Abstract
Several theoretical frameworks have suggested that anxiety/stress impairs cognitive performance. A competing prediction is made by attentional narrowing models that predict that stress decreases the processing of task-irrelevant items, thus benefiting performance when task-irrelevant information interferes with behavior. Critically, previous studies have not evaluated these competing frameworks when potent emotional manipulations are involved. Here, we used threat of bodily harm preceding a color-word Stroop task to test these claims. We found a basic effect of threat consisting of a slowing down of performance during neutral Stroop trials. Furthermore, both facilitation and interference scores were affected by threat of shock in a way that was consistent with a reduced-distractor effect. Taken together, we interpret our findings in terms of two opposing effects of stress on cognitive performance. Although partly consistent with the attentional narrowing hypothesis, both resource models and cognitive breadth models require revision in order to account for the results.
Keywords: Anxiety, Cognition, Stress, Attentional Narrowing
Emotion is a powerful determinant of behavior and often impairs task performance. For example, determining the orientation of a target visual stimulus was slower following emotional pictures (Hartikainen, Ogawa, & Knight, 2000) and the presence of a central unpleasant picture increased response times (RTs) when participants discriminated the orientation of peripheral bars (Erthal et al., 2005). Whereas the effect of fear (involving an emotion-laden stimulus) on cognitive performance is well described, the impact of anxiety/stress (involving temporally extended threat) is poorly understood.
Several theoretical frameworks have attempted to describe interactions between emotion and cognition. For instance, we have recently proposed a dual competition model in which high-threat items consume central processing resources that are shared with cognition, thereby impairing the execution of cognitive processes (Pessoa, 2009). Eysenck and colleagues have proposed that anxiety has adverse effects on processing efficiency (Eysenck & Calvo, 1992; Eysenck et al., 2007). Other frameworks have proposed that anxiety/stress leads to a narrowing of attention (Callaway, 1959; Callaway & Dembo, 1958; Easterbrook, 1959). Under this scenario, stress decreases the processing of task-irrelevant stimulus dimensions (Chajut & Algom, 2003). Resource models (e.g., Pessoa, 2009) and cognitive breadth models (e.g., Easterbrook, 1959) make competing predictions concerning the effects of anxiety on response-conflict tasks. On the one hand, resource theories predict that anxiety should increase interference-related effects of incongruent stimuli, whereas, on the other hand, attentional narrowing theories predict a reduction of these effects. The present study tested these alternative hypotheses by assessing the effects of anxiety on Stroop task performance (Stroop, 1935). Importantly, anxiety was induced by the threat of bodily harm (i.e., highly unpleasant electrical stimulation), a manipulation qualitatively different from previous studies of stress (e.g., Hochman, 1967, 1969, with time pressure; Keinan, et al., 1999, with exam pressure, etc.).
Method
Nineteen subjects participated in the experiment (age range =18–29 years; 10 males) and provided informed consent approved by the Institutional Review Board of Indiana University, Bloomington. Subjects performed a standard Stroop task in which the word “RED”, “BLUE”, “GREEN” or “YELLOW” was displayed on the screen either in the color indicated by the word (congruent condition) or in a randomly mismatching color (incongruent condition). Neutral stimuli consisted of a string of Xs that matched the color corresponding words in length (e.g., “XXX” was shown in red; “XXXX” was shown in blue).
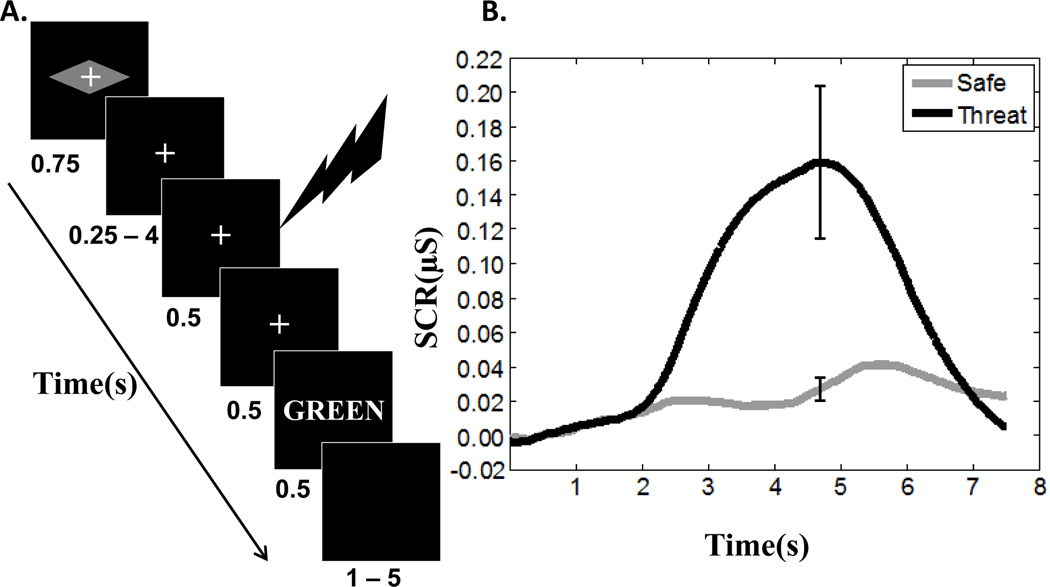
Trials started with a cue (rectangle or diamond) indicating the Anticipation condition (safe, threat) shown for 750 ms. Following a variable interval (range: 250 to 4000 ms), a 500-ms mild electrical stimulus was administered during threat trials involving shock (the onset of the shock occurred on average 2000 ms following the offset of the cue stimulus), and then followed by a 500-ms fixation display screen. In trials not involving shock, a 1000-ms fixation display screen was employed instead. Finally, the target stimulus containing colored words or a string of Xs appeared for 500 ms (Fig. 1A). In all trial, a fixation was shown starting at trial onset until the target stimulus. Note that during threat trials, participants had no information that could reveal whether they would receive a shock or not until the target stimulus appeared on the screen (at which point no shock could occur). Trials were separated from each other by a variable interval (range: 1000 to 5000 s). During the threat condition, shocks were administered 33% of the time. Subjects were instructed to respond as rapidly as possible, while avoiding errors. The schedule of stimulus presentation and data collection were controlled by Presentation software (Neurobehavioral Systems).
Figure 1. Experimental paradigm and skin conductance responses.
(A) An initial cue indicated whether the trial involved potential shock or not (threat and safe trials, respectively). In the former case, in 33% of the trials, an unpleasant electrical stimulus was administered for 500 ms during the cue-target interval. During the target period, a Stroop stimulus was presented and involved neutral, congruent, and incongruent conditions (for simplicity, not all task phases are displayed; see text). (B) For display purposes, untransformed SCRs were averaged as a function of condition. A robust effect of threat is apparent. The error bars represent the standard error of the mean.
Participants performed five “runs”, each comprising 36 trials and containing safe and threat trials randomly intermixed. Shocks were administered with an electrical stimulator (Coulbourn Instruments, PA) via two electrodes attached to the distal phalanges of the fourth and fifth fingers of the left hand. The level of stimulation was set separately by each individual at the beginning of the experimental session to a level that was characterized as “highly unpleasant but not painful”. Skin conductance response (SCR) data were also collected (BIOPAC Systems, Inc., CA) by using electrodes attached to the distal phalanges of the second and third finger of the left hand. Trials containing actual shocks were discarded from subsequent analysis. This was done because we were interested in investigating the impact of shock anticipation per se, not the influence of the physical shock itself. To balance trials, safe trials always followed physical-shock trials and were also discarded from subsequent analysis. In other words, trial balancing was done in such a way that all condition would be balanced once shock and safe trials following shocks were discarded. In this manner, each level of congruency was balanced and trials were presented such that no word or color was the same as in the preceding trial, thus minimizing priming effects (Mayr, Awh & Laurey, 2003). Note also that threat and safe trials were balanced (50% each) and that the cue type (rectangle vs. diamond) was counterbalanced across participants.
On each trial, the skin conductance response was calculated by subtracting a baseline (average signal between 0 and 1 s) from the peak amplitude during the 4–6 s time window following the cue onset (Prokasy & Raskin, 1973).
Results
We probed SCRs via a paired t test (threat vs. safe) given that congruency information was only available at the time of the target word. A significant difference was detected on loge-transformed data (t(18) =3.93, p<.01, Cohen's d=1.37). Given that trials were spaced apart by 7.5 s on average, clear differences in evoked responses were observed by selectively averaging the threat and safe conditions (Fig. 1B). To investigate potential effects of cognitive condition on SCRs during the threat condition, we performed a one-way repeated-measures ANOVA (neutral, congruent, incongruent), which revealed no discernible differences (F(2,18)=0.93, p=0.40, ηp2=0.05). In addition, during threat trials (pooled over cognitive condition), no differences in SCR were detected as a function of the cognitive condition of the previous trial (neutral, congruent, incongruent; F(2,18) = .35, p = .71, ηp2=0.02).
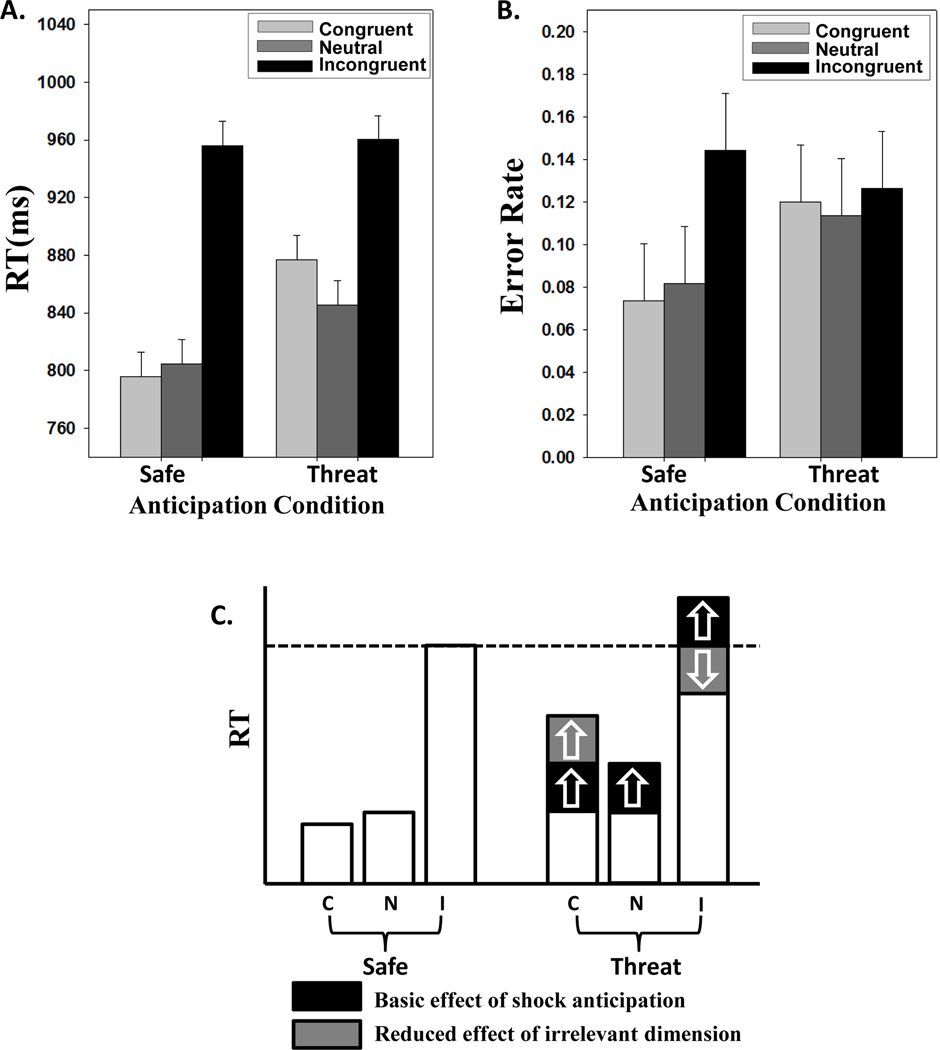
We interrogated reaction time (RT) and error rate data in terms of 2 Anticipation (safe, threat) × 3 Congruency (neutral, congruent, incongruent) repeated-measures ANOVAs. RT data from correct trials revealed a main effect of Anticipation (F(1,18) =12.26, p<.001, ηp2=0.41), a main effect of Congruency (F(2,36) =29.02, p<.01, ηp2=0.62), as well as interaction [F(2,36)=10.86, p<.01, ηp2=0.38] (Fig. 2A). Error rate data revealed moderate evidence for main effects (Anticipation: F(1,18)=3.50, p=.08, ηp2=0.16; Congruency: F(2,36)=2.55, p=.09, ηp2=0.12); a significant interaction was detected given an F value right at the critical boundary (F(2, 36)=3.23, p=.05, ηp2=0.15) (Fig. 2B).
Figure 2. Data and schematic interpretation.
(A) Reaction time data; (B) Error rate data (reported as a proportion). For both, error bars indicate 95% within-subject confidence intervals (Loftus & Masson, 1994). (C) A schematic interpretation of the results. The effect of shock anticipation was evidenced by a general slowing down of performance (red) and a reduction of task-irrelevant processing (blue). The latter tended to increase or decrease RT depending on whether the task-irrelevant information was congruent or incongruent, respectively. C, congruent; N, neutral; I, incongruent.
Additional tests were conducted to evaluate aspects of the RT data that informed the types of model consistent with the data. In particular, we probed facilitation and interference effects by performing additional 2 × 2 analyses. First, a 2 Anticipation (safe, threat) by Congruency (neutral, incongruent) revealed a significant interaction (F(1,18)=5.31, p<.05, ηp2=0.23). Second, a 2 Anticipation (safe, threat) by 2 Congruency (neutral, congruent) ANOVA revealed a significant interaction (F(1,18) = 4.55, p<.05, ηp2=0.20). To further probe interference, the contrast between incongruent trials (safe vs. threat) was evaluated, and did not reveal a significant difference (t(18)=0.30, p=.77, Cohen’s d=0.10). Finally, to evaluate the basic effect of threat, we contrasted neutral trials (safe vs. threat), which revealed a statistically significant difference (t(18) =3.14, p<.01; Cohen’s d=1.05).
Discussion
In the present study, we investigated the effects of anxiety/stress on cognition. The basic effect of threat on task performance was revealed by the slowing down during the neutral cognitive condition (threat slower than safe). The impact of threat was also revealed by changes in facilitation (congruent vs. neutral) and interference (incongruent vs. neutral) effects. Furthermore, RTs during the incongruent condition were the same irrespective of anxiety condition. Finally, our anxiety manipulation was effective in generating an emotional reaction, as evidenced by elevated skin conductance responses during threat vs. safe conditions.
The neutral cognitive condition allowed us to evaluate a basic effect of shock anticipation on task performance. A robust slowing down of performance (approximately 41 ms) was observed during safe vs. threat neutral trials. This effect is thus similar in nature to the impact of negative emotional stimuli (e.g., emotional pictures) on task performance in many paradigms (e.g., Erthal et al., 2005; Hartikainen, Ogawa, & Knight, 2000).
Next, we evaluated the influence of shock anticipation on interference by probing responses during incongruent trials. An Anticipation (threat, safe) by Congruency (neutral, incongruent) interaction was detected, such that the sizeable interference effect (incongruent vs. neutral) that was observed during safe trials decreased during the threat condition. Furthermore, our findings revealed that RTs during the incongruent condition were essentially unchanged by anticipation (threat vs. safe). In other words, unlike the slowing down during threat observed for both the neutral and congruent conditions, no discernible slowing down was observed when participants encountered conflicting information. One interpretation of these findings is that there exist two opposing contributions of shock anticipation on incongruent trials (see below).
We also evaluated the influence of shock anticipation on facilitation scores. Accordingly, we focused on the Anticipation (threat, safe) by Congruency (neutral, congruent) interaction, which revealed a cross-over interaction that suggested that a small trend towards facilitation during the safe condition (approximately 9 ms) was actually reversed during the threat condition (approximately 31 ms); note that it was not entirely surprising that a robust facilitation effect was not detected during safe trials given the baseline (“Xs”) condition employed here (for discussion, see MacLeod, 1991). This interaction pattern is consistent with the notion that the task-irrelevant congruent stimulus dimension received decreased processing during the threat condition.
Taken together, our findings revealed opposing effects of anxiety/stress on cognitive Stroop performance. We interpret these findings as summarized in Fig. 2C. One effect consisted of a slowing down of task performance. Although this effect was demonstrated during neutral trials, we suggest that this interference component was part of the RT during all threat trials. Another manifestation of shock anticipation involved the reduction of processing of the task-irrelevant dimension. We suggest that this reduced-distractor effect (Callaway, 1959; Callaway & Dembo, 1958; Easterbrook, 1959) was responsible for the reduction of both interference and facilitation effects during incongruent and congruent threat trials, respectively. We suggest that this effect actually opposed the generalized slowing down due to threat if we consider that the RTs during incongruent trials (safe vs. threat) essentially did not change. Thus, in particular, the reduction of interference during threat nullified the basic threat effect. Moreover, the reduction in facilitation (40 ms) almost exactly matches the observed basic effect of threat (41 ms).
What are the implications of our findings for models of cognitive-emotional interactions? The finding that shock anticipation did not increase response conflict poses a problem for resource-based proposals, such as the dual completion model (Pessoa, 2009) and possibly attentional control theory (Eysenck et al., 2007). According to these proposals, stress/anxiety impairs cognitive performance, especially when more demanding cognitive conditions are faced – in the present case, such an impact would have been expected during incongruent trials. For instance, the dual competition model makes this prediction because the processing of threat relies on cognitive resources needed to resolve conflict.
At the same time, our findings are partly consistent with the idea that stress induces a narrowing of attention (e.g. Easterbrook, 1959), given the effects of threat on interference and facilitation. Several studies have reported a reduction of conflict effects during stressful manipulations, including time pressure, threat to the ego, and aversive noise stimulation (e.g. Booth & Sharma, 2009; Callaway, 1959; Chajut & Algom, 2003; Hartley & Adams, 1974; Kluge, 1992; Renaud & Blondin, 1997). However, in our task, the neutral Stroop condition revealed a robust slowing down of RT during the threat condition. This effect is unlike those observed during other stress studies, in which no slowing down was observed during baseline conditions. In fact, several studies have reported, if anything, slightly faster RTs for baseline/neutral trials during high vs. low stress conditions (Chajut & Algom, 2003; Booth & Sharma, 2009). Thus, we suggest that our manipulation of anxiety/stress was qualitatively different from those of previous studies. First, typical stress manipulations (e.g., time pressure, exam pressure) do not involve the threat of physical harm. Second, stress manipulations that more closely approximate the present manipulation (e.g., loud noise) were applied in a “blocked” fashion, in contrast to our randomized (i.e., trial-by-trial) presentation. In summary, like resource models discussed previously, cognitive breadth models (e.g., attentional narrowing) appear to require revision.
An alternative interpretation of the opposing effects of shock anticipation on the processing of incongruent trials deserves consideration. The deployment of cognitive resources required for detection and resolution of response conflict may have decreased the impact of threat. This type of interaction would resemble the finding that a sufficiently strong attentional engagement away from an affective stimulus decreases its impact on behavior and the brain (Pessoa et al., 2002; Van Dillen et al., 2009), as well as the suggestion of a push-pull arrangement between emotion and cognition in general (Drevets & Raichle, 1998) or in the context of conflict processing (Wyble et al., 2008). The finding that SCRs were unaltered as a function of congruency suggests, however, that in the present study the cognitive manipulation was not sufficiently strong to down-regulate emotion.
Finally, it is instructive to consider the error rate data. The pattern of results is again consistent with the proposal that two opposing effects of threat were present in our task, as indicated by the 2 Anticipation by 3 Congruency interaction pattern (a post-hoc test of the basic effect of threat on neutral Stroop trials, revealed a t score just outside the decision boundary: t(18)=1.94, p=.07, Cohen’s d=0.63). Thus, the overall error rate pattern revealed an effect of threat that was qualified by an interaction. These results are of relevance in understanding the contributions of processing effectiveness (in addition to processing efficiency) during anxiety-related conditions (Eysenck et al., 2007).
In summary, our findings potentially suggest two opposing effects of anxiety/stress on cognitive performance, a basic slowing down of RT and a reduction of task-irrelevant processing. Both resource models and cognitive breadth models require revision in order to account for the present findings.
Acknowledgements
Support for this work was provided in part by the National Institute of Mental Health (R01 MH071589). We would like to thank Jong-Moon Choi and Brad Wyble for constructive feedback on the manuscript, and Michael Simmons and Jena Wierwille for assistance with running participants.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/emo
References
- Booth R, Sharma D. Stress reduces attention to irrelevant information: evidence from the Stroop task. Motivation and Emotion. 2009;33:412–418. [Google Scholar]
- Callaway E, Dembo D. Narrowed attention:a psychological phenomenon that accompanies a certain physiological change. Archives on Neurology and Psychiatry. 1958;79:74–90. [PubMed] [Google Scholar]
- Callaway E. The influence of amobarbital (amylobarbitone) and methamphetamine on the focus of attention. Journal of Mental Science. 1959;105:382–392. doi: 10.1192/bjp.105.439.382. [DOI] [PubMed] [Google Scholar]
- Chajut E, Algom D. Selective attention improves under stress: Implications for theories of social cognition. Journal of Personality and Social Psychology. 2003;85:231–248. doi: 10.1037/0022-3514.85.2.231. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. Cognition and Emotion. 1998;12:353–385. [Google Scholar]
- Easterbrook JA. The effect of emotion on cue utilization and the organization of behavior. Psychological Review. 1959;66:183–201. doi: 10.1037/h0047707. [DOI] [PubMed] [Google Scholar]
- Erthal FS, de Oliveira L, Mocaiber I, Pereira MG, Machado-Pinheiro W, Volchan E, Pessoa L. Load-dependent modulation of affective picture processing. Cognitive, Affective, & Behavioral Neuroscience. 2005;5:388–395. doi: 10.3758/cabn.5.4.388. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Calvo MG. Anxiety and performance: The processing efficiency theory. Cognition and Emotion. 1992;6:409–434. [Google Scholar]
- Hartikainen KM, Ogawa KH, Knight RT. Transient interference of right hemispheric function due to automatic emotional processing. Neuropsychologia. 2000;38:1576–1580. doi: 10.1016/s0028-3932(00)00072-5. [DOI] [PubMed] [Google Scholar]
- Hartley LR, Adams RG. Effects of noise on the Stroop test. Journal of Experimental Psychology. 1974;102(1):62–66. doi: 10.1037/h0035695. [DOI] [PubMed] [Google Scholar]
- Hochman SH. The effects of stress on Stroop color-word performance. Psychological Science. 1967;9:475–476. [Google Scholar]
- Hochman SH. Stress and response competition in Chinldren’s color-word performance. Perceptual and Motor Skills. 1969;28:115–118. doi: 10.2466/pms.1969.28.1.115. [DOI] [PubMed] [Google Scholar]
- Keinan G, Friedland N, Kahneman D, Roth D. The effect of stress on the suppression of erroneous competing responses. Anxiety, Stress and Coping. 1999;12:455–476. doi: 10.1080/10615809908249321. [DOI] [PubMed] [Google Scholar]
- Kluge S. Trading accuracy for speed: gender differences on a Stroop task under mild performance anxiety. Perceptual and Motor Skills. 1992;75:651–657. doi: 10.2466/pms.1992.75.2.651. [DOI] [PubMed] [Google Scholar]
- Loftus GR, Masson MEJ. Using confidence intervals in within-subject designs. Psychonomic Bulletin & Review. 1994;1:476–490. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: An integrative review. Psychological Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Mayr U, Awh E, Laurey P. Conflict adaptation effects in the absence of executive control. Nature Neuroscience. 2003;6(5):450–452. doi: 10.1038/nn1051. [DOI] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends in Cognitive Sciences. 2009;13(4):160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokasy WF, Raskin DC. Electrodermal activity in psychological research. New York, NY: Academic Press; 1973. [Google Scholar]
- Renaud P, Blondin JP. The stress of Stroop performance: physiological and emotional responses to color-word interference, task pacing, and pacing speed. International Journal of Psychophysiology. 1997;27:87–97. doi: 10.1016/s0167-8760(97)00049-4. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;28:643–662. [Google Scholar]
- Van Dillen L, Heslenfeld DJ, Koole S. Tuning down the emotional brain: An fMRI study of the effects of cognitive load on the processing of affective images. NeuroImage. 2009;45:1212–1219. doi: 10.1016/j.neuroimage.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Wyble B, Sharma D, Bowman H. Strategic regulation of cognitive control by emotional salience, a neural network model. Cognition and Emotion. 2008;22(6):1019–1051. [Google Scholar]