Abstract
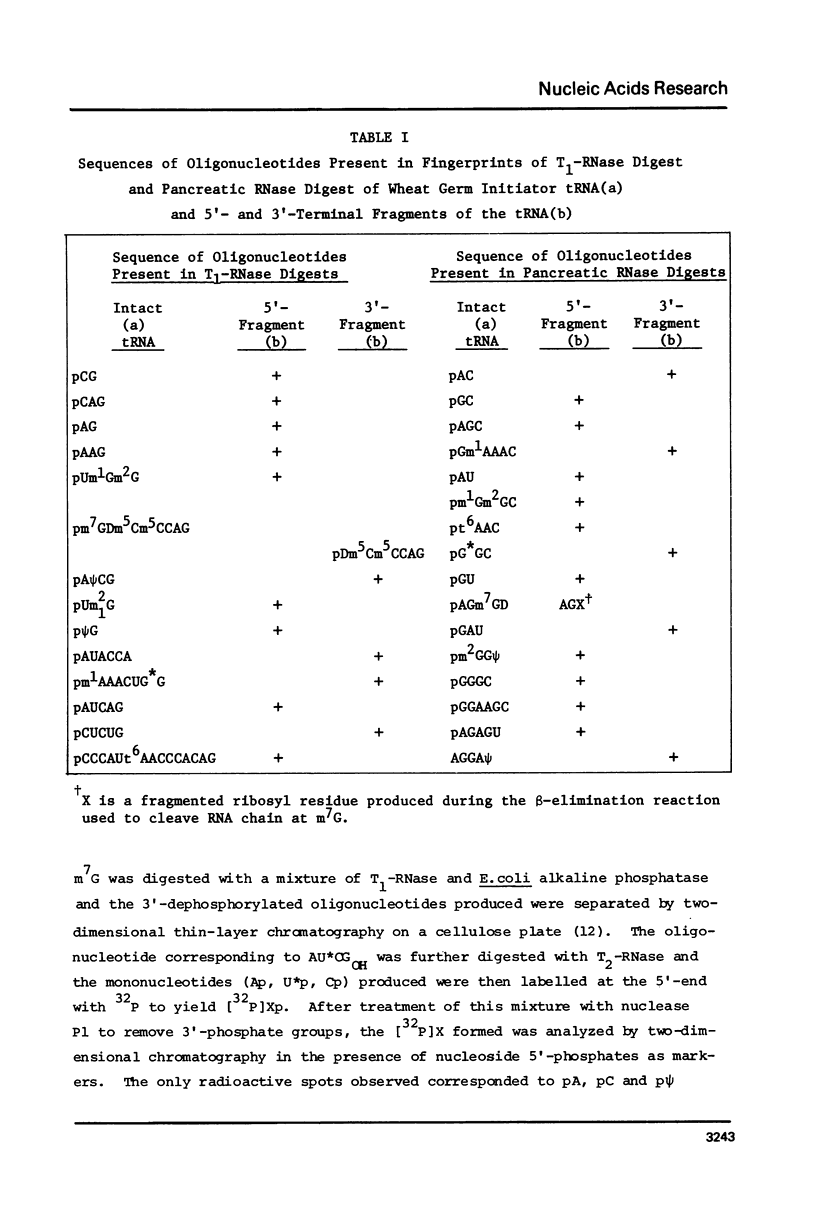
The primary sequence of wheat germ initiator tRNA has been determined using in vitro labelling techniques. The sequence is: pAUCAGAGUm1Gm2GCGCAG CGGAAGCGUm2GG psi GGGCCCAUt6AACCCACAGm7GDm5Cm5CCAGGA psi CGm1AAACCUG*GCUCUGAUACCAOH. As in other eukaryotic initiator tRNAs, the sequence -T psi CG(A)- present in loop IV of virtually all tRNA active in protein synthesis is absent and is replaced by -A psi CG-. The base pair G2:C71 present in all other initiator tRNAs recognized by E. coli Met-tRNA transformylase is absent and is replaced by U2:A71. Since wheat germ initiator tRNA is not formylated by E. coli Met-tRNA transformylase this implies a possible role of the G2:C71 base pair present in other initiator tRNAs in formylation of initiator tRNA species.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dube S. K., Marcker K. A., Clark B. F., Cory S. Nucleotide sequence of N-formyl-methionyl-transfer RNA. Nature. 1968 Apr 20;218(5138):232–233. doi: 10.1038/218232a0. [DOI] [PubMed] [Google Scholar]
- Gauss D. H., Sprinzl M. Compilation of tRNA sequences. Nucleic Acids Res. 1981 Jan 10;9(1):r1–23. [PMC free article] [PubMed] [Google Scholar]
- Ghosh K., Ghosh H. P., Simsek M., Raj Bhandary U. L. Initiator methionine transfer ribonucleic acid from wheat embryo. Purification, properties, and partial nucleotide sequences. J Biol Chem. 1974 Aug 10;249(15):4720–4729. [PubMed] [Google Scholar]
- Ghosh K., Grishko A., Ghosh H. P. Initiation of protein synthesis in eukaryotes. Biochem Biophys Res Commun. 1971 Feb 5;42(3):462–468. doi: 10.1016/0006-291x(71)90393-7. [DOI] [PubMed] [Google Scholar]
- Haselkorn R., Rothman-Denes L. B. Protein synthesis. Annu Rev Biochem. 1973;42:397–438. doi: 10.1146/annurev.bi.42.070173.002145. [DOI] [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Identification of features in 5' terminal fragments from reovirus mRNA which are important for ribosome binding. Cell. 1978 Jan;13(1):201–212. doi: 10.1016/0092-8674(78)90150-2. [DOI] [PubMed] [Google Scholar]
- Leis J. P., Keller E. B. Protein chain initiation by methionyl-tRNA. Biochem Biophys Res Commun. 1970 Jul 27;40(2):416–421. doi: 10.1016/0006-291x(70)91025-9. [DOI] [PubMed] [Google Scholar]
- Rich A., RajBhandary U. L. Transfer RNA: molecular structure, sequence, and properties. Annu Rev Biochem. 1976;45:805–860. doi: 10.1146/annurev.bi.45.070176.004105. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Silverman S., Heckman J., Cowling G. J., Delaney A. D., Dunn R. J., Gillam I. C., Tener G. M., Söll D., RajBhandary U. L. The nucleotide sequence of the initiator tRNA from Drosophila melanogaster. Nucleic Acids Res. 1979 Feb;6(2):421–433. doi: 10.1093/nar/6.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek M., RajBhandary U. L. The primary structure of yeast initiator transfer ribonucleic acid. Biochem Biophys Res Commun. 1972 Oct 17;49(2):508–515. doi: 10.1016/0006-291x(72)90440-8. [DOI] [PubMed] [Google Scholar]
- Simsek M., Ziegenmeyer J., Heckman J., Rajbhandary U. L. Absence of the sequence G-T-psi-C-G(A)- in several eukaryotic cytoplasmic initiator transfer RNAs. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1041–1045. doi: 10.1073/pnas.70.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarragó A., Monasterio O., Allende J. E. Initiator-like properties of a methionyl-tRNA from wheat embryos. Biochem Biophys Res Commun. 1970 Nov 9;41(3):765–773. doi: 10.1016/0006-291x(70)90079-3. [DOI] [PubMed] [Google Scholar]
- Wintermeyer W., Zachau H. G. A specific chemical chain scission of tRNA at 7-methylguanosine. FEBS Lett. 1970 Dec;11(3):160–164. doi: 10.1016/0014-5793(70)80518-x. [DOI] [PubMed] [Google Scholar]
- Yarwood A., Boulter D., Yarwood J. N. Methionyl-tRNAs and initiation of protein synthesis in vicia faba (L.). Biochem Biophys Res Commun. 1971 Jul 16;44(2):353–361. doi: 10.1016/0006-291x(71)90607-3. [DOI] [PubMed] [Google Scholar]