Abstract
Background
A major effect of low dose ethanol is impairment of hippocampus-dependent cognitive function. α4/δ-containing GABAAR’s are highly expressed within the dentate gyrus region of the hippocampus where they mediate a tonic inhibitory current that is sensitive to enhancement by low ethanol concentrations. These receptors are also powerful modulators of learning and memory, suggesting that they could play an important role in ethanol’s cognitive-impairing effects. The goal of the present study was to develop a high-throughput cognitive ethanol assay, amenable to use in genetically modified mice that could be used to test this hypothesis.
Methods
We developed a procedure where pre-exposure to a conditioning chamber is used to rescue the “immediate shock deficit.” Using this task, ethanol can be specifically targeted at the hippocampus-dependent process of contextual learning without interfering with pain sensitivity or behavioral performance.
Results
Validation of this task in C57Bl6 mice indicated that 1.0 g/kg ethanol and 10 mg/kg allopregnanolone disrupt contextual learning. Ro15-4513 reversed the effects of ethanol but not allopregnanolone, whereas it produced an impairment when given alone. The high-throughput nature of this task allowed for its application in a large cohort of α4 GABAAR KO mice. Loss of the α4 GABAAR subunit produced an enhanced sensitivity to the cognitive impairing effects of ethanol. This is consistent with the enhanced ethanol sensitivity of synaptic GABAARs that has been previously observed in the dentate gyrus in these mice, but inconsistent with the reduced ethanol sensitivity of extrasynaptic GABAAR’s observed in the same cells.
Conclusions
Overall, these findings are consistent with our hypothesis that ethanol acts directly at GABAA receptors to impair hippocampus-dependent cognitive function. Furthermore, validation of this high-throughput assay will allow for future studies to use anatomically and temporally restricted genetic manipulations to probe more deeply into the neural mechanisms of ethanol action on learning and memory circuits.
Keywords: ethanol, behavior, hippocampus, learning and memory, neurosteroid, Ro15-4513
INTRODUCTION
It is widely accepted that acute doses of ethanol produce profound behavioral and cognitive changes ranging from anxiolysis and impaired cognitive function at low doses to loss of consciousness and death at high doses. There is very little consensus, however, as to which of ethanol’s many neurophysiological effects underlies each of these changes. A major reason for this lack of consensus is the inherent difficulty of connecting putative mechanisms identified using isolated and simplified in-vitro preparations to the complex milieu of the intact brain. One approach to solving this problem is to study both the in vitro and in vivo effects of ethanol in transgenic mice where putative ethanol targets have been altered or genetically deleted (Lobo and Harris, 2008). Electrophysiology in organotypic slices from a defined brain region can be combined with behavioral tasks that are thought to rely on that brain region. The relevance of altered neurophysiological responsiveness to ethanol can then be related to changes in behavioral ethanol sensitivity. A current limitation of this approach is that despite increasingly widespread use of hippocampal slice preparations there is no standard high-throughput behavioral ethanol assay that directly tasks hippocampal function. The goal of the present study was to develop such an assay and then apply it in α4 GABAAR knockout (KO) mice, which have been extensively characterized at the electrophysiological level (Jia et al., 2008, Liang et al., 2008).
In a number of independent studies, ethanol has been shown to enhance tonic inhibitory currents via binding to extra-synaptic α4/δ-containing GABAARs at concentrations ranging from 3 to 30 mM (Spigelman et al., 2002, Wei et al., 2004, Hanchar et al., 2004, Wallner et al., 2003, Fleming et al., 2007, Sundstrom-Poromaa et al., 2002). However, these findings are not without controversy (Borghese and Harris, 2007, Korpi et al., 2007, Santhakumar et al., 2007), and their translation to the behavioral level using α4 and δ KO mice has produced equivocal results (Mihalek et al., 2001, Chandra et al., 2008). For example, ethanol’s acute effects on anxiety, locomotion and motor coordination were not found to be significantly altered in α4 KO mice (Chandra et al, 2008), despite a total loss of ethanol-mediated enhancement of tonic inhibition in the thalamus and hippocampus of these mice (Liang et al., 2008, Jia et al., 2008).
A major effect of low dose ethanol is impairment of cognitive function and the available evidence suggests that α4/δ-containing GABAARs are ideally situated to mediate this effect. Studies in both rodents and humans have found that low-to-moderate doses of ethanol preferentially disrupt hippocampus-dependent learning while sparing hippocampus-independent processes (Acheson et al., 1998, Lister et al., 1991, Matthews et al., 1995, Melia et al., 1996, Weitemier and Ryabinin, 2003, Berry and Matthews, 2004, Berry et al., 2009). At the neurophysiological level ethanol has been shown to disrupt in-vivo synaptic plasticity in the dentate gyrus region of the hippocampus and reduce the spatial specificity of hippocampal CA1 place cells (Givens and McMahon, 1995; Matthews et al, 1996; White and Best, 2000). The α4/δ-containing GABAARs are highly expressed at extrasynaptic locations in the dentate gyrus where they mediate a powerful tonic inhibitory current (Peng et al., 2002, Zhang et al., 2007, Wei et al., 2003, Fleming et al., 2007, Herd et al., 2008, Sun et al., 2004, Sperk et al., 1997, Glykys et al., 2008). Genetic deletion of either the δ or α4 GABAAR subunit produces a specific enhancement of hippocampus-dependent learning and memory, which indicates that the tonic inhibition mediated by these receptors negatively modulates hippocampus-dependent learning (Moore et al., 2010, Wiltgen et al., 2005). Based on all of these findings we therefore hypothesized that α4/δ-containing GABAAR’s may be involved in mediating the cognitive impairing effects of ethanol.
We developed a fear conditioning task where pre-exposure to a conditioning chamber is used to rescue an “immediate shock deficit” (Fanselow, 1990). Critically, this task allows researchers to pharmacologically target hippocampus-dependent learning during the pre-exposure period without affecting the amygdala-dependent formation of the context-shock association (Stote and Fanselow, 2004, Barrientos et al., 2002, Matus-Amat et al., 2004, Rudy et al., 2004).
When placed in a novel environment, such as a conditioning chamber, animals engage in an active exploratory process that allows them to integrate the various multimodal cues that characterize the chamber into a unified “contextual representation” (Rudy et al., 2004, Sanders et al., 2003). In context fear conditioning, the contextual representation serves as the conditional stimulus (CS) that becomes associated with the shock unconditional stimulus (US). The context-shock association is thought to form via synaptic strengthening of hippocampal inputs to the amygdala at sites of CS-US convergence (Maren and Fanselow, 1995). Efficient formation of this contextual CS requires an intact and properly functioning hippocampus and requires several minutes of exploration before it can support conditioning (Wiltgen et al., 2006). If an animal is shocked immediately after being placed into the conditioning chamber it is unable to acquire contextual fear, a phenomenon referred to as the immediate shock deficit (Fanselow, 1990). The ISD occurs because at the time of shock presentation the animal has not yet formed a functional contextual representation and therefore has no CS to associate with the shock. The ISD can be “rescued” if the animal is pre-exposed to the conditioning chamber prior to the immediate shock. This pre-exposure allows the animal to form a contextual representation that it can then rapidly retrieve when placed back in the chamber and given the immediate shock.
Utilizing this pre-exposure immediate shock procedure, ethanol can be administered just before the pre-exposure period and the level of fear produced by the immediate shock 24 hours later can then be used to determine its effects on hippocampus-dependent formation of the contextual CS. If ethanol impairs hippocampus-dependent contextual learning, it follows that mice lacking the ethanol-sensitive α4 GABAAR subunit would show reduced impairment of contextual learning due to ethanol injection. The current study tests this prediction in addition to validating the context pre-exposure immediate shock procedure as a highly sensitive assay of ethanol-induced behavioral/cognitive impairments. The effects of allopregnanolone (ALLO), a neurosteroid that enhances α4/δ-mediated tonic inhibition (Hosie et al., 2006, Meera et al., 2009, Mody, 2007), and Ro15-4513, a GABAAR inverse agonist and “alcohol antagonist” (Santhakumar et al., 2007, Suzdak et al., 1986, Wallner and Olsen, 2008) were also examined in this task in order to further characterize its underlying pharmacology.
MATERIALS AND METHODS
Subjects
Validation Study
Male C57Bl/6 mice ranging in age from 3–5 months were purchased from Taconic Farms and housed in UCLA’s Psychology Department vivarium on a 12 h light/12 h dark cycle with all experiments performed during the light phase. Mice were housed in groups of three to four and had free access to food and water. All animals were handled for two days before the start of the experiment to acclimate to the transport, experimenter, and restraint used in intraperitoneal (i.p.) injection. On the last day of handling, the animals were also weighed to determine injection volumes. The number of animals run over six separate replications totaled 169. At minimum, vehicle pre-exposed animals were included in each replication and there was no significant effect of replication on post-shock or context freezing. For presentation, the data are divided into two experiments. First, the effects of ethanol alone were analyzed and, subsequently, the effects of Ro15-4513 by itself, and in combination with ethanol and allopregnanolone, were examined. For analysis of the effects of ethanol on the context pre-exposure rescue of the immediate shock deficit the following groups were used (see Figure 1A for the experimental design): Saline Non-Pre-Exposed (Saline NP, n = 16), Ethanol Non-Pre-Exposed (EtOH NP, n = 8), Saline Pre-Exposed, (Saline PRE, n = 17), 1.0 g/kg Ethanol Pre-Exposed (EtOH PRE, n = 19) and 0.5 g/kg Ethanol Pre-Exposed (0.5 EtOH PRE, n = 14).
Figure 1. Experimental Design of the Context Pre-Exposure Rescue of the Immediate Shock Deficit Assay.
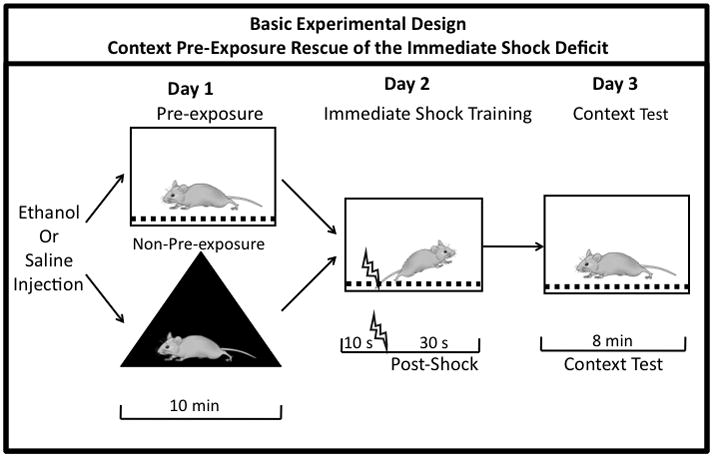
Saline or ethanol is administered to both the non-pre-exposed and pre-exposed groups on Day 1. To control for handling, the non-pre-exposed groups are placed in a distinctly different context in a different room, while the pre-exposed groups are placed in the conditioning context. On Day 2, both groups undergo immediate shock training in the conditioning context. Day 3 consists of an 8 minute context test in the conditioning context.
Two additional groups were run to determine if ethanol had any non-specific effects on the subsequent behavioral expression of freezing. These groups were given ethanol 30 minutes after either non-pre-exposure or pre-exposure: 1.0 g/kg Ethanol 30 minutes Post-Pre-Exposure (EtOH post NP, n = 8) and 1.0 g/kg Ethanol 30 minutes Post-Non-Pre-Exposure (EtOH post NP, n = 8). These data are presented in Supplementary Figure 1.
For analysis of the combined effects of Ro15-4513, ethanol and allopregnanolone on the context pre-exposure rescue of the immediate shock deficit the following 5 groups were used: 1) 3 mg/kg Ro15-4513 (Ro15-4513, n = 8), 2) 3 mg/kg Ro15 + 1.0 g/kg co-administered with ethanol (Ro + EtOH, n = 8), 3) 3 mg/kg Ro15-4513 + 10 mg/kg allopregnanolone (Ro + ALLO, n = 9), 4) 1.0 g/kg ethanol + 10 mg/kg allopregnanolone (EtOH + ALLO, n = 10) and 5) Saline + BCD (control for double injection, n = 6).
Breeding of α4 GABAA subunit KO mice
Heterozygous breeding pairs of hybrid C57Bl6/129 mice were used to produce α4 KO (−/−), α4 HET (+/−) and α4 WT (+/+) mice. In order to generate sufficient α4 WTs for 1.5 g/kg dose of ethanol female α4 HET mice were paired with male α4 WT mice. A total of 142 α4 HET, 54 α4 KO and 72 α4 WT male and female mice ranging in age from 4–7 months were used in the experiment. All housing and breeding occurred in UCLA’s Psychology Department vivarium. Animals were kept on a 12 h light/12 h dark cycle with all experiments performed during the light phase. Mice were housed in groups of two to five and had free access to food and water. All animals were handled and weighed one day before the start of the experiment to acclimate to the transport, experimenter, and restraint used in intraperitoneal (i.p.) injection. A subset of mice from the α4 HET × α4 WT breeders were injected with a 1.5 g/kg dose of ethanol and these data are presented in Supplementary Figure 3. In addition, the sensitivity of 3–4 month old 129 mice (purchased from Taconic Farms) to the cognitive impairing effects of a 1.0 g/kg or 1.5 g/kg dose of ethanol was examined (34 mice total, 9 saline controls, 9 1.0 g/kg ethanol, 8 1.5 g/kg ethanol and 6 NP). These data are presented in Supplementary Figure 2. The following groups are presented in the main experiment: α4 WT: Saline PRE (n = 23), EtOH Pre (n = 24) and NP (n = 14); α4 HET: Saline Pre (n = 48), EtOH Pre (n = 47) and NP (n = 31); α4 KO: Saline PRE (n = 17), EtOH Pre (n = 21) and NP (n = 14). Outliers (defined as being greater than 2 standard deviations away from their group mean) were removed, resulting in the removal of: one female α4 KO EtOH PRE and one male α4 HET NP.
Drugs and Injections
95% Ethyl alcohol (ethanol) (Pharmco, Brookfield, CT) was diluted in bacteriostatic 0.9% sodium chloride (Hospira, Lake Forest, IL) to a 16% w/v solution. 5α-Pregnan-3α-ol-20-one (allopregnanolone, ALLO) (Sigma Chemical, St. Louis, MO) was diluted to 4 mg/ml with 20% w/v (2-Hydroxypropyl)-β-cyclodextrin (abbreviated as BCD) (Sigma Chemical, St. Louis, MO) and sonicated for 90 min at 37°C. Ro15-4513 (Hoffman–LaRoche) administered alone was diluted in 20% w/v BCD to 0.48 mg/ml and sonicated for 90 min at 37°C to give the same injection volume as the 1.0 g/kg ethanol group. Ro15-4513 administered with ethanol (Ro15 + ethanol) was diluted directly in 16% w/v ethanol to 0.48 mg/ml. Ro15-4513 administered with ALLO (Ro15 + ALLO) was diluted in the 4 mg/ml ALLO solution to a final concentration of 1.2 mg/ml. For the EtOH + ALLO groups two i.p. injections were given one immediately following the other. An additional control, that received saline and BCD was used to control for receiving two injections in this group.
Apparatus
Pre-exposure (Day 1), training (Day 2), and testing (Day 2 and Day 3) took place in four identical chambers (Context A) (30 × 25 × 25 cm; Med-Associates Inc). An overlay of two white plastic panels created a continuous curve of side and back walls. The floor of each chamber was made up of 16 stainless steel rods of alternating diameter (0.4 and 1.0 cm) spaced 1.5 cm apart (center to center) wired to a shock generator and scrambler (Med-Associates Inc.) to deliver foot shock. The chamber was placed in a sound-attenuating cubicle. Each chamber was wiped down with 70% ethanol before conditioning and between animals. A metal pan containing a thin film of Windex was placed underneath the grid floors to provide an olfactory component to the context.
For Day 1 only, the non-pre-exposed animals were placed in a separate room with 4 structurally distinct chambers (Context B). Each of these four identical chambers had a white plastic floor (28 × 21 cm) and ceiling and two metal side walls (24 × 21 cm). The chambers were wiped down with a 1% acetic acid solution between each animal. A fan placed on the floor provided background noise (60 dB) and the room was brightly lit with overhead lights.
Procedure
On Day 1, animals were brought by cart to a holding room where they were left untouched in their home cages for 30 minutes. Subjects were then given drug or vehicle by i.p. injection and returned to their home cages for 10 min. Animals pre-exposed to the training context were placed in Context A for 10 min. Non-pre-exposed animals were handled and injected in the same fashion as the PRE group, but placed in Context B by a different experimenter than used in the PRE group. Animals were then returned to their home cages.
On Day 2 (24 h later), all animals were brought by cart to a holding room where they were left untouched in their home cages for 30 minutes. Subjects were placed in Context A for 10 s before foot shock (2 sec, 0.75 mA), remained in the chamber for 30 s post-shock (for a total of 42 s in the conditioning chamber) and then returned to their home cages. On Day 3, all subjects were brought by cart to a holding room where they were left untouched in their home cages for 30 minutes before being placed in Context A for an 8 min context test.
Activity and freezing were recorded using an automated near infrared (NIR) video tracking equipment and computer software (VideoFreeze, Med-Associates Inc.). Video was recorded at 30 frames per second and the software calculated the noise (standard deviation) for each pixel in a frame by comparing its grayscale value to previous and subsequent frames. This produced an “activity unit” score for each frame. Based on previous validation by a human observer, freezing was defined as sub-threshold activity (set at 19 activity units (AU)) for longer than 1 sec. Freezing was scored during the post-shock period for 27 seconds, the first 3 seconds of the 30 second post-shock interval were not analyzed as the animals are often still engage in the activity burst response to the shock during this period.
Measurement of Locomotor Activity During the Pre-Exposure
The activity unit score was also used to measure locomotor activity during the pre-exposure period, with activity averaged across the 10 minute pre-exposure. For all of the mice in the validation study the video was manually scored by a blind observer for crossovers, defined as the animal crossing the midline with all four paws, and rearings, defined as the animal raising its front paws off the floor for at least one second (excluding grooming). The automated AU measure was highly correlated with the sum of crossovers and rearings (R2 = 0.67, P < 0.0001), although it is unable to resolve these measures individually. This validates its use as a high-throughput measure of general locomotor activity. For a detailed analysis see Supplementary Figure 4. Context B did not have video tracking equipment so locomotor activity data was not available for the NP groups.
Statistical Analysis
All statistical analysis was conducted using SPPS version 13.0 (SPSS Inc, Chicago, IL). ANOVA was followed by planned least significant difference (LSD) post-hoc tests when appropriate. Differences were considered statistically significant at p < 0.05.
RESULTS
Effects of Ethanol on the Context Pre-Exposure Rescue of the Immediate Shock Deficit in C57BL6 mice
Overall, the results from the initial validation study indicate that 1.0 g/kg ethanol produced a clear disruption of the context pre-exposure rescue of the immediate shock deficit, but that sensitivity to this disruption was greater during the post-shock period relative to the context test. Early studies of the immediate shock deficit (Blanchard, 1976) and the pre-exposure rescue focused on post-shock freezing (Fanselow, 1986), whereas the majority of recent studies focused on freezing during a context test 24 hours later (Fanselow, 1990; Stote and Fanselow, 2004; Rudy et al, 2004). In order to systematically analyze both of these measures in the present study we performed a repeated measures ANOVA, with the post-shock and context test as the within-subject repeated measures, and pre-exposure condition (i.e. PRE vs. NONPRE) and ethanol dose as the between-subject factors (i.e. 1.0 g/kg vs. 0.0 g/kg). This analysis indicates that freezing, overall, is higher during the context test (F(1,55) = 56.072, p < 0.001) and that this increase interacts with both pre-exposure condition (F(1,55) = 9.675, p = 0.003) and ethanol dose (F(1,55) = 4.381, p = 0.047). The interaction of all three factors (post-shock vs. context test, pre-exposure condition and ethanol dose) was not significant (F(1,55) = 0.094, p = 0.760). Based on these interactions post-shock and context test freezing were analyzed separately as outlined below.
Analysis of Freezing During the Post-Shock Interval
In the saline-treated group, pre-exposure to the conditioning chamber rescued the immediate shock deficit, but 1.0 g/kg ethanol severely attenuated this effect (Figure 2A). Two-way ANOVA with ethanol dose (1.0 g/kg vs. 0.0 g/kg) and pre-exposure condition (PRE vs. NONPRE) as factors indicated significant main effects for dose (F(1,60) = 8.716, p = 0.005) and pre-exposure (F(1,60) = 35.315, p < 0.001), as well as a significant dose × pre-exposure interaction (F(1,60) = 8.734, p = 0.005). This interaction is driven by a significant effect of ethanol dose in the PRE (F(1,36) = 14.413, p = 0.001), but not the NP groups (F(1,24) = 0.000, p = 0.992). This indicates that ethanol specifically disrupted the context pre-exposure rescue, but had no effect in non-pre-exposed animals. Additionally, in order to determine if deficits could be seen at a lower dose of ethanol we administered .5 g/kg ethanol 10 minutes prior to the pre-exposure. This failed to produced a deficit relative to saline controls (t(29) = 1.127, p > 0.05, mean freezing = 19.8 +/− 3.5 SEM). Due to this lack of an effect we did not run .5 g/kg ethanol non-pre-exposure group and therefore this dose could not be included in the 2 × 2 ANOVA described above.
Figure 2. Effects of ethanol on contextual learning.
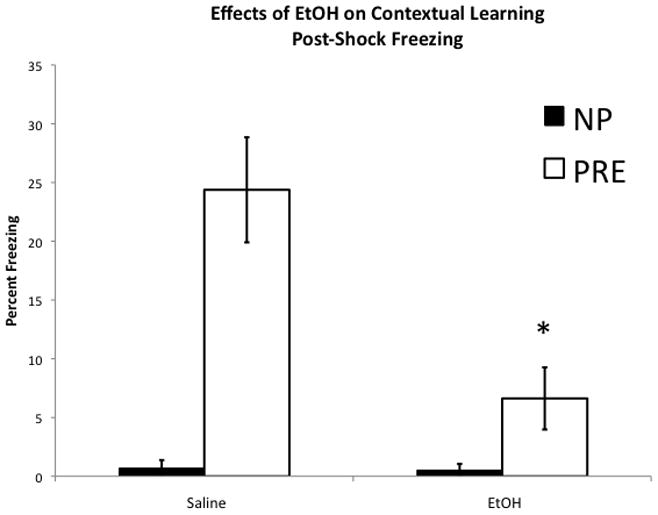
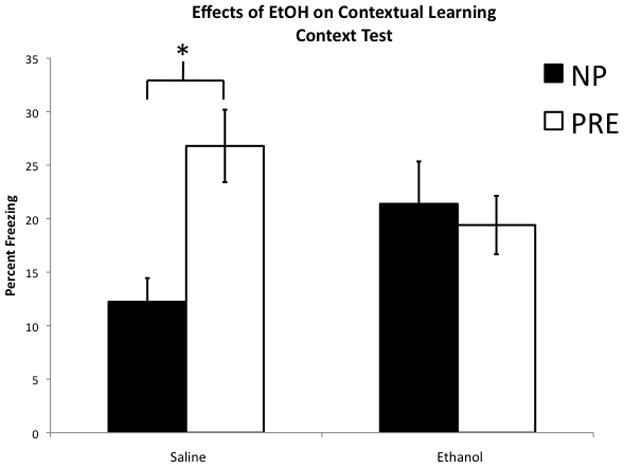
1.0 g/kg ethanol administered prior to pre-exposure on Day 1 disrupts the context pre-exposure rescue of the immediate shock deficit. A. Post-shock freezing: Non-pre-exposed groups show the immediate shock deficit as evidenced by a complete lack of freezing. Pre-exposure to the context rescued the immediate shock deficit in the Saline control group, however, ethanol severely attenuated the rescue; * indicates significant difference between Saline Pre and EtOH PRE, (p < 0.05). B. Context test freezing: Pre-exposure in the saline control group rescued the immediate shock deficit, but failed to do so in the Ethanol groups. * indicates significant difference between Saline NP and Saline PRE (p < 0.05). Error bars represent +/− SEM.
Analysis of Freezing During the 24-hour Context Test
Freezing during the context test showed a similar overall pattern to the post-shock freezing data, but the effect of ethanol was more complex, affecting both pre-exposed and non-pre-exposed groups (Figure 2B). Two-way ANOVA with ethanol dose and pre-exposure condition indicated a significant main effect for pre-exposure (F(1,60) = 5.095, p = 0.028), but not ethanol dose (F(1,60) = 0.00, p = 0.991), as well as a near significant trend for a dose × pre-exposure interaction (F(1,60) = 4.015, p = 0.05). Although the interaction did not quite reach the p < 0.05 cut-off, further break down of the data is justified by the repeated measures analysis described above, as the lack of a three-way repeated measures interaction argues that the pre-exposure × ethanol interaction is preserved at both time points. As can be seen in Fig. 2B, there is a significant effect of pre-exposure condition in the saline groups (F(1,33) = 11.559, p = 0.002) but not in the ethanol groups (F(1,33) = 0.026, p = 0.873). Thus, ethanol did block the pre-exposure rescue, e.g., it eliminated the difference between the pre-exposed and NP groups, but this was driven by an increase in the NP group and a slight decrease in the pre-exposed group. Ethanol failed, however, to block the pre-exposure rescue based on direct comparison to the Saline PRE group (F(1,36) = 2.38, p = 0.132). As was the case for the post-shock interval, the .5 g/kg ethanol group did not differ from Saline (t(29) = 0.955, p > 0.05; data not shown for the 0.5 g/kg group as described above, mean = 24.1 +/− 3.1 SEM).
Post-shock vs. context test freezing
Overall, this analysis indicates that post-shock freezing is a more sensitive measure of the ethanol-mediated disruption of the pre-exposure rescue, as the decreased freezing in EtOH PRE relative to the Saline PRE is significant only during this interval. Disruption of the pre-exposure rescue is still clearly observed during the context test, however it requires comparison with the non-pre-exposed ethanol group to be resolved. The theoretical implications of these findings are discussed in more detail below, but the practical implication is that only the post-shock interval is necessary to resolve the ethanol-mediated disruption of hippocampus-dependent contextual learning. In addition, focusing on post-shock freezing means that the assay can be shortened to two days, with a 10 minute exposure on Day 1 and 42 second immediate shock procedure on Day 2, thereby greatly increasing throughput.
Additional Control Groups
Two additional control groups were run to rule out any non-specific withdrawal effects of ethanol exposure. In these groups 1.0 g/kg ethanol was given either 30 minutes after the context pre-exposure (EtOH POST-PRE) or 30 minutes after exposure to the non-pre-exposure context (EtOH POST-NP). As shown in Figure S1, the EtOH POST-Pre groups did not differ from the Saline Pre group (F(1,20) = 1.292, p = 0.271) indicating that 1.0 g/kg ethanol does not produce any withdrawal effects that reduced freezing in this task. The EtOH POST-NP group did not differ from the Saline NP group (F(1,25) = .204, p < 0.656) indicating that 1.0 g/kg ethanol also does not produce withdrawal effects that increase freezing.
Combined effects of Ro15-4513, Ethanol and Allopregnanolone on the Context Pre-Exposure Rescue of the Immediate Shock Deficit
As shown in Figure 3B, ALLO (10 mg/kg), Ro15-4513 (3 mg/kg), and co-administration of ALLO with ethanol all produced similar impairments to ethanol given alone (overall ANOVA: (F(6,92) = 6.25, p < 0.001), p < 0.05 for all three LSD post-hoc comparisons relative to vehicle). Each of the 3 different vehicles (Saline, BCD, and Saline + BCD) did not significantly differ from each other and were therefore collapsed to simplify the analysis. Co-administration of Ro15-4513 with ethanol reversed the ethanol-mediated impairment (p > 0.05 relative to vehicle). Co-administration of Ro15-4513 with ALLO, in contrast, failed to reverse the impairment (p < 0.05 relative to vehicle). Overall, this data shows that ALLO produces a similar impairment as EtOH, but only the ethanol-mediated impairment was reversed by Ro15-4513. The fact that both Ro15-4513 and EtOH produced a deficit alone, but not when co-administered supports the idea that these drugs competitively interact. EtOH and ALLO both produced impairments when given alone, but they did not produce a larger impairment when administered together. This lack of an additive effect is most likely due to a floor effect as freezing levels below 10% were not observed for any pre-exposed group.
Figure 3. Effects of ethanol, allopregnanolone and Ro15-4513 on locomotor activity and contextual learning.
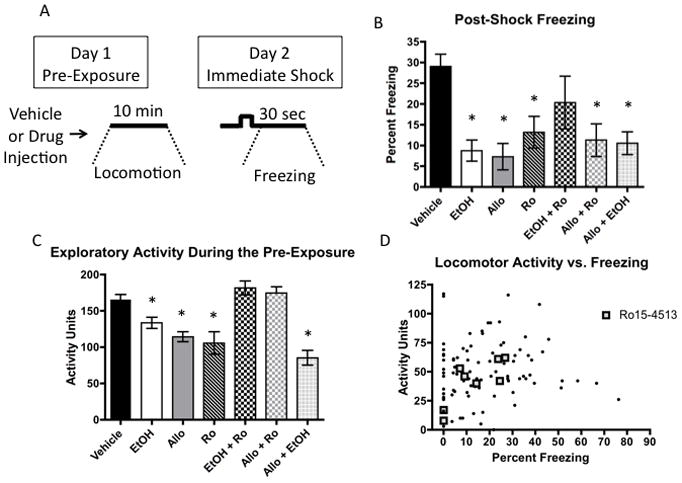
A. Experimental design: Locomotor activity is measured during the 10 min Pre-exposure and freezing is measured during the post-shock interval. B. Effects of different drug manipulations on post-shock freezing. * indicates a significant (p < 0.05) difference from Vehicle controls. C. Effects of different drug manipulations on locomotor activity during the pre-exposure period. * indicates a significant (p < 0.05) difference from Vehicle. D. Scatterplot of the correlation between locomotor activity during the pre-exposure and post-shock freezing. There was no overall correlation, however, within the Ro15-4513 group there significant (p < 0.05) positive correlation. Error bars represent +/− SEM.
Post-shock vs. context test freezing
Analysis of the context test again confirmed its decreased sensitivity to the pre-exposure pharmacological manipulations. The overall ANOVA for the context test did not reach statistical significance (F(6,92) = 1.760, p = .116), indicating that analysis of individual drug groups is not justified (For reference this data is shown in Supplementary Figure 5). Within-subject comparison of post-shock to context test freezing, however, showed a significant interaction with drug group (F(6,92) = 4.859. p < 0.001). This repeated measure interaction was driven by a significant increase in freezing from post-shock to the context test only in the groups that showed deficits in post-shock freezing: ALLO + Ro15-4513 (F(1,8) = 52.861, p < 0.001), ALLO (F(1,10) = 19.657, p = 0.001), ETOH 1 (F(1,18) = 26.474, p < 0.001), Ro15-4513 (F(1,7) = 15.831, p < 0.005) and ALLO + ETOH (F(1,9) = 12.526, p = 0.006). Whereas the vehicle and Ro 15-4513 + ETOH did now show a significant repeated measures interaction (F(1,33) = 1.369, p = 0.250, F(1,7) = 1.961, p = 0.204, respectively). Overall, this analysis again shows that the clear deficits observed in post-shock freezing are not observed during the context test. For reference the context test data is presented in Supplementary Figure 5.
Locomotor activity
An advantage of this task is that the locomotor effects of a drug can be assessed independently of its learning and memory effects. Figure 3C shows that there was a significant effect of drug group on locomotor activity scores during the pre-exposure period (F(6,95) = 12.974, p < 0.001). Locomotor activity was significantly reduced by 1.0 g/kg ethanol (p < 0.05), 10 mg/kg ALLO (p < 0.001), 3 mg/kg Ro15-4513 (p < 0.001), and EtOH + ALLO (p < 0.001). Ro15 + EtOH and Ro15 + ALLO did not differ from Vehicle. Manual scoring indicates that the decreased locomotor activity was driven primarily by a decrease in rearings (see Supplementary Figure 4 for a detailed analysis).
An alternative interpretation of our results is that impaired contextual learning was an indirect effect of reduced exploration during the pre-exposure, rather than a direct action on learning and memory circuits. If such an explanation were true then the level of exploratory activity should predict subsequent freezing. The lack of an overall correlation between locomotor activity during the pre-exposure and post-shock freezing strongly argues against such an indirect mechanism (R2 = 0.017, p = 0.20, Figure 3D). Within each drug group there were also no significant correlations except for the Ro15-4513 group, which showed a positive correlation (R2 = 0.612, p = 0.022). This suggests that the Ro15-4513-mediated learning impairment may have been due to an indirect effect on exploration, but that the ethanol and ALLO-mediated effects were not.
Application of the assay in α4 KO mice
Application of this assay in α4 KO mice revealed an enhancement of contextual learning combined with an increased sensitivity to the cognitive impairing effects of 1.0 g/kg ethanol (Figure 4A). A combined ANOVA of data from both males and females, all three genotypes (WT, HET, KO) and all three groups (NP, Saline Pre, EtOH Pre) with genotype, drug group and sex as factors indicated a significant effect of group (F(2,240) = 32.7, p < 0.001) and a genotype by group interaction (F(4,240) = 4.03, p = .004), but no overall effect of genotype or sex and no genotype by sex, group by sex or genotype by group by sex interactions. Collapsing across sex, the effect of genotype was then analyzed within each group and the effect of ethanol was compared within each genotype. Non-pre-exposed WT, HET and KO mice all showed the immediate shock deficit indicated by equivalent low levels of freezing (F(2,60) = .112, p = .895). The equivalent level of freezing in this group rules out genotype-induced non-specific differences in the tendency to freeze that could confound interpretation of the results. Saline pre-exposed α4 KO mice showed elevated freezing relative to WT mice (p < 0.05) indicating an enhancement of contextual learning. EtOH Pre-exposed α4 KO mice showed reduced freezing relative to EtOH Pre-exposed WT’s (p < 0.05). Within the α4 KO’s ethanol significantly reduced freezing (F(1,38) = 11.33, p < 0.01), whereas ethanol had no effect in the WT mice (F(1,47) = .459, p = 0.502). This indicates that α4 KO mice exhibit an enhancement of contextual learning but are significantly more impaired by ethanol than WT’s, which did not show an ethanol-mediated impairment at this dose. The HET animals showed an intermediate phenotype between WT’s and KO’s and did not differ significantly from either genotype for Saline Pre or EtOH Pre groups. The HET animals did, however, show a significant impairment by ethanol (F(1,95) = 10.46, p < 0.01). An ethanol mediated impairment in both WT’s and HET’s was evident after 1.5 g/kg EtOH indicating that a higher dose of ethanol was required to show an impairment in the WT mice (F(1,31) = 7.029, p < 0.01 and F(1,61) = 11.64, p < 0.01, respectively, see Supplementary Figure 3). Pure 129 mice also required 1.5 g/kg to show an impairment (see Supplementary Figure 2). This indicates that the lack of an ethanol-mediated cognitive impairment in the WT mice in this study is likely conferred by the hybrid C57×129 background as 129 mice are more resistant to ethanol than are C57 mice in this task.
Figure 4. Effects of ethanol on locomotor activity and contextual learning in α4 KO mice.
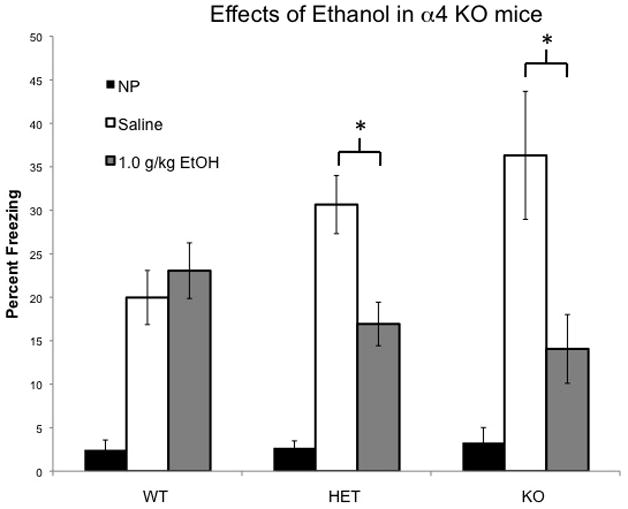
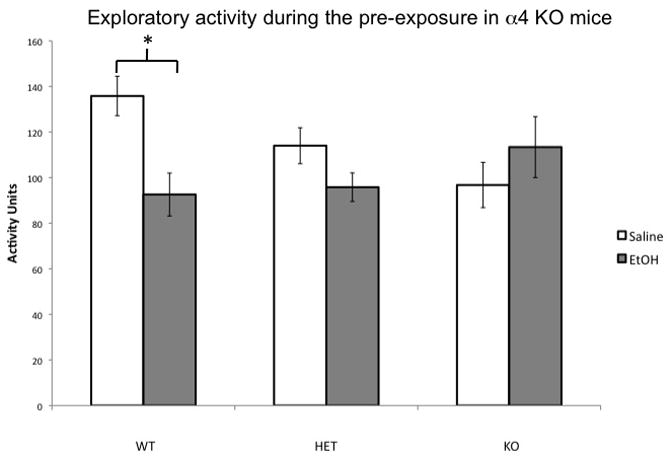
A. α4 KO mice show enhanced sensitivity to the ethanol-mediated learning impairment. NP groups were all low and did not differ. Ethanol reduced freezing in α4 HET and α4 KO mice (as indicated by *) but not α4 WT mice. Ethanol did not reduce freezing in α4 WT mice. B. Ethanol reduced locomotor activity in α4 WT (as indicated by *), but not in α4 HET or α4 KO. Error bars represent +/− SEM.
Locomotor Activity in α4 KO mice
As shown in Figure 4B, ethanol reduced locomotor activity in WT mice, but failed to do so in KO’s, which showed reduced activity relative to WT’s in the absence of ethanol. An ANOVA on the locomotor activity during the pre-exposure with genotype and drug group as factors indicated that there was a significant genotype by group interaction (F(2,181) = 4.128, p < 0.05), but no main effect of genotype (F(2,181) = .581, p = .561) or group (F(1,181) = 3.182, p = .076). Locomotor activity was decreased by ethanol in the α4 WT (F(1,38) = 11.128, p = 0.002) but not in α4 HET (F(1,95) = 3.164, p = .079) or α4 KO (F(1,38) = 1.115, p = 0.298) mice. In the Saline groups, locomotor activity was significantly lower in the α4 KO relative to α4 WT mice (p < 0.01). α4 HET mice showed an intermediate phenotype and did not differ between either A4 WT (p = 0.089) or α4 KO (p = 0.225).
Discussion
These findings show that a low-to-moderate dose of ethanol impairs hippocampus-dependent contextual learning in a novel, high-throughput cognitive assay. Ethanol-mediated disruption of hippocampal function is consistent with numerous findings from a wide range of tasks (Givens et al., 2000, Matthews and Morrow, 2000, Silvers et al., 2003). The disruption found with administration of 1.0 g/kg ethanol but not 0.5 g/kg ethanol is similar to previous studies with standard contextual fear conditioning when ethanol is administered before the context-shock pairings (Gould, 2003, Melia et al., 1996). The present findings, however, are the first to show that ethanol specifically disrupts contextual learning rather than interfering with the context-shock association, pain sensitivity, or behavioral performance. Application of this assay in transgenic mice showed that loss of the α4 GABAAR subunit produced an enhancement of contextual learning, consistent with previous findings (Moore et al, 2009). This enhancement was completely reversed by ethanol, with α4 GABAAR KO mice showing a paradoxical enhancement of sensitivity to the cognitive impairing effects of ethanol.
Validation of the assay in C57Bl/6 mice
Ethanol at a dose of 1.0 g/kg prior to the pre-exposure showed a clear disruption of the context pre-exposure rescue as measured by post-shock freezing, however, the data from the context test was less clear. Early studies of the immediate shock deficit (Blanchard, 1976) and the pre-exposure rescue focused on post-shock freezing (Fanselow, 1986), whereas the majority of recent studies focused on freezing during a context test 24 hours later (Fanselow, 1990; Stote and Fanselow, 2004; Rudy et al, 2004). Focusing on the post-shock data is sufficient for the purposes of the high-throughput hippocampus-dependent assay that was the intended goal of this study. However, for the broader theoretical significance as well as a more thorough vetting of this assay a discussion of the decreased sensitivity of the context test is warranted. The simplest explanation may be that that the dynamic range of the context test is reduced relative to the post-shock interval. As can be seen in Figure 2A vs. 2B, freezing levels are higher in the Saline NP group on the context test, whereas freezing in the Saline PRE group did not change. This means that even in the absence of learning about the conditioning context sensitivity to the pre-exposure rescue is reduced. In other words, the “floor” is higher on the context test resulting in a decrease in the overall dynamic range for observable behavioral deficits.
The overall pattern of ethanol’s effect is similar in the context test, i.e., ethanol prevented the pre-exposure rescue, as indicated by similar levels of freezing in the pre-exposed and NP groups. This lack of a difference, however, was driven by an increase in the EtOH NP groups and a decrease in the EtOH PRE groups relative to saline-treated controls. This finding may provide insight into the underlying mechanisms of the ethanol-mediated cognitive impairment. Rather than completely preventing the formation of the contextual representation it appears that ethanol may have produced a simplified or more general representation that is able to support a conditional fear response 24 hours after immediate shock training regardless of pre-exposure condition. This is consistent with a decreased ability to discriminate between the pre-exposure and non-pre-exposure contexts, which, though quite different, did have some structural similarities and, by design, involved identical handling procedures. The hippocampus in general (Frankland et al., 1998) and dentate gyrus, in particular, plays a critical role in context discrimination (McHugh et al., 2008). The dentate gyrus is also highly enriched in ethanol sensitive α4/δ-containing GABAAR receptors (Fleming et al., 2007, Herd et al., 2008, Sun et al., 2004, Sperk et al., 1997) and exhibits impaired synaptic plasticity at the dose of ethanol (1.0 g/kg) used in this study (Givens et al., 1995). Thus, impairment of dentate gyrus function is a plausible neurophysiological mechansim that could underlie the cognitive impairing effects of ethanol observed in this study.
ALLO produced a similar learning impairment as ethanol, consistent with previous findings that neurosteroids can have ethanol-like effects on neurophysiology and behavior (Brot et al., 1997, Matthews et al., 2002, Silvers et al., 2003, Tokunaga et al., 2003). Acute ethanol administration has been shown to increase brain neurosteroid levels in a dose and time-dependent manner and preventing this ethanol-mediated increase has been shown, in some cases, to block the effects of ethanol (Morrow et al., 2001, VanDoren et al., 2000). The ethanol-mediated learning impairment observed in the present study, however, does not appear to be driven by an indirect effect of ALLO for several reasons. First, the timing and route of ethanol administration used in the present study are inconsistent with an indirect ALLO-mediated effect. Increases in neurosteroids in C57Bl/6 mice were found specifically after oral ethanol consumption, but not i.p. injection (Finn et al., 2004) and, in rats, were shown to begin 20 minutes after injection and peak between 40–80 minutes (VanDoren et al., 2000). In the current study, male C57Bl/6 mice were placed in the conditioning chamber 10 minutes after injection on the pre-exposure day and were removed from the chamber at the time point that ethanol-mediated neurosteroid increases were reported to take effect (20 min). Second, the GABAAR partial inverse agonist Ro15-4513 specifically blocked the learning impairment produced by ethanol but not the impairment produced by ALLO. If the ethanol-mediated impairment was driven exclusively by its indirect effect on neurosteroid levels, then the failure of Ro15-4513 to block the effects of ALLO imply that it should also fail to block the effects of ethanol. This specific antagonism of ethanol’s effects also argues against a simple inverse agonist role for Ro15-4513. Instead, this data is consistent with the model that Ro15-4513 directly competes with ethanol for the same binding pocket on the GABAAR (Wallner and Olsen, 2008).
Despite its lack of efficacy at reversing the ALLO-mediated learning impairment, Ro15-4513 did reverse the overall ALLO-mediated decrease in locomotor activity. This dissociation suggests that the decrease in locomotion produced by ALLO is driven by different mechanisms than its cognitive impairing effects. Manual scoring indicated that the decreased locomotor activity for both ethanol and ALLO is driven by a decrease in rearing (Figure S4). This is consistent with previously reported effects in C57Bl6 mice: their horizontal activity is often unaffected by ethanol but the number of rearings is consistently decreased (Crabbe et al., 1994, Dudek et al., 1991, Phillips et al., 1989). The locomotor effects of ethanol and ALLO appear to be orthogonal to the learning impairments that they produce, as indicated by the lack of a correlation between locomotor activity and subsequent freezing.
Ro15-4513 produced a learning impairment when given alone, consistent with numerous studies that it has its own intrinsic effects on neurophysiology and behavior (Britton et al., 1988, Lister and Nutt, 1988). Ro15-4513 is a partial inverse agonist at BZ sensitive GABAA receptors but also shows agonist properties at α4/δ-containing BZ insensitive receptors (Suzdak et al., 1986, Liang et al., 2004). Behaviorally, it reduces seizure thresholds, increases anxiety and reduces exploration (Lister and Nutt, 1988). It decreased overall locomotor activity in this study and the amount of locomotor activity was positively correlated with the subsequent amount of freezing. This suggests that the learning impairment may be an indirect effect of reducing exploration due to enhanced anxiety rather than a direct effect on learning and memory circuits. These results are interesting in that Ro15-4513 does not have opposite effects to ethanol as it does in most studies, but instead has a similar effect. This provides further evidence for the specificity of the Ro15-4513–ethanol interaction, as an algebraic summation of the ethanol-mediated learning impairment with a non-specific Ro15-4513-mediated learning enhancement cannot account for the reversal. As expected, the combined administration of ethanol and ALLO also produces a deficit. The large decrease in locomotor activity in this group is suggestive of a synergistic interaction whereby the locomotor decrease is mediated by a common mechanism. Overall, these findings validate the context pre-exposure immediate shock procedure as a high-throughput cognitive ethanol assay that is ideally suited for application in transgenic mice.
Application in α4 KO mice
Application of this assay in α4 KO mice indicates that loss of the α4 GABAAR subunit produces an enhancement in hippocampus-dependent contextual learning and a paradoxical increased sensitivity to the cognitive impairing effects of ethanol. The enhanced learning is consistent with previous findings in α4 and δ GABAAR KO mice, providing further evidence that tonic inhibition mediated by α4/δ-containing receptors negatively modulate learning and memory processes (Wiltgen et al, 2005; Moore et al, 2009). The heterozygous animals showed an intermediate phenotype indicating that loss of the α4 subunit produces a graded effect on both the learning enhancement and the greater ethanol sensitivity. We have previously shown that α4 KO mice do not exhibit differences in ethanol metabolism or clearance, indicating that the present findings are not likely confounded by such differences (Chandra et al, 2008).
A potential confound of this study was that ethanol failed to produce a learning impairment in wild-type littermate controls at 1.0 g/kg. It is this lack of an effect which allowed for the detection of enhanced sensitivity in the α4 KO mice. We showed that this is likely due to the mixed 129 × C57 background of these mice and a reduced sensitivity to the ethanol-mediated cognitive impairment in 129 mice. Both pure 129 mice and α4 wild types required a higher dose of ethanol (1.5 g/kg) to show an impairment (Figures S2 and S3).
Analysis of the locomotor activity during the pre-exposure revealed two interesting findings. First, ethanol at 1.0 g/kg reduced locomotor activity in α4 WT mice, indicating that they are sensitive to the locomotor effects of ethanol at this dose but not its cognitive impairing effects. Second, locomotor activity is reduced in the α4 KO mice and they do not show a further reduction in activity in response to ethanol. This reduction in activity in α4 KO mice and their lack of sensitivity to the locomotor effects of ethanol is in contrast to a previous study in α4 KO mice (Chandra et al, 2008). Methodological and apparatus differences could account for this discrepancy, as the fear conditioning apparatus used in the present study is much smaller than a standard open field apparatus and exploration is occurring on top of a grid floor rather than a flat surface. Age could also be a factor as the mice in the present study were approximately two months older. Furthermore, the number of animals is much larger in the present study (268 overall), which may provide greater power to resolve subtle differences in locomotor activity. This pattern of results indicates a complete dissociation between the locomotor effects of ethanol and its impact on learning: α4 WT mice show the locomotor but not learning effect, whereas the α4 KO’s show the learning but not locomotor effect. This further underscores the orthogonal nature of these two ethanol-mediated effects, consistent with the genetic dissociations between ethanol’s many different behavioral effects (Phillips et al., 1989, Crabbe et al., 1994, Dudek et al., 1991). This finding is likely to be strain-dependent and therefore future studies involving multiple mouse strains will ultimately be required to fully test the extent of this dissociation. We would argue that the ability to obtain both measures, within the same animal and in a high-throughput manner, is a great advantage of the context pre-exposure immediate shock assay.
The enhanced sensitivity of α4 KO mice to the ethanol-mediated learning impairment is opposite to what one would predict based on the loss of tonic GABAAR sensitivity to ethanol in the dentate gyrus of these mice (Chandra et al., 2008, Liang et al., 2008). It is consistent, however, with their enhanced synaptic GABAAR sensitivity to ethanol, resulting from compensatory rearrangement of GABAAR subunits (Chandra et al., 2008, Liang et al., 2008). The locus of ethanol action on GABAAR’s is therefore different in WT’s vs. KO’s and the differential sensitivity to its cognitive impairing effects may speak to the functional significance of enhancing tonic vs. synaptic inhibition, respectively. A major function of the dentate gyrus is thought to be the integration of multi-modal sensory inputs from the lateral entorhinal cortex with spatial information from the medial entorhinal cortex (Manns and Eichenbaum, 2006). Tonic inhibition regulates overall membrane excitability, action potential back propagation and the time constant for temporal integration of excitatory inputs (Hausser and Clark, 1997, van den Burg et al., 2007, Semyanov et al., 2004). In contrast, the precise temporal dynamics of synaptic inhibition are necessary for entrainment of network activity in the gamma and theta frequency ranges (Whittington and Traub, 2003). These oscillations define the temporal windows for synaptic integration and allow for encoding of information related to spike timing, which is thought to play an essential role in hippocampal function (Axmacher et al., 2006, Lisman and Buzsaki, 2008). Thus, while both forms of inhibition affect integration of incoming information, synaptic inhibition also directly affects the spike timing-dependent fidelity of the information content itself. Disruption of the millisecond precision of synaptic inhibition by ethanol in α4 KO mice may therefore underlie their greater sensitivity to the ethanol-mediated cognitive impairment.
A similar pattern of reduced tonic inhibition and enhanced synaptic sensitivity to ethanol has been shown to occur after both acute and chronic intermittent ethanol exposure (Liang et al., 2007, Liang et al., 2006). It is therefore possible that a similar enhanced sensitivity to disruption of contextual learning might be present after CIE. Although future experiments would be required to test this possibility, there is evidence that chronic exposure to ethanol in adolescent rats results in a greater sensitivity to the cognitive impairing effects of ethanol in adulthood (White et al., 2000). Developmental down-regulation of the α4 subunit, ultimately resulting in ethanol sensitive synaptic GABAAR’s, could potentially be involved in this effect.
Conclusion
Overall, these results add to the growing literature that acute administration of ethanol at doses relevant to human consumption disrupts hippocampus-dependent learning and memory. This effect appears to be mediated by direct interaction of ethanol with GABAARs, although alternative explanations such as activation of adenosine receptors cannot be conclusively ruled out (Dar, 2001). The cognitive ethanol assay developed in this study provides a number of advantages over existing protocols: it is relatively simple and high-throughput, avoids potential confounding effects of drugs on shock sensitivity, and provides a pure measure of the locomotor effects of drug manipulations. Its application in α4 KO mice revealed an increased sensitivity to the ethanol-mediated cognitive impairment. This is in contrast to a previous study in which α4 KO mice did not show differential ethanol responsiveness in anxiety, locomotor or motor coordination tasks (Chandra et al., 2008). This suggests that the α4-subunit may be specifically involved in the cognitive impairing effects of ethanol. The enhanced, rather than decreased, ethanol sensitivity is consistent with the compensatory increase in synaptic GABAAR ethanol sensitivity in these mice (Liang et al, 2008). As is the case with all whole body, life time knock outs, however, these findings might tell us more about the functional consequences of the adaptation to the loss of the α4-subunit than the function of the α4 subunit itself. Future studies, utilizing anatomically and temporally restricted genetic manipulations, will be able to avoid such compensatory changes and probe more deeply into the neural mechanisms of ethanol action on learning and memory circuits.
Supplementary Material
This argues against any non-specific withdrawal effects induced by ethanol that may altered freezing levels. Error bars represent +/− SEM.
1.0 g/kg was not sufficient to disrupt contextual learning in 129 mice as it was for C57Bl6 mice. A higher dose of 1.5 g/kg was required to produce a deficit. Error bars represent +/− SEM.
A higher dose of 1.5 g/kg ethanol is required to produce an impairment in α4 WT and α4 HET mice. Error bars represent +/− SEM.
A. Manual scoring of horizontal crossing and rearing indicated indicated that these measures are correlated with each other (R2 = .2, p < 0.0001). B. Automated scoring is highly correlated with the sum of crossing and rearing (R2 = .673, p < 0.0001). C. Ro + EtOH showed increased horizontal crossings and ALLO + EtOH showed reduced crossings. D. EtOH, ALLO, Ro15-4513, ALLO + RO and EtOH + ALLO all showed reduced rearing relative to Vehicle. Ro + EtOH do not differ from Vehicle and show greater rearings relative to EtOH alone. * indicates a significant difference relative to Vehicle control.
Effects of drug manipulations on context test freezing.
Acknowledgments
Support: NIH grants: AA07680 and NS35985
Training grants: 5T32MH019384-14 and T32 MH019384-14
The authors would like to thank Claire Wang for her technical assistance and Dr. Martin Wallner for his pharmacological expertise and help in editing the manuscript. We would also like to thank Robin Davies and the Biochemsitry Media Lab at the University of Wisconsin for use of the mouse image.
Contributor Information
Jesse D. Cushman, Department of Psychology and Brain Research Institute, University of California Los Angeles.
Melissa D. Moore, Department of Molecular and Medical Pharmacology, Geffen School of Medicine, University of California Los Angeles.
Nate S. Jacobs, Department of Psychology and Brain Research Institute, University of California Los Angeles.
Richard W. Olsen, Department of Molecular and Medical Pharmacology, Geffen School of Medicine, University of California Los Angeles.
Michael S. Fanselow, Department of Psychology and Brain Research Institute, University of California Los Angeles.
References
- ACHESON SK, STEIN RM, SWARTZWELDER HS. Impairment of semantic and figural memory by acute ethanol: age-dependent effects. Alcohol Clin Exp Res. 1998;22:1437–42. doi: 10.1111/j.1530-0277.1998.tb03932.x. [DOI] [PubMed] [Google Scholar]
- AXMACHER N, MORMANN F, FERNANDEZ G, ELGER CE, FELL J. Memory formation by neuronal synchronization. Brain Res Rev. 2006;52:170–82. doi: 10.1016/j.brainresrev.2006.01.007. [DOI] [PubMed] [Google Scholar]
- BARRIENTOS RM, HIGGINS EA, SPRUNGER DB, WATKINS LR, RUDY JW, MAIER SF. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behav Brain Res. 2002;134:291–8. doi: 10.1016/s0166-4328(02)00043-8. [DOI] [PubMed] [Google Scholar]
- BERRY RB, CHANDRA D, DIAZ-GRANADOS JL, HOMANICS GE, MATTHEWS DB. Investigation of ethanol-induced impairment of spatial memory in gamma2 heterozygous knockout mice. Neurosci Lett. 2009;455:84–7. doi: 10.1016/j.neulet.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERRY RB, MATTHEWS DB. Acute ethanol administration selectively impairs spatial memory in C57BL/6J mice. Alcohol. 2004;32:9–18. doi: 10.1016/j.alcohol.2003.09.005. [DOI] [PubMed] [Google Scholar]
- BLANCHARD RJ, FUKUNAGA KK, BLANCHARD DC. Environmental control of defensive reactions to footshock. Bulletin of the Psychonomic Society. 1976;8:129–130. [Google Scholar]
- BORGHESE CM, HARRIS RA. Studies of ethanol actions on recombinant delta-containing gamma-aminobutyric acid type A receptors yield contradictory results. Alcohol. 2007;41:155–62. doi: 10.1016/j.alcohol.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRITTON KT, EHLERS CL, KOOB GF. Is ethanol antagonist Ro15-4513 selective for ethanol? Science. 1988;239:648–50. doi: 10.1126/science.3340849. [DOI] [PubMed] [Google Scholar]
- BROT MD, AKWA Y, PURDY RH, KOOB GF, BRITTON KT. The anxiolytic-like effects of the neurosteroid allopregnanolone: interactions with GABA(A) receptors. Eur J Pharmacol. 1997;325:1–7. doi: 10.1016/s0014-2999(97)00096-4. [DOI] [PubMed] [Google Scholar]
- CHANDRA D, WERNER DF, LIANG J, SURYANARAYANAN A, HARRISON NL, SPIGELMAN I, OLSEN RW, HOMANICS GE. Normal acute behavioral responses to moderate/high dose ethanol in GABAA receptor alpha 4 subunit knockout mice. Alcohol Clin Exp Res. 2008;32:10–8. doi: 10.1111/j.1530-0277.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRABBE JC, GALLAHER ES, PHILLIPS TJ, BELKNAP JK. Genetic determinants of sensitivity to ethanol in inbred mice. Behav Neurosci. 1994;108:186–95. doi: 10.1037//0735-7044.108.1.186. [DOI] [PubMed] [Google Scholar]
- DAR MS. Modulation of ethanol-induced motor incoordination by mouse striatal A(1) adenosinergic receptor. Brain Res Bull. 2001;55:513–20. doi: 10.1016/s0361-9230(01)00552-4. [DOI] [PubMed] [Google Scholar]
- DUDEK BC, PHILLIPS TJ, HAHN ME. Genetic analyses of the biphasic nature of the alcohol dose-response curve. Alcohol Clin Exp Res. 1991;15:262–9. doi: 10.1111/j.1530-0277.1991.tb01867.x. [DOI] [PubMed] [Google Scholar]
- FANSELOW MS. Associative vs topographical accounts of the immediate shock freezing deficit in rats: Implications for the response selection rules governing species-specific defensive reactions. Learning and Motivation. 1986;17:16–39. [Google Scholar]
- FANSELOW MS. Factors governing one-trial contextual conditioning. Animal Learning and Behavior. 1990;18:264–270. [Google Scholar]
- FINN DA, SINNOTT RS, FORD MM, LONG SL, TANCHUCK MA, PHILLIPS TJ. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience. 2004;123:813–9. doi: 10.1016/j.neuroscience.2003.11.017. [DOI] [PubMed] [Google Scholar]
- FLEMING RL, WILSON WA, SWARTZWELDER HS. Magnitude and ethanol sensitivity of tonic GABAA receptor-mediated inhibition in dentate gyrus changes from adolescence to adulthood. J Neurophysiol. 2007;97:3806–11. doi: 10.1152/jn.00101.2007. [DOI] [PubMed] [Google Scholar]
- FRANKLAND PW, CESTARI V, FILIPKOWSKI RK, MCDONALD RJ, SILVA AJ. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav Neurosci. 1998;112:863–74. doi: 10.1037//0735-7044.112.4.863. [DOI] [PubMed] [Google Scholar]
- GIVENS B, WILLIAMS JM, GILL TM. Septohippocampal pathway as a site for the memory-impairing effects of ethanol. Hippocampus. 2000;10:111–21. doi: 10.1002/(SICI)1098-1063(2000)10:1<111::AID-HIPO12>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- GLYKYS J, MANN EO, MODY I. Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci. 2008;28:1421–6. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOULD TJ. Ethanol disrupts fear conditioning in C57BL/6 mice. J Psychopharmacol. 2003;17:77–81. doi: 10.1177/0269881103017001702. [DOI] [PubMed] [Google Scholar]
- HANCHAR HJ, WALLNER M, OLSEN RW. Alcohol effects on gamma-aminobutyric acid type A receptors: are extrasynaptic receptors the answer? Life Sci. 2004;76:1–8. doi: 10.1016/j.lfs.2004.05.035. [DOI] [PubMed] [Google Scholar]
- HAUSSER M, CLARK BA. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron. 1997;19:665–78. doi: 10.1016/s0896-6273(00)80379-7. [DOI] [PubMed] [Google Scholar]
- HERD MB, HAYTHORNTHWAITE AR, ROSAHL TW, WAFFORD KA, HOMANICS GE, LAMBERT JJ, BELELLI D. The expression of GABAA beta subunit isoforms in synaptic and extrasynaptic receptor populations of mouse dentate gyrus granule cells. J Physiol. 2008;586:989–1004. doi: 10.1113/jphysiol.2007.146746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOSIE AM, WILKINS ME, DA SILVA HM, SMART TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–9. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- JIA F, CHANDRA D, HOMANICS GE, HARRISON NL. Ethanol modulates synaptic and extrasynaptic GABAA receptors in the thalamus. J Pharmacol Exp Ther. 2008;326:475–82. doi: 10.1124/jpet.108.139303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORPI ER, DEBUS F, LINDEN AM, MALECOT C, LEPPA E, VEKOVISCHEVA O, RABE H, BOHME I, ALLER MI, WISDEN W, LUDDENS H. Does ethanol act preferentially via selected brain GABAA receptor subtypes? the current evidence is ambiguous. Alcohol. 2007;41:163–76. doi: 10.1016/j.alcohol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- LIANG J, CAGETTI E, OLSEN RW, SPIGELMAN I. Altered pharmacology of synaptic and extrasynaptic GABAA receptors on CA1 hippocampal neurons is consistent with subunit changes in a model of alcohol withdrawal and dependence. J Pharmacol Exp Ther. 2004;310:1234–45. doi: 10.1124/jpet.104.067983. [DOI] [PubMed] [Google Scholar]
- LIANG J, SURYANARAYANAN A, ABRIAM A, SNYDER B, OLSEN RW, SPIGELMAN I. Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci. 2007;27:12367–77. doi: 10.1523/JNEUROSCI.2786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIANG J, SURYANARAYANAN A, CHANDRA D, HOMANICS GE, OLSEN RW, SPIGELMAN I. Functional consequences of GABAA receptor alpha 4 subunit deletion on synaptic and extrasynaptic currents in mouse dentate granule cells. Alcohol Clin Exp Res. 2008;32:19–26. doi: 10.1111/j.1530-0277.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- LIANG J, ZHANG N, CAGETTI E, HOUSER CR, OLSEN RW, SPIGELMAN I. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci. 2006;26:1749–58. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LISMAN J, BUZSAKI G. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophr Bull. 2008;34:974–80. doi: 10.1093/schbul/sbn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LISTER RG, GORENSTEIN C, FISHER-FLOWERS D, WEINGARTNER HJ, ECKARDT MJ. Dissociation of the acute effects of alcohol on implicit and explicit memory processes. Neuropsychologia. 1991;29:1205–12. doi: 10.1016/0028-3932(91)90034-6. [DOI] [PubMed] [Google Scholar]
- LISTER RG, NUTT DJ. RO 15-4513 and its interaction with ethanol. Adv Alcohol Subst Abuse. 1988;7:119–23. doi: 10.1300/J251v07n03_19. [DOI] [PubMed] [Google Scholar]
- LOBO IA, HARRIS RA. GABA(A) receptors and alcohol. Pharmacol Biochem Behav. 2008;90:90–4. doi: 10.1016/j.pbb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANNS JR, EICHENBAUM H. Evolution of declarative memory. Hippocampus. 2006;16:795–808. doi: 10.1002/hipo.20205. [DOI] [PubMed] [Google Scholar]
- MAREN S, FANSELOW MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci. 1995;15:7548–64. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS DB, MORROW AL. Effects of acute and chronic ethanol exposure on spatial cognitive processing and hippocampal function in the rat. Hippocampus. 2000;10:122–30. doi: 10.1002/(SICI)1098-1063(2000)10:1<122::AID-HIPO13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- MATTHEWS DB, MORROW AL, TOKUNAGA S, MCDANIEL JR. Acute ethanol administration and acute allopregnanolone administration impair spatial memory in the Morris water task. Alcohol Clin Exp Res. 2002;26:1747–51. doi: 10.1097/01.ALC.0000037219.79257.17. [DOI] [PubMed] [Google Scholar]
- MATTHEWS DB, SIMSON PE, BEST PJ. Acute ethanol impairs spatial memory but not stimulus/response memory in the rat. Alcohol Clin Exp Res. 1995;19:902–9. doi: 10.1111/j.1530-0277.1995.tb00965.x. [DOI] [PubMed] [Google Scholar]
- MATUS-AMAT P, HIGGINS EA, BARRIENTOS RM, RUDY JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24:2431–9. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEERA P, OLSEN RW, OTIS TS, WALLNER M. Etomidate, propofol and the neurosteroid THDOC increase the GABA efficacy of recombinant alpha4beta3delta and alpha4beta3 GABA A receptors expressed in HEK cells. Neuropharmacology. 2009;56:155–60. doi: 10.1016/j.neuropharm.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELIA KR, RYABININ AE, CORODIMAS KP, WILSON MC, LEDOUX JE. Hippocampal-dependent learning and experience-dependent activation of the hippocampus are preferentially disrupted by ethanol. Neuroscience. 1996;74:313–22. doi: 10.1016/0306-4522(96)00138-8. [DOI] [PubMed] [Google Scholar]
- MIHALEK RM, BOWERS BJ, WEHNER JM, KRALIC JE, VANDOREN MJ, MORROW AL, HOMANICS GE. GABA(A)-receptor delta subunit knockout mice have multiple defects in behavioral responses to ethanol. Alcohol Clin Exp Res. 2001;25:1708–18. [PubMed] [Google Scholar]
- MODY I. Extrasynaptic GABA(A) receptors in the crosshairs of hormones and ethanol. Neurochem Int. 2007 doi: 10.1016/j.neuint.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE MD, CUSHMAN J, CHANDRA D, HOMANICS GE, OLSEN RW, FANSELOW MS. Trace and contextual fear conditioning is enhanced in mice lacking the alpha4 subunit of the GABA(A) receptor. Neurobiol Learn Mem. 2010;93:383–7. doi: 10.1016/j.nlm.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORROW AL, VANDOREN MJ, PENLAND SN, MATTHEWS DB. The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res Brain Res Rev. 2001;37:98–109. doi: 10.1016/s0165-0173(01)00127-8. [DOI] [PubMed] [Google Scholar]
- PENG Z, HAUER B, MIHALEK RM, HOMANICS GE, SIEGHART W, OLSEN RW, HOUSER CR. GABA(A) receptor changes in delta subunit-deficient mice: altered expression of alpha4 and gamma2 subunits in the forebrain. J Comp Neurol. 2002;446:179–97. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- PHILLIPS TJ, FELLER DJ, CRABBE JC. Selected mouse lines, alcohol and behavior. Experientia. 1989;45:805–27. doi: 10.1007/BF01954056. [DOI] [PubMed] [Google Scholar]
- RUDY JW, HUFF NC, MATUS-AMAT P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci Biobehav Rev. 2004;28:675–85. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- SANDERS MJ, WILTGEN BJ, FANSELOW MS. The place of the hippocampus in fear conditioning. Eur J Pharmacol. 2003;463:217–23. doi: 10.1016/s0014-2999(03)01283-4. [DOI] [PubMed] [Google Scholar]
- SANTHAKUMAR V, WALLNER M, OTIS TS. Ethanol acts directly on extrasynaptic subtypes of GABAA receptors to increase tonic inhibition. Alcohol. 2007;41:211–21. doi: 10.1016/j.alcohol.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEMYANOV A, WALKER MC, KULLMANN DM, SILVER RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–9. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- SILVERS JM, TOKUNAGA S, BERRY RB, WHITE AM, MATTHEWS DB. Impairments in spatial learning and memory: ethanol, allopregnanolone, and the hippocampus. Brain Res Brain Res Rev. 2003;43:275–84. doi: 10.1016/j.brainresrev.2003.09.002. [DOI] [PubMed] [Google Scholar]
- SPERK G, SCHWARZER C, TSUNASHIMA K, FUCHS K, SIEGHART W. GABA(A) receptor subunits in the rat hippocampus I: immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- SPIGELMAN I, LI Z, BANERJEE PK, MIHALEK RM, HOMANICS GE, OLSEN RW. Behavior and physiology of mice lacking the GABAA-receptor delta subunit. Epilepsia. 2002;43(Suppl 5):3–8. doi: 10.1046/j.1528-1157.43.s.5.8.x. [DOI] [PubMed] [Google Scholar]
- STOTE DL, FANSELOW MS. NMDA receptor modulation of incidental learning in Pavlovian context conditioning. Behav Neurosci. 2004;118:253–7. doi: 10.1037/0735-7044.118.1.253. [DOI] [PubMed] [Google Scholar]
- SUN C, SIEGHART W, KAPUR J. Distribution of alpha1, alpha4, gamma2, and delta subunits of GABAA receptors in hippocampal granule cells. Brain Res. 2004;1029:207–16. doi: 10.1016/j.brainres.2004.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUNDSTROM-POROMAA I, SMITH DH, GONG QH, SABADO TN, LI X, LIGHT A, WIEDMANN M, WILLIAMS K, SMITH SS. Hormonally regulated alpha(4)beta(2)delta GABA(A) receptors are a target for alcohol. Nat Neurosci. 2002;5:721–2. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUZDAK PD, GLOWA JR, CRAWLEY JN, SCHWARTZ RD, SKOLNICK P, PAUL SM. A selective imidazobenzodiazepine antagonist of ethanol in the rat. Science. 1986;234:1243–7. doi: 10.1126/science.3022383. [DOI] [PubMed] [Google Scholar]
- TOKUNAGA S, MCDANIEL JR, MORROW AL, MATTHEWS DB. Effect of acute ethanol administration and acute allopregnanolone administration on spontaneous hippocampal pyramidal cell neural activity. Brain Res. 2003;967:273–80. doi: 10.1016/s0006-8993(02)04266-x. [DOI] [PubMed] [Google Scholar]
- VAN DEN BURG EH, ENGELMANN J, BACELO J, GOMEZ L, GRANT K. Etomidate reduces initiation of backpropagating dendritic action potentials: implications for sensory processing and synaptic plasticity during anesthesia. J Neurophysiol. 2007;97:2373–84. doi: 10.1152/jn.00395.2006. [DOI] [PubMed] [Google Scholar]
- VANDOREN MJ, MATTHEWS DB, JANIS GC, GROBIN AC, DEVAUD LL, MORROW AL. Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–9. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLNER M, HANCHAR HJ, OLSEN RW. Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci U S A. 2003;100:15218–23. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLNER M, OLSEN RW. Physiology and pharmacology of alcohol: the imidazobenzodiazepine alcohol antagonist site on subtypes of GABAA receptors as an opportunity for drug development? Br J Pharmacol. 2008;154:288–98. doi: 10.1038/bjp.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEI W, FARIA LC, MODY I. Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24:8379–82. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEI W, ZHANG N, PENG Z, HOUSER CR, MODY I. Perisynaptic localization of delta subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–61. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEITEMIER AZ, RYABININ AE. Alcohol-induced memory impairment in trace fear conditioning: a hippocampus-specific effect. Hippocampus. 2003;13:305–15. doi: 10.1002/hipo.10063. [DOI] [PubMed] [Google Scholar]
- WHITE AM, GHIA AJ, LEVIN ED, SWARTZWELDER HS. Binge pattern ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol. Alcohol Clin Exp Res. 2000;24:1251–6. [PubMed] [Google Scholar]
- WHITTINGTON MA, TRAUB RD. Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 2003;26:676–82. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- WILTGEN BJ, SANDERS MJ, ANAGNOSTARAS SG, SAGE JR, FANSELOW MS. Context fear learning in the absence of the hippocampus. J Neurosci. 2006;26:5484–91. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILTGEN BJ, SANDERS MJ, FERGUSON C, HOMANICS GE, FANSELOW MS. Trace fear conditioning is enhanced in mice lacking the delta subunit of the GABAA receptor. Learn Mem. 2005;12:327–33. doi: 10.1101/lm.89705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG N, WEI W, MODY I, HOUSER CR. Altered localization of GABA(A) receptor subunits on dentate granule cell dendrites influences tonic and phasic inhibition in a mouse model of epilepsy. J Neurosci. 2007;27:7520–31. doi: 10.1523/JNEUROSCI.1555-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This argues against any non-specific withdrawal effects induced by ethanol that may altered freezing levels. Error bars represent +/− SEM.
1.0 g/kg was not sufficient to disrupt contextual learning in 129 mice as it was for C57Bl6 mice. A higher dose of 1.5 g/kg was required to produce a deficit. Error bars represent +/− SEM.
A higher dose of 1.5 g/kg ethanol is required to produce an impairment in α4 WT and α4 HET mice. Error bars represent +/− SEM.
A. Manual scoring of horizontal crossing and rearing indicated indicated that these measures are correlated with each other (R2 = .2, p < 0.0001). B. Automated scoring is highly correlated with the sum of crossing and rearing (R2 = .673, p < 0.0001). C. Ro + EtOH showed increased horizontal crossings and ALLO + EtOH showed reduced crossings. D. EtOH, ALLO, Ro15-4513, ALLO + RO and EtOH + ALLO all showed reduced rearing relative to Vehicle. Ro + EtOH do not differ from Vehicle and show greater rearings relative to EtOH alone. * indicates a significant difference relative to Vehicle control.
Effects of drug manipulations on context test freezing.


