Abstract
Numerous neuroimaging studies have revealed that in young adults, remembering the past and imagining the future engage a common core network. Although it has been observed that older adults engage a similar network during these tasks, it is unclear whether or not they activate this network in a similar manner to young adults. Young and older participants completed two autobiographical tasks (imagining future events and recalling past events) in addition to a semantic-visuospatial control task. Spatiotemporal Partial Least Squares analyses examined whole brain patterns of activity across both the construction and elaboration of autobiographical events. These analyses revealed that that both age groups activated a similar network during the autobiographical tasks. However, some key age-related differences in the activation of this network emerged. During the construction of autobiographical events, older adults showed less activation relative to younger adults, in regions supporting episodic detail such as the medial temporal lobes and the precuneus. Later in the trial, older adults showed differential recruitment of a network of medial and lateral temporal regions supporting the elaboration of autobiographical events, and possibly reflecting an increased role of conceptual information when older adults describe their pasts and their futures.
Keywords: autobiographical memory, episodic simulation, aging, hippocampus, partial least squares
1.0 Introduction
A rapidly growing number of studies have begun to examine how memory is used to imagine or simulate possible future events (for reviews, see Buckner & Carroll, 2007; Schacter, Addis, & Buckner, 2007, 2008; Suddendorf & Corballis, 2007; Szpunar, 2010). These studies have revealed striking similarities between remembering the past and imagining the future. Neuropsychological evidence indicates that some amnesic patients with medial temporal lobe damage exhibit deficits in imagining future or novel events (Hassabis, Kumaran, Vann, & Maguire, 2007; Klein, Loftus, & Kihlstrom, 2002; Kwan, Carson, Addis, & Rosenbaum, 2010; Tulving, 1995; cf., Maguire, Vargha-Khadem, & Hassabis, 2010; Squire et al., 2010), and neuroimaging studies show that similar brain regions are active when remembering the past and imagining the future (Addis, Pan, Vu, Laiser, & Schacter, 2009; Addis, Wong, & Schacter, 2007; Botzung, Dankova, & Manning, 2008; Hassabis, Kumaran, & Maguire, 2007; Okuda et al., 2003; Szpunar, Watson, & McDermott, 2007; Weiler, Suchan, & Daum, 2010a). Such observations have led to the suggestion that a core brain network (also known as the autobiographical memory retrieval network, or the default network; henceforth referred to as the core network), including medial temporal lobe, posterior cingulate/retrosplenial cortex, lateral parietal cortex as well as medial prefrontal and lateral temporal cortices supports both past and future episodic events (cf., Addis, Pan, et al., 2009; Addis, Wong, et al., 2007; Buckner & Carroll, 2007; Hassabis, Kumaran, & Maguire, 2007; Hassabis & Maguire, 2007; Schacter & Addis, 2007; Schacter et al., 2007; Spreng, Mar, & Kim, 2009).
These observations have led us to propose the constructive episodic simulation hypothesis (Schacter & Addis, 2007, 2009), which holds that simulations of specific episodes in the past and future draw on similar information and rely on similar underlying processes, and that episodic memory supports the construction of future events through the extraction and recombination of stored information into a simulation of a novel event. Related theories, such as the scene construction hypothesis (Hassabis & Maguire, 2007) also emphasize the importance of retrieving relevant information from memory and integrating these ‘information components’ into coherent scenarios.
Despite the striking similarity of the neural network engaged by remembering and imagining, there are some important differences. It has been shown that the construction of future events differentially engages several regions of this network, including the medial temporal and prefrontal regions (Addis, Cheng, Roberts, & Schacter, in press; Addis, Pan, et al., 2009; Addis & Schacter, 2008; Addis, Wong, et al., 2007; Okuda et al., 2003). Other studies, however, report increased activation during remembering relative to imagining, in posterior visuospatial cortices (Addis, Pan, et al., 2009; Weiler, Suchan, & Daum, 2010b), medial parietal cortex (Hassabis, Kumaran, & Maguire, 2007; Weiler et al., 2010a) and medial temporal regions (Abraham, Schubotz, & von Cramon, 2008; Botzung et al., 2008; Okuda et al., 2003; Weiler et al., 2010a). However, it is important to note that in many studies reporting this past>future effect in medial temporal regions, the future event task did not require online construction of a novel future scenario (e.g., Abraham, Schubotz, & von Cramon, 2008; Botzung et al., 2008; see Addis et al., in press, for further discussion of this issue). Overall, it appears that actively imagining future events requires additional medial temporal resources, likely supporting recombination of details into a coherent episodic event, while remembering engages medial parietal regions supporting the increased episodic detail comprising representations of events that actually occurred (Johnson, Foley, Suengas, & Raye, 1988).
Motivated by these findings and the constructive episodic simulation hypothesis, we recently extended this research to cognitive aging (Addis, Wong, & Schacter, 2008). Research by Spreng and Levine (2006) had provided initial evidence of age-related changes in future simulation, with older adults generating future events that were closer to the present than those generated by younger adults (for evidence concerning age differences when older and younger adults think about their future hopes, aspirations, duties, and obligations, see Mitchell et al., 2009). We used an adapted version of a paradigm developed by Levine, Svoboda, Hay, Winocur, & Moscovitch (2002; see also St. Jacques & Levine, 2007), in which older and younger adults remembered past events or imagined future events in response to word cues. As demonstrated by Levine et al., memories of past events generated by older adults contained less episodic and more generic content than those remembered by young adults. We replicated this finding and also reported that the same pattern was evident for future events: older subjects provided fewer specific episodic details about imagined experiences (e.g., what, when, and where) than young adults and instead produced more general conceptual information, such as facts, associations, and the like. In a follow-up study, we reported similar results with a paradigm in which older and younger participants imagined past and future events using recombined elements of actual experiences (Addis, Musicaro, Pan, & Schacter, 2010). In both of these studies, the number of episodic details generated when describing past and future events were significantly correlated, and like young adults, older adults produced significantly more episodic details for events that had actually occurred relative to those that were imagined. There is evidence to suggest, however, that when required to recombine episodic details into a coherent future event, older adults are less able to integrate key details than younger adults (Addis et al., 2010).
Although the foregoing studies provide initial insights into cognitive aspects of age differences in past and future events, the neural underpinnings of these age differences remain unclear. Neuroimaging studies have investigated the neural basis of past autobiographical events in older adults. For example, Viard et al. (2007) demonstrated that when remembering past events (relative to a control task), older adults engaged many regions of the core network reported in studies with young adults (cf. Cabeza & St Jacques, 2007; Svoboda, McKinnon, & Levine, 2006), including bilateral hippocampal regions. More recently, Viard et al. (2011) reported that the same group of participants activated this same network, including the hippocampus (albeit at a subthreshold level for the right hippocampus), when thinking about already planned future events. In contrast to previous findings in young adults, no differential activation of the hippocampus was evident for future relative to past events, although it is important to note that participants were likely retrieving representations of these planned events, rather than constructing them, and that only half of these future events were classed as episodic in nature. However, it was found that the episodic qualities of past events correlated with hippocampal activity, while for future events it correlated with inferior frontal and lateral temporal activity. Although this study revealed some past-future differences within older adults, it cannot speak to the neural underpinnings in the age-related reductions of episodic content of past and future events due to the lack of a young control group
Although Viard et al. (2007) found that older adults engage bilateral hippocampus during past event retrieval, studies directly comparing neural activity in young and older adults have reported age-related differences. Maguire and Frith (2003) found that older adults engaged the left hippocampus to the same level as young adults; moreover, they also exhibited bilateral hippocampal activity, differentially recruiting the right hippocampus. In addition, more recent work has revealed some hippocampal differences during autobiographical memory retrieval (St. Jacques, Rubin, & Cabeza, in press). These findings highlight the need to compare directly old and young adults during both remembering the past and imagining the future.
The present study used fMRI to determine whether there are age-related changes in the neural activity associated with remembering the past and imagining the future. While previous fMRI studies suggest that older adults can engage regions comprising the core network when remembering and imagining, it remains unknown whether and how activation patterns during the active construction of past and future events differ in older and younger adults.
2.0 Materials and Methods
2.1 Participants
Twenty young and twenty older right-handed adults with no history of neurological or psychiatric impairment gave informed written consent in a manner approved by the Harvard and Massachusetts General Hospital Institutional Review Boards. All older adults had a Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975) score of 27 or higher, excluding dementia. Six young and six older adults were excluded due to technical difficulties, anatomical abnormalities detected during scanning, excessive motion, claustrophobia or insufficient responding. Thus, data from 14 young (8 males; mean age, M=19.50 years, SD=2.14) and 14 older adults (8 males; age, M=72.93 years, SD=4.98; Mini-Mental State Examination, M=29.07, SD=1.07) were analyzed. These older adults were significantly more educated (M=15.50 years, SD=3.35) than the younger adults (M=13.25 years, SD=1.55), t(18)=−2.28, p = .035.
2.2 Stimuli
Cue words were 125 nouns selected from the Clark and Paivio (2004) extended norms. All were high in Thorndike Lorge frequency (M = 1.55, SD= .42), imageability (M = 5.77, SD = .40) and concreteness (M = 6.86, SD= .29) to increase the likelihood that an event could be generated, and also so that all words could be used in all conditions in a counterbalanced design (i.e., only imageable words can be used in the control task; see below). The cue words were randomly divided into five lists of twenty-five words and Analyses of Variance (ANOVA) confirmed the lists did not differ significantly in frequency, F(4,120) = .26, p = .90, imageability F(4,120) = .56, p = .69 or concreteness F(4,120) = .33, p = .86. The word lists cycled through conditions (one list for past events, one for future events and three for the control task), and each participant was randomly assigned to a version.
2.3 Experimental tasks
The paradigm used here is a variation of that used in our previous study (Addis et al., 2007), with increased trial lengths as piloting revealed that older adults felt time-pressure when the trial length was 20 s as per our original paradigm (however, despite this increase, we focused our analyses on the initial 20 s). Prior to scanning, detailed instructions for each task and examples of specific events were provided. Participants completed 6 practice trials (2 for each condition) aloud, so that we could confirm task understanding and compliance. Discussion and feedback on task performance were provided to ensure task instructions were fully understood (i.e., what constituted a specific event) and participants did not undergo scanning unless it was ensured that this was the case. Moreover, participants were provided with reminders of the task instructions between each scanning run.
In this slow event-related design, participants completed 25 trials of each of 2 autobiographical tasks (past, future). Participants were instructed to generate temporally and contextually specific events, occurring over minutes or hours, but not more than one day (e.g., recalling a graduation day or special dinner; imagining a marriage proposal or upcoming anniversary). Future events had to be novel and plausible. Events were to be experienced from a field perspective (i.e., seeing the event from the perspective of being there) rather than an observer perspective (i.e., observing the self in the event from an external vantage point). Each trial began with the display of a cueing slide specifying the condition (remember or imagine an event), a time period (past or next few months) and a cue word for 28 s. Participants made a button press when an event was in mind, and continued to elaborate and flesh-out the event for the remainder of the 28 s.
Participants also completed 25 trials of a control task adapted from Addis et al. (2009). This task followed the same sequence as the autobiographical tasks, with a cueing slide displayed for 28 s that described the task (i.e., “create sentence, start with smallest”), followed by three cue words. Participants were required to order the three objects named by the cue words in terms of physical size (i.e., a size-discrimination task), and insert them into the following sentence: “X is smaller than Y is smaller than Z”. Thus, this control task construction phase required both the retrieval of information about the objects in order to make the size judgment and the manipulation and integration of this information into a sentence. Once subjects had silently said the sentence to themselves, they made a button-press, and for the remainder of the 28s they engaged in a semantic elaboration task (focusing on the semantic definition of each of the three words). Requiring the generation of as much detail as possible about these nouns (e.g., their semantic meaning, function and visual features) meant the control elaboration phase was goal-directed in the same way as past and future elaboration. Therefore, by this design, the control task contained processes similar to those recruited during the autobiographical event tasks: retrieval, manipulation and integration of information during construction, a decision that the construction phase is over, making a response, and finally the generation of as much semantic or visuospatial detail as possible for the remainder of the elaboration phase. Each trial was followed by a rating scale for the amount of detail generated (1=vague; 5=vivid), displayed for 8 s. A fixation cross was then displayed for a jittered duration (M = 4 s; range 2–6 s).
2.4 Data Acquisition
Detailed anatomical data were collected on a 1.5T Siemens Avanto MRI scanner using a multiplanar rapidly acquired gradient echo (MP-RAGE) sequence. Functional images (25 coronal oblique slices, 5mm thick) were acquired at an angle perpendicular to the long axis of the hippocampus in an interleaved fashion using a T2*-weighted echo planar imaging (EPI) sequence (TR=2000ms, TE=23ms, FOV=200mm, flip angle=90°). Note that for one older adult, data from 178 s of one run (four trials) were lost due to a scanner malfunction. Cues were projected on a screen viewed on a mirror incorporated into the head-coil. E-Prime software (Psychology Software Tools, Inc., Pittsburgh) was used for the presentation and timing of stimuli and collection of response data. Responses were made on an MR-compatible five-button box.
2.5 Post-Scan Interview
Immediately following scanning, a detailed post-scan interview was conducted. Cues shown in past and future trials during scanning were presented and participants were asked to describe the event generated during scanning. The specificity of every event was then determined by the experimenter (Williams, Healy, & Ellis, 1999). Specific autobiographical events were considered to be unique events not lasting more than one day that involved the participant; any events that did not meet this definition (e.g., past events that had occurred more than once in the past; future events that had already occurred in the past; non-personal events) were removed from the analysis. To ensure that this classification was made reliably and without bias, approximately half of the events across both groups (640 events) were blinded for group membership, and classified by another experimenter blind to group membership (RPR) and an independent researcher blind to group membership and hypothesis (AH). Reliability was high for both of these raters when compared with the classifications made at the time of the post-scan interview (by DRA); the same classification was given on 93% and 95% of trials; and Cohen’s Kappa indicates good (.75) to very good (.84) agreement between raters (Altman, 1991). During the post-scan interview, participants dated each event (temporal distance), and used 5-point scales to rate the emotional intensity (1=detached; 5= highly emotional) and personal significance (1=insignificant; 5=highly significant) of each event.
2.6 Data Pre-processing
All pre-processing of imaging data was performed using SPM2 (Wellcome Department of Cognitive Neurology, London, UK). Standard preprocessing of functional images was performed, including discarding the first four functional images to allow scanner equilibrium effects, rigid-body motion correction and unwarping, slice timing correction, spatially normalization to the Montreal Neurological Institute (MNI) template (resampled at 4×4×4 mm3 voxels) and spatial smoothing (using an 8mm full-width half maximum isotropic Gaussian kernel).
2.7 Data Analysis
Data were analyzed using Spatiotemporal Partial Least Squares (ST-PLS), a multivariate technique that identifies whole brain patterns of activity correlating with experimental design (i.e., tasks, groups) across the length of an event (Addis, McIntosh, Moscovitch, Crawley, & McAndrews, 2004; Lin et al., 2003; Lobaugh, West, & McIntosh, 2001; McIntosh, Chau, & Protzner, 2004). PLS is a robustly validated (McIntosh, Bookstein, Haxby, & Grady, 1996; McIntosh et al., 2004) and widely used analysis technique in cognitive neuroscience, including the study of autobiographical memory (Addis et al., 2004; Addis, Pan, et al., 2009; Burianova & Grady, 2007; Burianova, McIntosh, & Grady, 2010; Spreng & Grady, 2010; Spreng, Stevens, Chamberlain, Gilmore, & Schacter, 2010). PLS has also been widely used to assess age-related changes in neural activity (e.g., Cabeza et al., 1997; Della-Maggiore et al., 2000; Grady, McIntosh, & Craik, 2003; Grady et al., 2010; Rajah & McIntosh, 2008; Stevens, Hasher, Chiew, & Grady, 2008). The use of this technique is particularly appropriate in aging studies because unlike univariate event-related analyses, ST-PLS is not dependent upon assumptions about the shape and time course of the hemodynamic response function (HRF), and thus can thus be used to examine neural differences between age-groups wherever they emerge across the duration of the trial. Moreover, ST-PLS typically has increased power relative to univariate approaches (Addis, Pan, et al., 2009; McIntosh et al., 2004; McIntosh & Lobaugh, 2004); as the entire spatio-temporal pattern (i.e., whole-brain patterns of activity across all TRs entered into the analysis) is assessed in one analytic step rather computing a series of voxelwise statistical tests, correction for multiple comparisons is not required.
For this analysis, we used the non-rotated version of task ST-PLS (Addis, Pan, et al., 2009; McIntosh & Lobaugh, 2004; Rajah & McIntosh, 2008), enabling us to specify a priori non-orthogonal contrasts (as opposed to the data-driven version of rotated ST-PLS that identifies orthogonal latent variables). In the current study, two contrasts (design matrices) were specified. The first contrast (“condition contrast”) examined whether both age groups engaged the core network when generating past and future events, and thus specified an effect of autobiographical (past and future) > control task for both groups. The second contrast (“interaction contrast”) examined the interaction of the autobiographical > control effect with age-group. Specifically, conditions were weighted to test for opposite condition effects in the two age groups (i.e., autobiographical > control tasks in young; control > autobiographical tasks in older). In this way, we were able to identify brain networks that showed a reduced autobiographical > control effect in older relative to younger adults, or a cross-over interaction (i.e., regions recruited by young adults for the control task and by older adults for the autobiographical tasks).
A data matrix – containing all of the voxels across the length of each event following the onset of each trial (specified as a 10 TR or 20 s temporal window), across all subjects in both groups and all tasks – was constructed. MR signals from all TRs were normalized within trials with respect to the signal at the onset of the trial. The resulting data matrix was then cross-correlated with the contrasts in the design matrix. The dot product of the contrasts with the data matrix was computed, resulting in a matrix of voxel saliences. The weighted value of a salience can be either positive to negative, depending on whether the voxel exhibits a positive or negative relation to the specified contrast of conditions. In other words, voxels in which activity (increases or decreases) is associated with a negatively-weighted condition(s) (and other voxels showing the same pattern) will have negatively weighted saliences.
For each a priori contrast, the non-rotated analysis produced a series of dot product images (one for each 2 s TR) that displays the relative increases and decreases in whole-brain activity related to the positively and negatively weighted conditions. Moreover, brain scores for each condition in each contrast for each subject were also derived; these scores are analogous to factor scores in a factor analysis, as they indicate how much of the spatiotemporal brain pattern is expressed by a subject within a condition. Brain scores can be used in a number of ways. Examination of average brain scores for each condition (mean centered across groups) with confidence intervals indicates how reliably each condition in each group contributes to the spatiotemporal pattern associated with the contrast (if the confidence interval crosses zero, a condition is considered to not contribute reliably to the pattern) and whether groups express the pattern to differing degrees (if the confidence intervals of the groups do not overlap). Moreover, examining average brain scores across the TRs comprising the event (temporal brains cores) enables identification of the TR(s) at which the differentiation between conditions is maximal.
The statistical significance of each non-rotated contrast was determined using permutation testing (500 permutations were computed), conducted using the sums of squares of the dot product images, which is equivalent to the ‘singular value’ – the amount of covariance accounted for by the contrast (McIntosh & Lobaugh, 2004). This procedure involved randomly re-ordering the data matrix rows, re-running the non-rotated analysis, and determining the new singular value for each re-ordering. Thus, significance reflects the probability based on the number of times the singular value from the permuted data exceeds the original singular value (McIntosh et al., 1996). A threshold of p ≤ .05 was used. Note that unlike univariate analyses, the significance of whole brain patterns of activity are determined in one single analytic step and thus correcting for multiple comparisons is not necessary.
The reliability of the voxel saliences was computed in an independent step using bootstrap estimation of the standard errors. This procedure involves randomly resampling subjects with replacement, and computing the standard error of the saliences after a number of bootstrap samples (McIntosh et al., 1996). In the present study, this sampling and analysis procedure was carried out 300 times. Clusters of 5 or more voxels in which bootstrap ratios were greater than ±3 (roughly equal to a z-score, and a p-value of p < .003), were considered to represent reliable voxels (Addis et al., 2004). Note that for the main effect of condition, the effect was so robust, most of these saliences survived a more conservative threshold of 4.0 (roughly equivalent to a p-value of p < .0001), and for brevity only these saliences are reported here (Addis et al., 2004). Confidence intervals (95%) for the mean brain scores (collapsed across all TR) in each condition and each group also were calculated from the bootstrap (see Figures 1 and 4).
Figure 1.
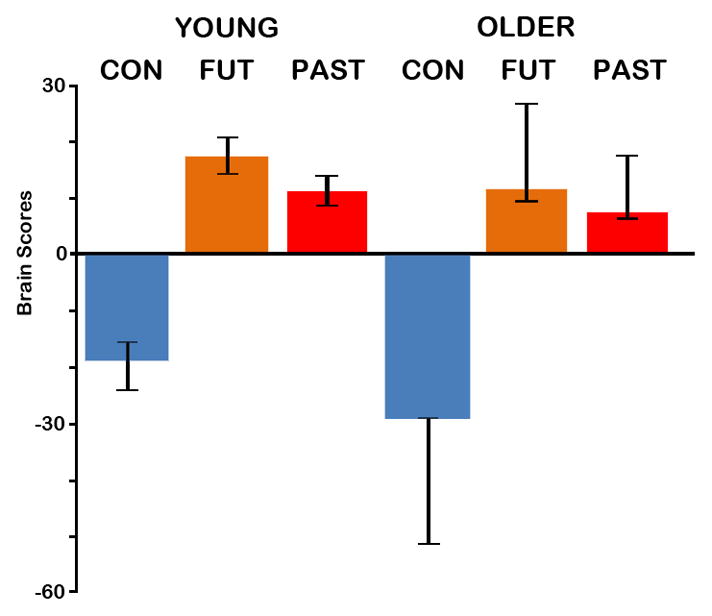
The average brain scores (mean-centered across groups) with 95% confidence intervals for control and autobiographical (past and future) tasks in young and older adults associated with the condition contrast (autobiographical > control tasks). Brain scores are a weighted average of activity across all voxels associated with particular conditions. This contrast was significant (p<.001). Con=Control task; Fut=Future task; Past=Past task.
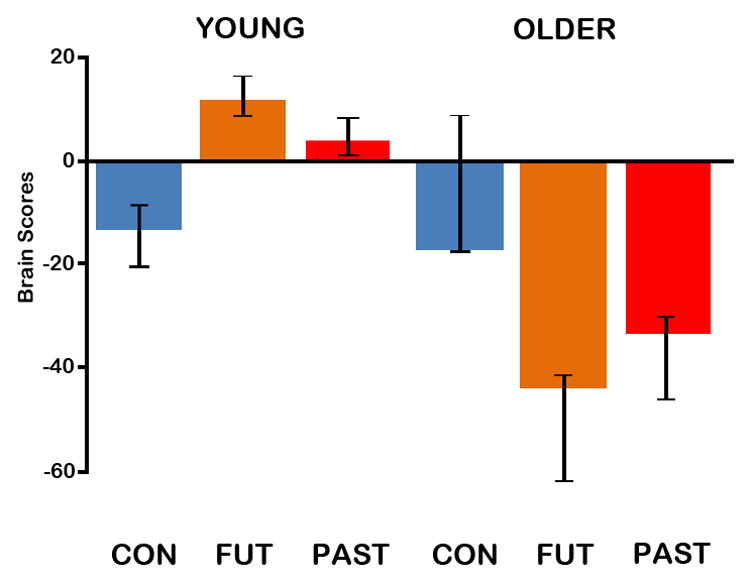
Figure 4.
The average brain scores (mean-centered across groups) with 95% confidence intervals for control and autobiographical (past and future) tasks in young and older adults associated with the interaction contrast (autobiographical > control tasks in young; control > autobiographical tasks in old). Brain scores are a weighted average of activity across all voxels associated with particular conditions. This contrast was significant (p = .05). Con=Control task; Fut=Future task; Past=Past task.
Follow-up behavior ST-PLS analyses were also computed. This form of ST-PLS analyzes the correlations between a behavioral measure and neural activity across the brain. In this case, three behavioral PLS analyses were computed, one each for different phenomenological ratings (detail, emotionality, personal significance). Data from the past and future conditions from both groups were entered into each of these analyses. Correlations across participants between activity in all brain voxels and behavior (ratings) for the past and future conditions within each condition for each group are computed (brain-behavior correlation matrix) and PLS is then used to contrast these correlations across conditions and groups. Resulting brain scores reflect the strength of the correlation between the phenomenological rating and the spatiotemporal pattern of activity. Voxel saliences indicate how strongly a particular voxel is associated with the behavior (rating) as well as other voxels exhibiting the same correlation. Statistical significance and reliability is assessed in the same way as task ST-PLS, described above. In this analysis, clusters of 5 or more voxels in which bootstrap ratios were greater than ±2 (roughly equal to a z-score, and a p-value of p < .045), are reported.
Temporal brain scores (average activation across the brain weighted by the contrast) were examined to identify the TR(s) in which the contrast was maximally evident (e.g., there was maximal differentiation between autobiographical and control tasks for the condition contrast). The peak voxel from each cluster (i.e., the voxel showing the highest bootstrap ratio) active during the maximal TRs are reported. HRFs from peak voxels of regions of interest evident at these TRs were extracted and plotted, to illustrate the effects indicated by the LVs.
3.0 Results
3.1 Trials entered into analyses
Only successfully completed trials were included in the analyses. For autobiographical events, this included trials on which participants successfully generated a specific autobiographical event, as indicated by collection of reaction time (RT) during scanning, participants describing the event during the post-scan interview, and the event being classified as specific. Additionally, trials for which the reaction time was excessively fast (i.e., within 3 s) were also excluded from analysis given that much slower RTs are typical for this task.
There was no group difference in the number of control trials contributed to the analyses (Young, M= 23.50, SD=3.94; Older, M=21.71, SD = 5.11; p = .31). However, older adults generated significantly fewer specific past and future events than younger adults, F(1,26) =42.77, p< .001. On average 27% (SD=14.81) of autobiographical events generated by older adults were generic in contrast to 1.62% (SD=1.65) of events generated by younger adults. Because this group difference in bin size could result in a bias in the analyses with older adults having just over 2/3 the number of autobiographical events than young adults, we randomly selected a subset of events for each younger participant to match a randomly selected older participant. The resulting bin sizes did not differ significantly across groups (Past: Young, M=14.92, SD=4.00, Older, M=14.71, SD=4.03; Future: Young, M=12.36, SD=5.67, Older, M=12.79, SD=5.55).
3.2 Behavioral Results
A mixed-model ANOVA of RT data (presented in Table 1) indicated there was no significant effect of condition (p = .09), and no significant effect of group (p = .12). However, there was a significant group x condition interaction, F(2,43) = 7.43, p = .003, reflecting significantly longer RTs for the control condition for older relative to younger adults.
Table 1.
Behavioral data according to age-group and condition
| Past | Future | Control | ||||
|---|---|---|---|---|---|---|
| Young | Old | Young | Old | Young | Old | |
| Reaction time (s)§ | 6.84 (1.62) | 7.24 (2.12) | 7.36 (2.40) | 7.41 (2.72) | 6.53 (1.40) | 9.35 (2.49) |
| Temporal distance (weeks) | 30.45 (20.37) | 75.53 (143.08) | 14.37 (6.33) | 17.83 (20.73) | n/a | n/a |
| Detail | 2.83 (0.45) | 2.81 (0.74) | 2.63 (0.39) | 2.73 (0.76) | n/a | n/a |
| Emotional intensity† | 2.19 (0.71) | 2.82 (0.57) | 2.49 (0.83) | 2.87 (0.54) | n/a | n/a |
| Personal significance† | 1.89 (0.70) | 2.48 (0.59) | 2.03 (0.78) | 2.66 (0.74) | n/a | n/a |
Note. Standard deviations are given in parentheses.
Significant group difference (p < .05)
Significant group x condition interaction (p < .01)
The estimated temporal distance, percentage of generic events and phenomenological ratings for the past and future trials are also presented in Table 1. For temporal distance, there was no significant effect of group (p=.24), condition (p=.06) or a significant group x condition interaction (p=.28). However, the high variance in temporal distance of past events for the older adults (see Table 1), and the trend towards a significant effect of condition, was largely attributable to three participants that had 1 or 2 past events that were over ten years from the present.
Consistent with a previous behavioral study (Addis et al., 2010) there was no age-related difference in subjective ratings of detail (p=.90). Older adults did, however, rate events as higher in emotional intensity, F(1,26)=4.48, p =.04, and personal significance, F(1,26)=6.16, p =.02, than did young adults. Although there were not significant main effects of condition (p values > .06), past events were rated as more detailed that future events, and future events rated as more emotional and significant than past events. No interaction effects were significant (p values > .18).
3.3 Non-Rotated ST-PLS – Condition Contrast
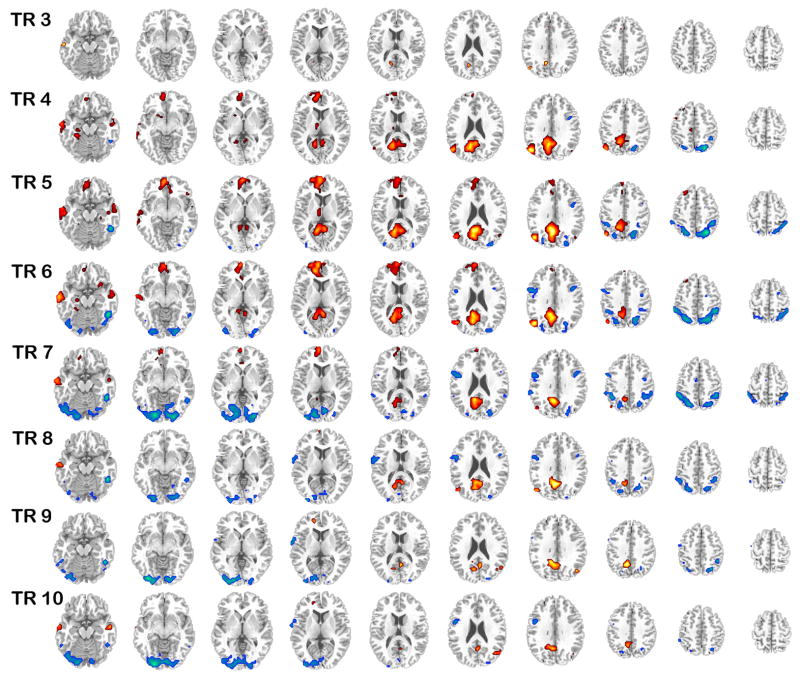
The contrast of autobiographical (past and future) tasks relative to the control task was significant, p < .001, explaining 30.49% of crossblock covariance. Examining the average brain scores with confidence intervals (Figure 1) confirmed that all conditions across both groups reliably contributed to this overall pattern, and there was no evidence of a group difference in the extent to which the groups expressed the autobiographical>control effect. Thus, the whole-brain patterns of activity associated with the autobiographical tasks and for the control task were evident in both groups to a similar degree. Examination of temporal brain scores indicated that the differentiation of autobiographical and control tasks was maximal during TRs 5 and 6, 8 – 12 s after task onset. This temporal pattern is also reflected in the brain activity associated with the autobiographical tasks occurring during TRs 5 and 6 in the majority of regions (as indicated in Figure 2).
Figure 2.
The brain regions identified by the condition contrast; activation associated with the autobiographical tasks (warm colors) and the control task (cool colors) is shown superimposed over a standard anatomical template and divided into 2 s TR (TRs 3 to 10 are shown). Images are thresholded using a bootstrap ratio of ±4 (equivalent to p < .0001).
The positive saliences associated with the autobiographical tasks are listed in Table 2 and shown in warm colors in Figures 2 and 3A. Young and older adults engaged a network of regions during both past and future events: bilateral medial parietal cortices (including posterior cingulate, precuneus and retrosplenial cortex extending into cuneus), lateral parietal cortex, medial temporal lobes including bilateral hippocampus and parahippocampal gyrus, lateral temporal cortex, and medial prefrontal cortex (including bilateral frontal poles). Examination of the HRFs extracted from regions comprising this network (see Table 2 for regions and coordinates; signal from selected regions are plotted in Figure 3A) illustrate the pattern identified by this LV: that both young and older adults show increased activity for autobiographical tasks relative to the control task. The brain scores also indicate that young adults recruit this network more strongly for future than past events, but older adults do not show this differentiation between the two autobiographical tasks. Although not evident in all regions, this future>past effect in the young is illustrated in the HRF data extracted from the precuneus.
Table 2.
Positive and negative saliences associated with the main effect of Condition
| Structure | BA | MNI | Peak BSR | Active TRs |
|---|---|---|---|---|
|
Positive Saliences: Autobiographical > Control Tasks
| ||||
| L Medial Frontal Gyrus † | 10/11 | −12 48 8 | 8.81 | 4,5,6*,7,8,9,10 |
| L Middle Frontal Gyrus | 8/9 | −24 32 48 | 6.16 | 4,5*,6 |
| R Inferior Frontal Gyrus | 47 | 48 28 −8 | 5.73 | 5* |
| R Inferior Frontal Gyrus | 47 | 32 16 −16 | 5.32 | 5* |
| L Inferior/Middle Temporal Gyrus | 21 | −64 −8 −20 | 8.89 | 3,4,5,6*,7,8,10 |
| R Middle Temporal Gyrus | 21 | 52 0 −28 | 10.49 | 4,5*,6,7,8,9,10 |
| L Thalamus | - | −8 −20 12 | 6.31 | 4,5* |
| L Hippocampus | - | −24 −20 −16 | 4.84 | 6* |
| L Parahippocampal Gyrus | 36 | −24 −40 −12 | 5.21 | 5*,6 |
| R Parahippocampal Gyrus ‡ | 36 | 28 −20 −24 | 5.34 | 4,5* |
| L Medial Parietal Cortex φ † | 23/29/30/31 | −4 −56 32 | 12.73 | 3,4,5*,6,7,8,9,10 |
| L Angular Gyrus | 39 | −48 −68 32 | 12.85 | 3,4*,5,6,7,8 |
| R Angular Gyrus | 39 | 44 −68 32 | 5.87 | 4,5,9*,10 |
| L Cerebellum | - | −24 −36 −20 | 4.98 | 6* |
| R Cerebellum | - | 48 −64 −44 | 6.24 | 4,5*,6 |
|
Negative Saliences: Control > Autobiographical Tasks
| ||||
| L Inferior Frontal Gyrus | 44 | −56 8 36 | −6.79 | 6*,7,8,9,10 |
| R Inferior Frontal Gyrus | 44 | 44 8 32 | −5.98 | 4,5,6*,7,8 |
| R Middle Frontal Gyrus | 6 | 24 −4 56 | −5.68 | 5,6* |
| R Inferior Temporal Gyrus | 20/37 | 52 −48 −16 | −9.22 | 4,5,6*,7,8,9,10 |
| R Inferior Parietal Lobule | 40 | 44 −52 56 | −8.36 | 4,5,6*,7,8,9 |
| L Superior Parietal Lobule | 7 | −24 −68 48 | −7.66 | 4,5,6*,7,8,9,10 |
| R Superior Parietal Lobule | 7 | 32 −68 48 | −8.84 | 4,5*,6,7,8,9,10 |
| L Middle Occipital Gyrus | 18 | −28 −92 20 | −4.22 | 6* |
| R Superior Occipital Gyrus | 19 | 32 −88 24 | −5.40 | 5* |
| L Cerebellum | - | −32 −72 −24 | −7.34 | 6,7,8,9,10* |
Note. Only clusters evident at least during TR5 and TR6 are shown here. For each cluster, the TRs of activation are noted, and the peak of activation (from which the bootstrap ratio and coordinates are taken) is indicated by an asterisk. Bootstrap ratios were greater than ±4 (roughly equivalent to a p-value of p < .0001), and had a spatial extent of at least 5 voxels (4 × 4 × 4 mm3). BA = Brodmann area; BSR = Bootstrap ratio; L = left; R = right;
Extends into right hippocampus;
Extends into precuneus, posterior cingulate, retrosplenial cortex;
Extends bilaterally.
Figure 3.
HRF plots (percent signal change) extracted from regions identified by the condition contrast associated with (A) the autobiographical tasks (left medial parietal cortex, xyz = −4 −56 32; left medial prefrontal cortex, xyz = −12 48 −8; right hippocampus, xyz = 28 −16 −20) and (B) the control task (left superior parietal lobule, xyz = −24 −68 48; right superior parietal lobule, xyz = 32 −68 48; right inferior temporal gyrus, xyz = 52 −48 16). Images of activation are superimposed over a standard anatomical template and thresholded using a bootstrap ratio of ±4 (equivalent to p < .0001). The crosshair indicates the location of this peak voxel, and the grey bar on the plot indicates the TR from which these peak voxels were identified (TR 5). BA = Brodmann area; L = left; R = right.
A distinct network of regions, including aspects of the dorsal attention network, was recruited by the control task relative to the autobiographical tasks, and again this pattern was evident in both young and older adults. The negative saliences associated with the control task are listed in Table 2 and shown in cool colors in Figure 3B. This pattern included primarily posterior visuospatial and semantic regions, such as bilateral middle occipital gyri, cuneus, inferior and superior parietal lobule, and bilateral inferior temporal gyri, as well as right middle frontal gyrus. The brain scores suggest that the older adults express this pattern more strongly than young adults (as evidenced by non-overlapping confidence intervals for the young and old in the control condition), and indeed, some of these regions were certainly more strongly engaged by older than younger adults (e.g., right inferior temporal gyrus).
Notably, despite the fact that the mean RT for the control condition was significantly slowed in older relative to younger adults (by approximately 2.8 s), there did not appear to be any delay in the older adult’s HRF in the control condition relative to young adults (Figure 3B). Even so, it is still possible that the delayed RT could have influenced the current results. According to McKiernan et al. (2006), increasing task difficulty is associated with increasing deactivations, including some regions of the core (default) network. If the slight delay in older adults’ RT reflects an increase in task difficulty, it is possible that the HRF associated with the control task in older adults is artifactually decreased, thereby inflating the neural distinction in the core network between the autobiographical and control tasks. In effect, this could result in the neural activity of older adults mapping more closely onto that of younger adults. It is notable that in many regions, such as the frontal poles and precuneus, older adults are actually showing increased activation in response to the control task, suggesting that artifactual deactivation is not underlying the autobiographical > control task effect in this group, at least not in all regions. However, to more definitively rule out this possibility, we correlated brains scores for the control condition with average control task RTs for each subject (Grady et al., 2003). Correlations were run collapsed across group, and separately for each group, but none of these correlations were significant (both groups, r = −0.20; p = 0.31; young group, r = −0.38, p = 0.18; older group, r = 0.09, p=0.77). Moreover, we confirmed that RT did not correlate with control task activity in medial temporal regions of interest for young (all p values > .271) or older (all p values > .637) groups.
3.4 Non-Rotated ST-PLS – Interaction Contrast
The interaction of the autobiographical > control effect with age-group was significant as determined by permutation tests (p = .05), accounting for 23.82% of the crossblock covariance. As the average brain scores with confidence intervals show (Figure 4), both groups reliably contributed to this interaction pattern. Although the brain scores show that the control condition in older adults does not contribute to this pattern, critically this LV indicates that young and older adults engage different regions during the past and future tasks. This interaction was evident in two ways: (1) there were regions recruited by younger adults during autobiographical events that were not recruited by older adults; and (2) during the autobiographical tasks, older adults engaged regions that were activated by young adults, but during the control task. Interestingly, these interaction patterns emerged at different time points.
Within the temporal window of the main effect of condition (TRs 4 to 6), the interaction effect for positive saliences was maximal, peaking at TR 5. These positive saliences (listed in Table 3 and shown in warm colors in Figures 5 and 6A) were associated with regions engaged by the young (but not older) adults for the autobiographical tasks relative to the control tasks, including bilateral precuneus, hippocampus, caudate and thalamus, left parahippocampal gyrus, middle temporal, angular and middle frontal gyri. As is evident in the HRF data included in Figure 6A to illustrate this effect, young adults showed an autobiographical >control task effect in these regions, while older adults show much less, if any, differentiation between these tasks. Additionally, the brain scores indicate that young adults engage these regions to a greater degree during the future versus past task. Although this pattern is not clearly evident in the medial temporal HRF data shown in Figure 6A, it is evident in other regions including the precuneus (as shown in Figure 3).
Table 3.
Positive and negative saliences associated with the Interaction effect
| Structure | BA | MNI | Peak BSR | Active TRs |
|---|---|---|---|---|
|
Positive saliences: Autobiographical > Control effect greater in young than old
| ||||
| L Middle Frontal Gyrus | 6 | −32 8 64 | 5.98 | 4,5,6*,7 |
| L Globus Pallidus | - | −12 8 −4 | 4.08 | 9* |
| L Caudate† | - | −8 4 −8 | 5.37 | 4,5*,6 |
| L Caudate | - | −20 −24 20 | 5.43 | 4*,5 |
| L Thalamus† | - | −4 −8 12 | 5.63 | 3,4,5* |
| L Hippocampus | - | −28 −24 −12 | 4.61 | 4*,5 |
| R Hippocampus | - | 24 −28 −12 | 4.05 | 5* |
| L Parahippocampal/Fusiform Gyrus | 37 | −32 −40 −12 | 4.60 | 5*,6 |
| L Parahippocampal Gyrus | 18 | −20 −52 0 | 6.20 | 4*,5,6,7 |
| L Middle Temporal Gyrus | 21 | −68 −36 −8 | 3.64 | 5* |
| L Precuneus† | 7 | −8 −60 44 | 6.18 | 3,4,5,6,7,8,9*,10 |
| L Angular Gyrus | 39 | −44 −68 28 | 6.07 | 3,4*,5,6,7,8,9,10 |
| L Cerebellum | - | −44 −60 −28 | 5.32 | 4*,5 |
| R Cerebellum | - | 36 −52 −24 | 4.87 | 4*,5 |
|
Negative saliences: Autobiographical > Control effect greater in old than young
| ||||
| R Middle Frontal Gyrus | 10 | 44 56 0 | −3.54 | 9* |
| R Inferior Frontal Gyrus | 44 | 52 8 20 | −5.09 | 5,6,7,8*,9,10 |
| R Claustrum | - | 28 20 4 | −4.62 | 8,9*,10 |
| R Uncus | 20 | 36 −4 −36 | −4.17 | 9*,10 |
| R Hippocampus | - | 32 −8 −24 | −3.94 | 9* |
| R Parahippocampal Gyrus | 19 | 24 −52 −8 | −3.17 | 9* |
| R Postcentral Gyrus | 2 | 56 −28 36 | −5.17 | 4,5,6* |
| B Paracentral Lobule | 5 | 0 −36 56 | −4.31 | 8*,9 |
| L Superior Temporal Gyrus | 22 | −60 −20 8 | −4.37 | 8*,9 |
| R Superior Temporal Gyrus | 13 | 56 −20 0 | −4.44 | 9*,10 |
| R Superior Temporal Gyrus | 42 | 52 −36 16 | −4.09 | 9*,10 |
| R Middle Temporal Gyrus | 37 | 48 −68 4 | −3.84 | 9* |
| L Inferior Temporal Gyrus | 20 | −40 0 −32 | −3.92 | 8,9*,10 |
| R Inferior Temporal Gyrus | 37 | 64 −52 −12 | −5.62 | 5,6,7*,8,9 |
| L Inferior Parietal Lobule | 40 | −64 −24 28 | −3.24 | 6,7*,9 |
| R Inferior Parietal Lobule | 40 | 44 −36 56 | −5.10 | 6,7,8,9*,10 |
| R Inferior Parietal Lobule | 40 | 52 −28 24 | −3.80 | 9* |
| L Superior Parietal Lobule | 7 | −16 −68 40 | −4.94 | 6,7,8,9* |
| R Superior Parietal Lobule | 7 | 32 −60 56 | −4.99 | 5,6*,7,8,9,10 |
| R Precuneus | 31 | 8 −76 20 | −4.46 | 6,7,8*,9 |
| B Cuneus/Lingual Gyrus | 18 | 0 −60 8 | −3.95 | 9* |
| R Lingual Gyrus | 18 | 20 −92 −12 | −3.57 | 9* |
| R Lingual Gyrus | 19 | 16 −72 −4 | −3.54 | 9* |
| L Fusiform Gyrus | 19 | −24 −84 −20 | −3.98 | 9*,10 |
| L Inferior Occipital Gyrus | 18 | −48 −76 −4 | −3.48 | 6*,7,8,9,10 |
| R Cerebellum | - | 12 −56 −28 | −5.38 | 5,6,7,8,9*,10 |
Note. All clusters reported were active during TR5 and TR9, though these regions may have been active for more TRs (listed) and activation may have peaked outside these TRs of interest. The peak of activation (from which the bootstrap ratio and coordinates are taken) is indicated by an asterisk. Bootstrap ratios were greater than ±3 (roughly equivalent to a p-value of p < .003), and had a spatial extent of at least 5 voxels (4 × 4 × 4 mm3). BA = Brodmann area; BSR = Bootstrap ratio; L = left; R = right.
Extends bilaterally.
Figure 5.
The brain regions identified by the interaction; activation associated with an autobiographical>control task effect in young but not old (warm colors) and an autobiographical>control task in old but not young (cool colors) is shown superimposed over a standard anatomical template and divided into 2 s TR (TRs 3 to 10 are shown). Images are thresholded using a bootstrap ratio of ±3 (equivalent to p < .003).
Figure 6.
HRF plots (percent signal change) extracted from regions identified by the interaction contrast associated with (A) an autobiographical>control task effect in young but not old during TR 5 (left hippocampus, xyz = −28 −24 −12; right hippocampus, xyz = 24 −28 −12; left parahippocampal gyrus, xyz = −32 −40 −12) and (B) an autobiographical>control task effect in old than young during TR 9 (left temporal pole, xyz = −40 0 −32; right superior temporal gyrus, xyz = 52 −36 16; right hippocampus, xyz = 32 −8 −24). Images of activation are superimposed over a standard anatomical template and thresholded using a bootstrap ratio of ±3 (equivalent to p < .003). The crosshair indicates the location of this peak voxel, and the grey bar on the plot indicates the TR from which these peak voxels were identified (TR 5 or TR 9). BA = Brodmann area; L = left; R = right.
The negative saliences (listed in Table 3 and shown in cool colors in Figures 5 and 6B) were associated primarily with regions in which older adults activated for the autobiographical tasks relative to the control task. In contrast, young adults either showed a reduced effect, no effect, or the opposite (control > autobiographical) effect in these regions (see Figure 6B for HRF data illustrating these different patterns). This particular interaction effect was evident later in the trial during TRs 6 to 10 (12–20 s after task onset) and peaking at TR 9. Thus, this effect mapped onto the elaboration phase of the trial (approximately 6 to 14 s after the average RT marking the beginning of elaboration). Regions with negative saliences included bilateral posterior visuospatial regions (e.g., inferior occipital, lingual and fusiform gyri, cuneus), lateral temporal and parietal cortices, right medial temporal lobe (including right hippocampus, parahippocampus gyrus and uncus) and right middle/inferior frontal gyrus. The brain scores indicate that, like young adults, older adults engage their network more for future than past events, although this trend did not reach significance (as the error bars are overlap to a degree). However, this future>past pattern is evident in the older adults’ HRF data plotted in Figure 6B.
3.5 Phenomenological Ratings and Neural Activity
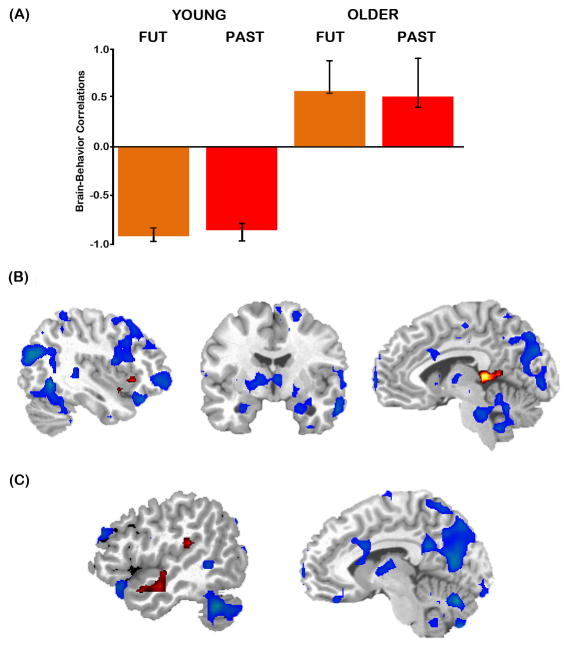
In order to determine whether the observed age-differences in neural activity were related to differences in the phenomenology of past and future autobiographical events, we conducted a series of follow-up behavioral PLS analyses. The behavioral PLS analyses for emotionality and personal significance ratings were not significant. However, the behavioral PLS analysis for detail resulted in a significant LV (p=.006), explaining 41.39% of the crossblock covariance. This LV indicated that while the regions associated with detail ratings did not differ across autobiographical event type (i.e., past and future), they differed significantly between young and older adults. Specifically, detail ratings were associated with one set of regions in young adults (past detail, r = −.85; future detail, r = −.92; note these regions were assigned negative saliences, thus negative correlations reflect a positive correlation of activity with detail), and a distinct set of regions in older adults (past detail, r = .51; future detail, r = .57; see Figure 7A).
Figure 7.
Results of the behavioral PLS analysis for detail. (A) The brain-behavior LV indicated that the regions correlated with past and future detail ratings differed significantly between young and older adults. (B) Regions in which activity correlated with detail ratings are shown in blue for young adults and red for older adults. At TR 5, regions correlated with detail in young included right lateral prefrontal and parietal cortex (left), bilateral hippocampus and right lateral temporal cortex (middle), and left medial parietal cortex and cerebellum (right). In older adults, detail correlated with insula (left) and retrosplenial cortex (right). (C) At TR 9, brain regions correlated with detail included left lateral temporal cortex for older adults and cerebellum for young adults (left) and left medial parietal cortex for young adults (right). Images of activation are superimposed over a standard anatomical template and thresholded using a bootstrap ratio of ±2 (equivalent to p < .045).
For brevity, we report patterns that emerged in TRs 5 and 9, given the aim of this behavioral PLS analysis was to explore whether the interaction pattern that emerged in these TRs was related to detail (see Table 4). At TR 5 (Figure 7B), young adults exhibited positive correlations between detail ratings for past and future events and activation in an extensive network of regions, including right superior parietal lobule, right temporal pole, left lateral temporal cortex, and left middle and inferior frontal gyri. Notably the clusters reported in Table 4 extended into a number of regions relevant with respect to episodic detail: bilateral hippocampus (xyz = −20 −32 −4; 20 −36 −4), right precuneus (xyz =4 −60 44) and cuneus (xyz =8 −76 20). However, for older adults, detail ratings were correlated with a distinct and more limited set of regions during TR 5: left inferior frontal gyrus, left thalamus (extending into retrosplenial cortex), left temporal pole, right insula and right cerebellum. A similar pattern emerged at TR 9 (Figure 7C), with detail ratings in young adults again correlating with activity in regions including medial parietal cortex (e.g., retrosplenial cortex extending into the precuneus), cuneus, medial temporal lobes (left parahippocampal gyrus, uncus), left lateral temporal cortex, and left middle frontal gyrus. In contrast, older adults continued to exhibit correlations in a more limited set of regions, such as left anterolateral temporal cortex, right insula and cerebellum.
Table 4.
Positive and negative saliences associated with the behavior PLS for detail
| TR | Structure | BA | MNI | Peak BSR |
|---|---|---|---|---|
| 5 | Positive saliences: Detail correlations in older adults | |||
|
| ||||
| L Inferior Frontal Gyrus | 44 | −60 12 12 | 4.30 | |
| L Thalamus†φ | - | −4 −32 4 | 4.26 | |
| R Cerebellum | - | 56 −60 −32 | 3.57 | |
| R Insula | 13 | 44 4 −8 | 3.30 | |
| L Superior Temporal Gyrus | 22 | −48 −4 0 | 3.17 | |
| 5 | Negative saliences: Detail correlations in young adults | |||
|
| ||||
| R Superior Parietal Lobule†‡Δ | 7 | 8 −68 60 | −8.44 | |
| R Superior Temporal Gyrus | 38 | 56 12 −24 | −7.14 | |
| R Superior Frontal Gyrus | 8 | 28 40 44 | −5.80 | |
| L Cerebellum | - | −28 −48 −52 | −5.06 | |
| L Superior Temporal Gyrus | 39 | −44 −52 4 | −4.81 | |
| L Middle Frontal Gyrus | 10 | −44 44 24 | −4.26 | |
| L Superior Temporal Gyrus | 22 | −60 −16 0 | −3.54 | |
| L Superior Temporal Gyrus | 38 | −32 16 −32 | −3.52 | |
| R Cerebellum | - | 16 −80 −52 | −3.33 | |
| L Middle Frontal Gyrus | 6 | −24 0 56 | −3.14 | |
| L Inferior Frontal Gyrus | 44 | −44 4 24 | −3.12 | |
| R Medial Frontal Gyrus | 8 | 8 36 48 | −3.06 | |
| L Middle Temporal Gyrus | 37 | −48 −68 4 | −2.89 | |
| L Precentral Gyrus | 6 | −60 0 8 | −2.87 | |
| L Fusiform Gyrus | 37 | −44 −44 −20 | −2.60 | |
| 9 | Positive saliences: Detail correlations in older adults | |||
|
| ||||
| L Inferior Temporal Gyrus | 20 | −48 −4 −36 | 3.44 | |
| L Inferior/Middle Temporal Gyrus | 20/21 | −44 −8 −16 | 3.29 | |
| R Insula | 13 | 44 0 −4 | 2.41 | |
| L Cerebellum | - | −12 −40 −12 | 2.41 | |
| 9 | Negative saliences: Detail correlations in young adults | |||
|
| ||||
| R Inferior Temporal Gyrus | 21 | 68 −12 −20 | −7.53 | |
| R Posterior Cingulate †Δφ | 23 | 4 −64 16 | −6.47 | |
| R Superior Frontal Gyrus | 6 | 4 4 60 | −6.46 | |
| R Middle Frontal Gyrus | 47 | 48 48 −8 | −5.77 | |
| L Middle Frontal Gyrus | 8 | −40 24 52 | −3.97 | |
| R Inferior Parietal Lobule | 40 | 56 −60 40 | −3.68 | |
| L Middle Frontal Gyrus | 46 | −48 40 28 | −3.55 | |
| R Precentral Gyrus | 6 | 60 −12 44 | −3.53 | |
| L Inferior Temporal Gyrus | 20 | −64 −12 −28 | −3.43 | |
| L Parahippocampal Gyrus | 36 | −28 −24 −28 | −3.02 | |
| L Medial Frontal Gyrus | 10 | −4 64 28 | −2.93 | |
| L Uncus | 28 | −16 −4 −32 | −2.89 | |
| L Hippocampus | - | −28 −28 −8 | −2.80 | |
| L Middle Occipital Gyrus | 19 | −48 −80 16 | −2.77 | |
| L Cerebellum | - | −4 −40 −28 | −2.73 | |
| R Cerebellum | - | 20 −32 −52 | −2.72 | |
| L Cuneus | 18 | −20 −96 12 | −2.63 | |
| R Putamen | - | 28 0 0 | −2.53 | |
| L Precentral Gyrus | 4 | −28 −24 72 | −2.50 | |
| R Superior Frontal Gyrus | 9 | 8 56 40 | −2.46 | |
| L Superior Parietal Lobule | 7 | −32 −56 48 | −2.44 | |
Note. All clusters listed here were active during TR5 and TR9, and for each cluster, the bootstrap ratio and coordinates from the peak voxel are reported. Bootstrap ratios were greater than ±2 (roughly equivalent to a p-value of p < .045), and clusters had a spatial extent of at least 5 voxels (4 × 4 × 4 mm3). BA = Brodmann area; BSR = Bootstrap ratio; L = left; R = right.
Extends bilaterally;
Extends into retrosplenial cortex;
Extends into cuneus and precuneus;
Extends into the hippocampus.
4.0 Discussion
Our results indicate that when remembering and imagining, older adults activate many of the same regions evident in young adults (Addis, Pan, et al., 2009; Addis, Wong, et al., 2007; Botzung et al., 2008; Hassabis, Kumaran, & Maguire, 2007; Okuda et al., 2003; Szpunar et al., 2007; Weiler et al., 2010a). The network common to both age groups included regions comprising the core network: bilateral medial and lateral parietal cortices, medial temporal (including hippocampus) and lateral temporal lobes, and frontopolar cortex. Recruitment of this network by older adults is broadly consistent with previous findings that the past and future events generated by older adults are not completely devoid of episodic detail: although older adults show a significant reduction in episodic content relative to younger adults, they do not perform at floor levels, generating approximately 30 to 40 episodic details per event (for a review, see Schacter, Gaesser, & Addis, 2010).
Despite common engagement of this network by both young and older adults in this study, and previous observations that the network engaged by older adults during remembering and imagining is qualitatively similar to that evident in young adults (Viard et al., 2011), our interaction analysis revealed that neural activity was not equivalent across age-groups in all regions. Moreover, by combining spatiotemporal partial least squares with a paradigm requiring the active construction of past and future events from generic cues, we were able to assess whether group differences in whole brain patterns of covariance emerged at particular times during the construction and elaboration process.
During construction, the interaction effect reflected a set of regions in which young adults exhibited an autobiographical >control task effect, while older adults showed reduced or no differentiation between these tasks. This age difference was evident in regions mediating episodic imagery, detail and the contextual content of autobiographical events in young adults: the hippocampus (Addis and Schacter, 2008), parahippocampal cortex (Szpunar, Chan, & McDermott, 2009) and the precuneus (Fletcher et al., 1995; Hassabis, Kumaran, & Maguire, 2007). In line with this finding, we also found that detail ratings correlated with activity in these regions in young, but not older, adults. Instead, detail ratings from older adults correlated with construction-related activity in left inferior frontal gyrus, left thalamus (extending into retrosplenial cortex), left temporal pole and the right insula. Taken together, these findings suggest that during construction, functional changes in precuneus and medial temporal regions may be related to age-related decreases in the episodic qualities of autobiographical events (Addis et al., 2008), and that the detail generated by older adults relies on a distinct set of regions.
With respect to the hippocampus, although this age-related decrease contrasts with previous findings of hippocampal activity in older adults during both past event retrieval (Maguire and Frith, 2003; St. Jacques et al., in press; Viard et al., 2007) and future event simulation (Viard et al., 2011), this apparent inconsistency likely reflects methodological differences. The current paradigm required participants to actively construct past and future events from generic cues, rather than retrieve events associated with personalized stimuli as in these other studies (Maguire and Frith, 2003; Viard et al., 2007, 2011). The provision of personal cues reduces the need for construction– a notable methodological difference, given that in the current study, age-related reductions in hippocampal activity emerged early during the construction phase.
Despite showing decreased engagement of some regions in the core network during the autobiographical tasks, older adults also showed increased activation of a number of regions relative to younger adults. These effects emerged later in the trial, during the elaboration phase when participants were instructed to flesh out their generated event with as much detail as possible. There was also an indication both in the brain scores and the HRF data that older adults showed a future>past effect (albeit non-significant) in this network, an effect evident in the network recruited by young adults both in the current and other studies (see Addis et al., in press, for a discussion). Although non-significant, this pattern does suggest that even when a particular population recruits an alternate network to complete autobiographical event tasks, the future task still requires more activation of that network for task completion.
One of the most interesting findings was a late recruitment of the right hippocampus in older adults. Although young adults engaged the right hippocampus during construction, older adults differentially activated this region during elaboration. This difference in latency of activation cannot be explained by RT, as the groups did not differ in RT for the autobiographical tasks. It is also unlikely to reflect hemodynamic factors; it has been shown the hemodynamic response is not significantly delayed in older adults (Aizenstein et al., 2004; Huettel, Singerman, & McCarthy, 2001), and thus a lag of 8 seconds (4 TRs) seems an unlikely cause of this difference. A more plausible interpretation is that older adults exhibit differential hippocampal engagement during elaboration. Interestingly, this result is consistent with Maguire and Frith’s (2003) finding of an age-related increase in right hippocampal activity in an autobiographical task that did not require construction and thus can be considered to focus on elaboration. Therefore, the current analysis revealed that the stage of event generation (construction versus elaboration) is crucial to understanding these age-related differences in medial temporal activity, with age-related reductions during construction and age-related increases during elaboration. However, even though older adults engage the hippocampus during the autobiographical tasks, this activity did not correlate with detail ratings, in contrast to the correlations between construction-related hippocampal activity and detail in young adults.
Of particular note was the differential recruitment of lateral temporal regions by older relative to younger adults, including left temporal pole (BA 20) and right superior temporal gyrus (BA 22). In these regions, young adults either showed no differentiation between the autobiographical and control tasks, or more activity for the control task. Interestingly, a recent meta-analysis examining age-related changes in neural activation found that older adults showed a reliable increase in one of these same regions (left anterior BA 20) across 12 studies of memory retrieval (Spreng, Wojtowicz, & Grady, 2010). Thus, this region is likely recruited more by older than younger adults during mnemonic tasks such as retrieval of specific autobiographical memories, as well as tasks that draw on mnemonic processes, such as future simulation (Schacter & Addis, 2007).
Viard et al. (2011) found that in older adults, lateral temporal cortex was associated with the content of future events, which they argue reflects the semantic content of event representations. We expand this finding to show that older adults differentially engage these regions relative to younger adults, and that this effect is specific to the elaboration phase of autobiographical events. Moreover, older adults’ subjective detail ratings correlated with activity in lateral temporal regions. Given the association of this region with conceptual autobiographical information (Graham, Lee, Brett, & Patterson, 2003), these findings can be considered consistent with behavioral studies showing that older adults include more conceptual detail in descriptions of specific events (Addis et al., 2008). It also suggests that detail ratings by older adults may have been based on the conceptual content of their events, which may also explain why their detail ratings correlate with a set of regions distinct from young adults, even during construction. Interestingly, Burianova and colleagues (2010) observed that the network supporting both autobiographical and semantic memory included middle temporal gyrus and temporal pole, and argued that retrieval of semantic knowledge supported by these regions is important for all forms of declarative memory including autobiographical memory. In that study, which also used PLS, lateral temporal regions were functionally connected with the hippocampus. In the present PLS study, older adults showed a pattern of connectivity during future elaboration that involved the same regions: the right hippocampus and lateral temporal regions. However, further work is needed to directly link these neural changes with age-related changes in the type of details comprising autobiographical event representations.
These neural differences could also reflect, at least in part, a more general difference in the way older adults approach autobiographical tasks such as this. For instance, older adults rated their future simulations as more emotional (see also Addis et al., 2008, 2010) and personally significant than did younger adults. It is likely that there are also other ways in which older adults’ future thought differs qualitatively from their younger counterparts (see Schacter, et al., 2010, for more discussion of this issue). More research exploring such age-related changes – and linking them with neural changes – is also needed.
To conclude, although both young and older engage the same network during the construction of past and future events, there are some crucial differences. During the construction of autobiographical events, older adults show less activation relative to younger adults in regions supporting episodic detail, such as the medial temporal lobes and precuneus. However, later in the trial, older adults show differential recruitment of a network of medial and lateral temporal regions supporting the elaboration of autobiographical events, and possibly reflecting the more conceptual way in which older adults describe their pasts and their futures.
Highlights.
Young and older adults constructed and elaborated past and future autobiographical events during an fMRI scanning session.
Both age groups engaged a similar set of regions during the autobiographical tasks relative to the control task.
There was an age-related reduction in activity during construction, in regions such as the medial temporal lobes.
Later during elaboration, older adults showed increased recruitment of lateral and medial temporal regions relative to younger adults.
Acknowledgments
The authors gratefully acknowledge the assistance of B. Gaesser, A. Gilmore, A.C. Holland, J. Paxton, R. Musicaro and L. Pan with participant recruitment, data collection and scoring. A.R. McIntosh, A. Protzner, M.N. Rajah and R.N. Spreng are thanked for guidance on PLS analyses. We also thank anonymous reviewers for helpful suggestions. This work was supported by the National Institute on Aging at the Institutes of Health (grant number AG08441 to D.L.S.) and the Royal Society of New Zealand (grant number UOA0810 to D.R.A.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham A, Schubotz RI, von Cramon DY. Thinking about the future versus the past in personal and non-personal contexts. Brain Research. 2008;1233:106–119. doi: 10.1016/j.brainres.2008.07.084. [DOI] [PubMed] [Google Scholar]
- Addis DR, Cheng T, Roberts RP, Schacter DL. Hippocampal contributions to the episodic simulation of specific and general future events. Hippocampus. doi: 10.1002/hipo.20870. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, McIntosh AR, Moscovitch M, Crawley AP, McAndrews MP. Characterizing spatial and temporal features of autobiographical memory retrieval networks: a partial least squares approach. Neuroimage. 2004;23:1460–1471. doi: 10.1016/j.neuroimage.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Addis DR, Musicaro R, Pan L, Schacter DL. Episodic simulation of past and future events in older adults: Evidence from an experimental recombination task. Psychology and Aging. 2010;25:369–376. doi: 10.1037/a0017280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Pan L, Vu M, Laiser N, Schacter DL. Constructive episodic simulation of the future and the past: distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia. 2009;47:2222–2238. doi: 10.1016/j.neuropsychologia.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Addis DR, Schacter DL. Effects of detail and temporal distance of past and future events on the engagement of a common neural network. Hippocampus. 2008;18:227–237. doi: 10.1002/hipo.20405. [DOI] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Age-related changes in the episodic simulation of future events. Psychological Science. 2008;19:33–41. doi: 10.1111/j.1467-9280.2008.02043.x. [DOI] [PubMed] [Google Scholar]
- Altman DG. Practical Statistics for Medical Research. London: Chapman and Hall; 1991. [Google Scholar]
- Aizenstein HJ, Clark KA, Butters MA, Cochran J, Stenger VA, Meltzer CC, Reynolds CF, Carter CS. The BOLD hemodynamic response in healthy aging. Journal of Cognitive Neuroscience. 2004;16:786–793. doi: 10.1162/089892904970681. [DOI] [PubMed] [Google Scholar]
- Botzung A, Denkova E, Manning L. Experiencing past and future personal events: functional neuroimaging evidence on the neural bases of mental time travel. Brain and Cognition. 2008;66:202–212. doi: 10.1016/j.bandc.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends in Cognitive Sciences. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Burianova H, Grady CL. Common and unique neural activations in autobiographical, episodic, and semantic retrieval. Journal of Cognitive Neuroscience. 2007;19:1520–1534. doi: 10.1162/jocn.2007.19.9.1520. [DOI] [PubMed] [Google Scholar]
- Burianova H, McIntosh AR, Grady CL. A common functional brain network for autobiographical, episodic, and semantic memory retrieval. Neuroimage. 2010;49:865–874. doi: 10.1016/j.neuroimage.2009.08.066. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, Jennings JM, Houle S, Craik FIM. Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. Journal of Neuroscience. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, St Jacques P. Functional neuroimaging of autobiographical memory. Trends in Cognitive Sciences. 2007;11:219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Clark JM, Paivio A. Extensions of the Paivio, Yuille, and Madigan (1968) norms. Behavior Research Methods, Instruments, and Computers. 2004;36:371–383. doi: 10.3758/bf03195584. [DOI] [PubMed] [Google Scholar]
- Della-Maggiore V, Sekuler AB, Grady CL, Bennett PJ, Sekuler R, McIntosh AR. Corticolimbic interactions associated with performance on a short-term memory task are modified by age. Journal of Neuroscience. 2000;20:8410–8416. doi: 10.1523/JNEUROSCI.20-22-08410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RS, Dolan RJ. The mind’s eye--precuneus activation in memory-related imagery. Neuroimage. 1995;2:195–200. doi: 10.1006/nimg.1995.1025. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FIM. Age-related differences in the functional connectivity of the hippocampus during memory encoding. Hippocampus. 2003;13:572–586. doi: 10.1002/hipo.10114. [DOI] [PubMed] [Google Scholar]
- Grady CL, Protzner AB, Kovacevic N, Strother SC, Afshin-Pour B, Wojtowicz M, Anderson JAE, Churchill N, McIntosh AR. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cerebral Cortex. 2010;20:1432–1447. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham KS, Lee ACH, Brett M, Patterson K. The neural basis of autobiographical and semantic memory: new evidence from three PET studies. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:234–254. doi: 10.3758/cabn.3.3.234. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Maguire EA. Using imagination to understand the neural basis of episodic memory. Journal of Neuroscience. 2007;27:14365–14374. doi: 10.1523/JNEUROSCI.4549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Vann SD, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proceedings of the National Academy of Sciences USA. 2007;104:1726–1731. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends in Cognitive Sciences. 2007;11:299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Singerman JD, McCarthy G. The effects of aging upon the hemodynamic response measured by functional MRI. Neuroimage. 2001;13:161–175. doi: 10.1006/nimg.2000.0675. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Foley MA, Suengas AG, Raye CL. Phenomenal characteristics of memories for perceived and imagined autobiographical events. Journal of Experimental Psychology: General. 1988;117:371–376. [PubMed] [Google Scholar]
- Klein SB, Loftus J, Kihlstrom JF. Memory and temporal experience: The effects of episodic memory loss on an amnesic patient’s ability to remember the past and imagine the future. Social Cognition. 2002;20:353–379. [Google Scholar]
- Kwan D, Carson N, Addis DR, Rosenbaum RS. Deficits in past remembering extend to future imagining in a case of developmental amnesia. Neuropsychologia. 2010;48:3179–3186. doi: 10.1016/j.neuropsychologia.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychology and Aging. 2002;17:677–689. [PubMed] [Google Scholar]
- Lin F, McIntosh AR, Agnew JA, Eden GF, Zeffiro TA, Belliveau JW. Multivariate analysis of neuronal interactions in the generalized partial least squares framework: simulations and empirical studies. Neuroimage. 2003;20:625–642. doi: 10.1016/S1053-8119(03)00333-1. [DOI] [PubMed] [Google Scholar]
- Lobaugh NJ, West R, McIntosh AR. Spatiotemporal analysis of experimental differences in event-related potential data with partial least squares. Psychophysiology. 2001;38:517–530. doi: 10.1017/s0048577201991681. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frith CD. Aging affects the engagement of the hippocampus during autobiographical memory retrieval. Brain. 2003;126:1511–1523. doi: 10.1093/brain/awg157. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Vargha-Khadem F, Hassabis D. Imagining fictitious and future experiences: evidence from developmental amnesia. Neuropsychologia. 2010;48:3187–3192. doi: 10.1016/j.neuropsychologia.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR, Bookstein FL, Haxby JV, Grady CL. Spatial pattern analysis of functional brain images using partial least squares. Neuroimage. 1996;3:143–157. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Chau WK, Protzner AB. Spatiotemporal analysis of event-related fMRI data using partial least squares. Neuroimage. 2004;23:764–775. doi: 10.1016/j.neuroimage.2004.05.018. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Lobaugh NJ. Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage. 2004;23:S250–S263. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, D’Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: an fMRI investigation. Neuroimage. 2006;29:1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Raye CL, Ebner NC, Tubridy SM, Frankel H, Johnson MK. Age-group differences in medial cortex activity associated with thinking about self-relevant agendas. Psychology and Aging. 2009;24:438–449. doi: 10.1037/a0015181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, Suzuki K, Kawashima R, Fukuda H, Itoh M, Yamadori A. Thinking of the future and past: the roles of the frontal pole and the medial temporal lobes. Neuroimage. 2003;19:1369–1380. doi: 10.1016/s1053-8119(03)00179-4. [DOI] [PubMed] [Google Scholar]
- Rajah MN, McIntosh AR. Age-related differences in brain activity during verbal recency memory. Brain Research. 2008;1199:111–125. doi: 10.1016/j.brainres.2007.12.051. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. On the nature of medial temporal lobe contributions to the constructive simulation of future events. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:1245–1253. doi: 10.1098/rstb.2008.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nature Reviews Neuroscience. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: concepts, data, and applications. Annals of the New York Academy of Science. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Gaesser B, Addis DR. Age-related changes in the episodic simulation of past and future events. In: Benjamin AS, editor. Successful Remembering and Successful Forgetting: A Festschrift in Honor of Robert A Bjork. New York: Psychology Press; 2010. pp. 505–525. [Google Scholar]
- Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. Journal of Cognitive Neuroscience. 2010;22:1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Levine B. The temporal distribution of past and future autobiographical events across the lifespan. Memory and Cognition. 2006;34:1644–1651. doi: 10.3758/bf03195927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim ASN. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Wojtowicz M, Grady CL. Reliable differences in brain activity between young and old adults: a quantitative meta-analysis across multiple cognitive domains. Neuroscience and Biobehavioral Reviews. 2010;34:1178–1194. doi: 10.1016/j.neubiorev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Squire LR, van der Horst AS, McDuff SGR, Frascino JC, Hopkins RO, Mauldin KN. Role of the hippocampus in remembering the past and imagining the future. Proceedings of the National Academy of Sciences USA. 2010;107:19044–19048. doi: 10.1073/pnas.1014391107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Levine B. Ageing and autobiographical memory for emotional and neutral events. Memory. 2007;15:129–144. doi: 10.1080/09658210601119762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StJacques PL, Rubin DC, Cabeza R. Age-related effects on the neural correlates of autobiographical memory retrieval. Neurobiology of Aging. doi: 10.1016/j.neurobiolaging.2010.11.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens WD, Hasher L, Chiew KS, Grady CL. A neural mechanism underlying memory failure in older adults. Journal of Neuroscience. 2008;28:12820–12824. doi: 10.1523/JNEUROSCI.2622-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suddendorf T, Corballis MC. The evolution of foresight: What is mental time travel, and is it unique to humans? Behavioral and Brain Sciences. 2007;30:299–313. doi: 10.1017/S0140525X07001975. [DOI] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, Chan JCK, McDermott KB. Contextual processing in episodic future thought. Cerebral Cortex. 2009;19:1539–1548. doi: 10.1093/cercor/bhn191. [DOI] [PubMed] [Google Scholar]
- Szpunar KK, Watson JM, McDermott KB. Neural substrates of envisioning the future. Proceedings of the National Academy of Sciences USA. 2007;104:642–647. doi: 10.1073/pnas.0610082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK. Episodic Future Thought. Perspectives on Psychological Science. 2010;5:142 –162. doi: 10.1177/1745691610362350. [DOI] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychology. 1995;25:1–12. [Google Scholar]
- Viard A, Chételat G, Lebreton K, Desgranges B, Landeau B, de La Sayette V, Eustache F, Piolino P. Mental time travel into the past and the future in healthy aged adults: an fMRI study. Brain and Cognition. 2011;75:1–9. doi: 10.1016/j.bandc.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Viard A, Piolino P, Desgranges B, Chételat G, Lebreton K, Landeau B, Young A, De La Sayette V, Eustache F. Hippocampal activation for autobiographical memories over the entire lifetime in healthy aged subjects: an fMRI study. Cerebral Cortex. 2007;17:2453–2467. doi: 10.1093/cercor/bhl153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler JA, Suchan B, Daum I. Foreseeing the future: occurrence probability of imagined future events modulates hippocampal activation. Hippocampus. 2010a;20:685–690. doi: 10.1002/hipo.20695. [DOI] [PubMed] [Google Scholar]
- Weiler JA, Suchan B, Daum I. When the future becomes the past: Differences in brain activation patterns for episodic memory and episodic future thinking. Behavioural Brain Research. 2010b;212:196–203. doi: 10.1016/j.bbr.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Williams JM, Healy HG, Ellis NC. The effect of imageability and predicability of cues in autobiographical memory. Quarterly Journal of Experimental Psychology A. 1999;52:555–579. doi: 10.1080/713755828. [DOI] [PubMed] [Google Scholar]