Abstract
Purpose
Liposomal doxorubicin (D) and bevacizumab (A) are active single agents in gynecologic and breast malignancies which share a resistance mechanism: up-regulation of hypoxia inducible factor (HIF-1α). We therefore added temsirolimus (T), which inhibits HIF-1α, to D and A (DAT). Trial objectives were assessment of safety, preliminary efficacy and identification of biologic response correlates.
Patients and Methods
Cycle length was 21 days, with IV D, A and T on day 1; T on days 8 and 15 (3+3 dose68 escalation design with expansion cohorts). Mutational assays for PIK3CA, BRAF, KRAS and immunhistochemistry for PTEN loss were performed.
Results
This report details 74 patients with gynecologic and breast malignancies who received at least one dose of drug on study. Median patient age: 52, (27-79); prior regimens: 4, (1–11). Responses: 1 (1.4%) complete response (CR), 14 (18.9%) partial responses (PR), and 13 (17.6%) with stable disease (SD) ≥ 6 months (total = 37.9%). The most common grade 1 toxicities were fatigue (27%) and anemia (20.2%). Notable grade 3/4 toxicities: thrombocytopenia (9.5%), mucositis (6.7%) and bowel perforation (2.7%). PIK3CA mutations or PTEN loss were identified in 25/59 (42.3%) of tested patients. Among these, nine (36%) achieved CR/PR and four (16%) had SD ≥ 6 months (CR+PR+SD ≥ 6 months = 52%).
Conclusions
DAT is well tolerated with manageable side effects. Responses observed warrant further evaluation. Mutational analyses were notable for a high percentage of responders with PI3K pathway aberrations.
Keywords: Liposomal doxorubicin, Bevacizumab, Temsirolimus, Phase I Trials, PI3K
INTRODUCTION
Anthracycline antibiotics have a broad spectrum of antineoplastic action. Liposomal doxorubicin (D) is a pegylated, liposomal encapsulated form of doxorubicin which has demonstrated activity in a number of solid tumors. In contrast to doxorubicin, D exhibits less non-specific drug delivery to normal tissues and is associated with lower peak plasma levels. These features account for it’s more tolerable side effect profile in comparison to free doxorubicin1, 2.
A number of resistance mechanisms mediate anthracycline resistance to chemotherapy3, 4. Recently, up regulation of the transcription factor hypoxia-inducible factor alpha (HIF-1α), with subsequent increases in the production of proteins that promote angiogenesis, anaerobic metabolism and other cellular survival pathways, has been demonstrated as an important mechanism of anthracycline resistance5-7.
Angiogenesis, the formation of new blood vessels from existing vasculature, is essential for tumor growth and metastasis8. Members of the vascular endothelial growth factor (VEGF) family of cytokines are among the most potent pro-angiogenic molecules. Bevacizumab (A), the most widely used VEGF inhibitor, is a chimeric murine / human IgG antibody that targets the VEGF ligand9. As with anthracyclines, multiple mechanisms have been described which confer resistance to bevacizumab. Central among them is hypoxia-induced HIF-1α upregulation10.
The phosphatidylinositol-3-kinase (PI3K) signaling pathway is crucial to many aspects of normal cell growth and survival. Accordingly, its dysregulation plays a pivotal role in carcinogenesis, the development of metastatic competence and therapy resistance. Consequently, there is great interest in the development of targeted inhibitors of key PI3K pathway molecules. Of particular interest to us during the development of this trial was the high prevalence of PI3K signaling abnormalities, including PIK3CA mutations and PTEN loss, described in both gynecologic and breast cancers11, 12. Temsirolimus (T) is a derivative of the drug Sirolimus, an inhibitor of the mammalian target of rapamycin (mTOR) complex13. mTOR is a critical downstream mediator of PI3K signaling, which when activated, modulates cell proliferation via a number of downstream targets11. In this manner, mTOR inhibitors have been shown to have significant anti-cancer properties. Importantly, mTOR inhibitors, particularly (T), also have potent HIF-1α inhibitory properties14.
Rationale for the combination of DAT
Each of the three drugs was chosen based on its proven anti-tumor activity in both gynecologic and breast malignancies. Additionally, because HIF-1α up-regulation is a key mediator of chemo-resistance to both D and A, we postulated that (T) could provide at least additive anti-tumor activity when administered in combination with D and A (DAT). Because these three agents have mostly non-overlapping toxicities, we anticipated that it would be possible to administer them together at near-maximal single agent doses.
PATIENTS AND METHODS
Study Design and Dosing
This was a single institution, phase I, open-label, sequential dose-escalation study with a standard 3 + 3 design open to all patient with solid tumors. It was institutional review board approved and all patients provided informed consent. This manuscript addresses the subset of patients with gynecologic and breast cancers that were treated on the study (N = 74 of the 117 total treated). All pathology was centrally confirmed at M. D. Anderson.
Primary end points were to establish the maximum tolerated dose (MTD) and characterize dose limiting toxicities (DLT). Secondary end points included a preliminary assessment of anti-tumor efficacy, safety profiling, and the establishment of biologic corollaries for prediction of tumor response and tolerability. Six dose levels were originally planned. As the MTD was not met at dose level six, the protocol was amended with the addition of an additional dose level (Table I).
Table I. Dose-Escalation Schedule (21-day cycle)*.
| Dose Level |
Bevacizumab (IV) [Day 1] (mg/kg) |
Liposomal doxorubicin (IV) [Day 1] (mg/m2) |
Temsirolimus (IV) [Days 1, 8, 15] (mg) |
|---|---|---|---|
| 1 | 5 | 10 | 12.5 |
| 2 | 5 | 20 | 12.5 |
| 3 | 5 | 20 | 25 |
| 4 | 10 | 20 | 25 |
| 5 | 15 | 20 | 25 |
| 6 | 15 | 30 | 25 |
| *7 | 15 | 40 | 25 |
The original protocol included dose levels 1 – 6, however at dose level 6 there were no DLTs, so the protocol was amended to include dose level 7. After mmultiple responses were seen in lower dose levels, the protocol was amended to include expansion cohorts for up to fifteen patients from malignancies in which response criteria were met. This resulted in expansion cohorts in uterine, ovarian, breast, cervix, colorectal, parotid, adrenocorticoid and malignant thymoma. No gynecologic or breast cancer patients were treated at dose level seven.
Drug administration was repeated on a 21 day cycle with all three drugs given on day one and T administered weekly on days eight and fifteen (Table I). If one patient in a cohort experienced a DLT during the first cycle, three additional patients were enrolled and treated at that dose level. If at any time more than 33% of patients in a cohort experienced a DLT, that cohort was closed to additional patients. Of note, early in the trial multiple significant responses were observed and the protocol was amended to allow for cohort expansions if specific response criteria were met. This resulted in cohort expansions in the following malignancies: uterus, ovary, breast, cervix, malignant thymoma, parotid, adrenocorticoid and colorectal.
Administration of (DAT) continued until unacceptable toxicity or disease progression occurred, or total cumulative anthracycline dose exceeded 550 mg/m2. Dose delays and reductions were left to the discretion of the treating physician. DLTs were defined as follows: Any grade three or four non-hematologic toxicity as defined in the NCI CTC v3.0 that was possibly, probably or definitely related to any of the three study medications, with the following exceptions: a) any grade four hematologic toxicity lasting less than two weeks, and b) any grade four nausea or vomiting lasting less than five days15. DLTs had to occur within the first cycle of treatment.
Eligibility Criteria
Key inclusion criteria were age ≥ twelve years; measurable, histologically documented solid tumors refractory to standard treatment or for which no standard therapy was available; Eastern Cooperative Oncology Group (ECOG) performance status ≤ two (exceptions required IRB approval); absolute neutrophil count ≥ 1.5 × 109/L; platelet count ≥ 100.0 × 109/L; serum creatinine < 3.0 mg/dl, alanine transferase (ALT) ≤ five times the upper limit of normal (ULN), with the exception of patients with significant liver metastases who were allowed to have values ≤ eight times the ULN; bilirubin ≤ 2.0 mg/dl, and cardiac left ventricular ejection fraction ≥ 50% without evidence of congestive heart failure (CHF). Key exclusion criteria were poorly controlled hypertension (systolic blood pressure > 150 mm Hg, diastolic pressure > 100 mm Hg), patients with clinically significant cardiovascular disease, prior cumulative doxorubicin dose > 300 mg/m2, and pregnancy. Prior exposure to anthracyclines and VEGF-inhibitors were not exclusion criteria for study entry, nor were patients with a history of venous thromboembolism excluded.
Assessment of Tumor Response
Tumor measurements were performed by a staff radiologist pre-treatment and every two cycles thereafter as well as by a departmental RECIST measurement team. Measurable target lesions were evaluated for response using Response Evaluation Criteria in Solid Tumors (RECIST)16, 17. For purposes of this report, prolonged stable disease (prolonged SD) was defined as trial enrollment without dose delays of more than two weeks in total for ≥180 days. Adverse events were recorded from day one through thirty days after the last dose and were graded based on the Common Terminology Criteria for Adverse Events, version 3.0 (CTCAEv3.0).
Molecular assays for biologic markers: PIK3CA, KRAS, BRAF mutations and PTEN loss
We included Clinical Laboratory Improvement Amendment (CLIA) certified mutational or immunohistochemical assays, as appropriate, for PIK3CA, KRAS and BRAF mutations as well as PTEN loss. The tests were performed within the Division of Pathology and Laboratory Medicine at M. D. Anderson. Archival formalin-fixed, paraffin-embedded tissue blocks or tissue from fine-needle aspiration or surgical biopsies were used to test for BRAF mutations. DNA was extracted from micro-dissected paraffin embedded tumor sections and analyzed using a polymerase chain reaction (PCR)-based DNA sequencing method for BRAF codons 468-474, codons 595-600, and mutations of exon 15 by pyro-sequencing as previously described18. Tests for PIK3CA and K-RAS mutations were performed using a similar method. Exons 9 (codons 532-554) and 20 (codons 1011-1062) for examined PIK3CA mutations, and codons 12, 13 and 61 were examined for KRAS mutations7. PTEN loss was assessed using immunohistochemistry12 (monoclonal mouse anti-human PTEN, clone 6H2.1, Dako®, Denmark)12.
At trial initiation, IRB approved pre- and post-treatment image-guided percutaneous tumor biopsies were offered to patients with the specified intention to identify molecular corollaries for response assessment. Mutational analyses for PIK3CA, BRAF, KRAS and PTEN loss as described above, and reverse phase proteomic analysis (RPPA) assays for more than 100 cell signaling proteins grouped by signaling system were planned. We found recruitment of patients for these biopsies to be difficult; to date there are 3 patients who have completed the pre- and post-treatment biopsies; as a result these batched tissue samples have not yet been processed for reporting purposes.
Statistical Analysis
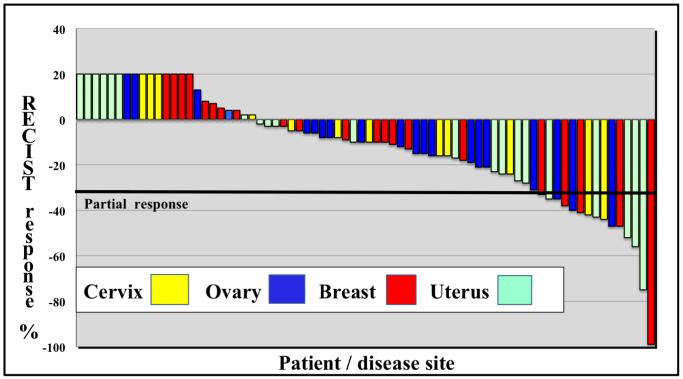
Descriptive statistics are provided for all end points using STATA v10.0, College Station, TX . Continuous measurements are summarized using mean, standard deviation, median, range, number of patients and percentages. Time to treatment failure (TTF) and overall survival (OS) were calculated using the method of Kaplan and Meier in days, from date of enrollment to disenrollment or death from any cause, whichever came first. Patients still on trial at the time of last assessment were censored. A waterfall plot depicting best RECIST responses by percent is presented in figure I.
Figure 1.
Individual patients (disease sites by color) are represented with vertical bars on the X axis. Best RECIST response (%) is depicted by on the Y axis. Patients with progressive disease as their best response are depicted as +20%.
RESULTS
Patient Characteristics and Disposition
Seventy-seven women with advanced, metastatic, chemo-refractory ovarian, uterine, cervix and breast malignancies were enrolled. Two of these had deteriorating functional status prior to dosing and were subsequently disenrolled. One patient voluntarily disenrolled prior to first dosing citing financial reasons. 74 patients were treated and evaluable. Demographic and clinical characteristics are summarized in Table II. The median age of patients was 52 years (range, 27 to 75 years). The median number of prior therapies for metastatic disease was four. 52 deaths occurred; 50 were attributed to disease progression and two were thought possibly due to adverse effects of one or more of the study drugs. The median number of completed cycles for all patients was five (range 0 – 20). For patients with SD or better, the median number of cycles completed was seven (range 2 – 27).
Table II. Baseline demographic and clinical data.
| Characteristic | # patients (%) |
|---|---|
| Race/ethnicity | 74 |
| White | 59 (79.7) |
| Black | 8 (10.8) |
| Hispanic | 4 (5.4) |
| SE Asian | 1 (1.4) |
| SW Asian | 2 (2.7) |
| Age, years | |
| Median (Range) | 52 (27-75) |
| ECOG performance score ¥ | |
| 0 | 18 (24.3) |
| 1 | 34 (45.9) |
| 2 | 20 (27) |
| 3§ | 2 (2.7) |
| Primary organ site | |
| Uterus | 21 (28.4) |
| Epithelial | 18 (24.3) |
| Sarcoma | 3 (4.1) |
| Ovary | 20 (27) |
| Epithelial | 16 (21.6) |
| Stromal | 4 (5.4) |
| Cervix | 13 (17.6) |
| Breast | 20 (27) |
| Median # of prior cytotoxic regimens (range) |
4 (1-11) |
| Median # of prior biologics (range) |
1 (0-3) |
ECOG = Eastern Cooperative Oncology Group
Principal Investigator (PI) override authorized for patient entry
Overall survival (OS) and Time to treatment failure (TTF)
70 out of 74 patients had survival information available. The remaining four were lost to follow up. Median overall survival was 214 days (95% confidence interval (CI) 185 – 312). At time of censoring, 67/74 (90.5%) were disenrolled. The overall median TTF was 112 days (95% CI 89 – 147).
Dose Escalation, DLT, Tolerability and MTD
Patients were enrolled in accordance with the planned 3 + 3 study design until dose level four, at which point our IRB approved expansion cohorts as described in the methods section; they were filled using dose levels shown in Table I (in diseases in which activity was observed, expansions were permitted at the highest dose level found to be safe as of that date.) This resulted in dose escalations in cancers of the ovary, endometrium and cervix. Dose escalation for the remaining three levels continued in accordance with the original escalation plan. There were two DLTs observed during the study; however both involved non-gynecologic cancer patients (grade four thrombocytopenia) within dose level seven (liposomal doxorubicin 40 mg/m2, bevacizumab 15 mg/kg and temsirolimus 25 mg). These were the first two patients treated at dose level seven and no further gynecologic or breast cancer patients were enrolled at that dose level. The MTD for the study was therefore level 6 (Table I).
Safety
All 74 (100%) patients experienced at least one adverse event that was at least possibly drug related. These events were mostly grade one or grade two and reversible. Grade two fatigue (27%), anemia (20.2%), neutropenia (18.5%) and mucositis (17.5%) were the most common events, requiring dose modification and trial discontinuation in seven (9.5%) and four (5.4%) patients, respectively. Grade three or four toxicities were: thrombocytopenia (9.5%), mucositis (6.7%), cardiac (4.1%), gastrointestinal (bowel perforation (2.7%)) and genitourinary (vesico-vaginal fistula (1.4%)). Of note, three of seven (43%) patients who experienced grade three to four thrombocytopenia were enrolled with baseline platelet levels less than 125 × 109/L. There were two (2.7%) possible treatment related events of fatal colonic perforation (dose levels 1 and 3). One of the patients had ovarian cancer; the other patient had endometrial cancer. Both patients had bulky pelvic tumor and both had completed pelvic radiotherapy; both events were associated with precipitous declines in each patient’s serum Ca-125 level.
Response data and efficacy
All patients are assessable for response. Five patients (6.7%) discontinued treatment for social reasons without formal restaging or evidence of clinical progression before completing two cycles. These patients were classified as having progressive disease (PD). Of the remaining patients, one (1.4%) had a complete response (CR) and fourteen (18.9%) had a partial response (PR) (CR + PR = 20.3%); thirteen patients (17.6%) had prolonged SD. Responses by disease site are detailed in Table III.
Table III. Response data by disease site and histology.
| Disease site histology |
patients treated # |
CR N (%) |
PR N (%) |
SD ≥ 6 months N (%) |
CR+PR+SD ≥ 6 months N (%) |
Median # cycles completed (range) |
|---|---|---|---|---|---|---|
| Uterus | 21 | 5 (1-20) | ||||
| Epithelial | 18 | 5 (27.8) | 4 (22.2) | 9 (50) | 6 (1-20) | |
| Leiomyosarcoma | 3 | 1 (0-2) | ||||
| Ovary | 20 | 4 (2-13) | ||||
| Epithelial (high grade) | 16 | 3 (18.8) | 4 (25) | 7 (43.8) | 6 (1-13) | |
| Epithelial (low grade) | 1 | |||||
| Granulosa | 2 | 4 (1-8) | ||||
| Sarcoma | 1 | 2 | ||||
| Cervix | π 13 | 3 (1-7) | ||||
| Squamous | 10 | 2 (15.4) | 1 (7.7) | 3 (23.1) | 3 (1-6) | |
| Adenocarcinoma | 3 | 2 (1-7) | ||||
| Breast | π 20 | 5 (1-12) | ||||
| Ductal | 11 | 1 (5) | 1 (5) | 2 (10) | 3 (15) | 4 (1-8) |
| Lobular | 1 | 1 (5) | 1 (5) | 2 (10) | 9 (7–12) | |
| Metaplastic | 8 | 2 (10) | 1 (5) | 4 (20) | 5 (4-8) |
prolonged SD defined as trial enrollment for more than or equal to 180 days
Organ specific histologic subtypes grouped to determine denominator
Among eighteen patients with epithelial uterine cancer (excluding three patients with stromal cancers), five (27.8%) had a PR and four patients (22.2%) had prolonged SD (PR + prolonged SD = 50%). Among sixteen patients with epithelial ovarian cancer (excluding four patients with non-high grade epithelial histologies), three (18.7%) had a PR and four patients (25%) had prolonged SD (PR + prolonged SD = 43.7%). Among twenty patients with breast cancer, one (5%) had a CR and four (25%) had a PR (CR + PR = 30%); four patients (20%) had prolonged SD (CR + PR + prolonged SD = 45%). Of interest is that three of the breast cancer responders (1 CR, 2 PRs) had metaplastic breast cancer, a notoriously chemo-refractory triple negative histologic subtype. Two of thirteen (15.4%) patients with cervical cancer achieved a PR and one (7.7%) had prolonged SD (PR + prolonged SD = 23.1%). Of note, five of the fifteen (33%) patients with a CR or PR had previously progressed on anthracycline chemotherapy (median cumulative dose 200 mg/m2; range 160 - 360).
Seven patients remained on study at time of censoring. The one patient (metaplastic breast cancer) who achieved a CR is still on study at 530 days. This patient was without evidence of disease after 6 cycles; after 8 cycles, D and A were discontinued and she has remained on maintenance T. Among all patients, median TTF was 112 days (95% CI 89 – 147). Among patients with a PR, average time to treatment failure (TTF) was 172 days (range 46 – 468; 95% C.I. 100 – 246). Patients with SD after two cycles had an average TTF equal to 133 days (range 42 – 272; 95% C.I. 112 – 154), and TTF for patients with PD was 42 days (range 6 – 89; 95% C.I. 29 – 55). Table V. details responses, OS and TTF by dose level.
Table V. Responses, overall survival (OS) and time to treatment failure (TTF) by dose level.
| Dose level |
Patients treated (#) |
Events | OS in days (CI) | TTF in days (CI) | PR/CR |
|---|---|---|---|---|---|
| 1 | 2 | 1 | 359.5 (78, NA) | 358.5 (61, NA) | 1 |
| 2 | 1 | 1 | 249 (NA, NA) | 138.5 (46, NA) | 0 |
| 3 | 4 | 4 | 209.5 (84, NA) | 82 (43, NA) | 0 |
| 4 | 5 | 4 | 271 (139, NA) | 158 (96, NA) | 1 |
| 5 | 34 | 33 | 312 (185, 391) | 133 (89, 183 | 7 |
| 6 | 24 | 20 | 188 (135, NA) | 90 (76, 160) | 6 |
Responses were seen among a variety of histologic subtypes. Notable characteristics of each responder are detailed in Table III. Estrogen receptor (ER) status was known among three of the four uterine cancer responders; all three of these were positive. ER status was known for all breast cancer patients. Three of the five breast cancer responders had ER positive tumors.
Molecular testing for PIK3CA , kRAS, bRAF mutations, PTEN loss and association with response
When archival cell blocks for patients were available, CLIA certified testing was performed for BRAF and PIK3CA mutations as well as PTEN loss. PIK3CA mutational status was known for 57/74 (77%) patients, and was positive in 16 (28%). PTEN status was known for 25/74 (33.8%) patients, and PTEN loss was identified in 11 (44%). BRAF status was known for 45/74 (60.8%) patients, and 2 (4.4%) were positive. KRAS status was known for 49/74 (66.2%) patients, and 8 (16.3%) were positive. Four (25%) of the sixteen patients with a PIK3CA mutation, five (45.5%) of eleven patients with PTEN loss, two (25%) of the eight patients with a KRAS mutation and one (50%) of the two patients with a BRAF mutation achieved a response. Among the fifteen responders (CR + PR), PIK3CA and PTEN status were known in nine (60%) and five (33.3%), respectively. Four (44.4%) of the nine responders for whom PIK3CA mutational status was known were positive, and three (60%) of the five responders for whom PTEN status was known were found to have PTEN loss. PIK3CA mutations or PTEN loss were identified in 25/59 (42.3%) tested patients. Among these, nine (36%) achieved CR/PR and four (16%) had SD ≥ 6 months (CR + PR + SD ≥ 6 months = 52%).
DISCUSSION
Treatment planning for patients with chemo-refractory gynecologic and breast cancers is challenging because there is little definitive data regarding optimal therapy in patients who have failed 1st line agents19-21. Additionally, the majority of such patients are heavily pre-treated and unable to tolerate full dose cytotoxic regimens. As insights into tumor resistance biology have improved, we are increasingly able to exploit these mechanisms to more effectively treat patients with chemo-refractory disease4, 10, 22-26. The biologic rationale for this combination was that each drug has proven efficacy as a single agent in multiple solid tumors, each is relatively tolerable with non-overlapping toxicities, and two of the three drugs (D and A), share HIF-1α as a resistance mechanism. In addition, PI3K pathway aberrations are common in breast and gynecologic cancers27, 28. This fact makes an mTOR inhibitor such as T a potentially ideal drug for use in combination with D and A29.
The overall response rate (ORR = CR + PR) in this population of heavily pre-treated, patients (median number of prior cytotoxic regimens = four) was 15/74 (20.3%). Responses by disease site among epithelial uterine, ovarian, breast and cervical carcinomas were 27.8%, 18.8%, 25% and 15.4%, respectively. When patients with prolonged SD are considered with responders, total response plus prolonged stable disease was 50%, 43.8%, 45% and 23.1%, respectively.
Whether the responses observed are due simply to the additive effects of the three drugs, or synergism resulting from T mediated HIF-1α inhibition is unknown. Unfortunately our plan for pre- and post-treatment tissue analyses did not result in an adequate number of tissue samples to be of use for answering this question. Future investigations should emphasize the obtainment of pre- and post-treatment samples so that biologic corollaries for a clinical response can be identified. Of particular interest in this study were the percentages of patients with PI3K pathway aberrations that achieved a response or prolonged SD; of 25 patients with either PIK3CA mutations or PTEN loss, nine (36%) achieved a PR, and the rate of response plus stable disease for at least six months was 52%.
This combination is relatively safe and well tolerated, with predictable and largely manageable adverse effects. As expected, the primary issue with tolerability was thrombocytopenia, and this was managed without the need for disenrollment in all but one patient. The incidence of mucositis was consistent with that seen with D alone, and was also manageable without disenrollment for all but one patient. Also notable were two colonic perforations. These are well described in association with A in ovarian cancer patients. The incidence of bowel perforation in our population was within the range of that described by previous investigations30,31, 32. The most serious toxicity was bowel perforation, seen in two patients with bulky disease and prior radiation treatment, both of whom showed a precipitous early fall in CA125. This significant problem might be due to bevacizumab and / or temsirolimus and / or rapid response and / or the disease itself.
Further study of DAT in larger populations of patients with gynecologic and breast malignancies is warranted. The recommended dose for phase II/III study is liposomal doxorubicin dosed at 20 – 30 mg/m2 every 21 days, bevacizumab 15 mg/kg every 21 days and temsirolimus 25 mg weekly using a 21 day cycle. Studies that include enrichment for patients with PIK3CA mutations or PTEN loss may be especially worthwhile.
Statement of translational relevance.
With an increasing number of newer, targeted and costly therapies available for the treatment of gynecologic and breast cancers, it is important that clinical correlative data be available for assistance in identifying subsets of patients most likely to benefit from exposure to these agents. This phase I study describes an active and tolerable regimen. Of translational relavence is the fact that correlative Clinical Laboratory Improvement Amendment (CLIA) certified assays for multiple cell signaling pathways suggest an association between specific signaling aberrations and clinical response to this regimen. In particular, the presence of PI3K pathway mutations (PIK3CA mutations as well as PTEN loss) correlate with response in heavily pre-treated gynecologic and breast cancer patients. While the small numbers in this study preclude the generation of strong conclusions regarding the use of these assays in response prediction, these results are compelling and warrant further study with an emphasis on pre- and post-treatment biologic corollaries.
Table IV. Complete response (CR) and partial response (PR) characterization by patient*.
| Response | Disease site |
Histology | Dose level |
# prior cytotoxic regimens |
Prior anthracycline history |
# cycles completed |
|
|---|---|---|---|---|---|---|---|
| 1 | PR (−75%) |
Uterus | Endometrioid | 6 | 2 | None | 6 |
| 2 | PR (−56%) |
Uterus | Endometrioid | 1 | 5 | Progression | 27 |
| 3 | PR (−43%) |
Uterus | Endometrioid | 5 | 4 | None | 8 |
| 4 | PR (−35%) |
Uterus | Endometrioid | 6 | 2 | None | 5 |
| 5 | PR (−32%) |
Uterus | Endometrioid | 5 | 8 | Progression | 10 |
| 6 | PR (−47%) |
Ovary | Undifferentiated | 4 | 2 | None | 13 |
| 7 | PR (−31%) |
Ovary | Serous + transitional |
5 | 4 | Progression | 8 |
| 8 | PR (−35%) |
Ovary | Clear cell | 5 | 2 | None | 6 |
| 9 | PR (−44%) |
Cervix | Squamous | 6 | 2 | None | 13 |
| 10 | (PR) (−42%) |
Cervix | Squamous | 6 | 2 | None | 8 |
| 11 | CR (−99%) |
Breast | Metaplastic | 5 | 3 | None | 8 |
| 12 | PR (−47%) |
Breast | Ductal | 6 | 8 | Progression | 7 |
| 13 | PR (−38%) |
Breast | Metaplastic | 5 | 1 | None | 5 |
| 14 | PR (−36%) |
Breast | Metaplastic | 5 | 3 | None | 4 |
| 15 | PR (−33%) |
Breast | Ductal | 6 | 11 | Progression | 5 |
Acknowledgement
This work was supported in part by Grant Number RR024148 from the National Center for Research Resources, a component of the NIH Roadmap for Medical Research (http://nihroadmap.nih.gov/clinicalresearch/overview=translational.asp)
References
- 1.Murphy CS. AD. Evolving approaches to metastatic breast cancer previously treated with anthracyclines and taxanes. Clinical Breast Cancer. 2009;9:58–65. doi: 10.3816/CBC.2009.s.006. [DOI] [PubMed] [Google Scholar]
- 2.Doxil Prescribing label. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails.
- 3.Lothstein L, Israel M, Sweatman TW. Anthracycline drug targeting: cytoplasmic versus nuclear--a fork in the road. Drug Resist Updat. 2001;4:169–77. doi: 10.1054/drup.2001.0201. [DOI] [PubMed] [Google Scholar]
- 4.Coley HM. Mechanisms and strategies to overcome chemotherapy resistance in metastatic breast cancer. Cancer Treat Rev. 2008 doi: 10.1016/j.ctrv.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62:3387–94. [PubMed] [Google Scholar]
- 6.Mi J, Zhang X, Rabbani ZN, Liu Y, Reddy SK, Clary BM, et al. RNA aptamer-targeted inhibition of NF-kappa B suppresses non-small cell lung cancer resistance to doxorubicin. Mol Ther. 2008;16:66–73. doi: 10.1038/sj.mt.6300320. [DOI] [PubMed] [Google Scholar]
- 7.Riganti C, Doublier S, Aldieri E, Orecchia S, Betta PG, Bosia A, et al. Asbestos induces doxorubicin resistance in MM98 mesothelioma cells via HIF-1{alpha} Eur Respir J. 2008 doi: 10.1183/09031936.00090407. [DOI] [PubMed] [Google Scholar]
- 8.Grothey AE, Ellis LM. Targeting angiogenesis driven by vascular endothelial growth factors using antibody-based therapies. Cancer J. 2008;14:170–7. doi: 10.1097/PPO.0b013e318178d9de. [DOI] [PubMed] [Google Scholar]
- 9.Bevacizumab Prescribing Label. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails.
- 10.Ellis LH, Hicklin DJ. Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res. 2008;14:6371–5. doi: 10.1158/1078-0432.CCR-07-5287. [DOI] [PubMed] [Google Scholar]
- 11.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 12.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, Hennessy BT, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–91. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Temsirolimus Prescribing Label http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails.
- 14.Pantuck AJ, Zeng G, Belldegrun AS, Figlin RA. Pathobiology, prognosis, and targeted therapy for renal cell carcinoma: exploiting the hypoxia-induced pathway. Clin Cancer Res. 2003;9:4641–52. [PubMed] [Google Scholar]
- 15.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Rubin P, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–81. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Verweij J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Benjamin RS, Choi H, Macapinlac HA, Burgess MA, Patel SR, Charnsangavej C, et al. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25:1760–4. doi: 10.1200/JCO.2006.07.3411. [DOI] [PubMed] [Google Scholar]
- 18.Zuo Z, Chen SS, Chandra PK, Galbincea JM, Soape M, Luthra R, et al. Application of COLD-PCR for improved detection of KRAS mutations in clinical samples. Mod Pathol. 2009;22:1023–31. doi: 10.1038/modpathol.2009.59. [DOI] [PubMed] [Google Scholar]
- 19.Fleming GF. Systemic chemotherapy for uterine carcinoma: metastatic and adjuvant. J Clin Oncol. 2007;25:2983–90. doi: 10.1200/JCO.2007.10.8431. [DOI] [PubMed] [Google Scholar]
- 20.Markman M. Optimal management of recurrent ovarian cancer. Int J Gynecol Cancer. 2009;19(Suppl 2):S40–3. doi: 10.1111/IGC.0b013e3181bf8143. [DOI] [PubMed] [Google Scholar]
- 21.Moroney JW, Wheler J, Hong DS, Naing A, Coleman RL, Kurzrock R, et al. Phase I clinical trials in 85 patients with gynecologic cancer: the M. D. Anderson Cancer Center experience. Gynecol Oncol. 2010;117:467–72. doi: 10.1016/j.ygyno.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bilyeu JD, Panta GR, Cavin LG, Barrett CM, Turner EJ, Arsura M, et al. Circumvention of nuclear factor kappaB-induced chemoresistance by cytoplasmic-targeted anthracyclines. Mol Pharmacol. 2004;65:1038–47. doi: 10.1124/mol.65.4.1038. [DOI] [PubMed] [Google Scholar]
- 23.Blagosklonny MV. Hypoxia-inducible factor: Achilles’ heel of antiangiogenic cancer therapy (review) Int J Oncol. 2001;19:257–62. doi: 10.3892/ijo.19.2.257. [DOI] [PubMed] [Google Scholar]
- 24.Blagosklonny MV. Antiangiogenic therapy and tumor progression. Cancer Cell. 2004;5:13–7. doi: 10.1016/s1535-6108(03)00336-2. [DOI] [PubMed] [Google Scholar]
- 25.Chaudhry P, Asselin E. Resistance to chemotherapy and hormone therapy in endometrial cancer. Endocr Relat Cancer. 2009;16:363–80. doi: 10.1677/ERC-08-0266. [DOI] [PubMed] [Google Scholar]
- 26.Duyndam MC, van Berkel MP, Dorsman JC, Rockx DA, Pinedo HM, Boven E. Cisplatin and doxorubicin repress Vascular Endothelial Growth Factor expression and differentially down-regulate Hypoxia-inducible Factor I activity in human ovarian cancer cells. Biochem Pharmacol. 2007;74:191–201. doi: 10.1016/j.bcp.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Samarnthai N, Hall K, Yeh IT. Molecular profiling of endometrial malignancies. Obstet Gynecol Int. 2010;2010:162363. doi: 10.1155/2010/162363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazzoletti M, Broggini M. PI3K/AKT/mTOR inhibitors in ovarian cancer. Curr Med Chem. 2010;17:4433–47. doi: 10.2174/092986710794182999. [DOI] [PubMed] [Google Scholar]
- 29.Janku F, Tsimberidou AM, Garrido-Laguna I, Wang X, Luthra R, Kurzrock R, et al. PIK3CA Mutations in Patients with Advanced Cancers Treated with PI3K/AKT/mTOR Axis Inhibitor. Mol Cancer Ther. 2011 Mar;10(3):558–65. doi: 10.1158/1535-7163.MCT-10-0994. Epub 2011 Jan 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia AA, Hirte H, Fleming G, Yang D, Tsao-Wei DD, Oza AM, et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol. 2008;26:76–82. doi: 10.1200/JCO.2007.12.1939. [DOI] [PubMed] [Google Scholar]
- 31.Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, McGuire W, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007;25:5180–6. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 32.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:5165–71. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]