Abstract
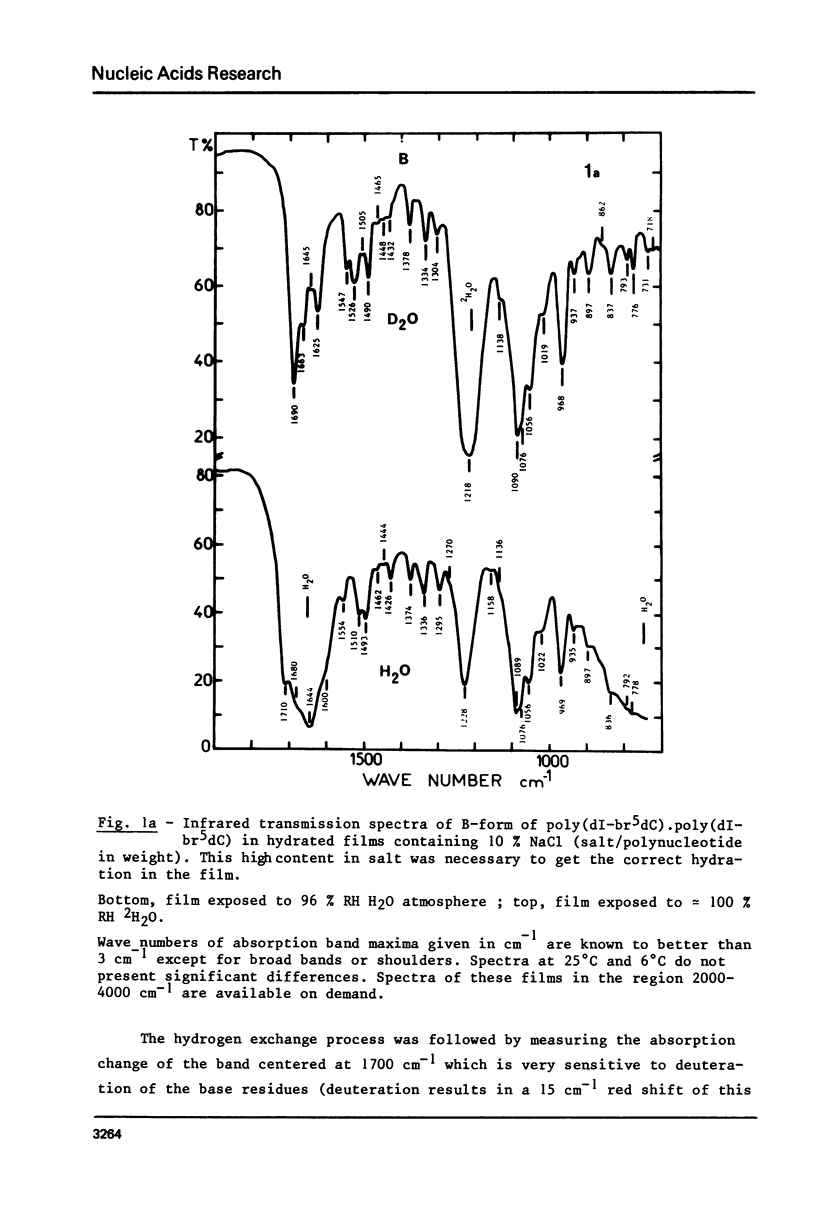
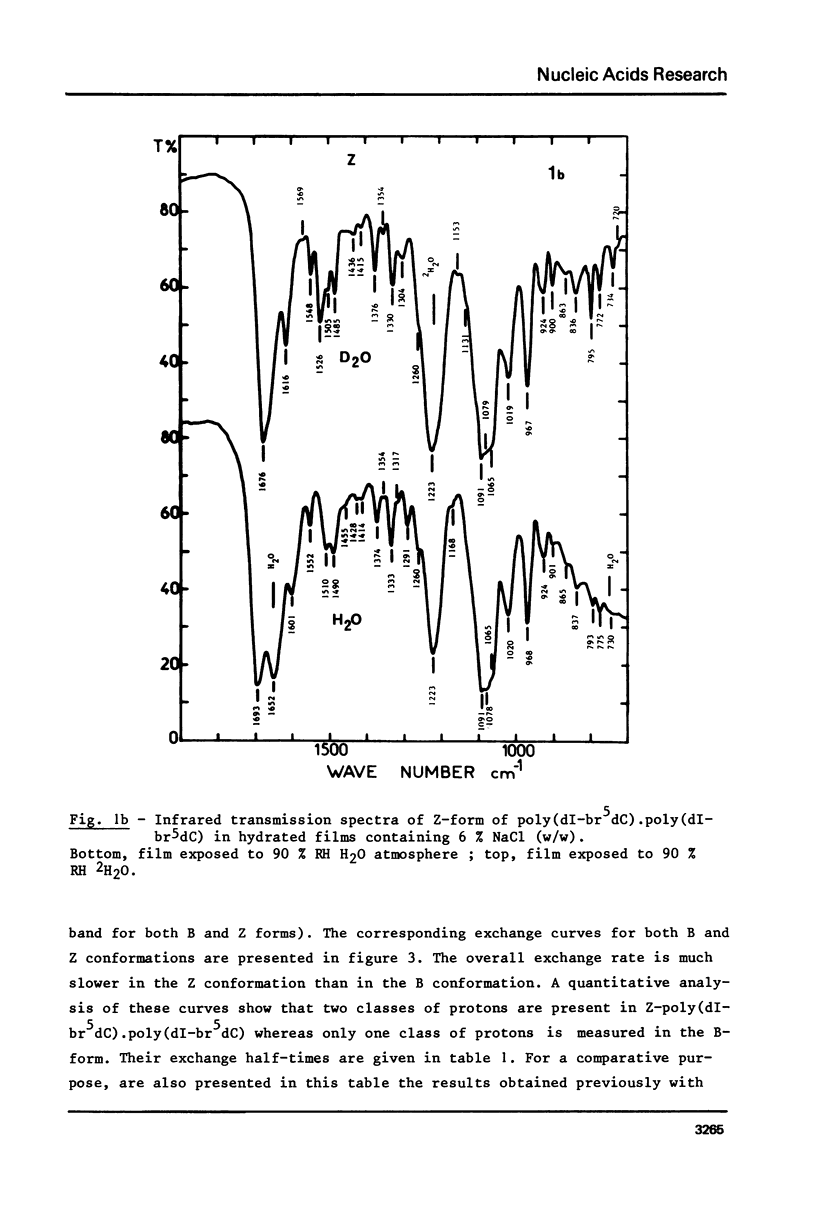
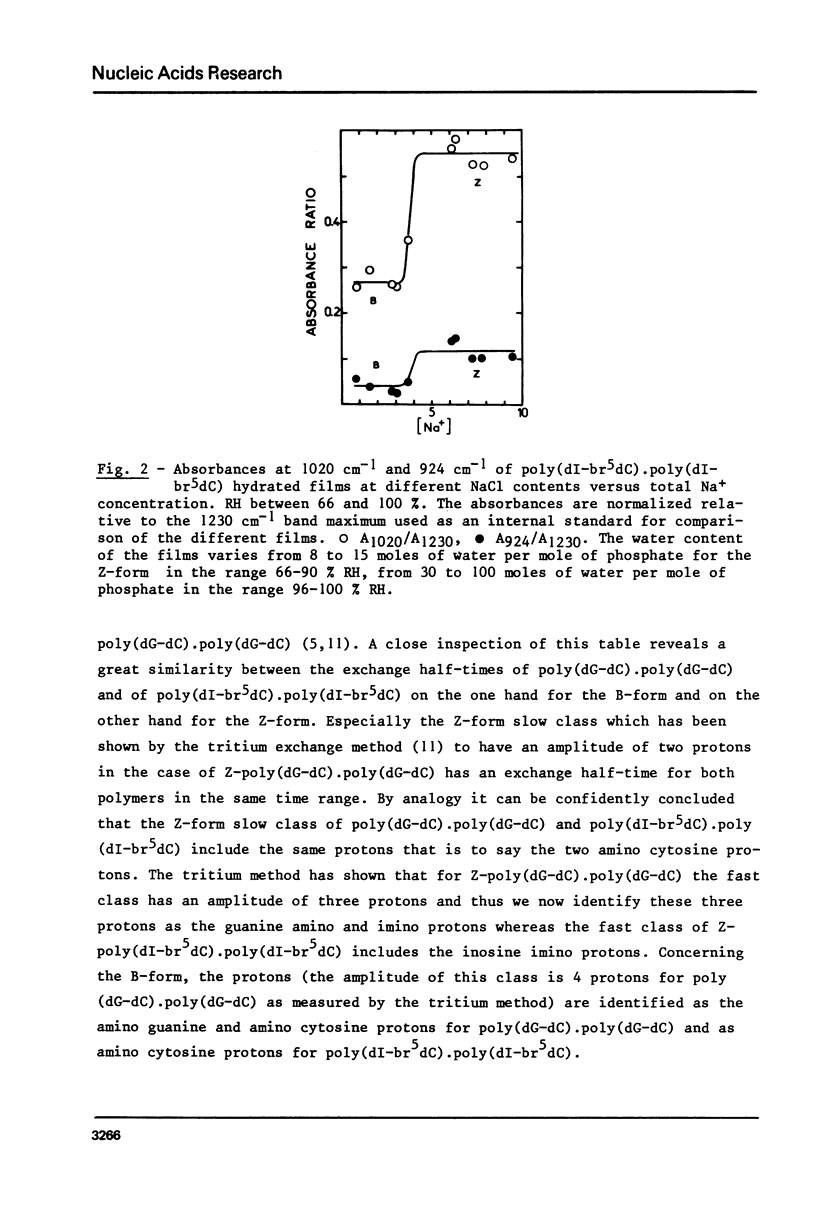
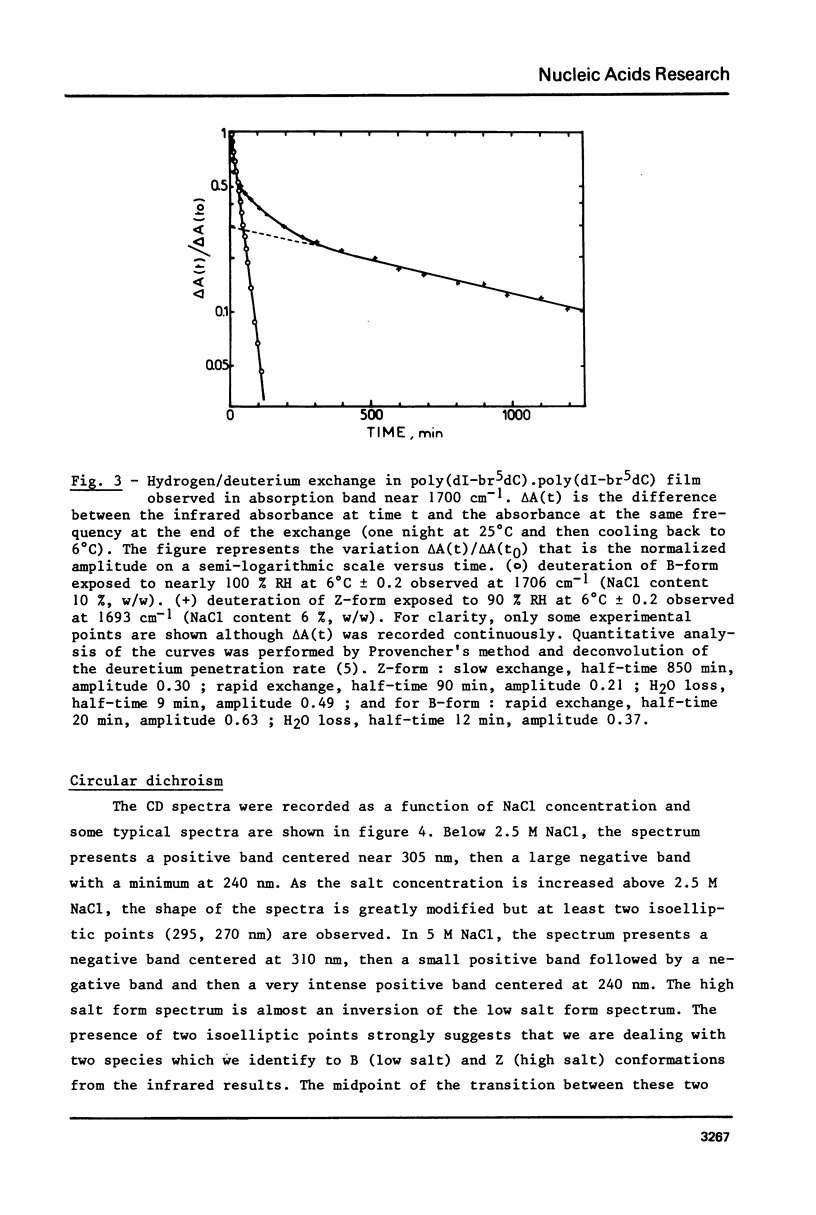
The study of poly(dI-br5dC).poly(dI-br5dC) films by infrared spectroscopy shows that in low salt concentration, the conformation of this polynucleotide belongs to the B-family and in high salt concentration to the Z-family. 31P nuclear magnetic resonance and circular dichroism confirm the existence of these two forms. By circular dichroism and ultraviolet absorption, it is shown that the equilibrium constant of the B reversible Z transition depends upon temperature. The deuteration rates of exchangeable protons involved in hydrogen bonds between base pairs were deduced from the changes in absorbance near 1700 cm-1. In the B-form, one class of protons is measured with an exchange half-time of 20 minutes. In the Z-form, two classes of protons are measured with very different exchange half-times, the exchange half-time of the slow protons being of the order of 850 minutes. By comparison of these results with those previously obtained for poly(dG-dC).poly(dG-dC), these very slow protons of these two Z-polynucleotides are identified as the cytosine amino protons. A quantitative description of the dynamic structure of the Z-form is presented.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Birdsall D. L., Leslie A. G., Ratliff R. L. Left-handed DNA helices. Nature. 1980 Feb 21;283(5749):743–745. doi: 10.1038/283743a0. [DOI] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behe M., Zimmerman S., Felsenfeld G. Changes in the helical repeat of poly(dG-m5dC) . poly(dG-m5dC) and poly(dG-dC) . poly(dG-dC) associated with the B-Z transition. Nature. 1981 Sep 17;293(5829):233–235. doi: 10.1038/293233a0. [DOI] [PubMed] [Google Scholar]
- Champeil P., Tran T. P., Brahms J. A new approach to the characterization of the B and A forms of DNA by I.R. spectroscopy. Biochem Biophys Res Commun. 1973 Dec 10;55(3):881–887. doi: 10.1016/0006-291x(73)91226-6. [DOI] [PubMed] [Google Scholar]
- Drew H., Takano T., Tanaka S., Itakura K., Dickerson R. E. High-salt d(CpGpCpG), a left-handed Z' DNA double helix. Nature. 1980 Aug 7;286(5773):567–573. doi: 10.1038/286567a0. [DOI] [PubMed] [Google Scholar]
- Englander S. W., Englander J. J. Hydrogen-tritium exchange. Methods Enzymol. 1972;26:406–413. doi: 10.1016/s0076-6879(72)26021-9. [DOI] [PubMed] [Google Scholar]
- Englander S. W., Kallenbach N. R., Heeger A. J., Krumhansl J. A., Litwin S. Nature of the open state in long polynucleotide double helices: possibility of soliton excitations. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7222–7226. doi: 10.1073/pnas.77.12.7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K. C., Van Zandt L. L., Prohofsky E. W. Displacements of backbone vibrational modes of A-DNA and B-DNA. Biophys J. 1979 Oct;28(1):27–32. doi: 10.1016/S0006-3495(79)85156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra C. K., Sarma M. H., Sarma R. H. Left-handed deoxyribonucleic acid double helix in solution. Biochemistry. 1981 Mar 31;20(7):2036–2041. doi: 10.1021/bi00510a046. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L., Pohl F. M. "Alternating B-DNA" conformation for the oligo(dG-dC) duplex in high-salt solution. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2508–2511. doi: 10.1073/pnas.76.6.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilet J., Blicharski J., Brahms J. Conformations and structural transitions in polydeoxynucleotides. Biochemistry. 1975 May 6;14(9):1869–1876. doi: 10.1021/bi00680a011. [DOI] [PubMed] [Google Scholar]
- Pilet J., Leng M. Comparison of poly(dG-dC).poly(dG-dC) conformations in oriented films and in solution. Proc Natl Acad Sci U S A. 1982 Jan;79(1):26–30. doi: 10.1073/pnas.79.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilet J., Szabo A. G., Maurizot J. C. Hydrogen exchange in hydrated films of proteins. Application to the E. coli lac repressor core. Biophys Chem. 1980 Dec;12(3-4):279–284. doi: 10.1016/0301-4622(80)80005-6. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Polymorphism of a synthetic DNA in solution. Nature. 1976 Mar 25;260(5549):365–366. doi: 10.1038/260365a0. [DOI] [PubMed] [Google Scholar]
- Ramstein J., Leng M. Salt-dependent dynamic structure of poly(dG-dC) x poly(dG-dC). Nature. 1980 Nov 27;288(5789):413–414. doi: 10.1038/288413a0. [DOI] [PubMed] [Google Scholar]
- Taillandier E., Taboury J., Liquier J., Sautière P., Couppez M. Structural transitions in DNAs and nucleohistones studied by I.R. spectroscopy. Biochimie. 1981 Nov-Dec;63(11-12):895–898. doi: 10.1016/s0300-9084(82)80282-4. [DOI] [PubMed] [Google Scholar]
- Teitelbaum H., Englander S. W. Open states in native polynucleotides. I. Hydrogen-exchange study of adenine-containing double helices. J Mol Biol. 1975 Feb 15;92(1):55–78. doi: 10.1016/0022-2836(75)90091-1. [DOI] [PubMed] [Google Scholar]
- Teitelbaum H., Englander S. W. Open states in native polynucleotides. II. Hydrogen-exchange study of cytosine-containing double helices. J Mol Biol. 1975 Feb 15;92(1):79–92. doi: 10.1016/0022-2836(75)90092-3. [DOI] [PubMed] [Google Scholar]
- Thamann T. J., Lord R. C., Wang A. H., Rich A. The high salt form of poly(dG-dC).poly(dG-dC) is left-handed Z-DNA: Raman spectra of crystals and solutions. Nucleic Acids Res. 1981 Oct 24;9(20):5443–5457. doi: 10.1093/nar/9.20.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wang A. J., Quigley G. J., Kolpak F. J., van der Marel G., van Boom J. H., Rich A. Left-handed double helical DNA: variations in the backbone conformation. Science. 1981 Jan 9;211(4478):171–176. doi: 10.1126/science.7444458. [DOI] [PubMed] [Google Scholar]



