Abstract
BACKGROUND
Transferrin (Tf) is a paradigmatic metalloprotein, which has been extensively studied in the past and still is a focal point of numerous investigation efforts owing to its unique role in iron homeostasis and enormous promise as a component of a wide range of therapies.
SCOPE OF REVIEW
Electrospray ionization mass spectrometry (ESI MS) is a potent analytical tool that has been used successfully to study various properties of Tf and Tf-based products, ranging from covalent structure and metal binding to conformation and interaction with their physiological partners.
MAJOR CONCLUSIONS
Various ESI MS-based techniques produce unique information on Tf properties and behavior that is highly complementary to information provided by other experimental techniques.
GENERAL SIGNIFICANCE
The experimental ESI MS-based techniques developed for Tf studies are not only useful for understanding of fundamental aspects of the iron-binding properties of this protein and optimizing Tf-based therapeutic products, but can also be applied to study a range of other metalloproteins.
1. Introduction
Transferrin (Tf) has a very special place among the plethora of metal-binding proteins because of the unique role played by iron, its prime binding target, in a variety of biological processes. It is virtually impossible to find a living organism on this planet whose life could be sustained in the absence of iron. Although this element is the second most abundant metal in the earth’s crust, its bioavailability is severely limited by the poor solubility characteristics of ferric ion (Fe3+), the predominant oxidation state of iron under aerobic conditions. The ability of Tf to bind ferric ion, as well as a range of other metal cations with very high affinity at neutral pH and promptly release it in response to certain chemical cues is remarkable by itself and absolutely critical for iron homeostasis in vertebrates. It probably is not an exaggeration to say that Tf has become a paradigmatic metalloprotein, and various aspects of its behavior provide important clues vis-à-vis general properties of a variety of other metalloproteins.
Understanding Tf behavior and properties has not only great fundamental value, but also significant practical importance. It is one of the very few plasma proteins that have direct access to cells. Furthermore, Tf is capable of traversing the blood-brain barrier (BBB) and the intestinal epithelial barrier via transcytosis, which makes it an attractive candidate for developing complex therapies targeting the central nervous system and orally administered macromolecular drugs. It is not, therefore, surprising that Tf has been and remains a prime target of extensive research efforts. Until the last decade, mass spectrometry (MS) was notably absent from the arsenal of experimental techniques used to investigate various aspects of Tf structure and interaction with its physiological partners. This situation has dramatically changed in the past several years, as an impressive battery of MS-based experimental methodologies have been developed to probe various aspects of Tf behavior, frequently delivering unique information complementary to that obtained with other, more traditional biophysical techniques. This review summarizes these developments and highlights the most important achievements.
2. Tf interaction with metals and synergistic anions: characterization of the protein-metal complex composition with ESI MS
Iron binding to low molecular weight organic or peptide siderophores is so strong, that the resulting complexes can be probed by MS using ionization techniques that usually do not preserve non-covalent complexes, such as Fast Atom Bombardment (FAB) [1]. Although the metal affinity of Tf rivals that of many low-molecular weight siderophores, the protein itself is too large to be desorbed by FAB, and the MS analysis of Tf can be accomplished only when electrospray ionization (ESI) is used as ionization technique. It was not long after the introduction of ESI MS that this technique had been applied to the analysis of various forms of recombinant Tf based on mass differences among them due to mutations [2] or variations in glycosylation [3]. However, Tf characterization in this early work was carried out under denaturing conditions and any information on the protein interaction with metal ions and/or other ligands was inevitably lost. The pioneering work by Fenselau with metallothioneins in the early 1990s demonstrated that the gentle nature of the ESI process allows the protein-metal complexes to be preserved upon their transition to the gas phase, thereby allowing both the nature of the metal(s) and the binding stoichiometry to be determined if the measurements are carried out under near-native conditions [4].
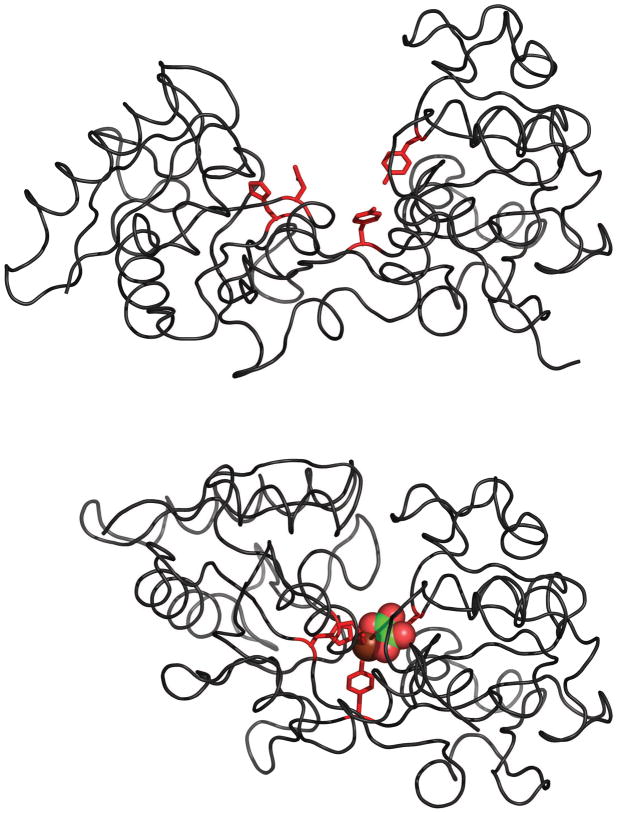
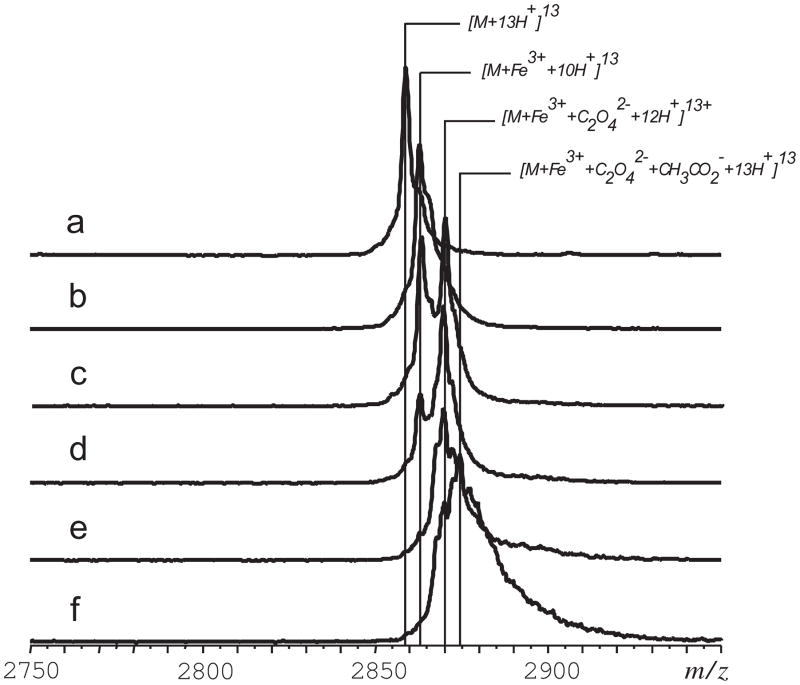
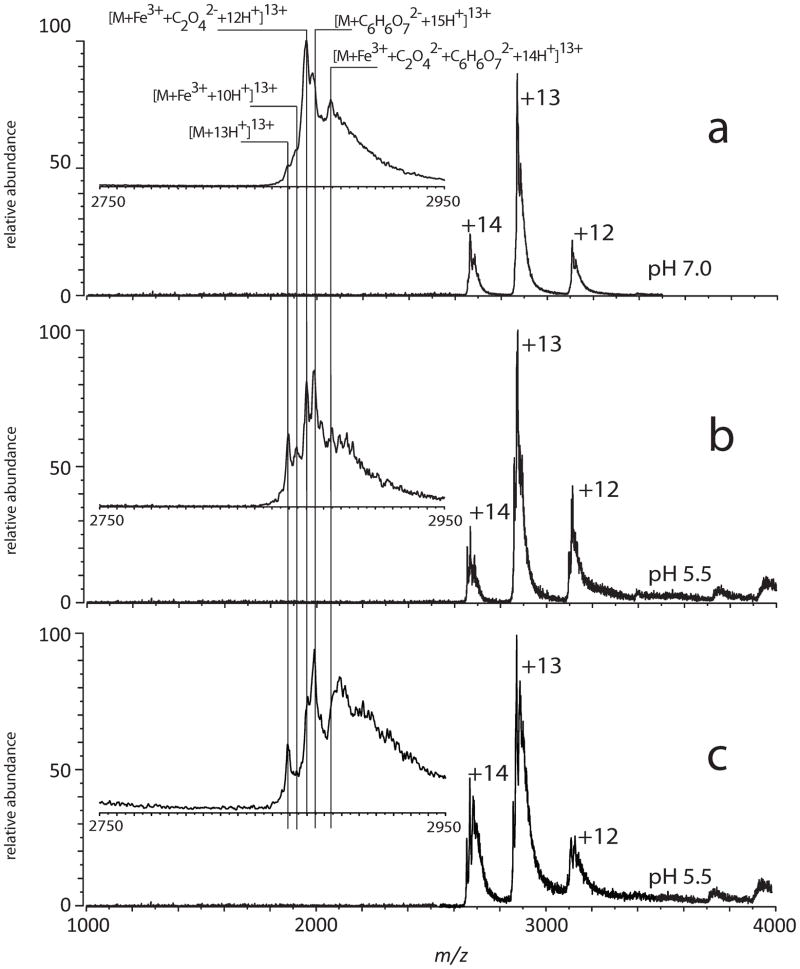
The initial application of ESI MS to study Tf-metal complexes was done with the iron-bound form of the N-lobe of human serum transferrin (hTf/2N, Figure 1) [5]. Despite the relatively large molecular weight of hTf/2N (>35 kDa), the mass resolution and accuracy offered by ESI MS allow both metal ion and synergistic anion to be identified (Figure 2). Although the ESI MS measurements produce a single mass readout, corresponding to the combined mass of the protein, metal ion and the synergistic anion, further manipulation of the complex ion in the gas phase allows the masses of both ligands to be measured. This is done by increasing the internal energy of the complex ion in the gas phase (via collisional activation in the ESI MS interface), which leads to partial dissociation of the complex. While the protein-metal binding is very strong and survives significant collisional excitation, dissociation of the synergistic anion is observed even following relatively modest excitation (Figure 2B). This dissociation process does not change the charge state distribution of hTf/2N ions, suggesting that the synergistic anion departs as a neutral species (an alternative process would result in inordinate enthalpic penalty due to electrostatic attraction between the protein polycation and oxalate di-anion in the gas phase).
Figure 1.
Crystal structures of the apo- (top) and holo-forms (bottom) of human serum transferrin showing details of iron binding: side chains coordinating Fe3+ are colored in red; both ferric ion and the synergistic anion are presented with space-fill models, and are colored by elements (Fe, chocolate red; C, green; and O, red). The PDB accession numbers are 1BTJ (apo-form [65]) and 1A8E (holo-form [66]).
Figure 2.
Dissociation of the ternary complex [hTf/2N·Fe3+·C2O42−] in the gas phase: ion peaks of a +13 charge state for control apo-hTf/2N in H2O/CH3OH/CH3CO2H (a) and holo-hTf/2N in 10 mM NH4CH3CO2 (b–f). The efficiency of ion collisional activation decreases from (b) to (f) following monotonic decrease of the skimmer potential and temperature in the ESI interface. Adapted with permission from [5].
It is noteworthy that these early measurements yielded a somewhat unexpected result: it was found that the mass of the synergistic anion was 18 amu higher than that of carbonate, consistent with the notion of oxalate being the synergistic anion. It may seem strange that oxalate, rather than carbonate, serves as a synergistic anion despite large excess of the latter in solution (all protein samples were kept in ammonium bicarbonate solution, while their exposure to oxalate was possible only during the expression step, where oxalate was a component of culture media). However, later studies revealed that oxalate was a much stronger binder compared to carbonate [6], which explains why it remained bound to Tf despite the presence of excess carbonate in the protein solution.
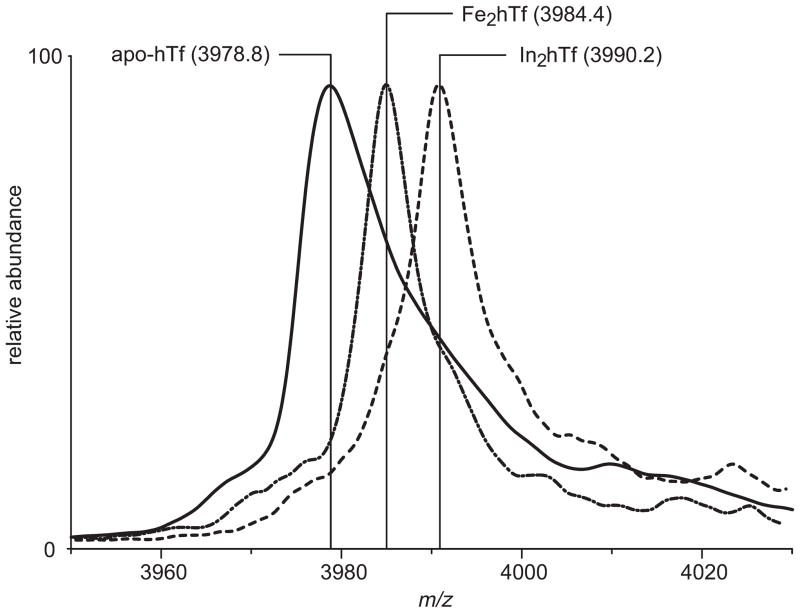
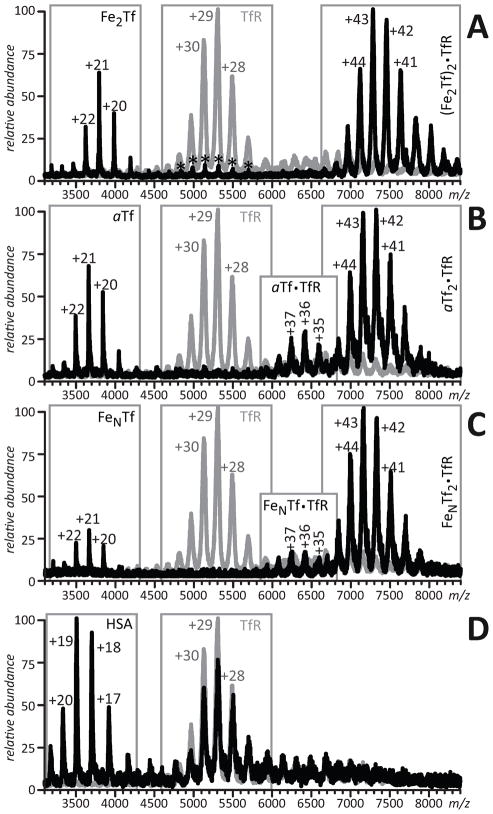
In addition to iron, Tf is capable of binding a range of other metals, and mass measurements of the protein-metal complex allow the bound metal to be identified and the binding stoichiometry to be determined [7]. Again, moderate collisional activation allows the synergistic anion to be removed from the ternary complex prior to the mass measurement, thereby making the data interpretation more straightforward, as can be seen for iron- and indium-bound forms of full-length human serum transferrin (hTf), Fe2hTf and In2hTf in Figure 3. It must be noted, however, that some Tf-metal complexes are extremely weak in the gas phase (e.g., hTf complex with Bi), and while these interactions can still be detected by ESI MS, this task is not straightforward [7].
Figure 3.
Molecular ions of hTf (charge state +20) in ESI mass spectra of apo-protein (solid line) and metal-saturated protein solutions (Fe3+, dash-dot line, and In3+, dashed line). Adapted with permission from [7].
3. Metal release from Tf: simultaneous monitoring of the ternary complex integrity and protein conformation with ESI MS
In addition to being able to characterize composition of non-covalent associations, such as Tf-metal complexes, ESI MS can also provide information on the integrity of the protein higher order structure. Proteins with intact tertiary structure are compact, and their solvent-exposed surface areas in solution are relatively small, compared to those of less structured (partially or completely unfolded) proteins. This leads to dramatic difference in the charge density of protein ions representing folded and unfolded proteins in ESI MS [8]. Proteins with intact higher order structure give rise to ionic distributions that are located in the higher m/z region of mass spectra, and the corresponding charge state distributions are relatively narrow. Less structured proteins give rise to ions with significantly higher charge density, and the corresponding ion peaks are usually located in the lower m/z region of ESI mass spectra. The charge state distribution of such species is typically much broader compared to their folded counterparts due to much higher conformational heterogeneity. It is not uncommon to observe in a single mass spectrum ionic signals emanating from both folded and (partially) unfolded forms of the protein in solution, in which case the ionic charge state distributions in ESI MS become bimodal [9]. Since each ESI MS measurement provides data on both masses and charges of all ionic species simultaneously, occurrence of conformational changes within the protein can be easily correlated with its ligand-binding competence (where the ligand could be a metal ion [10], a small organic molecule [11] or another biopolymer [12]).
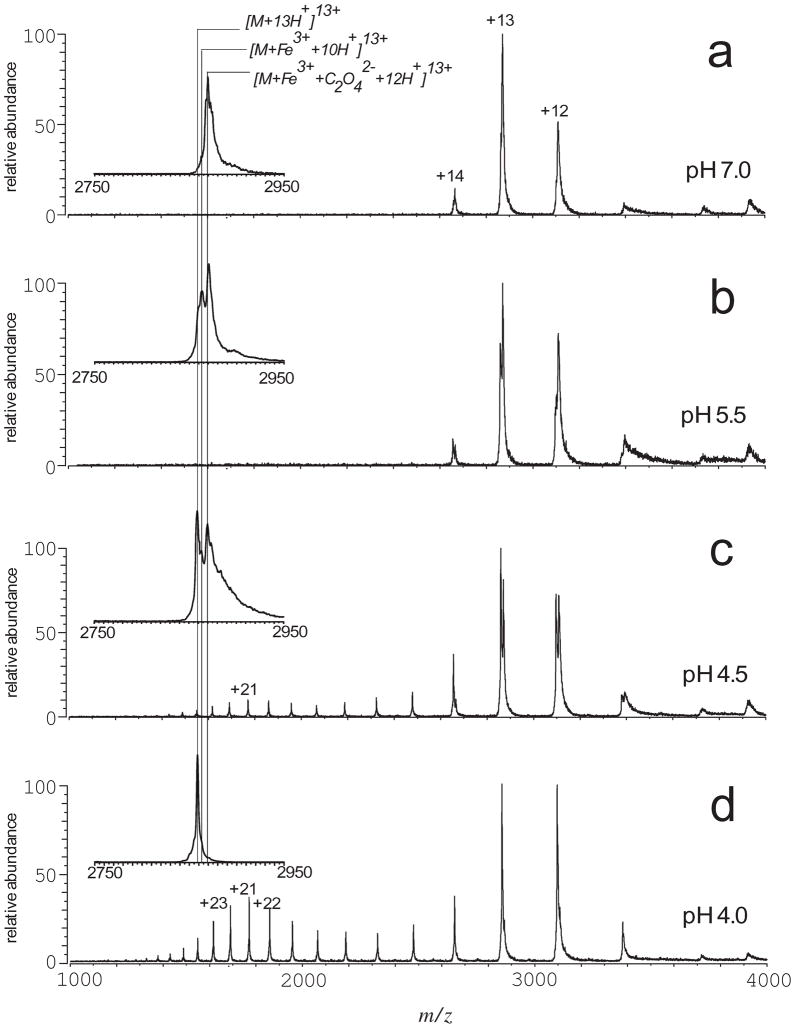
This unique feature of ESI MS was used in the early studies of acid-assisted metal ion release from hTf/2N [5]. Although ferric ion is known to dissociate from each lobe of Tf at endosomal pH [13, 14], ESI MS clearly demonstrated that the metal ion remained bound to the protein at pH 5.5 (Figure 4). The metal-protein dissociation became evident only when the solution was acidified to pH 4.5, one unit below the endosomal pH level. The ESI mass spectrum acquired at this pH also showed clear signs of partial unfolding of the protein, as indicated by the appearance of high charge-density ions (charge states > +14 in Figure 4C). Since no signs of loss of higher order structure could be seen at higher pH, a conclusion was made that there is clear correlation between acid-induced unfolding and the release of ferric ion from hTf/2N [5].
Figure 4.
Acid-induced dissociation of the [hTf/2N·Fe3+·C2O42−] complex in the absence of chelators and nonsynergistic anions. The ESI interface conditions used for these measurements are the same as those used to obtain a spectrum shown in Figure 1e. Insets show expanded view of protein ion peaks corresponding to the +13 charge state. Reproduced with permission from [5].
Although the protein fails to release the metal at endosomal pH, ESI MS data do suggest that the integrity of the ternary complex [hTf/2N·Fe3+·C2O42−] is compromised, as some dissociation of oxalate anion from the protein becomes evident (Figure 4B). Addition of iron chelating agents, such as citrate, to the protein solution results in facile removal of the metal from hTf/2N at endosomal pH without any visible signs of protein unfolding [5]. At the same time, citrate does not destabilize the integrity of the [hTf/2N·Fe3+·C2O42−] complex at neutral pH (Figure 5).
Figure 5.
Citrate-assisted iron removal from hTf/2N. The ESI mass spectra were obtained from 6 μM solutions of holo-hTf/2N in 10 mM NH4CH3CO2/0.03 mM (NH4)2C6H6O7 (a, b) and 10 mM NH4CH3CO2/0.15 mM (NH4)2C6H6O7 (c). The ESI interface conditions used in these measurements were identical to those used to obtain the mass spectrum shown in Figure 1e. Reproduced with permission from [5].
It has been known for quite some time that transferrin fails to release iron at endosomal pH in the absence of chelating agents [13, 14], and that various chelators display remarkable variability vis-à-vis facilitation of the protein-metal complex dissociation. A commonly accepted mechanistic model of this process based on earlier spectroscopic evidence [15] suggests that the anion binding to transferrin induces a conformational switch (closed → open), followed by metal dissociation from the open conformation (characteristic of its apo-form) [15]. Further refinement of this model led to an introduction of a dual pathway iron release mechanism, which included competitive anion binding to the iron coordination sites and to a specific anion site within the protein [16]. However, the results of ESI MS measurement indicate that the process of metal ion dissociation from hTf/2N cannot be understood without taking into account large-scale protein dynamics. The experiments discussed earlier in this section clearly suggest that the open state of the protein does become populated at endosomal pH even in the absence of chelating agents and non-synergistic anions (Figure 6). The protein retains the metal ion very effectively under these conditions, even though the synergistic anion does dissociate from the complex. At the same time, the open conformation facilitates access of the chelator (e.g., citrate) to the metal ion, leading to efficient iron removal. In contrast, iron release in the absence of the chelator does not occur until the protein becomes partially unfolded at pH well below the endosomal level (Figure 6).
Figure 6.
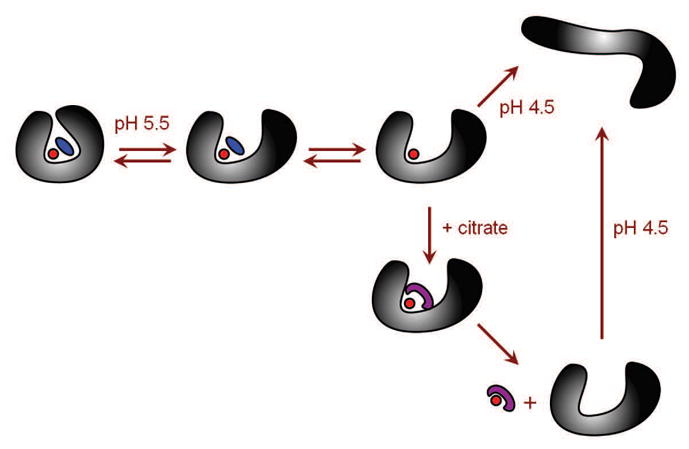
A mechanism of Fe3+ dissociation from hTf/2N based on the experimental evidence provided by ESI MS.
In order to rationalize the existence of the non-canonical forms of Tf (e.g., open conformation of the holo-protein at endosomal pH, see Figure 6), it is instructive to consider the available crystal structures of iron-loaded forms of hen ovotransferrin N-lobe [17] and camel lactoferrin [18], which had been forced to stay in the open conformation characteristic of the apo-form (by soaking the apoprotein crystals in concentrated Fe3+ solution followed by crystal drying). Coordination of Fe3+ ion in each case is provided by two Tyr residues that participate in metal coordination in the native (closed) conformation of the holo-protein. The rest of the coordination sphere is completed by small organic ligands. These alternative structural states of iron-bound proteins from Tf family were suggested to represent transient intermediate states on the iron-binding pathway, consistent with a hypothesis that apo-Tf actually samples both conformations (open and closed) at endosomal pH, although the open state is energetically favorable [19].
The studies of the role of protein conformational dynamics on iron dissociation from hTf/2N can be extended to a full-length protein [20]. Although the two ferric ions (bound to the N- and C-lobe of Tf) cannot be distinguished from each other on basis of mass measurement alone, it is possible to use ESI MS to study iron dissociation from the protein in the lobe-specific fashion. This can be done by using mutants where metal-binding affinity is dramatically enhanced in one of the lobes (e.g., by converting the dilysine trigger to a lock in the N-lobe of Tf [21] or abolishing a pH-sensitive element in the C-lobe [22]).
4. Detection of non-canonical forms of Tf with HDX MS
The existence of the non-canonical conformations of Tf (e.g., open conformation of the holo-form and closed conformation of the apo-form) is important not only for understanding the mechanism of protein-metal interaction, but also for Tf binding to its receptor (TfR). The latter is known to bind with high affinity to the holo-form of Tf at neutral pH and to the apo-form at endosomal pH, and these preferences are thought to be dictated by the conformational transitions within Tf as a function of pH. However, the existence of the non-canonical conformations of Tf complicates this picture, e.g. leading to the possibility of apo-Tf binding to TfR at neutral pH. Such interactions, if they indeed exist, must be taken into account when engineering advanced therapeutic strategies that utilize intracellular trafficking pathways [23]. This warranted further, more detailed investigation of the non-canonical forms of Tf that would provide more definitive proof of their existence beyond the circumstantial evidence produced in the early work [5, 7]. However, establishing the existence of these non-canonical states of Tf in solution is a difficult task since conventional experimental techniques provide limited information on the conformational dynamics of Tf. Application of NMR spectroscopy to obtain dynamic data on Tf molecules is limited by the relatively large size of Tf and the presence of paramagnetic ferric ions. Dynamic processes associated with metal dissociation from Tf molecules can be monitored using optical spectroscopic techniques, such as fluorescence; however, these studies usually provide very limited structural information. Detailed structural information can be deduced from X-ray crystallographic data, but this method is hardly suitable for characterization or indeed detection of transient metastable species.
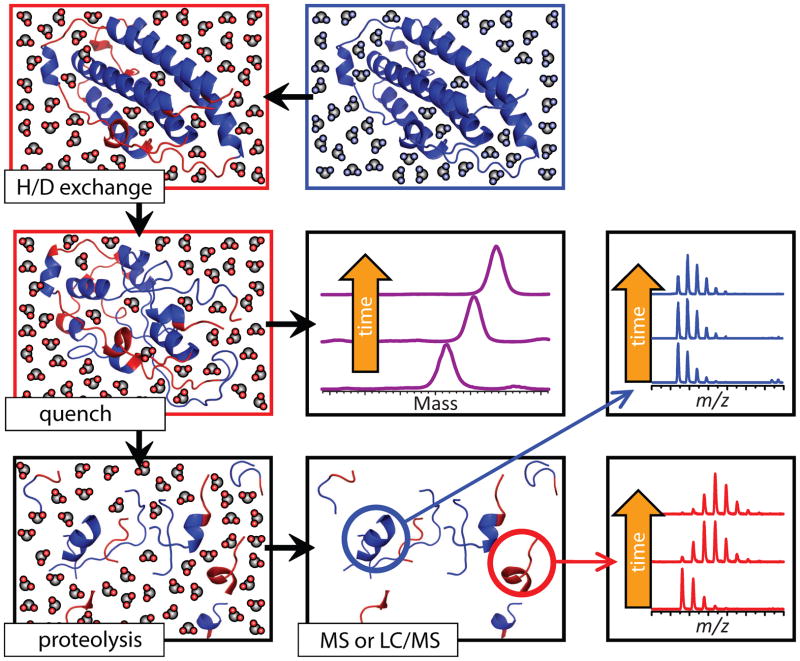
Both higher order structure and conformational dynamics of larger proteins can be probed using hydrogen/deuterium exchange (HDX) with MS detection [24, 25]. In a typical experiment, HDX is initiated by rapid dilution of the protein solution in D2O-based solvent system. This results in exchange of all labile hydrogen atoms (i.e., those attached to nitrogen atoms at the backbone amides and heteroatoms at polar/charged side chains), which are not protected by hydrogen bonding or else sequestered from the solvent in the protein interior. Some of these hydrogen atoms that are protected initially, may later become exposed to the solvent as a result of local structural fluctuations or transient unfolding events. Therefore, the hydrogen exchange level following brief HDX is determined mostly by the protein higher order structure, while HDX kinetics over extended periods of time reflect conformational dynamics.
Mass measurements of proteins undergoing HDX are usually carried out following rapid acidification of the protein solution to pH 2.5–3 and lowering the temperature to 0–4°C, which results in significant deceleration of the chemical (intrinsic) exchange rates of backbone amide hydrogen atoms. These conditions, known as “HDX quenching” or “slow exchange conditions,” also result in unfolding of most proteins. Since the intrinsic exchange rates of labile side chain hydrogen atoms are not decelerated as significantly as those for backbone amides [26], all information on the side chain protection is lost during this step, leaving a single HDX reporter for each amino acid residue (with the exception of proline, the only natural amino acid lacking a backbone amide hydrogen atom). Another fortunate consequence of quench-induced protein denaturation is dissociation of all non-covalently bound ligands (ranging from metal cations and small organic molecules to other biopolymers) from the protein. Therefore, measuring the protein mass under these conditions provides information only on the protein conformation and stability, rather than composition of complexes formed by the protein and its ligands (Figure 7). In addition to characterizing protein conformation and stability globally, the protein can be digested with an acidic protease (e.g., pepsin) under the slow exchange conditions, and MS (alone or in combination with liquid chromatography, LC) can be used to measure the deuterium content of each proteolytic fragment. This will produce information on protein conformation and dynamics at the local level.
Figure 7.
Schematic representation of HDX MS work flow to examine protein higher order structure and conformational dynamics. The exchange is initiated by placing the unlabeled protein into a D2O-based solvent system (e.g., by a rapid dilution). Unstructured and highly dynamic protein segments undergo fast exchange (blue and red colors represent protons and deuterons, respectively). Following the quench step (rapid solution acidification and temperature drop), the protein loses its native conformation, but the spatial distribution of backbone amide protons and deuterons across the backbone is preserved (all labile hydrogen atoms at side chains undergo fast back-exchange at this step). Rapid clean-up followed by MS measurement of the protein mass reports the total number of backbone amide hydrogen atoms exchanged under native conditions (a global measure of the protein stability under native conditions), as long as the quench conditions are maintained during the sample work-up and measurement. Alternatively, the protein can by digested under the quench conditions using acid-stable protease(s), and LC/MS analysis of masses of individual proteolytic fragments will provide information on the backbone protection of corresponding protein segments under the native conditions. Reproduced with permission from [67].
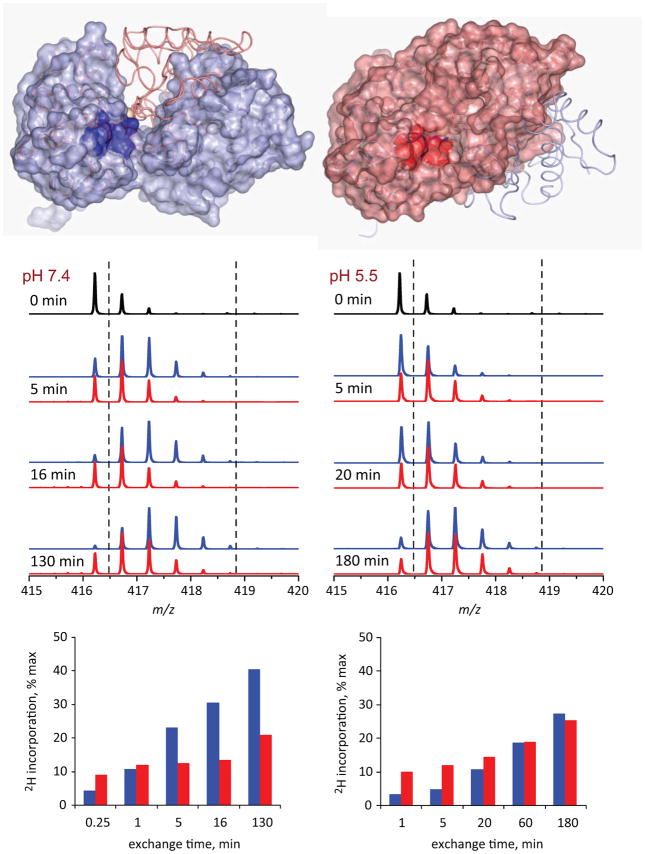
Using HDX MS to probe changes in solvent accessibility within various segments of hTf/2N following metal removal at both neutral and endosomal pH levels produced conclusive evidence for the existence of an iron-bound form of hTf/2N that adopts an open conformation in solution [27]. HDX MS of hTf/2N at neutral pH clearly identifies the protein segments whose structure and dynamics are affected by the presence of the metal [27]. Nearly all of them are located in the inter-domain cleft interface region (an example of one such peptide is shown in Figure 8), consistent with the notion of the iron-bound protein existing mostly in the closed conformation, where the solvent exposure of the inter-domain interface region is very limited compared to the apo-form of the protein (open conformation). However, this difference all but disappeared at endosomal pH (Figure 8), providing unequivocal evidence that the iron-bound state of hTf/2N exists at endosomal pH in the open conformation, just like the iron-free protein [27].
Figure 8.
Local HDX data for hTf/2N segment represented by peptic fragment (205–211). The top diagrams show localization of this segment within open (left) and closed (right) conformation of the protein. The mass spectra in the middle panel show evolution of the isotopic distribution of this peptide throughout the course of exchange reactions at neutral (left) and endosomal (right) pH. The red and blue traces correspond to peptides derived from holo- and apo-forms of hTf/2N, respectively. The two diagrams at the bottom show kinetics of deuterium incorporation within this peptide at neutral and endosomal pH.
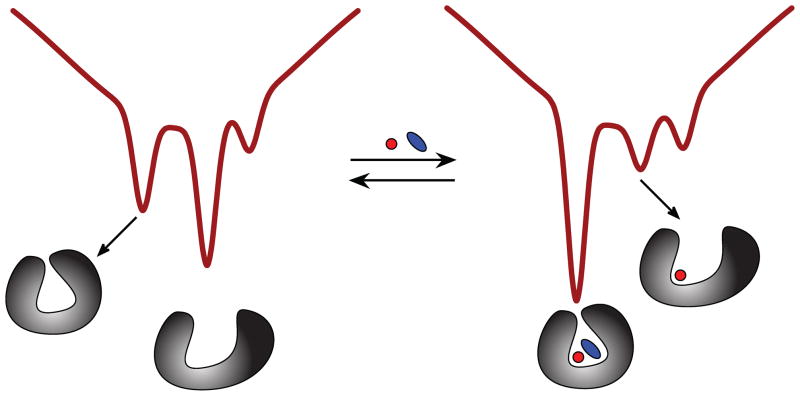
HDX MS was also used to obtain experimental evidence that the N-lobe of Tf samples a non-canonical state at neutral pH, where the holo-form of the protein was observed to exist in a metastable open conformation [28]. This was done by carrying out HDX MS measurements on a G65R mutant of hTf/2N. This mutation was created to mimic a naturally occurring variant (G394R) found in the homologous C-lobe, which results in much more facile removal of iron from the protein. Although direct ESI MS measurements clearly indicate that Fe3+ remains bound to the protein at neutral pH, its backbone protection is identical to that of the apo-form of hTf/2N [28]. The conclusions of the HDX MS work [27, 28] lend strong support to a conformational hopping model as a descriptor of Tf interaction with ferric ion [19]. In this model, two alternative conformations are sampled by both states of the protein (apo and holo), and metal binding and release always occurs through the open conformation (Figure 9).
Figure 9.
Schematic representation of the energy landscape for a single lobe of Tf at neutral pH showing the existence of two alternative conformations (open and closed) and the influence of metal binding on their relative Boltzmann weights.
5. Non-canonical interaction of Tf with the receptor: evaluating protein-protein interaction with ESI MS
Co-existence of two alternative conformations of Tf questions one of the fundamental assumptions of the commonly accepted model of its interaction with the receptor, namely the suggestion that TfR differentiates very effectively between the apo- and holo-forms of Tf at neutral and endosomal pH based on the conformation of the latter [29–31]. The ability of apo-Tf to sample the closed conformation (Figure 9) would necessarily mean that the presence of the metal is not an absolute prerequisite for Tf/TfR binding at neutral pH. However, most recent studies failed to detect apo-hTf binding to TfR at neutral pH [29, 32–35], despite earlier suggestions that apo-hTf is capable of interacting with TfR at neutral pH [36, 37]. As a result, the idea of apo-hTf/TfR association at neutral pH became increasingly apocryphal, and until recently this possibility was either ignored [38], or attributed to contamination of commercial “metal-free” hTf with iron.
ESI MS provides a unique capability to monitor protein-protein interactions under a variety of conditions, as the soft nature of ESI often preserves the integrity of large non-covalent macromolecular complexes [39–41]. When applied to Tf/TfR interactions [42], this approach readily detects formation of 2:1 Fe2Tf/TfR complexes (Figure 10A). However, formation of 2:1 protein-receptor complexes is also detected for both mono-ferric and iron-free forms of Tf (Figure 10B, C). Contamination of apo-Tf with iron cannot be invoked in this case as a cause of receptor binding, since this protein incorporated Y95F/Y188F/Y426F/Y517F mutations abolishing iron binding in both lobes [43]. Even though apo-Tf does bind TfR, as is unequivocally proved by ESI MS, this association is significantly weaker compared to the Fe2Tf/TfR interaction. This can be demonstrated by either using “competition” experiments (where several forms of Tf compete for binding to TfR) or “displacement” experiments, where incremental additions of Fe2Tf to solution containing apo-Tf and TfR results in facile replacement of the metal-free form of the protein from Tf/TfR complex with the diferric form [42]. In fact, the receptor affinity of apo-Tf at neutral pH can be estimated (0.2–0.6 μM) using a set of Tf variants in a series of competition and displacement experiments [42]. Consistent with current models of endosomal iron release from Tf, acidification of the protein solution results in a dramatic change of binding preferences, with apo-Tf becoming a preferred receptor binder [42].
Figure 10.
ESI mass spectra of 6μM TfR solutions in 100 mM NH4HCO3 containing 10 μM Fe2Tf (A), αTf (B), FeNTf (C), and HSA (D). Gray traces represent mass spectra of TfR in the absence of binding partners. Asterisks represent hTf dimers. Reproduced with permission from [42].
The conclusions of the experimental work reviewed in this section are consistent with conformational hopping model (Figure 9), but contradict the commonly accepted model of Tf/TfR interaction, which implies that the apo-Tf/TfR complex dissociates almost immediately upon exposure to the neutral environment at the cell surface. Indeed, the ESI MS measurements [42] provide very clear evidence that this complex remains intact at neutral pH, although iron-bound Tf (either di- or mono-ferric) displaces apo-Tf from TfR, making it available for the next cycle of iron binding, transport and delivery to tissues. Nevertheless, apo-Tf may still interfere with the cellular uptake of engineered Tf molecules whose TfR affinity is affected by various modifications (e.g., conjugation to cytotoxic molecules).
6. Tf-based therapies: characterization of properties and behavior of drug conjugates and fusion proteins with ESI MS
Tf is one of the very few plasma proteins that can enter the cell in the process of receptor-mediated endocytosis [44]. Since the rapidly growing malignant cells have dramatically elevated requirements for iron consumption, they over-express transferrin receptor (TfR), making Tf very attractive as a vehicle for selective delivery of cytotoxic agents to cancer cells [45–47]. Furthermore, the ability of Tf to cross the blood-brain barrier (BBB) through transcytosis [48] further increases its appeal by suggesting that it may be used to target brain tumors, which are notorious for being difficult to reach [49]. Tf was also evaluated as a potential delivery vehicle for orally administered biopharmaceutical products, and the initial results are promising [50, 51].
While covalent attachment of therapeutics to Tf (either through chemical conjugation [52–55] or by creating fusion proteins [50, 51, 56]) offers an elegant solution to the targeted drug delivery problem, it is also clear that these procedures can change the binding affinity of Tf for TfR. The receptor affinity can be affected either directly (e.g., by obstructing Tf/TfR interface) or indirectly (by changing Tf metal affinity), thereby limiting its ability to either cross the BBB or the intestinal epithelial barrier, or be internalized by the malignant cells. It is clear that successful implementation of the drug delivery strategy that utilizes Tf as a means of transporting the payload to its therapeutic target requires better understanding of the effects exerted by the conjugated drug on various properties of Tf.
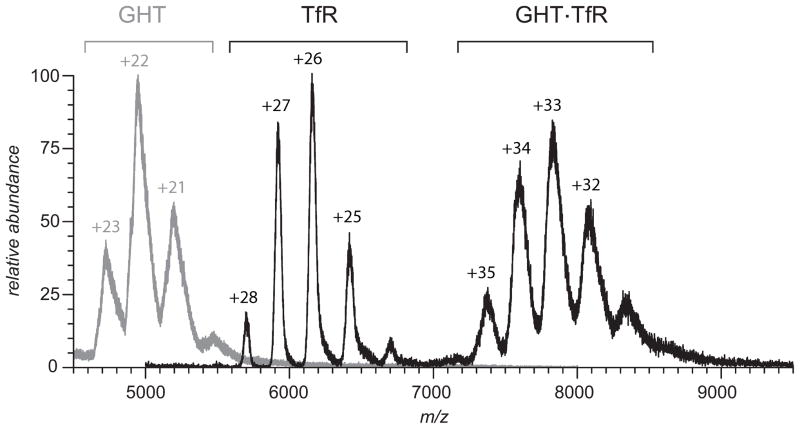
ESI MS will undoubtedly play a critical role in these efforts due to its ability not only to detect Tf-small molecule drug adducts [57, 58] and perhaps localize the modification sites [59, 60], but also provide a wealth of information on other aspects of Tf-based therapeutics, particularly their structure and interaction with TfR and other physiological partners and/or therapeutic targets. An example is shown in Figure 11, where ESI MS is used to study TfR binding to a human growth hormone-transferrin fusion protein (GHT), which has been shown recently to be a viable orally administered protein therapeutic [50]. The growth hormone is unable to traverse the intestinal epithelial barrier on its own, while TfR has been found in many parts of intestinal tract [61]. Therefore, the ability of GHT to reach the growth hormone’s physiological targets outside of the GI tract following oral administration critically depends upon its ability to bind to TfR. The ESI mass spectrum of the GHT/TfR mixture presented in Figure 11 clearly shows that transferrin maintains its ability to associate with the receptor despite the presence of a large (>20 kDa) payload attached to its N-terminus via a polypeptide linker.
Figure 11.
ESI MS of a mixture of a recombinant human growth hormone protein/human transferrin (GHT) and the soluble part of transferrin receptor (TfR) shows facile formation of a 1:1 complex under near-physiological conditions (130 mM ammonium acetate 20mM ammonium bicarbonate, pH 7.4). Signal corresponding to unbound TfR reflects the excessive amount of the receptor in solution, while no signal of unbound GHT could be detected (the gray trace represents the reference mass spectrum of free GHT in solution in the absence of TfR). Reproduced with permission from [68].
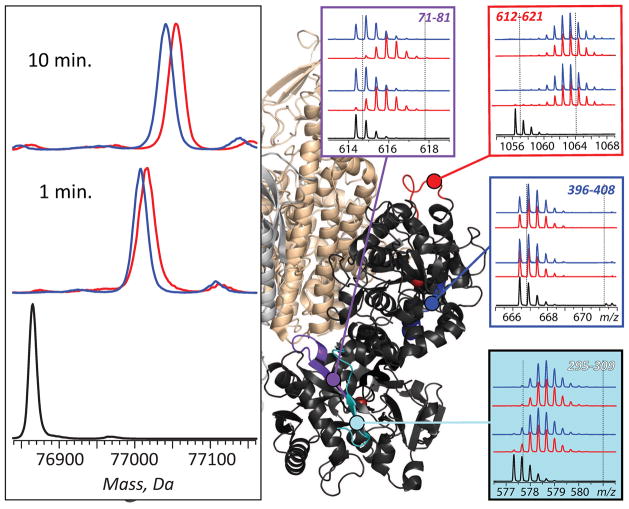
More detailed characterization of interactions of Tf-based therapeutics with TfR, as well as their intended therapeutic targets, can be obtained using HDX MS. Figure 12 illustrates the process of mapping the receptor binding interface on Fe2Tf surface by comparing the HDX kinetics (both global and at the peptide level) for the protein in its free and receptor-bound forms. Small, but detectable deceleration of global HDX can be seen for Fe2Tf in the presence of the receptor, although the local protection in the majority of the protein segments remains insensitive to the presence of the receptor. Nonetheless, deceleration of the local exchange kinetics can be observed within a few Tf segments in the presence of TfR (e.g., segment 71–81 in Figure 12) suggesting their intimate involvement in receptor binding. Since X-ray crystallography data are unavailable for Tf-drug conjugates or fusion proteins (let alone their complexes with TfR), HDX MS measurements are likely to play a vital role in rational design of the next generation of Tf-based therapies.
Figure 12.
Effect exerted by the receptor binding on Tf protection and localization of receptor interface with HDX MS. Left panel: HDX MS of Fe2Tf (global exchange) in the presence (blue) and the absence (red) of TfR. The exchange was carried out by diluting the protein stock solution 1:10 in exchange solution (100 mM NH4HCO3 in D2O, pH adjusted to 7.4) and incubating for a certain period of time as indicated on each diagram followed by rapid quenching (lowering pH to 2.5 and temperature to near 0°C). The black trace shows unlabeled protein. Right panel: isotopic distributions of representative peptic fragments derived from Fe2Tf subjected to HDX in the presence (blue) and the absence (red) of the receptor and followed by rapid quenching, proteolysis and LC/MS analysis. Dotted lines indicate deuterium content of unlabeled and fully exchanged peptides. Colored segments within the Fe2Tf/receptor complex show localization of these peptic fragments (based on the low-resolution structure of Tf/TfR [69]). Adapted with permission from [70].
8. Conclusions
Mass spectrometry is a relative newcomer in Tf characterization, but the past decade witnessed a dramatic expansion of the scope of studies where various MS-based techniques provide important and often unique information on various properties of this protein. These developments also benefit mass spectrometry itself, since many of the techniques that were initially developed to investigate Tf behavior can now be applied to study properties of a range of other metalloproteins [62–64]. Likewise, the suite of MS-based analytical techniques that are being developed now to support design and optimization of various Tf-based therapies are likely to be utilized in the near future to support development of other novel medicines that will employ other transport proteins for precise routing of drugs to their therapeutic targets.
Acknowledgments
This work was supported by a grant R01 GM061666 from the National Institutes of Health. The authors are grateful to Prof. Anne B. Mason (University of Vermont College of Medicine) for useful discussions of many aspects of the presented work over the last decade and for her generosity in providing Tf and TfR samples. The GHT sample was generously provided by Prof. Wei-Chiang Shen (University of Southern California School of Pharmacy, Los Angeles, CA).
List of abbreviations
- BBB
blood-brain barrier
- ESI
electrospray ionization
- FAB
fast atom bombardment
- Fe2hTf
diferric form of human serum transferrin
- GHT
human growth hormone-human serum transferrin fusion protein
- HDX
hydrogen/deuterium exchange
- hTf
human serum transferrin
- hTf/2N
N-lobe of human serum transferrin
- MS
mass spectrometry
- Tf
transferrin
- TfR
transferrin receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaltashov IA, Cotter RJ, Feinstone WH, Ketner GW, Woods AS. Ferrichrome: Surprising stability of a cyclic peptide Fe-III complex revealed by mass spectrometry. J Am Soc Mass Spectrom. 1997;8:1070–1077. [Google Scholar]
- 2.Woodworth RC, Mason AB, Funk WD, MacGillivray RTA. Expression and initial characterization of five site-directed mutants of the N-terminal half-molecule of human transferrin. Biochemistry. 1991;30:10824–10829. doi: 10.1021/bi00109a002. [DOI] [PubMed] [Google Scholar]
- 3.Mason AB, Miller MK, Funk WD, Banfield DK, Savage KJ, Oliver RWA, Green BN, MacGillivray RTA, Woodworth RC. Expression of glycosylated and nonglycosylated human transferrin in mammalian cells. Characterization of the recombinant proteins with comparison to three commercially available transferrins. Biochemistry. 1993;32:5472–5479. doi: 10.1021/bi00071a025. [DOI] [PubMed] [Google Scholar]
- 4.Yu X, Wojciechowski M, Fenselau C. Assessment of metals in reconstituted metallothioneins by electrospray mass spectrometry. Anal Chem. 1993;65:1355–1359. doi: 10.1021/ac00058a010. [DOI] [PubMed] [Google Scholar]
- 5.Gumerov DR, Kaltashov IA. Dynamics of iron release from transferrin N-lobe studied by electrospray ionization mass spectrometry. Anal Chem. 2001;73:2565–2570. doi: 10.1021/ac0015164. [DOI] [PubMed] [Google Scholar]
- 6.Halbrooks PJ, Mason AB, Adams TE, Briggs SK, Everse SJ. The oxalate effect on release of iron from human serum transferrin explained. J Mol Biol. 2004;339:217–226. doi: 10.1016/j.jmb.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 7.Zhang M, Gumerov DR, Kaltashov IA, Mason AB. Indirect detection of protein-metal binding: Interaction of serum transferrin with In3+ and Bi3+ J Am Soc Mass Spectrom. 2004;15:1658–1664. doi: 10.1016/j.jasms.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Konermann L, Douglas DJ. Acid-Induced unfolding of cytochrome c at different methanol concentrations: Electrospray ionization mass spectrometry specifically monitors changes in the tertiary structure. Biochemistry. 1997;36:12296–12302. doi: 10.1021/bi971266u. [DOI] [PubMed] [Google Scholar]
- 9.Kaltashov IA, Abzalimov RR. Do ionic charges in ESI MS provide useful information on macromolecular structure? J Am Soc Mass Spectrom. 2008;19:1239–1246. doi: 10.1016/j.jasms.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 10.van den Bremer ET, Jiskoot W, James R, Moore GR, Kleanthous C, Heck AJ, Maier CS. Probing metal ion binding and conformational properties of the colicin E9 endonuclease by electrospray ionization time-of-flight mass spectrometry. Protein Sci. 2002;11:1738–1752. doi: 10.1110/ps.0200502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao H, Kaltashov IA, Eyles SJ. Indirect assessment of small hydrophobic ligand binding to a model protein using a combination of ESI MS and HDX/ESI MS. J Am Soc Mass Spectrom. 2003;14:506–515. doi: 10.1016/S1044-0305(03)00135-1. [DOI] [PubMed] [Google Scholar]
- 12.Griffith WP, Kaltashov IA. Protein conformational heterogeneity as a binding catalyst: ESI-MS study of hemoglobin H formation. Biochemistry. 2007;46:2020–2026. doi: 10.1021/bi062032q. [DOI] [PubMed] [Google Scholar]
- 13.Aisen P. Transferrin, the transferrin receptor, and the uptake of iron by cells. Met Ions Biol Syst. 1998;35:585–631. [PubMed] [Google Scholar]
- 14.He QF, Mason AB. Molecular aspects of release of iron from transferrin. In: Templeton DM, editor. Molecular and cellular iron transport. Marcel Dekker, Inc; New York: 2002. pp. 95–123. [Google Scholar]
- 15.Nguyen SAK, Craig A, Raymond KN. Transferrin - the role of conformational changes in iron removal by chelators. J Am Chem Soc. 1993;115:6758–6764. [Google Scholar]
- 16.Muralidhara BK, Hirose M. Anion-mediated iron release from transferrins. The kinetic and mechanistic model for N-lobe of ovotransferrin. J Biol Chem. 2000;275:12463–12469. doi: 10.1074/jbc.275.17.12463. [DOI] [PubMed] [Google Scholar]
- 17.Mizutani K, Yamashita H, Kurokawa H, Mikami B, Hirose M. Alternative structural state of transferrin. The crystallographic analysis of iron-loaded but domain-opened ovotransferrin N-lobe. J Biol Chem. 1999;274:10190–10194. doi: 10.1074/jbc.274.15.10190. [DOI] [PubMed] [Google Scholar]
- 18.Khan JA, Kumar P, Srinivasan A, Singh TP. Protein intermediate trapped by the simultaneous crystallization process. Crystal structure of an iron-saturated intermediate in the Fe3+ binding pathway of camel lactoferrin at 2.7 Å resolution. J Biol Chem. 2001;276:36817–36823. doi: 10.1074/jbc.M104343200. [DOI] [PubMed] [Google Scholar]
- 19.Baker HM, Anderson BF, Baker EN. Dealing with iron: Common structural principles in proteins that transport iron and heme. Proc Natl Acad Sci U S A. 2003;100:3579–3583. doi: 10.1073/pnas.0637295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gumerov DR, Mason AB, Kaltashov IA. Interlobe communication in human serum transferrin: metal binding and conformational dynamics investigated by electrospray ionization mass spectrometry. Biochemistry. 2003;42:5421–5428. doi: 10.1021/bi020660b. [DOI] [PubMed] [Google Scholar]
- 21.He QY, Mason AB, Tam BM, MacGillivray RT, Woodworth RC. Dual role of Lys206-Lys296 interaction in human transferrin N-lobe: iron-release trigger and anion-binding site. Biochemistry. 1999;38:9704–9711. doi: 10.1021/bi990134t. [DOI] [PubMed] [Google Scholar]
- 22.Halbrooks PJ, He QY, Briggs SK, Everse SJ, Smith VC, MacGillivray RT, Mason AB. Investigation of the mechanism of iron release from the C-lobe of human serum transferrin: mutational analysis of the role of a pH sensitive triad. Biochemistry. 2003;42:3701–3707. doi: 10.1021/bi027071q. [DOI] [PubMed] [Google Scholar]
- 23.Lao BJ, Kamei DT. Improving therapeutic properties of protein drugs through alteration of intracellular trafficking pathways. Biotechnol Prog. 2008;24:2–7. doi: 10.1021/bp070080b. [DOI] [PubMed] [Google Scholar]
- 24.Englander SW. Hydrogen exchange and mass spectrometry: A historical perspective. J Am Soc Mass Spectrom. 2006;17:1481–1489. doi: 10.1016/j.jasms.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konermann L, Tong X, Pan Y. Protein structure and dynamics studied by mass spectrometry: H/D exchange, hydroxyl radical labeling, and related approaches. J Mass Spectrom. 2008;43:1021–1036. doi: 10.1002/jms.1435. [DOI] [PubMed] [Google Scholar]
- 26.Dempsey CE. Hydrogen exchange in peptides and proteins using NMR-spectroscopy. Progr Nucl Magn Res Spectrosc. 2001;39:135–170. [Google Scholar]
- 27.Bobst CE, Zhang M, Kaltashov IA. Existence of a noncanonical state of iron-bound transferrin at endosomal pH revealed by hydrogen exchange and mass spectrometry. J Mol Biol. 2009;388:954–967. doi: 10.1016/j.jmb.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason AB, Halbrooks PJ, James NG, Byrne SL, Grady JK, Chasteen ND, Bobst CE, Kaltashov IA, Smith VC, MacGillivray RTA, Everse SJ. Structural and functional consequences of the substitution of Glycine 65 with Arginine in the N-lobe of human transferrin. Biochemistry. 2009;48:1945–1953. doi: 10.1021/bi802254x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giannetti AM, Snow PM, Zak O, Bjorkman PJ. Mechanism for multiple ligand recognition by the human transferrin receptor. PLoS Biol. 2003;1:341–350. doi: 10.1371/journal.pbio.0000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aisen P. Transferrin receptor 1. Int J Biochem Cell Biol. 2004;36:2137–2143. doi: 10.1016/j.biocel.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Macedo MF, de Sousa M. Transferrin and the transferrin receptor: of magic bullets and other concerns. Inflamm Allergy Drug Targets. 2008;7:41–52. doi: 10.2174/187152808784165162. [DOI] [PubMed] [Google Scholar]
- 32.Dautry-Varsat A, Ciechanover A, Lodish HF. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983;80:2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lebrón JA, Bennett MJ, Vaughn DE, Chirino AJ, Snow PM, Mintier GA, Feder JN, Bjorkman PJ. Crystal structure of the hemochromatosis protein HFE and characterization of its interaction with transferrin receptor. Cell. 1998;93:111–123. doi: 10.1016/s0092-8674(00)81151-4. [DOI] [PubMed] [Google Scholar]
- 34.Giannetti AM, Halbrooks PJ, Mason AB, Vogt TM, Enns CA, Björkman PJ. The molecular mechanism for receptor-stimulated iron release from the plasma iron transport protein transferrin. Structure. 2005;13:1613–1623. doi: 10.1016/j.str.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 35.Yersin A, Osada T, Ikai A. Exploring transferrin-receptor interactions at the single-molecule level. Biophys J. 2008;94:230–240. doi: 10.1529/biophysj.107.114637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young SP, Bomford A, Williams R. The effect of the iron saturation of transferrin on its binding and uptake by rabbit reticulocytes. Biochem J. 1984;219:505–510. doi: 10.1042/bj2190505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mason A, He QY, Tam B, MacGillivray RA, Woodworth R. Mutagenesis of the aspartic acid ligands in human serum transferrin: lobe-lobe interaction and conformation as revealed by antibody, receptor-binding and iron-release studies. Biochem J. 1998;330:35–40. doi: 10.1042/bj3300035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hemadi M, Kahn PH, Miquel G, El Hage Chahine J-M. Transferrin’s mechanism of interaction with receptor 1. Biochemistry. 2004;43:1736–1745. doi: 10.1021/bi030142g. [DOI] [PubMed] [Google Scholar]
- 39.Loo JA. Studying noncovalent protein complexes by electrospray ionization mass spectrometry. Mass Spectrom Rev. 1997;16:1–23. doi: 10.1002/(SICI)1098-2787(1997)16:1<1::AID-MAS1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 40.Sharon M, Robinson CV. The role of mass spectrometry in structure elucidation of dynamic protein complexes. Annu Rev Biochem. 2007;76:167–193. doi: 10.1146/annurev.biochem.76.061005.090816. [DOI] [PubMed] [Google Scholar]
- 41.Heck AJR. Native mass spectrometry: a bridge between interactomics and structural biology. Nat Meth. 2008;5:927–933. doi: 10.1038/nmeth.1265. [DOI] [PubMed] [Google Scholar]
- 42.Leverence R, Mason AB, Kaltashov IA. Noncanonical interactions between serum transferrin and transferrin receptor evaluated with electrospray ionization mass spectrometry. Proc Natl Acad Sci USA. 2010;107:8123–8128. doi: 10.1073/pnas.0914898107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mason AB, Halbrooks PJ, Larouche JR, Briggs SK, Moffett ML, Ramsey JE, Connolly SA, Smith VC, MacGillivray RTA. Expression, purification, and characterization of authentic monoferric and apo-human serum transferrins. Protein Expr Purif. 2004;36:318–326. doi: 10.1016/j.pep.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 44.Dautry-Varsat A. Receptor-mediated endocytosis: the intracellular journey of transferrin and its receptor. Biochimie. 1986;68:375–381. doi: 10.1016/s0300-9084(86)80004-9. [DOI] [PubMed] [Google Scholar]
- 45.Singh M. Transferrin as a targeting ligand for liposomes and anticancer drugs. Curr Pharm Des. 1999;5:443–451. [PubMed] [Google Scholar]
- 46.Qian ZM, Li H, Sun H, Ho K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev. 2002;54:561–587. doi: 10.1124/pr.54.4.561. [DOI] [PubMed] [Google Scholar]
- 47.Daniels TR, Delgado T, Helguera G, Penichet ML. The transferrin receptor part II: Targeted delivery of therapeutic agents into cancer cells. Clin Immunol. 2006;121:159–176. doi: 10.1016/j.clim.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Smith MW, Gumbleton M. Endocytosis at the blood-brain barrier: From basic understanding to drug delivery strategies. J Drug Target. 2006;14:191–214. doi: 10.1080/10611860600650086. [DOI] [PubMed] [Google Scholar]
- 49.Jones AR, Shusta EV. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm Res. 2007;24:1759–1771. doi: 10.1007/s11095-007-9379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amet N, Wang W, Shen W-C. Human growth hormone-transferrin fusion protein for oral delivery in hypophysectomized rats. J Control Release. 2010;141:177–182. doi: 10.1016/j.jconrel.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bai Y, Ann DK, Shen W-C. Recombinant granulocyte colony-stimulating factor-transferrin fusion protein as an oral myelopoietic agent. Proc Natl Acad Sci U S A. 2005;102:7292–7296. doi: 10.1073/pnas.0500062102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Widera A, Bai Y, Shen W-C. The transepithelial transport of a G-CSF-transferrin conjugate in Caco-2 cells and its myelopoietic effect in BDF1 mice. Pharmaceutical research. 2004;21:278–284. doi: 10.1023/b:pham.0000016240.81059.ec. [DOI] [PubMed] [Google Scholar]
- 53.Shah D, Shen WC. Transcellular delivery of an insulin-transferrin conjugate in enterocyte-like Caco-2 cells. Journal of pharmaceutical sciences. 1996;85:1306–1311. doi: 10.1021/js9601400. [DOI] [PubMed] [Google Scholar]
- 54.Hartinger CG, Hann S, Koellensperger G, Sulyok M, Groessl M, Timerbaev AR, Rudnev AV, Stingeder G, Keppler BK. Interactions of a novel ruthenium-based anticancer drug (KP1019 or FFC14a) with serum proteins-significance for the patient. Int J Clin Pharmacol Ther. 2005;43:583–585. doi: 10.5414/cpp43583. [DOI] [PubMed] [Google Scholar]
- 55.Yazdi PT, Wenning LA, Murphy RM. Influence of cellular trafficking on protein synthesis inhibition of immunotoxins directed against the transferrin receptor. Cancer Res. 1995;55:3763–3771. [PubMed] [Google Scholar]
- 56.Amet N, Lee HF, Shen WC. Insertion of the designed helical linker led to increased expression of Tf-based fusion proteins. Pharm Res. 2009;26:523–528. doi: 10.1007/s11095-008-9767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pongratz M, Schluga P, Jakupec MA, Arion VB, Hartinger CG, Allmaier G, Keppler BK. Transferrin binding and transferrin-mediated cellular uptake of the ruthenium coordination compound KP1019, studied by means of AAS, ESI-MS and CD spectroscopy. J Anal At Spectrom. 2004;19:46–51. [Google Scholar]
- 58.Mendoza-Ferri MG, Hartinger CG, Mendoza MA, Groessl M, Egger AE, Eichinger RE, Mangrum JB, Farrell NP, Maruszak M, Bednarski PJ, Klein F, Jakupec MA, Nazarov AA, Severin K, Keppler BK. Transferring the concept of multinuclearity to ruthenium complexes for improvement of anticancer activity. J Med Chem. 2009;52:916–925. doi: 10.1021/jm8013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hartinger CG, Tsybin YO, Fuchser J, Dyson PJ. Characterization of platinum anticancer drug protein-binding sites using a top-down mass spectrometric approach. Inorg Chem. 2008;47:17–19. doi: 10.1021/ic702236m. [DOI] [PubMed] [Google Scholar]
- 60.Scotcher J, Clarke D, Weidt S, Mackay C, Hupp T, Sadler P, Langridge-Smith P. Identification of two reactive cysteine residues in the tumor suppressor protein p53 using top-down FTICR mass spectrometry. J Am Soc Mass Spectrom. 2011;22:888–897. doi: 10.1007/s13361-011-0088-x. [DOI] [PubMed] [Google Scholar]
- 61.Banerjee D, Flanagan PR, Cluett J, Valberg LS. Transferrin receptors in the human gastrointestinal tract. Relationship to body iron stores. Gastroenterology. 1986;91:861–869. doi: 10.1016/0016-5085(86)90687-6. [DOI] [PubMed] [Google Scholar]
- 62.Kaltashov IA, Zhang M, Eyles SJ, Abzalimov RR. Investigation of structure, dynamics and function of metalloproteins with electrospray ionization mass spectrometry. Anal Bioanal Chem. 2006;386:472–481. doi: 10.1007/s00216-006-0636-6. [DOI] [PubMed] [Google Scholar]
- 63.Griffith WP, Kaltashov IA. Mass spectrometry in the study of hemoglobin: from covalent structure to higher order assembly. Curr Org Chem. 2006;10:535–553. [Google Scholar]
- 64.Shi Y, Harvey I, Campopiano D, Sadler PJ. Niobium uptake and release by bacterial ferric ion binding protein. Bioinorg Chem Appl. 2010;2010:307578. doi: 10.1155/2010/307578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jeffrey PD, Bewley MC, MacGillivray RT, Mason AB, Woodworth RC, Baker EN. Ligand-induced conformational change in transferrins: crystal structure of the open form of the N-terminal half-molecule of human transferrin. Biochemistry. 1998;37:13978–13986. doi: 10.1021/bi9812064. [DOI] [PubMed] [Google Scholar]
- 66.MacGillivray RT, Moore SA, Chen J, Anderson BF, Baker H, Luo Y, Bewley M, Smith CA, Murphy ME, Wang Y, Mason AB, Woodworth RC, Brayer GD, Baker EN. Two high-resolution crystal structures of the recombinant N-lobe of human transferrin reveal a structural change implicated in iron release. Biochemistry. 1998;37:7919–7928. doi: 10.1021/bi980355j. [DOI] [PubMed] [Google Scholar]
- 67.Bobst CE, Kaltashov IA. Advanced Mass Spectrometry-Based Methods for the Analysis of Conformational Integrity of Biopharmaceutical Products. Curr Pharm Biotechnol. 2011 doi: 10.2174/138920111798357311. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaltashov IA, Bobst CE, Abzalimov RR, Wang G, Baykal B, Wang S. Advances and challenges in analytical characterization of biotechnology products: Mass spectrometry-based approaches to study properties and behavior of protein therapeutics. Biotechnol Adv. 2011 doi: 10.1016/j.biotechadv.2011.05.006. in press, accepted manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng Y, Zak O, Aisen P, Harrison SC, Walz T. Structure of the human transferrin receptor-transferrin complex. Cell. 2004;116:565–576. doi: 10.1016/s0092-8674(04)00130-8. [DOI] [PubMed] [Google Scholar]
- 70.Kaltashov IA, Bobst CE, Abzalimov RR. H/D exchange and mass spectrometry in the studies of protein conformation and dynamics: Is there a need for a top-down approach? Anal Chem. 2009;81:7892–7899. doi: 10.1021/ac901366n. [DOI] [PMC free article] [PubMed] [Google Scholar]