Abstract
Ixodes ticks harbour several human pathogens belonging to the order Rickettsiales, including Anaplasma phagocytophilum, the agent of human anaplasmosis. When ticks feed on A. phagocytophilum-infected mice, the pathogen enters the ticks' gut. The bacteria then migrate from the gut to infect the salivary glands of the ticks and are transmitted to the next host via the saliva. The molecular mechanisms that enable the migration of A. phagocytophilum from the gut to the salivary glands are poorly understood. Here we show that a secreted tick protein, P11, is important in this process. We show that P11 enables A. phagocytophilum to infect tick haemocytes, which are required for the migration of A. phagocytophilum from the gut to the salivary glands. Silencing of p11 impaired the A. phagocytophilum infection of tick haemocytes in vivo and consequently decreased pathogen infection of the salivary glands. In vitro experiments showed that P11 could bind to A. phagocytophilum and thus facilitate its infection of tick cells. This report provides new insights into A. phagocytophilum infection of ticks and reveals new avenues to interrupt the life cycle of Anaplasma and related Rickettsial pathogens.
Keywords: Anaplasma , haemocytes, Ixodes ticks, salivary gland protein
Introduction
Human anaplasmosis, caused by Anaplasma phagocytophilum, is an emerging tick-borne illness that has been reported in the United States, Europe and Asia (Bakken et al, 1994; Paulauskas et al, 2009; Zhan et al, 2010). A. phagocytophilum is an obligate intracellular pathogen, closely related to organisms of the genera Rickettsia and Ehrlichia, that primarily resides in the neutrophils of its mammalian hosts (Bakken & Dumler, 2000). Understanding how the organism infects and survives in a short-lived and hostile phagocytic cell such as the neutrophil has been a major research focus in the field. A. phagocytophilum uses the P-selectin glycoprotein ligand, PSGL-1, to enter the neutrophils (Herron et al, 2000). Once inside the neutrophils, the bacterium uses several strategies to pre-empt neutrophil killing mechanisms and delays neutrophil apoptosis to successfully survive and disseminate in the host (Carlyon & Fikrig, 2006; Rikihisa, 2010). Uninfected Ixodes scapularis ticks acquire the pathogen while feeding on A. phagocytophilum-infected mice. Once inside the ticks, the bacteria migrate from the gut to infect the secretory acini of the salivary glands (Hodzic et al, 1998), and here they persist through the moult to the next life stage of the tick. When an infected tick takes a blood meal, the bacteria replicate in the salivary glands and exit them, possibly with the tick saliva, to enter the mammalian host and invade the neutrophils to complete its life cycle.
A molecular understanding of the strategies used by the bacterium to infect the tick salivary gland is only beginning to unfold (Sukumaran et al, 2006; Sultana et al, 2010). When a tick feeds on an infected host, A. phagocytophilum predominantly contained in the morulae within the host neutrophils enters the gut along with the blood meal and infects the salivary gland within 24 h of feeding (Hodzic et al, 1998). Exactly how these obligate intracellular pathogens migrate from the gut to the salivary glands is not clear. In this report we show that the secreted salivary protein, P11, facilitates A. phagocytophilum infection of tick haemocytes, the blood cells of the tick, which is a critical step that possibly precedes infection of the salivary glands.
Results And Discussion
Anaplasma alters tick salivary gland gene expression
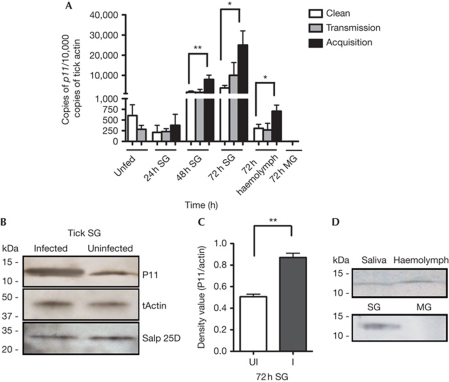
To assess the influence of A. phagocytophilum infection on the salivary gland transcriptome, a subset array of 200 secreted salivary gland genes was probed with amplified RNA generated from A. phagocytophilum-infected and uninfected nymphal I. scapularis salivary glands. The expression levels of several salivary gland genes were altered in the presence of A. phagocytophilum (supplementary Table S1 online). Quantitative PCR (qPCR) analysis revealed that DQ066011 expression in tick salivary glands was increased about 3.7-fold when uninfected nymphs fed on A. phagocytophilum-infected mice (acquisition of A. phagocytophilum), and 1.6-fold when A. phagocytophilum-infected nymphs fed on naive mice (transmission of A. phagocytophilum) compared with that in uninfected nymphs fed on naive mice (Fig 1A). In this study, we therefore characterized DQ066011 (henceforth referred to as p11) in the context of A. phagocytophilum infection of I. scapularis. p11 encodes an ∼11.8-kDa predicted mature protein. P11 also contains a signal peptide indicative of a secretory protein as assessed by SignalP3.0 (http://www.cbs.dtu.dk/services/SignalP/). Immunoblotting using rP11 antiserum recognized an approximately 12-kDa protein in nymphal salivary gland extracts (Fig 1B). Immunoblotting also confirmed the upregulation of P11 in the protein extracts of A. phagocytophilum-acquiring salivary glands (Fig 1B,C). p11 messenger RNA was also detected in the haemocytes of the fed nymphs and its expression was increased about twofold during A. phagocytophilum acquisition compared with that in the haemocytes of uninfected nymphs fed on naive mice and during A. phagocytophilum transmission (Fig 1A). P11 protein could also be detected in the A. phagocytophilum-acquiring tick haemolymph and tick saliva by immunoblotting (Fig 1D), suggesting that P11 is a secreted salivary protein. As P11 is expressed by haemocytes and salivary glands (Fig 1), it might have a dual role in Anaplasma infection of the salivary glands and haemocytes. Absence of tools to preferentially silence p11 in each of these tissues precludes our ability to address the tissue-specific roles of P11. p11 mRNA and protein were not detectable in the tick gut (Fig 1A,D).
Figure 1.
Tick SG protein P11 is induced during tick feeding on A. phagocytophilum-infected mice. (A) The expression profile of p11 in unfed nymph ticks, or in the SG of naive nymphal ticks feeding on clean mice (Clean); A. phagocytophilum-infected mice (Acquisition); or A. phagocytophilum-infected nymphal ticks feeding on naive mice (Transmission); p11 expression levels in the haemolymph of nymphs during acquisition and transmission. (B–D) Western blot assessment of P11 expression: during acquisition in tick SG (B,C); and in the haemolymph and MG of nymphal ticks during acquisition and in saliva from adult ticks (D). Tick actin and another unrelated tick SG protein Salp25D were used as loading control. Error bars show means±s.e.m. *P<0.05 and **P<0.01. The three independent experiments yielded similar results. MG, midgut; SG, salivary gland.
BLAST analysis of the protein sequence showed that P11 had 33% identity with Dickkopf (DKK)-related proteins (Zorn, 2001). DKK-related proteins bind to the LDL-receptor-related protein 5/6 via the carboxy-terminal cysteine-rich domain of DKK and regulate the Wnt signalling pathway (Li et al, 2002; Chen et al, 2008). Interestingly, P11 has 34% identity with the C-terminal cysteine-rich domain of DKK (supplementary Fig S1 online), suggesting a potential protein/ligand-binding domain on P11.
Silencing p11 reduces Anaplasma acquisition
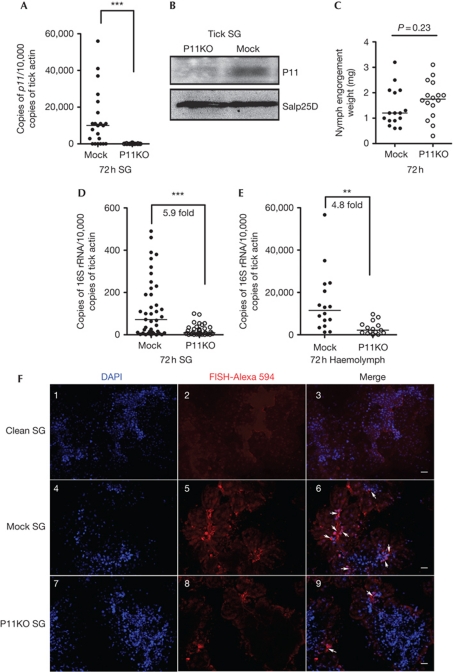
To examine whether P11 has a role in the acquisition of A. phagocytophilum by ticks, p11-deficient ticks were generated by RNA-mediated interference. The silencing efficiency of p11 was confirmed at the mRNA and protein levels by qPCR and immunoblotting, respectively (Fig 2A,B). The expression of Salp25D, an unrelated tick salivary protein, was not altered (Fig 2B). There was no impairment of tick feeding in P11-silenced ticks, as assessed by the comparable engorgement weights of buffer-injected and p11 double-stranded RNA (dsRNA)-injected ticks (Fig 2C). However, the A. phagocytophilum load in the salivary glands of p11-deficient ticks was significantly lower when compared with the control ticks (Fig 2D). Interestingly, the A. phagocytophilum load in the haemolymph was also decreased in the p11-deficient ticks when compared with control ticks (Fig 2E). RNA-fluorescence in situ hybridization analysis of the A. phagocytophilum burden in tick salivary glands also showed a decreased A. phagocytophilum burden in the p11-deficient tick salivary glands when compared with control ticks at 72 h post feeding on infected mice (Fig 2F). These results indicate that A. phagocytophilum infects the haemocytes and uses a secreted tick protein, P11, to facilitate its infection.
Figure 2.
Silencing p11 expression decreases A. phagocytophilum burden in tick SG and haemolymph. (A) Silencing of p11 expression. (B) Specificity of p11 silencing at the protein level. Tick SG protein Salp25D was used as control. (C) Tick feeding was not impaired after silencing p11 expression. (D,E) The levels of A. phagocytophilum 16S rRNA transcripts in tick SG (D) and haemolymph (E). (F) RNA-FISH microscopy of A. phagocytophilum in clean tick SG (Clean SG, panels 1–3), in mock tick SG (Mock SG, panels 4–6) and in P11-deficient tick SG (P11KO SG, panels 7–9). The horizontal line represents the median. **P<0.01 and ***P<0.001. The three independent experiments yielded similar results. Scale bar, 50 μm. MG, midgut; RNA-FISH, RNA-fluorescence in situ hybridization; SG, salivary gland.
P11 secreted into the bite site at the vector–host interface could also influence Anaplasma transmission to the host. We therefore determined whether pathogen survival in Ixodes ticks and transmission to mammalian host were also influenced by P11. However, when A. phagocytophilum-infected nymphal ticks were injected with p11 dsRNA, no significant difference in the bacterial load was observed in unfed and fed ticks. A. phagocytophilum load in the blood and skin of mice fed upon by either control or P11-deficient ticks was also comparable at all time points (supplementary Fig S2 online).
Anaplasma requires P11 for migration in ticks
A time-course assessment of the levels of A. phagocytophilum in the gut, haemolymph and salivary glands of feeding ticks was performed to determine the role of P11 in A. phagocytophilum infection of specific tissues. At 24 h of feeding, low bacterial load was observed in both control and p11-deficient ticks (supplementary Fig S3A online). However, the levels of A. phagocytophilum in the salivary glands and haemolymph of p11-deficient ticks at 48 and 72 h was significantly less than that in control ticks (supplementary Fig S3B,C online). In contrast, the levels of A. phagocytophilum in the gut did not show any change at any time point in both control and p11-deficient ticks (supplementary Fig S3D online). Ticks at 72 h post repletion also showed a significant decrease in A. phagocytophilum load in P11-deficient tick salivary glands compared with mock salivary glands (supplementary Fig S3E online). Taken together, these observations suggest that P11 is critical for Anaplasma infection of the haemocytes and salivary glands in the post-gut phase of the pathogen.
To further confirm the knockdown phenotype and exclude the possibility of off-target silencing, recombinant P11 protein purified from Drosophila S2 cells (rP11-DES) was injected into p11-deficient nymphal ticks that were then allowed to feed on A. phagocytophilum-infected mice for 72 h. The A. phagocytophilum burden was restored in the tick salivary glands and haemolymph of nymphs that received rP11-DES, when compared with nymphs that were injected with p11 dsRNA alone (supplementary Fig S3F,G online).
P11 is required for Anaplasma hemocyte infection
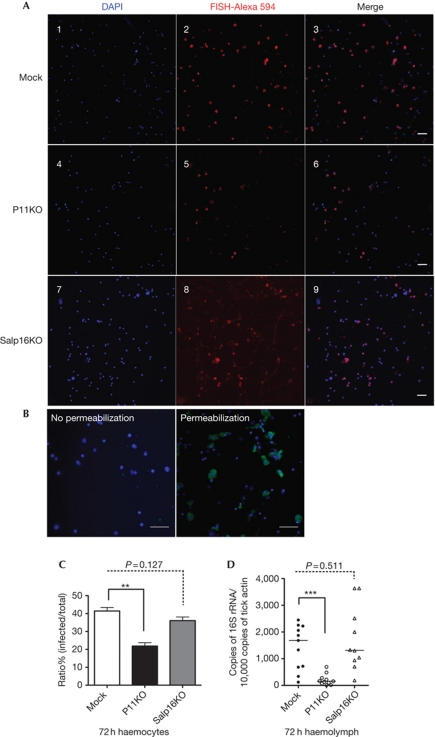
Tick haemolymph consists of haemocytes (circulating cells) and plasma (haemocyte-free) (Kuhn & Haug, 1994; Borovickova & Hypsa, 2005). Since the A. phagocytophilum burden was decreased in P11-deficient tick haemolymph, we determined whether A. phagocytophilum could infect tick haemocytes. Haemolymphs from mock and P11-deficient ticks were collected after 72 h of feeding and stained by RNA-fluorescence in situ hybridization. A red fluorescence signal, which indicated A. phagocytophilum, was observed in haemocytes from mock ticks (Fig 3A-3) and P11-deficient ticks (Fig 3A-6), but not in the uninfected ticks (supplementary Fig S4 online). Immunofluorescence staining of permeabilized and unpermeabilized haemocytes revealed A. phagocytophilum within the haemocytes (Fig 3B), confirming the entry of bacteria into the haemocytes. While 40% of haemocytes were infected by A. phagocytophilum in the mock group, the infection was decreased to about 20% in P11-deficient ticks (Fig 3C), showing that A. phagocytophilum infects tick haemocytes and P11 facilitates the infection. Another tick protein, Salp16, was earlier shown to enable A. phagocytophilum infection of tick salivary glands (Sukumaran et al, 2006). The mRNA of salp16 was not detectable in the tick haemolymph by qPCR analysis (supplementary Fig S5 online). We examined whether silencing salp16 expression would also influence infection of tick haemocytes. However, A. phagocytophilum infection of haemocytes was not altered in the absence of salp16 expression (Fig 3A-9,C,D), suggesting that salp16 specifically influenced salivary gland infection by mechanisms that remain to be elucidated.
Figure 3.
P11 is required for A. phagocytophilum infection of tick haemocytes. (A) (Panels 1–3) RNA-FISH images of A. phagocytophilum in mock group haemocytes (Mock); (panels 4–6) P11-deficient tick haemocytes (P11KO); (panels 7–9) Salp16-deficient tick haemocytes (Salp16KO). (B) A. phagocytopholum was detected in permeabilized tick haemocytes, but not in unpermeabilized haemocytes, by immunofluorescence analysis. (C) Quantification of A. phagocytophilum infection ratio in mock (Mock), P11-deficient (P11KO) and Salp16-deficient (Salp16KO) tick haemocytes. (D) Levels of A. phagocytophilum 16S rRNA transcripts in mock, p11-deficient (p11KO) and salp16-deficient (Salp16KO) tick haemolymph. Error bars show means±s.e.m. The horizontal line represents the median. **P<0.01 and ***P<0.001. The three independent experiments yielded similar results. Scale bar, 50 μm. RNA-FISH, RNA-fluorescence in situ hybridization.
P11 antibodies impair Anaplasma migration
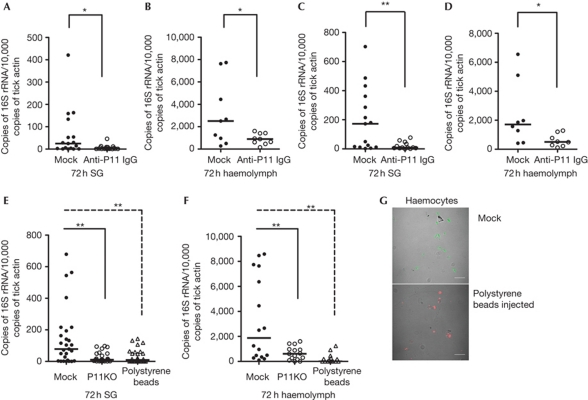
We also examined whether P11 antibodies could block A. phagocytophilum migration from the gut to the salivary gland. Purified rabbit anti-rP11 IgG was injected into tick haemocoel and the ticks fed on A. phagocytophilum-infected mice. The results showed that the A. phagocytophilum burden in the tick salivary glands and haemolymph was decreased in the anti-rP11 IgG-injected group compared with the control group (Fig 4A,B). However, there was no difference in the bacterial load of the midgut (supplementary Fig S6A online). Further, when A. phagocytophilum-infected C3H mice were passively immunized with rabbit anti-P11 serum and naive nymphal ticks fed on the mice, the A. phagocytophilum load was also decreased in the tick salivary glands and haemolymph (Fig 4C,D), but not in the midgut (supplementary Fig S6B online).
Figure 4.
A. phagocytophilum migration in tick SG and haemolymph was blocked by P11 antiserum and polystyrene beads. (A,B) Normal rabbit IgG (Mock) or purified rabbit anti-P11 IgG was injected into the haemocoel and ticks fed on A. phagocytophilum-infected mice. The levels of A. phagocytophilum 16S rRNA transcripts were compared in SG (A) and haemolymph (B). (C,D) A. phagocytophilum-infected mice were passively immunized by normal rabbit IgG (Mock) or purified rabbit anti-P11 IgG (P11 Ab), and the levels of A. phagocytophilum 16S rRNA were measured in tick SG (C) and haemolymph (D) after feeding for 72 h. (E,F) Polystyrene beads were injected into the haemocoel of clean nymphs and the ticks fed on A. phagocytophilum-infected mice. The levels of A. phagocytophilum 16S rRNA transcripts were compared in SG (E) and haemolymph (F). (G) Confocal microscopy image of A. phagocytophilum (Alexa488) in mock and polystyrene beads-injected haemocytes (red amine labelled). The horizontal line represents the median. *P<0.05 and **P<0.01. The three independent experiments yielded similar results. Scale bar, 50 μm. SG, salivary gland.
Inhibition of hemocyte phagocytosis impairs migration
Although the results obtained indicate that P11 facilitated the infection of haemocytes, it was important to show that this event preceded salivary gland infection and was indeed critical for salivary gland infection. Polystyrene beads impair phagocytosis of haemocytes (Elrod-Erickson et al, 2000). We therefore examined whether inhibiting the phagocytic ability of haemocytes by injection of polystyrene beads into tick haemocoel and preoccupying the haemocytes would impact A. phagocytophilum migration to salivary glands. Polystyrene beads were microinjected into the body of clean nymphal ticks that were then allowed to feed on A.phagocytophilum-infected mice, as described in Methods. The results showed that the A. phagocytophilum burden in tick haemocytes and salivary glands was significantly decreased in the polystyrene bead-injected group compared with the control group (Fig 4E,F). Confocal microscopy showed that haemocytes from the bead-injected nymphs had decreased bacterial burden (Fig 4G). However, there was no difference in the bacterial load in the midgut (supplementary Fig 6C online). As p11 silencing phenocopied haemocyte phagocytosis impairment (Fig 4), we posit that P11 facilitates A. phagocytophilum infection of haemocytes and precedes salivary gland infection.
P11 facilitates infection by binding to the bacteria
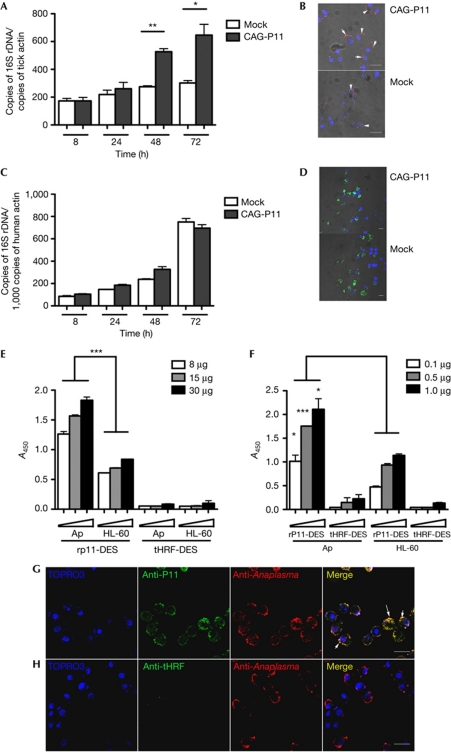
We then determined whether P11 could facilitate A. phagocytophilum infection in vitro by using HL-60 and I. ricinus tick cell lines. P11 was cloned into an expression vector (pCAG) with chicken actin promoter (Kurtti et al, 2008). The expression of P11 was confirmed by PCR and immunoblotting from protein extracts of cells at 2 days post transfection (supplementary Fig S7A,B online). qPCR assessment of A. phagocytophilum burden showed significantly higher burdens in tick cells that overexpressed P11 protein, when compared with the control group (Fig 5A). Assessment of bacterial burden by confocal microscopy also showed increased numbers of bacteria in cells expressing P11 (Fig 5B). However, in HL-60 cells, the pathogen burden was comparable between P11-overexpressed and control groups (Fig 5C,D). To confirm that P11 could facilitate A. phagocytophilum infection in vitro, bacteria were incubated with purified rP11-DES protein for 1 h before the infection of the I. ricinus tick cells and HL-60 cells. qPCR analysis showed that rP11-DES facilitated A. phagocytophilum infection of tick cells, but not that of HL-60 cells (supplementary Fig S7C,D online).
Figure 5.
P11 facilitate A. phagocytophilum infection by binding to the bacteria. (A,C) The levels of A. phagocytophilum 16S rRNA transcripts at different time points after infection of control (Mock) and p11-overexpressed (CAG-P11) I. ricinus tick cells (A) and HL-60 cells (C). (B,D) Confocal microscopy of A. phagocytophilum in control (Mock) and p11-overexpressed (CAG-P11) (B) I. ricinus tick cells and (D) HL-60 cells. (E,F) Dose-dependent increase in binding of the A. phagocytophilum-enriched fraction to rP11-DES (E), and that of rP11-DES to the A. phagocytophilum-enriched fraction (F). Tick salivary protein, tHRF, was used as control. (G,H) Confocal microscopy of infected tick cells shows direct interaction between P11 and A. phagocytophilum (G); tHRF was used as control (H). Error bars show means±s.e.m. *P<0.05, **P<0.01 and ***P<0.001. The three independent experiments yielded similar results. Scale bar, 20 μm.
To assess the mechanism by which tick protein P11 facilitates A. phagocytophilum infection in ticks, we examined whether P11 could interact with A. phagocytophilum and potentially facilitate the pathogen entry or survival in the salivary gland and haemocytes of ticks. The in vitro binding assay using rP11-DES suggested that P11 could bind to A. phagocytophilum-enriched fractions. Tick protein, tHRF, which was used as a control, did not show any binding with A. phagocytophilum-enriched fractions (Fig 5E,F). Confocal microscopy analysis showed that purified rP11-DES, but not tHRF, co-localized with A. phagocytophilum in tick cells in vitro, which indicated that there was direct interaction between A. phagocytophilum and P11 (Fig 5G,H). As P11 specifically enhanced the infection of tick cells, but not that of HL60 cells (Fig 5), we speculate that P11 might straddle an A. phagocytophilum membrane ligand and a tick haemocyte membrane ligand to enable infection of tick cells. However, attempts to demonstrate specific binding of P11 to tick cells were unsuccessful.
As P11 silencing, anti-P11 antibodies or impairment of haemocytes by latex beads had no influence on the ability of A. phagocytophilum to infect the midgut, the P11–Anaplasma interaction probably occurs in the post-midgut phase of acquisition. It is plausible that Anaplasma is extracellular, as it exits the midgut and interacts with the P11 secreted by haemocytes. Future efforts will delineate the molecular interactions between the bacterium and P11 during this event.
In the current study, we provide a new insight into a pathogen's strategy to migrate from the gut to the salivary glands through the immunocompetent milieu of the haemolymph (Eslin et al, 2009) and reveal that A. phagocytophilum infection of the haemocytes is critical for successful infection of the salivary glands. Ixodes ticks vector several ehrlichial and rickettsial human pathogens (Azad & Beard, 1998). The data presented in this study would inspire a similar investigation of related Anaplasma and Rickettsial pathogens to determine whether they might use similar molecular strategies to infect specific arthropod vectors. Pathogen acquisition by the vector is critical to maintain pathogen levels in the vector and in reservoir hosts in endemic areas. Targeting this pivotal step would therefore help thwart the life cycle of Anaplasma and Rickettsial vector-borne pathogens. Advancing our molecular understanding of how these pathogens infect their vectors is therefore a critical preamble to the development of new approaches to control the prevalence of infected vectors in endemic areas.
Methods
Cell lines, mice and ticks, detailed protocols for RNA-mediated interference, RNA and DNA extractions, RT–PCR, protein expression, immunoblotting and confocal microscopy. See supplementary information online.
A. phagocytophilum acquisition. For the acquisition experiments, control ticks (buffer-injected) and ticks injected with p11 dsRNA were allowed to feed on A. phagocytophilum-infected mice. Tick salivary glands, haemolymph and midgut were collected at different time points and pathogen burden was analysed by qPCR. Details can be found in supplementary information online.
Blocking A. phagocytophilum migration in ticks by anti-P11 antibodies. Purified rabbit anti-rP11 IgG (500 ng per tick) was injected into the haemocoel of naive nymphal ticks that were then allowed to feedon A. phagocytophilum-infected mouse, as described above. Normal rabbit IgG was injected as control. To further confirm the blocking of anti-P11 antibodies, A. phagocytophilum-infected C3H mice were passively immunized with 200 μl of normal rabbit serum (as control) or anti-P11 antiserum. At 24 h after immunization, naive nymphal ticks were placed on each mouse as described above. Details can be found in supplementary information online.
Micro-injection of rP11-DES and polystyrene beads into the tick haemocoel. Recombinant P11 protein (100 ng), purified from Drosophila expression system (rP11-DES), was injected into the tick haemocoel. The control ticks (Mock) received the injection buffer (10 mM Tris–HCl, pH 7.5, and 1 mM EDTA).
Red amine-conjugated polystyrene beads, 0.2 μm diameter (Molecular Probes, CA, USA), were washed twice with injection buffer (10 mM Tris–HCl, pH 7.5, and 1 mM EDTA) and re-suspended into their original volume. Beads of volume 10 nl were injected into the tick haemocoel.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank Debby Beck for technical support in the mice and tick experiments. We thank Lindsay Rollend for maintaining and providing uninfected and A. phagocytophilum-infected I. scapularis nymphal ticks. We thank Dr Lesley Bell-Sakyi and the University of Edinburgh for providing the I. ricinus (L.) cell line IRE/CTVM19. This work was supported by National Institutes of Health grant R01AI041440. E.F. is an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare that they have no conflict of interest.
References
- Azad AF, Beard CB (1998) Rickettsial pathogens and their arthropod vectors. Emerg Infect Dis 4: 179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken JS, Dumler JS (2000) Human granulocytic ehrlichiosis. Clin Infect Dis 31: 554–560 [DOI] [PubMed] [Google Scholar]
- Bakken JS, Dumler JS, Chen SM, Eckman MR, Van Etta LL, Walker DH (1994) Human granulocytic ehrlichiosis in the upper Midwest United States. A new species emerging? JAMA 272: 212–218 [PubMed] [Google Scholar]
- Borovickova B, Hypsa V (2005) Ontogeny of tick hemocytes: a comparative analysis of Ixodes ricinus and Ornithodoros moubata. Exp Appl Acarol 35: 317–333 [DOI] [PubMed] [Google Scholar]
- Carlyon JA, Fikrig E (2006) Mechanisms of evasion of neutrophil killing by Anaplasma phagocytophilum. Curr Opin Hematol 13: 28–33 [DOI] [PubMed] [Google Scholar]
- Chen L, Wang K, Shao Y, Huang J, Li X, Shan J, Wu D, Zheng JJ (2008) Structural insight into the mechanisms of Wnt signaling antagonism by Dkk. J Biol Chem 283: 23364–23370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod-Erickson M, Mishra S, Schneider D (2000) Interactions between the cellular and humoral immune responses in Drosophila. Curr Biol 10: 781–784 [DOI] [PubMed] [Google Scholar]
- Eslin P, Prevost G, Havard S, Doury G (2009) Immune resistance of Drosophila hosts against Asobara parasitoids: cellular aspects. Adv Parasitol 70: 189–215 [DOI] [PubMed] [Google Scholar]
- Herron MJ, Nelson CM, Larson J, Snapp KR, Kansas GS, Goodman JL (2000) Intracellular parasitism by the human granulocytic ehrlichiosis bacterium through the P-selectin ligand, PSGL-1. Science 288: 1653–1656 [DOI] [PubMed] [Google Scholar]
- Hodzic E, Fish D, Maretzki CM, De Silva AM, Feng S, Barthold SW (1998) Acquisition and transmission of the agent of human granulocytic ehrlichiosis by Ixodes scapularis ticks. J Clin Microbiol 36: 3574–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn KH, Haug T (1994) Ultrastructural, cytochemical, and immunocytochemical characterization of haemocytes of the hard tick Ixodes ricinus (Acari; Chelicerata). Cell Tissue Res 277: 493–504 [Google Scholar]
- Kurtti TJ, Mattila JT, Herron MJ, Felsheim RF, Baldridge GD, Burkhardt NY, Blazar BR, Hackett PB, Meyer JM, Munderloh UG (2008) Transgene expression and silencing in a tick cell line: a model system for functional tick genomics. Insect Biochem Mol Biol 38: 963–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Mao J, Sun L, Liu W, Wu D (2002) Second cysteine-rich domain of Dickkopf-2 activates canonical Wnt signaling pathway via LRP-6 independently of dishevelled. J Biol Chem 277: 5977–5981 [DOI] [PubMed] [Google Scholar]
- Paulauskas A, Radzijevskaja J, Rosef O (2009) Anaplasma in ticks feeding on migrating birds and questing ticks in Lithuania and Norway. Clin Microbiol Infect 15(Suppl 2): 34–36 [DOI] [PubMed] [Google Scholar]
- Rikihisa Y (2010) Anaplasma phagocytophilum and Ehrlichia chaffeensis: subversive manipulators of host cells. Nat Rev Microbiol 8: 328–339 [DOI] [PubMed] [Google Scholar]
- Sukumaran B, Narasimhan S, Anderson JF, DePonte K, Marcantonio N, Krishnan MN, Fish D, Telford SR, Kantor FS, Fikrig E (2006) An Ixodes scapularis protein required for survival of Anaplasma phagocytophilum in tick salivary glands. J Exp Med 203: 1507–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana H, Neelakanta G, Kantor FS, Malawista SE, Fish D, Montgomery RR, Fikrig E (2010) Anaplasma phagocytophilum induces actin phosphorylation to selectively regulate gene transcription in Ixodes scapularis ticks. J Exp Med 207: 1727–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan L et al. (2010) Anaplasma phagocytophilum from rodents and sheep, China. Emerg Infect Dis 16: 764–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn AM (2001) Wnt signalling: antagonistic Dickkopfs. Curr Biol 11: R592–R595 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.