of the TOA, however, had no effect on CTA learning and, if anything, disinhibited operant responses for sucrose and corn oil. The present experiment investigated the effects of the PBN and TOA lesions on ACE for sucrose or corn oil concentration pairs. If the orosensory properties of sucrose are processed through the PBN, lesions there will prevent an ACE for sucrose, but not for corn oil. The converse will occur if the orosensory properties of corn oil are processed through the oral trigeminal thalamus. The stimuli used were 0.06M vs. 1.0M sucrose and 1.5% vs. 25% corn oil emulsions.
3.1. Materials and Methods
3.1.1. Subjects
The subjects were 72 male Sprague-Dawley rats (Charles River, Wilmington, MA) weighing 275–300g at the beginning of this study. They were individually housed on a 12:12 h light:dark cycle with ad libitum access to tap water and standard laboratory diet [Rodent diet (W) 2018; Harlan Teklad, Madison, WI]. Once the experiment began, the rats were maintained on a food deprivation regimen as described below, with distilled water available at all times except when the rats were in the test chamber. Food was weighed and provided at least one hour after the daily session.
3.1.2. Surgery
The rats were divided into PBN lesions (PBNx, n=28), PBN surgical controls (n=6), TOA lesions (TOAx, n=20), TOA surgical controls (n=6), and naïve controls (n=12). Both the lesion and gastric cannula surgeries were conducted as described in the previous papers [1, 3]. After surgery, 5 PBNx, 1 TOAx, and 2 surgical control rats died during recovery. In all, rats had at least one month to recover before the experiments began.
3.1.3. Apparatus
The apparatus used was the same as in Experiment 1.
3.1.4. Procedure
The rats were run in two squads. The first iteration included 27 rats, 15 PBNx and 12 controls; the second iteration included 37 rats, 19 TOAx, 8 PBNx, and 10 controls. The rats in each surgical group were divided into an L-L and an L-H group for ACE testing. They were run in batches of 6, 3 in L-L chambers and 3 in L-H chambers. The procedures of this experiment were the same as those described in Experiment 1 except for the control of body weight. Body weight was maintained at 90% of their FFW for sucrose ACE, and 95% of their FFW for corn oil ACE. The rats were first trained for 14 days in sucrose ACE, using 0.06M - 0.06M for the L-L condition and 0.06M – 1.0M for the L-H condition. Body weight was then increased to 95% and the rats in the L-L and L-H conditions were reversed. They were then trained for 14 days in a corn oil ACE, using 1.5% corn oil emulsion as the L concentration and 25% as the H concentration. While the 1.5% corn oil solution failed to generate a great deal of licking in Experiment 1, in these rats, the concentration was effective.
3.1.5. Histology
At the end of the experiment, the rats were sacrificed with an overdose of pentobarbital sodium (150 mg/kg ip), then perfused transcardially with 0.9% saline solution followed by 4% buffered paraformaldehyde at 4° C. The brains were removed to paraformaldehyde and a few hours later cryoprotected in 20% sucrose in paraformaldehyde overnight, also at 4°C. Then they were blocked, and frozen sectioned coronally (50 µm) in three series. One series was mounted and stained with the cresyl violet. Another series was processed for immunohistochemical staining of NeuN [21], a neuron specific protein, using standard procedures [22]. In brief, free-floating sections were rinsed with 0.1M phosphate buffer saline (PBS), treated with 0.5% H2O2 in 0.1M PBS, and then rinsed again in 0.1M PBS. After incubating in a blocking solution, the sections were transferred without rinsing to the primary antibody solution consisting of mouse anti-NeuN (1:5,000; MAB-377, Chemicon International, Temecula, CA) for 24 hrs at 4°C. The tissue was rinsed again (4 X in 0.1M PBS) before being transferred to a secondary biotinylated horse anti-mouse IgG (1:1,000; BA-2000, Vector Laboratories) in BSA (A-3803, Sigma Chemical) and 0.3% Triton X-100/PBS for 2 hrs at room temperature. After four more rinses in PBS, the tissue was processed using an avidin-biotin-complex kit (1:200, Elite PK-6100, Vector Laboratories) for 2 hrs at RT. The sections were rinsed in PBS and then reacted in 0.05% DAB and 0.01% H2O2 dissolved in 0.175M sodium acetate for 2–5 min. The reaction was stopped by rinsing with PBS.
3.1.6. Statistical analysis
Only rats with accurately placed lesions (see below) and those that licked consistently in each session were included in the data analysis. There were no significant differences between non-surgical and surgical controls so the data from these two groups were combined. The data included the mean number of licks made for B1 and B2 and the latency to begin licking each bottle. The lick and latency data were averaged into 2-day blocks and were analyzed using mixed factorial ANOVAs varying group (L-L vs. L-H) or bottle (B1 vs. B2), and block (1–7) for each group (control, PBNx, and TOAx). Post hoc Newman-Keuls tests were conducted where appropriate.
3.2. Results
3.2.1. Lesions
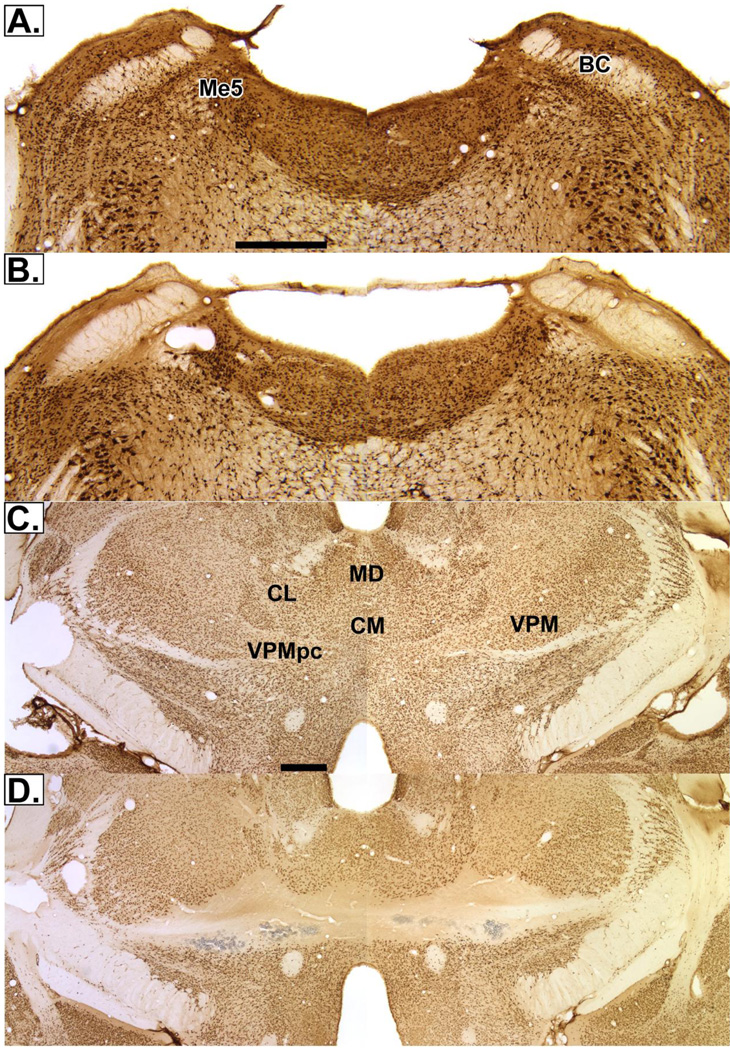
Twenty-three PBNx rats were tested in the ACE experiments; data from 8 were excluded from the statistical analysis due to poor lesion placement. These 8 rats also did not perform consistently; their licking was off and on across days. They all had large lesions that extended beyond the gustatory PBN rostrocaudally. In addition to the PBN, their lesions included the supratrigeminal area, locus coeruleus, and in some rats, the motor trigeminal nucleus. Therefore, it appeared that the large lesions prevented the rats from licking consistently. The 15 PBNx rats included had lesions centered in the gustatory PBN (Fig. 5B). The lesions included both medial and lateral PBN and, in some cases, extended to the supratrigeminal area. The lesions of the TOA (n=19) also were quite substantial. In 6 rats, they extended across the midline. These lesions damaged the entire orosensory area of the thalamus in the medial third of the ventroposteromedial nucleus (VPM) and the taste areas in the parvicellular part of VPM (VPMpc, Fig. 5D). In the other 13 rats, the lesions did not extend across the midline, but also included both the VPMpc and the intraoral trigeminal area. In these animals, the midline was spared but damage did extend into the parafascicular nucleus and the rostral end of the posterior nuclear group. Ultimately, due to misplaced lesions or inconsistent licking, 12 PBNx, 18 TOAx, and 20 controls contributed data to the sucrose ACE study; and 15 PBNx, 12 TOAx and 16 controls contributed data to the subsequent corn oil ACE study.
Fig. 5.
Digital photomicrographs of coronal sessions stained with NeuN. (A) PBN surgical control (B) PBN lesions (C) surgical control for TOA (D) TOA lesions. The images for the PBN used a 4× objective; those of the TOA, 2×. The bar in (A) and (C) equals 1.0mm. Abbreviations: BC, brachium conjunctivum; CL, central lateral nucleus; CM, central medial nucleus; MD, medial dorsal nucleus; Me5, mesencephalic trigeminal nucleus; VPM, ventroposteromedial nucleus; VPMpc, the parvicellular subdivision of VPM.
3.2.2. Sucrose ACE
Bottle 1
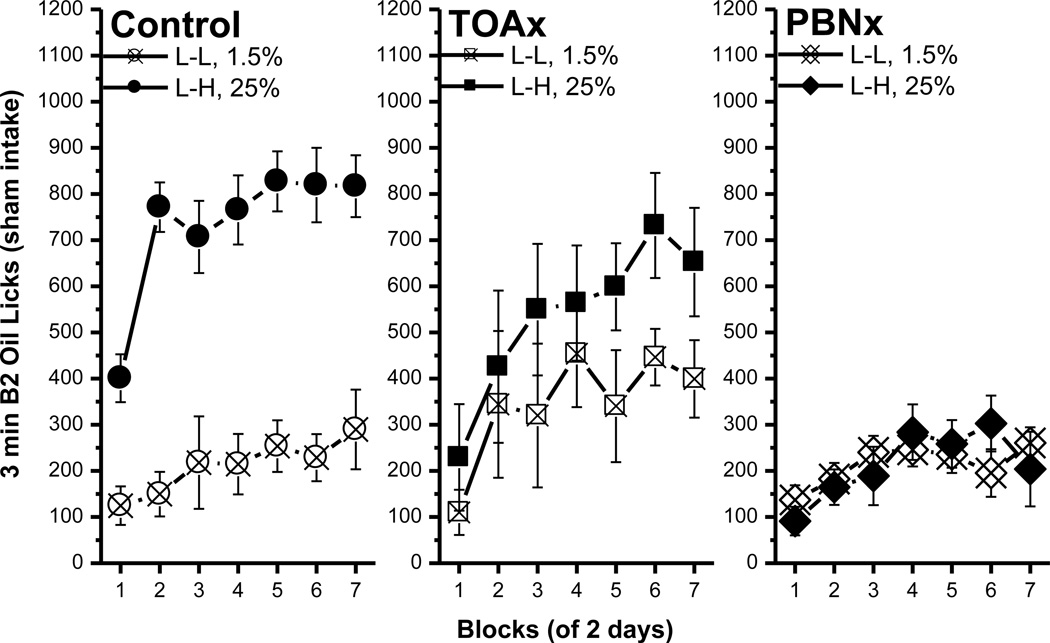
Consistent with the previous sham feeding study, the control rats in the L-H condition developed an ACE by making fewer licks for B1 0.06M sucrose than their L-L controls (Fig. 6, left panel). This observation was supported by a significant main effects of condition, F(1, 18)=9.53, p<0.007, and of block, F(6, 108)=15.14, p<0.0001. The condition × block interaction also was significant, F(6, 108)=6.75, p<0.0001, and post hoc tests revealed that B1 intake was reduced for rats in the L-H condition beginning with the 4th block, ps<0.05. The licking patterns and latency data of TOAx rats were essentially identical to the control rats (Fig. 6, middle panel). The B1 licks of the TOAx rats were significantly less in the L-H than in the L-L condition beginning with block 5 [condition, F(1, 16)=4.04, p=0.06; block, F(6, 96)=10.60, p<0.0001; interaction, F(6, 96)=8.59, p<0.0001; post hoc, p<0.02]. In contrast to the control and the TOAx groups, the PBNx rats did not show any contrast effect as measured with B1 licks (Fig. 6, right panel). A comparison between the groups showed that if anything, the L-H PBNx rats licked sucrose more rather than less on B1 [block, F(6, 60)=18.28, p<0.0001], particularly in block 6 and 7. This finding, however, was only evident only when assessed using a t-test (p<0.02). The main effect of condition was marginal, F(1, 10)=3.82, p=0.08, and the condition × block interaction was not significant, F(6, 60)=1.78, p<1. In sum, the control and the TOAx, but not the PBNx rats demonstrated a significant ACE for sucrose.
Fig. 6.
Comparisons of bottle 1 0.06M sucrose licks between the control, the TOAx and the PBNx rats. The data included B1 licks in the L-L and L-H conditions in each group. Mean (± SEM) number of 3 min licks (sham intake) made for the B1 over seven 2-day blocks for rats in the L-L or L-H condition. Both the control and the TOAx rats demonstrated an ACE for sucrose. The PBNx rats, on the other hand, failed to show an ACE for sucrose. (* indicates a significant difference between the L-L and L-H condition)
Comparisons of B1 licking across the control and lesion groups extend this conclusion. Comparisons across lesion groups in the L-L condition revealed that the control and TOAx group did not differ from each other in B1 licks, but both groups made significantly more B1 licks than the PBNx rats, particularly on blocks 6 and 7 [lesion × block interaction, F(12, 132)=1.94, p<0.04 with post hoc tests]. In the L-H conditions, B1 licks for the control and TOAx rats also did not differ significantly. The B1 licks in the L-H condition for the PBNx rats, however, were significantly higher than those emitted by both other groups [lesion × block interaction, F(12, 132)=2.81, p<0.002; last 4 blocks, post hoc, p<0.02; TOAx last 3 blocks, post hoc, p<0.03]. That is, unlike the control and the TOAx rats, the PBNx rats increased their B1 0.06M sucrose licks when they were expecting 1.0M sucrose in B2. Thus, lesions of the PBN, but not the TOA, prevent an ACE in B1 licks for sucrose.
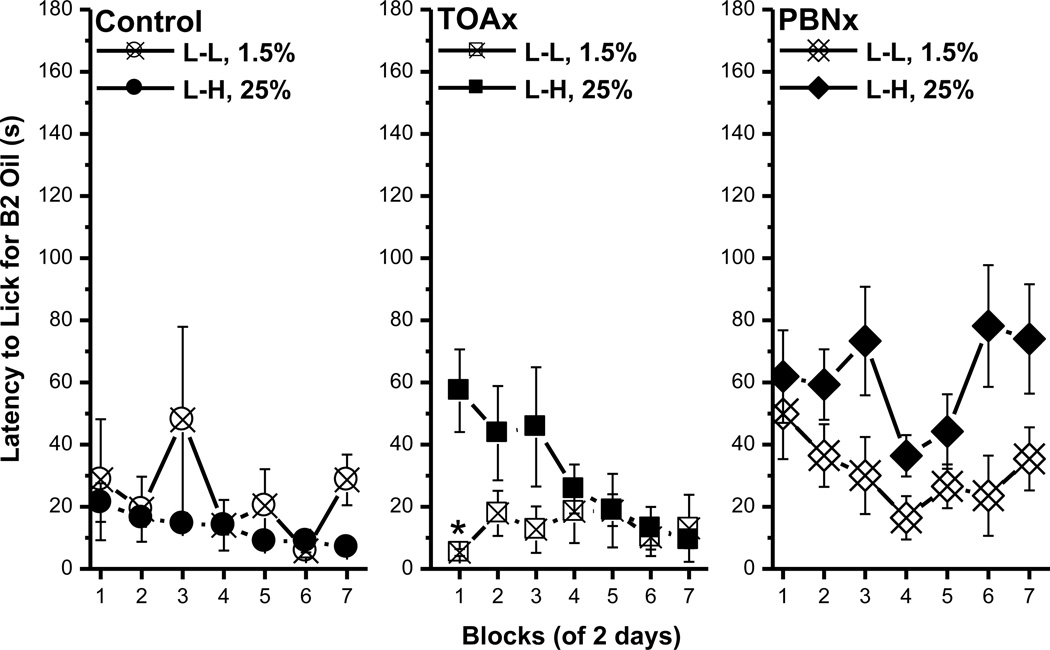
Latency to lick
The latency data in this experiment were consistent with those in Experiment 1A. On both B1 and B2, all rats initiated licking sooner as trials went on. For all three groups, no significant differences existed in the latency to lick B1 between the L-L and the L-H conditions (data not shown). For both the control and the TOAx rats, the latency to lick B2 was significantly shorter for 1.0M than for 0.06M sucrose [control, F(1, 18)=7.65, p<0.02; TOAx, F(1, 16)=14.9, p<0.002; Fig. 7, left and middle panels]. For the PBNx rats, however, there was no significant difference in the latency to lick B2 as a function of concentration (F<1; Fig. 7, right panel). Regarding the B1 data, these findings demonstrate that, while sucrose ACEs are readily obtained in lick frequency in control and TOAx rats, these contrast effects were not evident in the latency measure for these same subjects. The PBNx rats failed to exhibit an ACE for sucrose when using either lick number or latency as parameters. Finally, both control and TOAx rats, but not PBNx rats, demonstrated a magnitude of reinforcement effect by initiating licking more quickly for the high than the low concentration of sucrose.
Fig. 7.
Latency to the first lick on B2 for the control, the TOAx, and the PBNx rats. Mean (± SEM) second of latency to lick for the B2 over seven 2-day blocks for rats in the L-L (0.06M sucrose) and L-H (1.0M sucrose) conditions. Except for the PBNx group, rats in the L-H condition approached B2 sooner than the L-L group. (* indicates a significant difference between the L-L and L-H condition)
3.2.3. Corn oil ACE
Bottle 1 licks
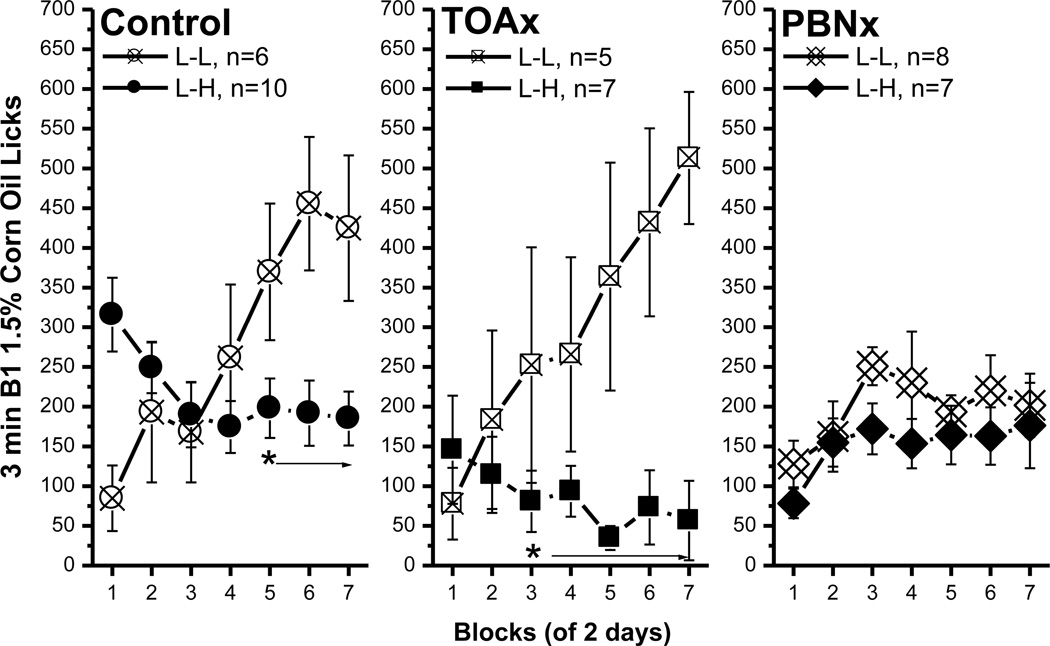
The control and the TOAx rats both developed an ACE for the 1.5% – 25% corn oil comparisons (Fig. 8, left and middle panels). For the controls, B1 licks increased across blocks during the L-L condition, becoming significantly higher than in the L-H condition beginning with block 5 [block, F(6, 84)=3.86, p<0.002; condition × block interaction, F(6, 84)=9.75, p<0.0001 ; post hoc, p<0.03; Fig. 8, control]. Note that, for the controls, the B1 licks in block 1 were significantly higher in the L-H than in the L-L condition (post hoc, p<0.006). This is likely a carryover effect from their experience during the sucrose ACE trials. The same pattern of behavior occurred in the TOAx group as rats in the L-H condition made fewer licks for B1 1.5% corn oil than did the TOAx L-L condition with the 3rd block [block, F(6, 60)=4.27, p<0.002; condition × block interaction, F(6, 60)=9.79, p<0.0001; post hoc, p<0.05; Fig. 8, TOAx]. Thus, the TOAx rats, like controls, form ACEs for corn oil.
Fig. 8.
Comparisons of bottle 1 1.5% corn oil licks between the control, the TOAx and the PBNx rats. The anticipatory contrast effect for 1.5% vs. 25% corn oil comparison. The data included B1 licks in the L-L and L-H conditions in each group. Mean (± SEM) number of 3 min licks (sham intake) made for the B1 over seven 2-day blocks for rats in the L-L or L-H condition. Both the control and the TOAx rats demonstrated an ACE for corn oil. The PBNx rats, on the other hand, failed to show an ACE for corn oil. (* indicates a significant difference between the L-L and L-H condition.
Although both the control and the TOAx rats showed an ACE for corn oil, the degree of the effect appears to be different. In the L-L condition, the number of B1 licks for the control and TOAx rats did not differ [F<1, p=0.43]. In the L-H condition, however, the B1 licks of the controls were significantly higher than for the TOAx rats during blocks 1, 2, 5 and 7 [group, F(2, 21)=4.08, p<0.04; group × block interaction, F(12, 126)=3.1, p<0.0008; post hoc, p<0.03]. This result indicates that the TOAx rats suppressed their 1.5% oil intake more than did the control rats. The behavior of the TOAx rats, however, is more like that obtained in naïve rats. The lesion difference, then, does not appear to be due to an augmented ACE in the TOAx rats, but to a reduced ACE in the control rats. The reduced magnitude of the corn oil ACE in the control rats (i.e., greater carry over from the sucrose study) may be because the control rats exhibited a greater sucrose ACE than the TOAx rats (see Fig. 6). We cannot tell, then, whether TOAx rats failed to carry over their prior experience in the sucrose ACE study because of a memory or motivational deficit, for example, or whether they simply failed to carry over their prior experience because the initial sucrose ACE was slower to develop and, therefore, less well acquired. Either way, TOAx rats readily acquire an ACE using disparate concentration pairs of corn oil.
For the PBNx rats, B1 licks in both L-L and L-H conditions increased somewhat over the first few blocks then leveled off [F(6, 78)=3.08, p<0.01]. Their subsequent exposure to B2, however, made no difference in B1 intake [F(1, 13)=1.34, p=0.27, Fig. 8, PBNx]. Thus, PBN lesions eliminate an ACE for another orosensory stimulus, corn oil. Furthermore, in either L-L or L-H conditions the PBNx rats increased licking for both 1.5% and 25% corn oil across blocks [L-L, F(6, 84)=3.81, p<0.003; L-H, F(6, 72)=3.40, p<0.006, see Fig. 9], but the averages did not differ between concentrations [L-L, F<1, p=0.7; L-H, F(1, 12)=1.86, p=0.2]. Thus the PBNx rats not only failed to show a contrast effect in B1 intake, but also failed to show a magnitude of reward effect in B2 intake. In fact, the PBNx rats licked 25% oil emulsion significantly less than either the control or the TOAx rats (post hoc, p<0.003).
Fig. 9.
Comparisons of bottle 2 corn oil licks between the control, the TOAx and the PBNx rats. The data included B2 licks in the L-L (1.5%) and L-H (25%) conditions in each group. Mean (± SEM) number of 3 min licks (sham intake) made for the B2 over seven 2-day blocks for rats in the L-L or L-H condition.
Latency to lick
For control subjects, the latency to lick was not sensitive to either contrast in B1 (data not shown) or a magnitude of reinforcement effect in B2 (Fig.10, control). During the first block rats with thalamic lesions approached B2 more slowly in the L-H than in the L-L condition, but by the last 4 blocks the difference disappeared [condition × block interaction, F(6, 60)=2.58, p<0.03, post hoc, p<0.02, Fig. 10, TOAx]. For the PBNx rats, there was no significant difference in B1 latency in the L-L or L-H conditions. Regarding the latency to lick B2, however, the PBNx rats exhibited a longer latency to lick 25% than 1.5% corn oil [F(1, 13)=10.11, p<0.008; Fig. 10, PBNx]. Thus, the PBNx rats exhibited no indication of a contrast effect for corn oil in either the number of licks made for the B1 solution or in the latency to lick the solution. They also failed to exhibit a magnitude of reward effect in either the lick or the latency measure. Indeed, these rats initiated licking more quickly for the lower than for the higher concentration of corn oil.
Fig. 10.
Latency to first lick of B2 for the control, the TOAx, and the PBNx rats. Mean (± SEM) second of latency to lick for the B2 over seven 2-day blocks for rats in the L-L (1.5% corn oil) and L-H (25% corn oil) groups. Except for the PBNx groups, there was no difference in approaching B2 between the L-L and L-H groups. (* indicates a significant difference between the L-L and L-H condition.)
3.3. Discussion
Bilateral ibotenic acid lesions centered on the gustatory zone of the PBN blocked sucrose ACE but, contrary to our initial prediction, they also eliminated oil ACE. In parallel, lesions of the TOA, which we initially predicted would disrupt ACE for oil but not sucrose, failed to affect contrast for either stimulus. Thus, the strongest version of our original hypothesis must be discarded (see more in General discussion). That said, while the PBN lesions prevented ACE for both sucrose and corn oil, the effects did differ. With a sucrose CS, the PBNx rats actually showed facilitation rather than suppression of B1 intake in the L-H vs. the L-L condition. With an oil CS, on the other hand, the PBNx rats showed neither an induction nor a contrast effect in B1 intake in the L-H condition. Lesions of the thalamic orosensory area (TOAx) failed to interfere with both operant or classical conditioning tasks that used gustatory stimuli, i.e. fixed ratio, progressive ratio, CTA, and ACE [1, 3]. Nevertheless, damage centered just medial to, but overlapping with the TOA lesions consistently disrupt saccharin-sucrose ACE [23–25].
Indeed, both chemical [23, 25] and electrolytic [24] lesions centered in the thalamic taste area (TTA) eliminate an ACE for sucrose. In the present study the thalamic lesions were larger than in those previous studies, but they spared not only sucrose ACE, but also corn oil ACE. Except for postingestive feedback, real vs. sham feeding, the ACE protocols did not differ materially between the previous and the present studies. In our two accompanying studies, sham feeding failed to alter the effects of lesions on taste guided operant tasks or CTA [1, 3]. This shifts attention to differences in lesion placement.
Our TOA damage was centered 0.5 mm lateral to the TTA, which we located electrophysiologically using NaCl presented on the tongue. In the prior experiments, in which ACE was blocked, the lesions also were taste-guided but centered more medially in the TTA itself. Although the different sets of lesions overlapped considerably, and they all damaged the gustatory area, there were differences. Because ibotenic acid lesions tend to extend dorsally along the pipette track, damage centered on TTA often included parts of the central medial (CM), medial dorsal (MD), and central lateral (CL) thalamic nuclei. By comparison, because the pipette tracks were more lateral, the TOA lesions usually spared more of these structures, at least unilaterally (Fig. 5D). Thus, based on the anatomical evidence, it is possible that these reticular and dorsomedial thalamic nuclei damaged with the TTA lesion are more important for supporting ACE than the TOA or the TTA per se.
The PBNx rats in this study failed to demonstrate an ACE for sucrose. This supports our hypothesis that the PBN is involved in processing the hedonic effects of oral sucrose. Furthermore, the PBNx rats licked more 0.06M sucrose (B1) when it predicted future access to 1.0M sucrose than when it predicted another bout with 0.06M. These data suggest that the failure to exhibit an ACE was not due to a simple inability to associate the first taste with the second, i.e. the predictive relationship between B1 0.06M – B2 1.0M sucrose did alter the responding of the PBNx rats, just not with the reduction in CS intake that is typical of an ACE (see General Discussion). Additionally, upon presentation of the 0.06M cue, the PBNx rats also must have remembered the ‘value’ of the expected reward. If the rats could not remember or associate the CS with the US, they would have shown neither contrast nor facilitation.
In contrast to the sucrose comparisons, the PBNx rats responded equivalently for B1 1.5% corn oil in the L-H and the L-L condition. Thus, ACE was eliminated, with no evidence of facilitation. Moreover, intake of 1.5% oil did not exceed 250 licks whereas the same PBNx rats made up to 360 licks for 0.06M sucrose. Thus the PBNx rats could have made more licks for B1 1.5% corn oil, but they did not. Similarly, the PBNx rats showed a magnitude of reward effect by making more licks for B2 1.0M vs. 0.06M sucrose, but these same rats failed to exhibit a magnitude of reward effect either in licks or latency for the disparate concentrations of corn oil. Indeed, the PBNx rats actually were faster to lick the low than the high concentration of corn oil. With sweet tastes, the development of an ACE depends on the reward value of the CS cue [8] as well as the reward disparity between the CS and the US [12]. Greater reward disparity between the CS and US is associated with more rapidly developing and more robust ACEs. Thus, our data suggest that, at least in this paradigm with three minutes access to the CS and the US, the PBNx rats may exhibit less drive for the CS (B1) and they may be insensitive to the US (B2) or inappropriately responsive to the reward disparity between 1.5% and 25% corn oil.
The failure of the PBNx rats to exhibit a corn oil ACE may be due to a reduced ability to discriminate between different concentrations. In other tasks, CS intensity appears to affect learning by PBNx rats. Specifically, PBNx rats were able to learn a conditioned flavor preference (CFP) as well as controls when the CS contained a higher concentration of Kool-Aid [26] but not when the concentration was reduced to 0.05% [24, 27–33]. The failure to show an ACE for the corn oil pairs may be due to the failure to appropriately identify the reward disparity between the CS and the US. Alternatively, the PBNx rats may be able to discriminate between different concentrations of corn oil but nevertheless fail to associate one with the other in a CS – US paradigm. Finally, with oil, we cannot rule out a simple failure to suppress intake of the corn oil CS when it predicts access to a higher concentration of corn oil in sham feeding rats. More effective oil concentration pairs may shed light on these possibilities. The relative effectiveness of the 5%–25% corn oil pair shown in Experiment 1B actually was determined after the conduct of these more time-consuming lesion analyses.
4. General Discussion
4.1. Summary of Parabrachial and Thalamic Lesion Effects
Operant responding, CTA, and ACE were examined in PBNx and TOAx rats to test whether sucrose and corn oil are processed through the same or different orosensory pathways. A summary of the data appears in Table 1. The results confirm that, when sucrose is the stimulus, rats with PBN lesions fail to respond in operant tasks, to learn a CTA, or to demonstrate an ACE. The same PBNx rats, however, licked corn oil emulsions normally during free access. They learned operant tasks for oil emulsions but performed them poorly, they learned a CTA to 100% corn oil, but they failed altogether to show an oil ACE with emulsions. Thus, the data fail to support the strong version of our hypothesis that the corn oil reward is independent of the central gustatory system. Nevertheless, bilateral PBN damage eliminated a rat’s ability to perform all 4 tasks with sucrose but only blunted the rat’s responsiveness to oil.
Table 1.
Comparisons of Bilateral Gustatory and Trigeminal Lesion Effects on Reward-Related Behaviors
| Rewarding effects of sucrose and corn oil are processed through different pathways. |
Free Access | PR | CTA | ACE | ||||
|---|---|---|---|---|---|---|---|---|
| Sucrose | Oil | Sucrose | Oil | Sucrose | Oil | Sucrose | Oil | |
| Normal | + | + | + | + | + | + | + | + |
| TOAx | + | + | + | + | + | + | + | + |
| PBNx | +/− | + | − | +/− | − | + | − | − |
Note. PR = progressive ratio; CTA = conditioned taste aversion; ACE = anticipatory contrast effect; + = can learn to perform the behavior; −= failed to perform the behavior; +/− = the performance of the behavior is impaired.
The thalamic arm of our hypothesis was even less well supported. Consistent with prior studies with lesions centered on the TTA [30, 34], we demonstrated that the TOA is not necessary for operant responding or for the acquisition of a conditioned aversion to sucrose. Lesions centered on the more medial TTA, however, do block sucrose ACE [23, 25], while our damage centered on the more lateral TOA (which included the TTA) did not. When the oral stimulus was corn oil, TOAx rats performed as well as the controls in all three behavioral tasks. Thus, at both the pontine and thalamic levels, the simplest version of the original hypothesis needs revision.
4.2 Thalamic lesion effects
The present experiments confirm and extend prior published reports that identified the pontine parabrachial nuclei as critical for several taste guided behaviors -- ACE, operant responding, and CTA. When ingesting corn oil, the same animals learned operant tasks and a CTA, but failed to demonstrate ACE. The thalamic relay for tongue taste, thermal, and tactile sensibility, however, had little influence on the same three tasks when rewarded by either sucrose or corn oil. Somewhat more medial thalamic taste area lesions had no effect on preference tests, CTA, or Na-appetite [24, 27, 33], but did block ACE and successive negative contrast effect [24, 25, 35]. The results did not support our hypothesis that the TOA is necessary for oil reward, but indicated that the TOA might process sucrose and oil differently.
An obvious reason for the failure of these TOA lesions to influence behavior is that they were misplaced, i. e. they did not damage neurons with gustatory, thermal, or tactile receptive fields on the tongue. While obvious, this explanation is not credible. Both anatomical and electrophysiological evidence has located tongue taste, thermal, and tactile responsive neurons in this medial extension of the thalamic VPM for more than 50 years [36–40]. Taste neurons are located most medially, then tongue thermal, and tongue tactile more laterally [41–43]. Other data demonstrate that these subregions project directly to the ventral edge of primary somatosensory cortex in the same modality specific order [38, 44]. This proves the area we damaged not only contains neurons that respond to gustatory and lingual somatosensory stimuli, but also that these cells form one limb of a classic thalamocortical circuit [45].
If we damaged the intended area of the thalamus, why did the lesions have so little effect on behavior? Most likely we were testing the wrong functions or alternative intraoral trigeminal pathways exist or both. As reviewed in the Discussion of the prior paper [3], alternative trigeminal pathways to the forebrain do exist but they have not been studied extensively and the investigations that do exist focus on pain. The trigeminal somatosensory subsystem that has garnered the most attention recently is associated with the vibrissae [46]. If this literature provides any guide, then lingual thalamocortical functions should be sensory discriminative rather than hedonic [47].
That said, it should be noted that the TOA lesion is not fully without effect. TOA lesions were competent in the three tasks. The performance of TOAx rats was somewhat different from the controls in the operant and ACE tasks. The TOAx rats demonstrated a tendency to respond more than the controls for both rewards, and this pattern was statically significant for sucrose. Unlike previous rats with damage centered on the TTA, the TOAx rats learned an ACE for sucrose. This significant contrast effect, however, developed more slowly than it did for the controls (see Fig. 6). Moreover, unlike the control rats, experience with the sucrose ACE did not appear to slow the development of the oil ACE for the TOAx rats (compare Figs. 6 and 8). Finally, during the sham feeding ACE protocols, the TOAx rats licked 25% oil less consistently (Fig. 9) but licked 1.0M sucrose as well as the controls. These results suggest that while TOAx rats can respond for both sucrose and oil reward in these tasks, the TOA contributes somewhat differently to sensory processing and associative function for sucrose and corn oil. Finally, it should be reiterated that rats with similar lesions of the TOA failed to avoid intake of a taste cue when paired with either morphine or cocaine (Nyland et al., in preparation). The TOA, then, may exhibit tonic inhibition of intake of a sweet, contribute to the normal acquisition of a sucrose ACE, and be essential for the establishment and/or the expression of a taste-morphine or taste-cocaine association.
4.3. Parabrachial lesion effects
For the PBNx rats the major differences between sucrose and corn oil was in their responsiveness during free access and their ability to learn a CTA. In both cases, the same rats that failed to respond normally for sucrose, performed like controls for corn oil. Thus, in the free access test, the PBNx rats failed to respond to 0.3 M sucrose. We have seen this previously, usually when the pontine lesions were extensive. Lacking any direct proof, the assumption was that virtually all the PBN taste neurons had been destroyed and thus the rats were unable to detect the sucrose. This parallels the effects of bilateral damage to the rostral nucleus of the solitary tract [48]. More commonly, however, PBNx rats actually over respond to sucrose, as they did in the present CTA experiment [3]. This was taken as evidence that the PBNx rats could detect the sucrose but, because they failed to acquire a CTA, they could not associate the taste with the consequences of a LiCl injection [49–51].
With free access, these PBNx rats ingested the same volume of a 25% corn oil emulsion as did the controls and they were able to acquire a learned aversion to 100% oil when it was paired with LiCl injections. With these data we might conclude that the PBN are not required for detecting oil in the oral cavity or for associating this stimulus with untoward consequences. Nevertheless, the poor operant performance for oil in PBNx rats [1], their failure to show a magnitude of reward effect in bottle 2 licking for oil, and their inability to exhibit an oil ACE militate against this inference.
When studying natural rewards, sucrose or saccharin are standard stimuli and, as a result, the parameters of these stimuli are well documented. Oil has been studied much less frequently and, until now, not at all for ACE. In this last set of experiments, the TOAx and PBNx rats were run first. Of the rats that would lick consistently, those in the Control and TOAx groups exhibited reasonable ACE with 1.5% corn oil as the low concentration. When we subsequently tested a variety of low concentrations in sham-feeding but otherwise normal rats (Expts. 1 A & B), however, the 1.5% oil did not sustain licking at a rate high enough to exhibit an ACE. In these rats, after many trials, 2.5% followed by 25% oil produced a modest ACE, but the phenomenon became robust only with 5% and 25% pairings and with further modifications of the food deprivation schedule. Given that an apparently suboptimal stimulus pair was used for testing, the failure to obtain an ACE with oil in the PBNx group should be viewed as preliminary.
The poor operant performance of the PBNx rats could be explained parsimoniously if the lesions decreased the reward value of the oil stimulus. This also could account for failure of the PBNx rats to acquire an oil ACE because these rats licked 1.5% and 25% oil emulsions at the same, low rates, i.e. 1.5% = 213.7±16.7, 25% = 213.4±28.1 (Bottle 2 data, Mean ± S.E.; Fig. 9, PBNx). The snag in this logic is that, given direct free access to a 25% oil emulsion, PBNx rats licked at the same, relatively high rate as did the controls, i. e. PBNx = 2657±450.1; Con =2165.4±637.9 [1].
The difference between an FR, PR, and ACE on one hand and free access on the other is that the former each involve learned contingencies and the latter does not. Prior studies using gustatory stimuli also concluded that bilateral lesions of the PBN interfered with the ability to associate a taste with contingent events, particularly those of a visceral or humoral nature, i.e. CTA and sodium appetite [49, 52, 53]. In the present series, however, PBNx rats did acquire a CTA to 100% corn oil, demonstrably a learned contingency [3]. This finding is consistent with other published data showing that PBNx rats also acquire a conditioned aversion when a trigeminal stimulus such as capsaicin serves as the CS [52]. Finally, while the PBNx rats in the present manuscript failed to exhibit a contrast effect, they look to have learned a contingency between the 0.06M and 1.0M sucrose solution in the ACE study because they made significantly more licks for the B1 solution when it predicted the H reward. The inability to establish a basic contingency, then, does not appear to be the problem.
To understand why PBNx rats might be able to associate two gustatory stimuli, but not a gustatory stimulus with visceral malaise, it is necessary to compare the procedural differences between the ACE and the CTA paradigm. The US in the CTA paradigm produces aversive effects whereas the US in the anticipatory contrast paradigm produces rewarding effects. That is, the association is taste-illness in the CTA paradigm, but is taste-taste (reward-reward) in the ACE. The interstimulus interval in a standard CTA task ranges from minutes to hours, while in this ACE study the interstimulus interval was zero seconds. Moreover, the mode by which the rats receive the CS and US differs across the two paradigms. In the CTA paradigm, the CS is approached and ingested, the US occurs via injection by the experimenter. In the ACE paradigm, on the other hand, both the CS and the US are actively consumed by the rat. These procedural differences add up to considerably more contiguity between the CS and the US in the ACE paradigm. In fact, in the ACE paradigm, every aspect of the procedure including the type of stimulus, the effects produced by the stimulus, and the way the stimulus is consumed is continuous for the rats. Thus, it is possible that rats with PBN lesions can establish the gustatory CS-US association in the ACE paradigm because of the close contiguity between the CS and the US. In fact, PBNx rats can learn to avoid 0.12M LiCl if it is offered as both the CS and US, i.e., the rats drink the LiCl [54], and they are able to acquire a conditioned flavor preference in which the CS and the US are actually experienced simultaneously [49, 52].
In the present CTA experiment, 100% corn oil (with the Tween80 emulsifier) was used as the CS because PBNx rats that failed to acquire an aversion to a gustatory stimulus nevertheless can learn to avoid putative trigeminal stimuli such as capsaicin and corn oil [52, 55]. In another pilot experiment, we tested a 16% emulsion and 100% corn oil as CSs. The PBNx group failed to learn an aversion to the former but did to the latter [56]. Due to the use of a cross over design and the fact that the rats in the pilot study were real feeding, this observation was difficult to interpret. It may be that the sensory properties of pure corn oil and a 16% or 25% emulsion are sufficiently different that parabrachial damage will disrupt acquiring a CTA to one but not the other. Even this possibility might be confounded by the operant conditioning data. In these experiments, we used both a 25% emulsion and pure corn oil as stimuli and the PBNx rats performed similarly if modestly for both [1].
Similar damage to the PBN, on the other hand, did block operant responding, CTA, and ACE for sucrose. In the same PBNx animals, corn oil produced mixed results. The PBNx rats sham ingested corn oil emulsions normally and learned to avoid an oil CS when made ill with LiCl. They also learned to respond for oil on both FR and PR schedules but their peak performance was dismal, at most 25% that of controls. Finally, one pair of oil emulsions failed to support ACE. Thus, the second half of the original hypothesis received limited support. Damaging the parabrachial nuclei, the source of both thalamic and limbic forebrain gustatory projections, interfered with two learning tasks guided by oil, but not with its non-contingent ingestion or with the development of a learned aversion.
The role of the PBN in the processing of sucrose and oil afferent activity may be linked by reward. Ingestion of sucrose or oil leads to an increased release of dopamine in the nucleus accumbens [15, 16]. Salamone [57, 58] has shown that rats with lesions in the nucleus accumbens fail to work for food. Thus far, we think of the accumbens as tracking reward, rather than mediating it [59], but PBN input to the nucleus accumbens may contribute to not only the accurate attribution of reward to a given stimulus, but also to the willingness to work for that reward on various schedules of reinforcement. This may be true whether responding for sucrose or oil reward. Anticipatory contrast, of course, also depends upon reward, in this case relative reward. So, while the PBNx rats appear to be able to associate the L with the H concentration in the ACE paradigm, this association fails to support something more akin to a reinforcement effect than to a contrast effect. In this light, some evidence suggests that dopamine in the nucleus accumbens also tracks the comparison of disparate rewards over time [59, 60]. Thus, the PBN – accumbens interaction may contribute to the comparison of disparate rewards over time, regardless of their nature (i.e., whether taste or not). Alternatively, the PBN may not be essential for the comparison of rewards, per se, but for the consequential shift from consummatory acceptance to avoidance. Of course, expression of the conditioned aversion to oil following oil-LiCl pairings also involves such a shift in the ingestive sequence, but in that case the behavior is sustained, apparently, by trigeminal, rather than gustatory, input. Strong trigeminal input, as with 100% corn oil, may circumvent the PBN.
These experiments put two related hypotheses at risk. First, are the rewarding properties of corn oil dependent on the intraoral trigeminal system? Second, if so, how does this trigeminal neural activity interact with the putative reward circuits in the brain? We demonstrated that sham-feeding rats can use oral sensory information about oil emulsions to guide complex behaviors. Gastrointestinal or metabolic feedback is not necessary. A standard thalamocortical loop, however, is not required to accomplish these tasks. The parabrachial nuclei interfere with some but not all of these oil guided behaviors. Because parabrachial lesions can block these same tasks when using taste stimuli, oil reward based solely on the gustatory system seems less likely. This leaves the oral trigeminal system as the most likely conduit for the sensory neural activity that constitutes oil reward.
As discussed earlier, the first central relays of the trigeminal system are more elaborate than the corresponding taste areas [3]. The paucity of electrophysiological data on the oral sensory effects of oil further complicates the issue. The problem is not framing testable hypotheses, but winnowing through an embarrassment of possibilities.
Research Highlights.
-
▶
Sham feeding rats show ACEs for both sucrose and corn oil.
-
▶
Lesions of the parabrachial nucleus eliminate ACE for both sucrose and corn oil.
-
▶
Lesions of the thalamic orosensory area spare sucrose and corn oil ACE.
-
▶
Both control and TOAx rats express an ACE, but the pattern between the two differs.
Acknowledgement
The authors thank Dr. Chris Freet for writing the computer programs for the ACE experiments, Han Li for making brain lesions, and Kathy Matayas and Nellie Horvath for histology. This study was supported by grant DK079182, DC00240, and DA012473 from the National Institute of Health, as well as a PA State Tobacco Settlement Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liang NC, Freet CS, Grigson PS, Norgren R. Pontine and thalamic influences on fluid rewards: I. Operant responding for sucrose and corn oil. 2011 doi: 10.1016/j.physbeh.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang NC, Norgren R, Grigson PS. Lesions of the parabrachial nuclei, but not the orosensory thalamus, disrupt operant responding for sucrose and corn oil in rats. Washington, DC: Society for Neuroscience; 2008. in abstract 197.20. [Google Scholar]
- 3.Liang N-C, Grigson PS, Norgren R. Pontine and thalamic influences on fluid rewards: II. sucrose and corn oil conditioned aversion. 2011 doi: 10.1016/j.physbeh.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flaherty CF. Incentive Relativity. New York: Cambridge Univ. Press; 1996. [Google Scholar]
- 5.Flaherty CF, Cheke S. Anticipation of incentive gain. Animal Learning & Behavior. 1982;10(2):177–182. [Google Scholar]
- 6.Lucas GA, Gawley DJ, Timberlake W. Anticipatory contrast as a measure of time horizons in the rats: Some methodological determinants. Animal Learning & Behavior. 1988;16(4):377–382. [Google Scholar]
- 7.Lucas GA, Timberlake W. Negative anticipatory contrast and preference conditioning: flavor cues support preference conditioning, and environmental cues support contrast. J Exp Psychol Anim Behav Process. 1992;18(1):34–40. doi: 10.1037//0097-7403.18.1.34. [DOI] [PubMed] [Google Scholar]
- 8.Flaherty CF, Turovsky J, Krauss KL. Relative hedonic value modulates anticipatory contrast. Physiol Behav. 1994;55(6):1047–1054. doi: 10.1016/0031-9384(94)90386-7. [DOI] [PubMed] [Google Scholar]
- 9.Flaherty CF, Rowan GA. Anticipatory contrast: Within-subjects analysis. Animal Learning & Behavior. 1985;13:2–5. [Google Scholar]
- 10.Flaherty CF, Coppotelli C, Grigson PS, Mitchell C, Flaherty JE. Investigation of the devaluation interpretation of anticipatory negative contrast. J Exp Psychol Anim Behav Process. 1995;21(3):229–247. doi: 10.1037//0097-7403.21.3.229. [DOI] [PubMed] [Google Scholar]
- 11.Timberlake W, Engle M. Decremental carryover effects of sucrose ingestion in the negative anticipatory contrast procedure in rats. J Exp Psychol Anim Behav Process. 1995;21(4):304–317. doi: 10.1037//0097-7403.21.4.304. [DOI] [PubMed] [Google Scholar]
- 12.Flaherty CF, Grigson PS, Cheke S, Hnat K. Deprivation State and Temporal Horizons in Anticipatory Contrast. J Exp Psychol Anim Behav Process. 1991;17(4):503–518. [Google Scholar]
- 13.Flaherty CF, Rowan GA. Successive, simultaneous, and anticipatory contrast in the consumption of saccharin solutions. J Exp Psychol Anim Behav Process. 1986;12(4):381–393. [PubMed] [Google Scholar]
- 14.Weatherford SC, Greenberg D, Gibbs J, Smith GP. The potency of D-1 and D-2 receptor antagonists is inversely related to the reward value of sham-fed corn oil and sucrose in rats. Pharmacol Biochem Behav. 1990;37(2):317–323. doi: 10.1016/0091-3057(90)90341-e. [DOI] [PubMed] [Google Scholar]
- 15.Liang NC, Hajnal A, Norgren R. Sham feeding corn oil increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1236–R1239. doi: 10.1152/ajpregu.00226.2006. [DOI] [PubMed] [Google Scholar]
- 16.Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2004;286(1):R31–R37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- 17.Smith GP. Current Protocols in Neuroscience. New York: Wiley; 1999. Sham feeding in rats with chronic, reversible gastric fistulas; pp. 1–6. [DOI] [PubMed] [Google Scholar]
- 18.Booth DA. Conditioned satiety in the rat. J Comp Physiol Psychol. 1972;81(3):457–471. doi: 10.1037/h0033692. [DOI] [PubMed] [Google Scholar]
- 19.Sclafani A, Nissenbaum JW. Is gastric sham feeding really sham feeding? Am J Physiol. 1985;248(3 Pt 2):R387–R390. doi: 10.1152/ajpregu.1985.248.3.R387. [DOI] [PubMed] [Google Scholar]
- 20.Waite P. Trigeminal sensory system. In: Paxinos G, editor. The rat nervous system. San Diego: Academic Press; 2004. pp. 817–851. [Google Scholar]
- 21.Jongen-Relo AL, Feldon J. Specific neuronal protein: a new tool for histological evaluation of excitotoxic lesions. Physiol Behav. 2002;76(4–5):449–456. doi: 10.1016/s0031-9384(02)00732-1. [DOI] [PubMed] [Google Scholar]
- 22.Mungarndee SS, Lundy RF, Jr, Norgren R. Expression of Fos during sham sucrose intake in rats with central gustatory lesions. Am J Physiol Regul Integr Comp Physiol. 2008;295(3):R751–R763. doi: 10.1152/ajpregu.90344.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reilly S, Bornovalova M, Trifunovic R. Excitotoxic lesions of the gustatory thalamus spare simultaneous contrast effects but eliminate anticipatory negative contrast: evidence against a memory deficit. Behav Neurosci. 2004;118(2):365–376. doi: 10.1037/0735-7044.118.2.365. [DOI] [PubMed] [Google Scholar]
- 24.Reilly S, Pritchard TC. Gustatory thalamus lesions in the rat: II. Aversive and appetitive taste conditioning. Behav Neurosci. 1996;110(4):746–759. [PubMed] [Google Scholar]
- 25.Schroy PL, Wheeler RA, Davidson C, Scalera G, Twining RC, Grigson PS. Role of gustatory thalamus in anticipation and comparison of rewards over time in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288(4):R966–R980. doi: 10.1152/ajpregu.00292.2004. [DOI] [PubMed] [Google Scholar]
- 26.Sclafani A, Azzara AV, Touzani K, Grigson PS, Norgren R. Parabrachial nucleus lesions block taste and attenuate flavor preference and aversion conditioning in rats. Behav Neurosci. 2001;115(4):920–933. doi: 10.1037/0735-7044.115.4.920. [DOI] [PubMed] [Google Scholar]
- 27.Flynn FW, Grill HJ, Schulkin J, Norgren R. Central gustatory lesions: II. Effects on sodium appetite, taste aversion learning, and feeding behaviors. Behav Neurosci. 1991;105(6):944–954. doi: 10.1037//0735-7044.105.6.944. [DOI] [PubMed] [Google Scholar]
- 28.Flynn FW, Grill HJ, Schwartz GJ, Norgren R. Central gustatory lesions: I. Preference and taste reactivity tests. Behav Neurosci. 1991;105(6):933–943. doi: 10.1037//0735-7044.105.6.933. [DOI] [PubMed] [Google Scholar]
- 29.Reilly S, Pritchard TC. Gustatory thalamus lesions in the rat: I. Innate taste preferences and aversions. Behav Neurosci. 1996;110(4):737–745. doi: 10.1037//0735-7044.110.4.737. [DOI] [PubMed] [Google Scholar]
- 30.Scalera G, Grigson PS, Norgren R. Gustatory functions, sodium appetite, and conditioned taste aversion survive excitotoxic lesions of the thalamic taste area. Behav Neurosci. 1997;111(3):633–645. [PubMed] [Google Scholar]
- 31.Reilly S. The role of the gustatory thalamus in taste-guided behavior. Neurosci Biobehav Rev. 1998;22(6):883–901. doi: 10.1016/s0149-7634(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 32.Reilly S, Pritchard TC. Gustatory thalamus lesions in the rat: III. Simultaneous contrast and autoshaping. Physiol Behav. 1997;62(6):1355–1363. doi: 10.1016/s0031-9384(97)00352-1. [DOI] [PubMed] [Google Scholar]
- 33.Reilly S, Trifunovic R. Progressive ratio performance in rats with gustatory thalamus lesions. Behav Neurosci. 1999;113(5):1008–1019. doi: 10.1037//0735-7044.113.5.1008. [DOI] [PubMed] [Google Scholar]
- 34.Reilly S, Trifunovic R. Gustatory thalamus lesions eliminate successive negative contrast in rats. Behav Neurosci. 1999;113(6):1242–1248. [PubMed] [Google Scholar]
- 35.Reilly S, Trifunovic R. Gustatory thalamus lesions eliminate successive negative contrast in rats: evidence against a memory deficit. Behav Neurosci. 2003;117(3):606–615. doi: 10.1037/0735-7044.117.3.606. [DOI] [PubMed] [Google Scholar]
- 36.Nomura T, Ogawa H. The taste and mechanical response properties of neurons in the parvicellular part of the thalamic posteromedial ventral nucleus of the rat. Neurosci Res. 1985;3(2):91–105. doi: 10.1016/0168-0102(85)90024-0. [DOI] [PubMed] [Google Scholar]
- 37.Norgren R, Leonard CM. Ascending central gustatory pathways. J Comp Neurol. 1973;150(2):217–237. doi: 10.1002/cne.901500208. [DOI] [PubMed] [Google Scholar]
- 38.Norgren R, Wolf G. Projections of thalamic gustatory and lingual areas in the rat. Brain Res. 1975;92(1):123–129. doi: 10.1016/0006-8993(75)90531-4. [DOI] [PubMed] [Google Scholar]
- 39.Ogawa H, Hayama T, Ito S. Response properties of the parabrachio-thalamic taste and mechanoreceptive neurons in rats. Exp Brain Res. 1987;68(3):449–457. doi: 10.1007/BF00249789. [DOI] [PubMed] [Google Scholar]
- 40.Lundy R, Norgren R. Gustatory System. In: Paxinos G, editor. The rat nervous system. New York: Academic; 2004. pp. 891–921. [Google Scholar]
- 41.Emmers R. Synaptic relationships between the ascending trigeminal fibers and the third order lingual relay neurons of the rat thalamus. Exp Neurol. 1975;48(3 Pt 1):586–594. doi: 10.1016/0014-4886(75)90015-1. [DOI] [PubMed] [Google Scholar]
- 42.Emmers R, Benjamin RM, Blomquist AJ. Thalamic localization of afferents from the tongue in albino rat. J Comp Neurol. 1962;118:43–48. doi: 10.1002/cne.901180104. [DOI] [PubMed] [Google Scholar]
- 43.Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. II. Thalamocortical projections. Brain Res. 1986;379(2):342–352. doi: 10.1016/0006-8993(86)90788-2. [DOI] [PubMed] [Google Scholar]
- 44.Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. I. Physiological properties and cytoarchitecture. Brain Res. 1986;379(2):329–341. doi: 10.1016/0006-8993(86)90787-0. [DOI] [PubMed] [Google Scholar]
- 45.Geddes RI, Han L, Baldwin AE, Norgren R, Grigson PS. Gustatory insular cortex lesions disrupt drug-induced, but not lithium chloride-induced, suppression of conditioned stimulus intake. Behav Neurosci. 2008;122(5):1038–1050. doi: 10.1037/a0012748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jadhav SP, Feldman DE. Texture coding in the whisker system. Curr Opin Neurobiol. 2010;20(3):313–318. doi: 10.1016/j.conb.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 47.Pfaffmann C, Norgren R, Grill HJ. Sensory affect and motivation. Ann N Y Acad Sci. 1977;290:18–34. doi: 10.1111/j.1749-6632.1977.tb39713.x. [DOI] [PubMed] [Google Scholar]
- 48.Shimura T, Norgren R, Grigson PS. Brainstem lesions and gustatory function: I. The role of the nucleus of the solitary tract during a brief intake test in rats. Behav Neurosci. 1997;111(1):155–168. [PubMed] [Google Scholar]
- 49.Reilly S, Grigson PS, Norgren R. Parabrachial nucleus lesions and conditioned taste aversion: evidence supporting an associative deficit. Behav Neurosci. 1993;107(6):1005–1017. doi: 10.1037//0735-7044.107.6.1005. [DOI] [PubMed] [Google Scholar]
- 50.Spector AC. Gustatory function in the parabrachial nuclei: implications from lesion studies in rats. Rev Neurosci. 1995;6(2):143–175. doi: 10.1515/revneuro.1995.6.2.143. [DOI] [PubMed] [Google Scholar]
- 51.Spector AC, Grill HJ, Norgren R. Concentration-dependent licking of sucrose and sodium chloride in rats with parabrachial gustatory lesions. Physiol Behav. 1993;53(2):277–283. doi: 10.1016/0031-9384(93)90205-t. [DOI] [PubMed] [Google Scholar]
- 52.Grigson PS, Reilly S, Shimura T, Norgren R. Ibotenic acid lesions of the parabrachial nucleus and conditioned taste aversion: further evidence for an associative deficit in rats. Behav Neurosci. 1998;112(1):160–171. [PubMed] [Google Scholar]
- 53.Spector AC, Norgren R, Grill HJ. Parabrachial gustatory lesions impair taste aversion learning in rats. Behav Neurosci. 1992;106(1):147–161. doi: 10.1037//0735-7044.106.1.147. [DOI] [PubMed] [Google Scholar]
- 54.Di Lorenzo P. Long-delay learning in rats with parabrachial pontine lesions. Chemical Senses. 1988;13(2) [Google Scholar]
- 55.Norgren R, Li B, Tesche J, Grigson PS. Learned aversions to corn oil in rats with lesions of the parabrachial nuclei. LaNapoule, France: Benjamin Franklin/Lafayette Seminar; 1999. [Google Scholar]
- 56.Norgren R, Li B, Tesche J, Grigson PS. Learned aversions to corn oil in rats with lesions of the parabraihial nuclei. LaNapoule, France: Benjamin Franklin/Lafayette Seminar; 1999. [Google Scholar]
- 57.Salamone JD, Correa M, Mingote S, Weber SM. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J Pharmacol Exp Ther. 2003;305(1):1–8. doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- 58.Salamone JD, Wisniecki A, Carlson BB, Correa M. Nucleus accumbens dopamine depletions make animals highly sensitive to high fixed ratio requirements but do not impair primary food reinforcement. Neuroscience. 2001;105(4):863–870. doi: 10.1016/s0306-4522(01)00249-4. [DOI] [PubMed] [Google Scholar]
- 59.Grigson PS, Hajnal A. Once is too much: conditioned changes in accumbens dopamine following a single saccharin-morphine pairing. Behav Neurosci. 2007;121(6):1234–1242. doi: 10.1037/0735-7044.121.6.1234. [DOI] [PubMed] [Google Scholar]
- 60.Genn RF, Ahn S, Phillips AG. Attenuated dopamine efflux in the rat nucleus accumbens during successive negative contrast. Behav Neurosci. 2004;118(4):869–873. doi: 10.1037/0735-7044.118.4.869. [DOI] [PubMed] [Google Scholar]