Abstract
Plastic bronchitis (PB) is a rare disease that often occurs in patients with congenital heart disease (CHD) who have undergone staged single ventricle palliation. It is characterized by the formation of rubbery “casts” in the airways. PB treatment frequently includes inhaled tPA. However, the efficacy of tPA to reduce cast burden is unknown. This is further complicated by our lack of knowledge of cast composition. We obtained spontaneously expectorated PB casts from children (n=4) with CHD and one adult patient with idiopathic PB. Pathological assessment was made from paraffin-preserved samples. Casts were treated with phosphate-buffered saline (PBS) or tPA. Cast response to tPA was assessed by changes in cast weight and the production of fibrin D-dimer. Independent of dose, tPA reduced cast weight compared with PBS-treatment (p=0.001) and increased D-dimer levels. Histological staining showed that PB casts from all patients were comprised of fibrin and contained notable numbers of lymphocytes. Cast composition did not change over time. Collectively, these data support that in our PB patients, casts are comprised of fibrin and are responsive to tPA treatment. This makes inhaled tPA a potentially viable option for symptomatic relief of PB while we work to unravel the complexity of PB pathogenesis.
Keywords: congential heart disease, Fontan physiology, pulmonary drug delivery
Background
Plastic bronchitis (PB) is a rare, primarily pediatric disease that may be a complication of congenital heart disease (CHD) or its surgical palliation by the Fontan procedure [7, 9, 12, 13, 16, 21, 29]. The hallmark of PB, is the formation of rubbery “casts” in the airway (figure 1) that have been reported to be comprised of mucin, fibrin or a mixture of both and can partially or completely obstruct the airways [19, 22]. They are usually spontaneously expectorated but can require urgent or emergent bronchoscopy for expeditious removal in cases of severe respiratory distress [21]. Plastic bronchitis is particularly problematic in children with Fontan physiology because it contributes to morbidity and mortality and there is no known effective pharmacotherapy [2, 13, 16, 27].
Figure 1.

Photograph of representative spontaneously expectorated cast from a patient with PB.
Plastic bronchitis has also been reported to occur in patients with pulmonary diseases such as asthma and cystic fibrosis and as a consequence of acute chest syndrome in sickle cell disease [2, 16]. In some cases, an underlying etiology is not apparent; these patients are classified as idiopathic. Unfortunately, PB is ill defined because the natural history of the disease is poorly understood and to date, only case reports exist in the literature. As such, the prevalence of PB is not known and the lack of prospective, longitudinal studies has hindered the advancement of our knowledge of PB etiology, pathogenesis and treatment.
Presently, PB treatment is anecdotal and patients often receive a number of drugs including inhaled corticosteroids, dornase alfa, N-acetylcysteine, bronchodilators and antibiotics. In some cases, fibrinolytic agents, inhaled urokinase or tissue plasminogen activator (tPA), both with unknown clinical safety and efficacy, have been used to reduce cast burden [7, 9, 22, 28, 29]. At the University of Michigan, inhaled tPA (Activase®, Genentech, S. San Francisco, CA USA) is commonly used in repeated doses for the treatment of acute PB. Based on our clinical dosing schemes and data from experimental models, we have a reasonable estimate of the amount of tPA that can be safely delivered to the airways in the absence of PB casts [10, 15, 25]. However, there is presently little evidence of the effectiveness of tPA for PB cast reduction [7, 9, 28]. These data are essential to demonstrate the utility of tPA for the treatment of PB.
A previously proposed taxonomy constructed using retrospective case reports of PB suggested that in children with CHD, most cases of PB are due to mucinous casts and that mucus hyper-secretion is the primary underlying cause of PB in these patients [16]. In addition, many of these cases describe casts as containing few or no cells or do not provide histological evidence of cast composition [1, 2, 4, 8, 18, 20, 22, 23]. Our observation of the University of Michigan’s Fontan population has been the production of what most often seems to be fibrin casts. Explanations for this difference include the possibility that cast composition changes over time and case reports only captured a single point in time during which casts were comprised of mucin or that pathologic assessments were incomplete. Resolution of this discrepancy is inherent to improving our understanding of the pathogenesis of PB and also has important implications in the treatment of patients with PB since fibrin casts would be more effectively treated with fibrinolytic rather than mucolytic therapy.
To this end, we undertook a prospective, longitudinal study of patients with PB to assess cast composition and to measure the responsiveness of casts to tPA ex vivo.
We report that our PB patients typically produce fibrin, cellular (lymphocytes) casts that are responsive to amounts of tPA that are consistent with those that may be safely delivered to the lungs [10, 15, 25].
Methods
Patient enrollment
The study was approved by the University of Michigan Health System’s Institutional Review Board (HUM00031493). Patients with active PB were considered eligible for participation. Written informed consent was obtained from adult patients and parents (or legal guardians) of patients less than 18 years of age. Written assent was obtained from patients aged 7 years or older. Following consent, a cast collection kit, consisting of a conical tube (50 mL) containing sterile phosphate buffered saline (PBS), overnight express mail supplies and instructions, and a medication use form, were provided to subjects. Patients were instructed to submit casts immediately after expectoration and to complete a medication use form for the 24 h prior to cast production. Upon receipt of a cast, patients were sent another collection kit for subsequent cast submission.
For Fontan patients, demographic information including age at date of operation (DOO), type of Fontan, underlying CHD, and onset of PB from DOO was extracted from the medical record.
Cast samples
Upon receipt, casts were rinsed in sterile PBS, photographed and weighed. Casts for pathological assessment were fixed in buffered formalin for 48 h after which they were transferred to ethanol (70%) and placed in cassettes in preparation for paraffin embedding. Clinical cast samples that were acquired during the study period were also assessed for composition. Routinely prepared histologic sections stained with hematoxylin and eosin were reviewed by two of us with expertise in pulmonary pathology (LS, JM). In addition, sections were specifically stained for mucin (Mucicarmine stain, Accustain®, Sigma Aldrich, St. Louis, MO) and fibrin using the Martius yellow-brilliant crystal scarlet blue (MSB) technique [3].
Cast for tPA testing was sectioned into equal parts based on weight (± 5%) and dried (37ºC) until dry weight was achieved. Dried samples were placed in sterile tubes (12 × 75 mm; BD Falcon, Franklin Lakes, NJ USA) that contained either sterile PBS or human recombinant tPA (0.5–5 μg; Activase®, Genentech) with human glu-plasminogen (0.5 μM; Molecular Innovations, Novi, MI). A tube containing PBS without cast was used as a negative control. Activase® was reconstituted according to the manufacturer’s instructions. An aliquot (10μL) was removed from each tube at 1, 2, 4, 8, and 24 h and was frozen (−80ºC) until the time of assay. Post-treatment cast was dried (37ºC) and the percent change in cast mass was calculated by subtracting the post-treatment dry weight from the pre-treatment dry weight and dividing it by the pre-treatment dry weight; this number was multiplied by 100.
Assays
Samples were thawed on ice at the time of assay. The amount of active tPA and fibrin D-dimer were measured by enzyme-linked immuno-sorbent assays (ELISA) that we developed and optimized in our laboratory using samples acquired in this study. Briefly, for the active tPA assay, the wells of Immulon II microtiter 96-well plates were coated with 100 μL/well of NeutrAvidin (1:1000, #A2666; Invitrogen, Carlsbad, CA) and incubated overnight (4º C). After plate washing (BioTek ELx405, Winooski, VT) with wash buffer (0.05% Tween-20 in 1X PBS, pH 7.2) blocking buffer (1X PBS with 0.1% Tween-20 and 1% BSA) was added to each well. After a second wash, 100 μL of human plasminogen activator inhibitor biotin (1:2000, #INTBIOPAI-A; Innovative Research, Novi, MI) was added to each well. Standards (recombinant human tPA, 0–2 ng/mL) and samples (100 μL/well) were added in duplicate to respective wells. Detection antibody (100μL of 1μg/mL; mouse anti-human tPA, Calbiochem, Gibbstown, NJ) was followed by 100 μL of a horseradish perioxidase (HRP) conjugated antibody (1μg/mL; goat anti-mouse IgG HRP; Southern Biotech, Birmingham, AB) and TMB substrate solution (100 μL/well). The reaction was stopped by the addition of sulfuric acid (100 μL of 2N). The D-dimer assay was prepared and performed in a similar manner except the plate was coated with an antibody to the murine Mab against human D-dimer IgG3K (1:500, #300 American Diagnostica, Stamford, CT) and a sheep anti-human fibrin fragment E antibody (1:500, #CL20065A Cedar Lane Labs, Burlington, NC) was used for detection. For both assays, plates were read at 450nm and the absorbance data were acquired using a ThermoMax microplate reader (Molecular Devices, Sunnyvale, CA). The active tPA and D-dimer concentrations of each sample were derived from the linear phase of each standard curve as analyzed by the reader’s software (Softmax PRO 4.1, Molecular Devices).
To investigate whether PB cast contained measurable amounts of active tPA, the main stem of two casts, one of which was from a patient receiving inhaled tPA, were sectioned (proximal to distal) and homogenized as previously described [15]. Prior to active tPA ELISA, proteins of ≥ 100kDa were cleared from the lysates by centrifugation using an Amicon Ultra centrifugal filter (Millipore, Billerica, MA) with a 100 kDa membrane.
Changes over time in cast weight and active tPA and D-dimer concentrations were determined by ANOVA followed by a Tukey-Kramer post hoc test when applicable. Differences in PBS and tPA treatment were compared using a Student’s t-test. A two-sided p-value of ≤ 0.05 was considered significant. Statistical analyses were performed using InStat (GraphPad Software, Inc., LaJolla, CA, USA).
Results
Patient characteristics and cast samples
Five patients were enrolled into the study. Four were children with CHD including three boys and one girl, three of whom had Fontan (fenestrated) physiology (Table 1).
Table 1.
Demographics of enrolled study patients
| Patient | Underlying CHD | Age at DOO (months) | Onset of PB from DOO (months) | Fontan Type |
|---|---|---|---|---|
| 1 | DILV | 19 | 54 | lateral tunnel |
| 2 | HLHS | 18 | 5.3 | lateral tunnel |
| 3 | HLHS | 31 | 80 | lateral tunnel |
| 4 | DORV | N/A | N/A | N/A |
| 5 | N/A | N/A | N/A | N/A |
CHD: congenital heart disease; DOO: date of Fontan operation; DILV = double inlet left ventricle; HLHS: hypoplastic left heart syndrome; DORV = double outlet right ventricle; N/A = not applicable.
A total of 20 cast samples were acquired from five patients over a nine-month period. For longitudinal pathological assessment, at least three serial cast samples from each of four patients (three Fontan and one idiopathic) were evaluated. Eleven cast samples from all five patients were tested for responsiveness to tPA.
Medication use
Anti-inflammatory and pulmonary delivered medication use for the 24 h prior to cast expectoration consisted primarily of inhaled corticosteroids and bronchodilators. Two patients routinely received dornase alfa (Pulmozyme®, Genentech) and one patient received inhaled tPA (Activase®, Genentech) on three occasions prior to cast expectoration.
Pathology
Histological staining showed that all studied casts were primarily comprised of fibrin (figure 2A). In addition, casts contained patchy cellular infiltrates in which lymphocytes predominated with a minor component of macrophages (figure 2B). Small whisps of mucin were evident in some casts which were located in the cast periphery (figure 2C). Other casts had no identifiable mucin on routinely stained or mucicarmine stained sections (figure 2B and D). These findings were consistent for all of the samples over time.
Figure 2.

Representative light micrographs of a MSB stained section (10X), in which fibrin stains red (A); a hemotoxylin and eosin stained section (600X), which highlights cellular content (B); and mucicarmine (mucin) stained sections which show a cast where mucin (dark pink) is localized in the cast periphery (arrows) (C) and one with no remarkable mucin (D).
Cast responsiveness to tPA
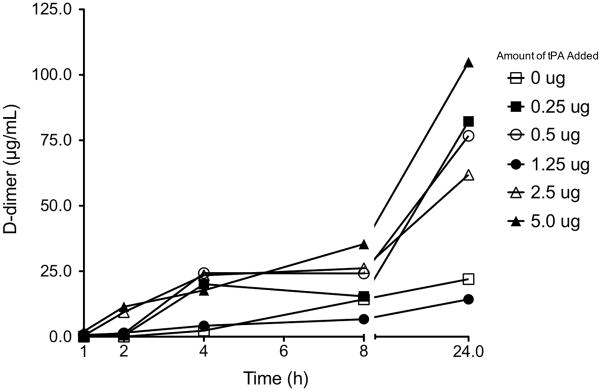
All doses of tPA tested reduced cast weight compared with PBS treatment (p < 0.05) but there was no tPA dose response (figure 3A). Independent of dose, tPA and PBS treatment reduced mean (+SD) cast mass by 68% (+17.3%) and 22% (+12.8%), respectively (figure 3B). In the presence of cast and added glu-plasminogen, tPA concentrations declined rapidly and to the same extent (mean + S.D., 83.4 + 4.7%) regardless of tPA dose within the first hour of incubation (figure 4A). Active tPA was also detected when cast was treated with PBS alone (figure 4B) suggesting that cast contained tPA. This may have contributed to the 22% decline in cast weight with PBS treatment (figure 3A and B). The presence of tPA in cast was confirmed by ELISA of homogenized cast sections (figure 5). As expected, the cast homogenate from a patient receiving inhaled tPA contained more active tPA than that from a patient who was not. However, the proximal to distal distribution pattern of tPA was similar. The tPA responsiveness and fibrin content of the PB cast samples was further evidenced by progressively increasing amounts of the fibrin degradation product, D-dimer, over the 24 h incubation period (figure 6).
Figure 3.
Percent cast weight reduction normalized to the ratio of the amount of added tPA to the dry weight of each cast sample (A). Each data point represents a section (n=40) from a whole cast. At least three sections from each of 11 cast samples were tested for tPA responsiveness using different amounts of tPA and PBS as a negative control. There was no evidence of a tPA dose response (p > 0.05 by ANOVA; also see discussion) so data were pooled and compared with PBS treatment (B). tPA treatment resulted in a greater reduction in cast weight compared with PBS (*p = 0.001 by unpaired Student’s t-test). Data are the mean (+SD) of 11 PBS-treated and 29 tPA-treated cast samples.
Figure 4.
Changes in active tPA concentration over time in the presence of glu-plasminogen and cast (A). The initial rapid decline in tPA concentration is indicative of plasminogen activation that occurred to a similar extent at all tPA doses. During incubation of cast with PBS, tPA was detectable at all time points (B). Standard deviations were removed for clarity. Data are the mean of 4–10 cast samples for each amount of tPA and PBS. The lower limit of detection of our tPA assay was 0.04 ng/mL.
Figure 5.
Amounts of active tPA varied regionally in PB casts from two patients, one who received inhaled tPA (A) within 24 h of cast expectoration and one who did not (B). Casts were sectioned into 1) proximal; 2) mid; and 3) distal sections and homogenized. The amount of tPA (μg) in each piece was normalized to protein content (μg) of the respective homogenate.
Figure 6.
Fibrin D-dimer increased over time with tPA treatment providing additional evidence of the tPA responsiveness and fibrin content of PB casts in our patients. These data also corroborate the presence of tPA in cast since D-dimer was evident in samples treated with PBS. In the absence of cast, D-dimer was not detected in PBS or at any of the tPA doses (data not shown). Data are the mean of three cast samples at each time point for each amount of tPA. Standard deviations were removed for clarity. The lower limit of detection of our D-dimer assay was 3.1 ng/mL.
Discussion
This is the first prospective, longitudinal study of PB cast pathology and cast responsiveness to tPA. The work is significant because it demonstrated that in a small cohort of patients, PB casts were consistently fibrin and most often cellular (lymphocytes). Mucin was evident in some casts but it was primarily localized in the cast periphery, which is not unexpected given that PB casts originate in the airways. Our findings introduce the concept that PB could be uniquely defined by the formation of fibrin casts in the airways.
To date, our knowledge and understanding of PB is based on case reports and a proposed taxonomy of PB casts [16]. The development of this taxonomy relied on case reports most of which do not provide evidence of cast composition. The prospective work presented here suggests that PB in patients with CHD is represented by the production of cellular, fibrin casts. This is evidenced by histological assessment, a reduction in cast weight following treatment with the fibrinolytic, tPA, and the production of the fibrin degradation product, D-dimer, by casts treated with tPA. However, we acknowledge that our small sample size from a single center may not be representative of the CHD population with PB. A larger, multi-center study is needed to confirm these findings. In addition, since our patients all received inhaled medications, including dornase alfa, tPA, and anti-inflammatory drugs, we cannot eliminate the possibility that these agents may have influenced cast composition and cellular content.
Interestingly, our data also showed measurable amounts of tPA in PB cast. Previous experimental studies have demonstrated the presence of a fibrinolytic system localized in bronchial epithelial cells. The functionality of this system may be important in the regulation of the micro-environment of the lungs [5, 17]. Our finding of the presence of tPA in PB cast further substantiates this and suggests that the pulmonary fibrinolytic system could be a factor in the development of PB. This warrants consideration in future studies and in the development of experimental models of PB.
The etiology of PB remains unclear and the natural history of the illness has not been characterized. Most often, it is observed as a rare complication of Fontan physiology but the prevalence and mechanism is unknown [11]. In this study, subjects consistently produced fibrin casts laden with lymphocytes. This composition is consistent with that of lymphatic fluid and supports the hypothesis that high pulmonary or intrathoracic lymphatic pressure and/or the presence of undetected lympho-bronchial fistulas could contribute to the etiology of PB [24]. However, why some patients with high pulmonary pressure develop PB and others do not remains unclear. In addition, while a reduction of pressure in the pulmonary venous circulation or improvement in cardiac output may alleviate symptoms it is not curative and illustrates that the pathophysiology of PB is much more complex than the presence of elevated Fontan pressures alone.
At present, inhaled tPA is anecdotally used to treat PB [7, 9, 28, 29]. In this study we showed that tPA, in amounts derived from experimental models, was effective in reducing cast mass ex vivo [10, 15, 25]. This is a reasonable approach for these initial experiments and while this is an encouraging result, much has yet to be done to optimize the pulmonary delivery of this therapeutic protein which appears to have a prolonged elimination in the lungs compared with that of the systemic circulation [15]. In addition, further characterization of the distribution of inhaled tPA in the presence of PB casts in the airways is needed to optimize dose. Notably, from this ex vivo study, the cast from a patient receiving inhaled tPA had more detectable tPA than the one from a patient who was not receiving tPA suggesting that inhaled tPA reached the site of action. Nevertheless, this finding needs to be interpreted with caution since we do not know the tPA content of this patient’s cast in the absence of inhaled tPA and whether the amounts of active tPA in cast vary across patients.
Our study also showed that increasing amounts of tPA did not improve cast weight reduction. In fact, there was no evidence of a tPA dose response using this end point. The absence of a dose-dependent effect is likely due to the Michaelis-Menten kinetics of plasminogen activation by tPA and limitations that exist in the ex vivo setting. This included the use of a fixed amount of plasminogen in the presence of a broad range of tPA doses and the likelihood of varying amounts of fibrin across cast samples [14, 26]. In an attempt to control for varying fibrin content, we started with equal wet weights rather than dry weights because, at the time the study was undertaken, we did not have information on cast content and wanted to avoid cast manipulation that could skew tPA response. Nevertheless, despite similar wet weights it is likely that fibrin content varied across cast samples of which even subtle differences can change the rate and extent of plasminogen activation and fibrinolysis [6]. This phenomenon is also evident in the measurement of fibrin D-dimer which also did not exhibit a tPA dose response. Therefore, until more elaborate in vivo and clinical data are available, a conservative approach to the pulmonary dosing of tPA may be warranted to minimize the potential for adverse events while we work towards an ideal dosing scheme.
In conclusion, these data support that in PB patients at the University of Michigan, casts are primarily comprised of fibrin and are responsive to tPA treatment. This finding is further evidenced by the ability of tPA to reduce cast mass making inhaled tPA a potentially viable option for symptomatic relief of PB while we work to unravel the complexity of PB pathogenesis.
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development (NICHD) grant no. R15 HD065594. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health.
Footnotes
This work was performed at the University of Michigan
References
- 1.Barber BJ, Burch GH, Tripple D, Balaji S. Resolution of plastic bronchitis with atrial pacing in a patient with fontan physiology. Pediatric cardiology. 2004;25:73–76. doi: 10.1007/s00246-003-0529-9. [DOI] [PubMed] [Google Scholar]
- 2.Brogan TV, Finn LS, Pyskaty DJ, Jr, Redding GJ, Ricker D, Inglis A, Gibson RL. Plastic bronchitis in children: a case series and review of the medical literature. Pediatr Pulmonol. 2002;34:482–487. doi: 10.1002/ppul.10179. [DOI] [PubMed] [Google Scholar]
- 3.Buk SJ. Simultaneous demonstration of connective tissue elastica and fibrin by a combined Verhoeff's elastic-Martius-scarlet-blue trichrome stain. Stain technology. 1984;59:1–5. doi: 10.3109/10520298409113822. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhari M, Stumper O. Plastic bronchitis after Fontan operation: treatment with stent fenestration of the Fontan circuit. Heart. 2004;90:801. doi: 10.1136/hrt.2003.029041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu EK, Cheng J, Foley JS, Mecham BH, Owen CA, Haley KJ, Mariani TJ, Kohane IS, Tschumperlin DJ, Drazen JM. Induction of the plasminogen activator system by mechanical stimulation of human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2006;35:628–638. doi: 10.1165/rcmb.2006-0040OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collen D, Lijnen HR. The fibrinolytic system in man. Crit Rev Oncol Hematol. 1986;4:249–301. doi: 10.1016/s1040-8428(86)80014-2. [DOI] [PubMed] [Google Scholar]
- 7.Costello JM, Steinhorn D, McColley S, Gerber ME, Kumar SP. Treatment of plastic bronchitis in a Fontan patient with tissue plasminogen activator: a case report and review of the literature. Pediatrics. 2002;109:e67. doi: 10.1542/peds.109.4.e67. [DOI] [PubMed] [Google Scholar]
- 8.DiCindio S, Theroux M, Costarino AT, Jr, Cook S, O'Reilly R. Plastic bronchitis: a case report. Paediatric anaesthesia. 2004;14:520–523. doi: 10.1111/j.1460-9592.2004.01265.x. [DOI] [PubMed] [Google Scholar]
- 9.Do TB, Chu JM, Berdjis F, Anas NG. Fontan patient with plastic bronchitis treated successfully using aerosolized tissue plasminogen activator: a case report and review of the literature. Pediatr Cardiol. 2009;30:352–355. doi: 10.1007/s00246-008-9312-2. [DOI] [PubMed] [Google Scholar]
- 10.Dunn JS, Nayar R, Campos J, Hybertson BM, Zhou Y, Manning MC, Repine JE, Stringer KA. Feasibility of tissue plasminogen activator formulated for pulmonary delivery. Pharm Res. 2005;22:1700–1707. doi: 10.1007/s11095-005-6335-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg DJ, Dodds K, Rychik J. Rare problems associated with the Fontan circulation. Cardiol Young. 20:113–119. doi: 10.1017/S1047951110001162. [DOI] [PubMed] [Google Scholar]
- 12.Goo HW, Jhang WK, Kim YH, Ko JK, Park IS, Park JJ, Yun TJ, Seo DM. CT findings of plastic bronchitis in children after a Fontan operation. Pediatr Radiol. 2008;38:989–993. doi: 10.1007/s00247-008-0937-3. [DOI] [PubMed] [Google Scholar]
- 13.Griffiths ER, Kaza AK, Wyler von Ballmoos MC, Loyola H, Valente AM, Blume ED, del Nido P. Evaluating failing Fontans for heart transplantation: predictors of death. Ann Thorac Surg. 2009;88:558–563. doi: 10.1016/j.athoracsur.2009.03.085. discussion 563–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoylaerts M, Rijken DC, Lijnen HR, Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982;257:2912–2919. [PubMed] [Google Scholar]
- 15.Lackowski NP, Pitzer JE, Tobias M, Van Rheen Z, Nayar R, Mosharaff M, Stringer KA. Safety of prolonged, repeated administration of a pulmonary formulation of tissue plasminogen activator in mice. Pulm Pharmacol Ther. 2010;23:107–114. doi: 10.1016/j.pupt.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madsen P, Shah SA, Rubin BK. Plastic bronchitis: new insights and a classification scheme. Paediatr Respir Rev. 2005;6:292–300. doi: 10.1016/j.prrv.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Nishiuma T, Sisson TH, Subbotina N, Simon RH. Localization of plasminogen activator activity within normal and injured lungs by in situ zymography. Am J Respir Cell Mol Biol. 2004;31:552–558. doi: 10.1165/rcmb.2004-0162OC. [DOI] [PubMed] [Google Scholar]
- 18.Onoue Y, Adachi Y, Ichida F, Miyawaki T. Effective use of corticosteroid in a child with life-threatening plastic bronchitis after Fontan operation. Pediatrics international: official journal of the Japan Pediatric Society. 2003;45:107–109. doi: 10.1046/j.1442-200x.2003.01659.x. [DOI] [PubMed] [Google Scholar]
- 19.Park JY, Elshami AA, Kang DS, Jung TH. Plastic bronchitis. Eur Respir J. 1996;9:612–614. doi: 10.1183/09031936.96.09030612. [DOI] [PubMed] [Google Scholar]
- 20.Peleg U, Schwartz S, Sirota G, Hochman I, Cohen D, Picard E. Persistent plastic bronchitis in a child after cardiac surgery. The Israel Medical Association journal: IMAJ. 2005;7:122–124. [PubMed] [Google Scholar]
- 21.Preciado D, Verghese S, Choi S. Aggressive bronchoscopic management of plastic bronchitis. Int J Pediatr Otorhinolaryngol. 74:820–822. doi: 10.1016/j.ijporl.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Quasney MW, Orman K, Thompson J, Ring JC, Salim M, Schoumacher RA, Watson D, Novick W, Deitcher SR, Joyner R. Plastic bronchitis occurring late after the Fontan procedure: treatment with aerosolized urokinase. Crit Care Med. 2000;28:2107–2111. doi: 10.1097/00003246-200006000-00074. [DOI] [PubMed] [Google Scholar]
- 23.Setzer N, Malvezzi L, McBride W. "Plastic bronchitis" complicating recovery from congenital heart surgery. The Journal of pediatrics. 2001;138:605. doi: 10.1067/mpd.2001.113004. [DOI] [PubMed] [Google Scholar]
- 24.Shah SS, Drinkwater DC, Christian KG. Plastic bronchitis: is thoracic duct ligation a real surgical option? Ann Thorac Surg. 2006;81:2281–2283. doi: 10.1016/j.athoracsur.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Stringer KA, Tobias M, Dunn JS, Campos J, Van Rheen Z, Mosharraf M, Nayar R. Accelerated dosing frequency of a pulmonary formulation of tissue plasminogen activator is well-tolerated in mice. Clin Exp Pharmacol Physiol. 2008;35:1454–1460. doi: 10.1111/j.1440-1681.2008.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suenson E, Lutzen O, Thorsen S. Initial plasmin-degradation of fibrin as the basis of a positive feed-back mechanism in fibrinolysis. Eur J Biochem. 1984;140:513–522. doi: 10.1111/j.1432-1033.1984.tb08132.x. [DOI] [PubMed] [Google Scholar]
- 27.Tzifa A, Robards M, Simpson JM. Plastic bronchitis; a serious complication of the Fontan operation. Int J Cardiol. 2005;101:513–514. doi: 10.1016/j.ijcard.2004.03.085. [DOI] [PubMed] [Google Scholar]
- 28.Wakeham MK, Van Bergen AH, Torero LE, Akhter J. Long-term treatment of plastic bronchitis with aerosolized tissue plasminogen activator in a Fontan patient. Pediatr Crit Care Med. 2005;6:76–78. doi: 10.1097/01.PCC.0000149320.06424.1D. [DOI] [PubMed] [Google Scholar]
- 29.Zaccagni HJ, Kirchner L, Brownlee J, Bloom K. A case of plastic bronchitis presenting 9 years after fontan. Pediatr Cardiol. 2008;29:157–159. doi: 10.1007/s00246-007-9127-6. [DOI] [PubMed] [Google Scholar]