Abstract
In fission yeast, meiotic mono-orientation of sister kinetochores is established by cohesion at the core centromere, which is established by a meiotic cohesin complex and the kinetochore protein Moa1. The cohesin subunit Psm3 is acetylated by Eso1 and deacetylated by Clr6. We show that in meiosis, Eso1 is required for establishing core centromere cohesion during S phase, whereas Moa1 is required for maintaining this cohesion after S phase. The clr6-1 mutation suppresses the mono-orientation defect of moa1Δ cells, although the Clr6 target for this suppression is not Psm3. Thus, several acetylations are crucial for establishing and maintaining core centromere cohesion.
Keywords: cohesin, acetylation, meiosis, chromosome segregation
Introduction
Duplicated chromosomes (sister chromatids) become connected during S phase through the action of a multisubunit complex called cohesin, which consists of four core subunits: two SMC (structural maintenance of chromosome) family ATPase proteins, Smc1 and Smc3, a kleisin family protein, Scc1, and Scc3 (called Psm1, Psm3, Rad21 and Psc3, respectively, in fission yeast; Onn et al, 2008; Peters et al, 2008; Nasmyth & Haering, 2009). Sister chromatid cohesion must be maintained throughout G2 phase until metaphase to identify the pair of chromosomes that must be separated at division. At the onset of anaphase, a protease called separase cleaves the Rad21 subunit, leading to the separation of sister chromatids. In meiosis, the Rad21 subunit is replaced by the meiosis-specific subunit Rec8, which is indispensable for meiosis-specific chromosome events, including monopolar attachment of sister chromatids (Watanabe et al, 2001). Whereas mitotic Rad21-containing cohesin localizes preferentially to the pericentromeric regions, meiotic Rec8-containing cohesin localizes additionally to the core centromere (Watanabe et al, 2001). The establishment of cohesion at the core centromere conjoins the two kinetochore domains and, thus, promotes mono-orientation at meiosis I, whereas the core regions might open to opposite sides when biorientation is favoured as in mitosis or meiosis II (Sakuno et al, 2009). Fission yeast Moa1, a meiosis-specific kinetochore protein, also has a crucial role in creating core centromere cohesion, presumably by assisting Rec8 action, although the molecular details of Moa1 function remain largely elusive (Yokobayashi & Watanabe, 2005).
During the mitotic cell cycle, the replication fork-associated acetyl transferase Eco1 has a crucial role in establishing cohesion in S phase (Uhlmann, 2009). Studies in budding yeast and humans have shown that a key function of Eco1 is the acetylation of two lysine residues of the Smc3 subunit of the cohesin complex (Ben-Shahar et al, 2008; Unal et al, 2008; Zhang et al, 2008; Rowland et al, 2009). Mutations of both lysine residues to non-acetylatable arginine cause cell lethality because of a cohesion defect as seen in eco1-deficient cells. Conversely, mutations of both lysine residues to asparagine or glutamine, which mimic the acetylated state, can sustain cell viability even in the absence of the ECO1 gene, which is otherwise an essential gene. A similar regulation has been shown between human Esco1 (an Eco1 orthologue) and Smc3 (Zhang et al, 2008). In budding yeast, class I histone deacetylase (HDAC), Hos1, removes the acetylation of Smc3 mostly at anaphase (Beckouet et al, 2010; Borges et al, 2010; Xiong et al, 2010). Because the de novo acetylation is crucial for cohesion establishment, its deacetylation at anaphase might enable the recycling of cohesin in the next cell cycle. A recent study in fission yeast also shows that K105 and K106 of Psm3 (corresponding to budding yeast Smc3-K112/K113) is acetylated by Eso1 (Eco1 orthologue; Feytout et al, 2011; supplementary Fig S1A online). The growth defect of eso1-H17 (temperature-sensitive allele of eso1+) or even eso1Δ cells is largely restored by expressing Psm3-K105Q, Psm3-K106Q or Psm3-KKQQ, in which K105 and/or K106 are mutated to the acetyl-mimic residue glutamine (supplementary Fig S1B online). Strikingly, fission yeast psm3-KKRR cells, in which both K105 and K106 on Psm3 are mutated to arginines, retain substantial viability and sister chromatid cohesion during mitosis (Feytout et al, 2011; supplementary Fig S1C–E online). Thus, Eso1 might have an additional acetylation target(s) that promotes the establishment of cohesion in a redundant capacity with Psm3-K105/K106 acetylation (Fig 1A). This second acetylation target might be another site(s) on Psm3 or completely distinct protein. Although the acetylation of cohesin has been studied during the mitotic cell cycle, its role in meiosis is totally unknown. Here we show that acetylation functions in the establishment and maintenance of cohesion at the core centromere in meiosis, which is crucial for setting up mono-orientation of sister kinetochores.
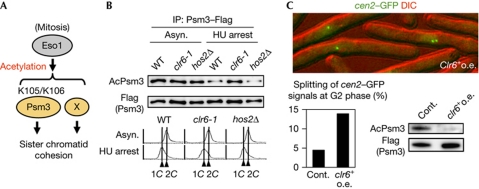
Figure 1.
Clr6 is a lysine deacetylase for the Psm3 subunit of cohesin. (A) Schematic depiction of the acetylation targets of Eso1 in establishing cohesion. X represents a putative acetylation target of Eso1 required for sister chromatid cohesion. (B) The indicated cells were cultured at 30°C and arrested by HU for 4 h. Psm3–Flag protein was immunopurified from the indicated asynchronous (asyn.) or HU-arrested cells. Samples were analysed by immunoblot using anti-AcPsm3 and anti-Flag antibodies (top). DNA content was measured by flow cytometry (bottom). (C) cdc25-22 cen2-green fluorescent protein (GFP) cells carrying pREP1 (Cont.) or pREP1-clr6+ (clr6+ o.e.) were cultured at 25°C in the absence of thiamine for 24 h and shifted to 36°C for 4 h to arrest at G2 phase. A photograph of clr6+ o.e. cells is shown at the top. The number of cells with two cen2–GFP dots was counted (n>220). Acetylation status of Psm3 was analysed by immunoblot as in B (supplementary Fig S3 online). Cont., control; DIC, differential interference contrast; HU, hydroxyurea; IP, immunoprecipitation; o.e., overexpression; WT, wild type.
Results And Discussion
Clr6 is the deacetylase of Psm3-K106 acetylation
As class I HDAC functions in the deacetylation of Smc3 in Saccharomyces cerevisiae, we envisaged the possibility that fission yeast class I HDAC (Clr6 or Hos2) might control cohesin deacetylation (Ekwall, 2005). Whereas hos2Δ cells are viable, clr6+ is indispensable for cell growth; thus, we used the temperature-sensitive mutant allele, clr6-1, for this assay (Grewal et al, 1998). Although the acetylation status of Psm3 was indistinguishable among the asynchronous cultures of wild-type and HDAC mutants, acetylation was significantly increased in clr6-1 cells compared with others if cells were arrested in early S phase (Fig 1B). Given that Psm3 acetylation declines during anaphase towards G1 phase (Feytout et al, 2011; supplementary Fig S2 online), these results indicate that clr6-1 cells show defects in Psm3 deacetylation mainly during anaphase. To examine whether Clr6 has an activity to antagonize Psm3 acetylation in vivo, we overexpressed Clr6 from a plasmid and examined Psm3 acetylation in G2-arrested cells. Indeed, acetylation levels of Psm3-K106 decreased substantially by the overexpression of Clr6, leading to defects in sister chromatid cohesion (Fig 1C; supplementary Fig S3 online). These results support the notion that Clr6 has the potential to antagonize the Eso1 function by removing Psm3 acetylation. Although the clr6-1 mutation did not suppress the growth defect in eso1-H17 cells (supplementary Fig S1F online), this might be tenable because clr6-1 itself impairs cell growth in a different context from Psm3 deacetylation.
Psm3 acetylation is required for mono-orientation
In fission yeast, sister chromatid cohesion at the core centromere has an essential role in the cosegregation of sister chromatids (mono-orientation) at meiosis I, and is prevented from being established during mitosis (Yokobayashi et al, 2003; Yokobayashi & Watanabe, 2005; Sakuno et al, 2009). In meiosis, cohesin is acetylated depending on Eso1 also during late S phase and persists until prophase (Fig 2A). To examine the requirement of cohesin acetylation for mono-orientation, we analysed meiotic chromosome segregation in the eso1-H17 mutant. We marked centromere 2 (cen2-green fluorescent protein) on only one of the two homologous chromosomes and monitored its segregation during meiosis I in recombination-deficient (rec12Δ) zygotes, as the linkage between homologous chromosomes indirectly affects chromosome orientation (Yokobayashi & Watanabe, 2005). Although eso1-H17 cells show intact biorientation of sister kinetochores in mitosis at the permissive temperature (supplementary Fig S4 online), mono-orientation of sister kinetochores is entirely abolished in meiosis I at the same temperature (Fig 2B). These results indicate that the acetylation of cohesin is crucial for mono-orientation at meiosis I, and might be the target for regulation of kinetochore orientation. We next examined whether acetylation of Psm3 is required for mono-orientation. Accordingly, mono-orientation is largely abolished in psm3-KKRR cells, but preserved intact in psm3-KKQQ cells (Fig 2B).
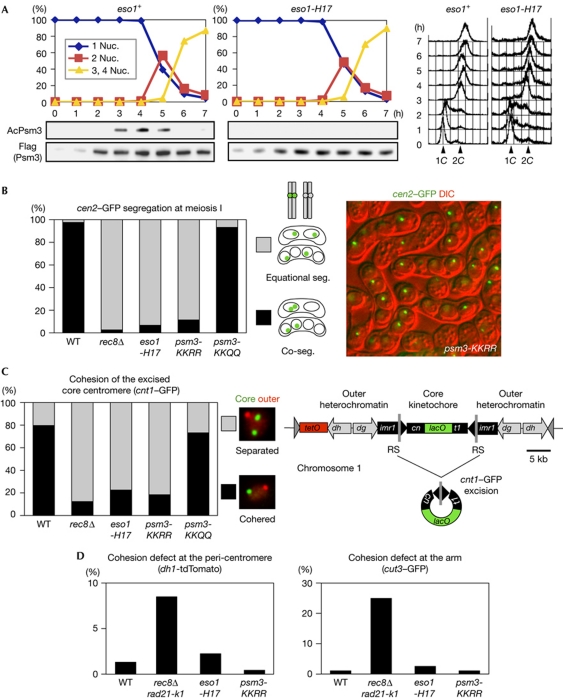
Figure 2.
Eso1-dependent acetylation of Psm3-K105/K106 is required for mono-orientation at meiosis I. (A) Haploid wild-type (WT) and eso1-H17 cells were induced to undergo synchronous meiosis by inactivating pat1-114 (Iino & Yamamoto, 1985). Cells were fixed and stained with 4,6-diamidino-2-phenylindole to monitor meiotic nuclear divisions. DNA content was measured by flow cytometry. Acetylation status of Psm3 was analysed by immunoblot. (B) Segregation (seg.) of heterozygous cen2-GFP at meiosis I was examined in the indicated rec12Δ zygotes at 26°C (n>230). Representative picture showing the cen2–GFP segregation patterns in psm3-KKRR cells. (C) Cohesion of the excised core centromere 1 in prophase I was examined in the indicated zygotes at 30°C (n>69). Schematic representation of centromere 1, in which a central core (cnt1) is surrounded by innermost repeats (imr1) and outer repeats (dg and dh), and its engineered version designed for excision (Sakuno et al, 2009). The split of cnt1 by an insertion is designated cn and t1. (D) Cohesion defect at pericentromeric and arm regions was examined in the indicated cells by arresting at prophase I (by the mei4Δ mutation) at 26°C. Strains marked with dh1-tdTomato or cut3–GFP were crossed with unmarked strains and the number of cells with two dot signals in a horsetail nucleus was counted (n>150). We used rec8Δ rad21-K1 as a cohesion defective control (Yokobayashi et al, 2003). DIC, differential interference contrast; nuc., nucleus.
We next examined whether the acetylation of Psm3 by Eso1 is indeed crucial for cohesion at the core centromere, the canonical mono-orientation pathway in fission yeast. To monitor this cohesion, we popped out the core centromere from the chromosome during prophase I and visualized the cohesion of the excised centromeres (Sakuno et al, 2009; Fig 2C). The results indicate that cohesion at the core centromere is abolished in psm3-KKRR cells or eso1-H17 cells as in rec8Δ cells, whereas it remains intact in psm3-KKQQ cells (Fig 2C). Chromatin immunoprecipitation assays indicate that the cohesion defect at the core centromere is not caused by displacement of Rec8 or Moa1 (supplementary Fig S5 online), suggesting that cohesion establishment itself is impaired in psm3-KKRR or eso1-H17 cells. By observing centromere-marked dh1-tdTomato in meiotic prophase, we confirmed that the cohesion at pericentromeres is preserved in psm3-KKRR or eso1-H17 cells (Fig 2D), consistent with the fact that sister chromatids segregate equationally rather than randomly in these cells (Fig 2B). Moreover, arm cohesion also remained largely intact during prophase in psm3-KKRR or eso1-H17 cells (Fig 2D). We conclude that the requirement of Psm3 acetylation for sister chromatid cohesion is most pronounced at the core centromere, which is essential for the mono-orientation of kinetochores at meiosis I.
Eso1 and Moa1 act at different stages in prophase I
It is unknown how the meiosis-specific kinetochore protein Moa1 contributes to core centromere cohesion (Yokobayashi & Watanabe, 2005; Sakuno et al, 2009). Considering the fact that acetylation of cohesin might be involved in this regulation, we next examined the functional relevance of Moa1 to the acetylation pathway. The Eso1 acetyltransferase is expressed throughout meiosis (Fig 3A), whereas acetylation of cohesin is detected during prophase I (Fig 2A, 3–5 h). In contrast, Moa1 is induced during late S phase (Yokobayashi & Watanabe, 2005; also see Fig 3C). By expressing eso1+ using the moa1+ promoter (Pmoa1), we could eliminate Eso1 expression during meiotic G1–S phase (Fig 3A, 0–3 h). We then expressed this Pmoa1–eso1+ allele in eso1-H17 cells, in which mono-orientation is intrinsically abolished. Consequently, the Pmoa1–eso1+ allele failed to restore the full level of mono-orientation in eso1-H17 cells, whereas the control Peso1–eso1+ allele fully restored mono-orientation (Fig 3B). These results indicate that the acetylation of cohesin by Eso1, which occurs mainly at the late S phase (Fig 2A), is important in establishing cohesion, at least, at the core centromere. Partial activity of the Pmoa1–eso1+ allele indicates that Eso1 was not completely abolished during S phase even in this allele, or that Eso1 could function also after S phase (see below).
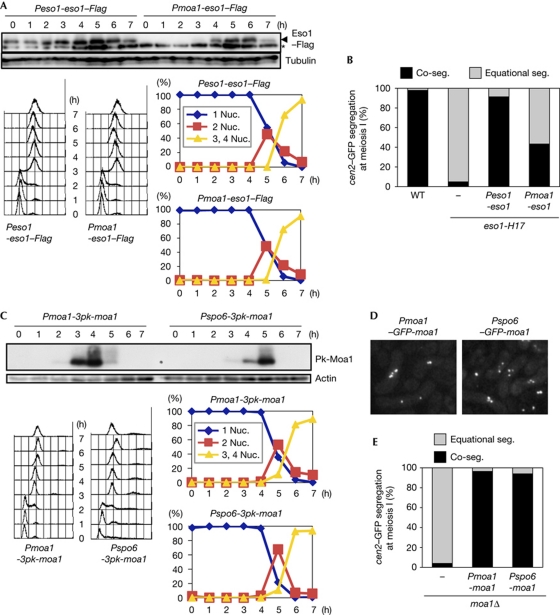
Figure 3.
Eso1–Psm3 acetylation functions during premeiotic S phase, whereas Moa1 functions after DNA replication. (A) Immunoblotting of Eso1–Flag using anti-FLAG antibody (top). The asterisk indicates a nonspecific band. The indicated cells were induced to undergo synchronous meiosis by pat1-114 inactivation. The progression of meiosis was monitored by measuring DNA content by flow cytometry (left bottom) and the number of nuclei (right bottom). (B) Segregation (seg.) of heterozygous cen2–GFP at meiosis I was examined in the indicated rec12Δ zygotes at 26°C (n>150). (C) Immunoblotting of 3Pk-Moa1 using anti-Pk antibody (top). The indicated cells were induced to undergo synchronous meiosis and examined as in A. (D) Representative pictures showing GFP–Moa1 localization at meiosis I in the indicated strains. (E) Segregation of heterozygous cen2–GFP at meiosis I was examined in the indicated rec12Δ zygotes at 30°C (n>150). Nuc., nucleus; WT, wild type.
Moa1 is produced during premeiotic S phase and loaded to kinetochores depending on DNA replication (Yokobayashi & Watanabe, 2005; Fig 3C). To examine whether Moa1 loading needs to be coupled to DNA replication for its function, we replaced the promoter of moa1+ with that of spo6+, which is activated long after premeiotic DNA replication but before meiosis I division (Watanabe et al, 2001). Indeed, the engineered Pspo6–moa1+ allele produced a physiological level of Moa1 protein after DNA replication (Fig 3C). Nevertheless, Moa1 localized properly at kinetochores before meiosis I division (Fig 3D), and perfectly fulfilled its function (Fig 3E). These results indicate that Moa1 works only after DNA replication in the ‘maintenance’ of cohesion, rather than in its ‘establishment’, which couples with replication-dependent cohesin acetylation by Eso1.
The Clr6 deacetylase antagonizes Eso1 in meiosis
We next examined the contribution of cohesin deacetylation to mono-orientation by testing the genetic interaction between clr6-1 and eso1-H17 mutations during meiosis. The mono-orientation defect in eso1-H17 cells was substantially (∼50%) suppressed by clr6-1 (Fig 4A). By performing the centromere excision assay, we confirmed that this suppression was the result of the recovery of cohesion at the core centromere (Fig 4B). Moreover, the mono-orientation defect in eso1-H17 cells was largely suppressed by psm3-KKQQ (∼95%) and again this suppression was mediated by the restored cohesion at the core centromere (Fig 4A,B). Because psm3-KKRR cells show complete impairment in mono-orientation as in eso1-H17 cells (Fig 2B), these results indicate that the defect of eso1-H17 in mono-orientation originates from the inability of Psm3 to be acetylated. To investigate whether the target of eso1-H17 suppression by clr6-1 is limited to Psm3 deacetylation, we examined the suppression in a psm3-KKRR background. clr6-1 suppressed eso1-H17 even in psm3-KKRR cells, albeit less efficiently than in psm3+ cells (Fig 4A). Thus, Clr6 antagonizes the Eso1 function in establishing mono-orientation by deacetylating not only Psm3 but also an unknown Eso1 target (see below).
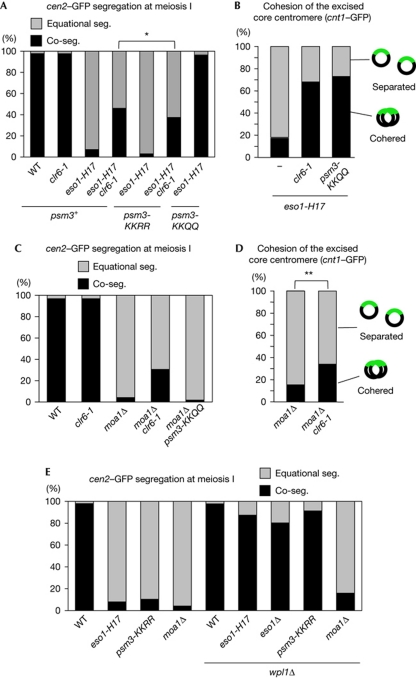
Figure 4.
The clr6-1 mutation suppresses the mono-orientation defect in eso1-H17 and moa1Δ cells. (A,C,E) Segregation (seg.) of heterozygous cen2-GFP at meiosis I was examined in the indicated rec12Δ zygotes (n>150). (B,D) Cohesion of the excised core centromere 1 in prophase I was examined in the indicated zygotes (n>79). Experiments in A, B and E were performed at 26°C, whereas those in C and D were performed at 30°C. *P<0.05; **P<0.01 (χ2-test). WT, wild type.
Acetylation in Moa1-dependent mono-orientation
Because of the crucial importance of the Psm3 acetylation for mono-orientation, we wondered whether Moa1 might be required to sustain the Psm3 acetylation. To examine this possibility, we introduced the psm3-KKQQ mutation into moa1Δ cells and monitored chromosome segregation at meiosis I. In contrast to eso1-H17 cells, the mono-orientation defect in moa1Δ cells was not suppressed at all by psm3-KKQQ. Strikingly, however, the moa1Δ defect was suppressed partially (∼30%) by clr6-1 (Fig 4C). The independence of this suppression and Psm3 acetylation was further validated by showing that clr6-1 could suppress moa1Δ even in the background of psm3-KKRR (supplementary Fig S6 online). The centromere excision assay confirmed that the mono-orientation restored by clr6-1 is mediated by the canonical cohesion pathway rather than another nonspecific effect on the kinetochores or spindle (Fig 4D). Consistently, suppression by clr6-1 is not observed in rec8Δ cells (supplementary Fig S6 online). These results indicate that an acetylation target(s) other than Psm3-K105/K106 contributes to cohesion at the core centromere at meiosis I, and that this acetylation might be decreased in moa1Δ cells by the action of Clr6 (Fig 5). Because eso1Δ is not completely suppressed by psm3-KKQQ in meiosis (supplementary Fig S7 online), Eso1 might be responsible for the acetylation of this non-Psm3-K105/K106 substrate (Fig 5). Accordingly, clr6-1 does not restore mono-orientation in eso1Δ psm3-KKQQ moa1Δ cells, whereas it suppresses eso1+psm3-KKQQ moa1Δ cells (supplementary Fig S8 online), indicating that the acetylation of the non-Psm3-K105/K106 substrate counteracted by clr6-1 in a moa1Δ background is indeed dependent on Eso1. Overall, these results imply that the antagonistic interaction between Eso1 and Clr6 in regulating cohesion is much more obvious in meiosis than in mitosis; the meiotic situation surrounding Eso1 and acetylation targets is reminiscent of that of mitosis (Figs 1A and 5).
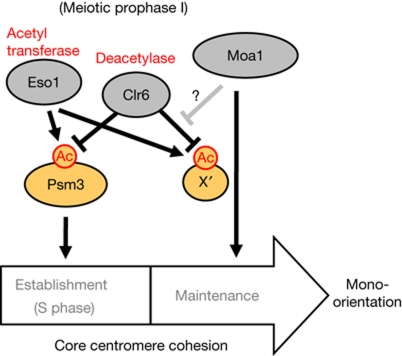
Figure 5.
Schematic depiction of the regulation of core centromere cohesion by acetylation during meiotic prophase I. X′ represents a putative acetylated protein required for core centromere cohesion. Moa1 might function to maintain the acetylation of X′ partly by antagonizing Clr6 and/or might function in parallel with this acetylation to maintain the cohesion.
It has been shown that the requirement of cohesin acetylation is counteracted by the Wapl protein (Ben-Shahar et al, 2008; Rowland et al, 2009; Sutani et al, 2009; Uhlmann, 2009; Feytout et al, 2011). We then examined whether deletion of the wpl1 gene (encoding Wapl homolog) can suppress the mono-orientation defect in Psm3 acetylation-defective or moa1Δ cells. Although the mono-orientation defect in eso1-H17, eso1Δ and psm3-KKRR cells was largely suppressed by wpl1Δ, that of moa1Δ cells was hardly suppressed (Fig 4E). These results reaffirm that cohesion defects in moa1Δ do not originate through Psm3 acetylation.
In summary, our results indicate that cohesion at the core centromere is established primarily by the Eso1-dependent acetylation of Psm3, but that the maintenance of this cohesion requires an additional acetylation of unidentified substrate(s) that can be antagonized by deacetylase Clr6 but might be protected by the meiosis-specific kinetochore factor Moa1 (Fig 5). Although further studies are required to validate this model, our study suggests that protein acetylation is an important target for the regulation of kinetochore orientation during meiosis I.
Methods
Yeast strains, plasmids and antibodies. Plasmids and antibodies are described in the supplementary information online; the genotypes of the strains are listed in supplementary Table S1 online. Methods used to generate the strains are described in supplementary methods online.
Medium. Complete medium yeast extract or minimal medium (MM) was used for the culture of Schizosaccharomyces pombe strains. Sporulation agar plate was used to induce meiosis and for microscopic examination. MM and MM-N (lacking nitrogen) containing 1% glucose were used for synchronous meiosis and for expressing clr6+ from the pREP1 plasmid.
Flow cytometry. Methanol-fixed cells were washed twice with 50 mM Na-citrate (pH 7.0) and treated with 100 μg/ml RNaseA for 3 h at 37°C. Cells were incubated with 10 μg/ml propidium iodide for 1 h at 4°C, and then analysed by FACS Calibur (BD Biosciences) after brief sonication.
Detection of Psm3 acetylation. To analyse the acetylation status of Psm3, anti-AcPsm3 antibodies were generated using acetylated peptides (TIGLK(AcK)DEY) spanning the Psm3 acetylation site at amino acid 106 of S. pombe. The Flag-tagged Psm3 was immunoprecipitated from cell extracts using anti-Flag M2 monoclonal antibody-conjugated agarose (Sigma), and analysed by immunoblot probed with anti-Flag M2 (Sigma) and anti-AcPsm3 antibodies.
Protein preparation and immunobloting. Cells were boiled in homogenization buffer for 5 min and then disrupted with glass beads using a Multi-bead shocker (Yasui Kikai). Total cell extracts were boiled in SDS-sample buffer and subjected to immunoblotting. The following antibodies were used: mouse monoclonal antibodies against Flag (1:1,000, M2; Sigma), Pk (1:1,000, MCA1360; Serotec) and TAT-1 (1:5,000), and goat polyclonal antibodies against actin (1:1,000, Santa Cruz).
Assay for sister chromatid cohesion at the core centromere. Cohesion of the excised core centromere 1 in prophase I (arrested by mei4Δ) was examined as previously described (Sakuno et al, 2009).
Chromosome segregation assays. To observe sister chromatid segregation during meiosis I, we cultured opposite mating-type cells, one marked with green fluorescent protein or tdTomato and the other unmarked, and mixed them before spotting them onto sporulation agar plate.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank Silke Hauf for critically reading the manuscript, the Yeast Genetic Resource Centre for yeast strains and all the members of our laboratory for their valuable support and discussion. This work was supported in part by the Japan Society for the Promotion of Science Research Fellowships (to A.K. and Y.Y.), Special Coordination Funds for Promoting Science and Technology (to T.S.), a Grant-in-Aid for Scientific Research on Priority Areas (to K.T. and T.S.), the Global COE Program and a Grant-in-Aid for Specially Promoted Research (to Y.W.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Footnotes
The authors declare that they have no conflict of interest.
References
- Beckouet F, Hu B, Roig MB, Sutani T, Komata M, Uluocak P, Katis VL, Shirahige K, Nasmyth K (2010) An Smc3 acetylation cycle is essential for establishment of sister chromatid cohesion. Mol Cell 39: 689–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar TR, Heeger S, Lehane C, East P, Flynn H, Skehel M, Uhlmann F (2008) Eco1-dependent cohesin acetylation during establishment of sister chromatid cohesion. Science 321: 563–566 [DOI] [PubMed] [Google Scholar]
- Borges V, Lehane C, Lopez-Serra L, Flynn H, Skehel M, Rolef Ben-Shahar T, Uhlmann F (2010) Hos1 deacetylates Smc3 to close the cohesin acetylation cycle. Mol Cell 39: 677–688 [DOI] [PubMed] [Google Scholar]
- Ekwall K (2005) Genome-wide analysis of HDAC function. Trends Genet 21: 608–615 [DOI] [PubMed] [Google Scholar]
- Feytout A, Vaur S, Genier S, Vazquez S, Javerzat JP (2011) Psm3 acetylation on conserved lysine residues is dispensable for viability in fission yeast but contributes to Eso1-mediated sister-chromatid cohesion by antagonizing Wpl1. Mol Cell Biol 31: 1771–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal SI, Bonaduce MJ, Klar AJ (1998) Histone deacetylase homologs regulate epigenetic inheritance of transcriptional silencing and chromosome segregation in fission yeast. Genetics 150: 563–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino Y, Yamamoto M (1985) Negative control for the initiation of meiosis in Schizosaccharomyces pombe. Proc Natl Acad Sci 82: 2447–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K, Haering CH (2009) Cohesin: its roles and mechanisms. Annu Rev Genet 43: 525–558 [DOI] [PubMed] [Google Scholar]
- Onn I, Heidinger-Pauli JM, Guacci V, Unal E, Koshland DE (2008) Sister chromatid cohesion: a simple concept with a complex reality. Annu Rev Cell Dev Biol 24: 105–129 [DOI] [PubMed] [Google Scholar]
- Peters JM, Tedeschi A, Schmitz J (2008) The cohesin complex and its roles in chromosome biology. Genes Dev 22: 3089–3114 [DOI] [PubMed] [Google Scholar]
- Rowland BD et al. (2009) Building sister chromatid cohesion: smc3 acetylation counteracts an antiestablishment activity. Mol Cell 33: 763–774 [DOI] [PubMed] [Google Scholar]
- Sakuno T, Tada K, Watanabe Y (2009) Kinetochore geometry defined by cohesion within the centromere. Nature 458: 852–858 [DOI] [PubMed] [Google Scholar]
- Sutani T, Kawaguchi T, Kanno R, Itoh T, Shirahige K (2009) Budding yeast Wpl1(Rad61)-Pds5 complex counteracts sister chromatid cohesion-establishing reaction. Curr Biol 19: 492–497 [DOI] [PubMed] [Google Scholar]
- Uhlmann F (2009) A matter of choice: the establishment of sister chromatid cohesion. EMBO Rep 10: 1095–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal E, Heidinger-Pauli JM, Kim W, Guacci V, Onn I, Gygi SP, Koshland DE (2008) A molecular determinant for the establishment of sister chromatid cohesion. Science 321: 566–569 [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Yokobayashi S, Yamamoto M, Nurse P (2001) Pre-meiotic S phase is linked to reductional chromosome segregation and recombination. Nature 409: 359–363 [DOI] [PubMed] [Google Scholar]
- Xiong B, Lu S, Gerton JL (2010) Hos1 is a lysine deacetylase for the smc3 subunit of cohesin. Curr Biol 20: 1660–1665 [DOI] [PubMed] [Google Scholar]
- Yokobayashi S, Watanabe Y (2005) The kinetochore protein Moa1 enables cohesion-mediated monopolar attachment at meiosis I. Cell 123: 803–817 [DOI] [PubMed] [Google Scholar]
- Yokobayashi S, Yamamoto M, Watanabe Y (2003) Cohesins determine the attachment manner of kinetochores to spindle microtubules at meiosis I in fission yeast. Mol Cell Biol 23: 3965–3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J et al. (2008) Acetylation of Smc3 by Eco1 is required for S phase sister chromatid cohesion in both human and yeast. Mol Cell 31: 143–151 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.