Abstract
Wnt proteins are secreted, lipid-modified glycoproteins that control animal development and adult tissue homeostasis. Secretion of Wnt proteins is at least partly regulated by a dedicated machinery. Here, we report a genome-wide RNA interference screen for genes involved in the secretion of Wingless (Wg), a Drosophila Wnt. We identify three new genes required for Wg secretion. Of these, Emp24 and Eclair are required for proper export of Wg from the endoplasmic reticulum (ER). We propose that Emp24 and Eca act as specific cargo receptors for Wg to concentrate it in forming vesicles at sites of ER export.
Keywords: cargo receptor, p24, Wnt secretion
Introduction
Wnt signalling is crucial for animal development and controls tissue homeostasis in the adult (Logan & Nusse, 2004). Wnt proteins can function as morphogens, being produced and secreted by a defined subset of cells and subsequently spreading in the tissue to induce target gene expression in receiving cells (Neumann & Cohen, 1996; Zecca et al, 1996).
It has recently become clear that Wnt signalling is already tightly controlled in ligand-producing cells by regulation of protein secretion (Port & Basler, 2010). For example, post-Golgi trafficking of the Drosophila Wnt protein Wingless (Wg) specifically requires the conserved transmembrane protein Wntless (Banziger et al, 2006; Bartscherer et al, 2006; Goodman et al, 2006; Port et al, 2008). Why Wg requires special help for Golgi exit and how Wntless provides it is unknown. Furthermore, it is not known whether Wg also requires the help of a specialized protein machinery to travel from other compartments of the secretory pathway, such as the endoplasmic reticulum (ER).
Here, we present a genome-wide RNA interference (RNAi) screen designed to detect genes that are required for secretion of Wg in cultured cells. After careful rescreening of candidates in vivo, we uncovered two genes—encoding the endosomal adaptor sorting nexin 3 and the transmembrane protein Éclair—that were required for normal Wg exocytosis. Sorting nexin 3 is part of an unconventional Retromer complex that controls Wntless levels in Wg-producing cells (Harterink et al, 2011). Éclair is a member of the p24 protein family and we found that another member of the same family, Emp24, is also involved in regulating Wg secretion. Our results indicate that Éclair and Emp24 are required for normal exit of Wg from the ER, probably by acting as cargo receptors.
Results And Discussion
A genome-wide screen for genes required for Wg secretion
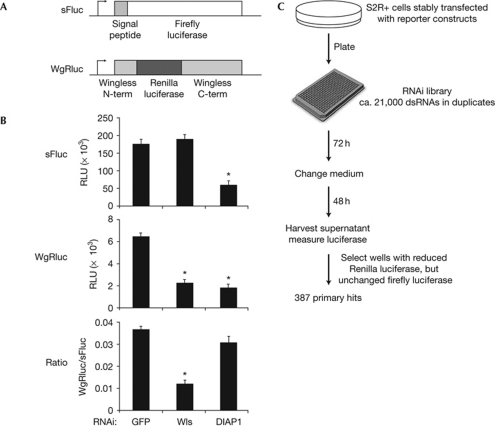
To screen for genes that are required for the secretion of Wg, we generated a Drosophila S2R+ cell line that stably expresses a Wg–Renilla luciferase (WgRluc) fusion protein (Fig 1A). WgRluc is secreted into the cell supernatant and is competent to trigger Wg signalling (data not shown). In addition, our reporter cells express a secreted form of Firefly luciferase (sFluc). To identify genes that are required for the secretion of Wg, but not secretion in general or cell survival, we screened for genes that if knocked down led to a reduction of WgRluc activity in the medium, without affecting sFluc activity. We used RNAi against Wntless as a positive control. Knockdown of Wntless in our reporter cells caused a substantial reduction of WgRluc activity in the medium, but did not change sFluc activity (Fig 1B). By contrast, interfering with the expression of Drosophila inhibitor of apoptosis 1 (diap1), a gene necessary for cell survival, caused a reduction in both WgRluc and sFluc levels. By using this assay we screened a state-of-the-art library of double-stranded RNAs (dsRNAs) targeting more than 14,000 Drosophila genes (Fig 1C). We assigned 387 genes as primary hits, based on the fact that their knockdown selectively reduced WgRluc activity in the supernatant of both duplicates (supplementary Table S1 online).
Figure 1.
A genome-wide RNAi screen for genes required for Wingless secretion. (A) Schematic diagram of the reporters used. The signal peptide of sFluc stems from influenza haemagglutinin. (B) Downregulation of Wntless reduces secretion of WgRluc, but not sFluc. Knockdown of anti-apoptotic diap1 causes cell death and a reduction of both reporters in the media. Data represent means±s.d. (n=4), t-test *P<0.01 compared with GFP controls. (C) Summary of the screening protocol. DIAP1, Drosophila inhibitor of apoptosis 1; dsRNA, double-stranded RNA; GFP, green fluorescent protein; RNAi, RNA interference; RLU, relative luciferase units; sFluc, secreted form of Firefly luciferase; Wls, Wntless; WgRluc, Wingless–Renilla luciferase.
Rescreening of selected candidate genes in vivo
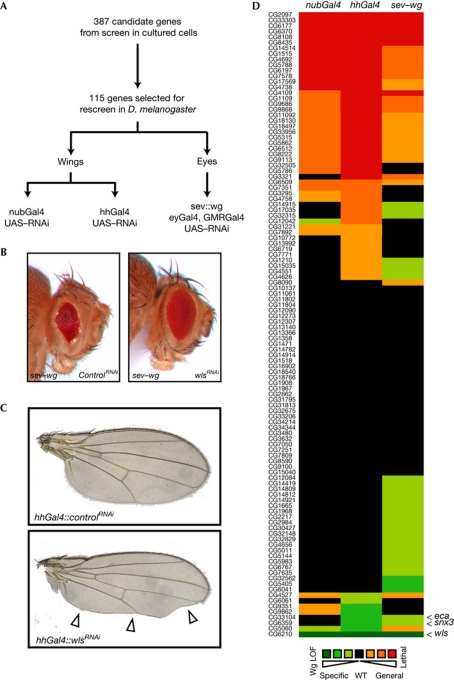
High-throughput screening produces a fraction of false-positive and false-negative results. Furthermore, RNAi technology has caveats such as off-target effects (Kulkarni et al, 2006). Therefore, it is crucial to carefully reevaluate results obtained from large-scale RNAi screens. We rescreened selected candidates from our tissue culture screen in flies using a sensitized system in the eye, as well as a system in which endogenous Wg signalling in the wing is affected. Importantly, we used independent reagents to knockdown gene expression in vivo, reducing the likelihood that artefacts related to the previously used dsRNA, such as off-target effects, would be repeated in our rescreen. Also, we now screened in cells that naturally secrete Wg, increasing the physiological relevance of our results. To optimize the rescreening we excluded genes with well-documented functions unrelated to protein secretion from further analysis. A total of 115 genes were chosen for further analysis (supplementary Table S2 online). We screened for inhibition of Wg secretion in the Drosophila eye and wing (Fig 2A). Overexpression of Wg in the developing eye under the control of the sevenless (sev) promoter disturbs the normal arrangement of the ommatidial facets and gives rise to a ‘rough’-eye phenotype (Brunner et al, 1997). This phenotype is suppressed when the secretion of the ectopic Wg protein is inhibited, as demonstrated by the effect of simultaneous knockdown of wntless by RNAi (Fig 2B). We expressed RNAi hairpin constructs targeting our candidate genes in the developing eye of sev–wg-expressing flies and screened for suppression of the rough-eye phenotype. Knockdown of 30 genes resulted in suppression of the sev–wg phenotype (Fig 2D; supplementary Table S2 online).
Figure 2.
Testing candidate genes for an effect on Wingless secretion in vivo. (A) Schematic illustration of the rescreening procedure. (B) Eyes of sev–wg-expressing flies coexpressing UAS–lacZ RNAi or UAS-wls RNAi induced by GMR–Gal4 and eyGal4. Inhibition of Wg secretion by reducing Wntless levels leads to a suppression of the sev–wg-induced rough-eye phenotype. (C) Wings of flies expressing UAS–lacZ RNAi or UAS–wls RNAi induced by hhGal4 in the posterior compartment of the developing wing. Strongly reduced Wg secretion caused by wls knockdown leads to loss of wing margin tissue (arrow heads). (D) Graphical summary of the results from the in vivo rescreen. For details, refer to supplementary Table S2 online. eca, éclair; hhGa14, hedgehog–Gal4; RNAi, RNA interference; nubGal4, nubbin–Gal4; sev, sevenless; snx3, sorting nexin 3; UAS, upstream activating sequence; LOF, loss of function; Wg, Wingless; wls, wntless; WT, wild type.
Next, we screened for potential Wg signalling components in the Drosophila wing. Inhibition of Wg signalling in the developing wing typically leads to the loss of wing margin tissue (Fig 2C), although this phenotype is not unique to defective Wg signalling. We expressed hairpin RNAi constructs in the wing imaginal disc using two Gal4 drivers. Whereas nubbin–Gal4 (nubGal4) leads to moderate expression in the whole wing pouch, hedgehog–Gal4 (hhGal4) results in strong expression in only the posterior compartment of the developing wing. We identified three genes that cause loss of wing margin tissue when knocked down with nubGal4 and eight genes when RNAi was induced by hhGal4 (Fig 2D; supplementary Table S2 online). Only three genes showed phenotypes associated with reduced Wg secretion in more than one assay: CG5060, sorting nexin 3 (snx3) and éclair (eca). Unfortunately, the RNAi line used to target CG5060 contains a stretch of 24 base pairs with perfect homology to the Notch gene. As reduced Notch signalling gives rise to wing margin defects, it is probably responsible for the notched phenotypes caused by the CG5060 RNAi. We could validate the remaining two genes, snx3 and eca, as new components of the Wg secretion machinery. Snx3 is part of an unconventional retromer complex that regulates Wntless levels and thereby promotes Wg secretion (Harterink et al, 2011). Éclair is a member of the p24 protein family that is involved in ER-to-Golgi trafficking, and thus potentially represents the first component in this early step of Wnt secretion. We then focused on the role of p24 proteins in Wg secretion.
The p24 proteins Éclair and Emp24 regulate Wg secretion
Éclair belongs to the evolutionarily conserved family of p24 transmembrane proteins that function in the early secretory pathway. As previous work has suggested that p24 proteins might function as hetero-oligomers or dimers (Fullekrug et al, 1999; Marzioch et al, 1999; Jenne et al, 2002), we tested all Drosophila p24 genes for a function in Wg secretion. There are nine p24 family members in flies: eclair, CG33105, emp24, CG9308, baiser, CG1967, CG9053, CG31787 and logjam (supplementary Fig S1 online). We were unable to detect transcripts from CG33105, CG9308 and CG31787 in wing imaginal discs by reverse transcription–PCR, suggesting that these genes are not expressed in this tissue (data not shown). This was supported by sequencing of total RNA from purified wing imaginal discs (C. Bauer & K. Basler, unpublished data). We used transgene-mediated RNAi to individually downregulate the expression of the six remaining p24 genes (supplementary Fig S2 online). Knockdown of baiser, CG1967, CG9053 or logjam in the developing wing or eye did not give rise to phenotypes indicative of reduced Wg secretion. However, downregulation of either eclair or emp24 expression moderately suppressed the sev–wg-induced rough-eye phenotype and frequently led to loss of wing margin tissue (supplementary Fig S3 online), suggesting that these genes are required for Wg secretion. To confirm that eclair and emp24, but not the other p24 genes, regulate Wg exocytosis we knocked down the expression of each gene in the posterior compartment of wing imaginal discs and stained for endogenous Wg protein. Knockdown of baiser, logjam, CG1967 and CG9053 did not result in any alterations in Wg staining (supplementary Fig S3 online). By contrast, Wg staining was stronger in cells with downregulated eclair or emp24 expression (Fig 3A,C). The increased levels of anti-Wg staining resulted from an accumulation of Wg inside the producing cells; staining exclusively extracellular Wg showed a reduction of Wg on the cell surface of eclairRNAi or emp24RNAi cells (Fig 3B,D). Elevated levels of Wg did not result from an increase in wg transcription, as expression of a wg–lacZ reporter was unchanged (supplementary Fig S4 online). Simultaneous knockdown of eclair and emp24 did not increase the defect in Wg secretion (data not shown). To confirm the role of p24 proteins in Wg secretion independent from RNAi, we used the ethylmethane sulphonate-induced allele eclair3R-45-33. We generated eclair3R-45-33/eclair3R-45-33 homozygous mutant clones in wing imaginal discs and stained for endogenous Wg protein. Mutant cells accumulated Wg protein, as observed with our RNAi lines (Fig 3E). We therefore conclude that the p24 proteins Éclair and Emp24 are required for proper Wg secretion.
Figure 3.
Emp24 and Éclair regulate Wingless secretion. (A–D) Downregulation of either emp24 (A,B) or eca (C,D) results in accumulation of Wg in producing cells. RNAi was expressed under the control of hhGal4 in the posterior compartment, which is marked by expression of UAS–CD8–GFP. Total Wg is shown in (A,C), whereas exclusively extracellular Wg is stained in B,D. (E) Clones of cells homozygous mutant for Éclair were induced by mitotic recombination. Wg protein accumulates inside Wg-expressing cells, as shown by staining total Wg protein. Quantification of the fluorescent signal in cells along the dorsal–ventral boundary is shown in the right panel. Scale bars, 50 μm. AU, arbitrary units; eca, éclair; GFP, green fluorescent protein; hhGa14, hedgehog–Gal4; RNAi, RNA interference; UAS, upstream activating sequence; Wg, Wingless.
Éclair and Emp24 govern ER exit of Wg
How might Éclair and Emp24 regulate Wg secretion? p24 proteins have been shown to act as cargo receptors for ER-to-Golgi transport of glycosyl phosphatidylinositol (GPI)-anchored proteins, but have also been shown to be important for biogenesis of retrograde COPI (coat protein complex I) vesicles at the cis-Golgi (Muniz et al, 2000; Aguilera-Romero et al, 2008). Whereas inhibition of COPI vesicle biogenesis would lead to a block of protein transport, because essential factors that mediate ER exit become mislocalized to the Golgi, loss of cargo receptors will specifically affect transport of their target proteins. Therefore, reduced Wg secretion in the absence of Éclair and Emp24 could be due to three reasons: (1) impaired vesicle biogenesis results in an overall block of ER-to-Golgi transport; (2) Éclair and Emp24 regulate transport of proteins essential for Wg secretion or Wg-binding proteins; (3) Éclair and Emp24 act as cargo receptors for Wg itself.
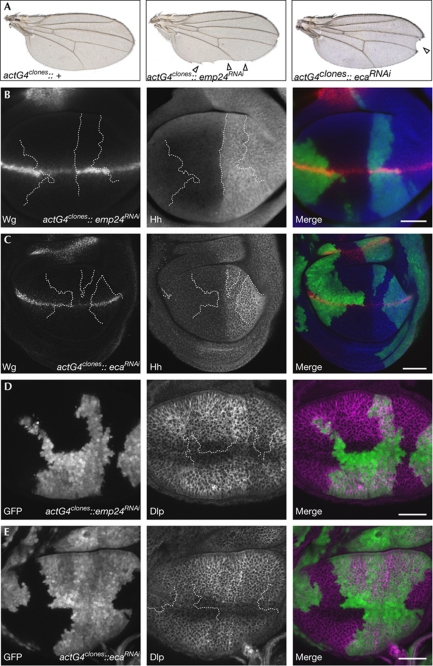
To address the first possibility, we analysed whether Éclair and Emp24 are also required for the secretion of two other morphogens that are expressed in wing imaginal discs: Hedgehog and Decapentaplegic (Dpp). We expressed RNAi hairpin constructs targeting eclair or emp24 in clones of cells using actGal4. These clones accumulate Wg and frequently give rise to Wg loss-of-function phenotypes in the adult wing (Fig 4A–C). By contrast, endogenous Hedgehog protein did not accumulate in RNAi-expressing clones, suggesting that it is insensitive to reduced levels of Éclair and Emp24 (Fig 4B,C). We then analysed whether Éclair or Emp24 are involved in Dpp signalling in wing imaginal discs, as has been suggested in Drosophila embryos (Bartoszewski et al, 2004). As antibodies that detect endogenous Dpp do not exist, we monitored the expression of two sensitive downstream events of Dpp signalling, phosphorylation of Mad protein and expression of the target gene brinker. Knockdown of eclair or emp24 had no effect on phosphorylation of Mad or expression of brinker, suggesting that Dpp signalling is not detectably altered in the wing imaginal discs (supplementary Fig S5 online). Together, these results show that neither Éclair or Emp24 are required for general protein secretion.
Figure 4.
Emp24 and Éclair are not required for general protein secretion in Drosophila. (A) Clones of cells expressing RNAi hairpins against emp24 or eca under the control of actin-Gal4 frequently lead to typical Wg loss-of-function phenotypes in adult wings (arrow heads). (B,C) In such clones, Wg accumulates in the wg-expression domain, whereas in Hh-expressing cells Hh does not accumulate (D,E). Clones induced by the same method do not accumulate Dlp along the dorsal–ventral boundary. However, slight Dlp accumulation is observed in emp24RNAi clones outside the Wg-expression domain. Clone boundaries are indicated by lines in B–E. Scale bars, 50 μm. Dlp, Dally-like protein; eca, éclair; GFP, green fluorescent protein; Hh, hedgehog; RNAi, RNA interference; Wg, Wingless.
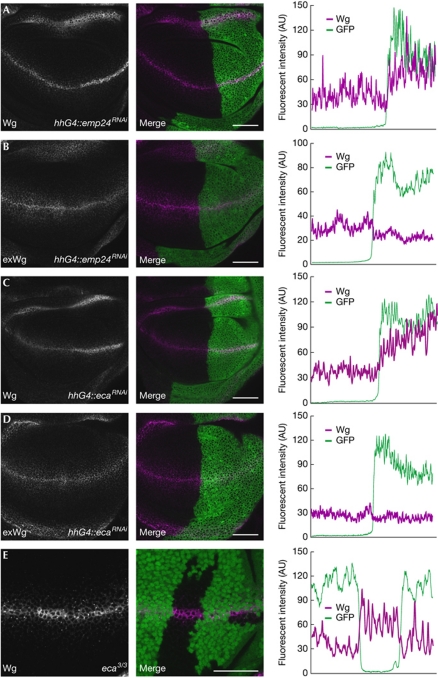
The second hypothesis predicts that Éclair and Emp24 affect Wg secretion indirectly by regulating a Wg-interacting protein. The acetyltransferase Porcupine is involved in Wg secretion by regulating the post-translational modification of Wg. However, Porcupine is thought to reside permanently in the ER and is therefore unlikely to be affected by ER-to-Golgi trafficking defects. By contrast, the Wg-interacting proteins Dally, Dally-like protein (Dlp) and Wntless transverse the secretory pathway, and their ER-to-Golgi transport could be regulated by Éclair and Emp24. In this case, in the absence of Éclair or Emp24 Dally, Dlp or Wntless might accumulate in the ER, where they could bind to and sequester Wg. Dally and Dlp are GPI-anchored proteins, making them likely candidates for an interaction with p24 s. We focused on Dlp, because an existing antibody allows the detection of endogenous protein in wing imaginal discs. Dlp protein levels are low in Wg-expressing cells and increase further away from the dorsal–ventral boundary. We reduced the levels of Éclair or Emp24 by using RNAi in clones of cells and stained for endogenous Dlp. Knockdown of Éclair or Emp24 did not lead to an increase of Dlp levels in Wg-expressing cells (Fig 4D,E). Another protein that interacts with Wg in the secretory pathway is Wntless (Banziger et al, 2006). We asked whether a reduction of Éclair or Emp24 sequesters Wntless in the ER. Again, we did not detect any increase of endogenous Wntless in cells with knocked down eclair or emp24 (Fig 5A,C). Instead, the punctuate staining of Wntless in Wg-producing cells was slightly reduced in RNAi-expressing cells (Fig 5B,D). We have previously shown that these punctuae represent an accumulation of Wntless in the Golgi that is induced by Wg (Port et al, 2008). Lowering Wg levels in secreting cells reduces Wntless levels in the Golgi, whereas providing more Wg leads to Golgi accumulation of Wntless. The fact that loss of Éclair or Emp24 causes a reduction rather than increase in Wntless levels in the Golgi indicates that less Wg reaches this compartment. Hence, the overall higher level of Wg in these cells probably reflects an accumulation in the ER. Consistent with this, we found that in cells expressing RNAi targeting eclair or emp24, Wg colocalizes extensively with the ER marker YFP–KDEL (Fig 5E–G).
Figure 5.
Emp24 and Éclair directly control endoplasmic reticulum export of Wingless. Knockdown of emp24 (A,B) or eca (C,D) does not cause any accumulation of Wntless. Instead, Wg-induced accumulation of Wntless along the dorsal–ventral boundary is reduced (B,D). A single confocal section is shown in A and C. Maximum intensity projections are shown in B and D to visualize spots of intense Wntless staining, and thresholded views of the same images are provided to compare the areas of most intense staining. Cells expressing RNAi hairpin constructs are marked in green in the merge images. (E–G) Wg accumulates in the ER in the absence of Emp24 or Éclair. RNAi constructs were expressed under the control of hhGal4 together with a UAS–YFP–ER marker. Samples were stained in parallel and imaged with identical settings, and single confocal views of similar regions of the wing disc are shown. (H) WgRluc specifically coimmunoprecipitates with Emp24–GFP. The indicated proteins were expressed in S2R+ cells, crosslinked and immunoprecipitated with specific antibodies. Scale bars, 50 μm in A–D and 5 μm in E–G. Ab, antibody; ER, endoplasmic reticulum; eca, éclair; GFP, green fluorescent protein; hhGa14, hedgehog–Gal4; IP, immunoprecipitation; RNAi, RNA interference; Rluc, Renilla luciferase; ROI, region of interest; UAS, upstream activating sequence; WB, Western blot; Wg, Wingless; WgRluc, Wg–Renilla luciferase; Wls, Wntless; WT, wild type; YFP, yellow fluorescent protein.
Together, these results argue for the third model, in which Éclair and Emp24 act as cargo receptors for Wg protein. Such a mode of action requires a physical interaction between the cargo and its receptor, and we therefore tested whether Wg could be coimmunoprecipitated with either Éclair or Emp24. Cargo–cargo receptor interactions are typically transient and have low affinity, making experimental validation of physical binding challenging. We used an established crosslinking protocol in cultured cells that was previously used to show interactions between p24 proteins and their protein cargo (Muniz et al, 2000). We failed to detect any binding of Éclair to Wg (data not shown). By contrast, we observed a robust and specific interaction between Emp24 and Wg (Fig 5H). The highly conserved mammalian orthologues of Éclair and Emp24 (TMED4/p25 71% identity, 84% similarity; TMED2/p24 63% identity, 80% similarity) have been demonstrated in several instances to form heteromeric complexes (Fullekrug et al, 1999; Marzioch et al, 1999; Jenne et al, 2002). Therefore, it is reasonable to propose that Éclair and Emp24 act together in a complex and Emp24 provides the interaction interface with Wg. For technical reasons, we probably did not detect the entire complex in our pull-downs: epitope-tagged Éclair protein was poorly expressed in S2 cells.
Together, our data support a model in which Éclair and Emp24 form a complex that recruits Wg into forming COPII vesicles at ER exit sites. Without this active recruitment Wg accumulates in the ER and Wg secretion is impeded. However, a fraction of Wg escapes the ER even in the absence of Éclair and Emp24, probably by bulk flow facilitated by the elevated levels of Wg, or by other cargo receptors that act in a partly redundant fashion. Until now, p24 proteins have been shown to act as cargo receptors for GPI-linked proteins in yeast and mammalian cells (Takida et al, 2008; Castillon et al, 2009). Our work shows that the Wnt protein Wg also depends on p24 proteins for ER export. A recent study in mammalian cells has shown that p24s and GPI-linked proteins partition into raft-like membrane microdomains in the ER, and clustering in these domains was found to be essential for their interaction (Bonnon et al, 2010). Interestingly, Wnt proteins are post-translationally modified by two different lipid adducts (Willert et al, 2003; Takada et al, 2006), and thereby become associated with detergent-resistant membrane microdomains (Zhai et al, 2004). Lipid modification of Wnts is important for protein secretion, although the details remain controversial (Port & Basler, 2010). It is tempting to speculate that lipid-modified Wnts and GPI-anchored proteins might be targeted to the same membrane microdomains. Stable association with these domains might depend on different members of the p24 family, which also provide the cytoplasmic signal to recruit coat proteins for vesicle formation (Dominguez et al, 1998). It will be important to untangle the exact role of the post-translational modification of Wnts for ER export, as well as other steps along the secretion route.
Methods
Genome-wide RNAi screen. The WgRluc construct was cloned by inserting the Renilla luciferase coding sequence between codons R32 and S36 of the Wg coding sequence (Zecca et al, 1996). S2R+ cells constitutively expressing secreted WgRluc and sFluc were produced by cotransfection of the corresponding plasmids and selection with blasticidine. The screen was performed at the Drosophila RNAi Screening Center (Harvard Medical School). In brief, 20 μl of cells (∼15,000 cells) in serum-free Schneider's medium were seeded in 384-well plates containing 0.1 μg dsRNA and incubated for 1 h. Medium was supplemented with 20 μl Schneider's medium containing 20% fetal calf serum and incubated for 3 days at 25°C. The plates were then centrifuged and the medium was aspirated and replaced by Schneider's medium containing 10% fetal calf serum. After incubation for 2 days further, plates were centrifuged and 10 μl medium was transferred to new plates. Activity of WgRluc and sFluc in the cell supernatant was assayed by using Dual-Glow luciferase substrate (Promega) and a plate reader (Analyst GT; Molecular Devices).
Drosophila stocks, in vivo RNAi and immunohistochemistry. Fly lines expressing RNAi hairpins were obtained from the Vienna Drosophila RNAi Center. To target emp24 and eclair we used lines 100274 and 101388, but lines 7038 and 49749 showed similar phenotypes. RNAi lines were crossed to the following fly strains: w, UAS–CD8–GFP;;hhGal4/Tm6b and w; nubGal4 and w; eyGal4, GMRGal4; sev–wg and w, hsp–Flp, UAS–CD8–GFP; sp/cyo; act>stop>Gal4. For ER colocalization hhGal4>UAS–YFP–ER flies (from S. Eaton, Dresden) were crossed to the respective RNAi strains or wild-type controls. Larva were collected at the late third-instar stage and dissected in PBS. Wing imaginal discs were stained using standard procedures (Port et al, 2008). Images were collected on a Zeiss LSM 710 confocal microscope using the sequential scanning mode. Images were processed using ImageJ and Adobe Photoshop. Quantification of fluorescent intensities was performed using the Plot profile function of ImageJ.
Crosslinking and coimmunoprecipitation. Drosophila S2R+ cells were transfected with Fugene HD (Roche) according to the manufacturer's instructions. After 48 h, cells were washed twice with PBS and then incubated with 1 mM dithiobis(succinimidylpropionate) (Pierce) for 20 min at 20°C. The crosslinking reaction was quenched by addition of glycine (50 mM final, 5 min, 20°C). Cells were lysed on ice for 30 min in 150 mM NaCl, 50 mM Tris–HCl (pH 8.0), 1% NP-40, 0.5% deoxycholic acid, protease inhibitor cocktail (Complete Mini, Roche). Lysate was incubated with green fluorescent protein antibody-conjugated protein-G sepharose beads. Beads were washed three times with lysis buffer and then immunoprecipitates were eluted by boiling in reducing SDS loading buffer. Samples were separated by SDS–PAGE, transferred onto nitrocellulose membranes and probed with specific antibodies.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank the staff of the Drosophila RNAi Screening Center for expert support during the screen; S. Eaton, M.A. Lilly, S. Luschnig, S. Bullock and the Vienna Drosophila RNAi Center for fly stocks and antibodies; G. Reim for experimental help and members of our lab for discussions. This work was supported by Forschungskredit Zurich (F.P.), European Molecular Biology Organization short-term fellowship (F.P.), Swiss National Science Foundation (K.B.) and the Kanton of Zurich (K.B.) and an ERC Advanced Investigator Grant (K.B.).
Footnotes
The authors declare that they have no conflict of interest.
References
- Aguilera-Romero A, Kaminska J, Spang A, Riezman H, Muniz M (2008) The yeast p24 complex is required for the formation of COPI retrograde transport vesicles from the Golgi apparatus. J Cell Biol 180: 713–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K (2006) Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 125: 509–522 [DOI] [PubMed] [Google Scholar]
- Bartoszewski S, Luschnig S, Desjeux I, Grosshans J, Nusslein-Volhard C (2004) Drosophila p24 homologues eclair and baiser are necessary for the activity of the maternally expressed Tkv receptor during early embryogenesis. Mech Dev 121: 1259–1273 [DOI] [PubMed] [Google Scholar]
- Bartscherer K, Pelte N, Ingelfinger D, Boutros M (2006) Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell 125: 523–533 [DOI] [PubMed] [Google Scholar]
- Bonnon C, Wendeler MW, Paccaud JP, Hauri HP (2010) Selective export of human GPI-anchored proteins from the ER. J Cell Sci 123: 1705–1715 [DOI] [PubMed] [Google Scholar]
- Brunner E, Peter O, Schweizer L, Basler K (1997) pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wg signal in Drosophila. Nature 385: 829–833 [DOI] [PubMed] [Google Scholar]
- Castillon GA, Watanabe R, Taylor M, Schwabe TM, Riezman H (2009) Concentration of GPI-anchored proteins upon ER exit in yeast. Traffic 10: 186–200 [DOI] [PubMed] [Google Scholar]
- Dominguez M, Dejgaard K, Fullekrug J, Dahan S, Fazel A, Paccaud JP, Thomas DY, Bergeron JJ, Nilsson T (1998) gp25L/emp24/p24 protein family members of the cis-Golgi network bind both COP I and II coatomer. J Cell Biol 140: 751–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullekrug J, Suganuma T, Tang BL, Hong W, Storrie B, Nilsson T (1999) Localization and recycling of gp27 (hp24γ3): complex formation with other p24 family members. Mol Biol Cell 10: 1939–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RM, Thombre S, Firtina Z, Gray D, Betts D, Roebuck J, Spana EP, Selva EM (2006) Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development 133: 4901–4911 [DOI] [PubMed] [Google Scholar]
- Harterink M et al. (2011) A SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required of Wnt secretion. Nat Cell Biol 13: 914–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenne N, Frey K, Brugger B, Wieland FT (2002) Oligomeric state and stoichiometry of p24 proteins in the early secretory pathway. J Biol Chem 277: 46504–46511 [DOI] [PubMed] [Google Scholar]
- Kulkarni MM, Booker M, Silver SJ, Friedman A, Hong P, Perrimon N, Mathey-Prevot B (2006) Evidence of off-target effects associated with long dsRNAs in Drosophila melanogaster cell-based assays. Nat Methods 3: 833–838 [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20: 781–810 [DOI] [PubMed] [Google Scholar]
- Marzioch M, Henthorn DC, Herrmann JM, Wilson R, Thomas DY, Bergeron JJ, Solari RC, Rowley A (1999) Erp1p and Erp2p, partners for Emp24p and Erv25p in a yeast p24 complex. Mol Biol Cell 10: 1923–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniz M, Nuoffer C, Hauri HP, Riezman H (2000) The Emp24 complex recruits a specific cargo molecule into ER-derived vesicles. J Cell Biol 148: 925–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann CJ, Cohen SM (1996) Distinct mitogenic and cell fate specification functions of wingless in different regions of the wing. Development 122: 1781–1789 [DOI] [PubMed] [Google Scholar]
- Port F, Basler K (2010) Wnt trafficking: new insights into Wnt maturation, secretion and spreading. Traffic 11: 1265–1271 [DOI] [PubMed] [Google Scholar]
- Port F, Kuster M, Herr P, Furger E, Banziger C, Hausmann G, Basler K (2008) Wg secretion promotes and requires retromer-dependent cycling of Wntless. Nat Cell Biol 10: 178–185 [DOI] [PubMed] [Google Scholar]
- Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S (2006) Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell 11: 791–801 [DOI] [PubMed] [Google Scholar]
- Takida S, Maeda Y, Kinoshita T (2008) Mammalian GPI-anchored proteins require p24 proteins for their efficient transport from the ER to the plasma membrane. Biochem J 409: 555–562 [DOI] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR III, Nusse R (2003) Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423: 448–452 [DOI] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G (1996) Direct and long-range action of a wingless morphogen gradient. Cell 87: 833–844 [DOI] [PubMed] [Google Scholar]
- Zhai L, Chaturvedi D, Cumberledge S (2004) Drosophila wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine. J Biol Chem 279: 33220–33227 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.