Abstract
Abnormalities of the hypothalamic-pituitary-adrenal (HPA) axis have been reported in subjects with Alzheimer’s disease (AD) and may include increased cerebrospinal fluid (CSF) cortisol concentrations. Moreover, presence of the APOE ε4 allele, which is an established risk factor for the development of AD, has been shown to associate with higher CSF cortisol levels, especially in AD sufferers. In this study, we examined whether TOMM40 variants, which have been reported to influence age of onset of AD, also had an effect on CSF cortisol levels, in healthy, cognitively intact individuals with or without APOE ε4. In our results, the increase in CSF cortisol associated with the presence of the APOE ε4 allele was only detected when a short TOMM40 poly-T variant, shown to associate with later age of onset of AD in ε4 carriers, was not when present. These results are consistent with previous reports (e.g., Roses et al. 2009) suggesting that TOMM40 poly-T variants influence the effects of APOE alleles.
Keywords: TOMM40 poly-T, APOE, cerebrospinal fluid, cortisol, Alzheimer’s disease, hippocampus
Introduction
Cortisol, a glucocorticoid, is released following stressful events, and is essential for human survival and for the ability of individuals to cope with stress. Stress response and the cortisol release are regulated via the hypothalamic-pituitary-adrenal (HPA) axis, which involves the hypothalamus, the anterior pituitary gland and the adrenal cortex (see Pomara et al., 2003; Roozendaal, 2002; Wingenfield & Wolf, 2010, for recent reviews).
Stress and cortisol release have also been found to influence memory and they have been associated in particular with poorer memory performance (Wolf, 2009). The neuronal substrate that connects HPA axis activity and memory performance is the hippocampus, which is considered critical for both episodic and spatial memory. The hippocampus has a high density of glucocorticoid receptors and has been implicated in the regulation of the HPA axis through negative feedback (de Kloet, 2003). Importantly, the hippocampus is also susceptible to structural damage if abnormally elevated release of glucocorticoids extends over time due to chronic stress or other factors (Lupien et al. 1998; Sapolsky, 2000). In turn, damage may provoke a reduction in the density of glucocorticoid receptors, or impaired function, which could result in less feedback inhibition and increased glucocorticoids release, thus propagating a vicious cycle that may lead to additional insult to the hippocampus.
Early decline of hippocampal function and a distinct loss of episodic memory are also among warning signs of incipient Alzheimer’s disease (AD) (Convit et al. 1993; Jack et al. 1997, 1999; Killianny et al. 2000). Some evidence also suggests an association between higher brain concentrations of glucocorticoids, HPA axis dysfunction and AD hippocampal pathology (see also Pomara et al. 2003, for a review). For instance, much higher levels of cortisol have been found in ventricular cerebrospinal fluid (CSF), post mortem, in pre-senile (under 65 years of age) AD subjects compared to age-matched controls (Swaab et al. 1994). Further evidence of an association between HPA axis abnormalities and AD comes from taking into consideration the APOE genotype. The apoliprotein E (apoE) has been found to regulate the synthesis of glucocorticoids (Poirier et al. 1995). Critically, the ε4 allele of the APOE gene, i.e., the gene which encodes the apoliprotein E, is considered the strongest risk factor for the development of late-onset AD (Blennow et al. 1996). Importantly, healthy individuals carrying the ε4 allele have been found to show structural and functional abnormalities of the hippocampus, consistent with preclinical brain changes related to AD (e.g., Crivello et al. 2010; Donix et al. 2010; Lu et al. 2011; Nierenberg et al. 2005).
A few recent studies have examined the relationship between APOE genotype, salivary cortisol concentrations and cognitive performance in elderly individuals, with differing outcomes (Beluche et al. 2010; Gerritsen et al. in press; Lee et al. 2008), but, to the best of our knowledge, only two studies so far have examined whether different forms of APOE are associated with varying degrees of cortisol concentrations (Fiocco et al. 2008; Peskind et al. 2001). Peskind et al. (2001) investigated CSF cortisol concentrations, which are thought to reflect brain levels more closely than plasma or salivary concentrations, in 64 AD sufferers and 34 healthy, nondemented, controls. They observed that CSF cortisol levels were higher in AD subjects than in controls (cf., Swaab et al. 1994) and, critically, when combining the two groups, they found that the APOE ε4/ε4 genotype was associated with higher CSF cortisol levels than the APOE ε3/ε4 genotype, which, in turn, was associated with higher CSF cortisol than the APOE ε3/ε3 genotype. The lowest cortisol level was observed in the APOE ε2/ε3 group, although this group did not significantly differ from the APOE ε3/ε3 group. This general pattern was stronger in AD sufferers than in controls. Fiocco et al. (2008), however, failed to detect an APOE effect on peripheral cortisol in a longitudinal study, although they observed that ε4 carriers (n=13) presented higher cortisol levels than non-ε4 carriers (n=50) at the initial visit, in one of two cohorts. These results suggest that brain cortisol levels, as reflected by CSF concentrations, may be affected by APOE genotype, whereas peripheral cortisol levels may not be.
Recently, a variable length deoxythymidine homopolymer (poly-T), rs10542523, in the TOMM40 gene, which is in linkage disequilibrium with APOE, has also been reported to modulate risk and onset age of late-onset AD (Caselli et al. 2010; Roses et al. 2009). Roses and colleagues (see also Lutz et al. 2010) have shown that APOE ε4 alleles are nearly exclusively linked to TOMM40 poly-T variants between 21 and 30 T residues in length (long variants; i.e., L), whereas APOE ε3 alleles may be linked to either short variants (20 or lower T residues in length; i.e., S) or very long variants (31 or over T residues in length; i.e., VL). In individuals carrying the ε3 allele, VL poly-T variants were found to associate with earlier late-onset AD onset age, including in APOE ε3/ε3 carriers, a genotype previously considered to confer neutral risk for late-onset AD. In contrast, S poly-T variants are found to be associated with later late-onset AD onset age in ε3/ε4 and ε3/ε3 individuals and are considered to result in a protective effect.
Given the recently reported association between APOE and TOMM40 in influencing risk and age of onset of late-onset AD, we raised the question as to whether TOMM40 poly-T variants might mediate the effects of APOE alleles on CSF cortisol concentrations, as initially reported by Peskind et al. (2001). We hypothesized that shorter TOMM40 poly-T variants, as for onset age of AD, should be protective against the deleterious effects of APOE ε4 and, thus, might moderate the increase in CSF cortisol associated with this allele. Roses and colleagues (2009) have suggested a number of mechanisms by which different TOMM40 poly-T variants may affect risk for AD, including interference with APOE transcription, but none of these mechanisms have yet been established. If our hypothesis is correct, it would be possible to advance an HPA axis-related mechanism to explain, at least in part, how TOMM40 variants influence AD risk.
Methods
Subjects
This study was approved by the Institutional Review Boards of the Nathan S. Kline Institute for Psychiatric Research and the New York University School of Medicine. Participants in the study were volunteers from the NY/NJ area who responded to advertisements in local newspapers and flyers. Some study participants were recruited from our active Memory Education and Research Initiative program sponsored in part by Rockland County Department of Health. All subjects signed a consent form prior to being examined. Compensation of up to $450 was provided to the study participants. The total number of 133 participants completed the baseline visit; 51 of these subjects took part in the optional lumbar puncture procedure where a CSF sample for cortisol determination was collected. Out of these 51 subjects, a total of three subjects were excluded because they showed gross structural abnormalities (e.g., infarction and severe ventriculomegaly) in the MRI, and one had a Mini-Mental State Exam (MMSE) score below 28. These subjects were originally recruited for a study on the relationship between major depressive disorder (MDD) and Aβ levels, which will be reported separately. For this reason, 28 of the 47 remaining subjects were diagnosed with MDD, and 19 were controls. Group demographics are reported in Table 1.
Table 1.
Demographics reported by TOMM40 and APOE genotypes combination; means (SD) are reported for Age, Years of Education, and MMSE; gender is expressed in proportion of females; MDD is expressed in proportion of subjects with MDD. None of these differences are significant at the 0.05 level.
| With S poly-T | Without S poly-T | |||
|---|---|---|---|---|
| ε4 positive (6) | ε4 negative (24) | ε4 positive (10) | ε4 negative (7) | |
| Age | 66.2 (6.6) | 67.3 (6.5) | 68.3 (6.8) | 65.8 (5.0) |
| Education | 18.3 (3.2) | 16.2 (2.7) | 16.8 (2.2) | 16.0 (2.4) |
| MMSE | 29.7 (0.5) | 29.5 (0.7) | 29.7 (0.5) | 30.0 (0.0) |
| Gender | 33% | 42% | 50% | 71% |
| MDD | 67% | 50% | 70% | 71.4% |
Note: S poly-T = short TOMM40 poly-T. Education = Years of education.
Cortisol Determination
CSF Cortisol was measured by the Clinical Translational Research Laboratory at Emory University Hospital, Atlanta, GA. Cortisol was measured by a chemiluminescent immunoassay available on the Beckman Access analyzer and calibrated against the European GC/MS reference method.
Description of the TOMM40 Polymorphic Assays
The TOMM40 determinations were performed By Polymorphic DNA Technologies, Inc. (Alameda, CA). Four polymorphisms were analyzed for each genomic sample: rs8106922, rs429358, rs7412, and rs10524523. The polymorphism rs10524523 is a homopolymer length polymorphism (polyT) located in an intronic region of TOMM40. In the human reference sequence, the number of T residues in the homopolymer, “N”, is 35, and the specific variation described by rs10524523 is a 19 base pair deletion, making the homopolymer T16 (N=16) the variant allele. In the Duke/Polymorphic DNA Technologies haplotyping work, other alleles of this homopolymer have been observed, with values of N ranging from 14 to 46 residues. For each genomic sample, PCR was used to amplify each polymorphism using 40 to 120 nanograms of genomic DNA per sample followed by bidirectional direct Sanger sequencing of the DNA templates on an ABI 3730xl sequencing platform and sequence data analysis.
Design and Analysis
The purpose of this study was to test whether shorter TOMM40 poly-T variants influenced the association between APOE alleles and changes in CSF cortisol levels. In Table 2 are summarized the APOE and TOMM40 genotypes combinations per group. APOE was defined by two levels: subjects in the ε4 carriers group had at least one ε4 allele (n= 16), whereas non-ε4 carriers had no ε4 alleles (n= 31). TOMM40 was also defined by two levels: short carriers had at least one S allele (n= 30), whereas non-short carriers had no S allele (n= 17). To examine the effect of APOE and TOMM40 on CSF cortisol, we conducted a 2 × 2 analysis of variance (ANOVA). In addition, we also conducted an analogous ANOVA on age, years of education, MMSE, gender and MDD status to test for covariates. In case of covariates, we planned to employ an analysis of covariance (ANCOVA) to replace the ANOVA.
Table 2.
List of individual combinations of TOMM40 and APOE genotypes. Numbers in parentheses indicate number of subjects.
| With S poly-T | Without S poly-T | ||
|---|---|---|---|
| ε4 positive (6) | ε4 negative (24) | ε4 positive (10) | ε4 negative (7) |
| ε2/ε4, S-L (1) | ε2/ε3, S-L (1) | ε2/ε4, L-VL (1) | ε2/ε3, VL-VL (1) |
| ε2/ε4, S-VL (1) | ε2/ε3, S-VL (3) | ε3/ε4, L-L (1) | ε3/ε3, VL-VL (6) |
| ε3/ε4, S-L (1) | ε2/ε3, S-S (5) | ε3/ε4, L-VL (8) | |
| ε3/ε4, S-S (2) | ε3/ε3, S-VL (11) | ||
| ε3/ε4, S-VL (1) | ε3/ε3, S-S (4) | ||
Note: S poly-T = short TOMM40 poly-T.
Procedure
The study was conducted over 4 visits, generally each 1 week apart. The first three visits were conducted at the Nathan Kline Institute, Orangeburg, NY and at the Clinical & Translational Science Institute, NYU Langone Medical Center. On visit 1, subjects were explained the study procedures and informed of their rights for the purpose of obtaining informed consent. Medical and psychiatric history and vital signs were obtained. Participants also underwent a psychiatric evaluation, and global cognitive status was assessed using the MMSE and the Clinical Dementia Rating. Blood was also drawn for routine laboratory tests and for APOE and TOMM40 genotyping. At visit 2, participants received an MRI scan of the head to quantify the magnitude of vascular brain pathology. At visit 3, subjects underwent a comprehensive neuropsychological assessment. On visit 4 a lumbar puncture was performed by a neuroradiologist. Subjects were asked to fast overnight prior to the lumbar puncture (LP) visit. After fasting, at 10 AM, 15 ml of clear CSF was collected into three polypropylene tubes using a fine 25G LP needle guided by fluoroscopy. Tubes were then immediately placed directly on ice for a maximum of 1 hour until samples were centrifuged at 4 degrees C at 1500 rpm for 10 minutes, then aliquots of 0.25 cc placed into 1.00 cc polypropylene cryogenic vials and labeled “A”, “B”, or “C”, and placed in Nunc 81-Cell Storage Boxes at −80 degrees C.
Results
The 2 × 2 ANOVAs did not show any significant effects of APOE, TOMM40 or an interaction on age, years of education, MMSE, gender and MDD status, therefore, we opted not to employ an ANCOVA to test the effects of APOE and TOMM40 variants on CSF cortisol. In addition, MDD status had no effect on CSF cortisol levels [t(45)=.682, p=.499].
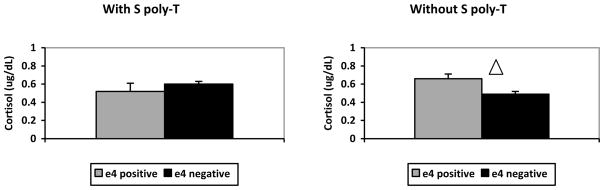
We conducted a 2 × 2 ANOVA on CSF cortisol to test our hypothesis. Results showed a significant interaction between APOE and TOMM40 [F(1,43)=5.948, p=.019], but no significant main effects (p>.05 for both APOE and TOMM40 main effects). Post-hoc comparisons, conducted with Fisher’s LSD to correct for multiple testing, showed no significant simple effect of carrying the ε4 allele on CSF cortisol concentrations when subjects also carried a S TOMM40 poly-T variant (p=.257) (Figure, left), whereas, we detected a significant simple effect of ε4 in subjects who did not carry a S poly-T variant (p=.028) (Figure, right). No other post-hoc comparison reached significance. Finally, as Peskind et al. (2001) reported that the lowest CSF cortisol level was associated with carrying the APOE ε2 allele, we compared CSF cortisol levels across subjects who carried the APOE ε2 allele (n=13) and subjects who did not (n=34), with a t-test. The results showed no difference across groups [t(45)=.073, p=.942], as both groups had the same level of CSF cortisol, 0.59 in ug/dL.
Figure.
Left: CSF cortisol concentrations in ug/dL in subjects with the S poly-T, as a function of APOE ε4. Right: CSF cortisol concentrations in ug/dL in subjects without the S poly-T, as a function of APOE ε4. e4 positive refers to subjects with APOE ε4; e4 negative refers to subjects without APOE ε4. △: indicates a significant difference.
Discussion
In our results, we found that the increase in CSF cortisol associated with carrying the APOE ε4 allele (Peskind et al. 2001) was only detected in subjects – cognitively intact and non-demented – who did not possess a S TOMM40 poly-T variant. These results are consistent with previous reports (e.g., Roses et al. 2009) suggesting that TOMM40 poly-T variants may modulate the effects of APOE alleles. In particular, our results suggest that the S poly-T variant of TOMM40 might have a “protective” effect against the effects of APOE ε4: when the S poly-T variant is present, elevations in CSF cortisol that have been reported in association with the APOE ε4 allele (Peskind et al.), and which might be damaging to hippocampal structure if extended over time (Lupien et al. 1998; Sapolsky, 2000), are not observed.
Our investigation of the effects of TOMM40 poly-T variants on CSF cortisol levels was based on evidence suggesting that TOMM40 poly-T variants may be associated with AD risk (e.g., Caselli et al. 2010; Roses et al. 2009) and, particularly, that shorter poly-T variants may be linked with lower risk and later age of onset for late-onset AD. Similarly, we recently provided evidence that the S TOMM40 poly-T variant is protective against the benzodiazepine-induced anterograde amnestic effects, showing that subjects who are homozygous for longer poly-T variants (e.g., VL-VL) are more likely to show decline in recall tasks after acute lorazepam administration, as compared subjects who are homozygous for the S variants (S-S), in the absence of APOE ε4 (Pomara et al. in press). Johnson et al. (2010) have also provided evidence that TOMM40 poly-T variants are linked to differences in brain volume. Johnson et al. utilized structural brain imaging to examine grey matter volume in healthy, APOE ε3 homozygous, adults (mean age of 57) and compared subjects who were homozygous for the VL TOMM40 poly-T variant (n = 33) to subjects who were homozygous for the S variant (n = 37). In their results, subjects with the VL-VL allele combination presented significantly less grey matter volume than controls in the ventral posterior cingulate and precuneus, which are regions of the brain associated with AD.
Taken together, this set of recent findings and our present results suggest that the TOMM40 gene may play a role in modulating AD and drug-toxicity risk, in combination with APOE. Although the exact neurobiological mechanisms by which TOMM40 would influence the emergence of AD are not entirely clear, Roses and colleagues (2009) have suggested a number of mechanisms, including exon skipping leading to malfunctioning Tom40 protein, and interference with APOE transcription, but none of these mechanisms have yet been established. Our findings implicate HPA axis dysfunction as a potential gateway through which the combined effects of APOE and TOMM40 may affect risk of AD and memory decline. Importantly, our findings may aid the early identification (i.e., before the emergence of symptoms of a dementing illness) of subjects in a clinical setting, who are at the highest risk for the development of AD. Specifically, our results suggest that subjects without the S TOMM40 poly-T who carry the APOE ε4 allele are at greater risk to have higher brain concentrations of cortisol than ε4-negative subjects with no S TOMM40 poly-T.
Our study was somewhat limited by small sample size, which forced us to group subjects in aggregated categories (i.e., whether they possessed a S poly-T variant of TOMM40 or not). A restriction of this approach is that we were limited to testing whether the presence of a protective S poly-T influenced (specifically, in this case, decreased) CSF cortisol levels. Future studies should overcome this limitation and examine in greater detail TOMM40 poly-T sub-groups (e.g., L-L vs. L-VL), as defined by differences in poly-T length.
Acknowledgments
We wish to thank Drs Ann M. Saunders and Allen D. Roses, Deane Drug Discovery Institute and Department of Medicine, Duke University, Durham, NC, for supervising the TOMM40 polymorphic assays determination.
Role of the Funding Source
This study was supported in part by an NIMH Grant (R01 MH080405) to NP. The NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of Interest
The authors report no conflict of interest.
Contributors
Davide Bruno wrote the paper and analyzed the data.
Jay J. Nierenberg analyzed the MRIs for subjects’ exclusion.
James C. Ritchie provided the cortisol quantitations.
Michael W. Lutz provided genetics support and counseling.
Nunzio Pomara supervised the project and supervised the data collection.
Suggested Reviewers
R. De Kloet
Leiden, The Netherlands
S.J. Lupien
Verdun, Canada
O.T. Wolf
Bochum, Germany
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beluche I, Carriere I, Ritchie K, Ancelin ML. A prospective study of diurnal cortisol and cognitive function in community-dwelling elderly people. Psychol Med. 2010;40:1039–1049. doi: 10.1017/S0033291709991103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, DeLeon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Saunders A, Lutz M, Huentelman M, Reiman E, Roses A. TOMM40, APOE, and age of onset of Alzheimer’s disease. Poster session presented at the Alzheimer’s Association International Conference on Alzheimer’s Disease; Honolulu, HI. Jul, 2010. [Google Scholar]
- Convit A, de Leon MJ, Golomb J, George AE, Tarshish CY, Bobinski M, Tsui W, De Santi S, Wegiel J, Wisniewski H. Hippocampal atrophy in early Alzheimer’s disease: anatomic specificity and validation. Psychiatr Q. 1993;64:371–387. doi: 10.1007/BF01064929. [DOI] [PubMed] [Google Scholar]
- Crivello F, Lemaître H, Dufouil C, Grassiot B, Delcroix N, Tzourio-Mazoyer N, Tzourio C, Mazoyer B. Effects of ApoE-epsilon4 allele load and age on the rates of grey matter and hippocampal volumes loss in a longitudinal cohort of 1186 healthy elderly persons. Neuroimage. 2010;53:1064–1069. doi: 10.1016/j.neuroimage.2009.12.116. [DOI] [PubMed] [Google Scholar]
- De Kloet ER. Hormones, brain and stress. Endocr Regul. 2003;37:51–68. [PubMed] [Google Scholar]
- Donix M, Burggren AC, Suthana NA, Siddarth P, Ekstrom AD, Krupa AK, Jones M, Martin-Harris L, Ercoli LM, Miller KJ, Small GW, Bookheimer SY. Family history of Alzheimer’s disease and hippocampal structure in healthy people. Am J Psychiatry. 2010;167:1399–406. doi: 10.1176/appi.ajp.2010.09111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiocco AJ, Poirier J, Joober R, Nair NPV, Lupien SJ. Acute and long-term associations between ApoE genetic polymorphism, cortisol levels, and declarative memory performance in older adults. Psychoneuroendocrinology. 2008;33:625–633. doi: 10.1016/j.psyneuen.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Gerritsen L, Comijs HC, Deeg DJH, Penninx BWJH, Geerlings MI. Salivary cortisol, APOE-ε4 allele and cognitive decline in a prospective study of older persons. Neurobiol of Aging. doi: 10.1016/j.neurobiolaging.2009.09.007. In Press. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu YC, Waring SC, O’Brien PC, Tangalos EG, Smith GE, Ivnik RJ, Kokmen E. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology. 1997;49:786–94. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E. Prediction of AD with MRI based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397e403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, La Rue A, Hermann BP, Xu G, Koscik RL, Jonaitas EM, Bendlin BB, Roses AD, Saunders AM, Lutz MW, Asthana S, Green RC, Sager MA. TOMM40 is associated with gray matter volume in middle-aged persons with APOE ε3/ε3 genotype. Poster session presented at the Alzheimer’s Association International Conference on Alzheimer’s Disease; Honolulu, HI. Jul, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killiany RJ, Gomez-Isla T, Moss M, Kikinis R, Sandor T, Jolesz F, Tanzi R, Jones K, Hyman BT, Albert MS. Use of structural magnetic resonance imaging to predict who will get Alzheimer’s disease. Ann Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- Lee BK, Glass TA, Wand GS, McAtee MJ, Bandeen-Roche K, Bolla KI, Schwartz BS. Apoliprotein E genotype, cortisol, and cognitive function in community-dwelling older adults. Am J Psychiatry. 2008;165:1456–1464. doi: 10.1176/appi.ajp.2008.07091532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PH, Thompson PM, Leow A, Lee GJ, Lee A, Yanovsky I, Parikshak N, Khoo T, Wu S, Geschwind D, Bartzokis G. Apoliprotein E genotype is associated with temporal and hippocampal atrophy rates in healthy elderly adults: A tensor-based morphometry study. J Alzheimers Dis. 2011;23:433–442. doi: 10.3233/JAD-2010-101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, se Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Lutz MW, Crenshaw DG, Saunders AM, Roses AD. Genetic variation at a single locus and age of onset for Alzheimer’s disease. Alzheimers Dement. 2010;6:125–131. doi: 10.1016/j.jalz.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierenberg J, Pomara N, Hoptman MJ, Sidtis JJ, Ardekani BA, Lim KO. Abnormal white matter integrity in healthy apoliprotein E epsilon4 carriers. Neuroreport. 2005;16:1369–1372. doi: 10.1097/01.wnr.0000174058.49521.16. [DOI] [PubMed] [Google Scholar]
- Peskind ER, Wilkinson CW, Petrie EC, Schellenberg GD, Raskind MA. Increased CSF cortisol in AD is a function of APOE genotype. Neurology. 2001;56:1094–1098. doi: 10.1212/wnl.56.8.1094. [DOI] [PubMed] [Google Scholar]
- Poirier J, Minnich A, Davignon J. Apoliprotein E, synaptic plasticity and Alzheimer’s disease. Ann Med. 1995;27:663–670. doi: 10.3109/07853899509019253. [DOI] [PubMed] [Google Scholar]
- Pomara N, Bruno D, Sidtis JJ, Lutz MW, Greenblatt DJ, Saunders AM, Roses AD. TOMM40 poly-T length regulates lorazepam-induced cognitive toxicity in healthy APOE e4-negative elderly. Journal of Clinical Psychopharmacology. doi: 10.1097/JCP.0b013e318222810e. in press. [DOI] [PubMed] [Google Scholar]
- Pomara N, Greenberg WM, Branford MD, Doraiswamy PM. Therapeutic implications of HPA axis abnormalities in Alzheimer’s disease: review and update. Psychopharmacol Bull. 2003;37:120–134. [PubMed] [Google Scholar]
- Roozendaal B. Stress and memory: Opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem. 2002;78:578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- Roses AD, Lutz MW, Amrine-Madsen H, Saunders AM, Crenshaw DG, Sundseth SS, Huentelman MJ, Welsh-Bohmer KA, Reiman EM. A TOMM40 variable-length polymorphism predicts the age of late-onset Alzheimer’s disease. Pharmacogenomics J. 2009;10:375–384. doi: 10.1038/tpj.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Raadsheer FC, Endert E, Hofman MA, Kamphorst W, Ravid R. Increased cortisol levels in aging and Alzheimer’s disease in postmortem cerebrospinal fluid. J Neuroendocrinol. 1994;6:681–687. doi: 10.1111/j.1365-2826.1994.tb00635.x. [DOI] [PubMed] [Google Scholar]
- Wingenfeld K, Wolf OT. HPA axis alterations in mental disorders: Impact on memory and its relevance for therapeutic interventions. 2010;00:1–9. doi: 10.1111/j.1755-5949.2010.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf OT. Stress ahnd memory in humans: Twelve years of progress? Brain Research. 2009;1293:142–154. doi: 10.1016/j.brainres.2009.04.013. [DOI] [PubMed] [Google Scholar]