Abstract
Purpose
To determine the response rate to oral capsular fenretinide in children with recurrent or biopsy proven refractory high-risk neuroblastoma.
Experimental Design
Patients received 7 days of fenretinide: 2475 mg/m2/day divided TID (<18 years) or 1800 mg/m2/day divided BID (≥18 years) every 21 days for a maximum of 30 courses. Patients with stable or responding disease after course 30 could request additional compassionate courses. Best response by course 8 was evaluated in Stratum 1 (measurable disease on CT/MRI +/− bone marrow and/or MIBG avid sites) and Stratum 2 (bone marrow and/or MIBG avid sites only).
Results
Sixty-two eligible patients, median age 5 years (range 0.6–19.9), were treated in Stratum 1 (n=38) and Stratum 2 (n=24). One partial response (PR) was seen in Stratum 2 (n=24 evaluable). No responses were seen in Stratum 1 (n=35 evaluable). Prolonged stable disease (SD) was seen in 7 patients in Stratum 1 and 6 patients in Stratum 2 for 4–45+ (median 15) courses. Median time to progression was 40 days (range 17–506) for Stratum 1 and 48 days (range 17–892) for Stratum 2. Mean 4-HPR steady state trough plasma concentrations were 7.25 µM (coefficient of variation 40–56%) at day 7 course 1. Toxicities were mild and reversible.
Conclusions
Although neither stratum met protocol criteria for efficacy, 1 PR + 13 prolonged SD occurred in 14/59 (24%) of evaluable patients. Low bioavailability may have limited fenretinide activity. Novel fenretinide formulations with improved bioavailability are currently in pediatric Phase I studies.
Keywords: fenretinide, neuroblastoma, Phase II, ANBL0321
INTRODUCTION
Retinoids are vitamin A derivatives that modulate growth and differentiation of normal and malignant cells (1). A previous Children’s Cancer Group (CCG) trial demonstrated that the addition of six courses of intermittent 13-cis-retinoic acid (13-cisRA) following induction chemotherapy and myeloablative chemotherapy demonstrated improved event-free survival (EFS) and overall survival (OS) in children with high risk neuroblastoma (2,3). A recent randomized Children’s Oncology Group (COG) study showed that adding immunotherapy with the anti-tumor cell disialoganglioside (anti-GD2) monoclonal antibody ch14.18 and cytokines to 13-cisRA maintenance further improves outcome (4). Despite these advances, only 45% children with high-risk neuroblastoma survive long-term (2,3), emphasizing the need for novel therapies.
The synthetic retinoid, fenretinide (N- (4-hydroxyphenyl) retinamide] or 4-HPR) inhibits growth of neuroblastoma (5–7), colorectal (8), prostate (9), breast (10), ovarian (11), small-cell lung cancer (12,13), and leukemia cell lines (14,15) at 1–10 µM concentrations in vitro. Importantly, 4-HPR is active against neuroblastoma cell lines resistant to 13-cisRA, alkylating agents, and etoposide (5,16), suggesting it may have activity against tumors resistant to standard neuroblastoma therapy. In contrast to the differentiating agent 13-cisRA, 4-HPR induces both apoptosis and non-apoptotic cell death (6,9,14). 4-HPR cytotoxic mechanisms may include increase of dihydroceramide production (16–19) or reactive oxygen species (16,20,21), inhibition of angiogenesis (17,22), or increased natural killer cell activity (23,24).
The CCG 09709 Phase I trial determined the maximal tolerated dose (MTD) of capsular fenretinide given for seven days every 21 days to children with solid tumors was 2475 mg/m2/day divided TID (25). The MTD achieved mean peak 4-HPR plasma levels (Day 7, continuous steady state [Css]) of 9.9 µM with minimal toxicity. Among 30 evaluable neuroblastoma patients, there was one complete response and 13 stable patients with disease for ≥8 courses. A Phase I adult trial using an identical schedule of capsular fenretinide determined an MTD of 1800 mg/m2/day divided BID based on a plateau in plasma levels rather than dose limiting toxicity, with variable peak plasma levels of 7.5 to 13 µM (26). Based on these data, this Phase II trial was designed to determine the response rate to capsular fenretinide (2475 mg/m2/day for ≤18 years of age or 1800 mg/m2/day for >18 years of age) in children with recurrent/refractory neuroblastoma. Response was assessed in two strata: ONE: disease measureable by CT/MRI, and TWO: disease evaluable by bone marrow morphology and/or iodine-131-meta-iodobenzylguanidine (131-I-MIBG) uptake.
PATIENTS AND METHODS
Eligibility
Patients ≤21 years at diagnosis of high risk neuroblastoma with recurrent or resistant/refractory disease were eligible after any number of prior therapies, including hematopoietic stem cell transplant and retinoids (excluding fenretinide). Patients were required to have at least one of the following: measurable soft tissue mass (≥20 mm on MRI/CT scan; ≥10 mm on spiral CT); MIBG avid tumor; or bone marrow metastases by routine morphology. Patients with prior relapse were eligible if they had ≥5 tumor cells/106 mononuclear cells by bone marrow immunocytology (27) on two serial marrows. Patients without prior relapse were required to have histologic confirmation of tumor sites on CT/MRI, and/or MIBG scans if bone marrow morphology was negative. Normal hepatic and renal function was required. Hematologic criteria were hemoglobin ≥7.5 mg/dl (transfusion allowed). All patients and/or guardian(s) signed written informed consent approved at local Institutional Review Boards in accordance with an assurance filed with and approved by the U.S. Department of Health and Human Services.
Treatment
Fenretinide (provided by National Cancer Institute) was given as intact 4-HPR (100 mg) capsules by mouth at a dose of 2475 mg/m2/day divided into three equal doses (≤18 years age) or 1800 mg/m2/day divided into two equal doses (>18 years age) for seven days followed by two weeks rest. Total dose administered (in milligrams) was reported for each course, and roadmaps submitted noted missed doses with comments on reason. Courses were repeated every 21 days, in the absence of progression, for maximum of 30 courses per protocol. Patients with stable or responding tumor could request additional compassionate access fenretinide. Patients off protocol therapy were followed until entry onto another COG therapeutic study, loss to follow-up, or death.
Response Criteria
Overall response was graded using a modification of the International Neuroblastoma Response Criteria using the RECIST criteria (28) to evaluate CT/MRI response of measurable tumor (≥30% decrease in sum of longest diameters) and Curie score (29) for MIBG response (relative Curie score ≤0.5). A complete bone marrow response was defined as no tumor by morphology on two serial samplings ≥3 weeks apart. CT/MRI, MIBG scans, and bone marrow slides were centrally reviewed for patients with an overall response of stable disease for >3 courses or better. Radiology and bone marrow reports were reviewed to confirm tumor sites at entry.
Pharmacokinetics
Heparinized blood samples were collected during Course 1 prior to the first dose and 6 hours later; prior to Day 4 morning dose; and prior to Day 7 morning dose and 6 hours later. Additional samples were collected before the Day 1 morning dose of Courses 2, 5, and 9; and before the Day 7 morning dose of Courses 4 and 8. Samples were wrapped in foil, immediately chilled in ice-water, and centrifuged to obtain plasma which was frozen in foil-wrapped polypropylene tubes at −70°C as described by Bugge et al (30)
Fenretinide, N-(4-methoxyphenyl) retinamide (4-MPR), and retinol were measured by reverse phase high performance liquid chromatography (HPLC) with UV absorbance detection using a modification of the assay of Formelli et al (31) under indirect yellow light. Plasma samples prepared in silanized amber microcentrifuge tubes were kept in the dark and cold. Retinol standard curve samples were prepared by adding known amounts of authentic compound to 500 µL of 5% serum albumin containing the internal standard. Plasma proteins were precipitated by adding 900 µL ice-cold acetonitrile and 100 µL ice-cold saturated potassium phosphate to each 500 µL plasma sample. After centrifugation, the supernatant was added to amber autosampler vials kept in the dark at room temperature until analyzed. HPLC separations were performed on a Phenomenex Luna C18(2) analytical column (100 mm × 4.6 mm i.d., 3 µ fitted with a Brownlee RP-18 precolumn (15 mm × 3.2 mm i.d.,7 µ) and eluted with a mobile phase composed of acetonitrile:water:glacial acetic acid (80:18:2) delivered at rate of 0.9 mL/min. The UV absorption wavelength and injection volume were 340 nm and 50 µL, respectively.
Statistical Considerations
The primary trial aim was evaluation of the response rate to capsular fenretinide. Patients were evaluable for response if they completed ≥2 courses or had tumor progression any time prior to completing 2 courses. A responder was defined as a best overall response of Complete (CR), Very Good Partial (VGPR), or Partial (PR) response after ≤8 courses. Response rates were assessed separately via a one-stage rule within Stratum 1: CT/MRI measurable tumor +/− other sites; and Stratum 2: MIBG avid tumor and/or tumor in bone marrow by morphology without CT/MRI measurable tumor. Fenretinide would be deemed effective if there were ≥5 responders among 25 evaluable patients in a given stratum (power of 91% to detect a 20% difference (30% vs. 10%) at significance level of 0.098). More than 25 patients were accrued to Stratum One since not all patients were evaluable for response, and some patients were reassigned from Stratum Two after review of tumor sites at entry. Patients with tumor detectable only by bone marrow immunocytology at entry (Stratum 3) were enrolled for descriptive analysis only until accrual was completed in other strata.
Toxicities were collected on patients who received at least one dose of fenretinide. The pharmacokinetics of fenretinide, metabolite 4-MPR, and plasma retinol levels were assessed via descriptive analyses of steady-state levels.
Progression-free survival (PFS) and overall survival (OS) were calculated using the method of Kaplan and Meier (32) with standard errors per Peto et al (33). Time to progression (TTP) was calculated from study enrollment date until the first occurrence of relapse/progression or death due to tumor, or last contact date if no progression occurred. Overall survival was calculated from study enrollment date until death from any cause, or date of last contact. Survival curves were compared using a log-rank test.
TTP was calculated from study enrollment date until the first relapse/progression. Median times to progression were compared using a two-sided Wilcoxon rank-sum test (34). P-values <0.05 were considered statistically significant.
RESULTS
Patient Characteristics
Sixty-five patients enrolled from May 12, 2003 to December 17, 2004. Characteristics of the 62 eligible patients are shown in Table 1. Three patients were ineligible: 1) (Stratum 3) enrolled, but failed to meet Stratum 3 eligibility (Stratum 2, for which the patient was eligible, was closed to accrual); 2) (Stratum 3) inadvertently enrolled prior to informed consent; 3) (Stratum 1) ineligible because oral etoposide was given the same day fenretinide was started; after one course went off protocol therapy due to progressive disease (PD).
Table 1.
Patient characteristics with 3-year PFS and OS by category.
| Characteristic | # Pts | 3-year PFS ± SE (%) |
p-value | 3-year OS ± SE (%) |
p-value |
|---|---|---|---|---|---|
| Overall | 62 | 6.1 ± 3.4 | N/A | 19.1 ± 5.7 | N/A |
| Gender | |||||
| Male | 39 | 5.3 ± 3.6 | 0.9087 | 20.5 ± 7.4 | 0.7101 |
| Female | 23 | 6.5 ± 6.3 | 16.1 ± 8.5 | ||
| Treatment & Patient Age | |||||
| ≤18 years old: 2475 mg/m2/day divided TID | 56 | 4.8 ± 3.3 | 0.398 | 15.0 ± 5.6 | 0.2755 |
| >18 years old: 1800 mg/m2/day divided BID | 6 | 16.7 ± 15.2 | 50.0 ± 20.4 | ||
| Stratum | |||||
| Stratum 1: ≥ 1 measurable lesion on CT or MRI | 38 | 8.1 ± 5.5 | 0.5603 | 16.0 ± 6.5 | 0.7827 |
| Stratum 2: BM tumor +/− ≥1 MIBG avid site | 24 | 4.2 ± 4.1 | 24.5 ± 10.6 | ||
| Tumor Sites at Study Entry | |||||
| Bone marrow only | 4 | 0 | 0.0211 | 0 | 0.0913 |
| MIBG only | 6 | 16.7 ± 15.2 | 40.0 ± 21.9 | ||
| CT/MRI only | 4 | 0 | 0 | ||
| BM, MIBG | 14 | 0 | 0.0218 | 23.1 ± 14.3 | 0.0072 |
| BM, CT/MRI | 2 | 0 | 0 | ||
| CT/MRI, MIBG | 13 | 15.4 ± 10.0 | 38.5 ± 13.5 | ||
| BM, MIBG, CT/MRI | 19 | 0 | 0 | ||
| Relapse/Recurrence Prior to Study Entry | |||||
| Yes | 52 | 2.0 ± 1.9 | 0.0005 | 10.5 ± 5.0 | 0.0026 |
| No | 10 | 26.7 ± 16.1 | 63.5 ± 17.2 | ||
| Prior Hematopoietic Stem Cell Transplanta | |||||
| Yes | 51 | 7.4 ± 4.1 | 0.4231 | 21.3 ± 6.7 | 0.4834 |
| No | 6 | 0 | 20.0 ± 17.9 | ||
| Prior Retinoid Therapya | |||||
| Yes | 44 | 4.5 ± 3.1 | 0.0583 | 14.9 ± 6.2 | 0.1683 |
| No | 13 | 12.5 ± 11.7 | 53.6 ± 18.3 | ||
5 patients with missing data. Pts = patients
The median age at enrollment was 5 years (range 0.6–19.9). Only three patients enrolled were less than 4 years of age. The median time from last prior retinoid use to study enrollment was 1.5 years (range 10 days–5.9 years). The median time from diagnosis to the start of fenretinide was 2.5 years (range 20 days–12.8 years). Three patients (2 in Stratum 2; 1 in Stratum 1) received more than 30 courses of fenretinide via compassionate release (Table 2).
Table 2.
Summary of 14 patients with response ≥SD (n = 59 evaluable patients)
| TUMOR SITES AT ENTRY: |
Overall Response (site responses) |
# Courses response maintained |
|||
|---|---|---|---|---|---|
| Stratum | MIBG Score | CT longest dimension |
Bone marrow |
||
| 2 | 3 | 0 | Negative | PR | 11 |
| 2 | 11 | 0 | Positivea (< 5%) | SD (SD on MIBG; CR in BM) | 15 |
| 2 | 15 | 0 | Negative | SD | 45+b |
| 2 | 7 | 0 | Positive | SD (MIBG sites resolved, not evaluable since radiated) | 35 |
| (all sites radiated C1) | (< 5%) | (5 courses compassionate) | |||
| 1 | 1 | 4 cm | Negative | SD | 30+c |
| 1 | 1 | 5 cm | Negative | SD | 30+d |
| 1 | 25 | 10.3 cm | Negative | SD | 24 |
| 1 | 0 | 4 cm | Negative | SD | 15 |
| 2 | 11 | 0 | Negative | SD | 9 |
| 1 | 15 | 10.9 | Negative | SD | 7 |
| 2 | 1 | 0 | Positive (<5–10%) | SD (CR at MIBG avid bone site; not evaluable since radiated) | 7 |
| 2 | 11 | 0 | Positive | SD | 7 |
| (< 5%) | |||||
| 1 | 1 | 6 cm | Negative | SD | 5 |
| 1 | 2 | 8.5 cm | Negative | SD | 4 |
BM slides not reviewed; reports were reviewed.
Centrally reviewed C1-C20; 15 compassionate courses. Alive with SD & no further therapy 13 months off fenretinide.
Completed C30 with SD; achieved CR on compassionate fenretinide 25 months later; last confirmed 50 months post C30; alive on fenretinide 53 months post C30.
Post C30 given 13-cis-retinoic acid for 3 months. Alive 57 months post C30. CR= complete response, PR=partial response, SD=stable disease, PD= progressive disease, BM=bone marrow; C= course
Response
There were 38 eligible (35 evaluable) patients enrolled on Stratum 1 and 24 eligible and evaluable patients on Stratum 2. Three patients on Stratum 1 were inevaluable: two patients went off therapy prior to completion of two courses, and one patient was unable to swallow capsules. There was one partial response in Stratum 2 and no responses in Stratum 1. Both strata had less than the 5 responses required to meet protocol criteria for effectiveness of fenretinide. Thirteen patients (seven on Stratum 1; six on Stratum 2) had stable disease for 4–45+ (median 15) courses, and had a median Curie score of 6.5 (range 0–25, excluding one patient radiated at all MIBG sites); with a median longest dimension for mass disease of 4 (range 0–10.9) cm. Three patients with SD after course 30 remained alive at last follow-up (Table 2). One of these 3 patients, with a history of prior PD at study entry, maintained SD 13 months after completing 45 courses of fenretinide (30 per protocol plus 15 additional courses) without other therapy. The second patient, with refractory tumor at study entry, maintained SD after course 30. This patient achieved CR after 25 months of compassionate fenretinide therapy, and maintained a CR on fenretinide therapy with last follow-up 50 months after ending course 30. The third patient, with refractory tumor at study entry, had SD after 30 courses of fenretinide, then received 13-cisRA for 3 months, and was alive 57 months off fenretinide therapy.
Toxicity
Grade 3 – 4 toxicities are summarized in Table 3; there were no toxic deaths. For each patient, only the worst toxicity grade per type across all courses was counted. No unexpected toxicities occurred. There was one death in a seven year old female ten days after completion of course 1 from hepatic failure presenting six days after the last dose of fenretinide. Autopsy found widespread tumor infiltration in the liver, which was felt to be the etiology of this event. No other predisposing factors were identified. No other patients had significant hepatic toxicity.
Table 3.
Toxicities of grade 3 or 4 (targeted toxicities indicated by *) in 62 eligible patients receiving at least one dose of fenretinide. Only the worst toxicity grade per type per patient across all courses was counted.
| Toxicity | ≤ 18 years age Dose of 2475 mg/m2/day (n=56) |
> 18 years age Dose of 1800 mg/m2/day (n=6) |
||
|---|---|---|---|---|
| Number of patients |
% patients | Number of patients |
% patients | |
| Rash* | 1 | 1.8 | 0 | 0 |
| Diarrhea* | 1 | 1.8 | 0 | 0 |
| Nausea* | 2 | 3.6 | 0 | 0 |
| Vomiting* | 1 | 1.8 | 0 | 0 |
| Bilirubin* | 4 | 7.1 | 0 | 0 |
| AST (SGOT)* | 2 | 3.6 | 0 | 0 |
| ALT (SGPT)* | 2 | 3.6 | 0 | 0 |
| Nyctalopia* | 0 | 0 | 1 | 16.7 |
| Abdominal pain* | 3 | 5.4 | 0 | 0 |
| Catheter-related infection | 1 | 1.8 | 0 | 0 |
| Anorexia | 1 | 1.8 | 0 | 0 |
| Muscle weakness | 1 | 1.8 | 0 | 0 |
| Inner ear/hearing | 1 | 1.8 | 0 | 0 |
| Epistaxis | 1 | 1.8 | 0 | 0 |
| Bone pain | 8 | 14.3 | 0 | 0 |
| Hemoglobin | 11 | 19.6 | 1 | 16.7 |
| Dyspnea (shortness of breath) | 1 | 1.8 | 0 | 0 |
| Infection without neutropenia | 2 | 3.6 | 0 | 0 |
| Hepatic enlargement | 1 | 1.8 | 0 | 0 |
| Liver dysfunction/failure (clinical) | 1 | 1.8 | 0 | 0 |
| Leukocytes (total WBC) | 5 | 8.9 | 0 | 0 |
| Hypoxia | 1 | 1.8 | 0 | 0 |
| Weight loss | 2 | 3.6 | 0 | 0 |
| Pleural effusion (non-malignant) | 1 | 1.8 | 0 | 0 |
| Lymphopenia | 6 | 10.7 | 0 | 0 |
| Neutrophils/granulocytes (ANC/AGC) | 5 | 8.9 | 0 | 0 |
| Platelets | 9 | 16.1 | 2 | 33.3 |
| Hypokalemia | 2 | 3.6 | 0 | 0 |
| Tumor pain | 1 | 1.8 | 0 | 0 |
| Hyponatremia | 2 | 3.6 | 0 | 0 |
| Transfusion: Platelets | 5 | 8.9 | 1 | 16.7 |
| Transfusion: PRBCs | 9 | 16.1 | 1 | 16.7 |
| Pain – Other | 1 | 1.8 | 0 | 0 |
Pharmacokinetics
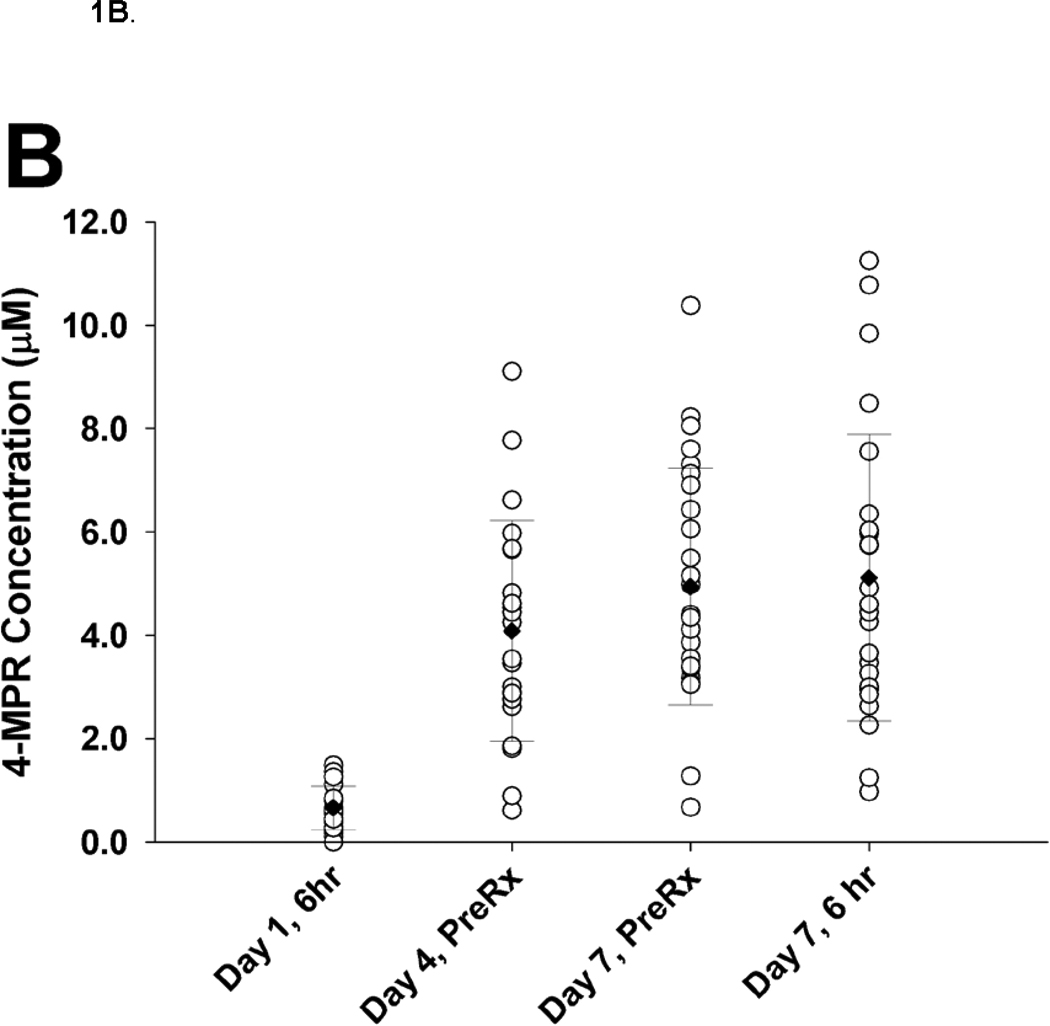
Twenty-eight patients submitted at least one specimen. Steady-state trough concentrations of 7.25 µM 4-HPR were achieved by day 4 and maintained through day 7 of course 1. There was substantial inter-patient variability (CV 40–56%) in 4-HPR plasma concentrations (Figure 1A). The 4-HPR accumulation factor of 2.8, determined by comparing mean 6 hour plasma concentrations measured on day 1 (3.07 µM) and day 7 (8.21 µM), suggests the 4-HPR plasma half-life was 18 hours. Steady-state trough concentrations of 4.8 µM 4-MPR were achieved on day 7 of course 1 (Figure 1B). There was substantial inter-patient variability (CV 44–61%) in 4-MPR plasma concentrations (Figure 1B). The mean retinol plasma concentration before treatment with fenretinide was 4.64 µM (range 0.58–9.12 µM), decreased by 97.3% (range 87.8–100%) after 4 days of fenretinide, and returned to 52% (range 13–87%) of the initial value before course 2 and to 46% (range 20–61%) of the initial value before course 5 (not shown).
Figure 1.
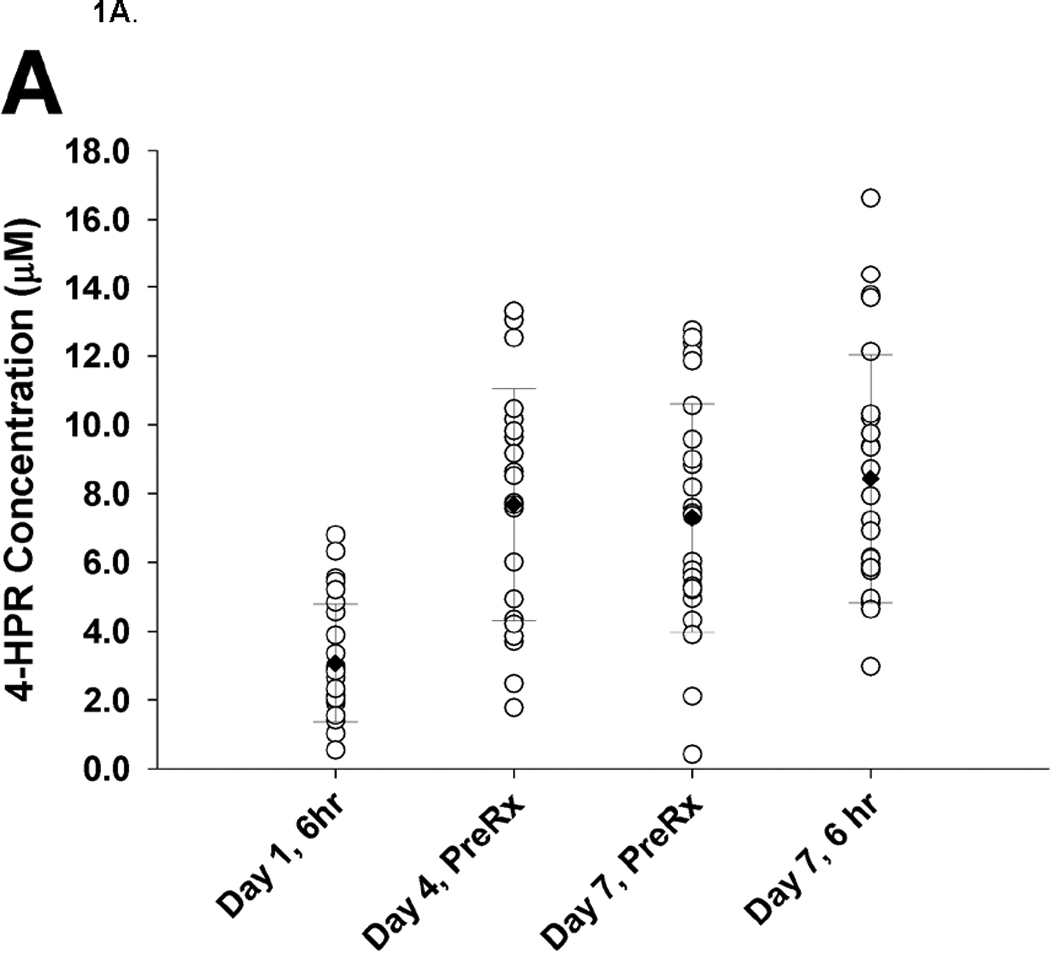
A. 4-HPR plasma concentrations (n = 28). Each circle represents one patient at a given timepoint
B. 4-MPR plasma concentrations (n = 28).
Survival Analysis
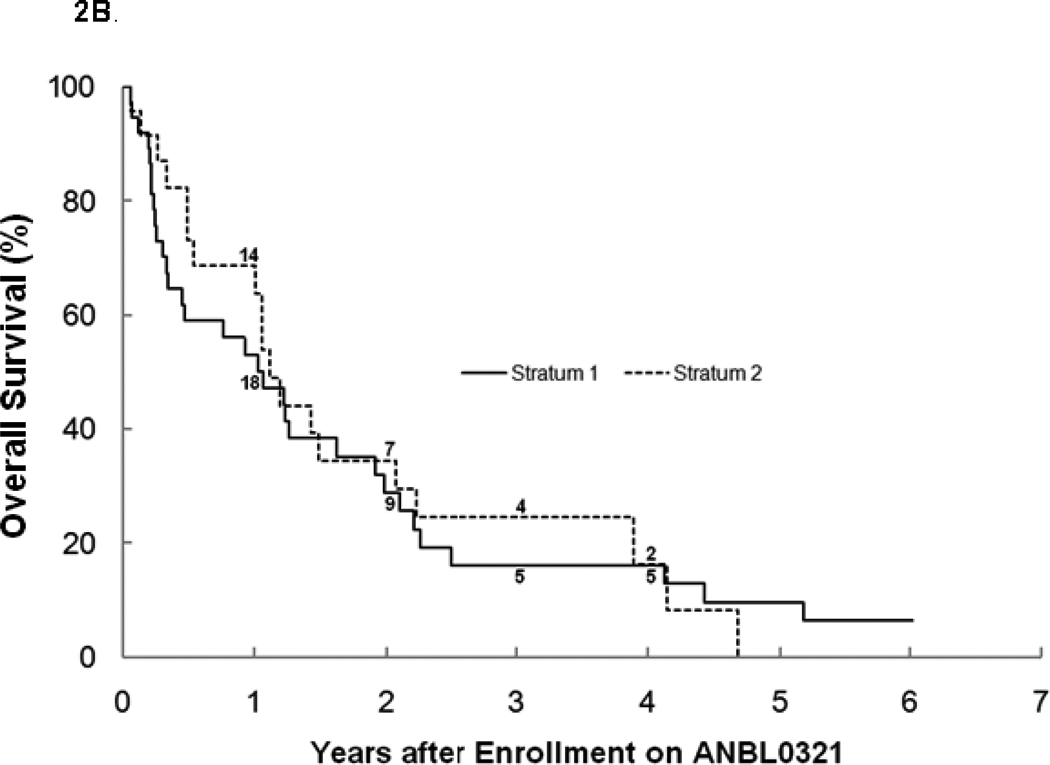
For all eligible patients, the 3-year PFS was 6.1% ± 3.4% and OS was 19.1% ± 5.7% (n=62; Table1). There was no significant difference between stratum 1 and 2 for PFS (3 year PFS: 8.1%±5.5% vs. 4.2%±4.1%; Figure 2a), OS (3 year OS: 16.0%±6.5% vs. 24.5% ±10.6%; Figure 2b), or median TTP (40 vs. 48 days). The median TTP for patients with bone marrow disease with or without other tumor sites at study entry (n=37) was significantly shorter (p=0.027) at 40 (range 17–892) days than for patients without bone marrow disease (n=20), who had a median TTP of 73 (range 23–506) days. Four patients with tumor limited to the bone marrow only at study entry progressed after 1, 1, 2, and 11 courses, with only one survivor 3 years from study entry (Figures 3a, 3b). There was no difference in median TTP for patients with MIBG avid sites at entry (n=47) versus patients (n=10) without MIBG avid sites (48 vs. 36.5 days, respectively).
Figure 2.
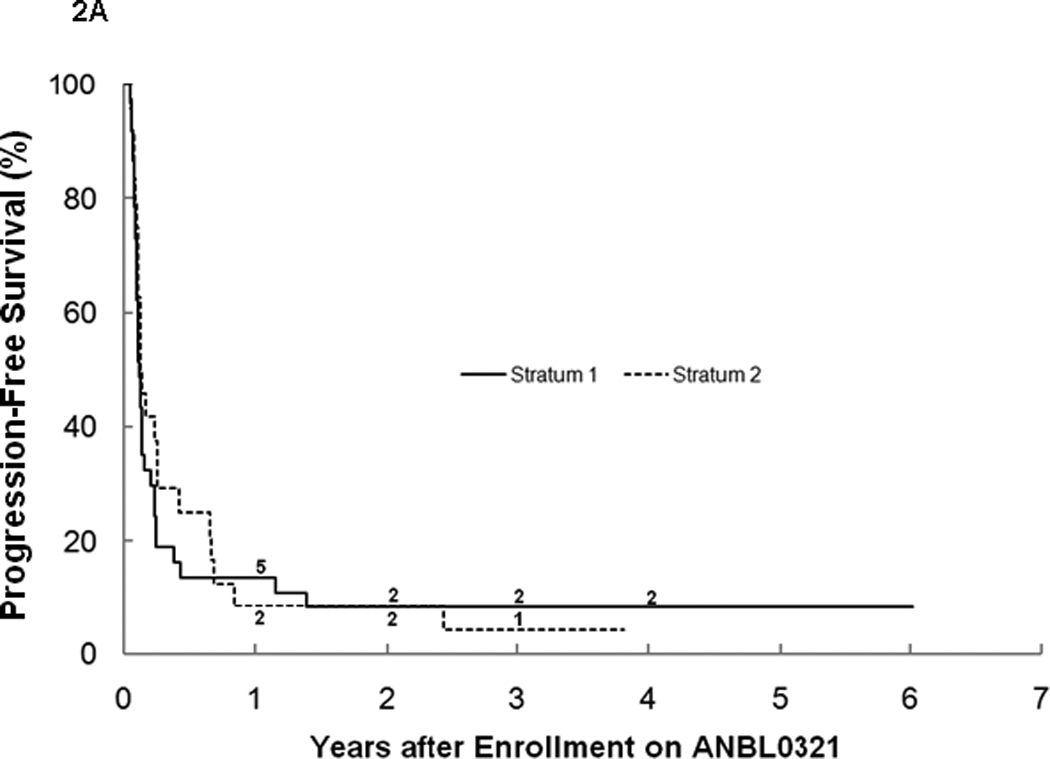
a. Progression-free survival for all eligible patients on ANBL0321 by stratum (n=62). The numbers at risk at the start of years 1–4 are given along the curves.
b. Overall survival for all eligible patients on ANBL0321 by stratum (n=62). The numbers at risk at the start of years 1–4 are given along the curves.
Figure 3.
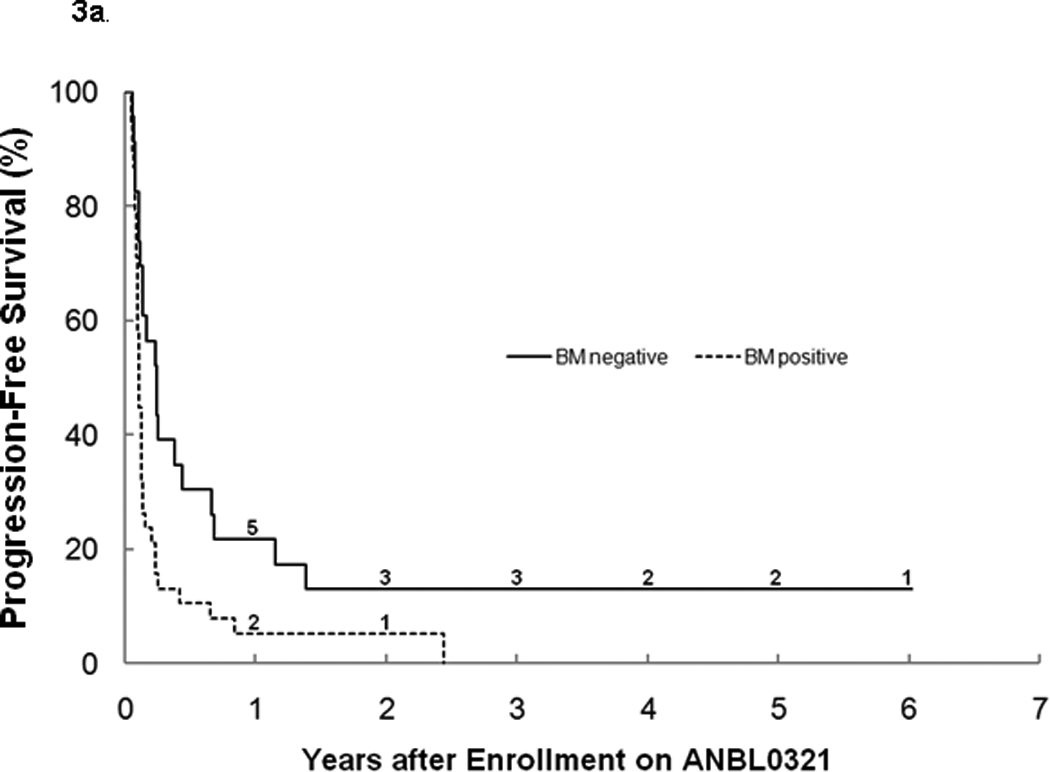
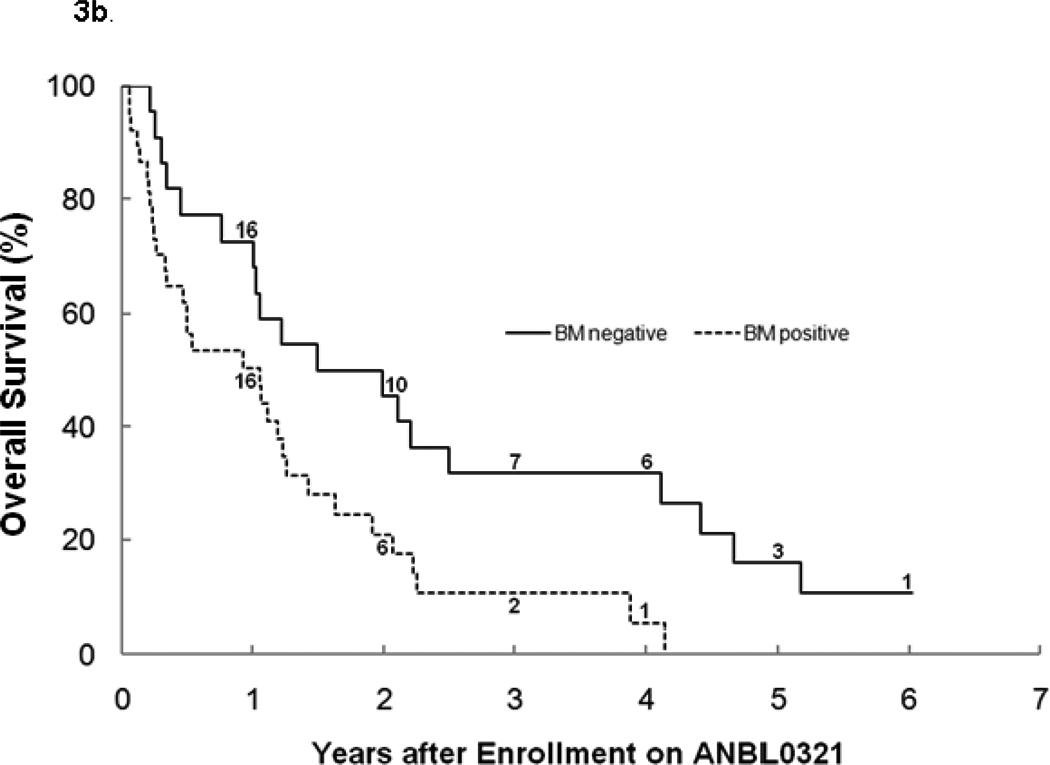
a. Progression-free survival by bone marrow involvement at study entry (Log-rank p=0.0078). The numbers at risk at the start of years 1–6 are given along the curves.
Outcome for patients older versus younger than 18 years was not significantly different. Patients without a history of previous tumor progression/relapse had significantly longer PFS (p = 0.0005) and OS (p = 0.0026) than patients treated after relapse/progression. No prior retinoid therapy was also associated with a trend towards higher PFS and OS (p=0.058 and p=0.168, respectively).
DISCUSSION
This Phase II trial was designed to determine the response rate of capsular fenretinide in children with refractory/resistant high-risk neuroblastoma, based on activity observed against neuroblastoma in pre-clinical models (5–7,35) and the Phase I CCG 09709 study (25). The trial utilized a novel design evaluating response in two different cohorts based on tumor sites at entry. It was hypothesized that fenretinide activity may differ against mass disease (stratum 1) versus disease limited to MIBG avid sites and bone marrow metastases (stratum 2). Stratum 2 patients have traditionally not been eligible for Phase II studies, which utilized the RECIST criteria (28). The RECIST criteria have not been shown to be associated with outcome, and may not be applicable to neuroblastoma, where bone and bone marrow are the most frequent and often only sites of relapse (36). In addition, agents with modest systemic toxicity are potentially suitable for treating minimal residual disease, non-measurable by RECIST criteria, that remains after completing front-line therapy. Responses in MIBG avid lesions were defined using the Curie scoring method (29), which has been validated for bone metastases and has shown prognostic value (37). Preliminary data from the COG A3973 trial for newly diagnosed high-risk neuroblastoma suggest that the Curie score at the end of induction chemotherapy is prognostic for EFS (38,39). Bone marrow response is difficult to quantify, due to patchy involvement. Neuroblastoma patients may also have minimal residual marrow disease (<5% tumor) variably detected on serial sampling. This study defined only complete response, SD, or PD in bone marrow. An ongoing retrospective study in the New Approaches to Neuroblastoma Consortium (NANT) will evaluate if these response criteria correlate with PFS and OS.
Neither stratum had sufficient responses to meet protocol criteria for efficacy. One of 59 (1.7%) patients with MIBG avid bone sites had a partial response. However, 13/59 (22%) patients had prolonged SD for ≥4 (median: 15; range 4–45+) courses and one eventually achieved a CR on further compassionate fenretinide therapy. Among 13 patients with SD, the high median Curie score and large median longest dimension of 4 cm at study entry may indicate that prolonged SD was possible in patients with significant tumor burden. However, we cannot definitively conclude that the prolonged SD observed is any different from the natural history without any therapy in this diverse patient population.
The two strata design based on tumor sites at protocol entry may not be superior to a single strata design to determine efficacy based on response. Two other COG Phase II studies (40, 41) using this same design found responses which met the statistical endpoint for efficacy in Stratum Two only. Additional studies are needed to resolve this issue. The two strata approach provides a framework for future clinical trials to better define the impact of disease burden on assessing drug activity in recurrent neuroblastoma.
The unplanned comparisons of survival by stratum, sites of tumor, or age were underpowered. However, marrow disease at entry was associated with significantly lower PFS and OS. The COG 09709 Phase I study of fenretinide also found this association with shorter time to progression in patients with bone marrow disease at entry (25). These data suggest that tumor sites may affect outcome after salvage therapy with novel agents. Patients with persistent refractory tumor (documented by histology) had significantly higher PFS and OS than patients with a history of prior relapse. Among patients with prolonged SD, 7 had prior relapse and 6 had refractory disease. The patient population on this study was heterogeneous in terms of sites of tumor, and whether they had recurrent progressive disease after prior responses or were primarily refractory to therapy; these factors will be critical to consider in future Phase II study design, since they potentially affect response rate and/or time-to-progression endpoints. Further data with larger numbers of patients are required to test these hypotheses.
Fenretinide steady-state pharmacokinetics confirmed Phase I CCG 09709 data (25) that steady state drug concentrations in the range associated with in vitro activity are achievable. However, intracellular biodistribution of fenretinide is complex, and much higher concentrations may be required in patient plasma than in cell culture to achieve cytotoxic intracellular drug concentrations. While it was recommended that the drug be given with high fat meals known to increase fenretinide bioavailability (42), wide inter-patient variability may be due to diet variations and/or incomplete disintegration of the gelatin capsules. Patients received 5–14 capsules per dose, which was challenging to administer to young children. Poor bioavailability of the capsular formulation may have limited efficacy.
Systemic toxicity was minimal. The death from hepatic failure was attributable to tumor progression. While the Phase I 09709 studies reported three reversible cases of pseudotumor cerebri at three dose levels (25), none occurred on this study. Despite significant retinol depletion, only 1/6 patients over 18 years reported nyctalopia. There were no cases in younger patients, which may be due to under-reporting.
Novel formulations of 4-HPR which optimize pharmacokinetics and feasibility of administration in children are currently being tested in pediatric and adult Phase I trials. A 4-HPR formulation packaged in LYM-X-SORB™ (LXS™) (43), a lipid matrix technology powder, was tolerated in doses up to 2210 mg/m2/day without dose-limiting toxicity in an ongoing NANT trial (44). Mean peak plasma levels were 15–20 µM versus 6–9 µM with the capsular formulation. An absorption plateau was observed, as seen with the capsule formulation. An intravenous 4-HPR emulsion formulation is also being tested in ongoing adult cancer trials and a pediatric neuroblastoma (NANT) trial, with clinically tolerable peak plasma levels up to 50 µM in adults (45,46). Results from these studies will help to determine if higher fenretinide plasma levels are tolerable and associated with improved anti-tumor activity.
The cumulative data with fenretinide support activity of this agent against neuroblastoma. The capsule formulation utilized in this study was suboptimal due to poor bioavailability, and difficulty administering to children, and is not recommended for future trials. However, this study provides important clinical response data for comparison to data obtained in future trials of novel fenretinide formulations that can achieve higher drug exposures that will be necessary to define the role of fenretinide in therapy for high-risk neuroblastoma.
Statement of translational relevance.
This phase II study assessed the activity of a capsular formulation of fenretinide in refractory/recurrent neuroblastoma. A novel study design utilized two strata: 1) RECIST-defined measurable disease by CT/MRI scans and 2) disease evaluable by non-RECIST methods, i.e. bone marrow morphology and semi-quantitative scoring of I-131MIBG avid disease. Other novel variables were identified that affected time to progression: history of prior relapse versus resistant tumor and tumor sites at study entry. Identification of variables affecting time to progression is critical for design of future studies using this endpoint. This study assessed the utility of a multi-strata phase II trial evaluating agents with minimal systemic toxicity but also minimal activity against mass disease and demonstrated sufficient activity of fenretinide at modest systemic exposures to justify ongoing trials of novel fenretinide formulations with higher bioavailability. Importantly this study documents responses and time to progression in both strata for comparison to ongoing and future phase II clinical trials in recurrent neuroblastoma.
Acknowledgements
The authors thank Jeanette Vandergiessen (protocol data manager) and Sharon Bergeron (protocol research nurse) for their efforts during the conduct of this study and COG staff Dina Willis and Rinat Sared for their dedication and efforts in the data management of this study.
Grant Support: This work was supported by the NIH grants U10 CA98413 (COG SDC grant), U10 CA98543 (COG Chair’s grant), CA82830, and NIH grant P30 CA15083: the Mayo Cancer Center support grant for the Pharmacology Shared Resource, T.J. Martell Foundation for Leukemia, Cancer and AIDS Research, The Bogart Pediatric Cancer Research Program, and by Cancer Prevention & Research Institute of Texas grant RP10072
Footnotes
Children’s Hospital Los Angeles (CHLA) holds patents and/or patent applications on anticancer therapies using the LYM-X-SORB™ (LXS™) and intravenous emulsion fenretinide formulations. These formulations have been licensed to the company CerRx, Inc. founded by two of the inventors, Drs. Barry Maurer and C. Patrick Reynolds (Texas Tech University, Lubbock, Texas). CHLA may benefit financially from the development and future use of these formulations of fenretinide.
This study was presented as an abstract (slide presentation) at Advances in Neuroblastoma Research, May 2006
REFERENCES
- 1.Gudas LJSM, Roberts AB. Cellular biology and biochemistry of the retinoids. In: Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids. New York: Raven Press; 1994. pp. 443–520. [Google Scholar]
- 2.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 3.Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: A Children's Oncology Group study. J Clin Oncol. 2009;27:1007–1013. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu AL, GIlman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;14:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds CP, Wang Y, Melton LJ, Einhorn PA, Slamon DJ, Maurer BJ. Retinoic-acid-resistant neuroblastoma cell lines show altered MYC regulation and high sensitivity to fenretinide. Med Pediatr Oncol. 2000;35:597–602. doi: 10.1002/1096-911x(20001201)35:6<597::aid-mpo23>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Di Vinci A, Geido E, Infusini E, et al. Neuroblastoma cell apoptosis induced by the synthetic retinoid N-(4-hydroxyphenyl)retinamide. Int J Cancer. 1994;59:422–426. doi: 10.1002/ijc.2910590322. [DOI] [PubMed] [Google Scholar]
- 7.Mariotti A, Marcora E, Bunone G, Costa A, Veronesi U, Pierotti MA, et al. N-(4-hydroxyphenyl)retinamide: a potent inducer of apoptosis in human neuroblastoma cells. J Natl Cancer Inst. 1994;86:1245–1247. doi: 10.1093/jnci/86.16.1245. [DOI] [PubMed] [Google Scholar]
- 8.Ziv Y, Gupta MK, Milson JW, Vladisavljevic A, Brand M, Fazio VW. The effect of tamoxifen and fenretinimide on human colorectal cancer cell lines in vitro. Anticancer Research. 1994;14:2005–2009. [PubMed] [Google Scholar]
- 9.Hsieh TC, Ng C, Wu JM. The synthetic retinoid N-(4-hydroxyphenyl) retinamide (4-HPR) exerts antiproliferative and apoptosis-inducing effects in the androgen-independent human prostatic JCA-1 cells. Biochem Mol Biol Int. 1995;37:499–506. [PubMed] [Google Scholar]
- 10.Kazmi SM, Plante RK, Visconti V, Lau CY. Comparison of N-(4-hydroxyphenyl)retinamide and all-trans-retinoic acid in the regulation of retinoid receptor-mediated gene expression in human breast cancer cell lines. Cancer Research. 1996;56:1056–1062. [PubMed] [Google Scholar]
- 11.Supino R, Crosti M, Clerici M, Warlters A, Cleris L, Zunino F, et al. Induction of apoptosis by fenretinide (4HPR) in human ovarian carcinoma cells and its association with retinoic acid receptor expression. Int J Cancer. 1996;65:491–497. doi: 10.1002/(SICI)1097-0215(19960208)65:4<491::AID-IJC17>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 12.Kalemkerian GP, Slusher R, Ramalingam S, Gadgeel S, Mabry M. Growth inhibition and induction of apoptosis by fenretinide in small-cell lung cancer cell lines. Journal National Cancer Institute. 1995;87:1674–1680. doi: 10.1093/jnci/87.22.1674. [DOI] [PubMed] [Google Scholar]
- 13.Ohlmann CH, Jung C, Jaques G. Is growth inhibition and induction of apoptosis in lung cancer cell lines by fenretinide [N-(4-hydroxyphenyl)retinamide] sufficient for cancer therapy? Int J Cancer. 2002;100:520–526. doi: 10.1002/ijc.10525. [DOI] [PubMed] [Google Scholar]
- 14.Delia D, Aiello A, Lombardi L, Pelicci PG, Grignani F, Formelli F, et al. N-(4-hydroxyphenyl)retinamide induces apoptosis of malignant hemopoietic cell lines including those unresponsive to retinoic acid. Cancer Res. 1993;53:6036–6041. [PubMed] [Google Scholar]
- 15.Ozpolat B, Tari AM, Mehta K, Lopez-Berestein G. Nuclear acid receptors are involved in N-(4-hydroxyphenyl) retinamide (Fenretinide)-induced gene expression and growth inhibition in HL-60 acute myeloid leukemia cells. Leuk Lymphoma. 2004;45:979–985. doi: 10.1080/1042819031000151833. [DOI] [PubMed] [Google Scholar]
- 16.Maurer BJ, Metelitsa LS, Seeger RC, Cabot MC, Reynolds CP. Increase of ceramide and induction of mixed apoptosis/necrosis by N-(4-hydroxyphenyl)-retinamide in neuroblastoma cell lines. J Natl Cancer Inst. 1999;91:1138–1146. doi: 10.1093/jnci/91.13.1138. [DOI] [PubMed] [Google Scholar]
- 17.Erdreich-Epstein A, Tran LB, Bowman NN, Wang H, Cabot MC, Durden DL, et al. Ceramide signaling in fenretinide-induced endothelial cell apoptosis. J Biol Chem. 2002;277:49531–49537. doi: 10.1074/jbc.M209962200. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Maurer BJ, Liu YY, Wang E, Allegood JC, Kelly S, et al. N-(4-Hydroxyphenyl)retinamide increases dihydroceramide and synergizes with dimethylsphingosine to enhance cancer cell killing. Mol Cancer Ther. 2008;7:2967–2976. doi: 10.1158/1535-7163.MCT-08-0549. [DOI] [PubMed] [Google Scholar]
- 19.Maurer BJ, Melton L, Billups C, Cabot MC, Reynolds CP. Synergistic cytotoxicity in solid tumor cell lines between N-(4-hydroxyphenyl)retinamide and modulators of ceramide metabolism. J Natl Cancer Inst. 2000;92:1897–1909. doi: 10.1093/jnci/92.23.1897. [DOI] [PubMed] [Google Scholar]
- 20.Oridate N, Suzuki S, Higuchi M, Mitchell MF, Hong WK, Lotan R. Involvement of reactive oxygen species in N-(4-hydroxyphenyl)retinamide-induced apoptosis in cervical carcinoma cells. J Natl Cancer Inst. 1997;89:1191–1198. doi: 10.1093/jnci/89.16.1191. [DOI] [PubMed] [Google Scholar]
- 21.Asumendi A, Morales MC, Alvarez A, Aréchaga J, Pérez-Yarza G. Implication of mitochondria-derived ROS and cardiolipin peroxidation in N-(4-hydroxyphenyl)retinamide-induced apoptosis. Br J Cancer. 2002;86:1951–1956. doi: 10.1038/sj.bjc.6600356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribatti D, Alessandri G, Baronio M, Raffaghello L, Cosimo E, Marimpietri D, et al. Inhibition of neuroblastoma-induced angiogenesis by fenretinide. Int J Cancer. 2001;94:314–321. doi: 10.1002/ijc.1441. [DOI] [PubMed] [Google Scholar]
- 23.Villa ML, Ferrario E, Trabattoni D, Formelli F, De Palo G, Magni A, et al. Retinoids, breast cancer and NK cells. Br J Cancer. 1993;68:845–850. doi: 10.1038/bjc.1993.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Z, Matsuura T, Popoff K, Ross AC. Effects of N-(4-hydroxyphenyl)-retinamide on the number and cytotoxicity of natural killer cells in vitamin-A-sufficient and -deficient rats. Nat Immun. 1994;13:280–288. [PubMed] [Google Scholar]
- 25.Villablanca JG, Krailo MD, Ames MM, Reid JM, Reaman GH, Reynolds CP. Phase I trial of oral fenretinide in children with high-risk solid tumors: a report from the Children's Oncology Group (CCG 09709) J Clin Oncol. 2006;24:3423–3430. doi: 10.1200/JCO.2005.03.9271. [DOI] [PubMed] [Google Scholar]
- 26.Jasti BR, LoRusso PM, Parchment RE, Wozniak AJ, Flaherty LE, Shields AF, et al. Phase I clinical trial of fenretinide (NSC374551) in advanced solid tumors [abstract] Proc Am Soc Clin Oncol. 2001;20:122a. (abstract # 485). [Google Scholar]
- 27.Seeger RC, Reynolds CP, Gallego R, Stram DO, Gerbing RB, Matthay KK. Quantitative tumor cell content of bone marrow and blood as a predictor of outcome in stage IV neuroblastoma: a Children's Cancer Group Study. J Clin Oncol. 2000;18:4067–4076. doi: 10.1200/JCO.2000.18.24.4067. [DOI] [PubMed] [Google Scholar]
- 28.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New Guidelines to Evaluate the Response to Treatment in Solid Tumors. Journal National Cancer Institute. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 29.Ady N, Zucker JM, Asselain B, Edeline V, Bonnin F, Michon J, et al. A new 123I-MIBG whole body scan scoring method--application to the prediction of the response of metastases to induction chemotherapy in stage IV neuroblastoma. Eur J Cancer. 1995;31A:256–261. doi: 10.1016/0959-8049(94)00509-4. [DOI] [PubMed] [Google Scholar]
- 30.Bugge CJL, Rodriguez LC, Vane FM. Determination of isotretinoin or etretinate and their major metabolites in human blood by reversed-phase high-performance liquid chromatography. J Pharm Biomed Anal. 1985;3:269–277. doi: 10.1016/0731-7085(85)80032-7. [DOI] [PubMed] [Google Scholar]
- 31.Formelli F, Clerici M, Campa T, Di Mauro MG, Magni A, Mascotti G, et al. Five-year administration of fenretinide: pharmacokinetics and effects on plasma retinol concentrations. J Clin Oncol. 1993;11:2036–2042. doi: 10.1200/JCO.1993.11.10.2036. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 33.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. Part II: Analysis and Examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mann HB, Whitney DR. On a test of whether one of two random variables is stochastically larger than the other. Annals of Mathematical Statistics. 1947;18:50–60. [Google Scholar]
- 35.Ponzoni M, Bocca P, Chiesa V, Decensi A, Pistoia V, Raffaghello L, et al. Differential effects of N-(4-hydroxyphenyl)retinamide and retinoic acid on neuroblastoma cells: apoptosis versus differentiation. Cancer Res. 1995;55:853–861. [PubMed] [Google Scholar]
- 36.DuBois SG, Kalika Y, Lukens JN, Brodeur GM, Seeger RC, Atkinson JB, et al. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J Pediatr Hematol Oncol. 1999;21:181–189. doi: 10.1097/00043426-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Matthay KK, Edeline V, Lumbroso J, Tanguy ML, Asselain B, Zucker JM, et al. Correlation of early metastatic response by 123I-metaiodobenzylguanidine scintigraphy with overall response and event-free survival in stage IV neuroblastoma. J Clin Oncol. 2003;21:2486. doi: 10.1200/JCO.2003.09.122. [DOI] [PubMed] [Google Scholar]
- 38.Yanik GA, Parisi MT, Shulkin B, Naranjo A, Kreissman S, London W, et al. Analyses of mIBG scoring as a prognostic indicator in patients with stage 4 neuroblastoma. A COG Children’s Oncology Group (A3973) report [abstract] Proc ANR. 2010 June;:102. (abstract #PL30). [Google Scholar]
- 39.Yanik GA, Parisi MT, Naranjo A, Matthay KK, London WB, McGrady PW, et al. MIBG scoring as a prognostic indicator in patients with stage IV neuroblastoma: A COG study [abstract #9516] ASCO Annual Meeting Proceedings. 2010;28:682s. [Google Scholar]
- 40.Shusterman S, London WB, Gillies SD, Hank JA, Voss SD, Seeger RC, et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children's Oncology Group (COG) phase II study. J Clin Oncol. 2010;28:4969–4975. doi: 10.1200/JCO.2009.27.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bagatell R, London WB, Wagner LM, Voss SD, Stewart CF, Maris JM, et al. Phase II study of irinotecan and temozolomide in children with relapsed or refractory neuroblastoma: a Children’s Oncology Group study. J Clin Oncol. 2011;29:208–213. doi: 10.1200/JCO.2010.31.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doose DR, Minn FL, Stellar S, Nayak RK. Effects of meals and meal composition on the bioavailability of fenretinide. J Clin Pharmacol. 1992;32:1089–1095. [PubMed] [Google Scholar]
- 43.Maurer BJ, Kalous O, Yesair DW, Wu X, Janeba J, Maldonado V, et al. Improved oral delivery of N-(4-hydroxyphenyl)retinamide with a novel LYM-X-SORB organized lipid complex. Clin Cancer Res. 2007;13:3079–3086. doi: 10.1158/1078-0432.CCR-06-1889. [DOI] [PubMed] [Google Scholar]
- 44.Marachelian A, Kang MH, Hwang K, Villablanca JG, Groshen S, Matthay KK, Maris J, DeSantes KB, Reynolds CP, Maurer BJ. Phase I study of fenretinide (4-HPR) oral powder in patients with recurrent or resistant neuroblastoma: New Approaches to Neuroblastoma Therapy (NANT) Consortium trial [abstract # 10009] ASCO Annual Meeting Proceedings. 2009;27:15s. [Google Scholar]
- 45.Kang MH, Marachelian A, Villablanca JG, Maris JM, Ames M, Reid JM, Matthay KK, Reynolds CP, Maurer BJ. Fenretinide (4-HPR) orally formulated in Lym-X-Sorb(LXS) lipid matrix or as an intravenous emulsion increased 4-HPR systemic exposure in patients with Recurrent or Resistant Neuroblastoma. A New Approaches To Neuroblastoma Therapy (NANT) Consortium Trial [abstract] Proc ANR. 2010 June;:123. (abstract # OR57) [Google Scholar]
- 46.Morbacher A, Gutierrez M, Murgo AJ, Kummar S, Reynolds CP, Maurer BJ, Groshen S, Vergara L, Kang MH, Yang AS. Phase I trial of fenretinide (4-HPR) intravenous emulsion for hematological malignancies [abstract] Blood. 2007;110:2581. [Google Scholar]


