Abstract
Purpose
Oncolytic viruses are self-amplifying anti-cancer agents that make use of the natural ability of viruses to kill cells. Adenovirus serotype 5 (Ad5) has been extensively tested against solid cancers, but less so against B cell cancers since these cells do not generally express the coxsackie and adenoviral receptor (CAR). To determine if other adenoviruses might have better potency, we “mined” the adenovirus virome of 55 serotypes for viruses that could kill B cell cancers.
Experimental Design
15 adenoviruses selected to represent Ad species B, C, D, E, and F were tested in vitro against cell lines and primary patient B cell cancers for their ability to infect, replicate in, and kill these cells. Select viruses were also tested against B cell cancer xenografts in immunodeficient mice.
Results
Species D adenoviruses mediated most robust killing against a range of B cell cancer cell lines, against primary patient marginal zone lymphoma cells, and against primary patient CD138+ myeloma cells in vitro. When injected into xenografts in vivo, single treatment with select species D viruses Ad26 and Ad45 delayed lymphoma growth.
Conclusions
Relatively unstudied species D adenoviruses have a unique ability to infect and replicate in B cell cancers as compared to other adenovirus species. These data suggest these viruses have unique biology in B cells and support translation of novel species D adenoviruses as oncolytics against B cell cancers.
Keywords: oncolytic, adenovirus, species D, lymphoma, myeloma
Introduction
B-cell malignancies can present as leukemias, myelomas, and lymphomas. In the United States there are over 300,000 patients afflicted by these diseases for which chemotherapy, radiation therapy, and stem cell transplantation are the main treatments (1, 2). The spectrum of B cell cancers are thought to arise at different stages of the ontogeny of normal B cells. For example, chronic lymphocytic leukemia and small lymphocytic lymphoma (CLL/SLL) may arise from intermediate and mature B-cells; non-Hodgkin’s lymphomas are thought to be generated from mature immunoblasts; and myeloma is thought to arise from differentiated plasma cells. While therapies have improved survival in many of these cancers, five year relative survival is still only 60% for lymphomas, 50% for leukemias, and 35% for myeloma. Therefore, complementary therapies are needed to address those cancers that resist current treatments.
Recent work has explored the use of viruses as cancer therapies (reviewed in (3)). Certain viruses have cancer killing or “oncolytic” capacity based on their intrinsic ability to kill cells. These oncolytic viruses have appeal because they are “self-amplifying” drugs, since each infected and killed cancer cell can produce thousands of progeny virions that can kill other cancer cells.
Adenoviruses (Ads) are non-enveloped DNA viruses that are among those being tested as oncolytic agents (3, 4). There are currently 55 serotypes of Ad that infect humans and these serotypes distribute into seven species (A–G). The vast majority of oncolytic testing has been focused on one virus, Ad serotype 5 (Ad5) from species C. Ad5 has been extensively tested for killing solid epithelial tumors in part due to its ability to infect these cells via the coxsackie and adenovirus receptor (CAR)(reviewed in (4)). While Ad5 can be quite potent particularly against epithelial solid tumors, 27 to 100% of humans are already immune to this virus. Therefore, its utility may be restricted as an oncolytic treatment for many patients.
Ad5 is markedly less potent against cells that do not express CAR including most of the cells of the hematologic system (5, 6). For this reason, Ad5 has not been applied extensively as an oncolytic against hematologic cancers. This ineffectiveness is curious, particularly in light of the fact that Ad5 and other Ad serotypes are frequently isolated from human lymphoid tissues and peripheral blood (reviewed in (7)). For example, species B, C, and E Ads have been isolated or detected in human adenoids, tonsils, and peripheral blood mononuclear cells (PBMCs). Consistent with this, species B viruses like Ad35 that bind CD46 are substantially better at infecting cells of hematologic origin (5, 6). Therefore, it is possible that other adenoviral serotypes may be better than Ad5 as oncolytics against hematologic malignancies.
Given there are 54 other serotypes of adenoviruses in seven biologically diverse species), we recently screened low seroprevalence Ads from species B, C, D, and F for oncolytic activity against the B cell cancer myeloma (8). In this work, we found that two species D viruses, Ad26 and 48, were more potent than all other viruses including species C Ad5. This was notable, since species D viruses make up 32 of the 55 known human Ads. Despite their abundance, this large and diverse group of viruses is also the least studied for basic virus biology and as oncolytics.
Given this and their high activity against B cell-derived myeloma cancers, we hypothesized that species D adenoviruses might have particular efficacy against B cell cancers, and that viral oncolysis might vary with the maturation state of the B cell cancer cells. To test these hypotheses, seven species D adenoviruses were compared to six viruses from species B, C, and E for their ability to kill B-cell cancers that arose from various stages of B cell ontogeny including chronic lymphocytic leukemia (CLL), different stages of B cell lymphoma, and myeloma.
Materials and Methods
Cell lines
The mouse myeloma cell line 5TGM1 and human Burkitt’s lymphoma line Raji were generously provided by Dr. Stephen Russell (Mayo Clinic). CHO-CD46 expressing cells were generously provided by Dr. Kah Whye Peng (Mayo Clinic). Human lymphoma RL and Hep3B human hepatocarcinoma cells were obtained from ATCC (American Type Culture Collection, Manassas, VA). Cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT). The human cancer cell lines Mec-1 and Mec-2 grew spontaneously from a patient with B-CLL (9). Mec-1 and 2 cells were maintained in IMDM + Glutamax supplemented with 10% FBS. Human cancer cell line A549 (lung carcinoma) from ATCC was maintained in DMEM supplemented with 10% FBS.
Patient samples
Peripheral blood mononuclear cells (PBMCs), CLL, lymphoma, multiple myeloma, and reactive follicular hyperplasia cell samples were collected at the Mayo Clinic (Rochester, MN) from patients after informed consent and institutional review board approval had been obtained. Mononuclear cells were isolated from post-apheresis leukocyte reduction chambers from healthy blood donors as described(10). Monocyte populations were isolated using CD14+ immunomagnetic bead selection followed by sequential isolation of CD3+ cells immunomagnetic selection for T cells (both from Miltenyi Biotec, San Diego, CA). Labeled cells were separated on an AutoMACS separator (Miltenyi Biotec) running the POSSEL program. Remaining cell populations after sequential isolation of monocyte and T cells were lymphoid cells that are predominantly B cells. Typical isolation purities for the positive selected cells are greater than 95% (10). Patient cells were maintained in culture in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 10% FBS.
Viruses
Ad4, Ad5, Ad6, Ad7, Ad11, Ad17, Ad24, Ad26, Ad28, Ad30, Ad35, Ad45, and Ad48 were obtained from the ATCC. Viruses were propagated in HEK 293 cells (also from ATCC). Viruses were purified by CsCl purification and quantitated by determining the optical density at 260nm (OD260).
Analysis of in vitro infection and killing by Trypan Blue uptake
Cell lines and/or patient cells were infected with 10,000 (VP) per cell for one hour at 4°C. Cells were then washed three times with Hank’s buffered saline solution (HBSS, Invitrogen, Carlsbad, CA) and placed in a 37°C incubator for the indicated length of time. Loss of membrane integrity was assessed by Trypan Blue uptake as described by Barry and colleagues (1990). For neuraminidase treated samples, cells were incubated with 5ug/ml neuraminidase (SIGMA, St. Louis, MO) at 4°C for one hour prior to incubation with virus.
Quantitative Real Time PCR (qPCR)
Virally infected cells were harvested at the indicated time points and DNA was isolated using DNeasy kit from Qiagen. 100ng of DNA was used in a real-time PCR assay using SybrGreen reagent to quantitate viral genomes in each sample. Samples were read using an ABI Prism 7900HT real-time instrument. A standard curve of the corresponding viral plasmid DNA was used for quantitation, using primers designed against hexon. Measurements were made in triplicate.
Cell Viability MTT Assay
To assess blocking of viral binding, 4×104 Hep3B cells were plated into each well of 96-well plates. Cells were then incubated in HBSS containing 500 μL of 200 μg/mL lectin from wheat germ agglutinin (SIGMA), 200 μg/ml mannose (SIGMA), or 22 mg/mL of cyclic 4-RGD peptide (Invitrogen) for one hour at 4°C. 4×107 of the indicated viral particles were then added to each well. After 7 days, cell viability was assessed by (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (SIGMA). N=3.
Blocking Receptor Binding
5×105 Hep3B cells were plated in each well of 24-well plates. 100 μL of 200 μg/mL lectin from wheat germ agglutinin, 200 μg/ml mannose, or 22 mg/mL of cyclic 4-RGD peptide were added and incubated with cells for one hour at 4°C. 5×108 particles of Ad26 were added to each well and left to incubate for 1 hour at 37°C. Wells were then washed three times with HBSS. DNA was isolated from the cells using DNeasy from Qiagen (Valencia, CA) and quantitated by real-time quantitative PCR. N=3.
Ad26 CD46 Receptor Blocking
4×104 human reactive follicular hyperplasia patient sample cells or Hep3B cells were plated into 96-well plates. Cells were then incubated with 1.5 μg of IgG1 mouse isotype antibody (BD Pharmingen–San Jose, CA), mouse anti-human CD46 from BD Pharmingen (anti-CD46a) or mouse anti-human CD46 antibody from AbD Serotec (Raleigh, NC)(CD46b) dialyzed in HBSS were added to each well. Plates were then incubated at 4°C for one hour. 4×107 of the indicated viral particles were then added to each well. After 7 days, cell viability was assessed by MTT assay or crystal violet staining. N=3.
Human lymphoma xenografts
3×106 RL (human lymphoma) cells were injected subcutaneously into the hind flank of 4–6 week-old nude mice (Harlan Sprague-Dawley – Indianapolis, IN). After palpable tumors formed (14 days), the mice were injected intratumorally with 3×1010 viral particles of wild-type virus in 100 μl total volume of HBSS. Control groups were injected with buffer alone. Tumors were observed every other day and measured weekly. Volume was calculated as ½ length × width × width. When animals were found with tumor volumes exceeding 2000 μl, ulcerated tumors, or greater than 20% weight loss, they were sacrificed. N = 6.
Animals
All animal experiments were carried out according to the provisions of the Animal Welfare Act, Public Health Service Animal Welfare Policy, the principles of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the policies and procedures of Mayo Clinic. Female nude mice (4–6 weeks old) were purchased from Harlan
Statistics
P values represent two-tailed t-test values unless otherwise indicated. P < 0.05 considered significant.
Results
Infection and Killing of PBMCs by Adenoviruses
Adenoviruses from species B, C, and E have frequently been identified in normal human PBMC and lymphoid tissues (7). Our previous testing of adenoviruses from species B, C, and D against bone marrow cells from myeloma patients found that species D Ads 26 and 48 were most potent at killing myeloma cells while largely sparing normal marrow cells (8). These data suggest that different Ad serotypes from different virus species may have unappreciated tropism for human hematologic cells.
To explore the ability of different Ads to infect and kill normal cells, PBMCs from healthy volunteers were enriched for CD14+ monocytes and CD3+ T cells by positive selection. The remaining cell population after sequential isolation of monocyte and T cells were predominantly (95%) B cells (10). These cells were then incubated with adenovirus representatives from different species. Ad serotype 5 (Ad5) was tested as a benchmark oncolytic since it is the only virus used to date in humans for virotherapy. Other adenoviruses were chosen based on the following criteria. First, their viral infection is well tolerated by humans. Viruses associated with cancer (species A) or obesity (species D Ad36) were excluded. Previously tested species F gastrointestinal viruses Ad40 and Ad41 were omitted as these were ineffective in all tests ((8) and data not shown). Second, new viruses that were tested here had to have low seroprevalence in humans to avoid neutralization in patients if they were translated into the clinic. Third, the viruses were genetically distinct and more likely to possess novel phenotypes that might be more effective against B cell cancers.
Each cell population was incubated with each of the Ads and cell viability was assessed by Trypan Blue exclusion over 7 days (Fig. 1). Addition of most of the viruses to CD14+ monocytes reduced viability by 20 to 45% as compared to controls. However, the untreated CD14+ monocytes lost 50% viability, so toxicity by the viruses was likely exaggerated by the non-optimal culture conditions. Treatment of CD3+ T cells with most of the Ads caused little loss of viability. The one exception was Ad11that reduced survival approximately 25%. Most of the viruses had little effect on CD3−/CD14− cells with the exception of Ad11 that reduced viability 30% (p = 0.0309). Ad26 also appeared to have some toxicity to these cells, however, this did not reach statistical significance. These data suggest that normal T and B cells are relatively resistant to infection by most Ad species, but that there is some evidence of cell killing by select viruses. This is consistent with previous tests in primary marrow cells (8). This is also consistent with observations of persistent association of Ads with lymphocytes (11).
Figure 1. Comparison of adenoviral serotypes for infection and killing of normal PBMCs.
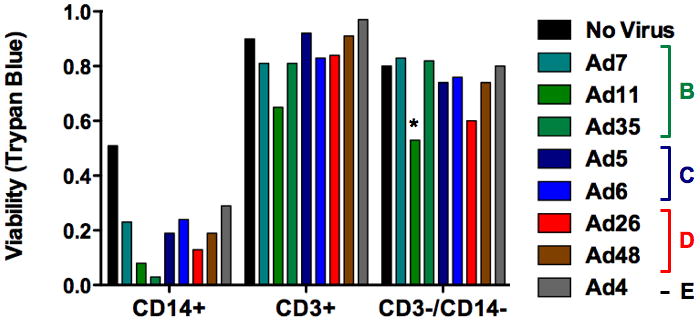
The indicated cells were incubated for seven days with the indicated wild-type adenoviruses and cell viability was assessed by Trypan Blue exclusion. MOI = 10,000 viral particles/cell.
Oncolytic Screen of Ad Species on CLL and Lymphoma Cell Lines
Our previous data suggested that species D viruses were most potent at killing primary patient myeloma cells while largely sparing normal cells (8). Myeloma arises from mature B cell plasma cells, the last step of B cell maturation. Given their activity against these mature B cell cancers, we tested select adenoviruses against B cell cancers arising from various stages of B cell maturation. These included chronic lymphocytic leukemia (CLL) and B cell lymphomas arising from various stages of B cell ontogeny.
Two viruses from each of species B, C, and D were first tested on B-cell cancer cell lines representing cancers arising at different stages of B cell development: MEC1, MEC2, Raji, and RL cells. MEC1 and MEC2 cells are human CLL cell lines that express mature B-cell markers CD19, CD20, CD21, and CD22. They are CD46 positive and coxsackie and adenovirus receptor (CAR) negative. These two CLL cell lines were derived from the same patient and differ mainly in the expression of CD23 and FMC7 (9). RL cells are human lymphoma cells that are EBV negative. RL cells are derived from the ascites of a patient with diffuse large cell lymphoma (12). Raji cells are an EBV-positive lymphoma cell line derived from a patient with Burkitt’s Lymphoma (13). Myeloma cell lines were tested previously (8).
Species B Ad11 and 35, species C Ad5 and 6, and species D Ad26 and 48 were incubated with the indicated cell lines and cell killing was monitored over time by loss of membrane integrity assessed by Trypan Blue uptake (Figure 2A–D). Under these conditions, species B viruses were largely ineffective against the CLL or lymphoma cell lines. Species C Ad5 and Ad6 mediated intermediate killing over time on all four cell lines, with Ad5 being slightly more effective than Ad6. Consistent with results on myeloma, species D viruses Ad26 and Ad48 were most effective in killing all of these B cell cancer cell lines with Ad26 generally being most effective.
Figure 2. Comparison of adenoviral serotypes for infection and replication in B-cell cancer cell lines.
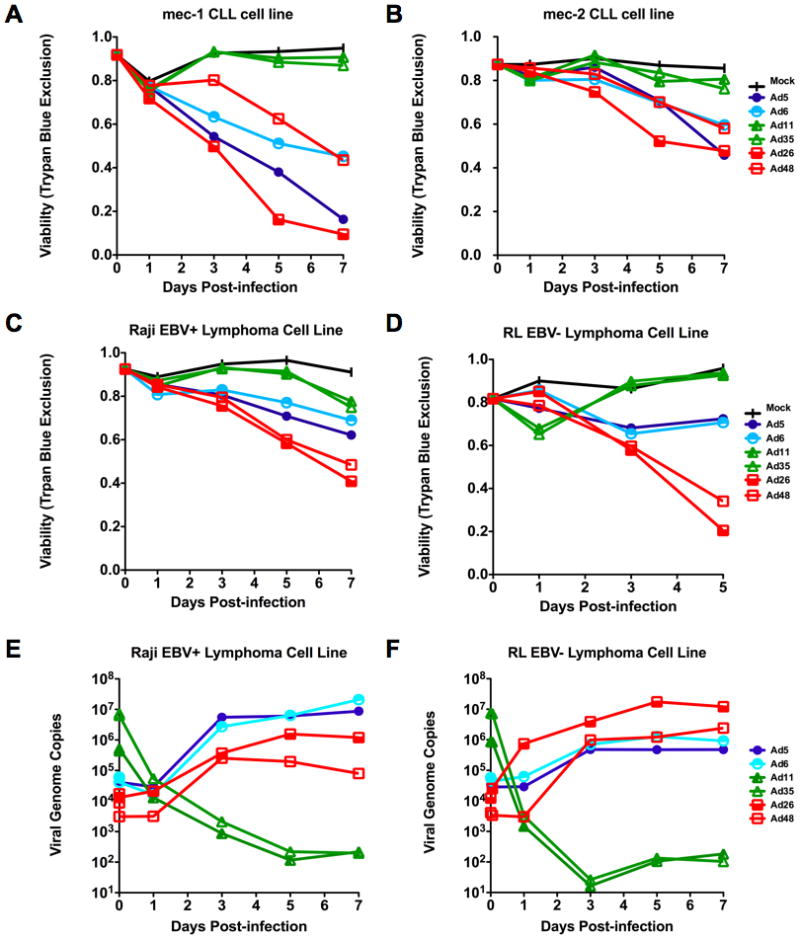
Comparison of cell killing of either MEC1 (A) or MEC2 (B) chronic lymphocytic leukemia cell lines by adenoviruses as assessed by Trypan Blue uptake following infection with virus at an MOI of 10,000 viral particles/cell. Killing of either Raji (C) or RL (D) human lymphoma cell lines by adenoviruses as assessed by Trypan Blue uptake. E and F. Real-time quantitative PCR showing viral genome copies present within cells over a one week time course following infection for cells in C and D. Species B (green), species C (blue), or species D (yellow and red). N=3.
Replication of Viruses in Lymphoma Cell Lines
In most cases, oncolysis depends on the virus proceeding through its life cycle. To test if oncolysis of these cell lines correlated with propagation of the virus life cycle, adenoviral genome replication was assessed by quantitative real time PCR (qPCR) at varied times after incubation with Epstein-Barr Virus (EBV) positive Raji lymphoma cells and with EBV-negative RL lymphoma cells. In addition to monitoring viral genomes associated with the cells, qPCR after one hour incubation at 4°C with the cells also provides a relative assessment of the number of virions that have successfully bound to the cells.
qPCR testing of each of the viruses incubated for one hour with Raji and RL cells revealed that the species B viruses Ad11 and Ad35 bound most effectively (Figure 2E and F). While these viruses were most effective at binding, they failed to replicate their DNA. This is consistent with both their inability to kill the cells and with previous observations on primary patient myeloma cells (8). In contrast, the species C and D viruses bound to lower degrees, but replicated their genomes and killed the cells. This higher replication and killing of cells by species C and D viruses is consistent with previous data in more mature myeloma cancer cells (8).
Oncolytic Testing on Primary Patient CLL and Lymphomas
While testing in cell lines provides useful guidance of potential therapy, there is no substitute for testing in primary patient cancers. To test this, CLL samples were collected from two patients and these were incubated with Ad5, 6, 11, 35, 26, and 48 (Figure 3A and B). In this case, cell killing was at most 50% over 7 days, but was markedly weaker than in the immortalized CLL cell lines. Again, the species D viruses Ad26 and Ad48 mediated the highest level of killing on these B cell cancers.
Figure 3. Comparison of adenoviral serotypes for infection and replication of primary B cell cancer cells.
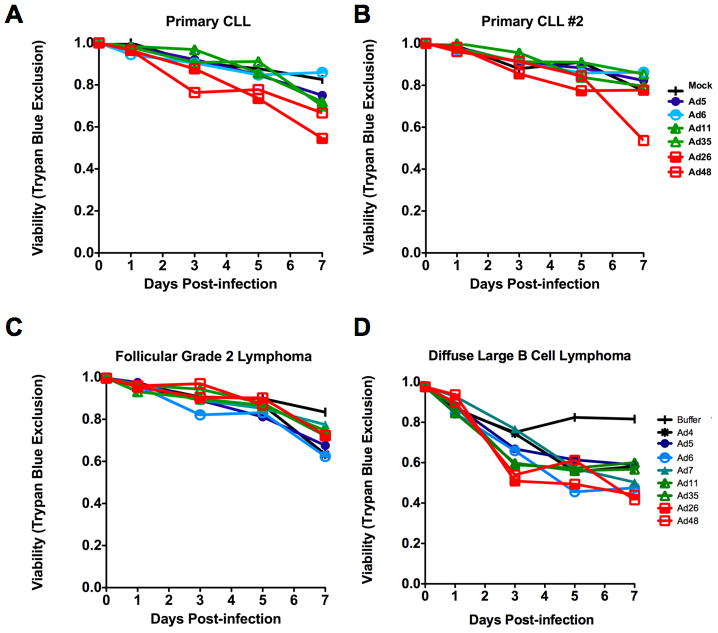
A and B. One week cell viability of two different CLL patient’s sample cells after infection with six different adenoviral serotypes. C. Viability of follicular grade 2 cancer lymphoma patient sample cells following infection by a panel of eight adenoviral serotypes. D. Viability of diffuse large B cell lymphoma patient sample cells following infection by panel of eight adenoviral serotypes. Experiments were performed using a viral MOI of 10,000 particles/cell.
Patient samples from two grades of lymphoma were tested with species B Ad7, Ad11 and Ad35, species C Ad5 and Ad6, species D Ad26 and Ad48, and also with species E Ad4. When tested on follicular lymphoma, the viruses only mediated 35% killing over 7 days (Figure 3C). Interestingly, the viruses that utilize CAR (Ad4, 5, and 6) were most potent against this particular type of solid tumor lymphoma. In contrast, when tested against diffuse large B lymphoma, many of the viruses had similar oncolytic activity with the most potent virus being Ad26 (Figure 3D).
Expanded Screen of Species D Adenoviruses
These observations in CLL, lymphoma, and myeloma indicated that the two species D viruses, Ad26 and Ad48, were generally most effective against cancers spanning B cell development. This observation is interesting because these viruses are largely unstudied despite constituting more than half of the 55 human Ads.
Based on this, we hypothesized: 1) that species D viruses might have a unique tropism for B cell cancer cells; 2) that this tropism might make them particularly useful as oncolytics in patients; and 3) that species D viruses might have differing abilities to kill B cell cancers from different steps of B cell ontogeny.
To test these hypotheses, a broader set of species D viruses including Ads 17, 24, 26, 28, 30, 45, and 48 were compared to species B Ads 7, 11, and 35, species C Ads 5 and 6, and species E Ad4. In agreement with results obtained using the smaller panel of viruses (Figure 2), all species D viruses were superior to species B, C, and E viruses for killing the RL EBV-negative lymphoma cells (Figure 4A). Species C and E viruses mediated intermediate levels of cell killing. Species B viruses again showed no significant killing effect.
Figure 4. Expanded comparison of adenoviral serotypes for infection and replication in B-cell cancer cells.
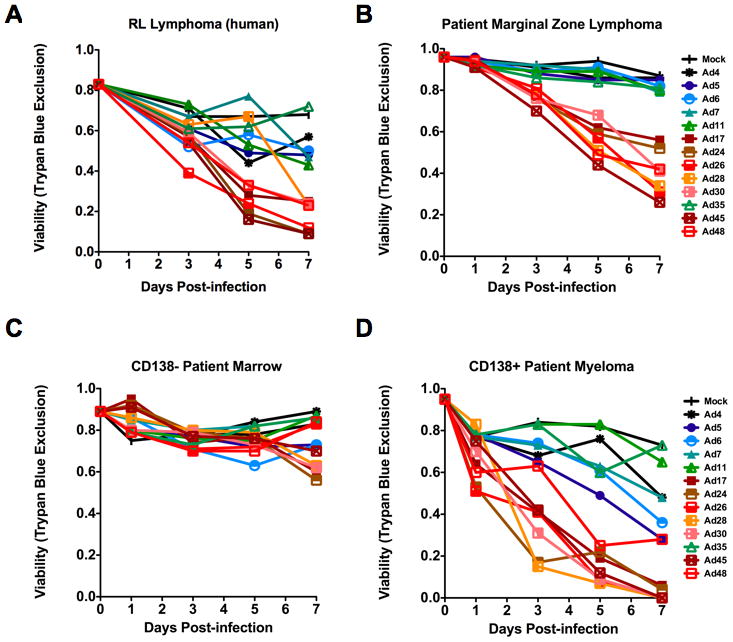
A 13-member panel of adenoviral serotypes are tested against two B cell cancers. Lymphoma - (A) RL human lymphoma (B) Patient marginal zone lymphoma. Myeloma – (C) non-malignant CD138- patient bone marrow cells (D) malignant C138+ patient bone marrow cells. Viability was assessed by Trypan Blue uptake following infection at an MOI of 10,000 viral particles/cell. Green = species B; blue = species C; yellow, orange, red = species D, and brown = species E.
When the 13 virus serotype panel of viruses was tested against primary marginal zone lymphoma cells from a patient, these cells were efficiently killed by all of the species D viruses. No other viruses were effective against these cells.
The 13 virus serotype panel was also tested on cells from a multiple myeloma patient. Testing on bone marrow samples from myeloma afforded the opportunity to compare viral cell killing on normal cells versus cancer cells since these cells can be separated by purification on CD138 antibody beads. When the 13 virus panel was tested on CD138-negative (Figure 4C) and CD138-positive marrow cells, the species D viruses were the most effective at killing the CD138+ malignant cells (Figure 4D). In contrast, markedly lower cell killing was observed in CD138- non-cancerous cells.
Due to the limited availability of patient sample cells and the large number of viruses being screened in the Trypan Blue viability assays, Figures 2–4 were each tested with only one sample. To compare the oncolytic activity of the viruses against the highly proliferative patient B-cell cancers statistically, data from the marginal zone lymphoma patient was pooled with that from multiple myeloma patients from Figures 4 and 7 as well as two additional multiple myeloma patient samples not included within the figures presented (Figure 5). While species B, C, or E viruses showed no consistent effect on the five patient samples, all of the species D viruses demonstrated significant cell killing (Ad17 p = 0.0031, Ad24 p = 0.0002, Ad26 p < 0.0001, Ad28 p < 0.0001, Ad30 p = 0.0004, Ad45 p < 0.0001, Ad48 p = 0.0003). Given the inherent heterogeneity between these patient samples, the higher activities of the species D viruses appears significant.
Figure 7. Receptor blocking during species D adenovirus infection.
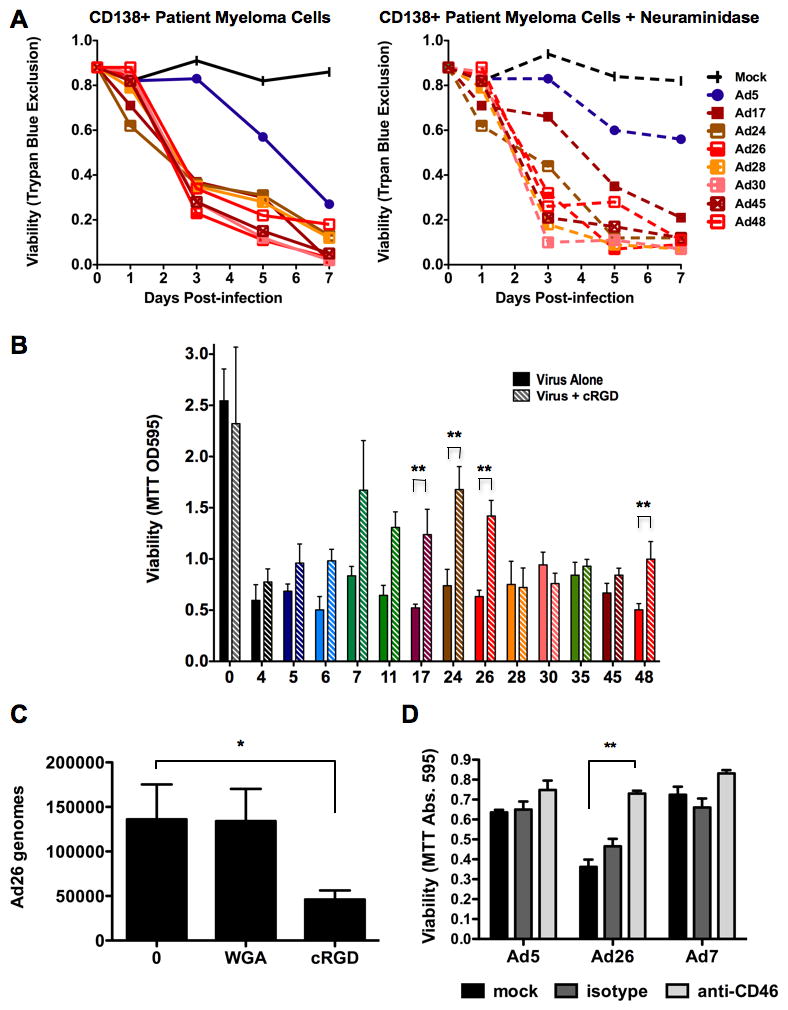
A. 13-panel adenoviral infection (MOI=1,000 viral particles/cell) of patient CD138+ multiple myeloma cells with (right) and without (left) prior treatment with neuraminidase to remove sialic acid. B. Viability of Hep3B cells one week following infection by the 13 adenoviruses (x-axis) in the presence or absence of cyclic RGD peptide. “0” = uninfected. P-value of two-tailed T-test. C. Effects of wheat germ agglutinin (WGA) or cyclic RGD on Ad26 binding to Hep3B cells as assessed by qPCR. “0” = uninfected. P-value of one-tailed T-test. D. Blocking CD46 binding of Ad26 to human patient reactive follicular hyperplasia cells by addition of isotype or anti-CD46 antibody. * = p < 0.05. ** = p < 0.01.
Figure 5. Pooled cell viability data from Five Patient B-cell cancers.
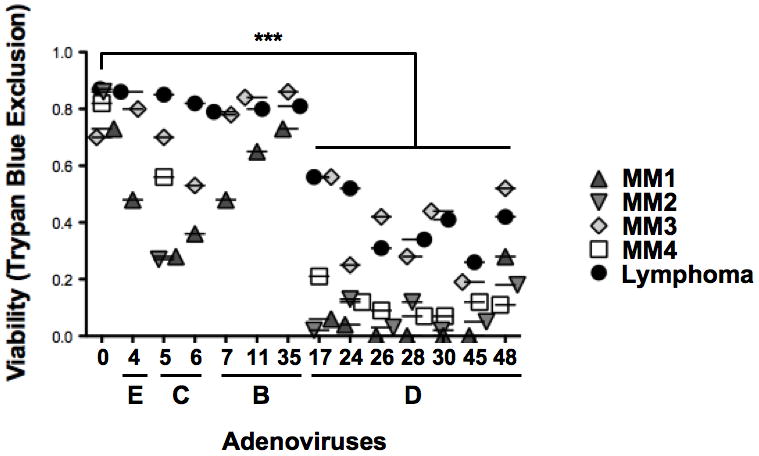
The marginal zone lymphoma patient sample data was pooled with cell killing data of four patient multiple myeloma samples. Individual viabilities (y-axis) from each experiment are presented as points above the viral serotype tested (x-axis).
In Vivo Testing of Ads in Mouse Xenografts of Human Lymphoma
To evaluate whether in vitro studies could be relevant to in vivo therapy, RL lymphoma tumors were initiated by subcutanous injection of the cells into the flanks of nude mice. Once tumors reached volumes of 200 mm3, groups of six mice were injected intratumorally a single time with 3×1010 virus particles (vp) of selected viruses. Since species D Ad26 and Ad45 were generally the most potent of the 13 tested Ads, these two viruses were tested. For comparison, another group of mice were injected with the same dose of Ad5 (species C) that was less effective in vitro. A final group was injected with buffer alone. Tumor volume was measured weekly (Figure 6). Under these conditions, both Ad26 and Ad45 mediated significant delay in tumor growth after only a single injection. In contrast, both buffer-treated and Ad5-treated tumors grew unchecked requiring animals to be sacrificed within two weeks.
Figure 6. In vivo comparison of select adenoviral serotypes for killing of lymphoma xenografts in nude mice.
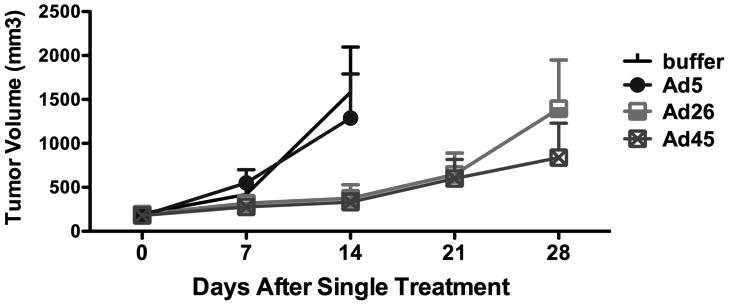
RL lymphoma tumors were initiated subcutaneously in nude mice. Groups of six tumor-bearing mice were injected intratumorally a single time with 3×1010 virus particles (vp) of the indicated adenoviruses. Tumor size was monitored over time after injection and mean tumor sizes are shown. Lines end when the first animal of the group had to be sacrificed to avoid misleading skewing of the lines as larger tumors are removed.
Receptor Utilization by Species D Adenoviruses
These data indicate that species D viruses have unique efficacy against B cell cancers spanning the ontogeny of normal B cells. One of the main differences between the different subgroups of adenovirus is their receptor specificity. Species C and E Ads are known to bind cellular CAR with high affinity and utilize their fiber proteins to then bind and enter cells via interactions of penton base with αv integrins (14, 15). In contrast, species B viruses bind CD46 and perhaps other receptors with their fibers (16–18).
Despite the fact that species D Ads represent more than half of all human adenoviruses, these viruses are diverse and relatively unstudied. Species D viruses can bind CAR, CD46, as well as sialic acid with their fiber proteins ((19–23) and Supplemental Figure 1). While they can bind all of these receptors, species D Ads have short fibers with only 8 repeats making them unable to efficiently enter cells using CAR (21). When Ad26 and 48 were vectored as replication-defective vaccines, receptor utilization studies suggested that they did not use CAR and only weakly used CD46 as a receptor (24). Sialic acid binding was not tested. From this, it is unclear whether all species D viruses use the same receptors and if receptor specificity of these viruses explains their unique ability to kill B cell cancers.
These data suggested that the subgroup D viruses might use sialic acid, CAR, or CD46 for binding and killing B cell cancers. An alternate possibility is that the viruses may be binding to αv integrins with RGD motifs on penton base (19). To test these possibilities, virus infection was assessed with and without incubation with agents that could block interactions with these receptors (Figure 7). Because a sialic acid binding motif of fiber is well conserved on these species D viruses (Supplemental Figure 1), they were tested for their ability to bind sialic acid by treating primary CD138+ myeloma cells with neuraminidase to remove cell surface sialic acid residues prior to incubation with the viruses (Figure 7A). Under these conditions, no obvious effects of removal of sialic acid were observed. To test the role of integrin binding by RGD motifs on the viruses, Hep3B cells were incubated with the cyclic RGD-4C peptide (25) prior to virus exposure and cell viability was measured 7 days later (Figure 7B). In contrast to the weak effects of neuraminidase, the RGD-4C peptide partially inhibited oncolysis by species D viruses Ad17, 24, 26, and 48 (p = 0.0076, p = 0.0019, p = 0.0011, and p = 0.0091 respectively), but not cell killing by species D viruses Ad28, 30, or 45 (p = 0.8717, p = 0.8914, and p = 0.0609 respectively). To test this further, Hep3B cells were incubated with wheat germ agglutinin (WGA) to block cell surface sialic acids or with cyclic RGD before addition of the potent Ad26 virus (Figure 7C). qPCR for viral genomes demonstrated no reduction in virus binding by WGA. In contrast, cRGD inhibited Ad26 binding more than 50% (p = 0.034). To test if CD46 binding was involved in virus killing, primary patient reactive follicular hyperplasia cells were incubated with a blocking CD46 antibody or with isotype control antibody before the addition of species B Ad7, species C Ad5, and species D Ad26. Consistent with previous results, Ad26 mediated more cell killing than Ad5 or Ad7. Incubation with the CD46 antibody reduced cell killing nearly 50% (p = 0.067). Combination of CD46 antibody and cyclic-RGD peptide on patient myeloma cells mediated complete protection against killing by Ad26, suggesting that both receptors are being utilized by the virus (Supplemental Figure 2).
Discussion
Following the first government approval for the use of oncolytic adenovirus in the treatment of head and neck cancers, researchers have been exploring the use of replication competent virus in other cancer treatments (3, 26). Ad5 is the most common vector being studied for this purpose. However, 27 to 100% of people in populations throughout the world are predicted to have neutralizing antibodies against Ad5 viral proteins (24, 27). The presumed neutralization of the therapeutic vector in a large group of individuals makes Ad5 a poor choice for an oncolytic in these patients. More recent studies have probed alternative less prevalent adenoviruses for efficacy against cancer cells (8, 28–32). In a recent study against myeloma, Ad5 and Ad6 species C viruses showed moderate anti-cancer effect against cell lines and primary multiple myeloma patient cells. However, Ad26 and Ad48 species D viruses were more proliferative in these cells (8). This is surprising considering species D viruses do not infect human solid tumor cancer cell lines (33). Based on these data, we hypothesized that uncommon species D viruses are superior to Ad5 for the treatment of B-cell cancers.
To test this hypothesis, species B, C, and D adenoviruses were tested against B lineage cancer cell lines and primary patient samples. As predicted by our previous myeloma study, Ad26 and Ad48 and all tested species D viruses were effective against all of the cell lines. When the viruses were tested against primary patient CLL, myeloma and lymphoma samples, the species D viruses were again most effective, but were not as active against CLL as against lymphoma and myeloma cells. Given that immortalized cell lines acquire additional modifications, it is not entirely surprising that a dichotomy in responses was observed between CLL cell lines and primary samples. Fortunately, the species D viruses remained active against most of the primary lymphoma samples and against all tested myeloma cancer cells. In future studies, it may be interesting to further characterize cells that are refractory to cell killing. For example, it would be useful to determine the precise changes in cellular receptor expression that occur as primary cells are converted to immortalized cell lines. In primary patient sample cells, refractory populations are likely non-malignant. Better sorting methods may reduce the number of these cells within samples.
These data suggest that species D adenoviruses are most effective against lymphomas and myelomas - the B cell cancers arising from the later stages of B cell ontogeny. To some degree, there appears to be correlation between the ability of the viruses to kill B cell cancers and their proliferative capacity. This is particularly notable in the ability of the viruses to kill immortalized CLL cell lines, but to have markedly less effect on primary CLL samples and slower growing lymphomas.
Slow growing B cell lymphomas and leukemias include: follicular lymphoma (the most common slow-growing NHL), CLL (also known as small lymphocytic lymphoma SLL), lymphoplasmacytic lymphoma, marginal zone lymphoma, mucosa-associated lymphoid tissue (MALT) lymphoma, and Waldenström macroglobulinemia. Fast growing NHLs include diffuse large B-cell lymphoma (the most common fast-growing NHL), AIDS-associated lymphoma, anaplastic large cell lymphoma, Burkitt lymphoma, central nervous system (CNS) lymphoma, lymphoblastic lymphoma, MALT lymphoma, mantle cell lymphoma, and peripheral T-cell lymphoma.
The CLL samples, and two of the three lymphoma samples obtained for this study were follicular grade 2 lymphoma and marginal zone lymphoma in the slow growing lymphoma group. The third lymphoma sample was a diffuse large lymphoma in the fast growing group. While the species D adenoviruses killed all of these cancers, there was no clear correlation between general cancer growth phenotype and oncolytic susceptibility. This could reflect the artificial in vitro testing that does not necessarily recapitulate the growth patterns of these cells in vivo. Future clinical testing will be needed to determine if an association with growth and oncolysis exists.
Because species D viruses were selective against lymphoma cell lines and patient cells, the two best performing species D viruses Ad26 and Ad45 were compared to Ad5 for in vivo efficacy against RL tumor xenografts. In this case, a single intratumoral injection of 3×1010 viral particles significantly slowed tumor growth and improved survival of these animals. Ad5 was ineffective against these tumors. Although single intratumoral injection of species D adenoviruses was unable to completely kill tumors, future studies using sequential treatment injections are likely to show additional therapeutic benefit. It will be interesting to assess the response of regressive tumors to these secondary treatments. If tumor cells that are resistant to infection and killing by one species D serotype persist and clonally expand, a serotype switch approach may demonstrate the greatest antitumor effect.
These data suggest that subgroup D viruses may have utility as oncolytics against myeloma and lymphoma. Moreover, species D viruses are the only fully replication competent adenoviruses to demonstrate efficacy against lymphoma. It should be noted that a conditionally replicating Ad5 carrying the melanoma differentiation-associated gene-7/interleukin-24 (MDA-7/IL-24) transgene was effective against a rare CAR expressing lymphoma (34). Subgroup C viruses also appear effective against myeloma; however, because of the seroprevalence of Ad5, either a subgroup D virus or Ad6 would seem a better choice of vector for future studies.
The work presented herein begs the question, what cellular factors expressed by B-cells and what viral factors related to species D viruses make species D viruses more oncolytic against B-cell cancers? Our data suggest that these viruses do not use sialic acid for oncolysis, but instead use a combination of CD46 and integrin as receptors. Neither CD46 nor integrin blockade completely inhibited species D virus cell killing. Therefore, the viruses may use both receptors in combination as suggested by the data from Supplemental Figure 2 or may utilize an as yet uncharacterized receptor for infection of these B cells.
Testing of viruses from species B, C, D, and E on normal PBMCs and on bone marrow cells here and previously(34) suggest that these non-cancerous cells are relatively resistant to lethal infection by the viruses. Previous analysis of viral binding and replication in marrow samples from myeloma patients indicated that species B Ad11 binds both “normal” CD138- marrow cells and cancerous CD138+ myeloma cells 100-fold more strongly than species C or D viruses. This is interesting given that Ad11 was almost always the only virus that showed any cytotoxicity against normal T and B cells. While Ad11 binds marrow cells well, it does not appear to replicate indicating that at least in marrow, this is not a productive infection. Whether it or other Ad serotypes from the diverse species tested here bind and undergo abortive or productive infections in PBMCs remains to be tested. While most of the viruses spared normal cells from killing, safe clinical translation into patients will likely require that these species D viruses be engineered conditionally-replicating adenoviruses (CRAds) for these B cell cancers. This may be achieved by mutation of E1A or B interactions with tumor suppressors or by putting early genes under control of cancer or cell-specific promoters (reviewed in (3)).
In summary, adenoviruses appear to interact with normal and cancerous hematologic cells. These observations have implications for interactions of these viruses with normal blood cells and for their use as oncolytic agents. In this regard, species D adenoviruses appear most robust at killing B cell cancers arising from later steps in B cell ontogeny. The specificity of this oncolytic phenotype is not precisely known but is likely due to a combination of viral fiber length and available cellular receptors. Conversion of these wild-type viruses to CRAds for cancer-specific or B cell-specific replication will likely be important next steps to maximize their efficacy and safety for clinical translation into humans.
Supplementary Material
Statement of Translational Relevance.
This work analyzes the potential use of novel adenovirus serotypes as therapeutic agents against B cell cancers. This work was performed in part using primary patient samples from myeloma and lymphoma patients to ensure that the observations are relevant to clinical translation. This work lays the foundation for generating conditionally-replicating adenoviruses engineered for selective systemic therapy against B cell cancers in human patients.
Acknowledgments
Funding: This project was supported by a Developmental Project to M.A.B. from the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (SPORE) in Lymphoma (P50 CA097274), a grant to M.A.B. from the Fraternal Order of Eagles Cancer Research Fund, and by the Predolin Foundation.
We would like to thank Mary Barry for her excellent technical assistance. We would also like to thank Dr. Allan Dietz and Peggy Bulur for purification of PBMCs from normal donors.
Footnotes
Conflicts of Interest: None
Authorship
C. Chen designed and performed experiments and evaluated data. J. Senac designed and performed experiments and evaluated data. E. Weaver provided vital new reagents. S. May performed experiments. D. Jelinek provided vital samples P. Greipp provided vital samples T. Witzig provided vital samples M. Barry designed experiments and evaluated data. The authors have no conflicts of interest to declare.
References
- 1.Altekruse SFKC, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK. SEER Cancer Statistics Review. 2010. National Cancer Institute; Apr 15, 2010. [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Cattaneo R, Miest T, Shashkova EV, Barry MA. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol. 2008;6:529–40. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barry MA, Hofherr SE, Chen CY, Senac JS, Hillestad ML, Shashkova EV. Systemic delivery of therapeutic viruses. Curr Opin Mol Ther. 2009;11:411–20. [PubMed] [Google Scholar]
- 5.Shayakhmetov DM, Papayannopoulou T, Stamatoyannopoulos G, Lieber A. Efficient gene transfer into human CD34(+) cells by a retargeted adenovirus vector. J Virol. 2000;74:2567–83. doi: 10.1128/jvi.74.6.2567-2583.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yotnda P, Onishi H, Heslop HE, Shayakhmetov D, Lieber A, Brenner M, et al. Efficient infection of primitive hematopoietic stem cells by modified adenovirus. Gene Ther. 2001;8:930–7. doi: 10.1038/sj.gt.3301488. [DOI] [PubMed] [Google Scholar]
- 7.Lichtenstein DL, Wold WS. Experimental infections of humans with wild-type adenoviruses and with replication-competent adenovirus vectors: replication, safety, and transmission. Cancer Gene Ther. 2004;11:819–29. doi: 10.1038/sj.cgt.7700765. [DOI] [PubMed] [Google Scholar]
- 8.Senac JS, Doronin K, Russell SJ, Jelinek DF, Greipp PR, Barry MA. Infection and killing of multiple myeloma by adenoviruses. Hum Gene Ther. 2010;21:179–90. doi: 10.1089/hum.2009.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stacchini A, Aragno M, Vallario A, Alfarano A, Circosta P, Gottardi D, et al. MEC1 and MEC2: two new cell lines derived from B-chronic lymphocytic leukaemia in prolymphocytoid transformation. Leuk Res. 1999;23:127–36. doi: 10.1016/s0145-2126(98)00154-4. [DOI] [PubMed] [Google Scholar]
- 10.Dietz AB, Bulur PA, Emery RL, Winters JL, Epps DE, Zubair AC, et al. A novel source of viable peripheral blood mononuclear cells from leukoreduction system chambers. Transfusion. 2006;46:2083–9. doi: 10.1111/j.1537-2995.2006.01033.x. [DOI] [PubMed] [Google Scholar]
- 11.Garnett CT, Talekar G, Mahr JA, Huang W, Zhang Y, Ornelles DA, et al. Latent species C adenoviruses in human tonsil tissues. J Virol. 2009;83:2417–28. doi: 10.1128/JVI.02392-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beckwith M, Longo DL, O’Connell CD, Moratz CM, Urba WJ. Phorbol ester-induced, cell-cycle-specific, growth inhibition of human B-lymphoma cell lines. Journal of the National Cancer Institute. 1990;82:501–9. doi: 10.1093/jnci/82.6.501. [DOI] [PubMed] [Google Scholar]
- 13.Epstein MA, Achong BG, Barr YM, Zajac B, Henle G, Henle W. Morphological and virological investigations on cultured Burkitt tumor lymphoblasts (strain Raji) Journal of the National Cancer Institute. 1966;37:547–59. [PubMed] [Google Scholar]
- 14.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins avb3 or avb5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–19. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 15.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–3. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 16.Segerman A, Lindman K, Mei YF, Allard A, Wadell G. Adenovirus types 11p and 35 attach to and infect primary lymphocytes and monocytes, but hexon expression in T-cells requires prior activation. Virology. 2006;349:96–111. doi: 10.1016/j.virol.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 17.Gaggar A, Shayakhmetov DM, Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nature medicine. 2003;9:1408–12. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- 18.Tuve S, Wang H, Ware C, Liu Y, Gaggar A, Bernt K, et al. A new group B adenovirus receptor is expressed at high levels on human stem and tumor cells. J Virol. 2006;80:12109–20. doi: 10.1128/JVI.01370-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnberg N, Kidd AH, Edlund K, Olfat F, Wadell G. Initial interactions of subgenus D adenoviruses with A549 cellular receptors: sialic acid versus alpha(v) integrins. J Virol. 2000;74:7691–3. doi: 10.1128/jvi.74.16.7691-7693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnberg N, Edlund K, Kidd AH, Wadell G. Adenovirus type 37 uses sialic acid as a cellular receptor. J Virol. 2000;74:42–8. [PMC free article] [PubMed] [Google Scholar]
- 21.Arnberg N. Adenovirus receptors: implications for tropism, treatment and targeting. Rev Med Virol. 2009;19:165–78. doi: 10.1002/rmv.612. [DOI] [PubMed] [Google Scholar]
- 22.Burmeister WP, Guilligay D, Cusack S, Wadell G, Arnberg N. Crystal structure of species D adenovirus fiber knobs and their sialic acid binding sites. J Virol. 2004;78:7727–36. doi: 10.1128/JVI.78.14.7727-7736.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seiradake E, Henaff D, Wodrich H, Billet O, Perreau M, Hippert C, et al. The cell adhesion molecule “CAR” and sialic acid on human erythrocytes influence adenovirus in vivo biodistribution. PLoS Pathog. 2009;5:e1000277. doi: 10.1371/journal.ppat.1000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol. 2007;81:4654–63. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koivunen E, Gay DA, Ruoslahti E. Selection of peptides binding to the alpha 5 beta 1 integrin from phage display library. J Biol Chem. 1993;268:20205–10. [PubMed] [Google Scholar]
- 26.Garber K. China approves world’s first oncolytic virus therapy for cancer treatment. Journal of the National Cancer Institute. 2006;98:298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- 27.Piedra PA, Poveda GA, Ramsey B, McCoy K, Hiatt PW. Incidence and prevalence of neutralizing antibodies to the common adenoviruses in children with cystic fibrosis: implication for gene therapy with adenovirus vectors. Pediatrics. 1998;101:1013–9. doi: 10.1542/peds.101.6.1013. [DOI] [PubMed] [Google Scholar]
- 28.Hemminki A, Kanerva A, Kremer EJ, Bauerschmitz GJ, Smith BF, Liu B, et al. A canine conditionally replicating adenovirus for evaluating oncolytic virotherapy in a syngeneic animal model. Mol Ther. 2003;7:163–73. doi: 10.1016/s1525-0016(02)00049-7. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann D, Bayer W, Heim A, Potthoff A, Nettelbeck DM, Wildner O. Evaluation of Twenty-One Human Adenovirus Types and One Infectivity-Enhanced Adenovirus for the Treatment of Malignant Melanoma. J Invest Dermatol. 2007 doi: 10.1038/sj.jid.5701131. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann D, Heim A, Nettelbeck DM, Steinstraesser L, Wildner O. Evaluation of twenty human adenoviral types and one infectivity-enhanced adenovirus for the therapy of soft tissue sarcoma. HumGene Ther. 2007;18:51–62. doi: 10.1089/hum.2006.132. [DOI] [PubMed] [Google Scholar]
- 31.Strauss R, Sova P, Liu Y, Li ZY, Tuve S, Pritchard D, et al. Epithelial phenotype confers resistance of ovarian cancer cells to oncolytic adenoviruses. Cancer Res. 2009;69:5115–25. doi: 10.1158/0008-5472.CAN-09-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shashkova EV, May SM, Barry MA. Characterization of human adenovirus serotypes 5, 6, 11, and 35 as anticancer agents. Virology. 2009 doi: 10.1016/j.virol.2009.08.038. Epub Sep 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen CY, Weaver EA, Khare R, May SM, Barry MA. Mining the Adenovirus Virome for Effective Oncolytics Against Solid Tumors. Cancer Gene Therapy. 2011 doi: 10.1038/cgt.2011.47. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qian W, Liu J, Tong Y, Yan S, Yang C, Yang M, et al. Enhanced antitumor activity by a selective conditionally replicating adenovirus combining with MDA-7/interleukin-24 for B-lymphoblastic leukemia via induction of apoptosis. Leukemia. 2008;22:361–9. doi: 10.1038/sj.leu.2405034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


