Abstract
The tandem Tudor-like domain-containing protein Spindlin1 has been reported to be a meiotic spindle-associated protein. Here we report that Spindlin1 is not associated with the spindle in mouse embryonic fibroblast cells during mitotic divisions. In interphase cells, Spindlin1 specifically localizes to the nucleoli. Moreover, Spindlin1 is a histone methylation effector protein that specifically recognizes H3K4 methylation. Finally, Spindlin1 localizes to the active ribosomal DNA (rDNA) repeats, and Spindlin1 facilitates the expression of rRNA genes.
Keywords: Spindlin1, histone methylation, nucleolus, rRNA, transcription
Introduction
Histone lysine methylation is a pivotal regulator of chromatin structure and function (Shilatifard, 2006; Allis et al, 2007; Bannister & Kouzarides, 2011; Zhu & Reinberg, 2011). In most cases, histone lysine methylations are specifically recognized by effector proteins through which they exert their functions (Yun et al, 2011). Similarly to RNA polymerase II-mediated messenger RNA (mRNA) transcription, RNA polymerase I-mediated rRNA gene transcription is regulated by histone lysine methylations (McStay & Grummt, 2008). Active rDNA repeats are enriched for H3K4 and H3K36 methylations, whereas repressed rDNA repeats are enriched for H3K9 methylation (McStay & Grummt, 2008). Many chromatin modifiers have been reported to regulate rRNA gene transcription. The histone demethylases KDM2A and KDM2B are nucleolar proteins that repress rRNA gene transcription (Frescas et al, 2007; Tanaka et al, 2010); interestingly, two other closely related histone demethylases, PHF2 and PHF8, also localize to nucleoli and activate rRNA gene transcription by demethylating H3K9me1/2 (Feng et al, 2010; Wen et al, 2010; Zhu et al, 2010). Although enzymes that modify histone methylation are known to influence rRNA gene transcription, little is known about the roles of effector proteins in mediating rRNA transcriptional regulation.
Spindlin1 was initially identified as a meiotic spindle-binding protein in mice (Oh et al, 1997). The human homologue of Spindlin1 has been reported to be a nuclear protein that transforms NIH3T3 cells on overexpression (Gao et al, 2005). The crystal structure of the human Spindlin1 protein shows that it has a fold resembling tandem Tudor-like domains (Zhao et al, 2007). Several Tudor-like domain-containing proteins have been characterized as histone lysine methylation effector proteins (Botuyan et al, 2006; Du et al, 2006; Huang et al, 2006; Kim et al, 2006; Lee et al, 2008), which raised the possibility that Spindlin1 might also be a histone methylation effector protein.
Here we report that human Spindlin1 specifically recognizes H3K4 methylation in vitro. Moreover, Spindlin1 is a nucleolar protein that localizes to active rDNA loci and facilitates the expression of rRNA genes.
Results And Discussion
Spindlin1 is a nucleolar protein
Spindlin1 has been reported to be a tandem Tudor-like domain-containing protein (Zhao et al, 2007) associated with the meiotic spindle (Oh et al, 1997). Because of our research interest in histone modification-mediated mitotic epigenetic inheritance (Xu & Zhu, 2010; Xu et al, 2010; Chen et al, 2011; Wu & Zhu, 2011), we decided to investigate whether Spindlin1 might be a histone lysine methylation-binding protein that specifies the kinetochore. To our surprise, indirect immunofluorescence staining with antibodies specific for Spindlin1 showed that little Spindlin1 was localized to the mitotic spindle or centromeres in prometaphase, anaphase and telophase mouse embryonic fibroblast cells (supplementary Fig S1A–C online). In contrast, Spindlin1 appeared to be enriched at nuclear compartments that showed little 4,6-diamidino-2-phenylindole (DAPI) staining in interphase cells (supplementary Fig S1D online), which indicates that Spindlin1 might be a nucleolar protein.
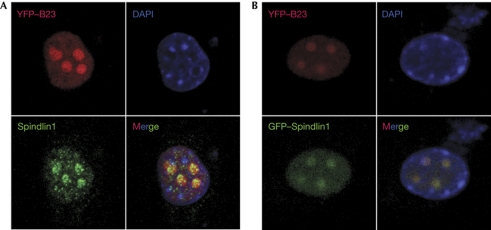
Staining for Spindlin1 in mouse embryonic fibroblasts transfected with a nucleolar marker, yellow fluorescent protein-fused B23 (Zhu et al, 2010), showed Spindlin1 to be highly enriched in the nucleoli (Fig 1A). To exclude the possibility that Spindlin1 staining in the nucleolus was the result of nonspecific antibody crossreactivity, green fluorescent protein-fused Spindlin1 was co-transfected with yellow fluorescent protein-fused B23, and the colocalization of the fluorescent proteins clearly showed that Spindlin1 was a nucleolar protein (Fig 1B).
Figure 1.
Spindlin1 is a nucleolar protein. (A) Endogenous Spindlin1 protein colocalizes with the nucleolar marker B23 in mouse fibroblast cells. (B) Green fluorescent protein (GFP)-tagged, ectopically expressed Spindlin1 fusion protein colocalizes with the nucleolar marker B23 in mouse fibroblast cells. DAPI, 4,6-diamidino-2-phenylindole; YFP, yellow fluorescent protein.
Spindlin1 recognizes H3K4 methylation
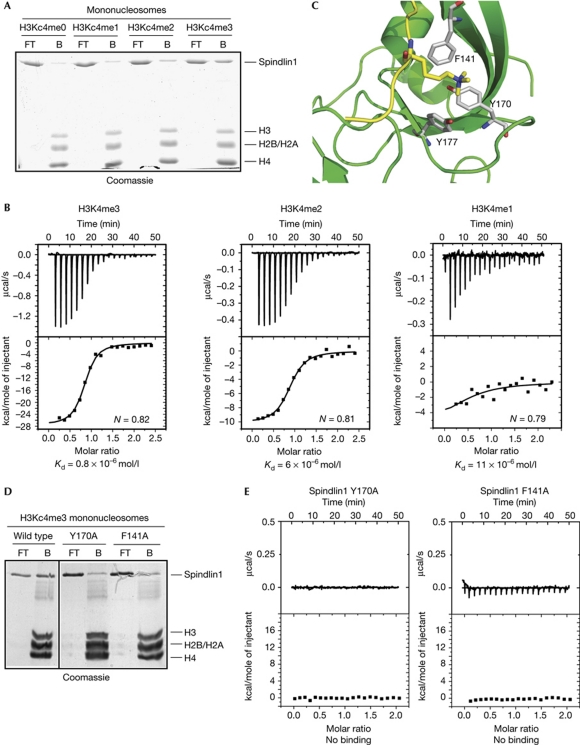
The crystal structure of Spindlin1 showed structural features similar to the Tudor-like domain (Zhao et al, 2007), indicating that Spindlin1 might be a methylated histone-binding protein. To characterize the role of Spindlin1 as an effector protein for histone modifications, pull-down experiments were performed using mononucleosomes (supplementary Fig S2A online) assembled using biotin-labelled 601 nucleosome positioning sequence (Lowary & Widom, 1998) and core histones containing various methylated lysine analogues (MLAs; supplementary Fig S2B online; Simon et al, 2007; Jia et al, 2009). Recombinant Spindlin1 was selectively pulled down using mononucleosomes containing methylated H3Kc4 (Fig 2A), but not methylated H3Kc9 or H3Kc27 (supplementary Fig S3A online).
Figure 2.
Spindlin1 is an H3K4me3-binding protein. (A) Nucleosome pulldown experiments showed that recombinant Spindlin1 recognized nucleosomes containing H3Kc4me2 and H3Kc4me3. (B) Isothermal titration calorimetry analysis showed binding between Spindlin1 and H3K4me3 peptide with a Kd of about 0.8 × 10−6 mol/l, binding between Spindlin1 and H3K4me2 peptide with a Kd of about 6 × 10−6 mol/l, and binding between Spindlin1 and H3K4me1 peptide with a Kd of about 11 × 10−6 mol/l. (C) A modelled structure of Spindlin1 and H3K4me3 complex, showing key residues caging the trimethylated H3K4. (D) Spindlin1 Y170A and F141A mutants showed reduced binding to mononucleosomes containing H3Kc4me3. (E) Spindlin1 Y170A and F141A mutants showed impaired interaction with H3K4me3 peptide.
The specific interaction between H3K4me3 and Spindlin1 was confirmed by isothermal titration calorimetry (ITC) experiments, which showed an interaction between recombinant Spindlin1 and H3K4me3 peptide, with a Kd of about 0.8 × 10−6 mol/l (Fig 2B). Spindlin1 showed a reduced affinity towards H3K4me2 peptide with a Kd of about 6 × 10−6 mol/l and a further reduced affinity towards H3K4me1 peptide with a Kd of about 11 × 10−6 mol/l (Fig 2B) and no apparent interaction with H3K4me0 peptide (supplementary Fig S3B online). In addition, H3K9me3 and H3K27me3 peptides did not show significant interactions with recombinant Spindlin1 protein (supplementary Fig S3C online).
These results show a direct interaction between H3K4 methylation and Spindlin1, which is in agreement with a recent report that showed significant enrichment of Spindlin1 using affinity purification with nucleosomes containing H3K4me3 (Bartke et al, 2010).
Using the crystal structures of Spindlin1 (Zhao et al, 2007) and H3K4me3–JMJD2A Tudor domain complex (Huang et al, 2006), we modelled a structure of the Spindlin1 and H3K4me3 complex using docking methods (Chen et al, 2003). The docking results showed that the trimethylated K4 inserts into a pocket composed of residues Y170, F141 and Y177 (Fig 2C). The predicted binding mode indicates that residues Y177, Y170 and F141 on Spindlin1 interact with the trimethylated K4 by cation-π interactions (Fig 2C).
Recombinant Spindlin1 proteins with Y170 or F141 mutated to alanine were then expressed and analysed for their binding abilities towards H3K4me3. As predicted by the structural data, Spindlin1 Y170A and F141A mutants showed greatly impaired interactions with H3K4me3, in both nucleosome pull-down (Fig 2D) and ITC (Fig 2E) experiments.
Spindlin1 localizes to rDNA loci
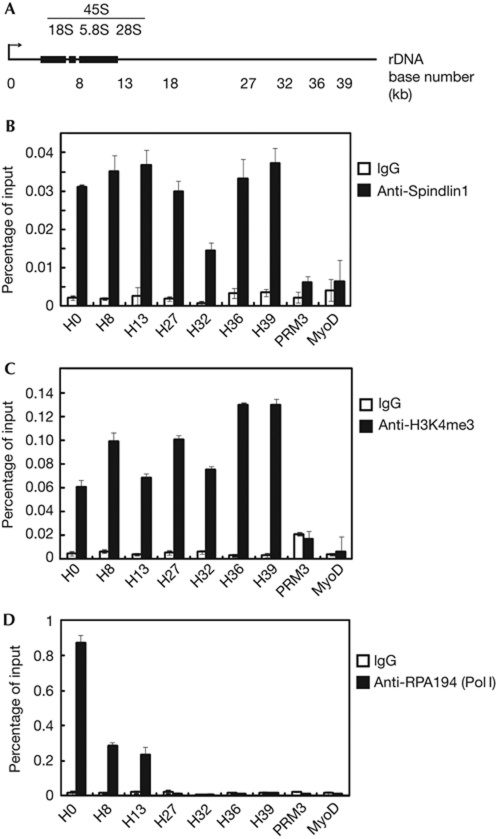
Spindlin1 is a nucleolar protein (Fig 1) that specifically recognizes H3K4me3 (Fig 2), which indicates that Spindlin1 might be localized to rDNA loci and that Spindlin1 might potentially regulate the transcription of rRNA genes. To validate the presence of Spindlin1 at rDNA loci (illustrated in Fig 3A), chromatin immunoprecipitation (ChIP) experiments were performed using antibodies specific for Spindlin1. These experiments confirmed that Spindlin1 was enriched at rDNA loci, but not at two inactive loci, PRM3 (specifically expressed during spermiogenesis; Grzmil et al, 2008) and MyoD (specifically expressed during myogenesis; Tapscott et al, 1988; Fig 3B). In addition, control ChIP experiments were also performed with antibodies specific for H3K4me3 (Fig 3C) and a Pol I subunit RPA194 (Fig 3D).
Figure 3.
Spindlin1 occupies ribosomal DNA (rDNA) loci. (A) Schematic illustration of a single human rDNA repeat. (B) Chromatin immunoprecipitation (ChIP) experiments using HeLa cells showed Spindlin1 occupancy at the rDNA loci. (C) The H3K4me3 profile at rDNA loci as determined using ChIP. (D) The distribution profile of Pol I at rDNA loci as determined using ChIP against RPA194, a Pol I subunit. Error bars represent the standard deviation calculated from three independent experimental repeats. IgG, immunoglobulin G.
Spindlin1 localizes to active rDNA repeats
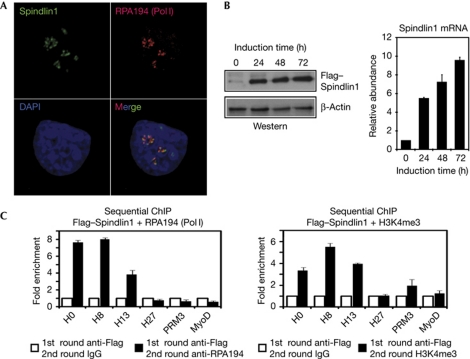
Ribosomal DNA contains both active repeats that are enriched for H3K4 methylation and inactive repeats that are enriched for H3K9 methylation (McStay & Grummt, 2008). To investigate whether Spindlin1 is localized at the active rDNA repeats, we performed co-staining using antibodies against Spindlin1 and RPA194. The immunostaining results showed that Spindlin1 and RPA194 were largely colocalized, which indicates that Spindlin1 was localized at Pol I-engaged, active rDNA repeats (Fig 4A).
Figure 4.
Spindlin1 occupies active ribosomal DNA (rDNA) repeats. (A) Endogenous Spindlin1 colocalizes with Pol I subunit RPA194. (B) Western blot analyses (left panel) and reverse transcription–polymerase chain reaction (right panel) verified the induction of ectopic Flag–Spindlin1 expression in HeLa cells. (C) Sequential chromatin immunoprecipitation (ChIP) showing co-occupancy of Flag–Spindlin1 with RPA194 (left panel) and H3K4me3 (right panel). Error bars represent the standard deviation calculated from three independent experimental repeats. DAPI, 4,6-diamidino-2-phenylindole; IgG, immunoglobulin G; mRNA, messenger RNA.
To further confirm that Spindlin1 localizes to active rDNA repeats, we decided to perform sequential ChIP experiments with a stable cell line expressing a Flag-tagged Spindlin1 using antibodies against Flag and RPA194 or H3K4me3. Attempts to establish a stable cell line that constitutively overexpresses Spindlin1 failed to yield a healthy cell line because continued overexpression of Spindlin1 led to apoptotic cell death, as previously reported (Yuan et al, 2008). Therefore, a stable HeLa cell line was established, in which an N-terminal Flag-tagged Spindlin1 was expressed under the control of a tetracycline-inducible promoter. In this cell line, Spindlin1 protein and mRNA levels were robustly induced by the addition of tetracycline (Fig 4B). Cells were collected for sequential ChIP after 72 h of tetracycline induction; RPA194 and H3K4me3 were robustly enriched at Flag–Spindlin-occupied chromatin (Fig 4C).
The above data collectively indicate that Spindlin1 was localized to active rDNA repeats, which is consistent with its role in recognizing H3K4 methylation.
Spindlin1 facilitates rRNA gene expression
H3K4 methylation recognition proteins, such as NURF (Wysocka et al, 2006), TFIID (Vermeulen et al, 2007), CHD1 (Flanagan et al, 2005; Sims et al, 2005) and Sgf29 (Vermeulen et al, 2010; Bian et al, 2011), are often involved in regulating transcription. Another nucleolar H3K4me3-binding protein, PHF8, has been reported to regulate the transcription of rRNA genes (Feng et al, 2010; Zhu et al, 2010). To investigate whether Spindlin1 also has a role in regulating rRNA gene transcription, the expression level of Spindlin1 was modulated to assess its effect on pre-rRNA production.
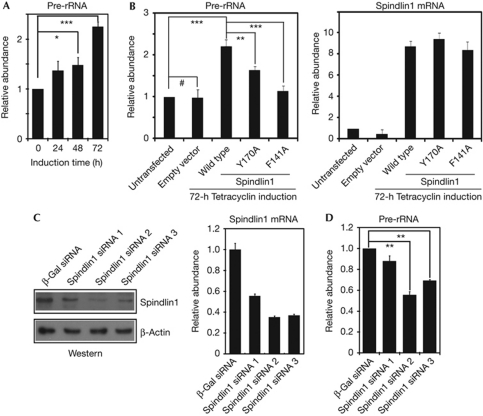
On induction of the Flag–Spindlin1 in the above-mentioned stable cells, pre-rRNA level was significantly elevated in a dose-dependent manner (Fig 5A). In contrast, pre-rRNA level did not change in cells stably transfected with the empty vector (Fig 5B). In addition, the Spindlin1 Y170A and F141A mutants significantly lost their abilities to stimulate the expression of pre-rRNA (Fig 5B). ChIP experiments showed that the rDNA occupancies of these mutants were slightly reduced (supplementary Fig S4A online). In this context, we point out that impaired histone modification binding activity does not necessarily fully abolish the chromatin localization of these mutant effector proteins. First of all, the binding affinity between effector proteins and histone modifications are typically at the μM range, which indicates that the role of such interactions is to stabilize the complex, rather than to serve as the initial recruitment. In addition, other properties of Spindlin1, such as its interaction with DNA (Zhao et al, 2007), its interaction with the endogenous Spindlin1 due to its dimeric structure (Zhao et al, 2007) and other potential protein partners, might also contribute to the partial chromatin localization of these mutants.
Figure 5.
Spindlin1 facilitates the expression of ribosomal RNA (rRNA) genes. (A) Reverse transcription–polymerase chain reaction (RT–PCR) results revealed elevated pre-rRNA levels upon Flag–Spindlin1 induction. (B) RT–PCR results showing impaired stimulation of pre-rRNA expression in cells expressing the Spindlin1 mutants (left panel); the expression levels of the Flag–Spindlin1 were also determined by RT–PCR (right panel). (C) Western blot analyses (left panel) and RT–PCR (right panel) verified the efficiency of Spindlin1 knockdown in HeLa cells. (D) RT–PCR results showed significantly reduced pre-rRNA levels on Spindlin1 knockdown. Error bars represent the standard deviation calculated from three independent experiment repeats. ***P<0.002; **P<0.01; *P<0.05; and #P>0.1. mRNA, messenger RNA; siRNA, small interfering RNA; β-Gal, β-galactosidase.
To further establish the role of Spindlin1 in rRNA gene transcription, Spindlin1 was knocked down in HeLa cells using three different pairs of small interfering RNA (siRNA). Spindlin1 protein and mRNA levels were significantly reduced, especially in cells treated with siRNA pair 2 and pair 3 (Fig 5C). Pre-rRNA level was significantly reduced in cells that showed efficient Spindlin1 knockdown (Fig 5D). Finally, control experiments showed that Spindlin1, but not other Spindlin family members such as Spindlin2A or Spindlin2B, was specifically targeted by the siRNA pair 3 (supplementary Fig S4B online).
These data collectively support a role for Spindlin1 in facilitating the expression of rRNA genes, which is consistent with its biochemical function in recognizing active chromatin modifications, H3K4me2 and H3K4me3 (Fig 2).
Spindlin1 belongs to the Spindlin family, which comprises Spindlin1, Spindlin2A, Spindlin2B and Spindlin3 (supplementary Fig S5 online). All these proteins are poorly studied at present; it would be interesting to investigate whether the other Spindlin family members are also chromatin modification effector proteins and/or whether they might also regulate the expression of rDNA.
Spindlin1 is not a subunit of a stable complex
Because Spindlin1 is a relatively small protein lacking apparent functional domains other than its H3K4me3-binding domain, it is possible that other proteins might associate with Spindlin1 to facilitate its role in transcriptional regulation. Therefore, we attempted to isolate a stable protein complex associated with Spindlin1. Under stringent washing conditions (0.5 M KCl), two protein bands appeared to be enriched in affinity purification eluates from cells expressing Flag-tagged Spindlin1 (supplementary Fig S6 online). Unfortunately, these proteins were identified using mass spectrometry as HSP70 and HSPA8, contaminants common to Flag-tag affinity purifications (supplementary Fig S6 online). This result indicates that Spindlin1-associated protein(s), if any, might be only weakly or transiently associated with Spindlin1. Further investigation to identify such factor(s) will help to deepen our understanding of how Spindlin1 facilitates rRNA gene expression.
Methods
Antibodies. Antibodies against Spindlin1 were purchased from ProteinTech Group (12105-1-AP); antibodies against H3K4me3 were purchased from Millipore (04-745); antibodies against H3K4me2 were purchased from Millipore (07-030); antibodies against PRA194 (Pol I subunit) was purchased from Santa Cruz (SC-48385); and antibodies against Flag were purchased from Sigma (F1804).
ChIP and RT–PCR experiments. Primers for rDNA loci used in ChIP experiments were synthesized according to previously reported sequences (O'Sullivan et al, 2002). The following ChIP primers were used for PRM3: forward primer, GAAGTTATCCTGACTCACAC, and reverse primer, CCAGAGCCCAGGCCACAGCC. The following ChIP primers were used for MyoD: forward primer, GAGAAGCTAGGGGTGAGGAA, and reverse primer, AGTCTGAATGCCCACCCACT.
The reverse transcription–polymerase chain reaction (RT–PCR) primers used for pre-rRNA and glyceraldehyde-3-phosphate dehydrogenase were described previously (Feng et al, 2010; Zhu et al, 2010). The following RT–PCR primers were used for Spindlin1: forward primer, ATGAAGACCCCATTCGGAAA, and reverse primer, TGTGGGATGTCCTCTTCTTC. Spindlin2A and 2B differ at only two bases in the coding region (supplementary Fig S5 online); the following RT–PCR primers were used for Spindlin2A/2B: forward primer, CAACGCACAGGAAGCCGAAG, and reverse primer, TGATGGGCTCATCTCCTTCC.
ChIP–PCR and RT–PCR experiments were performed in triplicate to calculate the standard deviation. A t-test was performed for all the RT–PCR experiments measuring the levels of pre-rRNA to calculate corresponding P values.
Stable cell line. Complementary DNA coding for a Flag-tagged human Spindlin1 was inserted into pcDNA4/TO (Invitrogen) and transfected into HeLa cells. Stable clones were selected using Zeocin (100 μg/ml, Invitrogen).
Methylated lysine analogue reactions and mononucleosome assembly. MLA reactions were performed as previously described (Simon et al, 2007). Core histones were assembled into mononucleosomes using biotin-labelled 601 nucleosome positioning sequence (Lowary & Widom, 1998).
Nucleosome pull-down assay. Recombinant Spindlin1 protein (10 μg) was incubated with 5 μg of biotin-labelled mononucleosomes captured using Streptavidin agarose (Promega) in buffer containing 20 mM Tris–HCl (pH 8.0), 150 mM NaCl and 0.1% NP40 at 4°C for 4 h. Unbound proteins were washed away with excess binding buffer, and proteins associated with the mononucleosomes were eluted using SDS–PAGE gel loading buffer.
Isothermal titration calorimetry. ITC experiments were performed using a MicroCal ITC titration calorimeter (ITC200, USA). Samples were prepared in titration buffer containing 50 mM HEPES (pH 8.0) and 150 mM NaCl. The reaction cell contained a degassed solution of 30 μM recombinant Spindlin1 and 20 2-μl aliquots of 300 μM H3K4me3, H3K9me3 or H3K27me3 peptides. Experimental data were analysed using the ORIGIN analysis software (MicroCal Software, USA).
Structural modelling. Using docking methodology (Chen et al, 2003), we constructed the complex of Spindlin1 with H3K4me3. The structure of H3K4me3 was extracted from the structure of JMJD2A Tudor domain–H3K4me3 complex (PDB code: 2GFA; Huang et al, 2006), and then was docked into the pocket of Spindlin1 (PDB code: 2NS2; Zhao et al, 2007) with ZDOCK program encoded in Discovery Studio (http://www.accelrys.com). The docking modes with the highest score were selected as candidates. After comparing with the binding mode of H3K4me3 in 2GFA, we selected one binding conformation of H3K4me3 in complex with Spindlin1.
RNA interference. siRNA pairs were transfected into HeLa cells using Lipofectamine 2000 (Invitrogen), and cells were collected for analysis 72 h after transfection. The following siRNA sequences were used:
Spindlin1 siRNA pair 1: sense, GGAUUCAGCAUGGGUGGAAdTdT and antisense, UUCCACCCAUGCUGAAUCCdTdT;Spindlin1 siRNA pair 2: sense, GCAAAGCAGUGGAACAUAUdTdT and antisense, AUAUGUUCCACUGCUUUGCdTdT; Spindlin1 siRNA pair 3: sense, GCAUUAUGCCUGAUUCCAAdTdT and antisense, UUGGAAUCAGGCAUAAUGCdTdT; and β-galactosidase siRNA pair: sense, AAGGCCAGACGCGAAUUAUdTdT and antisense, AUAAUUCGCGUCUGGCCUUdTdT.
Affinity purification. Affinity purification of Flag-tagged Spindlin1 was performed as previously described (Yuan et al, 2009). In brief, M2 anti-Flag agarose (Sigma) was equilibrated with buffer containing 20 mM Tris–HCl (pH 7.9), 1.5 mM MgCl2, 0.42 M NaCl and 0.2 mM phenylmethylsulphonyl fluoride and incubated overnight at 4°C with solubilized nuclear pellets derived from stably transfected cells. The resin was washed with buffer containing 20 mM Tris–HCl (pH 7.9), 1.5 mM MgCl2, 0.2 mM phenylmethylsulphonyl fluoride and 0.5 M KCl, and proteins were eluted with the same buffer supplemented with 0.1 mg/ml Flag peptide.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank Ting Li and Feng Shao at the National Institute of Biological Sciences, Beijing, for suggestions and reagents. This work was supported by the Chinese Ministry of Science and Technology 973 grants 2011CB812700 and 2011CB965300 (to B.Z.).
Footnotes
The authors declare that they have no conflict of interest.
References
- Allis CD, Jenuwein T, Reinberg D (2007) Epigenetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Bannister AJ, Kouzarides T (2011) Regulation of chromatin by histone modifications. Cell Res 21: 381–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke T, Vermeulen M, Xhemalce B, Robson SC, Mann M, Kouzarides T (2010) Nucleosome-interacting proteins regulated by DNA and histone methylation. Cell 143: 470–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian C et al. (2011) Sgf29 binds histone H3K4me2/3 and is required for SAGA complex recruitment and histone H3 acetylation. EMBO J 30: 2829–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botuyan MV, Lee J, Ward IM, Kim JE, Thompson JR, Chen J, Mer G (2006) Structural basis for the methylation state-specific recognition of histone H4-K20 by 53BP1 and Crb2 in DNA repair. Cell 127: 1361–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Li L, Weng Z (2003) ZDOCK: an initial-stage protein-docking algorithm. Proteins 52: 80–87 [DOI] [PubMed] [Google Scholar]
- Chen X, Xiong J, Xu M, Chen S, Zhu B (2011) Symmetrical modification within a nucleosome is not required globally for histone lysine methylation. EMBO Rep 12: 244–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du LL, Nakamura TM, Russell P (2006) Histone modification-dependent and -independent pathways for recruitment of checkpoint protein Crb2 to double-strand breaks. Genes Dev 20: 1583–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Yonezawa M, Ye J, Jenuwein T, Grummt I (2010) PHF8 activates transcription of rRNA genes through H3K4me3 binding and H3K9me1/2 demethylation. Nat Struct Mol Biol 17: 445–450 [DOI] [PubMed] [Google Scholar]
- Flanagan JF, Mi LZ, Chruszcz M, Cymborowski M, Clines KL, Kim Y, Minor W, Rastinejad F, Khorasanizadeh S (2005) Double chromodomains cooperate to recognize the methylated histone H3 tail. Nature 438: 1181–1185 [DOI] [PubMed] [Google Scholar]
- Frescas D, Guardavaccaro D, Bassermann F, Koyama-Nasu R, Pagano M (2007) JHDM1B/FBXL10 is a nucleolar protein that represses transcription of ribosomal RNA genes. Nature 450: 309–313 [DOI] [PubMed] [Google Scholar]
- Gao Y et al. (2005) Spindlin1, a novel nuclear protein with a role in the transformation of NIH3T3 cells. Biochem Biophys Res Commun 335: 343–350 [DOI] [PubMed] [Google Scholar]
- Grzmil P et al. (2008) Prm3, the fourth gene in the mouse protamine gene cluster, encodes a conserved acidic protein that affects sperm motility. Biol Reprod 78: 958–967 [DOI] [PubMed] [Google Scholar]
- Huang Y, Fang J, Bedford MT, Zhang Y, Xu RM (2006) Recognition of histone H3 lysine-4 methylation by the double tudor domain of JMJD2A. Science 312: 748–751 [DOI] [PubMed] [Google Scholar]
- Jia G et al. (2009) A systematic evaluation of the compatibility of histones containing methyl-lysine analogues with biochemical reactions. Cell Res 19: 1217–1220 [DOI] [PubMed] [Google Scholar]
- Kim J, Daniel J, Espejo A, Lake A, Krishna M, Xia L, Zhang Y, Bedford MT (2006) Tudor, MBT and chromo domains gauge the degree of lysine methylation. EMBO Rep 7: 397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Thompson JR, Botuyan MV, Mer G (2008) Distinct binding modes specify the recognition of methylated histones H3K4 and H4K20 by JMJD2A-tudor. Nat Struct Mol Biol 15: 109–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowary PT, Widom J (1998) New DNA sequence rules for high affinity binding to histone octamer and sequence-directed nucleosome positioning. J Mol Biol 276: 19–42 [DOI] [PubMed] [Google Scholar]
- McStay B, Grummt I (2008) The epigenetics of rRNA genes: from molecular to chromosome biology. Annu Rev Cell Dev Biol 24: 131–157 [DOI] [PubMed] [Google Scholar]
- O'Sullivan AC, Sullivan GJ, McStay B (2002) UBF binding in vivo is not restricted to regulatory sequences within the vertebrate ribosomal DNA repeat. Mol Cell Biol 22: 657–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh B, Hwang SY, Solter D, Knowles BB (1997) Spindlin, a major maternal transcript expressed in the mouse during the transition from oocyte to embryo. Development 124: 493–503 [DOI] [PubMed] [Google Scholar]
- Shilatifard A (2006) Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem 75: 243–269 [DOI] [PubMed] [Google Scholar]
- Simon MD, Chu F, Racki LR, de la Cruz CC, Burlingame AL, Panning B, Narlikar GJ, Shokat KM (2007) The site-specific installation of methyl-lysine analogs into recombinant histones. Cell 128: 1003–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims RJ, Chen CF, Santos-Rosa H, Kouzarides T, Patel SS, Reinberg D (2005) Human but not yeast CHD1 binds directly and selectively to histone H3 methylated at lysine 4 via its tandem chromodomains. J Biol Chem 280: 41789–41792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Okamoto K, Teye K, Umata T, Yamagiwa N, Suto Y, Zhang Y, Tsuneoka M (2010) JmjC enzyme KDM2A is a regulator of rRNA transcription in response to starvation. EMBO J 29: 1510–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapscott SJ, Davis RL, Thayer MJ, Cheng PF, Weintraub H, Lassar AB (1988) MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science 242: 405–411 [DOI] [PubMed] [Google Scholar]
- Vermeulen M et al. (2010) Quantitative interaction proteomics and genome-wide profiling of epigenetic histone marks and their readers. Cell 142: 967–980 [DOI] [PubMed] [Google Scholar]
- Vermeulen M et al. (2007) Selective anchoring of TFIID to nucleosomes by trimethylation of histone H3 lysine 4. Cell 131: 58–69 [DOI] [PubMed] [Google Scholar]
- Wen H, Li J, Song T, Lu M, Kan PY, Lee MG, Sha B, Shi X (2010) Recognition of histone H3K4 trimethylation by the plant homeodomain of PHF2 modulates histone demethylation. J Biol Chem 285: 9322–9326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Zhu B (2011) Split decision: why it matters? Front Biol 6: 88–92 [Google Scholar]
- Wysocka J et al. (2006) A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodelling. Nature 442: 86–90 [DOI] [PubMed] [Google Scholar]
- Xu M, Long C, Chen X, Huang C, Chen S, Zhu B (2010) Partitioning of histone H3-H4 tetramers during DNA replication-dependent chromatin assembly. Science 328: 94–98 [DOI] [PubMed] [Google Scholar]
- Xu M, Zhu B (2010) Nucleosome assembly and epigenetic inheritance. Protein Cell 1: 820–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H et al. (2008) Overexpression of SPINDLIN1 induces cellular senescence, multinucleation and apoptosis. Gene 410: 67–74 [DOI] [PubMed] [Google Scholar]
- Yuan W et al. (2009) Heterogeneous nuclear ribonucleoprotein L Is a subunit of human KMT3a/Set2 complex required for H3 Lys-36 trimethylation activity in vivo. J Biol Chem 284: 15701–15707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun M, Wu J, Workman JL, Li B (2011) Readers of histone modifications. Cell Res 21: 564–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q et al. (2007) Structure of human spindlin1. Tandem tudor-like domains for cell cycle regulation. J Biol Chem 282: 647–656 [DOI] [PubMed] [Google Scholar]
- Zhu B, Reinberg D (2011) Epigenetic inheritance: uncontested? Cell Res 21: 435–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z et al. (2010) PHF8 is a histone H3K9me2 demethylase regulating rRNA synthesis. Cell Res 20: 794–801 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.