Abstract
The mammary epithelial cell transitions from a, non-secreting to a terminally differentiated, secreting cell during lactation. Zinc (Zn) is a key modulator of phenotypic transition as it regulates over 300 biological functions including transcription, translation, energy transformation, intracellular signaling and apoptosis. In addition, Zn must be redirected from normal cellular functions into the secretory compartment, as many components of the secretory system are Zn-dependant and an extraordinary amount of Zn is secreted (1–3 mg Zn/d) into milk. Herein, we utilized a “systems biology” approach of genomic and proteomic profiling to explore mechanisms through which Zn is reallocated during phenotype transition in the lactating mammary gland from mice and cultured mammary cells. Nine Zn transporters play key roles in Zn redistribution within the network during lactation. Protein abundance of six Zip (Zip3, Zip5, Zip7, Zip8, Zip10, Zip11) and three ZnT (ZnT2, ZnT4, ZnT9) proteins was expanded > 2-fold during lactation, which was not necessarily reflected by changes in mRNA expression. Our data suggest that Zip5, Zip8 and Zip10 may be key to Zn acquisition from maternal circulation, while multiple Zip proteins reuptake Zn from milk. Confocal microscopy of cultured mammary cells identified the Golgi apparatus (modulated in part by ZnT5, Zip7 and Zip11) and the late endosomal compartment (modulated in part by ZnT2 and Zip3) as key intracellular compartments through which Zn is reallocated during lactation. These results provide an important framework for understanding the Zn transporting network through which mammary gland Zn pools are redistributed and secreted into milk.
Keywords: zinc, mammary gland, lactation, transporters, model, network
Introduction
The mammary gland is a phenotypically dynamic, hormonally responsive tissue which differentiates from a non-secreting, proliferative tissue to a highly differentiated secretory tissue during lactation. The morphological changes that occur in the mammary gland as it transitions to a secreting tissue have been well characterized (reviewed in (40)) and studies are continuing to reveal the molecular regulation of these morphological changes (reviewed in (8)). A key shift occurs during pregnancy and lactation as mammary epithelial cells (MECs) proliferate and terminally differentiate to establish a secretory epithelium. However, there is little understanding of how micronutrients regulate these phenotypic changes in MECs or are transferred into milk. Zinc (Zn) is a micronutrient that is required for over 300 key cellular processes including DNA and protein synthesis, mitosis, cell proliferation, apoptosis and bioenergetics serving both a structural and catalytic role. We recently determined that MECs accumulate Zn within mitochondria and the Golgi apparatus as the mammary gland transitions from a non-secreting to a secreting tissue (37). The relevance of Zn redistribution may reflect a requirement to redistribute Zn within the MEC to coordinate the transition from a proliferative to a terminally differentiated, secreting epithelium. In addition to cellular needs, MECs must transfer an extraordinary amount of Zn (~1–3 mg Zn/d) into milk to nourish the developing offspring (43). Inadequate Zn transfer into milk quickly results in severe Zn deficiency in infants which compromises growth and development, decreases cognitive development and increases morbidity and mortality (5, 52). However, the mechanisms responsible for regulating Zn redistribution in the MEC remain to be elucidated.
Mammalian Zn transport is regulated by two distinct families of Zn transporting proteins. Members of the ZnT family (ZnT1–10) transport Zn from the cytosol, either across the plasma membrane or into vesicles (41). In contrast, members of the Zip family (Zip1–14) facilitate cellular Zn uptake into the cytoplasm from across the cell membrane or from within intracellular compartments (16, 18). Although twenty-four Zn transporters have been identified in mammals, there is sparse information regarding the regulation of specific Zn transporters or their distinct cellular function. Our previous studies indicate that mammary gland Zn transport during lactation involves lactogenic hormone regulation (26, 37, 44). Thus far, expression of only four Zn transporters (ZnT1, ZnT2, ZnT4 and Zip3) has been described in the mammary gland (32). Localization of ZnT1, ZnT2 and ZnT4 at, or in proximity to, the apical (luminal) membrane of secreting MECs suggests all three participate in Zn transport into milk. ZnT1 is associated with the cell membrane (25, 27), while ZnT2 imports Zn into late endosomal/exocytotic vesicles which traffic to the cell membrane to efflux Zn in response to secretory cues (34, 44). The contribution of ZnT2 is evidenced by a point mutation in ZnT2 which is associated with a 75% reduction in milk Zn levels in women (10) and at least two single nucleotide polymorphisms (SNPs) which may play key roles modulating mammary gland Zn metabolism (45). ZnT2 also plays a role in expanding mitochondrial Zn pools which may modulate mammary gland phenotype specifically through alterations in bioenergetics and apoptosis (46). In addition, a spontaneous mutation in ZnT4 in mice (lethal milk, lm) results in ~35% reduction in milk Zn content as estimated by Zn analysis of stomach contents of pups suckled from lm dams (1). Curiously, mammary gland weight and milk volume in lm mice are significantly impaired, and pups suckled from lm dams die before weaning, illustrating a critical role for ZnT4 in mammary gland Zn metabolism. However, the role of ZnT4 in Zn secretion from the MEC is not understood as ZnT4 is localized to an intracellular compartment in rat MECs (27) and mutations in ZnT4 have not been associated with low Zn levels in human milk (10, 39). The only Zip protein to be specifically identified in the lactating mammary gland thus far is Zip3. Zip3 is localized in proximity to the apical cell membrane in MECs and traffics to the cell membrane following stimulation by the lactogenic hormone prolactin (26). Elevated milk Zn levels and changes in mammary gland morphology observed in Zip3-null mice are consistent with a role for Zip3 in Zn reuptake from milk for cellular functions essential to maintaining the differentiated epithelium (28).
Understanding the Zn transporting network, e.g., how MECs import, redistribute, utilize and ultimately secrete Zn, is important to understanding mammary gland function and improving both maternal and infant health. However, how Zn is reallocated during phenotypic transition is not currently understood. Herein, we combined immunohistochemistry and confocal microscopy with genomic and proteomic profiling to develop a comprehensive model of the Zn transporting network in the lactating mammary gland using a mouse model. We noted significant changes in abundance of numerous Zn transporters in lactating tissue reflecting the critical changes that are dependent upon Zn redistribution within the mammary gland during lactation. Determining the sub-cellular localization of these Zn transporters further permitted us to generate a model of the Zn transporting mechanisms through which the mammary gland integrates the Zn acquisition, redistribution and secretion through the Zn transporting network during lactation.
Materials and Methods
Animals
This study was approved by the IACUC Committee at the Pennsylvania State University, which is accredited by the American Association for the Accreditation of Laboratory Animal Care. Female C57BL/6 mice were obtained commercially (Charles River, Wilmington, MA) and housed individually in polycarbonate cages and fed a purified diet based on AIN93 containing 25 mg Zn/kg. Mice were maintained on a 12 h light/dark cycle under controlled temperature and humidity. Mice (n=6) were bred and allowed to deliver naturally. On lactation day (LD) 4–6, dams were removed from pups for 2 h to control for effects of suckling on Zn transporter expression and localization, and subsequently euthanized by CO2 asphyxiation. Mammary glands were removed and snap frozen in liquid nitrogen, stored in RNAlater or fixed in phosphate buffered paraformaldehyde (4%) until analysis. Tissues were taken from non-lactating mice (n=6) for comparison as noted below.
Localization of Zn transporters
To determine whether Zn transporters are localized to the cell membrane or within an intracellular compartment in lactating mammary gland, mammary glands from lactating mice were fixed, embedded in paraffin and sectioned (5 μm) as previously described (25, 28). Zinc transporter localization was examined by immunohistochemistry using the ImPRESS Peroxidase Polymer Detection Kit (Vector Labs, Burlingame, CA) or Vectastain ABC Kit (Zip13, Vector Labs) (Supporting Table 1) and counterstained with toluidine blue. Sections were examined by light microscopy (Leica DM IL LED, Leica Microsystems GmbH, Wetzlar, Germany) and images were captured using Leica Application Suite (version 3.6.0) at 40X magnification.
To overcome the limitations of immunohistochemistry and determine if Zn transporters localized intracellularly reside within the “secretory compartment” (endoplasmic reticulum, Golgi apparatus, late endosomes), we utilized confocal microscopy. Normal mouse mammary epithelial cells (HC11) were a gift from Dr. Jeffery Rosen (Baylor College of Medicine; Houston, Texas) and used with permission of Dr. Bernd Groner (Institute for Biomedical Research; Frankfurt, Germany). Cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum, insulin (5 μg/mL), epidermal growth factor (EGF, 10 ng/mL) and gentamycin. Cells were used between passage 40 and 45. Cells were seeded at ~30,000 cells/well onto glass coverslips and cultured until ~50% confluent (2 d). Cells were fixed in phosphate buffered-paraformaldehyde (4%), pH 7.4 or ice-cold 70% methanol for 10 min, washed in PBS, and briefly permeabilized with Triton X-100 (0.2% in PBS). Non-specific binding was blocked with 5% goat serum/1% bovine serum albumin in PBS for 20 min followed by incubation with antibody for 60 min at room temperature (Zip3, 1:1,000; Zip7, 1:50; Zip11, 1:50; ZnT5, 1:50; ZnT6, 1:50; ZnT8, 1:1,000; ZnT10, 1:50). Zinc transporter antibody was detected with either Alexa Fluor® 488- or Alexa Fluor® 568-conjugated anti-rabbit IgG (1 μg/mL, Invitrogen) for 45 min at room temperature shielded from light. Markers of the “secretory compartment” were as follows: calnexin, endoplasmic reticulum; p58, trans-Golgi apparatus; mannose 6-phosphate receptor, late endosomes. Cells were washed, mounted in ProLong Gold (Invitrogen), and sealed with nail polish. Immunofluorescent imaging was performed using an Olympus FV1000, with PlanApo 60X oil lens N.A. 1.42 and digital images were captured sequentially (FV10-ASW, version 4.5; Olympus) to eliminate potential interference between fluorochromes and images were saved as .tif files to maintain image quality. Co-localization analysis was performed through the use of the ‘Color Composite’ and ‘Colocalization’ functions in Image Pro Plus (version 4.5; Olympus) and co-localized pixels were pseudocolored yellow.
Real time relative RT-PCR
To determine if Zn transporter mRNA expression reflected phenotype transition, mRNA expression of all Zn transporters were assessed by real-time RT PCR. Total RNA was isolated from homogenized mammary gland following the manufacturer’s instructions (Life Technologies). RNA was electrophoresed through agarose (2%) and integrity was assessed by ethidium bromide staining. Gene specific primers were designed using Primer3 and synthesized by the Genomics Facility at The Pennsylvania State University (Supporting Table 2). Relative mRNA expression was determined by real time relative RT-PCR using the SYBR Green detection system (Perkin Elmer Applied Biosystems, Foster City, CA). cDNA synthesis, real-time relative PCR and data analysis were preformed as previously described (44). Each sample was analyzed in duplicate and normalized to β-actin using the following equation: ΔCtgene = Ctgene Ctβ-actin. The fold change in expression was calculated using the following equation: 2(ΔΔCt) where ΔΔCt = mean ΔCtgene in non-lactating gland mean ΔCtgene in lactating mammary gland. Values represent mean fold change ± SE, relative to non-lactating mice (set to 100%).
Immunoblot analysis
To determine effects of phenotype transition on Zn transporter protein abundance, mammary glands were homogenized in Hepes-EDTA buffer containing protease inhibitors and the total membrane fraction was isolated as previously described (27). The protein concentration of the total membrane fraction was determined using the Bradford assay (Bio-Rad). Total membrane proteins (50 μg) were diluted in Lammeli sample buffered containing dithiothreitol (100 μM) and heated at 95ºC for 5 min. Electrophoresis and immunoblotting was performed as previously described (27) with antibodies directed against each specific Zn transporter (Supporting Table 3). Proteins were detected with donkey, anti-rabbit IgG, donkey anti-goat IgG or donkey anti-chicken IgY conjugated to horseradish peroxidase (Amersham Pharmacia Biotech), visualized with Super Signal Femto Chemiluminescent Detection System (Pierce, Rockford, IL) and exposed to autoradiography film. Relative band density was quantified using the Carestream Gel Logic 212 Pro.
Model development of the Zn metallome in lactating mammary gland
Changes in protein abundance combined with sub-cellular localization of each transporter in the lactating mammary gland derived from data compiled from immunohistochemistry and confocal images were used to develop a model of the Zn transporting network. For clarity, arrows are used to signify movement of Zn from one compartment to another. To illustrate changes in Zn transporter protein abundance during the transition from a non-lactating to a lactating phenotype, we employed the use of a full color spectrum. Cool colors (e.g., purple and blue) represent a decrease in abundance while warm colors (orange and red) represent an increase in abundance relative to non-lactating mice (green). A change in color was employed if protein abundance was significantly different between phenotypes as analyzed below.
Statistical analysis
Data are expressed as mean ± SE and analyzed by t-test. If the variance was significantly different between phenotypes, Welch’s correction for unequal variance was used. Statistical analysis was conducted using GraphPad Prism 5.0 (San Diego, CA) and significance was demonstrated at p <0.05.
Results
Profiling of zinc transporters in non-lactating and lactating mammary gland
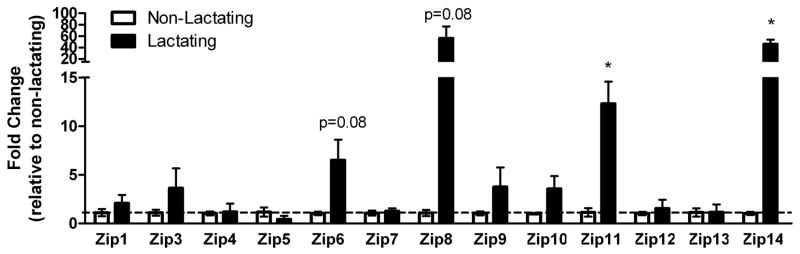
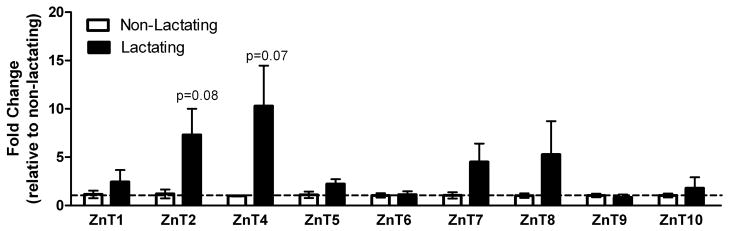
We first utilized a PCR-based molecular profiling screen to identify Zn transporters that were expressed in the mammary gland and determined if expression was different between non-lactating and lactating tissue (Figure 1A and 1B). Limited significant effects of phenotype on mRNA expression were detected. Expression of Zip11 and Zip14 was significantly higher in lactating compared with non-lactating mammary gland. While expression of Zip6 (p=0.08), Zip8 (p=0.08), ZnT2 (p=0.08) and ZnT4 (p=0.07) was higher in lactating mammary gland, these data did not reach statistical significance.
Figure 1.
Minimal changes in mRNA expression of zinc transporters are detected in the mammary gland of lactating mice. Changes in Zip (A) and ZnT (B) mRNA expression were compared between non-lactating and lactating mammary gland. Data represent mean fold change of mRNA expression relative to non-lactating mice ± SE, n = 4–6 mice/phenotype. Asterisk denotes significant effect of phenotype, p<0.05.
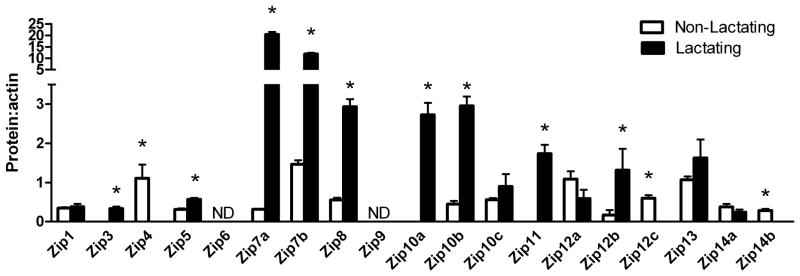
We next utilized immunoblotting to ascertain changes in Zn transporter protein abundance between non-lactating and lactating tissue. As illustrated in Figure 2, the profile of Zn transporter protein abundance changed dramatically following transition from a non-lactating to a lactating tissue. Four sub-families of Zip proteins are classified based on structural similarities (50): Zip subfamily I (Zip9), gufA subfamily (Zip11), Zip subfamily II (Zip1, Zip2, Zip3) and the LIV-1 subfamily (Zip4–6, Zip8, Zip10, Zip12–14). All proteins detected were of the predicted molecular mass, except where noted. Zip9 protein was not detected in the mammary gland. Zip11 protein was only detected in lactating tissue. We detected no change in Zip1 abundance while Zip3 protein was only detected in lactating tissue. Bioinformatic analysis (http://biogps.gnf.org) did not identify Zip2 expression in the mammary gland, nor were we able to obtain antibody against Zip2, thus we cannot conclusively detect or exclude the presence of Zip2 in mammary tissue at this time. Members of the LIV-1 subfamily can be sub-grouped into four clusters. Cluster A includes Zip4, Zip7 and Zip13. Zip4 was not detected in lactating mammary gland and abundance of Zip13 protein was not related to phenotype. We detected two isoforms of Zip7 protein (~50 and 150 kDa), the abundance of which were both greatly expanded (~8- and 65-fold higher) in lactating tissue. The predicted molecular mass of Zip7 is ~50kD suggesting the presence of a monomer and trimer in the mammary gland. Cluster B includes Zip5, Zip6 and Zip10. Zip6 protein was not detected in the mammary gland. These results were not due to the lack of immunoreactivity of the Zip6 antibody as we can detect Zip6 expression in mouse testes (13) and human breast cancer cells (35). Zip5 abundance was ~2.5-fold higher in lactating compared with non-lactating tissue. We detected three Zip10 isoforms (~42, 44 and 46 kDa). The abundance of the larger 44 and 46 kDa isoforms were ~7-fold higher in lactating compared with non-lactating tissue while abundance of the smaller 42 kDa isoform did not significantly change. The predicted molecular mass of Zip10 is ~95 kDa; however, a protein of equal abundance is routinely detected at ~45 kDa by the manufacturer (ProSci Inc, Poway, CA). This suggests that Zip10 may exist as a dimer. Cluster C includes Zip8 and Zip14. Abundance of two isoforms of Zip8 protein (~150 and 200 kDa), identical in size to what has been described previously in primary T-cells (3), was ~13-fold higher in lactating compared with non-lactating tissue, while the abundance of both isoforms of Zip14 (~66 and ~68 kDa), identical in size noted by Liuzzi et al (31) did not significantly change. Finally, we noted that there were two isoforms of Zip12 (~72 and ~35 kDa) in the non-lactating mammary gland. In the lactating mammary gland, the abundance of the 70 kDa protein was lower and the 35 kDa was eliminated. Interestingly, during lactation, we noted the appearance of two additional Zip12 proteins at ~48 kD and ~70 kDa. While to our knowledge there is no literature on Zip12, two isoforms of mouse Zip12 of ~72 and 76 kDa have been predicted (UniProt; http://www.uniprot.org/).
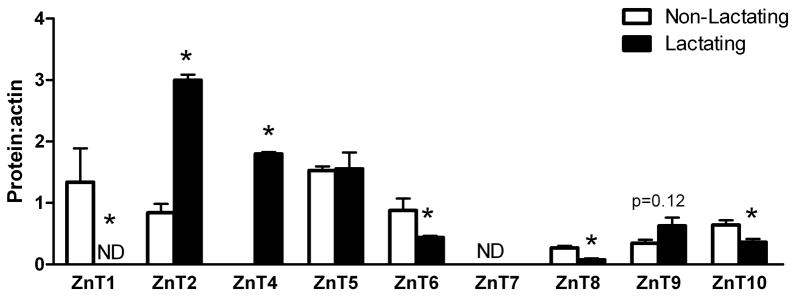
Figure 2.
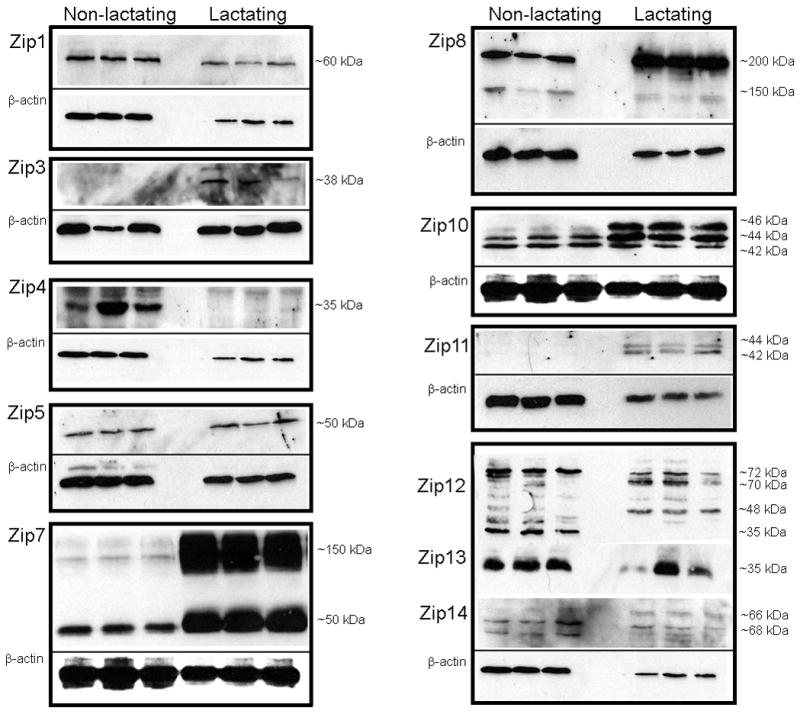
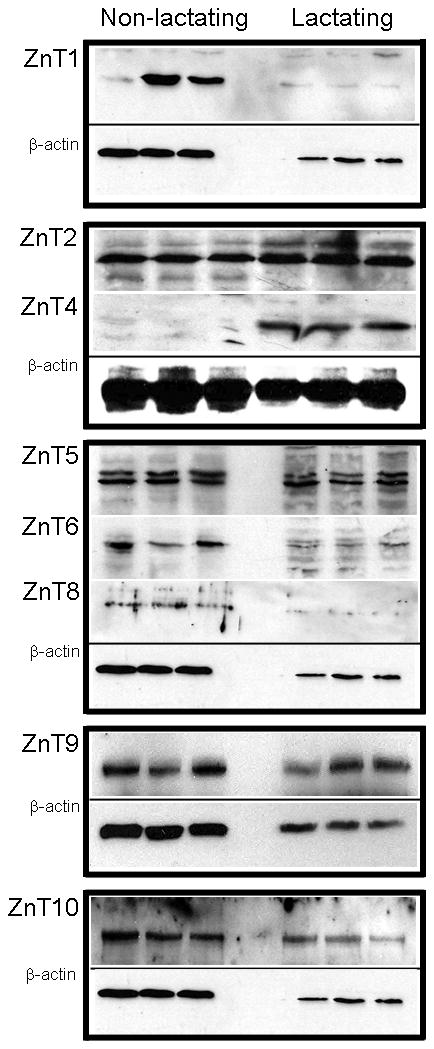
Zinc transporter protein abundance changes during the transition from a non-lactating to a lactating phenotype in mice. Representative immunoblots of Zip (A) and ZnT (C) transporters in total membrane fractions isolated from mammary gland (50 ug protein/lane, n=3 individual mice). Immunoblots were stripped and reprobed for β-actin to normalize for protein loading. Quantitative analysis of band density of Zip (B) and ZnT (D) proteins relative to β-actin documented a significant effect of phenotype on Zn transporter abundance. Data represent mean ratio of zinc transporter: β-actin ± SE, n=3 samples/phenotype as noted above. Immunoblotting was conducted at least twice. Asterisk denotes a significant effect of phenotype, p<0.05.
Members of the ZnT family of Zn exporters can be classified based on structural homology into three subfamilies ((41); http://www.ensembl.org): Subfamily I (ZnT1); Subfamily II (ZnT2, ZnT3, ZnT4, ZnT8, ZnT9 and ZnT10); and Subfamily III (ZnT5, ZnT6, ZnT7). ZnT1 protein was not detected in lactating mammary gland. In contrast, ZnT4 protein was only detected in lactating mammary gland. ZnT2 abundance was ~4-fold higher in lactating compared with non-lactating tissue, while ZnT8 and ZnT10 (~105 kDa) abundance was significantly lower in lactating mammary gland. While there is no literature on ZnT10, two isoforms of mouse ZnT10 of ~25 and ~50 kD are predicted (UniProt), suggesting the presence of a dimer in the mammary gland. Abundance of ZnT9 was ~2-fold higher in lactating tissue while ZnT5 abundance was not related to phenotype. In contrast, ZnT6 abundance was significantly lower in lactating mammary gland and ZnT7 was not detected in the mammary gland.
Zinc transporters are localized to specific domains and sub-cellular compartments in the lactating mammary gland
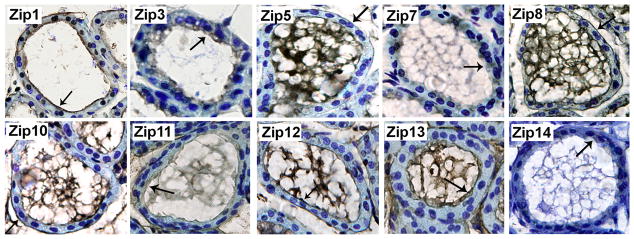
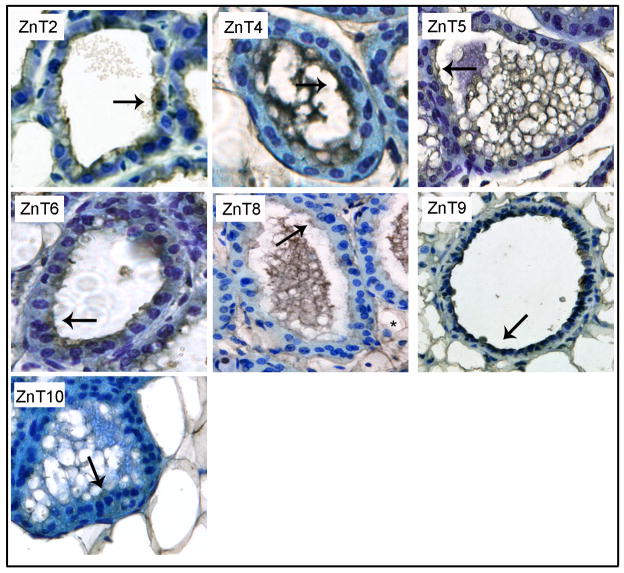
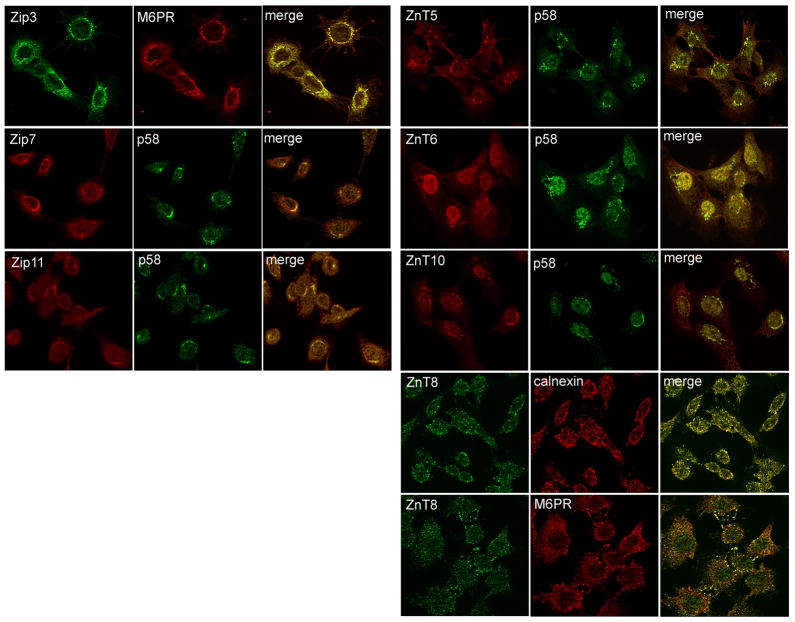
We next utilized immunohistochemistry to characterize the general localization (cell or intracellular membrane) of each Zn transporter that was detected in lactating mammary gland (Figure 3). Six Zip proteins were associated distinctly with the cell membrane (Zip1, Zip5, Zip8, Zip10, Zip12 and Zip13) of secreting MECs in the lactating mammary gland (Figure 3A). Three Zip proteins were primarily associated with the apical membrane (Zip1, Zip12 and Zip13) and three were detected on both the apical and basal membranes (Zip5, Zip8 and Zip10). The remaining four Zip proteins (Zip3, Zip7, Zip11 and Zip14) expressed in lactating mammary gland were localized intracellularly. To determine if these Zip proteins were localized to the “secretory compartment” (endoplasmic reticulum, Golgi apparatus, endosomes) we utilized confocal microscopy in cultured MECs (Figure 4). Our data indicated that Zip3 co-localized with the late endosomal marker M6PR. Zip7 and Zip11 were co-localized with the Golgi apparatus marker, p58. We were unable to localize Zip14 within compartments of the “secretory system” (data not shown). Our studies further determined that most members of the ZnT family expressed in lactating mammary gland were associated with the cell membrane of MECs in the lactating mammary gland (Figure 3B). ZnT4 was primarily localized at or proximal to the apical cell membrane of MECs in lactating mammary gland. ZnT2 displayed a diffuse intracellular staining pattern, consistent with previous reports of mitochondrial (46) and vesicular localization (34). ZnT9 was localized intracellularly but we were unable to localize ZnT9 within compartments of the “secretory system” (data not shown). A small amount of ZnT5 and ZnT6 was associated with the cell membrane of MECs in lactating mammary gland which was consistent with our detection of a minimal amount of staining proximal to the cell periphery in cultured MECs (Figure 4). In addition, ZnT5, ZnT6 and ZnT10 co-localized with the Golgi apparatus marker p58. ZnT8 partially co-localized with M6PR and the endoplasmic reticulum marker calnexin.
Figure 3.
Immunohistochemistry revealed the general localization of zinc transporters in mouse mammary epithelial cells during lactation. Representative mammary gland sections were immunostained for Zip (A) or ZnT (B) proteins. Zinc transporters were detected with DAB (brown) and counterstained with toluidine blue. Arrows illustrate predominant cellular localization in secreting mammary epithelial cells. Asterisk (ZnT8 and ZnT10) illustrates staining associated with adipocytes. Images were captured at 40X magnification.
Figure 4.
Confocal microscopy identified several Zn transporters associated with the “secretory compartment” in mammary epithelial cells. Double-immunofluorescence imaging of Zip3 (green) illustrated co-localization (merge, yellow) with the mannose 6-phosphate receptor (M6PR, red) a late endosome marker. Zip7, Zip11, ZnT5, ZnT6 and ZnT10 (red) were partially co-localized (merge, yellow) with (p58, green) a trans-Golgi apparatus marker. ZnT8 (green) co-localized with calnexin (red) an endoplasmic reticulum marker M6PR (red) in mammary epithelial cells. Images were collected at 40X magnification.
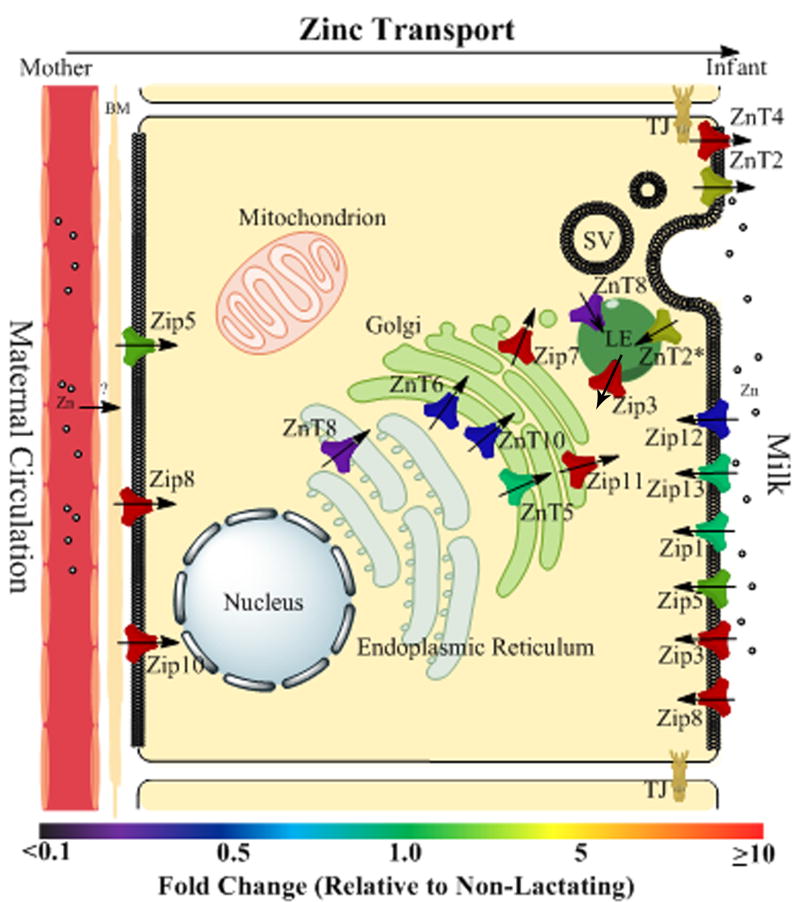
Modeling the zinc metallome of the mouse lactating mammary gland
These data were summarized in a model which illustrates changes in the Zn transporting network following the phenotypic transition to a lactating tissue (Figure 5). Based on these data our model predicts that three Zip proteins (Zip5, Zip8 and Zip10) integrate Zn import from maternal circulation. Once Zn is taken up by the cell, our data suggest that the Golgi apparatus is a specific Zn pool which is dramatically modified during lactation (through ZnT5, Zip7 and Zip11). Two pathways appear to participate in Zn transport from the cell into milk. Our data suggest that ZnT4 may mediate Zn efflux across the apical membrane. In addition, the late endosomal compartment contains a specific Zn pool (regulated through ZnT2 and Zip3) which plays a key role in vesicular Zn secretion. Interestingly, our data suggest that four Zip proteins (Zip1, Zip3, Zip12 and Zip13) integrate Zn import into the MEC from the secreted milk pool. Our model allows us to further predict that there may be limited dependence on several ZnT (ZnT1, ZnT6, ZnT7, ZnT8 and ZnT10) and Zip (Zip4, Zip6, Zip9, Zip12 and Zip14) proteins in the MEC during lactation.
Figure 5.
Model of the zinc transporting network during the transition from a non-lactating to a lactating phenotype. Sub-cellular localization and changes in protein expression (fold change) was complied to develop a model illustrating changes in the Zn transporting network during lactation. The localization of transporters was acquired from immunohistochemistry and confocal microscopy. The full color spectrum was developed to illustrate fold-changes in protein expression between phenotypes. Green reflects Zn transporter abundance in non-lactating mammary gland (set to 1). Lower wavelength colors (purple and blue) represent a decrease in abundance while higher wavelength colors (yellow and red) represent an increase in abundance relative to non-lactating mice, p<0.05. The direction of the arrow indicates the direction of Zn transport. SV, secretory vesicle; LE, late endosome, TJ, tight junction; BM, basal membrane.
Our data further illustrated that changes in Zn transporter mRNA expression are not always positively associated with changes in Zn transporter protein abundance (Table 1). Comparison of these data allow us to predict potential mechanisms (transcription, post-transcription, translation or degradation) which may responsible for regulating the function of specific Zn transporters in the mammary gland. It is interesting to note that Zn transporters which we identified as playing key roles in mammary gland Zn metabolism (ZnT2, ZnT4, ZnT5, Zip1, Zip3, Zip8, Zip10, Zip11 and Zip13) may be regulated through transcriptional mechanisms, while Zn transporters predicted to play a less important role during lactation may be regulated through other means.
Table 1.
Correlation between changes in mRNA expression and protein abundance in the lactating mammary gland relative to the non-lactating phenotype.
| Transporter | mRNA | Protein | Correlation | Predicted Regulation |
|---|---|---|---|---|
| Exporters | ||||
| ZnT1 | No change | Decreased | No | Degradation |
| ZnT2 | Increased | Increased | Yes | Transcription |
| ZnT4 | Increased | Increased | Yes | Transcription |
| ZnT5 | No change | No change | Yes | Transcription |
| ZnT6 | No change | Decreased | No | Degradation |
| ZnT7 | Increased | Not Detected | No | Degradation |
| ZnT8 | Increased | Decreased | No | Degradation |
| ZnT9 | No change | Increased | No | Stabilized |
| ZnT10 | No change | Decreased | No | Degradation |
| Importers | ||||
| Zip1 | No change | No change | Yes | Transcription |
| Zip3 | Increased | Increased | Yes | Transcription |
| Zip4 | No change | Decreased | No | Degradation |
| Zip5 | No change | Increased | No | Stabilized |
| Zip6 | Increased | Not Detected | No | Degraded |
| Zip7 | No change | Increased | No | Stabilized |
| Zip8 | Increased | Increased | Yes | Transcription |
| Zip9 | Increased | Not Detected | No | Degradation |
| Zip10 | Increased | Increased | Yes | Transcription |
| Zip11 | Increased | Increased | Yes | Transcription |
| Zip12 | No change | Decreased | No | Degradation |
| Zip13 | No change | No change | Yes | Transcription |
| Zip14 | Increased | No change | No | Post-transcription |
Discussion
The mammary gland phenotypically transitions from a proliferative, non-lactating tissue to a terminally differentiated, secretory tissue during lactation. In doing so, the MEC must switch from providing Zn for proliferative cellular functions to providing Zn for secretory functions and for secretion into milk (43). It has been presumed that the primary role of Zn in the MEC is to be transported vectorially from maternal blood directly through the MEC for secretion into the alveolar lumen. This implies that intracellular pathways are a series of “way-stations” as Zn moves through the MEC from one side to the other. However, our data indicate that the MEC also imports Zn from the milk pool once it has already been secreted and understanding the relevance of this process is critical to elucidating roles for Zn in MEC function. For example, previous studies indicate that Zn accumulates in specific organelles such as mitochondria and the Golgi apparatus (37) which is redistributed in response to hormonal cues. Either paradigm implies the existence of an intracellular Zn transporting “network” and the reallocation of Zn pools within the network to support alterations in cell function concomitant with Zn secretion into milk. This reallocation necessitates the complex integration of numerous Zn transporting mechanisms to maintain optimal cellular function while concurrently transferring an enormous amount of cationic charge. Our previous understanding of mammary gland Zn metabolism was restricted to loosely defined roles for ZnT1, ZnT2, ZnT4 and Zip3 (25–28). Herein, genomic and proteomic profiling elucidated the Zn transporters which modulate distinct intracellular compartments and play import roles in Zn metabolism during lactation. Although observational, these studies dramatically expand our understanding of the Zn transporting mechanisms and to our knowledge, this is the first delineation of the entire Zn metallome in a specific tissue, revealing how the mammary gland Zn network responds to lactogenic cues.
Of the fourteen Zn importers that have been identified in mammals (16) we detected expression of ten Zip proteins in the lactating mammary gland, dramatically expanding our current understanding beyond a role for Zip3. Zip5, Zip8 and Zip10 were localized to the basal membrane and their abundance was expanded by ~2-, 7- and 13-fold, respectively, in lactating tissue. This suggests they play a role in Zn acquisition form maternal circulation. Our detection of two Zip8 isoforms is consistent with previous reports in primary T-cells (3). In addition, we detected three Zip10 proteins around 50 kD, which is similar to the size of an isoform detected by the antibody manufacturer. While the relevance of multiple isoforms of Zip8 and Zip10 has not yet been determined, our results are consistent with Zn expansion during lactation (37) and suggests Zip5, Zip8 and Zip10 play key roles in this process. The minor expansion in Zip5 abundance does not appear to reflect increased expression of Zip5 mRNA suggesting that Zip5 is post-transcriptionally regulated consistent with reports of mRNA stabilization, increased translation or increased protein stability (53). In contrast, Zip8 and Zip10 expansion was positively correlated with mRNA expression, suggesting transcriptional regulatory elements which are lactation-dependent, similar to what we have previously observed for ZnT2 (44). Clearly, further studies are needed to understand the regulation of mammary gland Zn acquisition from maternal circulation. It is interesting to note that in addition to Zip3 (26, 28), multiple Zip proteins may play a role in Zn reuptake from milk. The precise contribution of Zn reuptake in mammary gland Zn metabolism is not understood but it clearly plays a critical role in mammary gland Zn metabolism Zip3-null mice (28) (as well as Zip1-null mice; SLK, unpublished observations) have serious defects in mammary gland morphology. Mammary gland function is dependent upon the maintenance of a highly differentiated secretory cell-type and we speculate that Zn signaling from the previously secreted milk pool, plays a novel and key role in regulating processes essential to maintaining differentiation such as modulating ATP biogenesis (29), regulating apoptosis (2) and regulating Zn-finger transcription factors such as Egr1 and Egr2 which are critical for cellular differentiation (7). Further studies are needed to explore the relevance of Zn reuptake on mammary cell function.
We previously identified the Golgi apparatus as a specific sub-cellular compartment in the MEC that accumulates Zn during lactation (37). Consistent with these observations, our current study suggests that ZnT5 may play a key role in this process as abundance was maintained during phenotypic transition. Zn accumulation in the Golgi apparatus seems logical given the function of the Golgi apparatus in secreting tissues. First, Zn incorporation into specific proteins which are components of the secretory machinery in the mammary cell, such as glutaminyl cyclases (14), glycosyltransferases (9, 38) and sialtransferases (15), must expand during lactation. Secondly, Zn may be specifically incorporated into proteins that are themselves secreted into milk such as caseins (19) and lactoferrin (4). On the other hand, the Golgi apparatus may act as a conduit for more loosely-bound or “labile” Zn which may associate with small molecular weight ligands such as citrate (54) which is secreted (30) perhaps through the porosomal complex (23) in humans (6, 33), mice (6) and cows (20). An alternative route for Zn transfer into milk may include Zn export directly from the Golgi apparatus through Zip11 or Zip7 (22), as abundance of both these Zip proteins was dramatically expanded during lactation. Our limited understanding of this process currently makes it difficult to envision a scenario whereby a copious amount of Zn would be exported from the Golgi apparatus prior to secretion as the amount of “free” Zn in the cytoplasm is negligible (11). However, the presence of an undefined cytoplasmic Zn pool available for export cannot be excluded. In fact, evidence that citrate is exported directly across the apical membrane into milk (47) suggests that a Zn-citrate complex may exist in the cytoplasm and provide this pool. Moreover, Zn is transported from the cytoplasm into late endosomes and exocytotic vesicles via ZnT2 (34) or perhaps directly across the apical membrane via ZnT4. The detection of ZnT4 associated with the apical membrane of the MEC in the lactating mammary gland is consistent with the observation that mice with a mutation in ZnT4 (lm mouse) have reduced milk Zn levels (21). Interestingly, our previous observations in a lactating rat model noted intracellular ZnT4 localization consistent with Golgi apparatus localization (39). Together, this suggests that ZnT4 may traffic from the Golgi apparatus to the apical membrane to participate in Zn export into milk, analogous to the role and regulation of ATP7A for copper transport (42). In addition, Zip7 plays a key role in providing Zn for tyrosine kinase activation (51). Thus, Zip7-mediated Zn movement may be critical not in milk Zn secretion but for activating intracellular signaling processes which are essential for maintaining a secretory phenotype such as the Jak2/Stat5 signaling cascade (12, 44).
Interesting information may also be gleaned from understanding which Zn transporters in the mammary gland are no longer required during lactation. For example, our data indicated that expression of numerous Zn transporters (Zip4, Zip6, Zip9, Zip12, Zip14, ZnT1, ZnT6, and ZnT7) is reduced or are not detected during lactation. The lack of Zip6 protein in the normal mammary gland is particularly interesting as Zip6 is over-expressed in estrogen receptor positive breast tumors (24, 35, 49–50). Further studies are required to explore the relevance of Zip6 expression in mammary gland Zn metabolism. A potentially interestingly switch occurs in the Golgi apparatus such that dependence upon ZnT6, ZnT7 (17, 48) and Zip9 (36) for Zn accumulation appears to be reduced in the lactating mammary gland, suggesting that Zn incorporation through ZnT5 may be the major pathway during lactation. This molecular switch may assist in the redirection of Zn for incorporation into lactation-specific Zn-requiring proteins or processes or for export from the Golgi apparatus for secretion though lactogenic-dependent transporters. It is intriguing to note that Zn import into the Golgi apparatus by ZnT7 is associated with metal transcription factor 1 (Mtf1) activation and increased expression of Zn-responsive genes such as ZnT1 and metallothionein (MT). Thus an interesting speculation is that ZnT7 repression during lactation may reduce Mtf1 activation, restricting Zn sequestration by MT and redirecting Zn away from specific Zn export mechanisms (i.e., ZnT1) towards Zn secretion mechanisms (i.e., ZnT2 and ZnT4). Additionally, we could not detect expression of MT in the mammary gland (NHM, unpublished observations) which is consistent with this hypothesis.
A positive relationship between changes in mRNA expression and protein abundance was detected for only nine Zn transporters. Intriguingly, eight of these transporters are directly associated with the expansion of critical Zn pools during lactation. This suggests that these transporters may be transcriptionally regulated by lactogenic factors, providing interesting further avenues for further investigation. A second important point is that there may be species-specific differences in mammary gland Zn metabolism. For example, ZnT1 expression in the mammary gland expands and is associated with the apical membrane in the lactating rat. In contrast, ZnT1 protein expression was not detected in the lactating mouse mammary gland. Additionally, ZnT4 is localized to an intracellular compartment in the lactating rat but is localized to the apical membrane in mice. Differences such as these may reflect differences in Zn speciation in milk as Zn is exclusively associated with proteins in rat milk (33) while human and mouse milk contain protein-bound Zn and Zn loosely associated with citrate. This suggests that mice may be a better model to explore the regulation of mammary gland trace element transport mechanisms in women compared with other species.
In summary, the results of this observational study have elucidated specific components of Zn transporting network in the MEC and identified key molecular mechanisms which are responsible for importing Zn from maternal circulation, transporting it into specific sub-cellular compartments and secreting it into milk. This information provides the foundation for exploring physiological, hormonal and regulatory factors responsible for modulating normal mammary gland Zn metabolism during lactation to improve breast health and infant development and may provide novel avenues of investigation to understand breast disease.
Supplementary Material
Acknowledgments
Contract grant sponsor: NIH; Contract grant number: R01HD058614.
The authors gratefully acknowledge the constructive input of members of Young Ah Seo, Stephen Hennigar and Dr. David I Soybel. All confocal microscopy was done at the Cytometry Facility, University Park (Huck Institutes of the Life Sciences, The Pennsylvania State University). This work was supported by R01HD058614 to SLK.
Literature Cited
- 1.Ackland ML, Mercer JF. The murine mutation, lethal milk, results in production of zinc-deficient milk. J Nutr. 1992;122:1214–1218. doi: 10.1093/jn/122.6.1214. [DOI] [PubMed] [Google Scholar]
- 2.Anchordoquy J, Picco S, Seoane A, Anchordoquy J, Ponzinibio M, Mattioli G, Garcia PP, Furnus C. Analysis of apoptosis and DNA damage in bovine cumulus cells after exposure in vitro to different zinc concentrations. Cell Biol Int. 2011;35:593–597. doi: 10.1042/CBI20100507. [DOI] [PubMed] [Google Scholar]
- 3.Aydemir TB, Liuzzi JP, McClellan S, Cousins RJ. Zinc transporter ZIP8 (SLC39A8) and zinc influence IFN-γ expression in activated human T cells. J Leukoc Biol. 2009;86:337–348. doi: 10.1189/jlb.1208759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babina S, Kanyshkova T, Buneva V, Nevinsky G. Lactoferrin is the major deoxyribonuclease of human milk. Biochemistry. 2004;69:1006–1015. doi: 10.1023/b:biry.0000043543.21217.b3. [DOI] [PubMed] [Google Scholar]
- 5.Bhatnagar S, Taneja S. Zinc and cognitive development. Br J Nutr. 2001;85:S139–S145. doi: 10.1079/bjn2000306. [DOI] [PubMed] [Google Scholar]
- 6.Blakeborough P, Salter D, Gurr M. Zinc binding in cow’s milk and human milk. Biochem J. 1983;209:505–512. doi: 10.1042/bj2090505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle K, Hadaschik D, Virtue S, Cawthorn W, Ridley S, O’Rahilly S, Siddle K. The transcription factors Egr1 and Egr2 have opposing influences on adipocyte differentiation. Cell Death Differ. 2009;16:782–789. doi: 10.1038/cdd.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brisken C, Rajaram R. Alveolar and lactogenic differentiation. J Mammary Gland Biol Neoplasia. 2006;11:239–248. doi: 10.1007/s10911-006-9026-0. [DOI] [PubMed] [Google Scholar]
- 9.Bushway A, Park C, Keenan T. Effect of pregnancy and lactation on glycosyltransferase activities of rat mammary gland. Int J Biochem. 1979;10:147–154. doi: 10.1016/0020-711x(79)90109-5. [DOI] [PubMed] [Google Scholar]
- 10.Chowanadisai W, Lonnerdal B, Kelleher SL. Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency. J Biol Chem. 2006;281:39699–39707. doi: 10.1074/jbc.M605821200. [DOI] [PubMed] [Google Scholar]
- 11.Colvin R, Holmes W, Fontaine C, Maret W. Cytosolic zinc buffering and muffling: their role in intracellular zinc homeostasis. Metallomics. 2010;2:306–317. doi: 10.1039/b926662c. [DOI] [PubMed] [Google Scholar]
- 12.Creamer BA, Sakamoto K, Schmidt JW, Triplett AA, Moriggl R, Wagner K-U. Stat5 promotes survival of mammary epithelial cells through transcriptional activation of a distinct promoter in akt1. Mol Cell Biol. 2010;30:2957–2970. doi: 10.1128/MCB.00851-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croxford T, McCormick N, Kelleher S. Moderate zinc deficiency reduced testicular Zip6 and Zip10 abundance and impairs spermatogenesis in mice. J Nutr. 2011;141:359–365. doi: 10.3945/jn.110.131318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cynis H, Rahfeld J-U, Stephan A, Kehlen A, Koch B, Wermann M, Demuth H-U, Schilling S. Isolation of an isoenzyme of human glutaminyl cyclase: retention in the golgi complex suggests involvement in the protein maturation machinery. J Mol Biol. 2008;379:966–980. doi: 10.1016/j.jmb.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 15.Dalziel M, Huang R, Dall’Olio F, Morris J, Taylor-Papadimitriou J, Lau J. Mouse ST6Gal sialyltransferase gene expression during mammary gland lactation. Glycobiology. 2001;11:407–412. doi: 10.1093/glycob/11.5.407. [DOI] [PubMed] [Google Scholar]
- 16.Eide DJ. Zinc transporters and the cellular trafficking of zinc. Biochimica Biophys Acta. 2006;1763:711–722. doi: 10.1016/j.bbamcr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Fukunaka A, Suzuki T, Kurokawa Y, Yamazaki T, Fujiwara N, Ishihara K, Migaki H, Okumura K, Masuda S, Yamaguchi-Iwai Y, Nagao M, Kambe T. Demonstration and characterization of the heterodimerization of ZnT5 and ZnT6 in the early secretory pathway. J Biol Chem. 2009;284:30798–30806. doi: 10.1074/jbc.M109.026435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaither LA, Eide DJ. Functional expression of the human hZIP2 zinc transporter. J Biol Chem. 2000;275:5560–5564. doi: 10.1074/jbc.275.8.5560. [DOI] [PubMed] [Google Scholar]
- 19.Harzer G, Kauer H. Binding of zinc to casein. Am J Clin Nutr. 1980;35:981–987. doi: 10.1093/ajcn/35.5.981. [DOI] [PubMed] [Google Scholar]
- 20.Hoac T, Lundh T, Purup S, Önning G, Sejrsen K, Åkesson B. Separation of selenium, zinc, and copper compounds in bovine whey using size exclusion chromatography linked to inductively coupled plasma mass spectrometry. J Agric Food Chem. 2007;55:4237–4243. doi: 10.1021/jf070169x. [DOI] [PubMed] [Google Scholar]
- 21.Huang L, Gitschier J. A novel gene involved in zinc transport is deficient in the lethal milk mouse. Nat Genet. 1997;17:292–297. doi: 10.1038/ng1197-292. [DOI] [PubMed] [Google Scholar]
- 22.Huang L, Kirschke CP, Zhang Y, Yu YY. The ZIP7 gene (Slc39a7) encodes a zinc transporter involved in zinc homeostasis of the golgi apparatus. J Biol Chem. 2005;280:15456–15463. doi: 10.1074/jbc.M412188200. [DOI] [PubMed] [Google Scholar]
- 23.Jena BP. Porosome: The secretory portal in cells. Biochemistry. 2009;48:4009–4018. doi: 10.1021/bi9002698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasper G, Armin A, Weiser A, Rump A, Sparbier K, Dahl E, Hartmann A, Wild P, Schwidetzky U, Castaños-Vélez E, Lehmann K. Expression levels of the putative zinc transporter LIV-1 are associated with a better outcome of breast cancer patients. Int J Cancer. 2005;117:961–973. doi: 10.1002/ijc.21235. [DOI] [PubMed] [Google Scholar]
- 25.Kelleher SL, Lonnerdal B. Zinc transporters in the mammary gland respond to marginal zinc and vitamin A intake during lactation in rats. J Nutr. 2002;132:3280–3285. doi: 10.1093/jn/132.11.3280. [DOI] [PubMed] [Google Scholar]
- 26.Kelleher SL, Lonnerdal B. Zip3 plays a major role in zinc uptake into mammary epithelial cells and is regulated by prolactin. Am J Physiol Cell Physiol. 2005;288:C1042–1047. doi: 10.1152/ajpcell.00471.2004. [DOI] [PubMed] [Google Scholar]
- 27.Kelleher SL, Lonnerdal B. Zn transporter levels and localization change throughout lactation in rat mammary gland and are regulated by Zn in mammary cells. J Nutr. 2003;133:3378–3385. doi: 10.1093/jn/133.11.3378. [DOI] [PubMed] [Google Scholar]
- 28.Kelleher SL, Lopez V, Lonnerdal B, Dufner-Beattie J, Andrews GK. Zip3 (Slc39a3) functions in zinc reuptake from the alveolar lumen in lactating mammary gland. Am J Physiol Regul Integr Comp Physiol. 2009;297:R194–201. doi: 10.1152/ajpregu.00162.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemire J, Mailloux R, Appanna VD. Zinc toxicity alters mitochondrial metabolism and leads to decreased ATP production in hepatocytes. J Appl Toxicol. 2008;28:175–182. doi: 10.1002/jat.1263. [DOI] [PubMed] [Google Scholar]
- 30.Linzell J, Mepham T, Peaker M. The secretion of citrate into milk. J Physiol. 1976;260:739–750. doi: 10.1113/jphysiol.1976.sp011541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci USA. 2006;103:13612–13617. doi: 10.1073/pnas.0606424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liuzzi JP, Bobo JA, Cui L, McMahon RJ, Cousins RJ. Zinc transporters 1, 2 and 4 are differentially expressed and localized in rats during pregnancy and lactation. J Nutr. 2003;133:342–351. doi: 10.1093/jn/133.2.342. [DOI] [PubMed] [Google Scholar]
- 33.Lönnerdal B, Stanislowski A, Hurley L. Isolation of a low molecular weight zinc binding ligand from human milk. J Inorg Biochem. 1980;12:71–78. doi: 10.1016/s0162-0134(00)80044-6. [DOI] [PubMed] [Google Scholar]
- 34.Lopez V, Kelleher SL. Zinc transporter-2 (ZnT2) variants are localized to distinct sub-cellular compartments and functionally transport zinc. Biochem J. 2009;422:43–52. doi: 10.1042/BJ20081189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez V, Kelleher SL. Zip6-attenuation promotes epithelial-to-mesenchymal transition in ductal breast tumor (T47D) cells. Exp Cell Res. 2010;316:366–375. doi: 10.1016/j.yexcr.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Matsuura W, Yamazaki T, Yamaguchi-Iwai Y, Masuda S, Nagao M, Andrews G, Kambe T. SLC39A9 (ZIP9) regulates zinc homeostasis in the secretory pathway: characterization of the ZIP subfamily I protein in vertebrate cells. Biosci Biotechnol Biochem. 2009;73:1142–1148. doi: 10.1271/bbb.80910. [DOI] [PubMed] [Google Scholar]
- 37.McCormick N, Velasquez V, Finney L, Vogt S, Kelleher SL. X-Ray fluorescence microscopy reveals accumulation and secretion of discrete intracellular zinc pools in the lactating mouse mammary gland. PLoS One. 2010;5:e11078. doi: 10.1371/journal.pone.0011078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menzies KK, Nicholas KR. The expression of [beta]-1,3 galactosyltransferase and [beta]-1,4 galactosyltransferase enzymatic activities in the mammary gland of the tammar wallaby (Macropus eugenii) during early lactation. Biochim Biophys Acta. 2007;1770:115–120. doi: 10.1016/j.bbagen.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Michalczyk A, Varigos G, Catto-Smith A, Blomeley RC, Ackland ML. Analysis of zinc transporter, hZnT4 (SIc30A4), gene expression in a mammary gland disorder leading to reduced zinc secretion into milk. Hum Genet. 2003;113:202–210. doi: 10.1007/s00439-003-0952-2. [DOI] [PubMed] [Google Scholar]
- 40.Neville M. Classic Studies of Mammary Development and milk Secretion: 1945 – 1980. J Mammary Gland Biol Neoplasia. 2009;14:193–197. doi: 10.1007/s10911-009-9151-7. [DOI] [PubMed] [Google Scholar]
- 41.Palmiter RD, Huang L. Efflux and compartmentalizatin of zinc by members of the SLC30 family of solute carriers. Pflugers Arch. 2004;447:744–751. doi: 10.1007/s00424-003-1070-7. [DOI] [PubMed] [Google Scholar]
- 42.Petris MJ, Mercer JFB. The Menkes protein (ATP7A:MNK) cycles via the plasma membrane both in basal and elevated extracellular copper using a C-termianl di-leucine endocytic signal. Hum Mol Genet. 1999;8:2107–2115. doi: 10.1093/hmg/8.11.2107. [DOI] [PubMed] [Google Scholar]
- 43.Picciano M, Guthrie H. Copper, iron, and zinc contents of mature human milk. Am J Clin Nutr 1976 Mar. 1976;29:242–254. doi: 10.1093/ajcn/29.3.242. [DOI] [PubMed] [Google Scholar]
- 44.Qian L, Seo Y, Lopez V, Kelleher S. ZNT2 is regulated by prolactin through activation of the JAK2-STAT5 signaling pathway. Am J Physiol Cell Physiol. 2008;297:C369–377. doi: 10.1152/ajpcell.00589.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seo Y, Kelleher S. Functional analysis of two single nucleotide polymorphisms in SLC30A2 (ZnT2): Implications for mammary gland function and breast disease in women. Physiol Genomics. 2010;42A:219–227. doi: 10.1152/physiolgenomics.00137.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seo Y, Lopez V, Kelleher S. A histidine-rich motif mediates mitochondrial localization of ZnT2 to modulate mitochondrial function. Am J Physiol Cell Physiol. 2011;300:C1479–1489. doi: 10.1152/ajpcell.00420.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shennan DB, Peaker M. Transport of milk constituents by the mammary gland. Physiological Rev. 2000;80:925–951. doi: 10.1152/physrev.2000.80.3.925. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki T, Ishihara K, Migaki H, Matsuura W, Kohda A, Okumura K, Nagao M, Yamaguchi-Iwai Y, Kambe T. Zinc transporters, ZnT5 and ZnT7, are required for the activation of alkaline phosphatases, zinc-requiring enzymes that are glycosylphosphatidylinositol-anchored to the cytoplasmic membrane. J Biol Chem. 2005;280:637–643. doi: 10.1074/jbc.M411247200. [DOI] [PubMed] [Google Scholar]
- 49.Taylor KM. A distinct role in breast cancer for two LIV-1 family zinc transporters. Biochem Soc Trans. 2008;036:1247–1251. doi: 10.1042/BST0361247. [DOI] [PubMed] [Google Scholar]
- 50.Taylor KM, Morgan HE, Smart K, Zahari NM, Pumford S, Ellis IO, Robertson JF, Nicholson RI. The emerging role of the LIV-1 subfamily of zinc transporters in breast cancer. Mol Med. 2007;13:396–406. doi: 10.2119/2007-00040.Taylor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor KM, Vichova P, Jordan N, Hiscox S, Hendley R, Nicholson RI. ZIP7-mediated intracellular zinc transport contributes to aberrant growth factor signaling in antihormone-resistant breast cancer cells. Endocrinology. 2008;149:4912–4920. doi: 10.1210/en.2008-0351. [DOI] [PubMed] [Google Scholar]
- 52.Walker C, Black R. Zinc for the treatment of diarrhoea: effect on diarrhoea morbidity, mortality and incidence of future episodes. Int J Epidemiol. 2010;39 (Suppl 1):i63–69. doi: 10.1093/ije/dyq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weaver BP, Dufner-Beattie J, Kambe T, Andrews GK. Novel zinc-responsive post-transcriptional mechanisms reciprocally regulate expression of the mouse Slc39a4 and Slc39a5 zinc transporters (Zip4 and Zip5) Biol Chem. 2007;388:1301–1312. doi: 10.1515/BC.2007.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zulak I, Keenan T. Citrate accumulation by a golgi apparatus-rich fraction from lactating bovine mammary gland. Int J Biochem. 1983;15:747–750. doi: 10.1016/0020-711x(83)90203-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.