Abstract
BACKGROUND
Children raised in institutional settings experience marked deprivation in social and environmental stimulation. This deprivation may disrupt brain development in ways that increase risk for psychopathology. Differential hemispheric activation of the frontal cortex is an established biological substrate of affective style that is associated with internalizing psychopathology. Previous research has never characterized the development of frontal EEG asymmetry in children or evaluated whether adverse rearing environments alter developmental trajectories.
METHODS
A sample of 136 children (mean age=23 months) residing in institutions in Bucharest, Romania and a sample of community controls (n=72) participated. Half of institutionalized children were randomized to a foster care intervention. Electroencephalogram (EEG) data were acquired at study entry and at ages 30, 42, and 96 months. A structured diagnostic interview of psychiatric disorders was completed at 54 months.
RESULTS
Children exhibited increases in right relative to left hemisphere frontal activation between the second and fourth years of life, followed by an increase in left relative to right hemisphere activation. Children reared in institutions experienced a prolonged period of increased right hemisphere activation and a blunted rebound in left frontal activation. Foster care placement was associated with improved developmental trajectories, but only among children placed before 24 months. The development trajectory of frontal EEG asymmetry in early childhood predicted internalizing symptoms at 54 months.
CONCLUSIONS
Exposure to adverse rearing environments can alter brain development, culminating in heightened risk for psychopathology. Interventions delivered early in life have the greatest potential to mitigate the long-term effects of these environments.
Keywords: institutionalization, deprivation, childhood adversity, brain development, frontal EEG asymmetry, electroencephalogram (EEG)
Frontal areas of the cerebral cortex are differentially lateralized for processing positive and negative stimuli and underlie behavioral and expressive responses to emotional information [1, 2]. The left frontal region is activated by positive emotional stimuli and promotes approach behavior, whereas the right frontal region is activated by negative stimuli and underlies withdrawal and avoidance behavior [1–4]. Activation of these neural systems can be assessed using electroencephalogram (EEG). Alpha power derived from scalp locations over the frontal cortex is used as a measure of regional activation [1, 5, 6].
Individual differences in relative hemispheric activation of the frontal cortex—as indexed by EEG alpha power—are associated with a variety of dispositional characteristics, including emotional reactivity, social behavior, and psychopathology [7]. Adults with frontal EEG asymmetry (FEA)—indexing right greater than left hemispheric activation—are more behaviorally inhibited [8] and experience greater negative affect in response to emotional stimuli than individuals without this pattern of asymmetry [9, 10]. FEA has been observed in adults with major depression [11–13], suicidality [14], and anxiety [15]. This pattern of FEA develops early in life and is associated in children with behavioral inhibition, social reticence, and heightened reactivity to maternal separation [16–20]. FEA is common in children of depressed mothers [21–23].
FEA has been conceptualized as a stable trait representing individual differences in affective style and approach/withdrawal responses to emotional stimuli [1, 7, 11, 24]. Indeed, FEA is stable over several weeks in adults [25]. We know virtually nothing, however, about how FEA develops in children. Children with right greater than left FEA at 3 months of age were observed to have a similar pattern of asymmetrical EEG activation at 3 years [21]. However, we are unaware of research that has examined developmental trajectories of FEA in children or identified predictors of individual differences in these trajectories.
Exposure to adverse rearing conditions early in life is one factor that may influence the development of FEA. Poor maternal caregiving in infancy is associated with right greater than left FEA in young children [26, 27], and this pattern has also been observed in maltreated adolescents [28]. Heritability estimates of FEA are quite low [29], which suggests substantial developmental plasticity in the neural systems underlying approach/withdrawal behavior. Although the development of FEA is likely shaped, at least in part, by environmental experiences, we are unaware of previous research that has examined this possibility using longitudinal data with repeated measurements of FEA.
We address this gap in the literature in the current report. Specifically, we examine developmental trajectories of FEA from infancy through middle childhood and determine whether variation in developmental trajectories is predicted by early environmental experiences. We also investigate whether changes in FEA over time predict internalizing psychopathology in early childhood. We examine these questions using data from the Bucharest Early Intervention Project (BEIP), a longitudinal study of children reared in institutional settings in Romania and a comparison sample of community-reared children. Institutional rearing is characterized by psychosocial deprivation and neglect [30] and has lasting effects on social development and mental health [30–36]. Prior work in this sample suggests that institutionalization is associated with abnormal neural development, as indexed by high low-frequency power and low high-frequency power in the EEG signal of children exposed to institutionalization [37]. If the environment does, in fact, alter the development of hemispheric activation patterns in the frontal cortex, we expect to observe these effects in this population.
Methods and Materials
Sample
The BEIP is a longitudinal study of a sample of children raised from early infancy in institutions in Bucharest, Romania. The BEIP is the only randomized controlled trial of foster care (FC) among institutionalized children [38]. A sample of 136 children (aged 6–30 months) was recruited from institutions in Bucharest. An age- and gender-matched sample of 72 community-reared children was recruited from pediatric clinics in Bucharest. Assessments of health, cognitive ability, and brain development were completed at baseline and at 30, 42, and 96 months. Psychiatric disorders were assessed at 54 months.
Participants were selected from each of the six institutions for young children in Bucharest. Physical examinations were completed on 187 children residing in these institutions. Of this group, 51 were excluded for medical reasons (e.g., Down syndrome) [38]. The remaining 136 children had lived in an institution for at least half of their lives (M=89.0%). Following baseline assessments, half of the children (n=68) residing in institutions were randomized to a FC intervention and half (N=68) remained in institutional care. No differences were found between the intervention and control group in gender distribution, age, birth weight, or percentage of life spent in the institution (Table 1). The mean age at FC placement was 22.97 months. By the 96-month assessment, 19 children were lost to follow-up, primarily due to adoption or reintegration with their biological parents. The study design and methods are described elsewhere [38].
Table 1.
Baseline Characteristics of Children in the Bucharest Early Intervention Project
| Community Control (n=72) | Foster Care (n=68) | Usual Care (n=68) | ||||
|---|---|---|---|---|---|---|
| Female, No. (%) | 41 | (56.9) | 33 | (49.3) | 35 | (51.5) |
| Age, mean (SD), months | ||||||
| Age at Institutionalization | -- | -- | 2.6 | (3.9) | 2.4 | (3.0) |
| Age at Study Entry | 19.3 | (7.1) | 20.9 | (7.1) | 20.8 | (7.7) |
| Birth Weight, mean (SD), grams | 3333 | (459.3) | 2733 | (576.2) | 2847 | (570.2) |
| Gestation Age, mean (SD), weeks | 37.8 | (1.4) | 37.0 | (2.4) | 37.6 | (1.5) |
| Head Circumference for age percentile, mean (SD), cm. | 52.4 | (25.6) | 32.3 | (28.1) | 25.2 | (22.7) |
| Height for age percentile, mean (SD), cm. | 53.6 | (27.7) | 25.7 | (22.5) | 26.9 | (23.1) |
| Weight for age percentile, mean (SD), kg. | 47.2 | (29.6) | 18.2 | (19.4) | 22.7 | (24.6) |
| Weight for height percentile, mean (SD), kg. | 54.0 | (28.0) | 30.1 | (27.2) | 37.3 | (29.6) |
| Duration of Institutionalization, mean (SD), weeks | -- | -- | 85.2 | (23.0) | 87.9 | (17.9) |
The BEIP was initiated with support from the Secretary of State for Child Protection in Romania. Study procedures were approved by local commissions on child protection in Bucharest, the Romanian ministry of health, an ethics committee including appointees from government and Bucharest University academic departments, and the institutional review boards of the institutions of the three principal investigators. A description of procedures employed to ensure ethical integrity has been published previously [39–41].
Intervention
Because FC was virtually non-existent in Bucharest at the beginning of the study, investigators created a network of foster homes with Romanian collaborators [38, 42]. Foster parents were recruited through advertising and were trained using a manual adapted for the study. FC was supported by social workers in Bucharest who received weekly consultation from U.S. clinicians. Social workers assisted foster parents in managing problem behaviors and facilitated the establishment of warm, supportive, and committed relationships with their foster children. The intervention is described in detail elsewhere [38, 42].
EEG Methods
Children completed resting electroencephalogram (EEG) assessment at baseline and at 30, 42, and 96 months. EEG was recorded using a lycra stretchable cap with tin electrodes sewn into it. EEG was recorded at 12 scalp sites (F3, F4, Fz, C3, C4, P3, P4, Pz, O1, O2, T7, and T8) and the right and left mastoids. EEG was collected with reference to the vertex (Cz). An anterior midline site (AFz) served as the ground. The scalp underlying each electrode was gently abraded and electrolytic conducting gel was inserted into the space between the scalp and electrode. Impedances measured at each electrode site were considered acceptable if they were ≤10 Ωs. Channels were digitized at 512 Hz using a 12-bit A/D converter (±2.5 V input range) and Snap-Master acquisition software (HEM Data Corporation, Southfield, MI). One channel of vertical electrooculogram (EOG) was recorded using tin electrodes placed above and below the left eye to record blinks and eye movements. The EEG and EOG signals were amplified by factors of 5000 and 2500, respectively, using custom amplifiers from SA Instrumentation Company (San Diego, CA). Amplifier filter settings were 0.1 Hz (high pass) and 100 Hz (low pass). A 50-μV 10-Hz signal was input into each of the channels prior to EEG recording and recorded for calibration purposes.
A standard protocol used for EEG collection in young children was used at baseline, 30, and 42 months [43]. An experimenter placed numerous colored balls in a bingo wheel and spun the wheel for nine 10-second trials. After each trial, the experimenter stopped spinning the wheel and changed the balls to maintain the child’s attention. The EEG signal was recorded for the entire 3-minute period, but only data from epochs in which the wheel was being spun were analyzed. At 96 months, EEG was recorded while the children sat quietly in a chair, alternating one-minute epochs of eyes open and closed for six minutes. Data presented here are from epochs with eyes closed (results were unchanged with eyes open).
Processing and analysis of the EEG signal was performed using the EEG Analysis System from James Long Company (Caroga Lake, NY). Epochs containing blinks or eye movement or in which the EEG signal exceeded ±250-μV were excluded from analysis. EEG channels were re-referenced to an average mastoids reference and spectrally analyzed using a discrete Fourier transform with a 1-sec Hanning window with 50% overlap between adjacent windows. Consistent with prior research specifying EEG frequency bands in early childhood [43–45], spectral power in the following frequency bands was computed at baseline, 30, and 42 months: theta (3–5 Hz), alpha (6–9 Hz), and beta (10–18 Hz). At 96 months, power was computed using different age-appropriate frequency bands: theta (4–6Hz), alpha (7–12Hz), and beta (13–20Hz).
We computed a standard metric of FEA [1, 5, 11] using the left (F3) and right electrodes (F4) over frontal scalp regions. Asymmetry was calculated by subtracting the natural logarithm of power in the left frontal lead from that in the right lead (ln [F4] – ln [F3]). Because alpha power is inversely associated with cortical activation, values above 0 reflect greater activation in left relative to right frontal regions and values below 0 reflect greater activation in right relative to left frontal regions. We computed an asymmetry index for homologous parietal leads (ln [P4] – ln [P3]) to evaluate whether observed associations were specific to frontal regions [5, 26, 27].
EEG data at baseline were collected from 166 children who were at least 9 months of age, and from 105, 90, and 143 children at the 30-month, 42-month, and 96-month assessments, respectively. EEG data were unavailable from a subset of children at each time point because of fussiness during placement of the cap, parent refusal, or excessive noise across channels. There were no significant group differences in the rate of attrition or data loss at any time point.
Psychiatric Assessment
Psychiatric disorders at 54 months were assessed using a structured diagnostic interview for young children, the Preschool Age Psychiatric Assessment (PAPA) [46, 47]. This instrument collects information from a caregiver and generates DSM-IV diagnoses [48]. The PAPA has comparable reliability as diagnostic interviews utilized with older children [46]. The interview was translated into Romanian and back-translated into English. Biological or foster mothers completed the assessment for non-institutionalized children. An institutional caregiver provided information for children living in institutions. Caregivers who worked with the child regularly and knew the child well were selected to complete the interview. If the child had a favorite caregiver, agreed upon by staff consensus, that caregiver completed the assessment.
Statistical Analysis
We used multilevel modeling to characterize the development of FEA over time and to identify predictors. A series of two-level models (observations over time nested within persons) were estimated. This approach allowed us to simultaneously estimate the variance in FEA both within and between individuals over time [49]. We first estimated an unconditional growth model that predicted FEA by Time, with baseline coded as zero, and subsequent time points coded as the number of months from baseline. This model was specified as follows:
In this model, π0i (the intercept) represents the level of FEA at baseline, π1i is the effect of Time, and eti represents time-specific residual variance in FEA for child i at time t. β00 represents the average FEA at baseline across children, β10 represents the average slope (rate of change over time), and r0i and r1i represent the random effects, or individual deviations from these mean values. We added a quadratic term for Time to the model to determine the functional form of the growth trajectory and tested the difference in model fit between the linear and quadratic model using a chi-square test. The quadratic model was a better fit to the data (see Results) and was specified as follows:
In this model β20 represents average quadratic growth across children, which was modeled as a fixed effect (i.e., did not vary across children). Including a random effect to the quadratic term did not significantly improve model fit. With a quadratic effect of Time that is equal for all individuals, the linear slope is interpreted as the rate of linear change at the intercept (because it is the effect of π1i when all other predictors equal zero). This effectively determines the “tilt” of the curve defined by the quadratic term. However, because the quadratic effect was found to be equal for all individuals, variability in the linear slope captures all individual differences in the rate of change in FEA over time.
We next examined Level 2 (time-invariant) predictors of FEA. Specifically, we examined the association of institutional rearing with FEA intercepts (value at baseline) and slopes (linear change over time). This model was specified as follows:
In this model, β01 represents the effect of institutionalization on FEA at baseline, and β11 represents the effect of institutionalization on the rate of linear change in FEA over time. We also evaluated whether, among the institutionalized group, the FC intervention and timing of placement predicted FEA slopes. Multilevel modeling was conducted using the Hierarchical Linear Modeling (HLM 6.0) software system [50] using full maximum likelihood estimation with robust standard errors and heteroscedastic Level 1 time-specific residuals.
Finally, we evaluated whether changes in FEA from baseline to 42 months were related to psychopathology at 54 months. Linear regression models were estimated that included terms for baseline and 42-month FEA to evaluate whether residualized change in FEA predicted 54-month psychopathology. Analyses controlled for birth weight and head circumference.
RESULTS
Development of Frontal EEG asymmetry
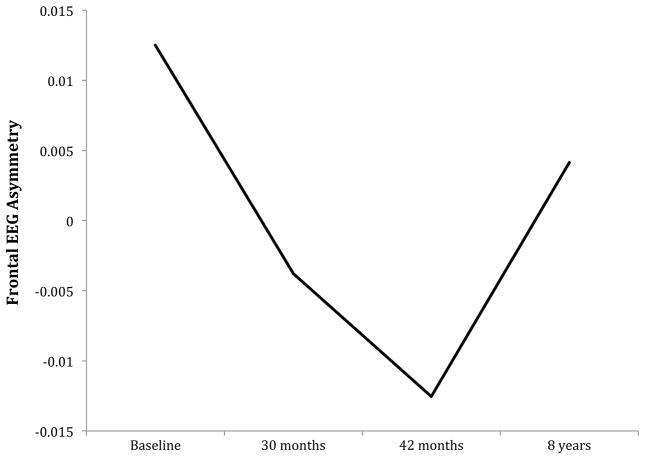
We plotted the average values for FEA at each assessment for the entire sample, which suggested a non-linear growth trajectory of FEA over time (Figure 1). To confirm this, we compared the fit of an unconditional model that included only Time as a Level 1 predictor to a model that included Time and Time2. The model that included a quadratic term was a better fit to the data, χ2 (3) = 31.7, p < .001. The quadratic model indicated a significant effect of both Time, β10 = −.05, p < .001, and Time2, β20 = .01, p < .001. This model also revealed significant variance components for the intercept, r0i = .08, p < .001, and slope, r1i = .03, p < .001, of FEA, indicating significant variability in baseline values and changes in FEA over time across children. Correlation coefficients for FEA at each assessment are shown in Table 2.
Figure 1.
Asymmetry in alpha power in frontal cortical areas (see Methods section for details) in entire sample. Higher alpha power reflects lower cortical activity; asymmetry values greater than zero reflect left greater than right frontal activity and values less than zero reflect right greater than left frontal activity.
Table 2.
Correlations of frontal EEG asymmetry across development.
| Baseline1 | 30 months | 42 months | 96 months | |
|---|---|---|---|---|
| Baseline | -- | .22* | .18* | −.07 |
| 30 months | -- | -- | .12 | .05 |
| 42 months | -- | -- | -- | .01 |
| 96 months | -- | -- | -- | -- |
Children ranged in age from 6–30 months at baseline.
Significant at .05 level, 2-sided test
Institutional Rearing and the Development of Frontal EEG asymmetry
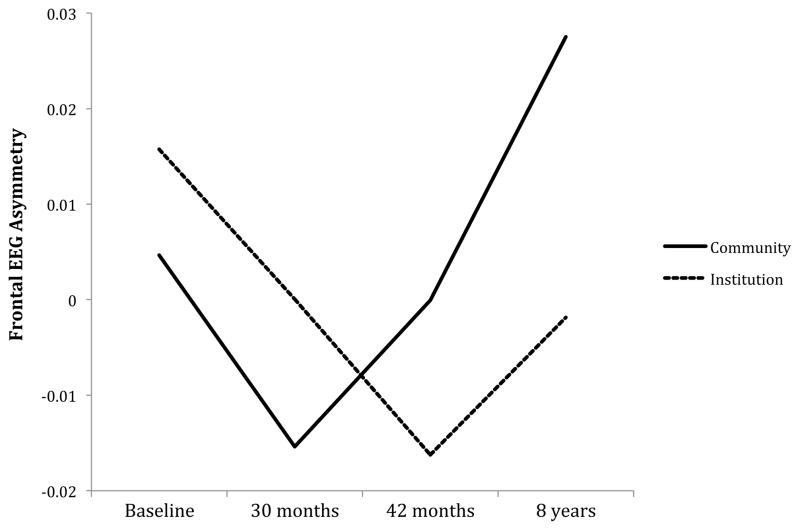
To determine whether institutionalization was associated with the development of FEA, we added a dummy variable coding whether each child had been raised in an institution at Level 2. This model revealed significant associations between institutionalization and FEA intercepts, β01 = .04, p = .024, and slopes, β11 = −.02, p = .014. Although children raised in institutions had left greater than right frontal activity at baseline compared to control children, they exhibited a growth trajectory characterized by increasing frontal EEG activity in the right relative to the left hemisphere. Visual inspection of the growth trajectories revealed that children in both groups experienced an initial increase in right relative to left hemisphere frontal EEG activity followed by an increase in left relative to right hemisphere activity (Figure 2). The initial increase in right hemisphere frontal EEG activity occurred for a longer period of time in institutionalized children, however, and the subsequent rebound in left frontal EEG activity was less marked for children exposed to institutionalization. By 96 months, children raised in the community exhibited fairly pronounced FEA characterized by left greater than right hemisphere EEG activity, whereas institutionalized children exhibited a pattern of right greater than left hemispheric activity (Table 3).
Figure 2.
Asymmetry in alpha power in frontal cortical areas (see Methods section for details), separately for children reared in institutional settings and children raised by families in the community. Higher alpha power reflects lower cortical activity; asymmetry values greater than zero reflect left greater than right frontal activity and values less than zero reflect right greater than left frontal activity.
Table 3.
Alpha absolute power in frontal scalp regions and frontal EEG asymmetry in children exposed to institutionalization and children raised in the community across development.
| Baseline1 | 30 months | 42 months | 96 months | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | Mean | (SD) | Mean | (SD) | |
| Absolute Alpha Power in Right Frontal Scalp Region (F4) | ||||||||
| Institutionalized | 3.64 | (.57) | 3.82 | (.41) | 3.78 | (.39) | 56.96 | (32.4) |
| Foster Care Group | 3.73 | (.50) | 3.88 | (.39) | 3.84 | (.38) | 60.48 | (37.19) |
| Institutional Care Group | 3.61 | (.59) | 3.81 | (.40) | 3.76 | (.42) | 53.38 | (26.62) |
| Community Controls | 3.82 | (.45) | 3.94 | (.41) | 3.83 | (.47) | 66.55 | (28.48) |
|
| ||||||||
| Absolute Alpha Power in Left Frontal Scalp Region (F3) | ||||||||
| Institutionalized | 3.60 | (.61) | 3.82 | (.40) | 3.85 | (.41) | 56.95 | (31.07) |
| Foster Care Group | 3.69 | (.48) | 3.88 | (.37) | 3.89 | (.41) | 60.00 | (33.55) |
| Institutional Care Group | 3.58 | (.65) | 3.79 | (.35) | 3.81 | (.45) | 53.83 | (28.32) |
| Community Controls | 3.80 | (.47) | 4.00 | (.42) | 3.83 | (.41) | 65.77 | (29.37) |
|
| ||||||||
| EEG Frontal Asymmetry | ||||||||
| Institutionalized | .010 | (.08) | −.000 | (.05) | −.016 | (.07) | −.002 | (.09) |
| Foster Care Group | .005 | (.05) | −.000 | (.04) | −.018 | (.07) | .002 | (.08) |
| Institutional Care Group | .016 | (.05) | −.000 | (.04) | −.014 | (.07) | −.006 | (.09) |
| Community Controls | .004 | (.04) | −.015 | (.03) | .000 | (.05) | .028 | (.09) |
|
| ||||||||
| Proportion of Children with Right Greater than Left Frontal Activation | ||||||||
| N | %2 | N | %2 | N | %2 | N | %2 | |
| Institutionalized | 54 | 41.5 | 57 | 50.0 | 61 | 62.2 | 51 | 50.5 |
| Foster Care Group | 28 | 44.4 | 28 | 47.5 | 31 | 59.6 | 26 | 49.1 |
| Institutional Care Group | 26 | 38.8 | 29 | 52.7 | 30 | 65.2 | 25 | 52.1 |
| Community Controls | 20 | 37.7 | 25 | 65.8 | 15 | 51.7 | 10 | 38.5 |
The mean age of participants at baseline was 22.97 months.
Proportion represents the number of children with right greater than left frontal activation divided by the total number of children with valid EEG data at that time point.
Institutionalization was unassociated with baseline values of parietal EEG asymmetry, β01 = .01, p = .669, or with changes in parietal asymmetry over time, β11 = −.01, p = .774, suggesting regional specificity.
Foster Care Effects on the Development of Frontal EEG asymmetry
We evaluated whether the intervention was associated with FEA growth trajectories by including a variable for FC at Level 2. This model was estimated only among children exposed to institutionalization. We were interested only in the association of FC with the slope of FEA, because all children were still in institutional care at baseline. The FC intervention was unrelated to the growth trajectory of FEA over time, β11 = .01, p = .663. Next, we examined whether FC impacted the development of FEA among children placed at an early age. To do so, we created three dummy variables coding different cut-points for timing of placement: 6, 12, and 24 months. These variables were added one at a time to the model. This analysis revealed a significant effect of the FC intervention on FEA among children placed before 24 months, β11= .02, p = .039. Institutionalized children who were placed into FC before 24 months exhibited a more favorable growth trajectory of FEA than children placed after 24 months (i.e., a shorter increase in right frontal activity relative to left).
FC was unassociated with baseline values of parietal EEG asymmetry, β01 = −.00, p = .980, or with changes in parietal EEG asymmetry over time, β11 = −.01, p = .531. Timing of placement was also unassociated with changes in parietal EEG asymmetry (p-values > .300).
Changes in Frontal EEG asymmetry and Psychopathology
To determine whether changes in FEA were associated with the onset of psychopathology, we predicted internalizing symptoms (anxiety, depression) and externalizing symptoms (ADHD, oppositional defiant disorder, and conduct disorder) at 54 months in a model that included baseline and 42-month FEA. Controlling for baseline asymmetry, 42-month FEA was significantly associated with internalizing symptoms at 54 months, β= −9.4, p = .041, but not externalizing symptoms, β = −5.5, p = .589.
DISCUSSION
Asymmetrical hemispheric EEG activation recorded over frontal scalp regions is a well-established biological substrate of affective style and approach/withdrawal behavior associated with mental health and social functioning [1–3, 5, 6, 11]. Although asymmetrical EEG activation in the frontal cortex has been documented in young children [5, 21, 23], we are unaware of a previous study that examined the developmental trajectory of FEA or identified factors that influence patterns of development. We provide novel evidence for developmental changes in FEA from infancy through middle childhood. Specifically, we find an increase in right relative to left hemisphere frontal EEG activation between the second and fourth years of life followed by a subsequent increase in left relative to right hemisphere activation into middle childhood. Moreover, our findings suggest that the caregiving environment early in life influences developmental trajectories of FEA. The lack of associations between the rearing environment and parietal EEG asymmetry suggests regional specificity to neural circuits in the frontal cortex. FEA development from infancy through early childhood is associated with internalizing symptoms, suggesting that the developmental course of relative hemispheric activation in the frontal cortex has important implications for mental health.
To our knowledge, we provide the first evidence documenting the developmental trajectory of FEA in early to middle childhood. Our results indicate that children exhibit a relatively greater increase in right relative to left frontal EEG activation during the second and third years of life. EEG activation in the right frontal cortex underlies withdrawal behavior in response to aversive stimuli [1–3], and in infants this pattern has been observed during the approach of a stranger and following maternal separation [4, 17]. Although the specific neural mechanisms underlying FEA are unknown, it has recently been proposed that asymmetry is related to affective style because of the role of the left prefrontal cortex in inhibiting the amygdala [51]. Pizzagalli and colleagues [52] find resting alpha activity in the left dorsolateral prefrontal and medial orbitofrontal cortex underlie motivated approach behavior in response to appetitive stimuli. Increased activation in the right frontal cortex beginning at age two may reflect an adaptive developmental change in withdrawal tendencies to novelty as the child’s experiences expand to encompass environments outside the home and interactions with people other than their primary caregivers. A concomitant increase in numerous aspects of effortful control—including delaying and inhibiting behavior—has also been observed among children during this developmental period [53] and is thought to reflect maturation of the prefrontal cortex [54]. Together with our findings, these patterns suggest normative increases in behavioral inhibition in two- to three-year-olds that are mediated by developmental changes in the prefrontal cortex.
We also demonstrate an association between early-life caregiving experiences and the development of FEA. First, institutionalized children exhibited greater left than right frontal activation at baseline. This finding indicates a greater tendency for approach behavior among institutionalized children during infancy, a period in which responsive caregiving is critical to scaffold neural development. This pattern may reflect an adaptive response to the institutional environment where there is a scarcity of individualized social attention from caregivers. Second, children exposed to institutional rearing experienced a prolonged period of increased right relative to left hemisphere EEG activation in early childhood and a blunted rebound in left frontal EEG activity. By age 96 months, children raised in the community exhibited considerably greater left relative to right frontal activation, whereas children reared in institutions exhibited greater activation in the right frontal cortex compared to the left. These differences in brain development likely reflect a greater propensity for withdrawal behavior in response to novelty among institutionalized relative to non-institutionalized children [5, 8]. As children raised in institutions become older, they accumulate increasing experiences of insensitive unresponsive caregiving [55]; thus, withdrawal from novel stimuli may be an adaptive response to an aversive environment. The neural underpinnings of this pattern of withdrawal, however, are associated with increased risk of internalizing psychopathology. Although right greater than left activation appears normative in early childhood, a delayed rebound of left frontal activation may be a marker of psychopathology risk.
Our results are consistent with evidence linking poor maternal caregiving in infancy to FEA in children [26, 27] as well as an extensive literature linking poor caregiving to elevated stress reactivity in animals [56–59]. We extend this work by showing that an adverse rearing environment is associated with the developmental trajectoryof relative hemispheric activation in the frontal cortex. These findings add to a growing literature documenting that early deprivation alters brain development in ways that increase risk for psychopathology. Abnormalities in the neural processing of facial emotion have frequently been observed in maltreated children [60, 61], and we recently found that these deficits predict the onset of externalizing problems in institutionally-reared children [62]. In previous work we also observed abnormal development of high-frequency brain electrical activity among children reared in institutions—suggesting a delay in maturation of the cerebral cortex—that predicted the onset of ADHD [63]. That pattern raises the question of whether the frequency band used to define alpha is appropriate for the institutionalized group. Although our data cannot speak directly to this issue, the frequency band used to define alpha from baseline to 42 months incorporates normative alpha in children aged 5–51 months [43], increasing confidence that this frequency band is appropriate. The current findings build on previous work by documenting a neurodevelopmental mechanism linking early-life deprivation to the onset of internalizing psychopathology. Together, these studies provide compelling evidence suggesting that adverse environmental conditions in early life may become biologically embedded, resulting in lasting changes in neural development that may ultimately manifest as psychopathology.
FC placement had a positive effect on FEA trajectories, but only among children placed before 24 months. This suggests that the brain circuits underlying hemispheric EEG activation in the frontal cortex are responsive to environmental input relatively early in life, and become less susceptible to these influences around two years of age. Early-life experiences have been important predictors of FEA in previous studies [23, 26–27]. The beneficial effects of FC for children placed before 24 months is consistent with previous reports from this sample which also found substantial benefit for children placed before 24 months in other developmental domains, including cognitive ability and attachment security [64, 65]. Children placed before 24 months also exhibit greater increases in alpha power by age 8 relative to children placed later [66]. Together, these findings suggest a possible sensitive period during which the environment has a particularly pronounced effect on brain development—particularly the development of high-frequency brain electrical activity and hemispheric activation patterns in the frontal cortex. An important caveat related to these findings is our inability to disentangle age at placement from duration of institutionalization. One explanation for the observed results is that the neural circuits underlying asymmetrical frontal EEG activation are most sensitive to environmental input during the first two years of life; an alternative explanation is that these neural systems are more profoundly affected in children who experience more chronic deprivation and therefore require additional time to “catch-up.” These effects are difficult to disentangle in our sample but highlight an important topic for future research. At the very least, our findings highlight the importance of intervening as early as possible to reduce the long-term effects of adverse rearing environments on brain development.
Deprived rearing environments are associated with a developmental trajectory of FEA characterized by a prolonged period of greater activation in the right hemisphere as compared to the left. This developmental pattern predicts the onset of internalizing psychopathology in early childhood. Exposure to adverse caregiving environments early in life can alter the trajectory of brain development in children, culminating in heightened risk for psychopathology. Interventions delivered early in the life-course have the greatest potential to mitigate the long-term effects of these adverse environments.
Acknowledgments
This research was supported by a grant from the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development (Charles A. Nelson, Network Chair) and research support from the National Institute of Mental Health (MH091363 to Charles A. Nelson and MH092526 to Katie A. McLaughlin). We thank Sebastian Koga for overseeing the project in Romania; Hermi Woodward and members of the MacArthur Foundation Research Network on Early Experience and Brain Development for input regarding the conceptualization, design, and implementation of the project; the caregivers and children who participated in this project; and the Bucharest Early Intervention Project staff for their tireless work on our behalf. We also thank Kevin King for expert consultation on statistical analysis.
Footnotes
Disclosure: The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davidson RJ. Emotion and affective style: Hemispheric substrates. Psychological Science. 1992;3:39–43. [Google Scholar]
- 2.Davidson RJ, Fox NA. Asymmetrical brain activity discriminates between positive and negative affective stimuli in human infants. Science. 1982;218(4578):1235–1237. doi: 10.1126/science.7146906. [DOI] [PubMed] [Google Scholar]
- 3.Davidson RJ, Ekman P, Saron CD, Senulis JA, Friesen WV. Approach-withdrawal and cerebral asymmetry: Emotional expression and brain physiology. Journal of Personality and Social Psychology. 1990;58:330–341. [PubMed] [Google Scholar]
- 4.Fox NA, Davidson RJ. Electronencephalogram asymmetry in response to the approach of a stranger and maternal separation in 10-month-old infants. Developmental Psychology. 1987;23:233–240. [Google Scholar]
- 5.Fox NA. If it’s not left, it’s right. American Psychologist. 1991;46:863–872. doi: 10.1037//0003-066x.46.8.863. [DOI] [PubMed] [Google Scholar]
- 6.Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain and Cognition. 1992;20:121–151. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- 7.Davidson RJ. Cerebral asymmetry and emotion: Conceptual and methodological considerations. Cognition and Emotion. 1993;7:115–138. [Google Scholar]
- 8.Sutton S, Davidson RJ. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological Science. 1997;8:204–210. [Google Scholar]
- 9.Tomarken AJ, Davidson RJ, Henriques JB. Resting frontal brain asymmetry predicts affective responses to films. Journal of Personality and Social Psychology. 1990;59:791–801. doi: 10.1037//0022-3514.59.4.791. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler RE, Davidson RJ, Tomarken AJ. Frontal brain asymmetry and emotional reactivity: A biological substrate of affective style. Psychophysiology. 1993;30:82–89. doi: 10.1111/j.1469-8986.1993.tb03207.x. [DOI] [PubMed] [Google Scholar]
- 11.Gotlib IH, Ranganathand C, Rosenfeld JP. Frontal EEG alpha asymmetry, depression, and cognitive functioning. Cognition and Emotion. 1998;12:449–478. [Google Scholar]
- 12.Bell IR, Schwartz GE, Hardin EE, Baldwin CM, Kline JP. Differential resting quantitative electroencephalographic alpha patterns in women with environmental chemical intolerance, depressives, and normals. Biological Psychiatry. 1998;43:376–388. doi: 10.1016/s0006-3223(97)00245-x. [DOI] [PubMed] [Google Scholar]
- 13.Henriques JB, Davidson RJ. Regional brain electrical asymmetries discriminate between previously depressed and healthy control subjects. Journal of Abnormal Psychology. 1990;99:22–31. doi: 10.1037//0021-843x.99.1.22. [DOI] [PubMed] [Google Scholar]
- 14.Graae F, Tenke C, Bruder G, Rotheram MJ, Piacentini J, Castro-Blanco D, Leite P, Towey J. Abnormality of EEG alpha asymmetry in female adolescent suicide attempters. Biological Psychiatry. 1996;40:706–713. doi: 10.1016/0006-3223(95)00493-9. [DOI] [PubMed] [Google Scholar]
- 15.Blackhart GC, Minnix JA, Kline JP. Can EEG asymmetry patterns predict future development of anxiety and depression? A preliminary study. Biological Psychology. 2006;72:46–50. doi: 10.1016/j.biopsycho.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Fox NA, Coplan RJ, Rubin KH, Porges SW, Calkins SD, Long JM, Marshall TR, Stewart S. Frontal activation asymmetry and social competence at four years of age. Child Development. 1995;66:1770–1784. [PubMed] [Google Scholar]
- 17.Davidson RJ, Fox NA. Frontal brain asymmetry predicts infants’ response to maternal separation. Journal of Abnormal Psychology. 1989;98:127–131. doi: 10.1037//0021-843x.98.2.127. [DOI] [PubMed] [Google Scholar]
- 18.Henderson HA, Marshall PJ, Fox NA, Rubin KH. Psychophysiological and behavioral evidence for varying forms and functions of nonsocial behavior in preschoolers. Child Development. 2004;75:251–263. doi: 10.1111/j.1467-8624.2004.00667.x. [DOI] [PubMed] [Google Scholar]
- 19.Shankman SA, Tenke CE, Bruder GE, Durbin CE, Hayden EP, Klein DN. Low positive emotionality in young children: Association with EEG asymmetry. Development and Psychopathology. 2005;17:85–98. doi: 10.1017/s0954579405050054. [DOI] [PubMed] [Google Scholar]
- 20.Buss KA, Malmstadt Schumacher JR, Dolski I, Kalin NH, Goldsmith HH, Davidson RJ. Right frontal brain activity, cortisol, and withdrawal behavior in 6-month olds. Behavioral Neuroscience. 2003;117:11–20. doi: 10.1037//0735-7044.117.1.11. [DOI] [PubMed] [Google Scholar]
- 21.Jones NA, Field T, Davalos M, PIckens J. EEG stability in infants/children of depressed mothers. Child Psychiatry and Human Development. 1997;28:59–70. doi: 10.1023/a:1025197101496. [DOI] [PubMed] [Google Scholar]
- 22.Field T, Fox NA, PIckens J, Nawrocki T. Relative right frontal EEG activation in 3- to 6-month old infants of “depressed mothers”. Developmental Psychology. 1995;31:358–363. [Google Scholar]
- 23.Jones NA, Field T, Fox NA, Lundy B, Davalos M. EEG activation in 1-month-old infants of depressed mothers. Development and Psychopathology. 1997;9:491–505. doi: 10.1017/s0954579497001260. [DOI] [PubMed] [Google Scholar]
- 24.Vuga M, Fox NA, Cohn JF, George CJ, Levenstein RM, Kovacs M. Long-term stability of frontal electronencephalographic asymmetry in adults with a history of depression and controls. International Journal of Psychophysiology. 2006;59:107–115. doi: 10.1016/j.ijpsycho.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Tomarken AJ, Davidson RJ, Wheeler RE, Kinney L. Psychometric properties of resting anterior EEG: Temporal stability and internal consistency. Psychophysiology. 1992;29:576–592. doi: 10.1111/j.1469-8986.1992.tb02034.x. [DOI] [PubMed] [Google Scholar]
- 26.Hane AA, Henderson HA, Reeb-Sutherland BC, Fox NA. Ordinary variations in human maternal caregiving in infancy and biobehavioral development in early childhood: A follow-up study. Developmental Psychobiology. 2010;52:1–10. doi: 10.1002/dev.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hane AA, Fox NA. Ordinary variations in maternal caregiving influence human infants’ stress reactivity. Psychological Science. 2006;17:550–556. doi: 10.1111/j.1467-9280.2006.01742.x. [DOI] [PubMed] [Google Scholar]
- 28.Miskovic V, Schmidt LA, Georgiades K, Boyle MH, MacMillan HL. Stability of resting frontal electronencelphalogram (EEG) asymmetry and cardiac vagal tone in female adolescents exposed to child maltreatment. Developmental Psychobiology. 2009;51:474–487. doi: 10.1002/dev.20387. [DOI] [PubMed] [Google Scholar]
- 29.Anokhin AP, Heath AC, Myers E. Genetic and environmental influences on frontal EEG asymmetry: A twin study. Biological Psychology. 2006;71:289–295. doi: 10.1016/j.biopsycho.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson CA. A neurobiological perspective on early human deprivation. Child Development Perspectives. 2007;1:13–18. [Google Scholar]
- 31.Rutter M, Kreppner JM, O’Connor TG English Romanian Adoptees (ERA) Study Team. Specificity and heterogeneity in children’s responses to profound institutional privation. British Journal of Psychiatry. 2001;17:97–103. doi: 10.1192/bjp.179.2.97. [DOI] [PubMed] [Google Scholar]
- 32.Rutter M English Romanian Adoptees (ERA) Study Team. Developmental catchup, and deficit, following adoption after severe global early privation. Journal of Child Psychology and Psychiatry. 1998;39:465–476. [PubMed] [Google Scholar]
- 33.O’Connor T, Marvin RS, Rutter M, Olrick JT, Britner PA The English and Romanian Adoptees Study Team. Child-parent attachment following early institutional deprivation. Development and Psychopathology. 2003;15:19–38. doi: 10.1017/s0954579403000026. [DOI] [PubMed] [Google Scholar]
- 34.Kreppner JM, Rutter M, Beckett C, Castle J, Colvert E, Groothues C, Hawkins A, O’Connor TG, Steves SE, Sonuga-Barke EJS. Normality and impairment following profound early institutional deprivation: A longitudinal follow-up into early adolescence. Developmental Psychology. 2007;43:931–946. doi: 10.1037/0012-1649.43.4.93. [DOI] [PubMed] [Google Scholar]
- 35.Zeanah CH, Egger HL, Smyke AT, Nelson CA, Fox NA, Marshall PJ, Guthrie D. Institutional rearing and psychiatric disorders in Romanian preschool children. American Journal of Psychiatry. 2009;166:777–785. doi: 10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]
- 36.Zeanah CH, Smyke AT, Koga SF, Carlson E The Bucharest Early Intervention Project Core Group. Attachment in institutionalized and community children in Romania. Child Development. 2005;76:1015–1028. doi: 10.1111/j.1467-8624.2005.00894.x. [DOI] [PubMed] [Google Scholar]
- 37.Marshall PJ, Fox NA the BEIP Core Group. A comparison of the electroencephalogram between institutionalized and community children. Journal of Cognitive Neuroscience. 2004;16:1327–1338. doi: 10.1162/0898929042304723. [DOI] [PubMed] [Google Scholar]
- 38.Zeanah CH, Nelson CB, Fox NA, Smyke AT, Marshall PJ, Parker SW, Koga S. Designing research to study the effects of institutionalization on brain and behavioral development: The Bucharest Early Intervention Project. Development and Psychopathology. 2003;15:885–907. doi: 10.1017/s0954579403000452. [DOI] [PubMed] [Google Scholar]
- 39.Miller FG. The randomzied controlled trial as a demonstration project: An ethical perspective. American Journal of Psychiatry. 2009;166:743–745. doi: 10.1176/appi.ajp.2009.09040538. [DOI] [PubMed] [Google Scholar]
- 40.Millum J, Emanuel EJ. The ethics of international research with abandoned children. Science. 2007;318:1874–1875. doi: 10.1126/science.1153822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeanah CH, Koga SF, Simion B, Stanescu A, Tabacaru CL, Fox NA, Nelson CA. Ethical considerations in international research collaboration: The Bucharest Early Intervention Project. Infant Mental Health Journal. 2006;27:559–576. doi: 10.1002/imhj.20107. [DOI] [PubMed] [Google Scholar]
- 42.Smyke AT, Zeanah CH, Fox NA, Nelson CA. A new model of foster care for young children: the Bucharest early intervention project. Child and Adolescent Psychiatric Clinics of North America. 2009;18:721–734. doi: 10.1016/j.chc.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology. 2002;113:1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- 44.Gasser T, Rousson V, Schreiter Gasser U. EEG power and coherence in children with educational problems. Journal of Clinical Neurophysiology. 2003;20:273–282. doi: 10.1097/00004691-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Cochin S, Barthelemy C, Roux S, Martinea J. Electroencephalographic activity during perception of motion in childhood. European Journal of Neuroscience. 2001;13:1791–1796. doi: 10.1046/j.0953-816x.2001.01544.x. [DOI] [PubMed] [Google Scholar]
- 46.Egger HL, Erkanli A, Keeler G, Potts E, Walter BK, Angold A. Test-retest reliability of the Preschool Age Psychiatric Assessment (PAPA) Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45:538–549. doi: 10.1097/01.chi.0000205705.71194.b8. [DOI] [PubMed] [Google Scholar]
- 47.Egger HL, Ascher BH, Angold A. The Preschool Age Psychiatric Assessment: Version 1.1. Department of Psychiatry and Behavioral Sciences, Center for Developmental Epidemiology; Duke University Medical Center; Durham, NC: 1999. [Google Scholar]
- 48.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 1994. (DSM-IV) [Google Scholar]
- 49.Raudenbush S, Bryk A. Hierarchical linear models: Applications and data analysis methods. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- 50.Raudenbush S, Bryk A, Cheong YF, Congdon R, du Toit M. HLM 6: Hierarchical linear and nonlinear modeling. Scientific Software International; Lincolnwood, IL: 2004. [Google Scholar]
- 51.Davidson JRT. Affective style, psychopathology, and resilience: brain mechanisms and plasticity. American Psychologist. 2000;55:1196–1214. doi: 10.1037//0003-066x.55.11.1196. [DOI] [PubMed] [Google Scholar]
- 52.Plaisier I, de Brujin JG, de Graaf R, ten Have M, Beekman AT, Penninx BW. The contribution of working conditions and social support to the onset of depressive and anxiety disorders among male and female employees. Social Science and Medicine. 2007;64:401–410. doi: 10.1016/j.socscimed.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 53.Kochanska G, Murray KT, Harlan ET. Effortful control in early childhood: Continuity and change, antecedents, and implications for social development. Developmental Psychology. 2000;36:220–232. [PubMed] [Google Scholar]
- 54.Miller EK. The prefrontal cortex and cognitive control. Nature Reviews Neuroscience. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- 55.Smyke AT, Koga S, Johnson DE, Fox NA, Marshall PJ, Nelson CA, Zeanah CH the BEIP Core Group. The caregiving context in institution-reared and family-reared infants and toddlers in Romania. Journal of Child Psychology and Psychiatry. 2007;48:210–218. doi: 10.1111/j.1469-7610.2006.01694.x. [DOI] [PubMed] [Google Scholar]
- 56.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 57.Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fear. Proceedings of the National Academy of Sciences. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 59.Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Development and Psychopathology. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 60.Pollak SD, Cicchetti D, Klorman R, Brumaghim JT. Cognitive brain event-related potentials and emotion processing in maltreated children. Child Development. 1997;68:773–787. doi: 10.1111/j.1467-8624.1997.tb01961.x. [DOI] [PubMed] [Google Scholar]
- 61.Pollak SD, Klorman R, Thatcher JE, Cicchetti D. P3b reflects maltreated children’s reactions to facial displays of emotion. Psychophysiology. 2001;38:267–274. [PubMed] [Google Scholar]
- 62.Slopen N, McLaughlin KA, Fox NA, Zeanah CH, Nelson CA. Alterations in neural processing of facial emotions and psychopathology in children exposed to institutional rearing. under review. [Google Scholar]
- 63.McLaughlin KA, Fox NA, Zeanah CH, Sheridan MA, Marshall PJ, Nelson CA. Delayed maturation in brain electrical activity explains the association between early environmental deprivation and symptoms of attention-deficit/hyperactivity disorder (ADHD) Biological Psychiatry. 2010;68:329–336. doi: 10.1016/j.biopsych.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nelson CA, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: The Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- 65.Smyke AT, Zeanah CH, Fox NA, Nelson CA, Guthrie D. Foster care enhances quality of attachment among young institutionalized children. Child Development. 2010;81:212–223. doi: 10.1111/j.1467-8624.2009.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vanderwert RE, Marshall PJ, Nelson CA, Zeanah CH, Fox NA. Timing of intervention affects brain electrical activity in children exposed to severe psychosocial neglect. PLoS One. 2011;5(7):e11415. doi: 10.1371/journal.pone.0011415. [DOI] [PMC free article] [PubMed] [Google Scholar]